Published online Apr 7, 2023. doi: 10.3748/wjg.v29.i13.1982
Peer-review started: January 9, 2023
First decision: February 7, 2023
Revised: February 20, 2023
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: April 7, 2023
Processing time: 87 Days and 17 Hours
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease worldwide. Reduced activity and slower metabolism in the elderly affect the balance of lipid metabolism in the liver leading to the accumulation of lipids. This affects the mitochondrial respiratory chain and the efficiency of β-oxidation and induces the overproduction of reactive oxygen species. In addition, the dynamic balance of the mitochondria is disrupted during the ageing process, which inhibits its phagocytic function and further aggravates liver injury, leading to a higher incidence of NAFLD in the elderly population. The present study reviewed the manifestations, role and mechanism of mitochondrial dysfunction in the progression of NAFLD in the elderly. Based on the understanding of mitochondrial dysfunction and abnormal lipid metabolism, this study discusses the treatment strategies and the potential therapeutic targets for NAFLD, including lipid accumulation, antioxidation, mitophagy and liver-protecting drugs. The purpose is to provide new ideas for the development of innovative drugs for the prevention and treatment of NAFLD.
Core Tip: The elderly are prone to a series of pathological damages such as liver fibrosis due to the decline of liver regeneration ability and immune response dysfunction. In addition, liver metabolism imbalance and mitochondrial dysregulation play a key role in the development of non-alcoholic fatty liver disease (NAFLD). And a treatment strategy for NAFLD was proposed in terms of abnormal lipid metabolism, mitophagy, and anti-oxidation, which provided new ideas for the development of innovative drugs for the prevention and treatment of NAFLD.
- Citation: Wang D, Ji DC, Yu CY, Wu DN, Qi L. Research progress on the mitochondrial mechanism of age-related non-alcoholic fatty liver. World J Gastroenterol 2023; 29(13): 1982-1993
- URL: https://www.wjgnet.com/1007-9327/full/v29/i13/1982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i13.1982
In the past several decades, ageing has become a research hot spot with the understanding of cancer and metabolic disorder-related diseases. The ageing liver is also becoming a public health problem[1]. Due to the decline in the regenerative ability of the liver and dysfunctions in the immune response, older people are more likely to suffer from non-alcoholic fatty liver disease (NAFLD), acute and chronic liver injury, liver fibrosis and other diseases. Studies have reported that the prevalence of NAFLD increases in the elderly, with a prevalence of < 30% in people under 40 years of age and > 50% in people over 60 years of age[2].
Currently, it is believed that the mechanism of development of NAFLD includes increased production of fat, increased dietary free fatty acid (FFA) levels, β-oxidative damage and dysfunction in very low density lipoprotein synthesis[3]. However, reduced activity and changes in diet structure lead to a continuous increase in body fat in the elderly. These factors lead to the accumulation of TGs in the liver and eventually cause age-related NAFLD[4]. Studies have reported that the accumulation of TG droplets in hepatocytes is not a harmful process in itself. On the contrary, it is considered an adaptive response to excessive lipid uptake or the production of fat[5], and this imbalance in TG synthesis and breakdown causes fatty degeneration of the liver[6]. In addition, the structural and functional changes in the mitochondria have been shown to be related to the pathogenesis of NAFLD. Ultramicroscopic analyses have demonstrated a disordered morphology of hepatocyte mitochondria in elderly patients with NAFLD, and the damage to the structure and function led to fatty degeneration of the liver and other injuries[3]. The changes in mitosis and fusion of mitochondria during ageing lead to the inhibition of mitochondrial phagocytosis[7]. Cell function can be affected further if the damaged mitochondria are not cleared in time[8].
Age-related diseases are the major challenge in the 21st century. Delaying ageing is a significant challenge that people want to overcome. Ageing has become a very serious risk factor. Ageing starts with molecular damage and eventually leads to the dysfunction of cells, tissues and organs[9]. Clinically, the core of biological ageing is the increased vulnerability to death. The structural and functional changes in the mitochondria have been proven to be related to the pathogenesis of NAFLD, the loss of mitochondrial DNA (mtDNA) in hepatocytes affects function, leading to hepatic steatosis and other injuries[10]. The present study reviewed the manifestations, role and mechanism of mitochondrial dysfunction in the progression of NAFLD in the elderly. In addition, the study discusses the treatment strategies for NAFLD based on the understanding of mitochondrial dysfunction and abnormal lipid metabolism to provide new ideas for the development of innovative drugs for the prevention and treatment of NAFLD.
Lipid metabolism is an important and complex biochemical process in the human body. The liver has a strong ability to synthesize fat, but it cannot store fat. Fatty degeneration of the liver occurs when fat is not transported in time. Simultaneously, the body decomposes fat through the action of various enzymes to maintain the dynamic balance of lipid metabolism. An imbalance in lipid metabolism ensues when there is a problem in decomposition. Therefore, in the event of fatty liver-related diseases, the primary consideration is to identify dysfunctions in the lipid metabolism pathway[11].
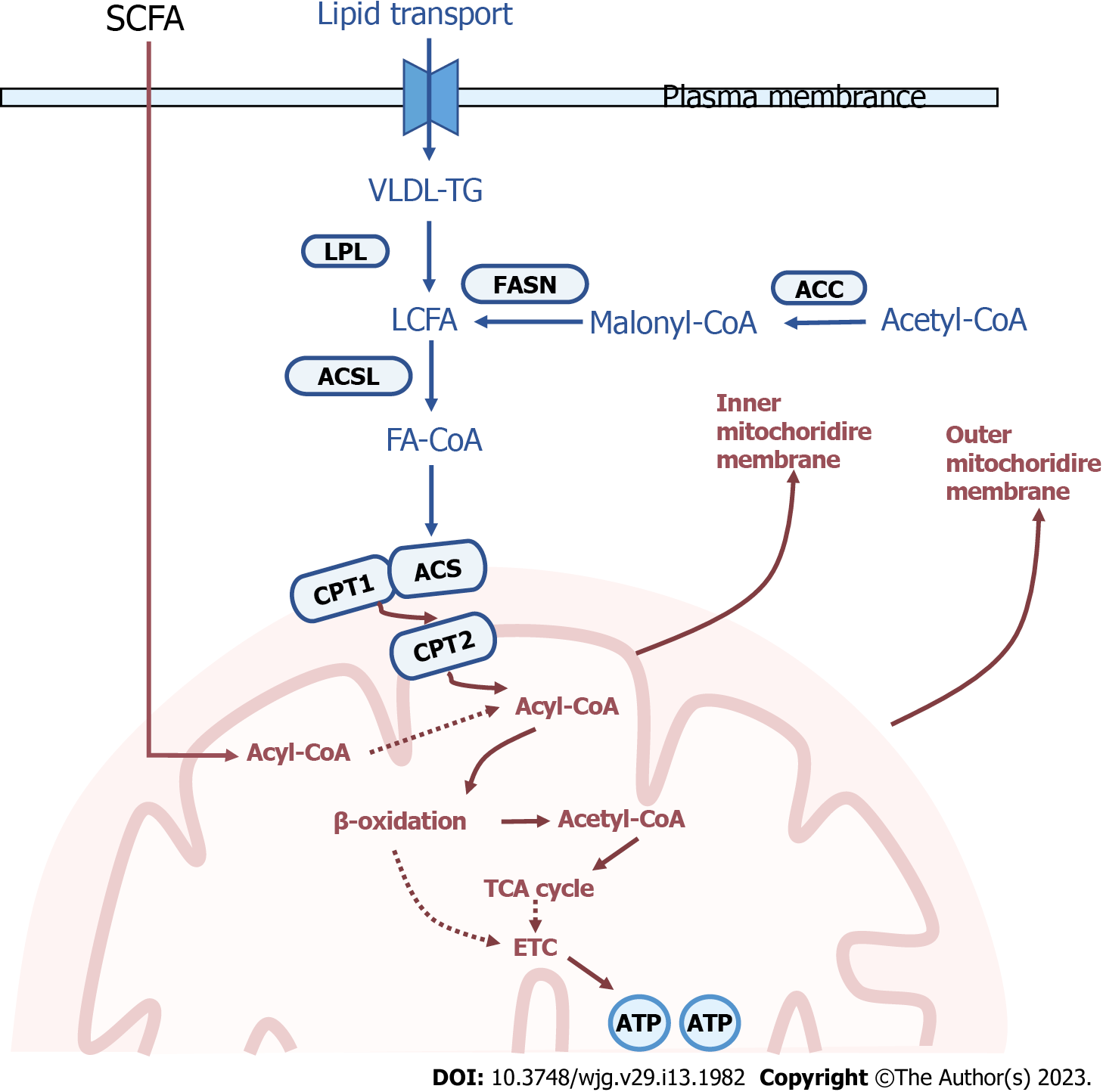
Mitochondria ensure a continuous supply of energy and metabolites to the organism through the aerobic oxidation of fatty acids, and the transfer of fatty acids into the mitochondria for β-oxidation needs to be regulated[12]. In the mitochondria, the rate of lipid oxidation is significantly related to the entry of long-chain fatty acids into the matrix as well as β-oxidation. The long-chain acyl-CoA synthetase catalyses the fatty acids to synthetize the acyl-coenzyme A. Considering that the inner mitochondrial membrane (IMM) is impermeable to coenzyme A, the exchange of coenzyme A and carnitine is essential. The enzyme carnitine palmitoyltransferase-1 (CPT-1) is located on the outer mitochondrial membrane (OMM) and catalyses the reversible transfer of acyl-coenzyme A groups (with a chain length of C12 to C18) to L-carnitine to form acylcarnitine esters[13]. This newly generated acylcarnitine is subsequently transferred into the mitochondrial matrix in exchange for free carnitine. This translocation step is mediated by carnitine-acylcarnitine translocase (gene name: SLC25A20). Once in the mitochondrial matrix, acyl groups are transferred back to coenzyme A via CPT2. Human CPT1 exists in three forms: Liver subtype (CPT1A), muscle subtype (CPT1B)[14] and cerebral subtype (CPT1C)[15]. CPT1A and CPT1B have similar functions but are expressed in different tissues. The deficiency of CPT1A is associated with increased levels of L-carnitine in plasma, which provides a clue for the diagnosis of CPT1A deficiency. At the cellular level, the lack of CPT1A activity in the liver leads to the inability to generate acylcarnitines that enter the mitochondria for oxidation. Therefore, CPT1 has become an important target in the regulation of mammalian lipid metabolism.
Abnormal lipid metabolism in the early stage of NAFLD induces oxidative stress, and mitochondrial dysfunction is also an important aspect of oxidative stress. Damage to the mtDNA is the main cause of mitochondrial structural damage. Gene damage occurs due to a direct attack on mtDNA by various lipid peroxidation products, resulting in the reduction of the synthesis of its encoded mitochondrial complex and ATP synthase. Gene damage directly affects mitochondrial function. Lipotoxicity-induced oxidative stress disorder reflects the close relationship between abnormal lipid metabolism and mitochondrial dysfunction (Figure 1).
In addition to various enzymes, nuclear receptors can also affect the β-oxidation of fatty acids[16]. Steatosis is one of the pathological characteristics of NAFLD, and fat accumulation leads to the activation of the nuclear receptor, peroxisome proliferator-activated receptors (PPARs)[17]. Studies conducted in 1990 revealed that drug experiments could cause the proliferation of liver peroxisomes, through which PPAR was discovered and later designated as PPARα (NR1C1). Subsequently, two additional PPARs were identified, namely, PPARβ/δ (NR1C2) and PPARγ (NR1C3). The expression organs and functions of the three PPARs are very different, PPARα is mainly expressed in the liver and brown adipose tissue[18]; PPARβ/δ is widely expressed in vivo, with relatively high expression in brain, stomach and intestine[19]; PPARγ expression is highest in white and brown adipose tissue[20]. PPARα is highly expressed in the liver, which is the main regulatory protein involved in hepatic β-oxidation, and is considered to be an important target for regulating dyslipidemia. Studies have shown that mitochondrial β-oxidation is significantly reduced in the liver of PPARα deficient mice[16]. Fatty liver can be improved by the activation of PPARα and the enhancement of hepatic fatty acid β-oxidation. Studies that investigated liver tissues from patients with NAFLD and non-alcoholic steatohepatitis (NASH)[21] reported that the expression of PPARα decreased with the progression of fibrosis. PPARα stimulants lead to decreased expression of substances associated with hepatic steatosis by enhancing mitochondrial β-oxidation. PPARα is involved in mitochondrial β-oxidation, with CPT1 as the key enzyme, allowing fatty acids to reach the mitochondrial matrix through the IMM and subsequently be metabolised. Abnormal PPARα expression can increase the levels of fatty acids and store them in the liver as triglycerides. PPARβ has been reported infrequently and has multiple functions and distributions, mainly being involved in wound healing. PPARγ is an important transcription factor for cell differentiation.
Lipid droplets (LDs) have a unique structure and exist in almost all cells. They are specialised organelles for storing lipids in cells. LDs provide energy for cells, aid in biofilm synthesis[22], and prevent FFA-induced lipotoxicity and its influx into toxic lipid species[23]. The accumulation of LDs in non-adipose tissue is a pathological feature of metabolic diseases such as obesity, diabetes and atherosclerosis[24]. In most cases, LDs exist in the form of an emulsion, which is the dispersed phase of water in oil in an aqueous solution. When large amounts of LDs are present, cells synthesize neutral lipids, which then form droplets dispersed in the aqueous phase[25]. The mechanism of the biogenesis of LDs remains unclear. The mechanisms underlying LDs biogenesis remain unknown, but studies suggest that LDs are simple structures surrounded by endoplasmic reticulum (ER)-derived phospholipid monolayers with dynamically changing proteome modifications on the surface[26]. A group of proteins, including seipin or lipid storage-inducing transmembrane proteins, are thought to be important for the formation of LDs. According to recent research, LDs are created de novo by neutral lipid deposition between ER leaflets and directed LD germination from the ER's outer leaflet into the cytoplasm[27].
LDs play a core role in cellular metabolism and homeostasis. Along with enhanced synthesis, decreased catabolism of LDs is another potential source of hepatic steatosis. There are two main pathways for the turnover of LDs in hepatocytes: conventional lipolysis and autophagy[28]. Lipolysis involves three key lipases, namely, hormone-sensitive lipase, adipose triglyceride lipase and monoacylglycerol lipase, which catalyse the rapid lipolysis of adipose tissue. In addition, some enzymes can directly interact with LDs to remove FAs from the triglyceride backbone stored in LDs one at a time. Furthermore, proteins also play an important role in the liver because the regulation of enzymes requires the participation of numerous hormones and growth factors through a variety of signal transduction pathways[29]. In addition to the above soluble lipases, hepatocytes also utilise the autophagy pathway to decompose LDs. Autophagy is mediated by lysosomes, and the formation of a special double-layer membrane vesicular structure during the process is called an autophagosome. In recent years, researchers have progressed in identifying the mechanism of other organelle-selective autophagy processes, such as mitochondrial phagocytosis and peroxidase phagocytosis[30]. Studies have found that lipid phagocytosis can be both non-selective and selective. Selective autophagy refers to the selective recognition and degradation of lipids, regulation of hepatocyte fat metabolism, and maintenance of intracellular lipid homeostasis.
Under normal circumstances, triglycerides are transported into the liver for lipid metabolism and are absorbed by hepatocytes. The main causes of NAFLD during ageing are an imbalance in hepatic metabolism, obesity, malnutrition and insulin resistance. However, there are few reports on the mechanism of development of age-related NAFLD, which needs further research in the future.
Obesity and metabolic abnormalities can be observed in elderly patients. The fat content in the body continues to increase with advancing age. Most of the fat exists in the form of triglycerides. The colour of the liver is closely related to the triglyceride content[31]. In addition to obesity, a low-quality diet and reduced activity in older patients can lead to malnutrition and muscle loss. Moreover, a low-quality diet can lead to an insufficient caloric supply and low protein levels, leading to an increase in the serum levels of FFAs. Furthermore, severe insulin resistance in skeletal muscles during ageing can induce the upregulation of the cholesterol regulatory element-binding protein 1, which inhibits β-oxidation and leads to fat deposition in the liver. Molecular substances related to NAFLD interfere with the cascade reaction of the insulin signalling pathway and aggravate insulin resistance. Studies have also reported that compared with young individuals, glycogen synthesis in the muscles of elderly individuals decreases by 45%, the liver fat re-synthesis rate is twice higher, and the fasting blood triacylglycerol level is significantly higher, which increases by approximately three times after food[32].
Mitochondria, which account for about one-fifth of the cell volume, are the organelles that mainly produce ATP in eukaryotic cells[33]. Almost all energy in the human body is generated by the tricarboxylic acid cycle and the electron transport chain of mitochondria, which is produced in the form of ATP. Mitochondria have inner and outer bilayers. The permeability of the inner membrane is lower than that of the outer membrane. The enzymes involved in the electron transport chain and ATP generation process are located in the inner membrane. An important feature of human ageing is a decrease in mitochondrial function in various tissues. Therefore, mitochondrial defects or dysregulation play a key role in ageing as well as diseases such as cancer[34].
Mitochondria are dynamic organelles that continuously undergo fusion/division, and their homeostatic balance is essential for the maintenance of function. Many genes associated with mitochondrial homeostasis are human disease genes. Mitochondrial dynamics are regulated by two opposite processes, fusion and fission. The processes are essential to regulate mitochondrial morphology, number, function and sub-cellular distribution, as well as to maintain mitochondrial homeostasis to cope with stress[35]. Mitochondrial dynamics vary according to the developmental stage, age, cell type, environmental factors and genetic background. Mitochondrial homeostasis is affected during ageing. The structure of the mitochondria becomes abnormal with advancing age, biogenesis is reduced, and mtDNA mutation is increased[36].
During ageing, the changes in mitochondrial fission and fusion lead to the inhibition of mitochondrial phagocytosis, and further damage to cellular function occurs if the damaged mitochondria are not cleared in time. The process of mitochondrial fusion requires the inner and outer membrane proteins, optic atrophy 1 (OPA1) protein and mitotic fusion proteins (MFN1 and MFN2)[37]. Deletion of the mitotic fusion proteins blocks the fusion of the inner and outer membrane proteins (IMM and OMM), while deletion of OPA1 blocks the fusion of the IMM but not the OMM[38]. The genetic disappearance of OPA1 changes the morphology and activity of lysosomes, thus inducing the accumulation of autophagic substrates. Mitochondrial fission is mediated by several proteins, with mitochondrial motility-related proteins being central. When mitochondria divide, dynamin-related protein 1 (DRP1) is located in the proteins and factors on the OMM to form a ring structure. The ring is divided into two mitochondrial cristae through the GTP enzyme activity of DRP1. After cell division, DRP1 returns to the cytoplasm to participate in the next mitochondrial division[39].
Mitochondria maintain their morphology and function in cells through continuous fusion and division[40]. Studies have found that the mitochondria in the liver of mice with NAFLD become smaller and fragmented[41]. Steatosis and inflammatory reaction in the liver are aggravated in mice with a knockdown of the MFN2 gene that controls mitochondrial fusion, promoting the progression of liver fibrosis and liver cancer. Wang et al[42] reported that mice with NAFLD and hepatic mitochondrial DRP1 gene knockout had mitochondrial fission disorder, which directly promoted ER stress and hepatocyte death. In the pathological state of age-related NAFLD, oxidative stress in the liver aggravates mitochondrial functional and structural damage. The damage can manifest as an imbalance in the regulation of mitochondrial quality, such as the splitting of mitochondria, increased autophagy and fusion, and reduced biosynthesis, thereby affecting liver function and energy metabolism and promoting NAFLD[43,44].
The acellular production of reactive oxygen species (ROS) is an inevitable process. Under normal circumstances, oxygen metabolism generates an adequate amount of ROS required for detoxification. An excessive amount of toxins will lead to tissue damage and inflammation; however, cells have several defense systems to fight them. Most data indicate that oxidative damage increases in older adults. Some studies have reported that the antioxidant defense capacity decreases with age, while others have not reported any changes[45]. Compared with other rodents, naked mole rats, a long-lived rodent, have an increased amount of ROS. However, these rats also have an increased free radical scavenging ability, which does not decline with age[46]. Intracellular ROS may also play important roles in intracellular signaling, which may be beneficial to the ageing process. Therefore, in our opinion, ROS are not necessarily harmful, and the balance between ROS and scavenging may be the key.
It has been found that the mitochondrial respiratory chain and β-oxidation are gradually impaired in hepatocytes of NAFLD, which induces the generation of excess amounts of ROS[47]. Superoxide anions, hydroxyl radicals, peroxy radicals, and other non-radicals that can produce free radicals are all members of the family of free radicals known as ROS[48]. The overproduction of ROS is related to oxidative damage to lipids, DNA and proteins[49,50]. According to the multiple parallel hit theory, oxidative stress is considered a major factor in liver injury and disease progression[51]. Oxidative stress has increasingly become one of the important pathological causes of the development of NAFLD, and it is the link between simple steatosis and symptoms of NASH[52].
Lipids are one of the main sources of mitochondrial ROS in NAFLD. Mitochondria can produce ROS during oxidative phosphorylation (OXPHOS), which can trigger mitochondrial dysfunction (such as DNA, proteins, lipids and other molecules) by interacting with mitochondria and cellular components[53]. Mitochondrial function and ROS production are considered important regulators of lifespan. Mitochondrial dysfunction leads to the overproduction of ROS. Oxidative damage may be involved in various pathological processes. At present, an imbalance in the mitochondrial redox state is considered the main cause of cell damage. The interaction between mitochondria and peroxisomes regulates metabolic and redox signaling pathways through the mitochondrial delivery system[54]. Mitochondrial ROS are considered an important factor in the development of liver disease. The production of mitochondrial ROS may lead to fatal cell damage due to the impairment of several bioenergetic responses involved in OXPHOS[55]. In addition, due to its proximity to ETC, mtDNA is extremely prone to oxidative damage, leading to DNA breakage and mutation[56].
The understanding of the physiological role of autophagy in mammals has increased in the past decade. Autophagy is associated with many physiological processes[57]. The main function of autophagy is to degrade endogenous biomacromolecules and recycle cellular substances. There are three main forms of autophagy[58], namely, microautophagy, macroautophagy and chaperone-mediated autophagy. The difference lies in the mode of transportation. Autophagy has become a potential anti-ageing mechanism[59]. Autophagy declines with age and the expression of several key indicators in the autophagy pathway (such as ATG5 and ATG7) show a downward trend in the brain of older people[60]. Caloric restriction and exercise can enable autophagy to delay aging-associated degeneration[61].
The ageing of cellular mitochondria gradually makes it inefficient and potentially toxic. An acute injury can increase the permeability of the mitochondrial membrane, thus triggering apoptosis or necrosis. Autophagy inhibits inflammation through phagocytosis of dysfunctional or damaged mitochondria, preventing unnecessary cell loss[62]. Autophagy can not only regulate lipid metabolism and insulin resistance[63] but also degenerative diseases caused by the reduced expression of autophagy or mitophagy genes, including inflammation and cell population death due to lack of quality control. A growing body of evidence also shows that hepatic autophagy is impaired in NAFLD[64], thus confirming the theory that the combination of mitochondrial dysfunction and insufficient autophagy may lead to a variety of age-related diseases, and making autophagic flux a potential pharmacological target in NAFLD.
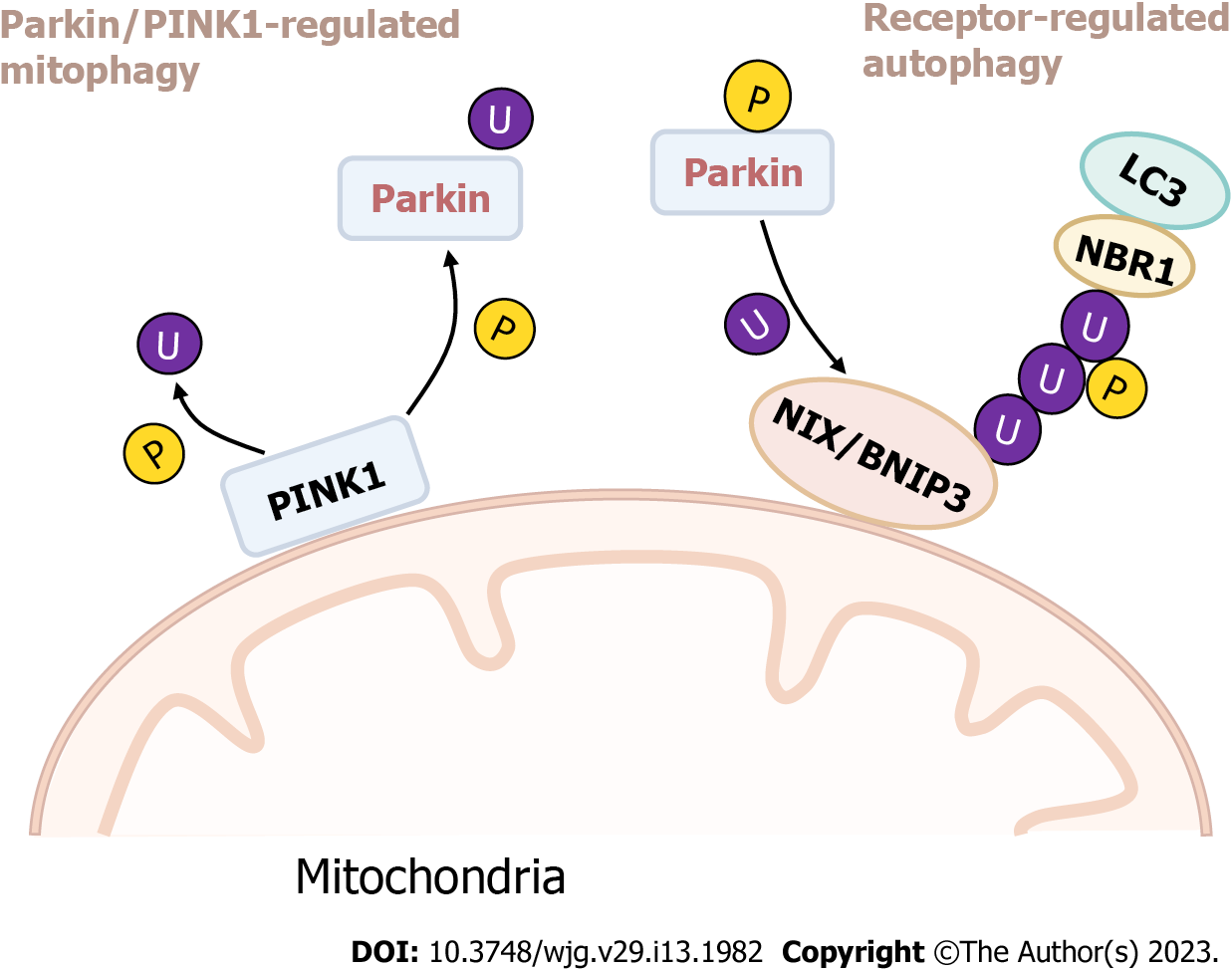
Presently, two main mechanisms are known to regulate mitochondrial autophagy, namely, mitophagy regulated by parkin/PINK1[65] and receptor-regulated autophagy[66]. Parkin is an E3 ubiquitin ligase located in the downstream. PINK1 is a serine/threonine kinase located in the upstream. Autophosphorylation of PINK1 is essential for ubiquitin recognition and can promote the translocation of phosphorylation for parkin from the cytosol to mitochondria. In the event of mitochondrial damage, the membrane potential decreases, leading to the accumulation of PINK1 in the OMM, attracting parkin to attach to mitochondria and causing further autophagy. In addition, Nip3-like protein X (NIX) and Bcl-219 kDa interacting protein (BNIP3) are outer membrane proteins associated with autophagy and apoptosis under hypoxia, which can act as mitochondrial autophagy receptors. Studies have confirmed that NIX can also participate in the process of parkin-dependent mitochondrial autophagy. It can be ubiquitinated as a substrate of parkin and recruit the LC3 adaptor protein NBR1 to target mitochondria to autophagosomes and induce mitochondrial autophagy (Figure 2)[67].
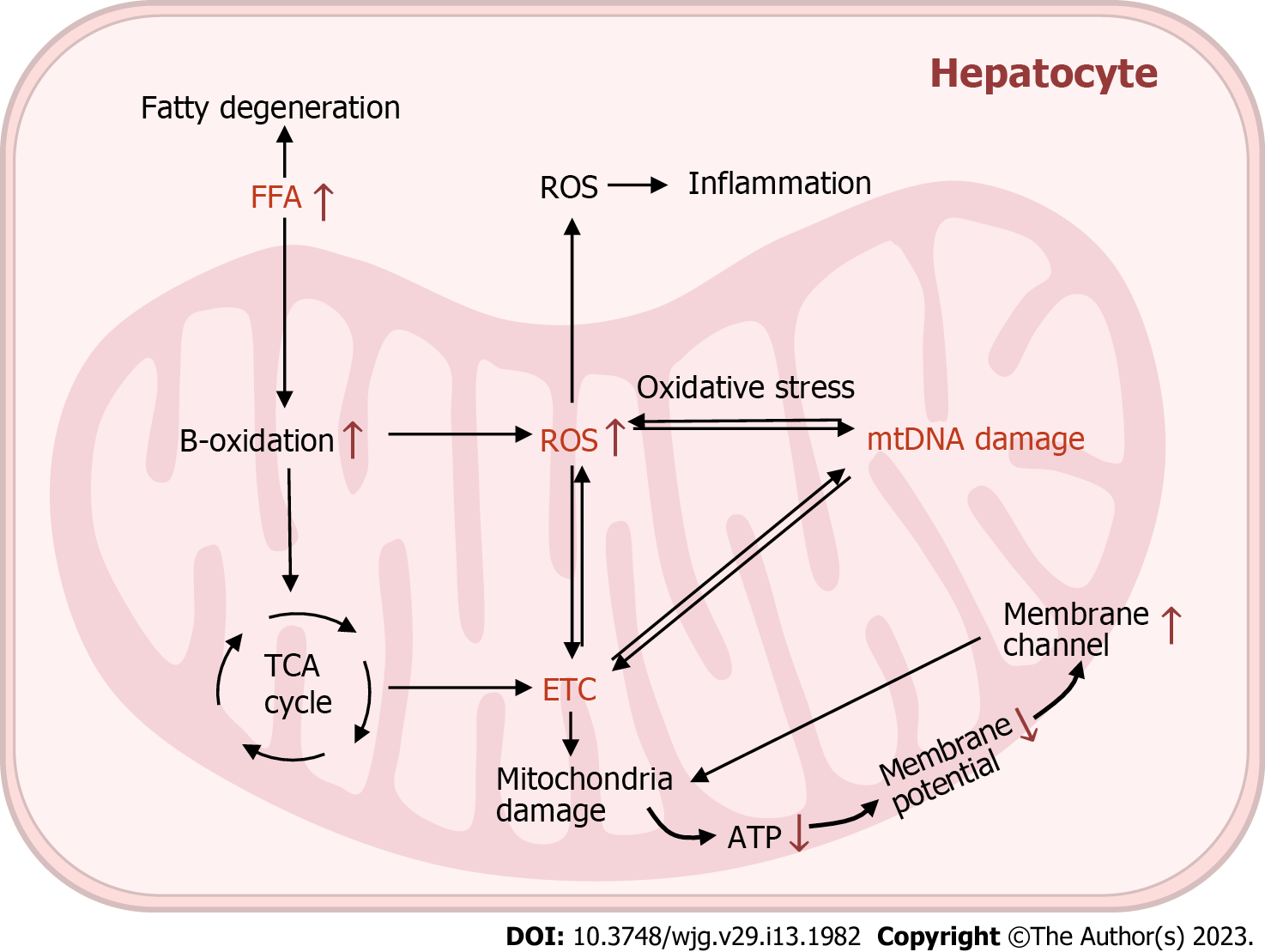
Mitochondrial dysfunction is characterised by an imbalance in mitochondrial homeostasis, ROS overproduction, autophagy abnormalities, respiratory chain disorders and mtDNA damage. A large number of studies have demonstrated structural and functional alterations in the mitochondria of hepatocytes in NAFLD (Figure 3)[68-70].
Appreciation of the role played by the gut microbiome has increased rapidly in recent years[71-73]. Comprehensive studies on the aetiology of NAFLD induced by excess nutrition and obesity have shown that changes in the gut microbiome are crucial in the development of NAFLD[74]. Dysregulation of gut flora has been shown to be an important factor in age-related pathology.
Mitochondria regulate intestinal function, including barrier defense of the intestinal epithelial cells[75-77]. Studies have shown that mitochondrial dysfunction in colonic epithelial cells induced by the use of dinitrophenol leads to dysfunction of the intestinal barrier[78], suggesting that mitochondrial dysfunction impairs the functional integrity of intestinal epithelial cells. MitoTEMPO, an antioxidant that targets mitochondria, inhibits these barrier defects, suggesting that mitochondrial stability is important for maintaining intestinal barrier function[71]. Unbalanced gut flora may contribute to the development and progression of multiple diseases. Recently, disorders of the intestinal flora have been implicated in liver diseases, such as hepatitis, cirrhosis and NAFLD[74].
Currently, there is no approved drug treatment for NAFLD. Hannah et al[79] found that a 3%-5% weight loss in patients with NAFLD slowed the progression of the condition. Although lifestyle changes through appropriate diet and exercise have been shown to be beneficial, most patients find it difficult to achieve and maintain the same. Therefore, identifying effective therapeutic drugs is an active area of research. Clinically, the pharmacological agents for NAFLD mainly include lipid-lowering drugs, liver-protecting drugs, antioxidants, autophagy inhibitors and insulin sensitizers.
The disorder of fat metabolism in the human body is one of the important reasons for the development of NAFLD. Aiming at the accumulation of liver fat, the PPAR ligand can be considered a possible therapeutic agent for NAFLD. Regulating lipid metabolism and reducing blood lipids is also a popular approach for the treatment of NAFLD[80]. The latest evidence from preclinical and clinical studies confirms the potential of PPAR ligands to treat this series of liver diseases. For example, elafibranor, a PPAR agonist[81], can increase mitochondrial fatty acid oxidation and oxidative phosphorylation and reduce the flow of fatty acids from adipose tissue to the liver. Presently, a phase III clinical trial (NCT02704403) has been completed.
Hepatoprotective drugs are commonly used as adjuvant in the treatment of NAFLD, which can not only protect the liver but also resist oxidation, inflammation and fibrosis and prevent the probability of malignant transformation of liver disease. Common hepatoprotective drugs include polyene phosphatidylcholine and obeticholic acid (OCA). Polyene phosphatidylcholine has anti-oxidative and anti-inflammatory effects that aid in reducing liver cell damage and even apoptosis and can effectively target the pathological symptoms caused by NAFLD. The effect of its combination with metformin in the treatment of patients with NAFLD and NASH is significantly better than that of monotherapy[82]. OCA is a farnesoid X receptor agonist. As a synthetic lipophilic bile acid, OCA can effectively reduce hepatic steatosis in mice[83].
Vitamin E is an antioxidant and has been proven one of the more effective drugs for the treatment of NAFLD. Clinical studies have reported significant improvement in blood lipid biochemical indicators and histopathological changes in the liver after oral administration of VE at 800 IU/d for 96 wk. The results indicated that VE was beneficial for the treatment of NAFLD and NASH[84]. Sanyal et al[85] found that oral VE at 800 IU/d for 2 years could improve hepatic steatosis and inflammatory injury. Lv et al[86] hypothesised that the accumulation of ROS was the process of quiescent cells entering the cell cycle. Through experiments, they confirmed that the expression of cell cycle regulators P16 and P38 was related to the production of ROS, which provided a feasible therapeutic target for scavenging ROS and improving NAFLD.
Improving mitophagy has been a hot topic in the treatment of NAFLD in recent years. Wu et al[87] found that a high-fat diet induced an increase in the expression of a mixed series of protein kinase-like domains (MLKL). MLKL can combine with liver mitochondria to stimulate the production of ROS and transfer it to the autophagosome membrane to inhibit the autophagy of injured cells and exacerbate liver injury. Therefore, MLKL-knockout models can improve the autophagy defect of injured hepatocytes and alleviate the progression of NAFLD. Studies have suggested the feasibility of inducing autophagy in the treatment of NAFLD and the application of related targets. However, excessive induction of autophagy may aggravate liver injury; therefore, maintaining the balance between them is the key to treating NAFLD.
In summary, as the central link of energy metabolism, mitochondria play an important role in maintaining the normal structure and function of age-related NAFLD. There is a concomitant and progressive relationship in the process of age-related NAFLD, from fatty degeneration to a series of pathological lesions, such as inflammation and liver fibrosis. However, whether a mitochondrial disorder of hepatocytes is the cause of the progression of age-related NAFLD or the result of abnormal lipid metabolism and lipotoxicity cannot be fully confirmed[88]. The occurrence of mitochondrial dysfunction is closely related to the disorders of dynamic balance, mtDNA deletion, excessive production of ROS, abnormal mitophagy and abnormal fatty acid oxidation. A better understanding of the mechanism of mitochondrial dysfunction will be helpful to develop new therapeutic strategies for NAFLD. In the next few years, the availability of therapeutic options for NAFLD is expected to curb the rising trend of related diseases. More rigorous experiments should be designed to validate the results and develop more targeted drugs that regulate mitochondrial function to treat NAFLD, thus achieving the transformation from basic theory to clinical practice.
We wish to thank Professor Gai XD from College of Basic Medicine for their critical comments on the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grgurevic I, Croatia; Sawazaki H, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Stahl EC, Haschak MJ, Popovic B, Brown BN. Macrophages in the Aging Liver and Age-Related Liver Disease. Front Immunol. 2018;9:2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, Chan FK, Chan HL. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Ding HR, Wang JL, Ren HZ, Shi XL. Lipometabolism and Glycometabolism in Liver Diseases. Biomed Res Int. 2018;2018:1287127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Lu J, Xu M, Xu Y, Li M, Wang T, Zhang J, Xu B, Sun J, Dai M, Bi Y, Wang W, Ning G. Association between history of abortion and nonalcoholic fatty liver disease in middle-aged and elderly Chinese women. Ann Epidemiol. 2013;23:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 386] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 501] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 7. | Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res Int. 2014;2014:238463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 8. | Klionsky DJ. Why do we need autophagy? Autophagy. 2018;14:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kirkwood TB. A systematic look at an old problem. Nature. 2008;451:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205-14218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 362] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (2)] |
| 11. | Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 697] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 12. | Cloonan SM, Kim K, Esteves P, Trian T, Barnes PJ. Mitochondrial dysfunction in lung ageing and disease. Eur Respir Rev. 2020;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Schlaepfer IR, Joshi M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 14. | Maples JM, Brault JJ, Witczak CA, Park S, Hubal MJ, Weber TM, Houmard JA, Shewchuk BM. Differential epigenetic and transcriptional response of the skeletal muscle carnitine palmitoyltransferase 1B (CPT1B) gene to lipid exposure with obesity. Am J Physiol Endocrinol Metab. 2015;309:E345-E356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Rodríguez-Rodríguez R, Miralpeix C, Fosch A, Pozo M, Calderón-Domínguez M, Perpinyà X, Vellvehí M, López M, Herrero L, Serra D, Casals N. CPT1C in the ventromedial nucleus of the hypothalamus is necessary for brown fat thermogenesis activation in obesity. Mol Metab. 2019;19:75-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Biswas D, Ghosh M, Kumar S, Chakrabarti P. PPARα-ATGL pathway improves muscle mitochondrial metabolism: implication in aging. FASEB J. 2016;30:3822-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 333] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 18. | Poulsen Ll, Siersbæk M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 19. | Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017;13:279-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 20. | Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 2021;114:154338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 388] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 21. | Liss KH, Finck BN. PPARs and nonalcoholic fatty liver disease. Biochimie. 2017;136:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 22. | Hashemi HF, Goodman JM. The life cycle of lipid droplets. Curr Opin Cell Biol. 2015;33:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Nguyen TB, Olzmann JA. Lipid droplets and lipotoxicity during autophagy. Autophagy. 2017;13:2002-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Khor VK, Shen WJ, Kraemer FB. Lipid droplet metabolism. Curr Opin Clin Nutr Metab Care. 2013;16:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Henne WM, Reese ML, Goodman JM. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018;37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 26. | Bersuker K, Peterson CWH, To M, Sahl SJ, Savikhin V, Grossman EA, Nomura DK, Olzmann JA. A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes. Dev Cell. 2018;44:97-112.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 27. | Gao Q, Binns DD, Kinch LN, Grishin NV, Ortiz N, Chen X, Goodman JM. Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J Cell Biol. 2017;216:3199-3217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Schulze RJ, McNiven MA. Lipid Droplet Formation and Lipophagy in Fatty Liver Disease. Semin Liver Dis. 2019;39:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Schott MB, Rasineni K, Weller SG, Schulze RJ, Sletten AC, Casey CA, McNiven MA. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J Biol Chem. 2017;292:11815-11828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Barneda D, Christian M. Lipid droplet growth: regulation of a dynamic organelle. Curr Opin Cell Biol. 2017;47:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Eliasen K, Patursson EJ, McAdam BJ, Pino E, Morro B, Betancor M, Baily J, Rey S. Liver colour scoring index, carotenoids and lipid content assessment as a proxy for lumpfish (Cyclopterus lumpus L.) health and welfare condition. Sci Rep. 2020;10:8927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 33. | Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 750] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 34. | Annesley SJ, Fisher PR. Mitochondria in Health and Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 35. | Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C, Lezza AM. A comparison among the tissue-specific effects of aging and calorie restriction on TFAM amount and TFAM-binding activity to mtDNA in rat. Biochim Biophys Acta. 2014;1840:2184-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest. 2017;127:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Meyer JN, Leuthner TC, Luz AL. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology. 2017;391:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 384] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 38. | Cho B, Cho HM, Jo Y, Kim HD, Song M, Moon C, Kim H, Kim K, Sesaki H, Rhyu IJ, Sun W. Constriction of the mitochondrial inner compartment is a priming event for mitochondrial division. Nat Commun. 2017;8:15754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 39. | Schrepfer E, Scorrano L. Mitofusins, from Mitochondria to Metabolism. Mol Cell. 2016;61:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 40. | Jia ZW. The Mechanism of Mitochondria Quality Control in Mammals. Zhongguo Xibao Shengwuxue Xuebao. 2017;39:373-380. [DOI] [Full Text] |
| 41. | Hernández-Alvarez MI, Sebastián D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, Plana N, Veiga SR, Hernández V, Vasconcelos N, Peddinti G, Adrover A, Jové M, Pamplona R, Gordaliza-Alaguero I, Calvo E, Cabré N, Castro R, Kuzmanic A, Boutant M, Sala D, Hyotylainen T, Orešič M, Fort J, Errasti-Murugarren E, Rodrígues CMP, Orozco M, Joven J, Cantó C, Palacin M, Fernández-Veledo S, Vendrell J, Zorzano A. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell. 2019;177:881-895.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 42. | Wang L, Li X, Hanada Y, Hasuzawa N, Moriyama Y, Nomura M, Yamamoto K. Dynamin-related protein 1 deficiency accelerates lipopolysaccharide-induced acute liver injury and inflammation in mice. Commun Biol. 2021;4:894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Navarro CDC, Figueira TR, Francisco A, Dal'Bó GA, Ronchi JA, Rovani JC, Escanhoela CAF, Oliveira HCF, Castilho RF, Vercesi AE. Redox imbalance due to the loss of mitochondrial NAD(P)-transhydrogenase markedly aggravates high fat diet-induced fatty liver disease in mice. Free Radic Biol Med. 2017;113:190-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Lohr K, Pachl F, Moghaddas Gholami A, Geillinger KE, Daniel H, Kuster B, Klingenspor M. Reduced mitochondrial mass and function add to age-related susceptibility toward diet-induced fatty liver in C57BL/6J mice. Physiol Rep. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim Biophys Acta. 2015;1847:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 46. | Saldmann F, Viltard M, Leroy C, Friedlander G. The Naked Mole Rat: A Unique Example of Positive Oxidative Stress. Oxid Med Cell Longev. 2019;2019:4502819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Liu X, Zhang J, Ming Y, Chen X, Zeng M, Mao Y. The aggravation of mitochondrial dysfunction in nonalcoholic fatty liver disease accompanied with type 2 diabetes mellitus. Scand J Gastroenterol. 2015;50:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Kausar S, Wang F, Cui H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 49. | Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1215] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 50. | Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 801] [Article Influence: 160.2] [Reference Citation Analysis (1)] |
| 51. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 52. | Polimeni L, Del Ben M, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol. 2015;7:1325-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 53. | Kowald A, Kirkwood TB. Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proc Natl Acad Sci U S A. 2011;108:10237-10242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Lismont C, Nordgren M, Van Veldhoven PP, Fransen M. Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol. 2015;3:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413-C422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC, Price DA, Chinnery PF. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet. 2011;43:806-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Xu F, Hua C, Tautenhahn HM, Dirsch O, Dahmen U. The Role of Autophagy for the Regeneration of the Aging Liver. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Schneider JL, Cuervo AM. Liver autophagy: much more than just taking out the trash. Nat Rev Gastroenterol Hepatol. 2014;11:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 59. | Madrigal-Matute J, Cuervo AM. Regulation of Liver Metabolism by Autophagy. Gastroenterology. 2016;150:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 60. | Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:14164-14169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 61. | Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 62. | Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 939] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 63. | Wu WKK, Zhang L, Chan MTV. Autophagy, NAFLD and NAFLD-Related HCC. Adv Exp Med Biol. 2018;1061:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Chao X, Wang H, Jaeschke H, Ding WX. Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. 2018;38:1363-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 65. | Palikaras K, Daskalaki I, Markaki M, Tavernarakis N. Mitophagy and age-related pathologies: Development of new therapeutics by targeting mitochondrial turnover. Pharmacol Ther. 2017;178:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 819] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 67. | Gao F, Chen D, Si J, Hu Q, Qin Z, Fang M, Wang G. The mitochondrial protein BNIP3L is the substrate of PARK2 and mediates mitophagy in PINK1/PARK2 pathway. Hum Mol Genet. 2015;24:2528-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 68. | Ajaz S, McPhail MJ, Gnudi L, Trovato FM, Mujib S, Napoli S, Carey I, Agarwal K. Mitochondrial dysfunction as a mechanistic biomarker in patients with non-alcoholic fatty liver disease (NAFLD). Mitochondrion. 2021;57:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 69. | Meex RCR, Blaak EE. Mitochondrial Dysfunction is a Key Pathway that Links Saturated Fat Intake to the Development and Progression of NAFLD. Mol Nutr Food Res. 2021;65:e1900942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 70. | Fouret G, Gaillet S, Lecomte J, Bonafos B, Djohan F, Barea B, Badia E, Coudray C, Feillet-Coudray C. 20-Week follow-up of hepatic steatosis installation and liver mitochondrial structure and activity and their interrelation in rats fed a high-fat-high-fructose diet. Br J Nutr. 2018;119:368-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Fulbright LE, Ellermann M, Arthur JC. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017;13:e1006480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 72. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1173] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 73. | Bodogai M, O'Connell J, Kim K, Kim Y, Moritoh K, Chen C, Gusev F, Vaughan K, Shulzhenko N, Mattison JA, Lee-Chang C, Chen W, Carlson O, Becker KG, Gurung M, Morgun A, White J, Meade T, Perdue K, Mack M, Ferrucci L, Trinchieri G, de Cabo R, Rogaev E, Egan J, Wu J, Biragyn A. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 74. | Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic Fatty Liver Disease and the Gut-Liver Axis: Exploring an Undernutrition Perspective. Gastroenterology. 2022;162:1858-1875.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 75. | Berger E, Rath E, Yuan D, Waldschmitt N, Khaloian S, Allgäuer M, Staszewski O, Lobner EM, Schöttl T, Giesbertz P, Coleman OI, Prinz M, Weber A, Gerhard M, Klingenspor M, Janssen KP, Heikenwalder M, Haller D. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat Commun. 2016;7:13171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 76. | Crakes KR, Santos Rocha C, Grishina I, Hirao LA, Napoli E, Gaulke CA, Fenton A, Datta S, Arredondo J, Marco ML, Sankaran-Walters S, Cortopassi G, Giulivi C, Dandekar S. PPARα-targeted mitochondrial bioenergetics mediate repair of intestinal barriers at the host-microbe intersection during SIV infection. Proc Natl Acad Sci U S A. 2019;116:24819-24829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Khaloian S, Rath E, Hammoudi N, Gleisinger E, Blutke A, Giesbertz P, Berger E, Metwaly A, Waldschmitt N, Allez M, Haller D. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn's disease recurrence. Gut. 2020;69:1939-1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 78. | Wang A, Keita ÅV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, Yates R, Dicay M, Beck PL, MacNaughton WK, Söderholm JD, McKay DM. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol. 2014;184:2516-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 79. | Hannah WN Jr, Harrison SA. Lifestyle and Dietary Interventions in the Management of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 80. | Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med (Berl). 2009;87:679-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 81. | Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: Current and emerging. J Hepatol. 2018;68:362-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 82. | Liu BB, Xu KS. Drug therapy for non-alcoholic fatty liver disease. Zhongguo Shiyong Neike Zazhi. 2019;39:214-217. [DOI] [Full Text] |
| 83. | Kunne C, Acco A, Duijst S, de Waart DR, Paulusma CC, Gaemers I, Oude Elferink RP. FXR-dependent reduction of hepatic steatosis in a bile salt deficient mouse model. Biochim Biophys Acta. 2014;1842:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2464] [Article Influence: 164.3] [Reference Citation Analysis (2)] |
| 86. | Lv F, Li N, Kong M, Wu J, Fan Z, Miao D, Xu Y, Ye Q, Wang Y. CDKN2a/p16 Antagonizes Hepatic Stellate Cell Activation and Liver Fibrosis by Modulating ROS Levels. Front Cell Dev Biol. 2020;8:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Wu X, Poulsen KL, Sanz-Garcia C, Huang E, McMullen MR, Roychowdhury S, Dasarathy S, Nagy LE. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J Hepatol. 2020;73:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 88. | Schröder T, Kucharczyk D, Bär F, Pagel R, Derer S, Jendrek ST, Sünderhauf A, Brethack AK, Hirose M, Möller S, Künstner A, Bischof J, Weyers I, Heeren J, Koczan D, Schmid SM, Divanovic S, Giles DA, Adamski J, Fellermann K, Lehnert H, Köhl J, Ibrahim S, Sina C. Mitochondrial gene polymorphisms alter hepatic cellular energy metabolism and aggravate diet-induced non-alcoholic steatohepatitis. Mol Metab. 2016;5:283-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |