Published online Apr 21, 2023. doi: 10.3748/wjg.v29.i15.2272
Peer-review started: October 26, 2022
First decision: December 12, 2022
Revised: January 19, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: April 21, 2023
Processing time: 169 Days and 21.8 Hours
Intestinal ultrasound (IUS) is a non-invasive, real-time, cross-sectional imaging tool that can be used at the point-of-care to assess disease activity in patients with Crohn’s disease or ulcerative colitis. IUS promotes quick and impactful treatment decisions that can modify disease progression and enhance patient compliance. This review will summarize the technical aspects of IUS, the evidence to support the use of IUS in disease activity monitoring, the comparison of IUS to current standard of care monitoring modalities such as colonoscopy and calprotectin, and the optimal positioning of IUS in a tight-control monitoring strategy.
Core Tip: Intestinal ultrasound (IUS) is a non-invasive, real-time, cross-sectional imaging tool that is currently underutilized for direct disease activity monitoring in Crohn’s disease (CD) and ulcerative colitis (UC). IUS is the optimal point-of-care method to monitor disease activity in patients with CD or UC with excellent patient compliance and comparison to such traditional monitoring modalities as calprotectin, colonoscopy, and magnetic resonance enterography.
- Citation: Dolinger MT, Kayal M. Intestinal ultrasound as a non-invasive tool to monitor inflammatory bowel disease activity and guide clinical decision making. World J Gastroenterol 2023; 29(15): 2272-2282
- URL: https://www.wjgnet.com/1007-9327/full/v29/i15/2272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i15.2272
The current conventional approach to inflammatory bowel disease (IBD) monitoring in the United States includes routine patient visits to the gastroenterology clinic every 3-6 mo with more urgent or frequent visits based on symptomatology. During these visits, an assessment of the patient’s current disease status is undertaken via history and physical examination. After the visit, the evaluation typically continues with biomarkers such as C-reactive protein and fecal calprotectin (FC), colonoscopy, and magnetic resonance enterography (MRE) though results are often delayed and compliance rates low. This traditional, fragmented model of IBD monitoring is not well suited to detect subclinical inflammation or prevent disease progression, and has contributed to a therapeutic effectiveness ceiling of 30%[1].
Intestinal ultrasound (IUS) is a non-invasive, real-time, cross-sectional imaging tool that is currently underutilized for direct disease activity monitoring in Crohn’s disease (CD) and ulcerative colitis (UC)[2]. Use of IUS at the point-of-care in a tight monitoring model can add significant value to the routine clinic visit by allowing the treating gastroenterologist to accurately and immediately assess disease activity and treatment responsiveness, something that is sorely lacking in the current traditional IBD care model. Furthermore, IUS allows for the easy assessment of transmural healing (TH), a target beyond simple mucosal healing (MH) that may impact long-term disease outcomes.
In this review, we discuss key concepts to understand how IUS utilization will impact care delivery for patients with IBD and achieve disease modification. We will review the technical aspects of IUS, evidence for detection of disease activity and treatment responsiveness, comparison to current standard of care management tools such as MRE, FC, and ileo colonoscopy, impact on shared understanding and real-time decision making, optimal utilization, and future applications.
Use of IUS as a cross-sectional tool for disease activity monitoring starts with a minimum requirement of a modern ultrasound machine available for regular use. Two probes are needed with ability to assess color Doppler signal (CDS) - a lower-frequency convex array probe (3-5 MHz) for global evaluation of the entire bowel and assessment of complications with ability for deeper penetration, and a higher-frequency linear array probe (5-15 MHz) for visualization of the five bowel wall layers and measurement of bowel wall thickness (BWT) to the level of 0.1 millimeters (mm)[3].
Fasting or bowel preparation prior to IUS is unnecessary for optimal visualization of the bowel and the examination can be performed without any prior planning. Intravenous [Contrast Enhanced Ultrasound (CEUS)] or oral contrast [Small Intestinal Contrast Ultrasound (SICUS)] are not beneficial during point-of-care evaluation in IBD activity monitoring and treatment response assessment. The value of CEUS and SICUS, based on local availability and expertise, is in the assessment of suspected complications. CEUS is a valuable tool in the detection of and differentiation of phlegmon and abscesses compared to traditional non-contrast IUS[4]. SICUS adds further value over IUS in the evaluation of proximal small bowel (proximal ileal and jejunal) lesions and detection of stenoses, but the ingestion of oral contrast leads to extended time for completion of the examination that is no longer suitable for use as a point-of-care assessment tool[5,6].
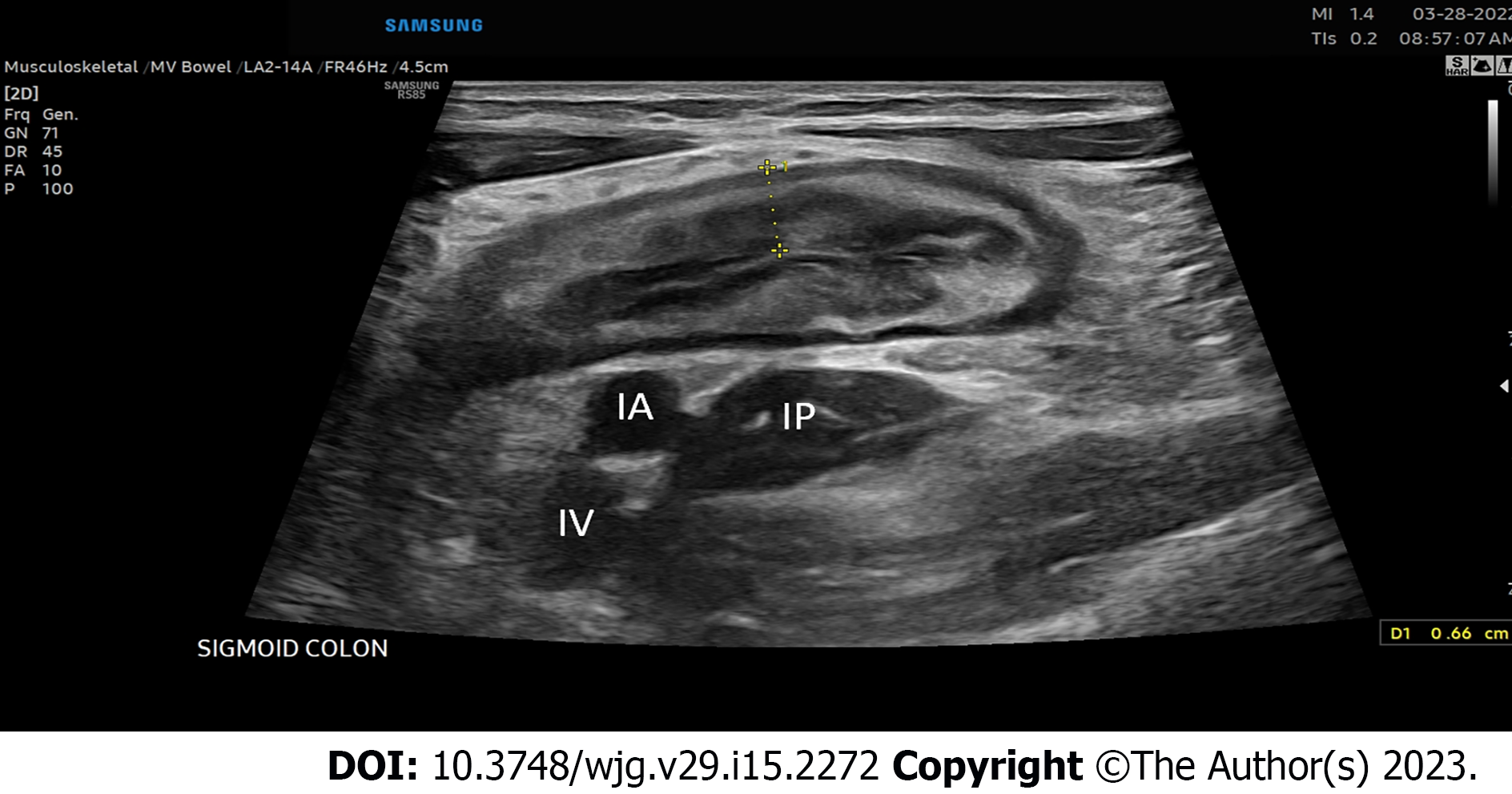
IUS examination should begin with an initial assessment of the distal sigmoid colon using the convex probe beginning in the left lower quadrant of the abdomen or left suprapubic region with identification of the iliac vessels and left iliopsoas muscle, fanning the probe up with the use of graded compression to identify air within the lumen of the distal sigmoid colon and visualization of the subsequent bowel wall layers (Figure 1).
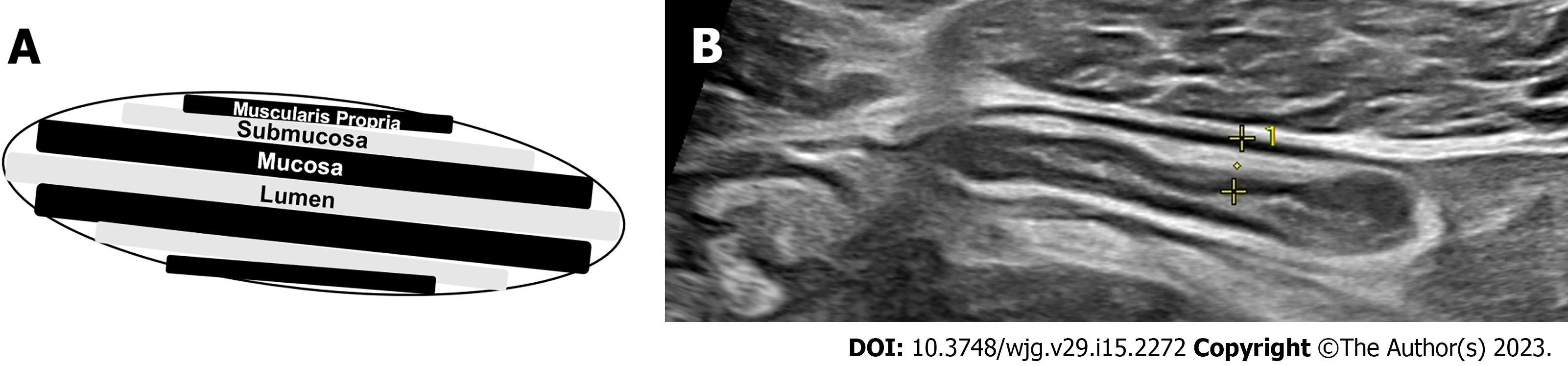
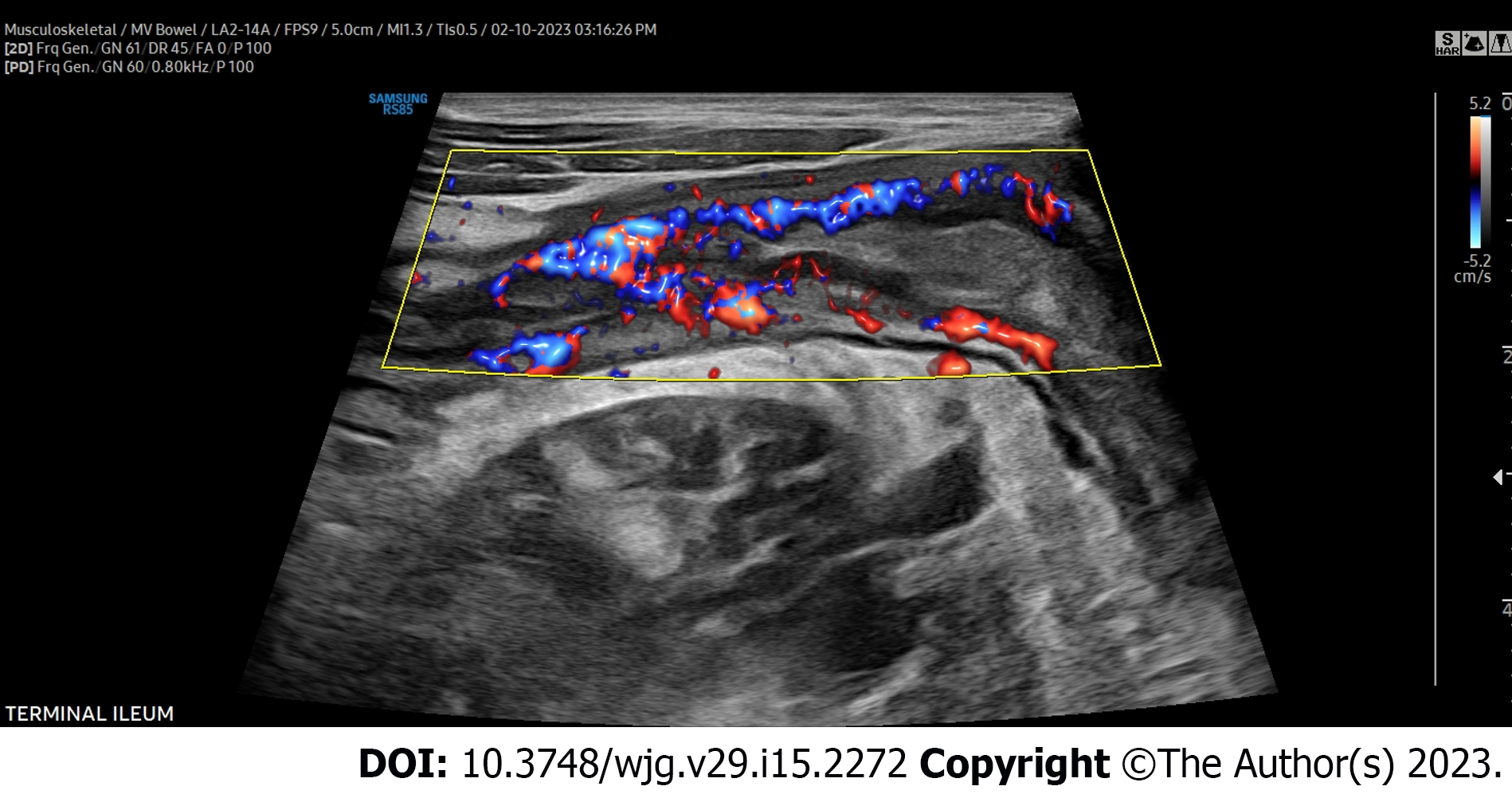
The colon should then be tracked proximally visualizing the retroperitoneal descending colon to the left inferior border of the intercostals or just below to denote the splenic flexure, then to identify the transverse colon utilizing the thick wall of the stomach as an anatomical landmark, then to the hepatic flexure, downward toward the right lateral hemiabdomen to visualize the ascending colon, cecum, and ultimately to the right lower quadrant of the abdomen and/or right suprapubic region to identify the ileocecal valve, and terminal ileum, again using the right lower quadrant iliac vessels and iliopsoas muscle as a landmark for proper identification of the terminal ileum superior. The remainder of the proximal small intestine should be scanned in a “lawn-mower” technique, sweeping up and down over the mid-abdomen, to visualize the remaining proximal small bowel. Differentiation of the large intestine and small intestine from one another during this process is essential and done easily based on the hypermotility and movement of intestinal contents within the small bowel compared to that of the colon. Once the global initial assessment of the bowel is complete with a convex probe, careful repeat assessment of the entire colon and small bowel should be performed for IBD activity assessment with the linear high-frequency probe. Measurements for BWT should be performed for each segment in both longitudinal and transverse planes, with an average of measurements taken at least 1 cm apart denoting the thickness between the lumen-mucosal interface and the muscularis-serosal interface (Figure 2). CDS assessment of the bowel wall for hyperemia should be performed in each segment with use of the modified Limberg score (Figure 3). The other two parameters that should be assessed in each segment are inflammatory fat (iFat) and the presence or loss of bowel wall layer stratification.
The primary bowel segments assessed during every IUS examination are the sigmoid colon, descending colon, transverse colon, ascending colon, cecum, and terminal ileum[7]. Visualization of the rectum can be challenging and typically only performed with adequate resolution for disease activity assessment in patients with lower body mass indices and full bladders at the time of examination. Even with visualization of the rectum, normal values are not well defined, thus making it even harder for use at the point-of-care for disease activity assessment without benchmark with another non-invasive biomarker such as FC or a recent endoscopic evaluation. Additionally, the proximal small bowel beyond 30 cm of the ileum remains challenging and even an expert utilizing the proper technique may miss proximal ileal or jejunal inflammation relying on evaluation with IUS alone (Table 1).
| Optimal bowel segments | Limited bowel segments |
| Sigmoid colon | Rectum |
| Descending colon | Splenic flexure |
| Transverse colon | Proximal ileum |
| Ascending colon | Jejunum |
| Ileocecal valve | |
| Terminal ileum |
IUS assessment of BWT is the primary and most important measure of disease activity in both CD and UC and has been shown to correlate with endoscopic inflammation[8]. In adults, a BWT cut-off > 3 mm in any segment is the most consistent individual IUS parameter that correlates with active endoscopic disease, with a sensitivity and specificity of 88%-89% and 93%-96% respectively[9,10]. When assessing the colonic bowel segments in patients with IBD, the per patient sensitivity and specificity of IUS to detect disease activity is 90% and 96% respectively[11]. UC is a transmural disease and thus IUS is an excellent option to monitor disease activity non-invasively and assess disease extension in patients with ulcerative proctitis[12]. In a retrospective study of patients with UC, BWT alone was shown to correlate with the Mayo endoscopic score[13]. In children, there is no agreed upon cut-off for BWT that correlates with endoscopic activity, however a BWT < 2 mm is almost always normal[14].
After BWT, hyperemia assessed by CDS is the next most important measure of disease activity. Assessment of CDS alone has been shown to correlate with CD clinical activity[15-17]. When combined with increased BWT, increased hyperemia is almost always representative of active disease. In fact, in development of both CD and UC IUS scores with endoscopy, multivariable analysis demonstrates that often the only independent predictors of endoscopic activity are BWT and hyperemia[18-20]. Additional characteristics of inflammation in IBD are focal disruption of the bowel wall layers, and hypoechoic or hyperechoic changes in the layers representing a loss of bowel wall stratification. Inflammatory mesenteric fat wrapping is seen, appearing hyperechoic encasing the inflamed bowel.
There have been more than twenty IUS activity scores developed for adults with CD and UC, with the most common and important parameters being BWT, followed by both hyperemia and bowel wall stratification[21]. Only a few recently developed IUS scores have been validated with a standardized endoscopic score. They are simple and can be used at the point-of-care for clinical decision making without significant time for calculation (Table 2)[19,20,22-24]. Only two scores specific to children with IBD have been developed, one CD and one UC, and only the UC score is validated with an endoscopic score[24,25]. A novel IUS score recently developed by a group of international experts, the International Bowel Ultrasound Group (IBUS)-SAS (score 0-100), is yet to be validated and is more complex. Although its clinical utility may be limited, it may serve as a novel score for disease monitoring and treatment response in clinical trials[26].
| IUS scoring system | BWT (mm) | iFAT | Modified LS | LBWS | Other |
| Crohn’s disease indices | |||||
| SUS-CD (0-5)[19] | 0: < 3.0 | 0: LS 0 | |||
| 1: 3.0-4.9 | 1: LS 1-2 | ||||
| 2: 5.0-7.9 | 2: LS 3 | ||||
| 3: ≥ 8.0 | |||||
| Simple US score[20] | 0.957 (BWT) | 0.859 (LS) | |||
| Ulcerative colitis indices | |||||
| MUC[21,24] | 1.4 (BWT) | 0: LS 0 | |||
| 2: LS 1-3 | |||||
| UC-IUS (0-7)[25] | 0: ≤ 2 | 0: Absent | 0: LS 0 | 0: No loss | |
| 1: 2.1-3.0 | 1: Present | 1: LS 1 | 1: Loss is present | ||
| 2: 3.1-4.0 | 2: LS 2-3 | ||||
| 3: > 4 | |||||
| Civitelli UC index (0-4)[26] (pediatric) | 0: ≤ 3 | 0: LS 0 | 0: No loss | Abnormal haustrations (0: Normal, 1: Abnormal) | |
| 1: > 3 | 2: LS 1-3 | 1: Loss is present | |||
IUS is a reliable tool to monitor treatment response in both CD and UC. Improvement in all IUS parameters (BWT, hyperemia, iFat, bowel wall stratification) can be seen within 12 wk of treatment initiation, with faster changes seen in certain patients and in the colon as compared to the ileum. (30) How to interpret these changes, including defining transmural remission, and the timing of repeat IUS assessments remains an evolving concept.
In patients with CD prescribed anti-inflammatory IBD therapy, a large multicenter German study demonstrated that improvement in all IUS measures can be seen at both 3 and 12 mo[27]. TH, defined as a BWT ≤ 3 mm and absent hyperemia by CDS, has been shown to be achievable in approximately 25%-31% of adult patients with CD after 2 years of treatment[28-32]. The STARDUST sub-study recently evaluated the effect of ustekinumab on TH monitored by IUS and demonstrated increasing TH rates to a total of 24.1% by week 48[33]. In 40 adult patients with endoscopically active CD initiating anti-tumor necrosis factor therapy, an 18% decrease in BWT 4-8 wk post-induction predicted endoscopic response [area under the receiver operating characteristic curve (AUROC) =0.77, odds ratio (OR) = 10.80, P = 0.012] and a BWT cut-off of 3.2 mm was accurate to detect endoscopic remission (AUROC = 0.94, OR = 39.42, P < 0.0001) at weeks 12-32[34]. Lastly, a recent multicenter Italian study of adult patients with CD treated with various biologic therapies demonstrated higher rates of TH (defined as normalization of all IUS parameters) with improvement in IUS in 53% at 3 mo and 64% by 1 year with a number needed to treat of 3.6 patients in order to achieve TH. Compared to MH alone, TH is associated with improved outcomes and a decreased risk of long-term disease progression[29,30,35,36].
Similarly, there is evidence in patients with UC to support the use of IUS as a monitoring tool of treatment response, and in fact, changes on IUS may be seen quicker in patients with UC compared to those with ileal CD. Evidence comes from a large multicenter German study of patients with UC who experienced clinical relapse and were treated with anti-inflammatory therapies; there was improvement in the colon BWT as early as 2 wk and BWT improvement continued 12 wk after treatment initiation[37]. In a study of 74 patients with UC, those who did not have a significant treatment response on IUS by 3 mo, measured by a significantly increased BWT to > 6 mm and presence of hyperemia, were at increased risk of continued severe endoscopic activity at 15 mo[38]. In 27 patients with UC treated with tofacitinib, BWT correlated with Mayo score (r = 0.68, P < 0.0001) and UC Endoscopic Index for Severity (r = 0.73, P < 0.0001) at induction and week 8 after treatment initiation. A decrease in BWT was more pronounced in patients with endoscopic response with a decrease in BWT of 32% from baseline being accurate for endoscopic response (AUROC = 0.87) and a BWT cut-off of 2.8 mm being the most accurate value to detect endoscopic remission (AUROC = 0.87)[39]. Utilizing IUS at baseline in patients with UC can also predict disease course. A Milan Ultrasound Criteria score > 6.2 at baseline in a study of 98 patients with UC was predictive of a negative disease course (need for corticosteroids, change in therapy, hospitalization, or colectomy) at 1.6 years after treatment initiation[40].
While it is critical to control IBD activity during pregnancy, the tools for tight control disease activity monitoring are limited. IUS provides significant value for the precise monitoring of disease activity in the colon throughout all three trimesters and in the terminal ileum during the first two trimesters. In an Australian cohort of 90 pregnant women with IBD, 127 IUS examinations were performed. Adequate ileal views for disease activity assessment were obtained in 93% of patients at less than 20 wk gestational age, but in only 56% of patients at 20-26 wk. In contrast, adequate colonic views were obtained in 91% of all IUS examinations[41]. In this cohort, BWT was compared to FC as the current reference standard for disease activity monitoring during pregnancy and BWT was found to positively correlate with FC (r = 0.26, P = 0.03) and had a sensitivity of 74%, specificity of 83% and negative predictive value of 90%. Similarly, in a small Dutch cohort of 38 pregnant women with IBD, 27 of whom were followed with serial IUS examinations during pregnancy, feasibility to assess the terminal ileum significantly decreased from 91.3% in the first trimester to 21.7% in the third trimester (P < 0.0001). When compared to FC and clinical activity combined, IUS was able to distinguish active from quiescent disease with 84% sensitivity and 98% specificity[42].
In children, use of IUS as a non-invasive tool to monitor disease activity is of even more importance than in adults, as younger children often require sedation for MRE and there is a need to reduce the risk of radiation exposure from repeated CT scans. As previously stated, there is a clear consensus that a cut-off value for normal BWT in children is not 3 mm, but more likely to be in the range of 2-2.5 mm[14]. Similar to adults, IUS can be used to monitor treatment response in children with CD treated with infliximab. In a study of 28 children with newly diagnosed ileal CD, BWT, hyperemia and involved segment length all significantly decreased as early as 2 wk after infliximab initiation and there was a strong correlation between CDS and FC (r = 0.71, P < 0.0001). Linear mixed models from this study demonstrated that BWT, hyperemia, and involved segment length continue to decrease over the course of 6 mo after infliximab initiation[43]. In a small pilot study of 13 children with small bowel CD undergoing infliximab induction, bowel wall hyperemia decreased in all but one patient post-induction (P = 0.01), indicating that hyperemia assessed by CDS may be the earliest IUS measure to normalize post-induction[44].
IUS is an accurate tool for monitoring postoperative CD recurrence in the neo-terminal ileum after ileocolic resection. BWT greater than 3-3.5 mm is accurate to detect recurrence based on ileocolonoscopy with a sensitivity of 90%-100%[45]. In a study using both traditional IUS and CEUS to assess CD recurrence, 90 patients, 62 of which had severe recurrence (Rutgeerts score i3 or i4), underwent IUS, CEUS, and endoscopy. A BWT > 5 mm, without any additional parameters, demonstrated 100% specificity to detect recurrence and a BWT > 6 mm was 95.7% specific to detect severe recurrence. The addition of bowel wall contrast enhancement ≥ 70% to either BWT ≥ 5 or 6 mm was the most accurate to the Rutgeerts score with an AUROC of 0.89 for detecting recurrence[46].
Overall IUS, MRE, and CTE have similar accuracy for the diagnosis of CD complications such as strictures, abscesses, and fistulae[47]. In the largest study to date, the prospective multi-center METRIC trial included 284 patients, 233 of which had small bowel CD, and demonstrated that IUS and MRE are comparable for detection of disease in the terminal ileum. IUS had a sensitivity and specificity of 92% and 84% while MRE had a sensitivity and specificity of 97% and 96%. MRE was superior to IUS for the detection of small bowel disease extent with a sensitivity of 80% compared to 70% for IUS. Similarly, in a multicenter Italian study of 234 adult patients with CD, IUS and MRE had comparable accuracy with a sensitivity and specificity to detect inflammation of 96% and 97% for IUS and 96% and 94% for MRE. MRE was more accurate than IUS to define small bowel disease extension (r = 0.69) and detect fistulae (k = 0.67), but comparable for detection of strictures (k = 0.82) and abscesses (k = 0.88)[48]. As such, the updated guidelines from the European Crohn’s and Colitis Organization (ECCO)-European Society for Gastrointestinal and Abdominal Radiology and ECCO-European Society for Pediatric Gastroenterology, Hepatology, and Nutrition now propose the use of IUS in the diagnostic evaluation for adult and pediatric CD[49,50].
Follow-up analysis from the METRIC trial for observer agreement demonstrated that IUS and MRE are similar, with IUS performing slightly better than MRE numerically. Interobserver agreement for MRE was modest for new diagnosis [68% (k = 0.36)] and relapsed patients [78% (k = 0.56)] and only slight for colonic assessment for new diagnosis [61% (k = 0.21)] and relapsed patients [60% (k = 0.20)][51]. Interobserver agreement for IUS was higher than MRE in the small bowel for new diagnosis [82% (k = 0.64)] and for relapsed patients [81% (k = 0.63)] and in the colon for new diagnosis [64% (k = 0.27)] and relapsed patients [78% (k = 0.56)][52]. Furthermore, a retrospective study in children with IBD demonstrated that MRE is not accurate for the assessment of colonic disease, with the simplified Magnetic Resonance Index of Activity unable to identify severe lesions in colonic segments[53].
When determining the optimal tight control monitoring tool, perhaps the most important aspect to consider is patient preference and compliance. IUS is one of the most preferred monitoring tool by patients with IBD. In a survey of 121 Australian patients with a formal diagnosis of IBD, IUS scored highly acceptable for disease activity monitoring (mean 9.20 ± 1.37) compared to colonoscopy (7.94 ± 2.30), FC (8.17 ± 1.96), serum sampling (8.87 ± 1.62) and alternative forms of cross-sectional imaging (8.67 ± 1.60)[54].
Beyond preference, use of IUS for IBD monitoring enhances shared understanding and increases the ability of providers to make major treatment decisions during routine clinic visits. In a study of patients randomized to ultrasound-driven IBD care vs non-ultrasound driven IBD care, patients who underwent IUS reported better understanding of all aspects of their disease and symptoms, and were more confident in their ability to make informed decisions about managing their disease[55]. Furthermore, gastroenterologists altered management by changing medications in 47% of patients in the ultrasound group compared to just 22% in the non-ultrasound group (P = 0.002). Based on disease activity, providers were more likely to change therapy when patients had an IUS compared to when they did not (P = 0.024).
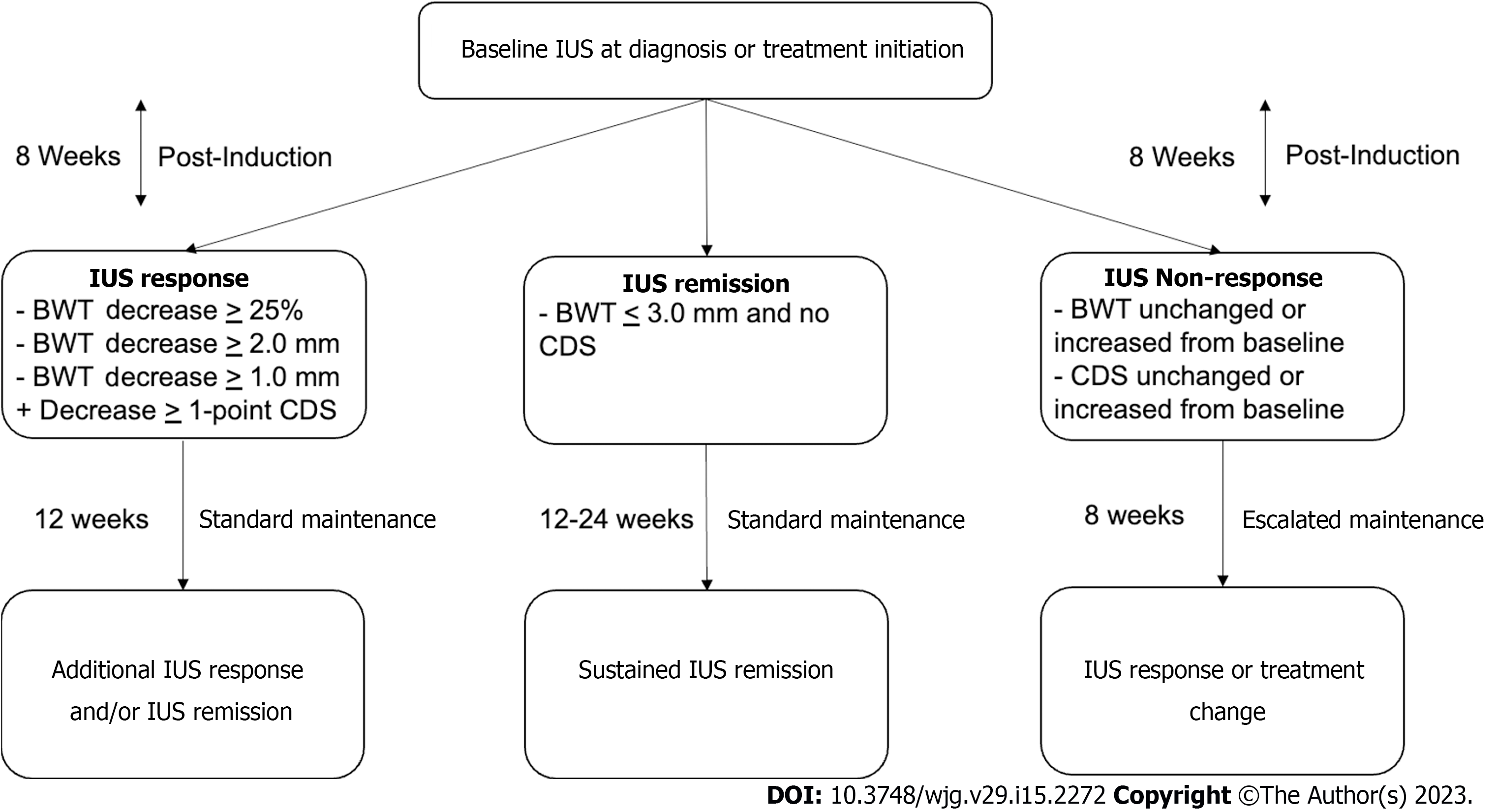
Debate over the timing and utilization of IUS for tight control monitoring, as well as the definition of treatment response and remission, is ongoing. Multiple studies demonstrate a decrease in BWT by week 4 after therapy initiation, but the longitudinal effect of response reassessment at this early timepoint is still unclear[43,56]. A panel of gastroenterologists from the IBUS developed an expert consensus statement and defined: (1) IUS response as a decrease in BWT by 25% from baseline or 2.0 mm, or greater than 1.0 mm with a decrease in CDS by the modified Limberg score of one grade; and (2) IUS remission as a BWT ≤ 3 mm and normal CDS or absence of hyperemia[57].
Here we propose a practical tight control monitoring algorithm based on IUS (Figure 4). IUS should be repeated post-induction at week eight, regardless of biologic therapy, to assess change in BWT and hyperemia by CDS. If there is no change or an increase in BWT and/or hyperemia, optimization of therapy (dose or interval escalation) should be considered. Repeat IUS should then occur again in eight weeks to reassess response. If again there is no change or worsening, repeat ileocolonoscopy assessment and subsequent therapy discontinuation and switch should be considered. If there is IUS response (as defined above), then the interval to repeat IUS should be extended to 12 wk. Repeat IUS should be performed then every 12 wk until one year and if complete IUS transmural remission is achieved, then IUS can be performed every 6 mo for subsequent monitoring and confirmation of sustained transmural remission.
Eventually, the use of IUS at the point-of-care may be able to quantify the proportion of active vs chronic inflammation in patients with IBD. This will aid in treatment decision making, guiding choices toward either an anti-inflammatory or anti-fibrotic therapy, with or without surgery. In a study of 35 CD patients who underwent sheer wave elastography (SWE) within 1 wk of surgical resection, a cut-off value of 22.5 kPa was 69.6% sensitive and 91.7% specific with an AUROC of 0.82 to discriminate between mild/moderate and severe fibrosis based on histopathology[58]. Future studies validating cut-offs to discriminate degrees of fibrosis and active inflammation are still needed, but in the future provides will be able to personalize management for patients based on where they fall on the active inflammation vs fibrosis pathway utilizing SWE[59,60]. Additionally, use of hand-held IUS in combination with artificial intelligence will be able to help patients monitor both their active inflammation and fibrosis remotely for further tight control in the future[61].
IUS is an accurate, non-invasive cross-sectional imaging tool to assess IBD activity in real time. Incorporation of IUS into a tight control monitoring strategy promotes quick and impactful treatment decisions that can modify disease progression and enhance patient compliance. Specific populations of patients with IBD, especially children and pregnant women, would benefit significantly from the increased use of IUS for disease monitoring. Continued advances in technology will likely allow for enhanced stratification of active vs chronic inflammation at the point-of-care and enable remote monitoring for even tighter control. Ultimately, an IUS-based tight control monitoring algorithm and prediction tool may be used for early treatment decisions to achieve sustained deep remission and disease modification.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giannetti A, Italy; Zhou W, China S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Kayal M, Ungaro RC, Bader G, Colombel JF, Sandborn WJ, Stalgis C. Net Remission Rates with Biologic Treatment in Crohn's Disease: A Reappraisal of the Clinical Trial Data. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 2. | Bryant RV, Friedman AB, Wright EK, Taylor KM, Begun J, Maconi G, Maaser C, Novak KL, Kucharzik T, Atkinson NSS, Asthana A, Gibson PR. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut. 2018;67:973-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Kucharzik T, Kannengiesser K, Petersen F. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol. 2017;30:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Ripollés T, Martínez-Pérez MJ, Paredes JM, Vizuete J, García-Martínez E, Jiménez-Restrepo DH. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn's disease and other abdominal conditions. Eur J Radiol. 2013;82:e525-e531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Calabrese E, Maaser C, Zorzi F, Kannengiesser K, Hanauer SB, Bruining DH, Iacucci M, Maconi G, Novak KL, Panaccione R, Strobel D, Wilson SR, Watanabe M, Pallone F, Ghosh S. Bowel Ultrasonography in the Management of Crohn's Disease. A Review with Recommendations of an International Panel of Experts. Inflamm Bowel Dis. 2016;22:1168-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, Bianco R, Gallus S, Bianchi Porro G. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn's disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Parente F, Greco S, Molteni M, Cucino C, Maconi G, Sampietro GM, Danelli PG, Cristaldi M, Bianco R, Gallus S, Bianchi Porro G. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther. 2003;18:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF, Hollerweger A, Sporea I, Saftoiu A, Maaser C, Hausken T, Higginson AP, Nürnberg D, Pallotta N, Romanini L, Serra C, Gilja OH. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018;39:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Dong J, Wang H, Zhao J, Zhu W, Zhang L, Gong J, Li Y, Gu L, Li J. Ultrasound as a diagnostic tool in detecting active Crohn's disease: a meta-analysis of prospective studies. Eur Radiol. 2014;24:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Antonelli E, Giuliano V, Casella G, Villanacci V, Baldini V, Baldoni M, Morelli O, Bassotti G. Ultrasonographic assessment of colonic wall in moderate-severe ulcerative colitis: comparison with endoscopic findings. Dig Liver Dis. 2011;43:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | van Wassenaer EA, de Voogd FAE, van Rijn RR, van der Lee JH, Tabbers MM, van Etten-Jamaludin FS, Kindermann A, de Meij TGJ, Gecse KB, D'Haens GR, Benninga MA, Koot BGP. Bowel ultrasound measurements in healthy children - systematic review and meta-analysis. Pediatr Radiol. 2020;50:501-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Yekeler E, Danalioglu A, Movasseghi B, Yilmaz S, Karaca C, Kaymakoglu S, Acunas B. Crohn disease activity evaluated by Doppler ultrasonography of the superior mesenteric artery and the affected small-bowel segments. J Ultrasound Med. 2005;24:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Sjekavica I, Barbarić-Babić V, Krznarić Z, Molnar M, Cuković-Cavka S, Stern-Padovan R. Assessment of Crohn's disease activity by doppler ultrasound of superior mesenteric artery and mural arteries in thickened bowel wall: cross-sectional study. Croat Med J. 2007;48:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Karoui S, Nouira K, Serghini M, Ben Mustapha N, Boubaker J, Menif E, Filali A. Assessment of activity of Crohn's disease by Doppler sonography of superior mesenteric artery flow. J Crohns Colitis. 2010;4:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Sævik F, Eriksen R, Eide GE, Gilja OH, Nylund K. Development and Validation of a Simple Ultrasound Activity Score for Crohn's Disease. J Crohns Colitis. 2021;15:115-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Ripollés T, Poza J, Suarez Ferrer C, Martínez-Pérez MJ, Martín-Algíbez A, de Las Heras Paez B. Evaluation of Crohn's Disease Activity: Development of an Ultrasound Score in a Multicenter Study. Inflamm Bowel Dis. 2021;27:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 20. | Allocca M, Fiorino G, Bonovas S, Furfaro F, Gilardi D, Argollo M, Magnoni P, Peyrin-Biroulet L, Danese S. Accuracy of Humanitas Ultrasound Criteria in Assessing Disease Activity and Severity in Ulcerative Colitis: A Prospective Study. J Crohns Colitis. 2018;12:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Goodsall TM, Nguyen TM, Parker CE, Ma C, Andrews JM, Jairath V, Bryant RV. Systematic Review: Gastrointestinal Ultrasound Scoring Indices for Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:125-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Allocca M, Filippi E, Costantino A, Bonovas S, Fiorino G, Furfaro F, Peyrin-Biroulet L, Fraquelli M, Caprioli F, Danese S. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: external validation. United European Gastroenterol J. 2021;9:438-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Bots S, Nylund K, Löwenberg M, Gecse K, D'Haens G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J Crohns Colitis. 2021;15:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Civitelli F, Di Nardo G, Oliva S, Nuti F, Ferrari F, Dilillo A, Viola F, Pallotta N, Cucchiara S, Aloi M. Ultrasonography of the colon in pediatric ulcerative colitis: a prospective, blind, comparative study with colonoscopy. J Pediatr. 2014;165:78-84.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Kellar A, Wilson S, Kaplan G, DeBruyn J, Tanyingoh D, Novak KL. The Simple Pediatric Activity Ultrasound Score (SPAUSS) for the Accurate Detection of Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2019;69:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Novak KL, Nylund K, Maaser C, Petersen F, Kucharzik T, Lu C, Allocca M, Maconi G, de Voogd F, Christensen B, Vaughan R, Palmela C, Carter D, Wilkens R. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn's Disease. J Crohns Colitis. 2021;15:609-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 27. | Kucharzik T, Wittig BM, Helwig U, Börner N, Rössler A, Rath S, Maaser C; TRUST study group. Use of Intestinal Ultrasound to Monitor Crohn's Disease Activity. Clin Gastroenterol Hepatol. 2017;15:535-542.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 28. | Castiglione F, Testa A, Rea M, De Palma GD, Diaferia M, Musto D, Sasso F, Caporaso N, Rispo A. Transmural healing evaluated by bowel sonography in patients with Crohn's disease on maintenance treatment with biologics. Inflamm Bowel Dis. 2013;19:1928-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 29. | Castiglione F, Imperatore N, Testa A, De Palma GD, Nardone OM, Pellegrini L, Caporaso N, Rispo A. One-year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49:1026-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 30. | Fernandes SR, Rodrigues RV, Bernardo S, Cortez-Pinto J, Rosa I, da Silva JP, Gonçalves AR, Valente A, Baldaia C, Santos PM, Correia L, Venâncio J, Campos P, Pereira AD, Velosa J. Transmural Healing Is Associated with Improved Long-term Outcomes of Patients with Crohn's Disease. Inflamm Bowel Dis. 2017;23:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (115)] |
| 31. | Paredes JM, Ripollés T, Cortés X, Martínez MJ, Barrachina M, Gómez F, Moreno-Osset E. Abdominal sonographic changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn's Disease. Dig Dis Sci. 2010;55:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Paredes JM, Moreno N, Latorre P, Ripollés T, Martinez MJ, Vizuete J, Moreno-Osset E. Clinical Impact of Sonographic Transmural Healing After Anti-TNF Antibody Treatment in Patients with Crohn's Disease. Dig Dis Sci. 2019;64:2600-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Kucharzik T, Wilkens R, D'Agostino MA, Maconi G, Le Bars M, Lahaye M, Bravatà I, Nazar M, Ni L, Ercole E, Allocca M, Machková N, de Voogd FAE, Palmela C, Vaughan R, Maaser C; STARDUST Intestinal Ultrasound study group. Early Ultrasound Response and Progressive Transmural Remission After Treatment With Ustekinumab in Crohn's Disease. Clin Gastroenterol Hepatol. 2023;21:153-163.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 34. | de Voogd F, Bots S, Gecse K, Gilja OH, D'Haens G, Nylund K. Intestinal Ultrasound Early on in Treatment Follow-up Predicts Endoscopic Response to Anti-TNFα Treatment in Crohn's Disease. J Crohns Colitis. 2022;16:1598-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Bachour SP, Shah RS, Lyu R, Nakamura T, Shen M, Li T, Dane B, Barnes EL, Rieder F, Cohen B, Qazi T, Lashner B, Achkar JP, Philpott J, Holubar SD, Lightner AL, Regueiro M, Axelrad J, Baker ME, Click B. Test Characteristics of Cross-sectional Imaging and Concordance With Endoscopy in Postoperative Crohn's Disease. Clin Gastroenterol Hepatol. 2022;20:2327-2336.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Vaughan R, Tjandra D, Patwardhan A, Mingos N, Gibson R, Boussioutas A, Ardalan Z, Al-Ani A, Gibson PR, Christensen B. Toward transmural healing: Sonographic healing is associated with improved long-term outcomes in patients with Crohn's disease. Aliment Pharmacol Ther. 2022;56:84-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Maaser C, Petersen F, Helwig U, Fischer I, Roessler A, Rath S, Lang D, Kucharzik T; German IBD Study Group and the TRUST&UC study group; German IBD Study Group and TRUST&UC study group. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 38. | Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, Sampietro G, Gallus S. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig Dis. 2009;27:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | de Voogd F, van Wassenaer EA, Mookhoek A, Bots S, van Gennep S, Löwenberg M, D'Haens GR, Gecse KB. Intestinal Ultrasound Is Accurate to Determine Endoscopic Response and Remission in Patients With Moderate to Severe Ulcerative Colitis: A Longitudinal Prospective Cohort Study. Gastroenterology. 2022;163:1569-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 40. | Allocca M, Dell'Avalle C, Craviotto V, Furfaro F, Zilli A, D'Amico F, Bonovas S, Peyrin-Biroulet L, Fiorino G, Danese S. Predictive value of Milan ultrasound criteria in ulcerative colitis: A prospective observational cohort study. United European Gastroenterol J. 2022;10:190-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Flanagan E, Wright EK, Begun J, Bryant RV, An YK, Ross AL, Kiburg KV, Bell SJ. Monitoring Inflammatory Bowel Disease in Pregnancy Using Gastrointestinal Ultrasonography. J Crohns Colitis. 2020;14:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 42. | De Voogd F, Joshi H, Van Wassenaer E, Bots S, D'Haens G, Gecse K. Intestinal Ultrasound to Evaluate Treatment Response During Pregnancy in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2022;28:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Dillman JR, Dehkordy SF, Smith EA, DiPietro MA, Sanchez R, DeMatos-Maillard V, Adler J, Zhang B, Trout AT. Defining the ultrasound longitudinal natural history of newly diagnosed pediatric small bowel Crohn disease treated with infliximab and infliximab-azathioprine combination therapy. Pediatr Radiol. 2017;47:924-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Dolinger MT, Choi JJ, Phan BL, Rosenberg HK, Rowland J, Dubinsky MC. Use of Small Bowel Ultrasound to Predict Response to Infliximab Induction in Pediatric Crohn's Disease. J Clin Gastroenterol. 2021;55:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Rispo A, Imperatore N, Testa A, Nardone OM, Luglio G, Caporaso N, Castiglione F. Diagnostic Accuracy of Ultrasonography in the Detection of Postsurgical Recurrence in Crohn's Disease: A Systematic Review with Meta-analysis. Inflamm Bowel Dis. 2018;24:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Martínez MJ, Ripollés T, Paredes JM, Moreno-Osset E, Pazos JM, Blanc E. Intravenous Contrast-Enhanced Ultrasound for Assessing and Grading Postoperative Recurrence of Crohn's Disease. Dig Dis Sci. 2019;64:1640-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther. 2011;34:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 48. | Castiglione F, Mainenti PP, De Palma GD, Testa A, Bucci L, Pesce G, Camera L, Diaferia M, Rea M, Caporaso N, Salvatore M, Rispo A. Noninvasive diagnosis of small bowel Crohn's disease: direct comparison of bowel sonography and magnetic resonance enterography. Inflamm Bowel Dis. 2013;19:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1159] [Article Influence: 193.2] [Reference Citation Analysis (0)] |
| 50. | van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 51. | Bhatnagar G, Mallett S, Quinn L, Beable R, Bungay H, Betts M, Greenhalgh R, Gupta A, Higginson A, Hyland R, Ilangovan R, Lambie H, Mainta E, Patel U, Pilcher J, Plumb A, Porté F, Sidhu H, Slater A, Tolan D, Zealley I, Halligan S, Taylor S. Interobserver variation in the interpretation of magnetic resonance enterography in Crohn's disease. Br J Radiol. 2022;95:20210995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Bhatnagar G, Quinn L, Higginson A, Plumb A, Halligan S, Tolan D, Lapham R, Mallett S, Taylor SA; METRIC study investigators. Observer agreement for small bowel ultrasound in Crohn's disease: results from the METRIC trial. Abdom Radiol (NY). 2020;45:3036-3045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Lepus CA, Moote DJ, Bao S, Mosha MH, Hyams JS. Simplified Magnetic Resonance Index of Activity Is Useful for Terminal Ileal but not Colonic Disease in Pediatric Crohn Disease. J Pediatr Gastroenterol Nutr. 2022;74:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Rajagopalan A, Sathananthan D, An YK, Van De Ven L, Martin S, Fon J, Costello SP, Begun J, Bryant RV. Gastrointestinal ultrasound in inflammatory bowel disease care: Patient perceptions and impact on disease-related knowledge. JGH Open. 2020;4:267-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Friedman AB, Asthana A, Knowles SR, Robbins A, Gibson PR. Effect of point-of-care gastrointestinal ultrasound on decision-making and management in inflammatory bowel disease. Aliment Pharmacol Ther. 2021;54:652-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Ripollés T, Martínez MJ, Barrachina MM. Crohn's disease and color Doppler sonography: response to treatment and its relationship with long-term prognosis. J Clin Ultrasound. 2008;36:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Ilvemark JFKF, Hansen T, Goodsall TM, Seidelin JB, Al-Farhan H, Allocca M, Begun J, Bryant RV, Carter D, Christensen B, Dubinsky MC, Gecse KB, Kucharzik T, Lu C, Maaser C, Maconi G, Nylund K, Palmela C, Wilson SR, Novak K, Wilkens R. Defining Transabdominal Intestinal Ultrasound Treatment Response and Remission in Inflammatory Bowel Disease: Systematic Review and Expert Consensus Statement. J Crohns Colitis. 2022;16:554-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 58. | Chen YJ, Mao R, Li XH, Cao QH, Chen ZH, Liu BX, Chen SL, Chen BL, He Y, Zeng ZR, Ben-Horin S, Rimola J, Rieder F, Xie XY, Chen MH. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn's Disease. Inflamm Bowel Dis. 2018;24:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Keshavarzi NR, Rubin JM. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33:2115-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Lu C, Gui X, Chen W, Fung T, Novak K, Wilson SR. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn's Disease Strictures. Inflamm Bowel Dis. 2017;23:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Rispo A, de Sire R, Mainenti PP, Imperatore N, Testa A, Maurea S, Ricciolino S, Nardone OM, Olmo O, Castiglione F. David Against Goliath: Direct Comparison of Handheld Bowel Sonography and Magnetic Resonance Enterography for Diagnosis of Crohn's Disease. Inflamm Bowel Dis. 2023;29:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |