Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2380
Peer-review started: September 18, 2022
First decision: November 26, 2022
Revised: January 26, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 28, 2023
Processing time: 218 Days and 11.1 Hours
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease with complex causes. The main pathological changes were intestinal mucosal injury. Leucine-rich repeat-containing G protein coupled receptor 5 (LGR5)-labeled small intestine stem cells (ISCs) were located at the bottom of the small intestine recess and inlaid among Paneth cells. LGR5+ small ISCs are active proliferative adult stem cells, and their self-renewal, proliferation and differentiation disorders are closely related to the occurrence of intestinal inflammatory diseases. The Notch signaling pathway and Wnt/β-catenin signaling pathway are important regulators of LGR5-positive ISCs and together maintain the function of LGR5-positive ISCs. More importantly, the surviving stem cells after intestinal mucosal injury accelerate division, restore the number of stem cells, multiply and differentiate into mature intestinal epithelial cells, and repair the damaged intestinal mucosa. Therefore, in-depth study of multiple pathways and transplantation of LGR5-positive ISCs may become a new target for the treatment of UC.
Core Tip: Intestinal mucosal injury is an important pathological change in ulcerative colitis (UC), and Leucine-rich repeat-containing G protein coupled receptor 5 (LGR5)-positive intestinal stem cells play an important role in the repair of intestinal mucosal injury. Through in-depth study of multiple signals, LGR5-positive intestine stem cell transplantation therapy may become an important means to treat UC.
- Citation: Zheng L, Duan SL. Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis. World J Gastroenterol 2023; 29(16): 2380-2396
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2380.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2380
Ulcerative colitis (UC) is a chronic, relapsing inflammatory disease of the intestinal tract[1]. The course of the disease is prolonged and often brings heavy physiological, psychological and economic burdens to patients. Clinical remission based on symptom improvement does not alter the natural course of UC, and mucosal healing has been the primary therapeutic target of UC in recent years[2]. However, studies have shown that up to 40% of patients who achieve clinical and endoscopic remission still have persistent histological inflammation, which is associated with a higher risk of clinical recurrence of UC, receiving colectomy, and dysplasia[3].
Intestinal stem cells (ISCs) are important adult stem cells that drive the daily renewal of the intestinal epithelium through constant self-renewal, proliferation, and differentiation. ISCs are mainly located in intestinal recesses and play an important role in the repair of damaged intestinal mucosa[4]. In mammals, the gut consists of small villi that extend into the gut cavity and small intestine crypts that sink deep into the lining of the intestine. Leucine-rich repeat-containing G protein coupled receptor 5 (LGR5) is an important marker of ISCs[5]. Under the action of multiple signaling pathways in the body, LGR5-positive ISCs repair damaged intestinal mucosa and maintain intestinal homeostasis through self-renewal and differentiation potential. However, the internal mechanism of how multiple different signaling pathways interact with each other to synergistically regulate LGR5 cells with differentiation potential in UC remains unclear[6]. In this paper, the concept, location, quantity and cycle of ISCs, the repair mechanism of intestinal mucosa by ISCs, the renewal of colon epithelial cells and the regulation of nutritional molecules in damage repair were reviewed to further provide evidence-based medical evidence for the treatment objectives of UC.
Stem cells have the capacity for lifelong self-renewal. They are cells that can produce a variety of highly differentiated progeny and can respond differently to changes in their internal environment[7]. Morphologically, the cells at the bottom were counted as "one" in the longitudinal section of the lacunae. The ISCs were approximately located at the fourth layer of cells but fluctuated between the second layer and the seventh layer[8]. Stem cells have three levels of structure, each with different properties and functions. Stem cells have a long cycle. In general, stem cells undergo asymmetric division, but during development or after injury, they undergo symmetrical division and divide into two progeny stem cells to increase the number of stem cells. Normally, the excess stem cells produced by symmetrical division are eliminated by apoptosis or rapid differentiation[9]. After some lacunae die after toxic injury, such as radiation or chemotherapy, the remaining potential stem cells begin to exercise their stem cell potential and undergo symmetrical division to regenerate lacunae[10]. The lacunae also divide to produce more lacunae until the intestinal mucosa returns to normal.
Intestinal epithelial tissue is one of the most active self-renewing tissues in adult mammals. Intestinal epithelial cells renew every 5 d, and this process mainly depends on the continuous division and replenishment of ISCs. ISCs are a type of adult stem cell that are mainly distributed in the recesses of the intestine in mammals[11]. ISCs have asymmetric division, self-renewal, and pluripotency; that is, they proliferate and differentiate into a variety of cell types, including absorbent cells, goblet cells, intestinal endocrine cells, and Pan's cells. Each crypt of the intestinal mucosa contains 4 to 6 independent ISCs[12]. Morphologically, the count begins with cells at the base of the crypt, and the ISCs are located in the fourth layer of the crypt, where the stem cells have a very active cell cycle. ISCs first differentiate into transient extender cells, which are daughter cells with limited ability to divide and circulate[13]. The transient expansion cells settled at the base of the crypt for approximately 48 to 72 h, then gradually migrated upward, underwent approximately 6 rounds of cell division, and finally differentiated into terminal cells[14]. Studies have shown that small intestine recess stem cells can rapidly differentiate and repair damage in a small intestine radiation injury model under the action of insulin-like growth factor and hepatocyte growth factor[15]. Some scholars studied Drosophila intestinal mucosal damage induced by sodium glucan sulfate and found that the damaged intestine could secrete signaling proteins to accelerate the division of ISCs to promote mucosal repair[16].
Each gut stem cell is coated with special protein receptors that selectively bind to or adhere to other "signaling" molecules. These cell surface receptors are known as stem cell markers. Currently, Musasi-1, telomerase reverse transcriptase (TERT) and ID14 are the main markers found in ISCs[17]. Musas-1 is a neural RNA-binding protein but has been shown to be a selective marker of ISCs in addition to the nervous system[18]. Some studies found that Musashi-1-positive cells were found in the small intestine of mice, and Musashi-1 was significantly increased in intestinal specimens of mice after reflex injury[19]. TERT is a ribonuclear protease complex. Studies have shown that immunohistochemical TERT-positive cells are mainly distributed in the base of the small intestine crypt, 4-7 cells away from the bottom of the crypt, and some cells are distributed in the interstitium surrounding the crypt[20]. ID14, a new gene found in Xenopus laevis, encodes a protein containing 315 amino acids[21]. Adult ID14 is mostly found in the intestine but is only weakly expressed in the stomach, lung and testis. Its expression in the intestine does not begin until the metamorphosis stage, which is closely related to the differentiation of adult intestinal epithelial cells[22].
ISCs continuously increase the number of stem cells through asymmetric division to promote the self-renewal and repair of damaged intestinal tissues to maintain the dynamic balance of the intestinal mucosa[23]. Stem cells divide asymmetrically to form a daughter cell identical to the mother cell and a daughter cell capable of differentiation[24]. During this process of division, the stem cell DNA double strand tends to enter daughter cells that are identical to the mother cell so that the daughter cells that maintain the characteristics of the stem cell retain the mother strand DNA, thus maintaining the stability of the gene[25].
Intestinal activity is innervated by the sympathetic, parasympathetic, and enteric nervous systems. The sympathetic and parasympathetic plexuses can promote the proliferation and regeneration of intestinal mucosal epithelial cells and accelerate the division of crypt cells through growth factors and inflammatory mediators[26]. The enteric nervous system consists of the intermuscular plexus and submucosal plexus, and most of its neurons are located in the intestinal wall[27]. It has been observed that chemical resection of the intestinal intermuscular nerve plexus can accelerate the proliferation of ISCs, indicating that the intermuscular nerve plexus has an inhibitory effect on intestinal mucosal cell renewal[28].
The intestinal epithelium is a single layer of cell epithelium covering the intestinal lining. As an important organ in mammals, the intestinal epithelium is responsible for digestion, absorption and resistance to intestinal pathogenic microorganisms[29]. Structurally, the epithelium of the small intestine is composed of a large number of repeating units called crypt villi[30]. The intestinal villi are composed of multiple differentiated cells that penetrate into the intestinal cavity to perform digestive and absorption functions, and the base of each villus encloses multiple intestinal recesses, each containing proliferative ISCs[31]. To avoid cytopathies caused by constant contact with external stimuli in the intestinal cavity, the small intestine epithelium is constantly renewing itself, and most cells renew themselves every 4-5 d on average. In line with this physiological function, small ISCs located in crypts have the ability of lifelong self-renewal, making the small intestinal epithelium an important model for adult stem cell research[32].
In the small intestine recess, small ISCs divide every 24 h on average, generating transient amplifying cells (TA cells) while renewing themselves[33]. Fast proliferating cells have a cell division cycle of approximately 12 h, migrating up the recess while performing several fast divisions[34]. In the process of upward migration, the descendant cells gradually differentiated into two types of cells, namely, the secretory lineage and the absorptive lineage. Secretory cells mainly include Paneth cells, goblet cells, and enteroendocrine cells, while absorptive cells mainly refer to intestinal epithelial cells. In contrast to the goblets, intestinal secretory cells and intestinal epithelial cells, which continue to migrate upward into the villi to perform their functions and reach the apex of the villi and undergo apoptosis within 3 to 5 d, Paneth cells migrate downward to the base of the crypt and survive for 3 to 6 wk[35].
LGR5-labeled small ISCs not only mediate the normal self-renewal of the small intestine epithelium but also act as the initiation cells of inflammatory cells in the case of mutation, seriously affecting life and health[36]. Since the self-renewal and repair rate of the small intestine epithelium is very fast, the imbalance of its renewal regulation easily leads to epithelial damage. LGR5-labeled small ISCs mediate the daily renewal of the small intestine epithelium, so the relationship between LGR5+ small ISCs and inflammation has received extensive attention[37].
Studies have shown that overactivation of the Wnt signaling pathway induces the release of inflammatory cytokines. Consistent with this, the vast majority of patients with UC carry the inactivated adenomatosis polyposis coli (APC) gene mutation. Using a mouse model, specific knockout of the APC gene in LGR5-labeled small ISCs resulted in a massive release of inflammatory cytokines in the short term[38]. Further studies showed that LGR5-positive cells in UC patients consistently produced all other cell types throughout the tumor tissue while self-renewing, demonstrating the tumor stem cell properties of LGR5-labeled cells[39].
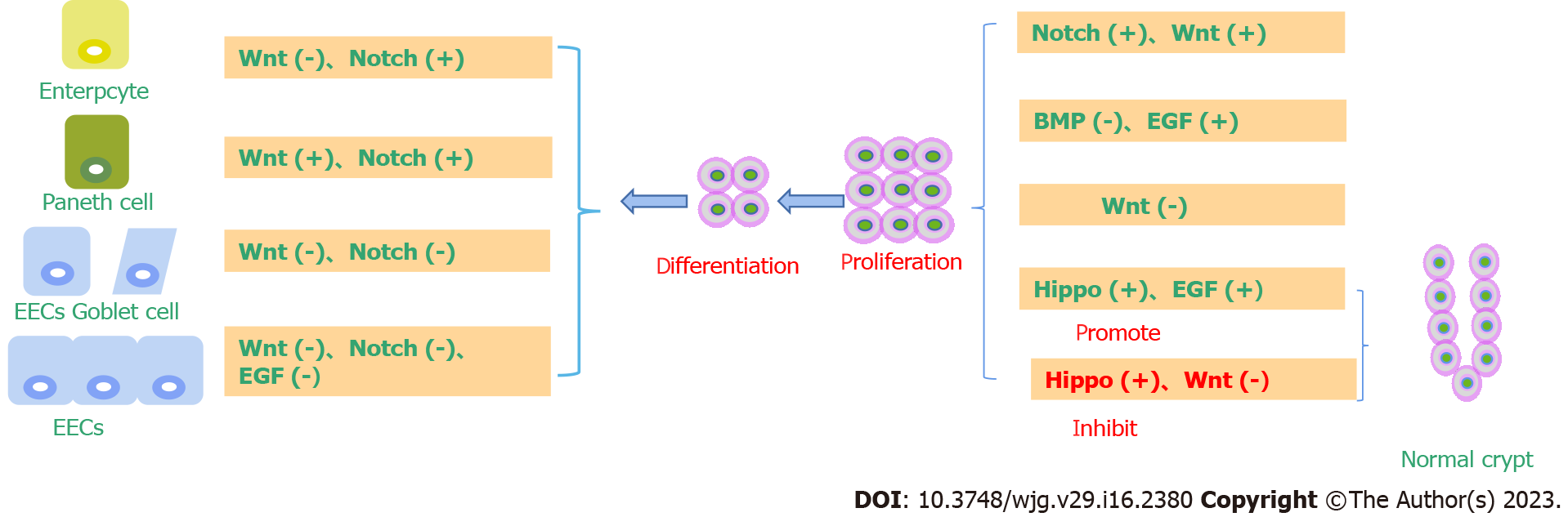
The microenvironment refers to the surrounding environment where stem cells are located under physiological conditions and is usually composed of stem cells themselves, surrounding cells and the extracellular matrix[40]. Cell-to-cell contact in the microenvironment and the existence of various growth factors in the microenvironment coregulate the self-renewal and differentiation of stem cells[41]. LGR5+ small ISCs live in a specific environment, namely, at the bottom of the small intestine recess, mosaic among Pan's cells[42]. Paneth cells, TA cells, and peripheral mesenchymal cells together constitute a unique microenvironment for small ISCs, in which a variety of cell pathways, including the Wnt, Notch, epidermal growth factor (EGF), and bone morphogenetic protein (BMP) signaling pathways, cooperate to regulate the proliferation and differentiation of intestinal epithelial cells and repair after injury[43].
The Wnt signaling pathway is a highly conserved signaling pathway that regulates cell proliferation, cell fate determination and cell differentiation and plays a crucial role in embryonic development and adult stem cell maintenance[44]. Mutations in the Wnt signaling pathway are closely related to the occurrence of many diseases, especially colorectal cancer.
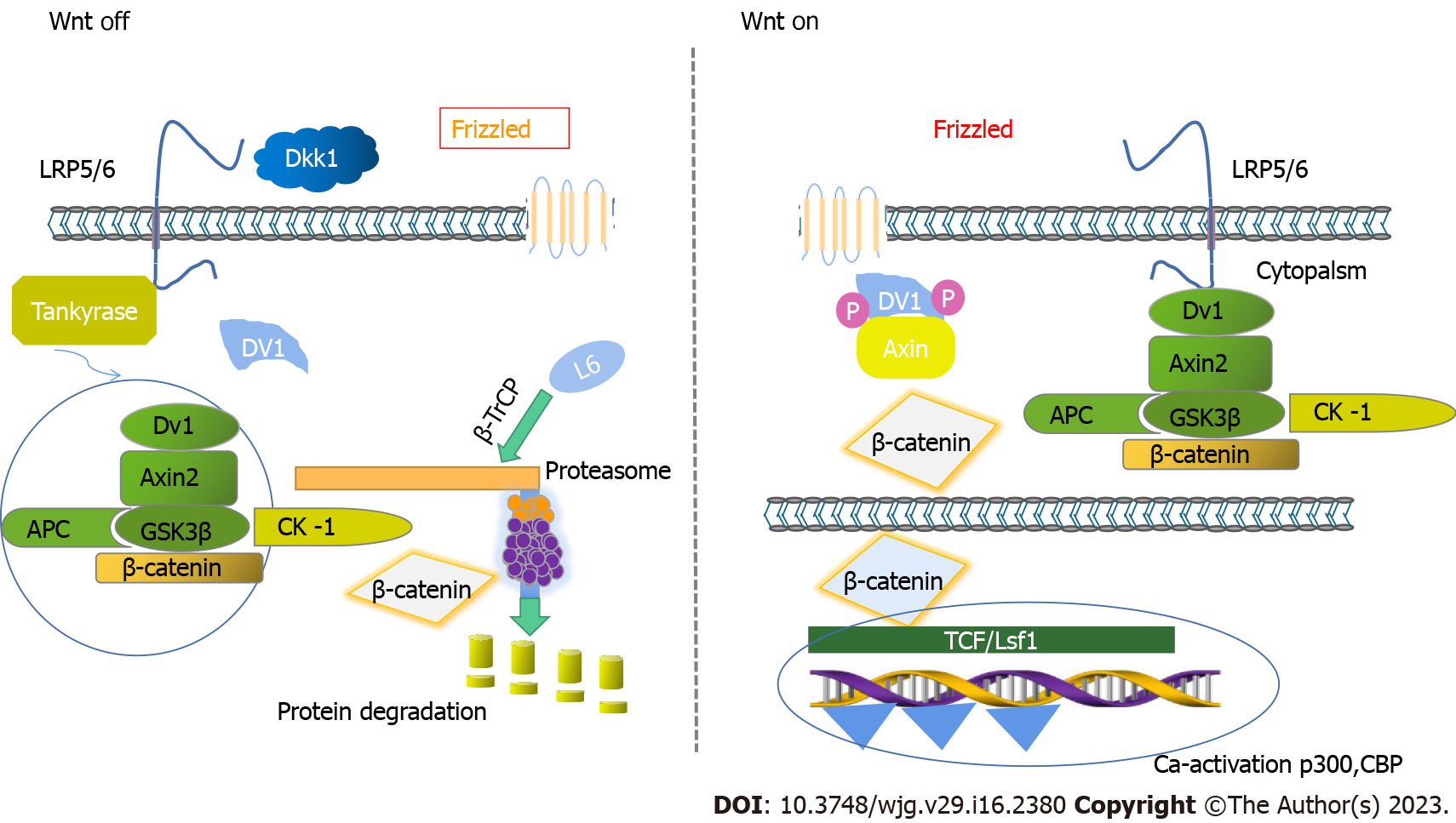
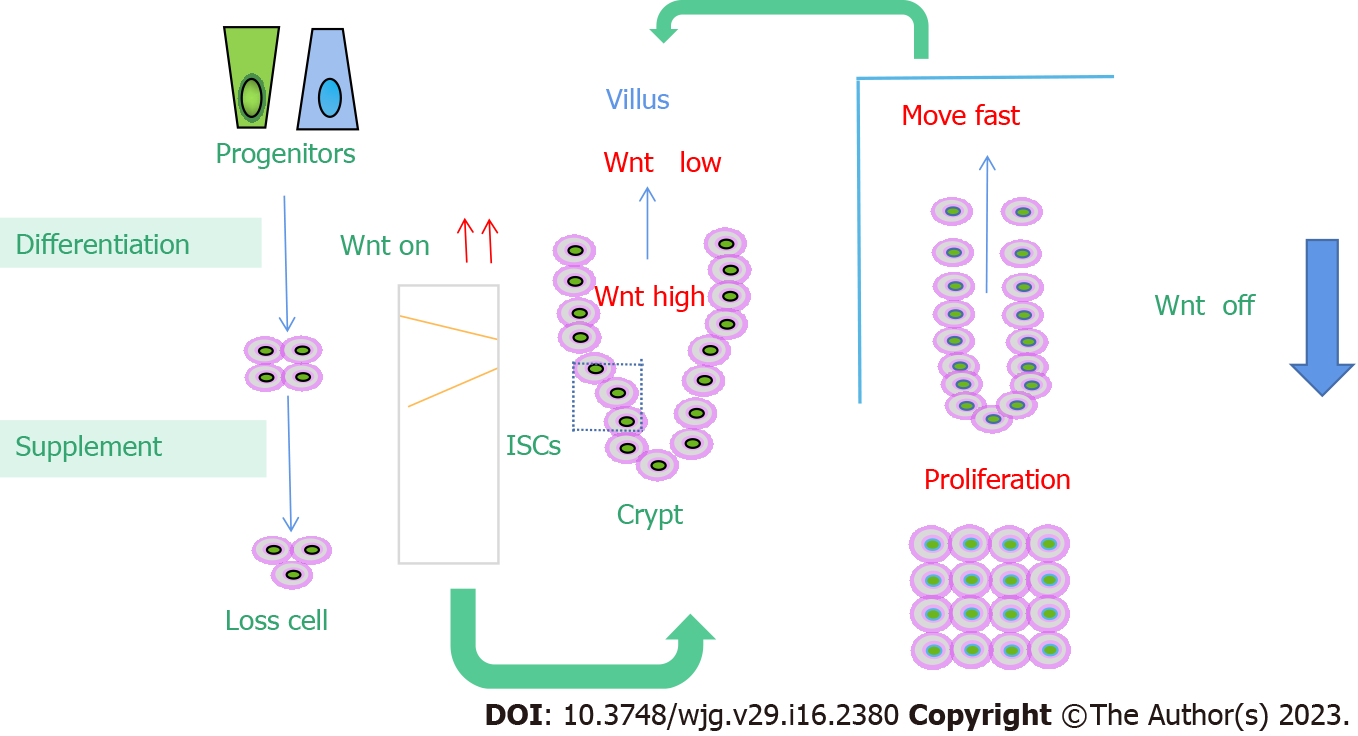
The Wnt signaling pathway plays a key role in the dry maintenance of small ISCs[45]. The first event that prompted the link of Wnt signaling to small ISCs was the discovery of a large number of APC gene mutations in colorectal cancer. As an important inhibitory factor of Wnt signaling, APC plays an important role in regulating Wnt signal strength. Mutation of the APC gene leads to overactivation of Wnt signaling[46]. Therefore, the overactivation of Wnt signaling may be closely related to the occurrence of colorectal cancer. In mouse models, APC gene mutation or deletion leads to the development of colorectal cancer. Both T cell factor 4 (TCF4) gene knockout and beta-catenin gene knockout will result in rapid loss of proliferative stem cell regions in the crypt[47]. All of this evidence suggests that activation of Wnt signaling promotes the dryness of small ISCs[48]. In line with this, Wnt signaling activity in the small intestinal epithelium decreased in a gradient along the crypt-villus axis, with the highest Wnt signaling activity at the base of the crypt[49]. The Wnt ligand is mainly secreted by Panzzled cells and peripheral mesenchymal cells at the base of the crypt. LGR5-labeled small ISCs actively express Frizzled receptors to transmit Wnt signals[50]. A series of target genes downstream of Wnt signaling mediate its physiological function. A large part of the abovementioned small ISC stem cells are direct target genes of Wnt signaling, including LGR5, achaete-scute family bHLH transcription factor 2, and Musashi-1. Other target genes of Wnt signaling, including Myc, play an important role in the occurrence of colorectal cancer[51] (Figure 1).
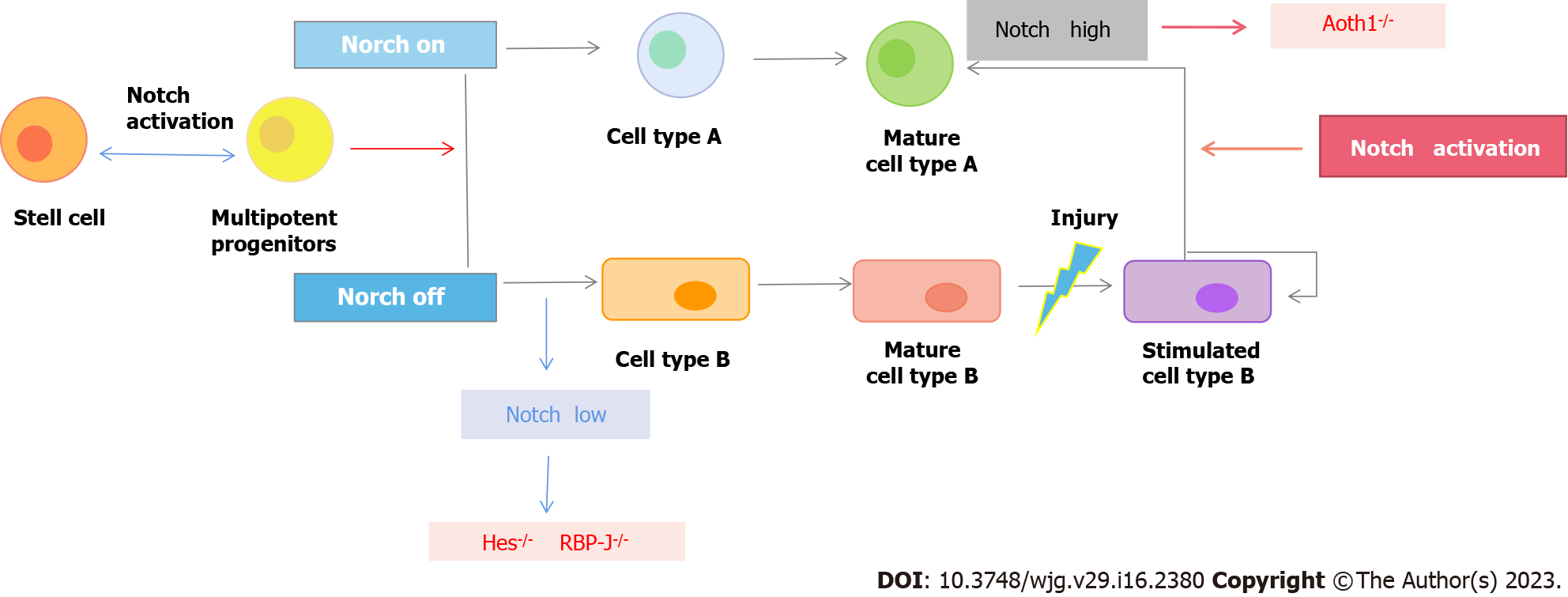
Notch signaling is a functionally conserved signaling family that exists widely in multicellular animals (metazoans). Notch signaling is mainly transmitted through cell-cell contact and plays an important role in physiological processes such as cell proliferation, stem cell maintenance, cell fate determination, differentiation and apoptosis[52].
Unlike most cellular pathway transduction processes, Notch signaling does not rely on a second messenger (secondary messengers). Posttranslational Notch protein is localized to the cell membrane as an active receptor after O-Fut-mediated glycosylation and PC5-mediated protease cleavage[53]. When ligands located near the cell membrane, such as Dll1, Dll4 and jagged1, bind to the Notch receptor, the Notch receptor is sequentially cleaved by ADAM and gamma-secretase-mediated protease[54]. The Notch receptor NICD (Noch intracellular domain) is released. Gamma-secretase-mediated protease cleavage may occur at the cell membrane or at the surface of endosome membranes containing NICDs, but NICDs produced by the latter usually enter the proteasome degradation pathway[55]. The released NICD is transferred into the nucleus, where it interacts with the DNA binding protein CSL (an acronym for C BF-1/RBPJ-κ in Homo sapiens / Mus musculus respectively, S uppressor of Hairless in Drosophila melanogaster, L ag-1 in Caenorhabditis elegans) and recruits a transcriptional coactivator to activate the expression of downstream target genes[56].
The Notch receptor is a single transmembrane protein that mainly includes Notch1, Notch2, Notch3 and Notch4 members in mammals. The Notch receptor extracellular end contains 29 to 36 EGF-like repeats, which may mediate Notch receptor and ligand interactions[57]. In mammals, Notch signaling ligands also contain multiple members, including Jagged1, Jagged2, Dll1, Dll3 and Dll4. The interaction of multiple ligands with multiple receptors increases the complexity of the Notch signaling pathway[58] (Figure 2).
BMP is a transforming growth factor (TGF-β). TGF-β is an important member of the TGF-β superfamily[59]. By regulating the activity of downstream genes, they play an important role in mesoderm formation, nervous system differentiation, bone development and cancer occurrence. BMP signal transmission occurs mainly through the specific binding of BMP protein to the BMP receptor (BMPR) on the cell membrane. Meanwhile, regulated Smads (R-SMAD) are regulated by activated type I receptors (BMPR1), which detach Smad molecules from cell membrane receptors[60]. After binding Smad4 [Common-mediator Smad (Co-SMAD)] in the cytoplasm, it enters the nucleus and coregulates the transcription of target genes with the participation of other DNA-binding proteins[61].
In contrast to Wnt signaling activity, BMP signaling activity increased gradually along the crypt-villus axis. In the small intestine, BMP ligands, including BMP2 and BMP4, are mainly secreted by mesenchymal cells around the crypt and inside the villi, while the BMP receptor Bmpr1a is expressed throughout the small intestine epithelium. Because peripheral mesenchymal cells also secrete BMP ligand inhibitors, including Noggin and Gremlin1, the BMP signal intensity in the crypt is low[62].
The Hedgehog (Hh) signaling pathway is essential for embryonic development and cell growth and differentiation after embryogenesis[63]. Among mammals are three Hh family members: Sonic Hh, Indian Hh (Ihh), and Desert Hh. Ihh is the main Hh protein expressed in the intestine. It acts on mesenchymal cells through paracrine signaling by differentiated epithelial cells and negatively regulates the proliferation of crypt columnar cells by increasing BMP signals[64]. In addition, Ihh inhibits the lamina propria immune response. Without causing any damage to the upper cortex, Ihh knockout activates an immune response similar to the wage-healing response, epithelial remodeling, and recruitment of fibroblasts and macrophages[65]. Therefore, the decreased expression of Ihh caused by the injury or dysfunction of the upper cortex, thus triggering the damage repair of the interstitial cells, may be one of the main mechanisms of the wound healing response[66].
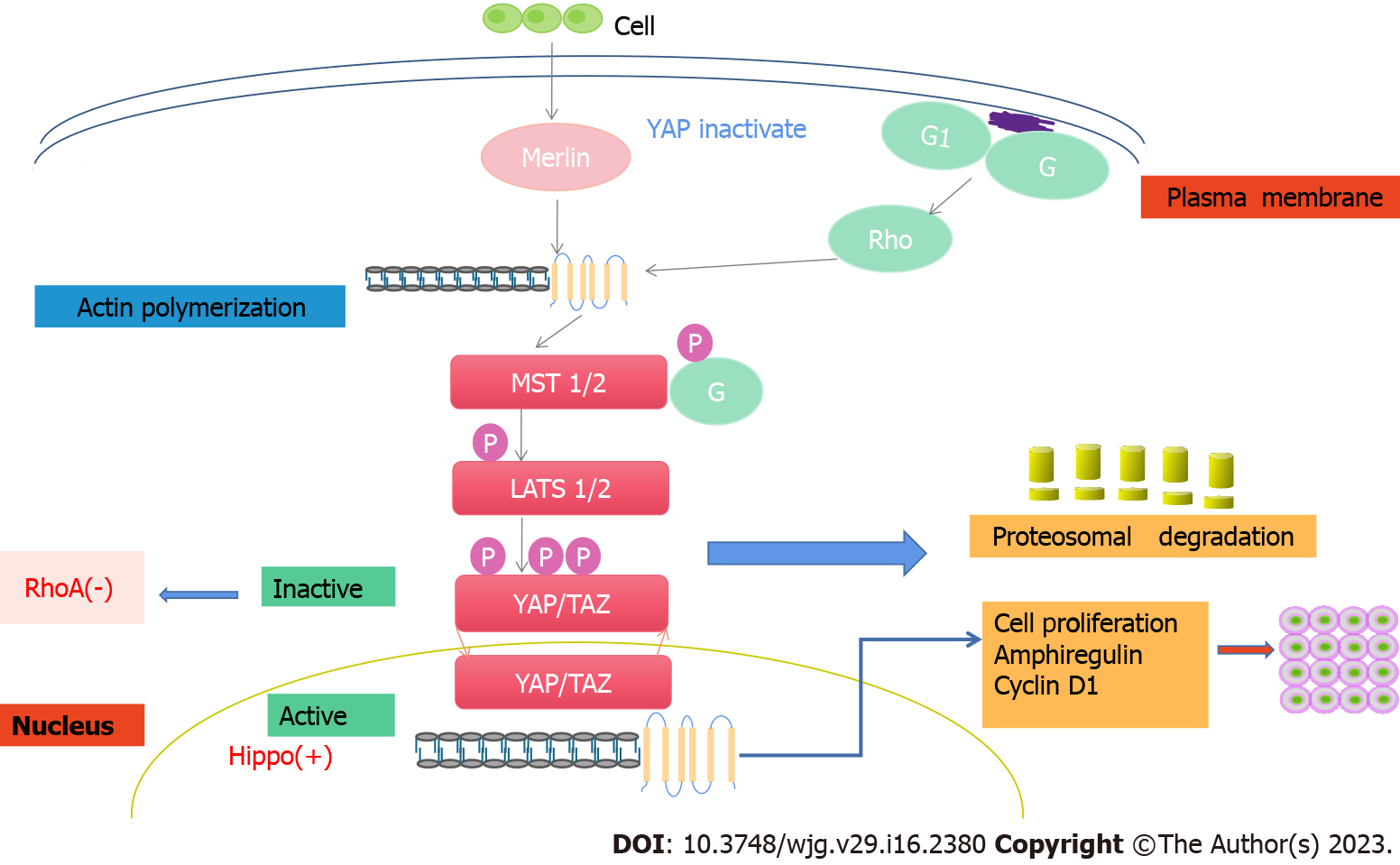
The Hippo signaling pathway is a newly discovered cell signal transduction pathway whose main part is a kinase chain. Among them, kinase MST1/2 (mammalian Sterile 20-like kinases 1/2) can phosphorylate and activate LATS1/2 (large tumor suppressor 1/2)[67]. LATS1/2 phosphorylates and inhibits the key kinase Yes-associated protein (YAP)/Tafazzin (TAZ). YAP/TAZ are two homologous transcription cofactors that mediate most of the physiological and pathological functions of Hippo signaling pathways[68]. YAP is expressed in both the small intestine and large intestine, with low expression in the small intestine but high expression in the colon, especially in the terminal colon. At the cellular level, YAP was localized in the cytoplasm in intestinal villi and upper crypt cells and in the nucleus in LGR5+ ISCs at the bottom of crypts and was expressed at low levels in Pan's cells, indicating that YAP activity was negatively correlated with the degree of differentiation of intestinal epithelial cells[69].
The Hippo signaling pathway plays an important role in regulating the differentiation of ISCs. The proliferative ability of mouse intestinal epithelial-specific YAP transgenic stem cells increased, while the differentiation ability decreased[70]. Consistently, MST1/2 knockout in the mouse gut promoted stem cell proliferation, accompanied by abnormal crypt cell differentiation and reduced goblet cells[71]. Some studies have found that LATS1/2 double knockout in the intestine promotes the proliferation of crypt cells, an increase in ISCs, and the differentiation of goblet cells[72]. Further study showed that YAP/TAZ could cooperate with Klf4 to promote the differentiation of crypt cells into goblet cells. Regarding the effect of Hippo signaling pathway inhibition on goblet cell differentiation, the results of the above two experiments were different. Some scholars believe that this is because the intestine-specific gene transfer method they used can mildly express YAP exogenically or inhibit MST1/2 and LATS1/2, so that YAP can not only promote ISC proliferation but also cause differences in the activity of goblet cell differentiation. These results indicate that the regulatory effect of YAP on ISCs is closely related to its activity level[73] (Figure 3).
The intestinal mucosa is covered with a thick layer of mucous substances containing a variety of antimicrobial molecules, which can play a protective role in the intestinal mucosa. It can lubricate the intestine and resist the invasion of microorganisms, pathogens and other harmful substances, acting as a chemical barrier and mechanical barrier protection[74]. One possible reason for the aggravation of UC is that the number of goblet cells in the intestinal mucosa is reduced, and the function of intestinal mucus secretion is impaired. Colon mucosal epithelial cells are mainly composed of goblet cells (GCs), secretory cells that secrete a large number of mucoproteins (MUCs) and intestinal trefoil factors (ITFs) and human intestinal resistin-like molecule β (resistin-like molecule β, RELM-β)[75].
The main component of the mucin layer is mucin, which is a high molecular weight glycoylated protein secreted by GCs. It is an important bioactive peptide that can coat bacteria and prevent direct contact between bacteria and epithelial cells[76]. Therefore, mice with insufficient mucus secretion easily develop UC, and studies have shown that MUC2-deficient mice or MUC2 gene mistranslation mice can spontaneously develop UC[77].
Studies have shown that the synthesis of MUC2 in the colon during MUC activity is 40% less than that in the normal colon, indicating that the decrease in mucin in the colon mucosa is one of the reasons for the weakening of intestinal mucosal barrier function and the pathogenesis of UC[78]. The Notch signaling pathway is one of the important ways to maintain the proliferation and differentiation of colon epithelial cells. Overactivation of Notch leads to increased expression of the transcription factor HE-1 in human colon cell lines, inhibits Hath-1 expression, and subsequently inhibits the differentiation of intestinal epithelial cells into goathous cells, resulting in a decrease in secretory cells and formation of the intestinal mucosal layer[79].
Chronic intestinal inflammation can cause a large number of white blood cells to infiltrate the intestinal mucosa, including neutrophils and macrophages, which can produce inflammatory factors and excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS), causing an intestinal mucosal oxidative stress response and intestinal mucosal damage together with an inflammatory response[80]. ROS content was positively correlated with the occurrence and development of UC. ROS consist of a variety of components, including peroxide, hydroxyl and a large amount of hydrogen peroxide. RNS include nitric oxide, nitrogen dioxide and peroxynitrite[81].
When UC occurs, colon mucosa produces a large number of inflammatory molecules and activates a large number of inflammatory response pathways, which jointly promote the production of a large number of peroxides and accumulate in the intestine, self-sustaining and amplifying intestinal oxidative stress, forming a vicious cycle[82]. A large number of ROS can destroy the structure of intestinal endothelial cytoskeleton proteins and cause intestinal mucosal barrier dysfunction. Finally, the structure and function of the intestinal mucosal barrier are damaged, which affects the protective effect of the intestinal tract[83]. ROS can also increase the permeability of the cell membrane. On the one hand, ROS can cause the inflow of extracellular Ca2+ into the cell to promote the apoptosis of intestinal cells; on the other hand, ROS can cause a peroxidation reaction with the cell membrane to damage the normal structure and function of intestinal mucosal cells and further lead to the impairment of intestinal mucosal function[84].
The intestinal mucosal barrier has selective permeability. When the intestinal mucosal barrier is destroyed, mucosal inflammation can cause necrosis and shedding of epithelial cells, which increases intestinal mucosal permeability[85]. Structural damage to epithelial cells leads to changes in the tight connective structure and loss of protective effects so that various pathogenic substances in the intestinal cavity are absorbed into the body[86]. The intestinal immune system is repeatedly stimulated and misidentified with these harmful substances, which continuously activates intestinal macrophages and tissue lymphocytes and further stimulates or aggravates the release of inflammatory factors in the intestine, thus continuously initiating an excessive intestinal immune inflammatory response and ultimately damaging the intestinal mucosal barrier, resulting in the loss of protective function of the intestinal mucosal barrier and damage to intestinal tissues[87]. A large number of studies have shown that a large number of epithelial cells in the inflammatory site of the intestinal mucosa in UC patients suffer from apoptosis, and the resulting tight connection injury is considered to be an important cause of UC[88].
It was found that the goblet cells and mucus secreted by intestinal mucosa in patients with UC were reduced. Tight junctions are occlusive links formed by the binding of the outer layer of the adjacent intestinal epithelial cell membrane by specific transmembrane proteins[89]. Tight junctions are mainly composed of tight junction proteins, including Occludin, the claudin family, Zonula occludens (ZO), the ZO family and junctional adhesion molecule (JAM), which are important structures of epithelial barrier function and play a decisive role in intestinal mucosal permeability by JAM[90]. As a transmembrane tight junction protein, Occludin can form the paracellular tight junction structure and is an important structural and functional protein involved in signal regulation of tight junction formation. Studies have shown that the silencing of occludin genes can increase the cell bypass permeability of intestinal epithelial cells, resulting in an increase in macromolecules and harmful substances in the intestine[91]. Several studies have shown that occludin gene expression in the colon mucosa of UC patients decreases, resulting in a decrease in occludin protein synthesis[92]. Claudins are one of the transmembrane proteins of intestinal epithelial cells. The extracellular part of Claudins acts as a ligand and interacts with transmembrane lectin receptors of adjacent epithelial cells to bind, thus filling the cellular gap and maintaining the tight connection function of the intestinal mucosa[93]. ZOs act as an "assembly platform" for tight junctions that link transmembrane proteins and the cytoskeleton to recognize and transmit signals[94]. The decreased expression of ZOs in intestinal mucosal epithelial cells indicated increased intestinal permeability and damage to the intestinal mucosal barrier[95]. Tight junctions are regulated mainly by protein phosphorylation. When the intestinal mucosa is stimulated by inflammation or oxidative stress, Occludin and ZO-1 phosphorylation are deactivated, and reallocation of the Occludin-Zo-1 complex affects the normal structure of tight junctions of intestinal epithelial cells, resulting in increased intestinal permeability and damage[96].
LGR5-labeled small ISCs are the most recognized small ISCs. The LGR5-labeled cells are located at the bottom of the crypt base columnar cell (CBC), which is also called the crypt base CBC because of its small size and elongated shape[97]. As early as 1974, the CBC stem cell model was proposed. According to the theory, CBC cells are small ISCs that live in a microenvironment formed by Paneth cells[98]. Once their offspring leave this microenvironment, they begin to differentiate into a variety of differentiated cells[99]. It was not until 2007, when the CBC cell-specific marker LGR5 was identified, that the theory was experimentally confirmed[100]. In the LGR5-enhanced green fluorescent protein (EGFP)-IRES-Cre ERT2 gene knockout mouse model, CBC cells were labeled with EGFP fluorescent protein. Lineage tracing experiments subsequently demonstrated that the progeny of CBC cells could differentiate into any kind of cell in the small intestinal epithelium, and such lineage markers could persist in the small intestinal epithelium, demonstrating the small ISCs property of CBC cells. EGFP fluorescent protein-labeled CBC cells were isolated using flow cytometry and were encapsulated in Matrigel for in vitro stem cell culture in the presence of three growth factors (EGF, Noggin, and R-spondin). Individual LGR5+ cells can grow into organoids, which closely resemble the structure and cellular composition of the intestinal epithelium in vivo. LGR5+ small ISCs can both self-renew and generate all progeny differentiated cells. This evidence suggests that LGR5-labeled cells represent small ISCs[101].
LGR5-labeled small ISCs are actively dividing stem cells that divide every 24 h on average. LGR5+ cells produce transient multiplication cells while generating new small ISCs[102]. TA cells migrate upward and differentiate gradually during subsequent rapid division. The present study suggests that self-renewal of LGR5+ small ISCs follows a "neutral competition" model. LGR5+ small ISCs can maintain their dry properties only when they are located in a microenvironment composed of Pan cells[103]. Because LGR5+ small ISCs divide symmetrically, the progeny cells forced out of the microenvironment due to space crowding will differentiate into TA cells, while the progeny cells left in the microenvironment will retain their stem cell properties[104].
The "+ 4 stem cell" model is another theory about the localization of small ISCs. The + 4 cells refer to the cells placed fourth from Paneth cells at the bottom of the crypt and are considered small ISCs because of their label retention ability[105]. Marker retention means that after marking the DNA of +4 cells, these markers remain in + 4 cells for a long time afterward and do not disappear with cell division[106]. This marker retention property is often thought to be unique to stem cells. At present, markers of + 4 stem cells have been identified, including Bmi and Lrig1, Hopx and Tert, etc. However, the specificity of these markers has been under great controversy. Studies have shown that cells at the bottom of the small intestine recess all express these genes; that is, the expression of these genes is not substantively specific[107].
Significant expansion of LGR5+ small ISCs was detected under normal physiological conditions after BMP signaling was blocked by directly inducing the small intestinal epithelial receptor Bmpr1a, which specifically knocked out BMP signaling[108]. Specific knockout of the Bmpr1a receptor in LGR5+ small ISCs also led to rapid expansion of stem cell groups. In vitro culture and in vivo lineage tracing experiments showed that the self-renewal and proliferation abilities of LGR5+ small ISCs were significantly enhanced after BMP signaling inactivation[109]. In the case of long-term BMP signal inactivation, continuous and unrestricted expansion of LGR5+ small ISCs will lead to malignant proliferation of the small intestinal epithelium and the appearance of small intestinal polyps[110]. These phenotypes are very similar to the symptoms of human juvenile intestinal polyps. Finally, the radiation damage model also verified the upregulation of stem cell function after BMP signal inactivation[111]. That is, after BMP signal inactivation, a certain dose of radiation damage can only lead to the apoptosis of some LGR5+ small ISCs, while the remaining LGR5+ small ISCs actively participate in the process of damage repair, thus greatly accelerating the process of radiation damage repair[112].
The relationship between BMP signaling inactivation and self-renewal disturbances in the small intestine epithelium has long been noted. This is because inactivated mutations in the BMP signaling pathway, including the BMPR1A and SMAD4 genes, are found in most human genetic juvenile polyps[113]. In the small intestine, the BMP ligands BMP2 and BMP4 are mainly secreted by mesenchymal cells in the intestinal villi and mesenchymal cells around the small intestine recess. The BMP inhibitors Noggin and Gremlin1 are mainly secreted by mesenchymal cells around the crypts of the small intestine[114]. This secretion pattern results in higher BMP signaling activity in the villi and lower BMP signaling in the crypts of the small intestine. Similarly, however, cells in the mesenchyme responding to BMP stimulation should also be in a state of BMP signaling activation due to the abundance of BMP ligands in the mesenchyme[115]. In juvenile intestinal polyps, BMP signaling inactivation means that all cells no longer respond to BMP signaling, and malignant proliferative intestinal polyps appear[116]. Therefore, it is not clear whether BMP signaling in epithelial cells or in mesenchymal cells plays an important role in inhibiting the appearance and growth of small intestinal polyps. Earlier studies using a Noggin transgenic overexpression mouse model and a systemic Bmpr1a knockout mouse model failed to solve this problem[117].
In the small intestine, the Wnt/β-catenin signaling pathway is thought to be critical for maintaining ISCs self-renewal and proliferation. Wnt was highly expressed in the stem cell area and around proliferating cells in the small intestine, and it decreased gradientally upward with the intensity of differentiation. Genes expressed in intestinal epithelial stem/progenitor cells, such as those that label ISCs LGR5 and Olfm4, are regulated by Wnt signals[118]. There are 19 different Wnt genes expressed in the small intestine. The main cell source of classical Wnt, such as Wnt3, Wnt6 and Wnt9b, is epithelial cells, not classical Wnt[119]. For example, Wnts2b, Wnt4, Wnt5a, Wnt5b, and Wnt antagonists [secreted frizzled related protein (SFRP)-1, SFRP-2, Dickkopf (DKK)2, and DKK-3] are derived from mesenchymal cells. Wnt secreted by epithelial or stromal cells first binds to the coreceptors LRP5/6 and Frizzled on the cell membrane in crypt cells, causing increased expression of β-catenin. Activated beta-catenin further binds to the nuclear transcription factor TCF4 to drive gene expression that supports stem cell maintenance, proliferation, and differentiation[120]. Blocking the Wnt/β-catenin signaling pathway leads to the stagnation of intestinal epithelial cell proliferation. Previous studies have demonstrated that knockout of TCF, DKK1 (Wnt antagonist), Ctnnb1 (β-catenin gene), or c-Myc (Wnt target gene) can significantly affect the proliferation of intestinal epithelial cells in mice. TCF4 knockout in embryonic intestinal epithelial cells resulted in no proliferation in the intervillus region of the small intestine in neonatal mice, while induced knockout of TCF4 and Ctnnb1 blocked crypt proliferation in adult mice[121]. In contrast, the addition of the Wnt agonist R-spondin (roof plate-specific spondin) or the elimination of APC resulted in small intestine or colorectal hyperplasia. Meanwhile, the deletion of Wnt key mediators ring finger protein 43 and zinc and ring finger 3 will also cause intestinal proliferation. Therefore, Wnt signaling plays an important role in the dry maintenance, proliferation and differentiation of small ISCs[122].
Although a large number of studies have confirmed that Wnt secreted by interstitial cells is essential in small intestine development, formation, and damage repair, the evidence that secreted Wnt regulates small intestine homeostasis in mice remains unclear[123]. Wnt3 derived from Pan's cells is necessary for the in vitro culture of LGR5-labeled ISCs organoids. Other studies have shown that Wnt generated from epithelial or mesenchymal cells supports intestinal epithelial growth in organoids in vitro[124]. Some scholars have demonstrated that purified stromal cells can support the formation of epithelial organoids that knock out Wnt3. However, mouse models with Paneth cells removed or Wnt3 knocked out in intestinal epithelial cells showed no obvious phenotype, and the types of interstitial cells secreting Wnt in the in vivo small ISCs microenvironment, as well as the mechanism of inducing secretion, remain unclear, so more in vivo evidence is needed to provide support[125] (Figure 4).
Notch receptors and ligands are expressed only at the crypt site, and their signaling activity plays an important role in the self-renewal and differentiation of the small intestine epithelium[126]. First, Notch signaling regulates the differentiation process of small ISCs. In the process of upward migration, TA cells gradually differentiate into two types of cells, namely, secretory cells and absorptive cells. This differentiation process is mainly regulated by the Notch signaling pathway. Notch activation inhibited cell differentiation toward the secretory type and promoted cell differentiation toward the attractor type. Specific inhibition of Notch signaling in the small intestinal epithelium can rapidly transform all small intestinal crypt cells into secretase cell types by Notch receptor, ligand knockout, or gamma-secretase inhibitor treatment[127]. Conversely, activation of Notch signaling in the small intestinal epithelium significantly inhibited secretory cell differentiation. Second, Notch signaling regulates the self-renewal of small ISCs. In the small ISCs microenvironment, the ligands for Notch signaling are mainly provided by Paneth cells, and Notch receptors are actively expressed in small ISCs[128]. Using the mouse model, cells with high Notch signaling activity were specifically labeled. Using lineage tracing experiments, it was found that small ISCs belong to a type of cell with high Notch signaling, and these cells can form all cell types in the small intestine epithelium[129].
The Wnt and Notch signaling pathways are two highly conserved signal transduction pathways that exist widely in multicellular animals. They regulate many life processes through different mechanisms and play an important role in cell proliferation, differentiation, and intestinal homeostasis[130]. However, the specific mechanism by which these two signaling pathways interact to regulate ISC activity and differentiation direction remains unclear. A large number of studies have reported crosstalk between Wnt and Notch signaling pathways[131]. The mechanisms are discussed as follows: (1) Wnt protein regulates downstream through binding to some Notch receptors, including Dfrizzled2, patched, shaggy, etc., hairy and patched genes are expressed, and Dfrizzled2 and patched genes can mediate the Wnt pathway itself[132]; (2) Dvl can antagonize the Notch pathway through its direct interaction with Notch intracellular domain (NIC)[133]; (3) NIC can increase the activation potential of lymphoid enhancer factor under the action of some promoters[134]; (4) GSK-3β phosphorylates NIC, prevents its degradation by the proteasome, and prolongs its half-life[135]; and (5) C promoter binding factor-1 can promote the expression of some genes encoding Fz[136]. In addition to the direct crossover between pathways, there are also many indirect (for example, some pathways in both pathways are involved in the regulation of cyclin D1 and p21 expression) and mechanistic associations[137] (Figure 5).
In conclusion, intestinal mucosal injury is an important pathological change in UC. ISCs proliferation and differentiation are the main cytological basis for intestinal mucosal renewal. ISCs participate in normal physiological processes and some pathological processes of the intestine. They are located at the base of the crypts of the intestinal mucosa, which is the cell bank of ISCs. All cells of the intestinal epithelium were derived from crypt stem cells. Meanwhile, LGR5-positive ISCs are significantly regulated by the Notch signaling pathway and Wnt/β-catenin signaling pathway, which jointly maintain the function of LGR5-positive ISCs. More importantly, the surviving stem cells after intestinal mucosal injury accelerate division, restore the number of stem cells, multiply and differentiate into mature intestinal epithelial cells, and repair the damaged intestinal mucosa. Therefore, in-depth study of multiple pathways and transplantation of LGR5-positive ISCs may become a new target for the treatment of UC.
We would like to thank our colleagues in the Institute of Digestive Disease for their help and support in this research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peter L Lakatos, Canada; Nakajima N, Japan; Reddy NNR, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 226] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 2. | Li H, Ye XF, Su YS, He W, Zhang JB, Zhang Q, Zhan LB, Jing XH. Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis. Chin J Integr Med. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Antonelli E, Villanacci V, Bassotti G. Novel oral-targeted therapies for mucosal healing in ulcerative colitis. World J Gastroenterol. 2018;24:5322-5330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Xie J, Li L, Deng S, Chen J, Gu Q, Su H, Wen L, Wang S, Lin C, Qi C, Zhang Q, Li J, He X, Li W, Wang L, Zheng L. Slit2/Robo1 Mitigates DSS-induced Ulcerative Colitis by Activating Autophagy in Intestinal Stem Cell. Int J Biol Sci. 2020;16:1876-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Zhang N, Chen Y, Huang C, Wei M, Li T, Lv Y, Song Q, Mo S. Adipose-derived mesenchymal stem cells may reduce intestinal epithelial damage in ulcerative colitis by communicating with macrophages and blocking inflammatory pathways: an analysis in silico. Aging (Albany NY). 2022;14:2665-2677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kazama S, Kishikawa J, Tanaka T, Hata K, Kawai K, Nozawa H, Ishihara S. Immunohistochemical Expression of CD133 and LGR5 in Ulcerative Colitis-associated Colorectal Cancer and Dysplasia. In Vivo. 2019;33:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Zheng L, Duan SL, Wen XL, Dai YC. Molecular regulation after mucosal injury and regeneration in ulcerative colitis. Front Mol Biosci. 2022;9:996057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Iwaya M, Ota H, Nakajima T, Uehara T, Riddell R, Conner J. Most colitis associated carcinomas lack expression of LGR5: a preliminary study with implications for unique pathways of carcinogenesis compared to sporadic colorectal carcinoma. BMC Cancer. 2021;21:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kim Y, Lee YS, Kang SW, Kim S, Kim TY, Lee SH, Hwang SW, Kim J, Kim EN, Ju JS, Park YY, Kweon MN. Loss of PKM2 in Lgr5(+) intestinal stem cells promotes colitis-associated colorectal cancer. Sci Rep. 2019;9:6212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Zhu P, Zhu X, Wu J, He L, Lu T, Wang Y, Liu B, Ye B, Sun L, Fan D, Wang J, Yang L, Qin X, Du Y, Li C, Ren W, Wu X, Tian Y, Fan Z. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. 2019;20:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Østvik AE, Svendsen TD, Granlund AVB, Doseth B, Skovdahl HK, Bakke I, Thorsvik S, Afroz W, Walaas GA, Mollnes TE, Gustafsson BI, Sandvik AK, Bruland T. Intestinal Epithelial Cells Express Immunomodulatory ISG15 During Active Ulcerative Colitis and Crohn's Disease. J Crohns Colitis. 2020;14:920-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front Immunol. 2021;12:767456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 13. | Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Björklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 559] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 14. | Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 15. | Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2018;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 16. | Nyström EEL, Martinez-Abad B, Arike L, Birchenough GMH, Nonnecke EB, Castillo PA, Svensson F, Bevins CL, Hansson GC, Johansson MEV. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021;372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 17. | Wan Y, Yang L, Jiang S, Qian D, Duan J. Excessive Apoptosis in Ulcerative Colitis: Crosstalk Between Apoptosis, ROS, ER Stress, and Intestinal Homeostasis. Inflamm Bowel Dis. 2022;28:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 18. | Neurath MF, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol. 2019;41:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Zhao X, Cui DJ, Yang LC, Yuan WQ, Yan F. Long Noncoding RNA FBXL19-AS1-Mediated Ulcerative Colitis-Associated Intestinal Epithelial Barrier Defect. Tissue Eng Regen Med. 2022;19:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Wang K, Ding Y, Xu C, Hao M, Li H, Ding L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology. 2021;10:1923910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Yao D, Zhou Z, Wang P, Zheng L, Huang Y, Duan Y, Liu B, Li Y. MiR-125-5p/IL-6R axis regulates macrophage inflammatory response and intestinal epithelial cell apoptosis in ulcerative colitis through JAK1/STAT3 and NF-κB pathway. Cell Cycle. 2021;20:2547-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, Ge W, Wu J, Du P, Chen Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 23. | Joly A, Rousset R. Tissue Adaptation to Environmental Cues by Symmetric and Asymmetric Division Modes of Intestinal Stem Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Sei Y, Feng J, Chow CC, Wank SA. Asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am J Physiol Gastrointest Liver Physiol. 2019;316:G64-G74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 893] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 26. | Zeve D, Stas E, de Sousa Casal J, Mannam P, Qi W, Yin X, Dubois S, Shah MS, Syverson EP, Hafner S, Karp JM, Carlone DL, Ordovas-Montanes J, Breault DT. Robust differentiation of human enteroendocrine cells from intestinal stem cells. Nat Commun. 2022;13:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Zhang C, Zhou Y, Zheng J, Ning N, Liu H, Jiang W, Yu X, Mu K, Li Y, Guo W, Hu H, Li J, Chen D. Inhibition of GABAA receptors in intestinal stem cells prevents chemoradiotherapy-induced intestinal toxicity. J Exp Med. 2022;219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 29. | Sugimoto S, Sato T. Establishment of 3D Intestinal Organoid Cultures from Intestinal Stem Cells. Methods Mol Biol. 2017;1612:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Li C, Zhou Y, Wei R, Napier DL, Sengoku T, Alstott MC, Liu J, Wang C, Zaytseva YY, Weiss HL, Wang Q, Evers BM. Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation. Cell Mol Gastroenterol Hepatol. 2023;15:931-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 32. | Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, Ali N, Sureban SM, Qante M, Wang TC, Bronze MS, Houchen CW. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget. 2015;6:30876-30886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Janeckova L, Fafilek B, Krausova M, Horazna M, Vojtechova M, Alberich-Jorda M, Sloncova E, Galuskova K, Sedlacek R, Anderova M, Korinek V. Wnt Signaling Inhibition Deprives Small Intestinal Stem Cells of Clonogenic Capacity. Genesis. 2016;54:101-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Nakaya T, Ogawa S, Manabe I, Tanaka M, Sanada M, Sato T, Taketo MM, Nakao K, Clevers H, Fukayama M, Kuroda M, Nagai R. KLF5 regulates the integrity and oncogenicity of intestinal stem cells. Cancer Res. 2014;74:2882-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Aden K, Bartsch K, Dahl J, Reijns MAM, Esser D, Sheibani-Tezerji R, Sinha A, Wottawa F, Ito G, Mishra N, Knittler K, Burkholder A, Welz L, van Es J, Tran F, Lipinski S, Kakavand N, Boeger C, Lucius R, von Schoenfels W, Schafmayer C, Lenk L, Chalaris A, Clevers H, Röcken C, Kaleta C, Rose-John S, Schreiber S, Kunkel T, Rabe B, Rosenstiel P. Epithelial RNase H2 Maintains Genome Integrity and Prevents Intestinal Tumorigenesis in Mice. Gastroenterology. 2019;156:145-159.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Wang X, Bootsma H, Kroese F, Dijkstra G, Pringle S. Senescent Stem and Transient Amplifying Cells in Crohn's Disease Intestine. Inflamm Bowel Dis. 2020;26:e8-e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Suzuki K, Murano T, Shimizu H, Ito G, Nakata T, Fujii S, Ishibashi F, Kawamoto A, Anzai S, Kuno R, Kuwabara K, Takahashi J, Hama M, Nagata S, Hiraguri Y, Takenaka K, Yui S, Tsuchiya K, Nakamura T, Ohtsuka K, Watanabe M, Okamoto R. Single cell analysis of Crohn's disease patient-derived small intestinal organoids reveals disease activity-dependent modification of stem cell properties. J Gastroenterol. 2018;53:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Mokrowiecka A, Veits L, Falkeis C, Musial J, Kordek R, Lochowski M, Kozak J, Wierzchniewska-Lawska A, Vieth M, Malecka-Panas E. Expression profiles of cancer stem cell markers: CD133, CD44, Musashi-1 and EpCAM in the cardiac mucosa-Barrett's esophagus-early esophageal adenocarcinoma-advanced esophageal adenocarcinoma sequence. Pathol Res Pract. 2017;213:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Cox CB, Storm EE, Kapoor VN, Chavarria-Smith J, Lin DL, Wang L, Li Y, Kljavin N, Ota N, Bainbridge TW, Anderson K, Roose-Girma M, Warming S, Arron JR, Turley SJ, de Sauvage FJ, van Lookeren Campagne M. IL-1R1-dependent signaling coordinates epithelial regeneration in response to intestinal damage. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Seltana A, Cloutier G, Reyes Nicolas V, Khalfaoui T, Teller IC, Perreault N, Beaulieu JF. Fibrin(ogen) Is Constitutively Expressed by Differentiated Intestinal Epithelial Cells and Mediates Wound Healing. Front Immunol. 2022;13:916187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Shen Y, Ma J, Yan R, Ling H, Li X, Yang W, Gao J, Huang C, Bu Y, Cao Y, He Y, Wan L, Zu X, Liu J, Huang MC, Stenson WF, Liao DF, Cao D. Impaired self-renewal and increased colitis and dysplastic lesions in colonic mucosa of AKR1B8-deficient mice. Clin Cancer Res. 2015;21:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Yin L, Zeng X, Li J, Yin Y, Wang Q, Yang H. Dietary High Dose of Iron Aggravates the Intestinal Injury but Promotes Intestinal Regeneration by Regulating Intestinal Stem Cells Activity in Adult Mice With Dextran Sodium Sulfate-Induced Colitis. Front Vet Sci. 2022;9:870303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Beaumont M, Andriamihaja M, Armand L, Grauso M, Jaffrézic F, Laloë D, Moroldo M, Davila AM, Tomé D, Blachier F, Lan A. Epithelial response to a high-protein diet in rat colon. BMC Genomics. 2017;18:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Samanta S, Chaudhuri AG. Guanylin and uroguanylin: a promising nexus in intestinal electrolyte and fluid homeostasis. J Physiol Pharmacol. 2021;72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 409] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 47. | Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 48. | Zhou Y, Xu J, Luo H, Meng X, Chen M, Zhu D. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022;525:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 49. | Rim EY, Clevers H, Nusse R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu Rev Biochem. 2022;91:571-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 251] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 50. | Chatterjee A, Paul S, Bisht B, Bhattacharya S, Sivasubramaniam S, Paul MK. Advances in targeting the WNT/β-catenin signaling pathway in cancer. Drug Discov Today. 2022;27:82-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 51. | Russell JO, Monga SP. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol. 2018;13:351-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 52. | Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, Chu Q. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 579] [Article Influence: 193.0] [Reference Citation Analysis (1)] |
| 53. | Li L, Tang P, Li S, Qin X, Yang H, Wu C, Liu Y. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy. Med Oncol. 2017;34:180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 54. | Chen W, Liu Y, Chen J, Ma Y, Song Y, Cen Y, You M, Yang G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int Immunopharmacol. 2021;99:107938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 55. | Vargas-Franco D, Kalra R, Draper I, Pacak CA, Asakura A, Kang PB. The Notch signaling pathway in skeletal muscle health and disease. Muscle Nerve. 2022;66:530-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 56. | Sprinzak D, Blacklow SC. Biophysics of Notch Signaling. Annu Rev Biophys. 2021;50:157-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 57. | Shim YS, Lee HS, Hwang JS. Aberrant Notch Signaling Pathway as a Potential Mechanism of Central Precocious Puberty. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Castro RC, Gonçales RA, Zambuzi FA, Frantz FG. Notch signaling pathway in infectious diseases: role in the regulation of immune response. Inflamm Res. 2021;70:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Liu M, Goldman G, MacDougall M, Chen S. BMP Signaling Pathway in Dentin Development and Diseases. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 60. | Thielen NGM, van der Kraan PM, van Caam APM. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 61. | Wang Y, Liu S, Yan Y, Li S, Tong H. SPARCL1 promotes C2C12 cell differentiation via BMP7-mediated BMP/TGF-β cell signaling pathway. Cell Death Dis. 2019;10:852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Xin Z, Wang J, Li S, Sun C, Jiang W, Xin Q, Qi T, Li K, Zhang Z, Luan Y. A review of BMP and Wnt signaling pathway in the pathogenesis of pulmonary arterial hypertension. Clin Exp Hypertens. 2022;44:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 504] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 64. | Zeng X, Ju D. Hedgehog Signaling Pathway and Autophagy in Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 65. | Ingham PW. Hedgehog signaling. Curr Top Dev Biol. 2022;149:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 66. | Quaglio D, Infante P, Di Marcotullio L, Botta B, Mori M. Hedgehog signaling pathway inhibitors: an updated patent review (2015-present). Expert Opin Ther Pat. 2020;30:235-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Wang M, Dong Y, Gao S, Zhong Z, Cheng C, Qiang R, Zhang Y, Shi X, Qian X, Gao X, Guan B, Yu C, Yu Y, Chai R. Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea. Cell Mol Life Sci. 2022;79:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Wang S, Zhou L, Ling L, Meng X, Chu F, Zhang S, Zhou F. The Crosstalk Between Hippo-YAP Pathway and Innate Immunity. Front Immunol. 2020;11:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 69. | Deng F, Wu Z, Zou F, Wang S, Wang X. The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury. Front Cell Dev Biol. 2022;10:894737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 70. | Ouyang T, Meng W, Li M, Hong T, Zhang N. Recent Advances of the Hippo/YAP Signaling Pathway in Brain Development and Glioma. Cell Mol Neurobiol. 2020;40:495-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 666] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 72. | Yuan L, Mao Y, Luo W, Wu W, Xu H, Wang XL, Shen YH. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J Biol Chem. 2017;292:15002-15015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 73. | Morice S, Danieau G, Rédini F, Brounais-Le-Royer B, Verrecchia F. Hippo/YAP Signaling Pathway: A Promising Therapeutic Target in Bone Paediatric Cancers? Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Yao Y, Kim G, Shafer S, Chen Z, Kubo S, Ji Y, Luo J, Yang W, Perner SP, Kanellopoulou C, Park AY, Jiang P, Li J, Baris S, Aydiner EK, Ertem D, Mulder DJ, Warner N, Griffiths AM, Topf-Olivestone C, Kori M, Werner L, Ouahed J, Field M, Liu C, Schwarz B, Bosio CM, Ganesan S, Song J, Urlaub H, Oellerich T, Malaker SA, Zheng L, Bertozzi CR, Zhang Y, Matthews H, Montgomery W, Shih HY, Jiang J, Jones M, Baras A, Shuldiner A, Gonzaga-Jauregui C, Snapper SB, Muise AM, Shouval DS, Ozen A, Pan KT, Wu C, Lenardo MJ. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185:1172-1188.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 75. | Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr. 2020;11:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 397] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 76. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3312] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 77. | Engevik MA, Danhof HA, Ruan W, Engevik AC, Chang-Graham AL, Engevik KA, Shi Z, Zhao Y, Brand CK, Krystofiak ES, Venable S, Liu X, Hirschi KD, Hyser JM, Spinler JK, Britton RA, Versalovic J. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 78. | Li J, Zhang L, Wu T, Li Y, Zhou X, Ruan Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J Agric Food Chem. 2021;69:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 79. | Liang L, Liu L, Zhou W, Yang C, Mai G, Li H, Chen Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin Sci (Lond). 2022;136:291-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 80. | Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1566] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 81. | Wei L, Li Y, Chang Q, Guo G, Lan R. Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. Exp Anim. 2021;70:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Zhang Z, Tang Y, Fang W, Cui K, Xu D, Liu G, Chi S, Tan B, Mai K, Ai Q. Octanoate Alleviates Dietary Soybean Oil-Induced Intestinal Physical Barrier Damage, Oxidative Stress, Inflammatory Response and Microbial Dysbiosis in Large Yellow Croaker (Larimichthys Crocea). Front Immunol. 2022;13:892901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 83. | Xu S, Li L, Wu J, An S, Fang H, Han Y, Huang Q, Chen Z, Zeng Z. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulating SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front Immunol. 2021;12:625627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 84. | Li J, Zhang H, Wang G. Correlations between inflammatory response, oxidative stress, intestinal pathological damage and intestinal flora variation in rats with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2020;24:10162-10168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Zhang X, Peng B, Zhang Y, Lu X, Cao Y. Biliary Drainage Reduces Intestinal Barrier Damage in Obstructive Jaundice by Regulating Autophagy. Contrast Media Mol Imaging. 2022;2022:3301330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Parigi SM, Larsson L, Das S, Ramirez Flores RO, Frede A, Tripathi KP, Diaz OE, Selin K, Morales RA, Luo X, Monasterio G, Engblom C, Gagliani N, Saez-Rodriguez J, Lundeberg J, Villablanca EJ. The spatial transcriptomic landscape of the healing mouse intestine following damage. Nat Commun. 2022;13:828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 87. | Liu X, Sun R, Li Z, Xiao R, Lv P, Sun X, Olson MA, Gong Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch Biochem Biophys. 2021;711:109019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 88. | Zhang YN, Chang ZN, Liu ZM, Wen SH, Zhan YQ, Lai HJ, Zhang HF, Guo Y, Zhang XY. Dexmedetomidine Alleviates Gut-Vascular Barrier Damage and Distant Hepatic Injury Following Intestinal Ischemia/Reperfusion Injury in Mice. Anesth Analg. 2022;134:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M, Yu Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11:997-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 90. | Maria-Ferreira D, Nascimento AM, Cipriani TR, Santana-Filho AP, Watanabe PDS, Sant Ana DMG, Luciano FB, Bocate KCP, van den Wijngaard RM, Werner MFP, Baggio CH. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci Rep. 2018;8:12261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 91. | Hou Q, Huang J, Ayansola H, Masatoshi H, Zhang B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front Immunol. 2020;11:623691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 92. | Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O'Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 853] [Article Influence: 85.3] [Reference Citation Analysis (1)] |
| 93. | Villablanca EJ, Selin K, Hedin CRH. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression? Nat Rev Gastroenterol Hepatol. 2022;19:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 94. | Ma Y, Yin Z, Li L, Chen B, Dai H, Wu D, Cong J, Ye L, Liao C, Ye Z, Huang Z. Food antigens exacerbate intestinal damage and inflammation following the disruption of the mucosal barrier. Int Immunopharmacol. 2021;96:107670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Xue M, Ji X, Liang H, Liu Y, Wang B, Sun L, Li W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018;9:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 96. | Haussner F, Chakraborty S, Halbgebauer R, Huber-Lang M. Challenge to the Intestinal Mucosa During Sepsis. Front Immunol. 2019;10:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 97. | Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 546] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 98. | Cao W, Li M, Liu J, Zhang S, Noordam L, Verstegen MMA, Wang L, Ma B, Li S, Wang W, Bolkestein M, Doukas M, Chen K, Ma Z, Bruno M, Sprengers D, Kwekkeboom J, van der Laan LJW, Smits R, Peppelenbosch MP, Pan Q. LGR5 marks targetable tumor-initiating cells in mouse liver cancer. Nat Commun. 2020;11:1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 99. | de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 496] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 100. | Ma Z, Guo D, Wang Q, Liu P, Xiao Y, Wu P, Wang Y, Chen B, Liu Z, Liu Q. Lgr5-mediated p53 Repression through PDCD5 leads to doxorubicin resistance in Hepatocellular Carcinoma. Theranostics. 2019;9:2967-2983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, Dijkgraaf GJ, Piskol R, de Sauvage FJ. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 583] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 102. | Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 1162] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 103. | Fumagalli A, Oost KC, Kester L, Morgner J, Bornes L, Bruens L, Spaargaren L, Azkanaz M, Schelfhorst T, Beerling E, Heinz MC, Postrach D, Seinstra D, Sieuwerts AM, Martens JWM, van der Elst S, van Baalen M, Bhowmick D, Vrisekoop N, Ellenbroek SIJ, Suijkerbuijk SJE, Snippert HJ, van Rheenen J. Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell. 2020;26:569-578.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 104. | Ishikawa K, Sugimoto S, Oda M, Fujii M, Takahashi S, Ohta Y, Takano A, Ishimaru K, Matano M, Yoshida K, Hanyu H, Toshimitsu K, Sawada K, Shimokawa M, Saito M, Kawasaki K, Ishii R, Taniguchi K, Imamura T, Kanai T, Sato T. Identification of Quiescent LGR5(+) Stem Cells in the Human Colon. Gastroenterology. 2022;163:1391-1406.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 105. | Fatehullah A, Terakado Y, Sagiraju S, Tan TL, Sheng T, Tan SH, Murakami K, Swathi Y, Ang N, Rajarethinam R, Ming T, Tan P, Lee B, Barker N. A tumour-resident Lgr5(+) stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat Cell Biol. 2021;23:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 106. | Zhu X, Yang M, Lin Z, Mael SK, Li Y, Zhang L, Kong Y, Zhang Y, Ren Y, Li J, Wang Z, Yang B, Huang T, Guan F, Li Z, Moses RE, Li L, Wang B, Li X, Zhang B. REGγ drives Lgr5(+) stem cells to potentiate radiation induced intestinal regeneration. Sci China Life Sci. 2022;65:1608-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 108. | Kobayashi H, Gieniec KA, Wright JA, Wang T, Asai N, Mizutani Y, Lida T, Ando R, Suzuki N, Lannagan TRM, Ng JQ, Hara A, Shiraki Y, Mii S, Ichinose M, Vrbanac L, Lawrence MJ, Sammour T, Uehara K, Davies G, Lisowski L, Alexander IE, Hayakawa Y, Butler LM, Zannettino ACW, Din MO, Hasty J, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Wang TC, Enomoto A, Takahashi M, Worthley DL, Woods SL. The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis. Gastroenterology. 2021;160:1224-1239.e30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 109. | Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51:1357-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 110. | Wang S, Chen YG. BMP signaling in homeostasis, transformation and inflammatory response of intestinal epithelium. Sci China Life Sci. 2018;61:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Ye W, Takabayashi H, Yang Y, Mao M, Hibdon ES, Samuelson LC, Eaton KA, Todisco A. Regulation of Gastric Lgr5+ve Cell Homeostasis by Bone Morphogenetic Protein (BMP) Signaling and Inflammatory Stimuli. Cell Mol Gastroenterol Hepatol. 2018;5:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Li Y, Liu Y, Liu B, Wang J, Wei S, Qi Z, Wang S, Fu W, Chen YG. A growth factor-free culture system underscores the coordination between Wnt and BMP signaling in Lgr5(+) intestinal stem cell maintenance. Cell Discov. 2018;4:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Li K, Wu H, Wang A, Charron J, Mishina Y, Habib SL, Liu H, Li B. mTOR signaling regulates gastric epithelial progenitor homeostasis and gastric tumorigenesis via MEK1-ERKs and BMP-Smad1 pathways. Cell Rep. 2021;35:109069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, Li B, Han JJ, Chen YG. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat Commun. 2017;8:13824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 115. | Palikuqi B, Rispal J, Klein O. Good Neighbors: The Niche that Fine Tunes Mammalian Intestinal Regeneration. Cold Spring Harb Perspect Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Bayne RA, Donnachie DJ, Kinnell HL, Childs AJ, Anderson RA. BMP signalling in human fetal ovary somatic cells is modulated in a gene-specific fashion by GREM1 and GREM2. Mol Hum Reprod. 2016;22:622-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Sunkara RR, Mehta D, Sarate RM, Waghmare SK. BMP-AKT-GSK3β Signaling Restores Hair Follicle Stem Cells Decrease Associated with Loss of Sfrp1. Stem Cells. 2022;40:802-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 118. | Kim S, Shin YC, Kim TY, Kim Y, Lee YS, Lee SH, Kim MN, O E, Kim KS, Kweon MN. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 175] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 119. | Takahashi T, Shiraishi A. Stem Cell Signaling Pathways in the Small Intestine. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 120. | Zhang H, Lin M, Dong C, Tang Y, An L, Ju J, Wen F, Chen F, Wang M, Wang W, Chen M, Zhao Y, Li J, Hou SX, Lin X, Hu L, Bu W, Wu D, Li L, Jiao S, Zhou Z. An MST4-pβ-Catenin(Thr40) Signaling Axis Controls Intestinal Stem Cell and Tumorigenesis. Adv Sci (Weinh). 2021;8:e2004850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 121. | Chen S, Zheng Y, Ran X, Du H, Feng H, Yang L, Wen Y, Lin C, Wang S, Huang M, Yan Z, Wu D, Wang H, Ge G, Zeng A, Zeng YA, Chen J. Integrin αEβ7(+) T cells direct intestinal stem cell fate decisions via adhesion signaling. Cell Res. 2021;31:1291-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 122. | Young MA, Daly CS, Taylor E, James R, Clarke AR, Reed KR. Subtle Deregulation of the Wnt-Signaling Pathway Through Loss of Apc2 Reduces the Fitness of Intestinal Stem Cells. Stem Cells. 2018;36:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Zhou JY, Lin HL, Wang Z, Zhang SW, Huang DG, Gao CQ, Yan HC, Wang XQ. Zinc L-Aspartate enhances intestinal stem cell activity to protect the integrity of the intestinal mucosa against deoxynivalenol through activation of the Wnt/β-catenin signaling pathway. Environ Pollut. 2020;262:114290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 124. | Li B, Lee C, Cadete M, Zhu H, Koike Y, Hock A, Wu RY, Botts SR, Minich A, Alganabi M, Chi L, Zani-Ruttenstock E, Miyake H, Chen Y, Mutanen A, Ngan B, Johnson-Henry KC, De Coppi P, Eaton S, Määttänen P, Delgado-Olguin P, Sherman PM, Zani A, Pierro A. Impaired Wnt/β-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 2019;10:743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 125. | Zhou JY, Wang Z, Zhang SW, Lin HL, Gao CQ, Zhao JC, Yang C, Wang XQ. Methionine and Its Hydroxyl Analogues Improve Stem Cell Activity To Eliminate Deoxynivalenol-Induced Intestinal Injury by Reactivating Wnt/β-Catenin Signaling. J Agric Food Chem. 2019;67:11464-11473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 126. | Ogaki S, Shiraki N, Kume K, Kume S. Wnt and Notch signals guide embryonic stem cell differentiation into the intestinal lineages. Stem Cells. 2013;31:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Teratani-Ota Y, Yamamizu K, Piao Y, Sharova L, Amano M, Yu H, Schlessinger D, Ko MS, Sharov AA. Induction of specific neuron types by overexpression of single transcription factors. In Vitro Cell Dev Biol Anim. 2016;52:961-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, Moreno-Serrano M, Iqbal AM, Bauer-Rowe KE, Imada S, Ulutas MS, Mylonas C, Whary MT, Levine SS, Basbinar Y, Hynes RO, Mino-Kenudson M, Deshpande V, Boyer LA, Fox JG, Terranova C, Rai K, Piwnica-Worms H, Mihaylova MM, Regev A, Yilmaz ÖH. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell. 2019;178:1115-1131.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 129. | Kurokawa K, Hayakawa Y, Koike K. Plasticity of Intestinal Epithelium: Stem Cell Niches and Regulatory Signals. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 130. | Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 764] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 131. | Chakrabarti R, Celià-Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A, Hwang J, Peng J, Nixon B, Grady JJ, DeCoste C, Gao J, van Es JH, Li MO, Aifantis I, Clevers H, Kang Y. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 132. | Aspal M, Zemans RL. Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 133. | Rishikaysh P, Dev K, Diaz D, Qureshi WM, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014;15:1647-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 134. | Li L, Shi X, Shi Y, Wang Z. The Signaling Pathways Involved in Ovarian Follicle Development. Front Physiol. 2021;12:730196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 135. | Zhou X, Guo Y, Yang K, Liu P, Wang J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J Ethnopharmacol. 2022;282:114662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 136. | Noorbakhsh N, Hayatmoghadam B, Jamali M, Golmohammadi M, Kavianpour M. The Hippo signaling pathway in leukemia: function, interaction, and carcinogenesis. Cancer Cell Int. 2021;21:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, Park O, Ishitani T, Jho EH, Gao B, Yang Y. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |