Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1838
Peer-review started: November 20, 2022
First decision: January 2, 2023
Revised: January 12, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: March 28, 2023
Processing time: 128 Days and 1.5 Hours
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), can trigger autoimmunity in genetically predisposed individuals through hyperstimulation of immune response and molecular mimicry. Here we summarise the current knowledge about auto-immune liver diseases (AILDs) and SARS-CoV-2, focusing on: (1) The risk of SARS-CoV-2 infection and the course of COVID-19 in patients affected by AILDs; (2) the role of SARS-CoV-2 in inducing liver damage and triggering AILDs; and (3) the ability of vaccines against SARS-CoV-2 to induce autoimmune responses in the liver. Data derived from the literature suggest that patients with AILDs do not carry an increased risk of SARS-Cov-2 infection but may develop a more severe course of COVID-19 if on treatment with steroids or thiopurine. Although SARS-CoV-2 infection can lead to the development of several autoimmune diseases, few reports correlate it to the appearance of de novo manifestation of immune-mediated liver diseases such as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) or AIH/PBC overlap syndrome. Different case series of an AIH-like syndrome with a good prognosis after SARS-CoV-2 vaccination have been described. Although the causal link between SARS-CoV-2 vaccines and AIH cannot be definitively established, these reports suggest that this association could be more than coincidental.
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a possible trigger of autoimmunity. Patients with autoimmune liver diseases (AILDs) were considered at higher risk of SARS-CoV-2 infection and more susceptible to severe coronavirus disease 2019 (COVID-19) due to genetic background and immunosuppressive treatments. Case reports documenting autoimmune hepatitis-like syndromes after the COVID vaccine started to emerge, raising worries about a possible risk of unwanted immunological side effects, especially in individuals predisposed to autoimmune disorders. We herein discuss the consequences of SARS-CoV-2 infection in patients with AILDs and the role of the vaccines in inducing liver autoimmunity.
- Citation: Sgamato C, Rocco A, Compare D, Minieri S, Marchitto SA, Maurea S, Nardone G. Autoimmune liver diseases and SARS-CoV-2. World J Gastroenterol 2023; 29(12): 1838-1851
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1838
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA, enveloped, beta coronavirus causing coronavirus disease 2019 (COVID-19), which has developed into a pandemic infection in a short time since its first appearance in Wuhan, China, in December 2019[1,2]. Since then, SARS-CoV-2 has caused more than 620 million infections, with a death toll exceeding 6.5 million as of October 31, 2022, according to the World Health Organization. Although the most common presentation of COVID-19 includes respiratory symptoms such as dry cough and shortness of breath[3], gastrointestinal symptoms, including diarrhoea, anorexia, nausea and or vomiting, and abdominal pain, have been variably reported in up to half of the cases[4,5]. Furthermore, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT), hallmarks of liver injury, have been widely recognised as major components of COVID-19[6]. Tissue injury is almost certainly multifactorial and related to several mechanisms, such as direct hepatocellular lesions, immune-mediated liver damage due to the severe inflammatory response, or development of endotheliopathy with hypoxic or ischaemic injury[7-9].
Furthermore, SARS-CoV-2 has also been identified as an instrumental trigger of autoimmunity. Hyper-stimulation of the immune system or molecular mimicry of the virus with human self-components may lead to the synthesis of multiple autoantibodies that could initiate autoimmune diseases in genetically predisposed individuals[10,11].
In this review, we summarised the current knowledge regarding the risk of SARS-CoV-2 infection and the course of COVID-19 in patients with autoimmune liver diseases (AILDs), as well as the role of SARS-CoV-2 in inducing de novo AILDs. Finally, since various case reports documenting autoimmune hepatitis (AIH)-like syndromes after receiving the COVID-19 vaccine started to emerge, we reviewed the literature to analyse the role of vaccines in inducing autoimmune responses in the liver.
ALDs are a heterogeneous group of inflammatory liver disorders mediated by autoimmunity, including AIH, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and overlapping syndromes. Patients with AILDs could theoretically present an increased risk of SARS-CoV-2 infections and poor outcomes due to lifelong immunosuppressive treatments taken to delay the progression to cirrhosis and liver failure.
However, the clinical impact of SARS-CoV-2 infection on the population with AILDs remains unclear. Preliminary data derived from regional observational studies driven by referral centres suggested that patients with AILDs did not carry a specific risk of becoming infected by SARS-CoV-2. In the earliest reports from Northern Italy, an area particularly affected by the virus's spread, the observed incidence of the infection in a cohort of 148 AILDs patients (133 AIH, 11 autoimmune sclerosing cholangitis, 2 PSC/AIH and 2 PBC/AIH) did not significantly differ from that estimated in the general population (43 vs 38 cases, respectively)[12]. Similarly, Verhelst et al[13] found a very limited SARS-CoV-2 infection ratio (1.2%) in a Belgian cohort of 110 patients with AIH[13]. More recently, a survey involving 1779 AILDs patients from 20 European countries detected a rate of COVID-19 (2.2%) equivalent to the period prevalence of the general population, thus indicating that patients with AILDs are not at elevated risk of developing COVID disease[14]. Interestingly, people suffering from autoimmune diseases do not have a higher risk of being positive for COVID-19, as indicated by a study performed in Milan that applied both a test-negative [odds ratio (OR): 0.86, 95% confidence interval (CI): 0.76–0.96] and population (OR: 0.98, 95%CI: 0.90–1.08) case–control design[15].
Regarding the impact of AILDs on the COVID-19 course, early phone-based surveys including patients with AIH (n = 73) and PBC (n = 68) found a rate of infection of 3.6% with a favourable outcome of COVID-19 in most cases[16]. Likewise, patients under immunosuppressive treatment for AIH show a disease course probably comparable to that reported in the non-immunosuppressed population[17]. Data combined from 3 large-scale international reporting registries, namely the European Association for the Study of the Liver supported COVID-Hep registry, the European Reference Network on Hepatological Diseases (ERN RARE-LIVER) and the American Association for the Study of Liver Diseases supported SECURE-cirrhosis registry, showed that patients with AIH, despite immunosuppressive treatment, did not present an increased risk of adverse outcomes including hospitalisation (76% vs 85%; P = 0.06), admission in an intensive care unit (29% vs 23%; P = 0.240), or death (23% vs 20%; P = 0.643) when compared to other causes of chronic liver disease and matched cases without the liver disease[18]. Another retrospective study collecting data on 110 patients with AIH and COVID-19 from 34 centres in Europe and the Americas confirmed that AIH was not a risk factor for severe COVID-19 (15.5% vs 20.2%, P = 0.231) and all-cause mortality (10% vs 11.5%, P = 0.852) than other causes of chronic liver disease. Still, continued immunosuppression during COVID-19 was associated with a lower rate of liver injury (P = 0.009; OR: 0.26; 95%CI: 0.09-0.71)[19]. However, in another study including 254 AIH patients, the same author observed that the use of systemic glucocorticoids (adjusted OR: 4.73, 95%CI: 1.12-25.89) and thiopurine (adjusted OR: 4.78, 95%CI: 1.33-23.50) was associated with worse COVID-19 outcome compared to patients who were not on immunosuppressive therapy[20].
These results might aid medical decisions when dealing with SARS-CoV-2 infection in immunocompromised patients. Although current expert recommendations advocate against changes in immunosuppressive treatments in patients with AIH before and after SARS-CoV-2 infection[21], data derived from larger case series support the notion of maintaining remission with the lowest effective glucocorticoid dose in AIH patients during the COVID-19 pandemic.
Nonetheless, it should be considered that cirrhotic patients, irrespective of the cirrhosis aetiology, once infected with SARS-CoV-2, are more vulnerable than the general population or patients without cirrhosis. Indeed, they have a higher risk of decompensation of liver disease and developing a more severe COVID-19 with a mortality rate of up to 30%[22,23].
Increasing evidence indicates that SARS-CoV-2 may have a trigger effect of, possibly pre-existing, autoimmune disease, including Guillain-Barré syndrome, systemic lupus erythematosus, myasthenia gravis, large vessel vasculitis and thrombosis, Graves' disease, sarcoidosis and inflammatory arthritis[24].
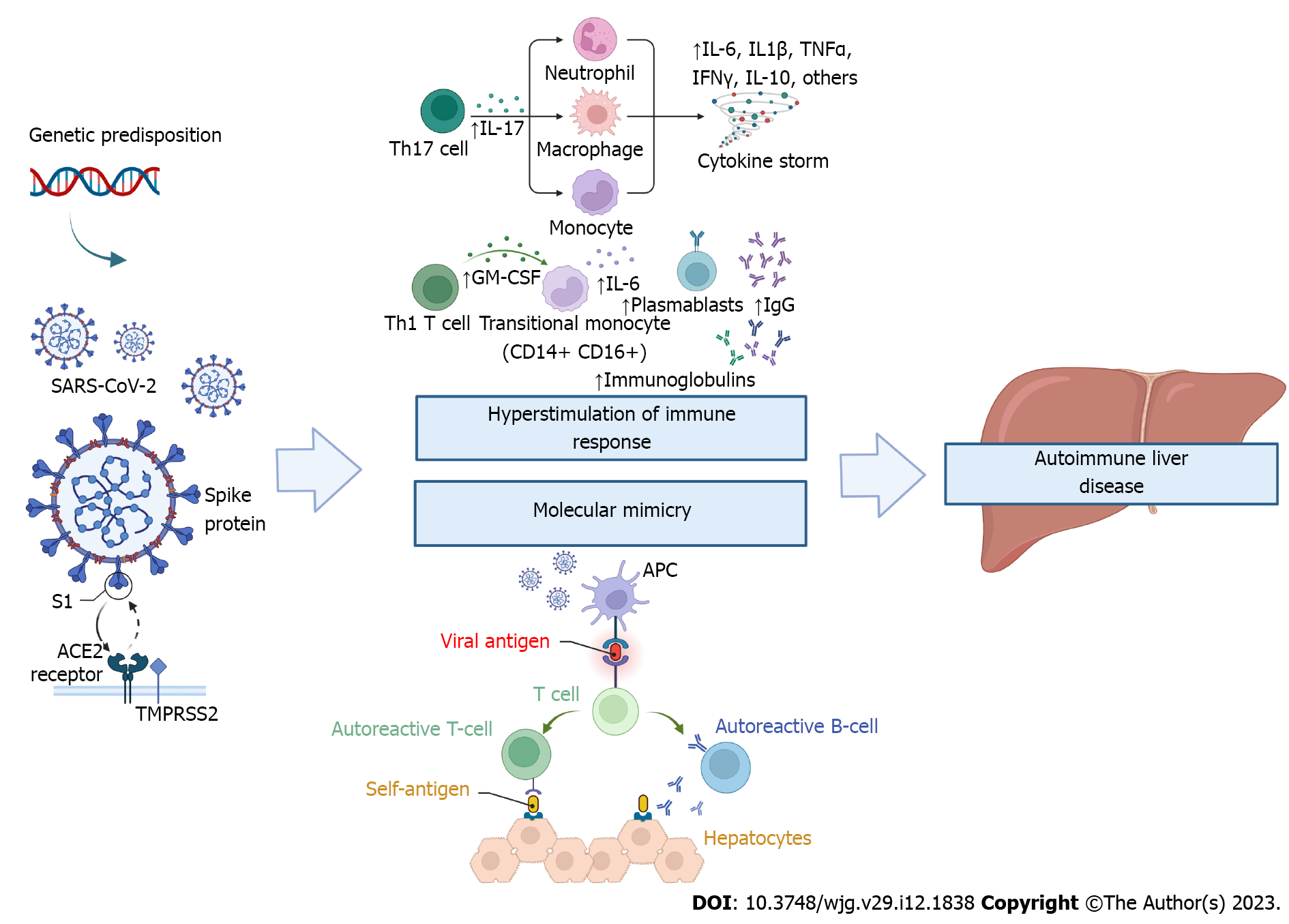
It is well-known that SARS-CoV-2 infection can result in hyperstimulation of the immune system, with increased serum concentration of pro-inflammatory cytokines, particularly monocyte chemoattractant protein 1, interferon-inducible protein-10, interleukin (IL)-6, IL-1β, IL-8, IL-10, IL-17, tumour necrosis factor, granulocyte-macrophage colony-stimulating factor, also referred to as "cytokine storm" or "cytokine release syndrome"[25]. In addition, due to structural homology between some components of SARS-CoV-2 and human proteins[11], the immune responses raised against the virus can cross-react with self-proteins, a concept commonly termed molecular mimicry[26]. These factors may contribute to the development of multiple autoantibodies with a trigger effect on autoimmunity (Figure 1).
In patients with SARS-CoV-2 infection and especially severely ill patients compared with those with mild or moderate disease, numerous autoantibodies, including antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies, antiphospholipid antibodies, have been detected[27,28]. Since systemic autoimmunity is known to initiate from generalised polyclonal B cell activation, the presence of autoantibodies in such kinds of patients may indicate a pre-AID[29].
However, interestingly, there have been very few reports suggesting that SARS-CoV-2 infection might reflect 'a final hit' leading to de novo manifestation of immune-mediated liver diseases such as PBC[30] or AIH/PBC overlap syndrome[31].
PBC developed concomitantly with Guillain-Barrè syndrome following SARS-CoV-2 infection in a 44-year-old obese and hypertensive woman already suffering from Hashimoto's thyroiditis. During her hospitalisation for severe bilateral interstitial pneumonia, she developed an important rise in serum gamma-glutamyl-transferase (GGT) with initially normal alkaline phosphatase (ALP) levels that progressively increased later.
As a result of the ALP increase combined with high-titre anti-mitochondrial (AMA) positivity (1:640), the patient underwent a liver biopsy showing histological findings consistent with an initial phase (stage I in Scheuer and Ludwig classifications) of PBC.
A case of AIH/PBC overlap syndrome has been described in a 57-year-old man suffering from hypertension, beta-thalassemia minor, and diabetes. He developed arthralgias, elevated liver enzymes and hyper hyperferritinemia one month after recovering from moderate COVID-19 disease. Although a liver biopsy was not performed, the patient presented a concomitant seropositivity for AMA and anti-double-stranded DNA antibodies (anti-ds DNA) that can be considered the serological profile of AIH/PBC[32].
On the other hand, liver involvement has been frequently reported in COVID-19. Abnormal liver function test, mainly elevated ALT, hypoalbuminemia, and elevated GGT, has been described in 14.8-53% of SARS-CoV-2 infected patients and up to 78% of those with severe manifestations of COVID-19 disease[8] being independent risk factors for adverse clinical outcomes such as intensive care unit admission, use of invasive mechanical ventilation or longer hospital stay[33].
The pathogenic mechanisms of liver injury during COVID-19 are not fully understood. SARS-CoV-2 cell entry is mediated by the interaction of the virus's spike (S) protein with the host angiotensin-converting enzyme 2 receptor (ACE2). S protein is then cleaved by the transmembrane serine protease 2, allowing the internalisation by endocytosis and the release of the viral genome from the endosome[10]. The liver is a possible target for SARS-CoV-2 due to a high expression, almost in the same percentage of type 2 pneumocytes, of ACE2 receptors in cholangiocytes and, to a lesser extent, hepatocytes[34].
However, rather than direct cytotoxicity derived from active viral replication of SARS-CoV-2 in the liver, current data suggest that tissue injury is likely due to immune dysregulation or cytokine storm and development of endotheliopathy and microthromboses with hypoxic or ischaemic injury[35,36]. Exacerbation of underlying liver disease or drug-induced liver injury can also contribute[37,38].
Given the dramatic socio-economical effect of the pandemic, vaccines against SARS-CoV-2 have been rapidly developed. In December 2020, two mRNA vaccines (BNT 162b2 Pfizer-BioNTech[39] and mRNA-1273 Moderna[40]) and one adenovirus (ADV) vector-based vaccine (ChAdOx1 nCOV-19 Oxford University/Astra Zeneca)[41] obtained approval under Emergency Use Listing from the most important drug regulatory agencies. As of October 31, 2022, approximately 68% of the global population has received at least one dose of the anti-SARS-CoV-2 vaccine, with 12.9 billion doses being administered worldwide[42].
Both the mRNA and ADV vaccines encode the intracellular production of the SARS-CoV-2 spike protein, the primary target for neutralising antibodies generated from natural infection and triggering both innate and adaptive responses. Via their recognition by innate intracellular sensors, including toll-like receptors 3, 7, 9 and inflammasome components, vaccines stimulate innate immunity through cellular activation and consequent release of interferon I or other pro-inflammatory cytokines, thus promoting differentiation of CD4+ and CD8+ T cells into effector and memory subsets[43].
Although the safety profiles of all vaccines have been extensively studied in large placebo-controlled trials, after their widespread diffusion, case reports and case series describing a range of liver diseases following COVID-19 vaccination started to emerge[44].
In the summer of 2021, Bril et al[45] first described an AIH-like syndrome developed after vaccination against SARS-CoV-2[45]. AIH is an inflammatory disease of the liver characterised by circulating autoantibodies, mainly ANA, elevated serum globulin levels, specific histological alterations and response to immunosuppressive therapy. The clinical picture is non-specific and varies among patients, ranging from asymptomatic liver enzyme elevation to acute liver failure[46]. SARS-CoV-2 vaccination has also been linked to other forms of immune-mediated liver injury, such as AIH-PBC overlap syndrome[47] or a specific CD8+ T-cell-dominant hepatitis[48].
Cases of de novo AIH or vaccine-induced AIH relapse after vaccination against SARS-CoV-2 are reported in Table 1[45,49-78]. A summary of patient information and disease presentation is shown in Table 2. Laboratory data, treatment, and outcome are reported in Table 3.
| Ref. | Sex, age | Comorbidity | Vaccine | Time onset after vaccination | Symptoms | Antibodies | Histology | Treatment | Outcome |
| Bril et al[45] | F, 35 | None | Pfizer-BioNTech | 7 d after dose I | Jaundice, pruritus, choluria | ANA, anti- Ds-DNA | Compatible with AIH | Prednisone | Remission |
| Ghielmetti et al[49] | M, 63 | Type II diabetes, ischemic heart disease | Moderna | 7 d after dose I | Jaundice, fatigue, anorexia | ANA | Typical for AIH | Prednisone | Remission |
| Avci and Abasiyanik[50] | F, 61 | Hashimoto thyroiditis, hypertension | Pfizer-BioNTech | 30 d after dose I | Jaundice, malaise, fatigue,anorexia | ANA, ASMA | Compatible with AIH | Prednisone + AZA | Remission |
| Mahalingham et al[51] | F, 32 | OLT post-AIH, hypertension | Pfizer-BioNTech | 21 d after dose III | / | SLA/LP | Compatible with AIH | Methylprednisolone | Remission |
| Erard et al[52] | F, 80 | Pfizer-BioNTech | 10 d after dose II | All asthenia, pruritus, jaundice | ANA | Compatible with AIH | Steroids | Remission | |
| F, 73 | Moderna | 21 d after dose I | ANA | Steroids | Remission | ||||
| F, 68 | AstraZeneca | 20 d after dose I | ANA, ASMA | Listing for liver transplantation | Death after 3 d | ||||
| Garrido et al[53] | F, 65 | Polycythaemia Vera | Moderna | 14 d after dose I | Abdominal pain, jaundice, pruritus, acholia, choluria | ANA | Compatible with AIH | Prednisolone | Remission |
| Goulas et al[54] | F, 52 | / | Moderna | 14 d after dose I | Malaise, jaundice | ANA, ASMA | Prednisolone + AZA | Remission | |
| Cao et al[55] | F, 57 | AIH | CoronaVac | 14 d after dose I | Choluria, acholia, jaundice | ANA | Compatiblewith AIH | Methylprednisolone + AZA + UDCA | Remission |
| Vuille-Lessard et al[56] | F, 76 | Hashimoto thyroiditis, urothelial ca. | Moderna | 3 d after dose I | Choluria, fatigue, jaundice | ANA, ASMA, ANCA | Typical for AIH | Prednisolone + AZA | Remission |
| Suzuki et al[57] | F, 80 | GERD | Pfizer-BioNTech | 10 d after dose II | Jaundice | ANA | Compatible with AIH | Prednisone | Remission |
| F, 75 | Dyslipidemia | Pfizer-BioNTech | 4 d after dose II | Choluria | ANA, AMA | Prednisone | Remission | ||
| F, 78 | Primary biliary cholangitis | Pfizer-BioNTech | 7 d after dose I | Fever, malaise | ANA, AMA | Prednisone | Remission | ||
| Tan et al[58] | F, 56 | / | Moderna | 42 d after dose I | Jaundice, malaise, anorexia | ANA, AMA | Compatible with AIH | Budenoside | Remission |
| Zhou et al[59] | F, 36 | Primary sclerosing cholangitis, ulcerative colitis | Moderna | 11 d after dose I | / | ANA, Ds-DNA | Compatible with AIH | Prednisone + Azathioprine | Remission |
| Kang et al[60] | F, 27 | / | Pfizer-BioNTech | 8 d after dose II | Nausea, vomiting, headache, fever, dark urine | ANA | Compatible with AIH | Prednisolone | Remission |
| Rocco et al[61] | F, 80 | Hashimoto thyroiditis Glomerulonephritis | Pfizer-BioNTech | 7 d after dose II | Jaundice | ANA | Typical for AIH | Prednisone | Remission |
| Londoño et al[62] | F, 41 | Premature ovarian failure | Moderna | 7 d after dose II | Choluria, jaundice, epigastric pain, nausea, and vomiting | ANA, AMA, SLA/LP | Typical for AIH | Prednisone | Remission |
| McShane et al[63] | F, 71 | / | Moderna | 4 d after dose I | Jaundice | ASMA | Compatible with AIH | Prednisolone | Remission |
| Clayton-Chubb et al[64] | M, 36 | Hypertension | AstraZeneca | 26 d after dose I | Fever | ANA | Compatible with AIH | Prednisolone | Remission |
| Palla et al[65] | F, 40 | Sarcoidosis | Pfizer-BioNTech | 30 d after dose II | / | ANA | Compatible with AIH | Prednisolone | Remission |
| Rela et al[66] | F, 38 | Hypothyroidism | AstraZeneca | 20 d after dose I | Jaundice, fever, fatigue | ANA | Compatible with AIH | Prednisolone | Remission |
| M, 62 | Diabetes | AstraZeneca | 16 d after dose I | Anorexia, fever, jaundice | / | Prednisolone | Death after 3 wk | ||
| Camacho-Domínguez et al[67] | M, 79 | / | AstraZeneca | 15 d after dose I | Abdominal pain, pruritus, acholia, choluria | ANA, ASMA | Compatible with AIH | Hydrocortisone; Prednisone; Prednisolone | Remission |
| Zin Tun et al[68] | M, 47 | / | Moderna | 3 d after dose I and 3 d after dose II | Malaise, jaundice | ANA | Compatible with AIH | Prednisolone | Remission |
| Torrente et al[69] | F, 46 | Hypothyroidism | AstraZeneca | 21 d after dose I | / | ANA | Compatible with AIH | Prednisone + AZA | Remission |
| Fimiano et al[70] | F, 63 | Hypothyroidism | Pfizer-BioNTech | 54 d after dose III | Abdominal pain, nausea, jaundice, choluria | - | Compatible with AIH | Methylprednisolone + AZA | Remission |
| Izagirre et al[71] | M, 72 | Ischemic heart disease, 30 gr/day alcohol consumption | Pfizer-BioNTech | 46 d after dose II | / | ANA | Compatible with AIH | Prednisone+ Azathioprine | Remission |
| F, 62 | Celiac disease | AstraZeneca | 4 d after dose II | / | ANA | Prednisone+ AZA | Remission | ||
| F, 72 | / | Pfizer-BioNTech | 14 d after dose II | / | ANA | Prednisone | Remission | ||
| F, 59 | Hypothyroidism | Pfizer-BioNTech | 9 d after dose I | / | ANA | Not performed | No treatment received | Spontaneous improvement | |
| Hasegawa et al[72] | F, 82 | Hepatitis C virus | Pfizer-BioNTech | 7 d after dose I | Malaise | ANA | Compatible with AIH | Prednisone | Remission |
| Shroff et al[73] | F, 59 | / | Moderna | 31 d after dose I | / | ANA | Compatible with AIH | Steroids | Remission |
| Efe et al[74] | M, 53 | / | Pfizer-BioNTech | 30 d after dose II | Abdominal pain, pruritus, erythematous skin eruption | / | Compatible with AIH | Prednisolone | Developed HE and liver transplantation |
| Shahrani et al[75] | F,59 | Dyslipidemia | AstraZeneca | 12 d after dose II | Jaundice | / | Compatible with AIH | Prednisolone | Remission |
| F,72 | / | Pfizer-BioNtech | 10 d after booster | Jaundice | AMA | Compatible with AIH | Prednisolone | Remission | |
| Nik et al[76] | F,63 | Primary sclerosing cholangitis, ulcerative colitis | AstraZeneca | 14 d after dose I | Jaundice, pruritus | ANA | Compatible with AIH | Prednisolone | Death after 2 wk for sepsis |
| Mekritthikrai et al[77] | F,52 | Dyslipidemia, hypertension | Coronavac | After dose II | Jaundice | ANA, ASMA | Compatible with AIH | Prednisolone + AZA | Remission |
| Mathew et al[78] | F, late 20s | Headache | AstraZeneca | 10 d after dose I | Jaundice | ANA | Compatible with AIH | Prednisolone | Remission |
| Classification | |
| Number of patients | 40 |
| Females, n (%) | 32 (83) |
| Age (years, mean ± SD) | 58.7 ± 16 |
| Race/ethnicity, n (%) | |
| Caucasian | 11 (27) |
| Asian | 3 (8) |
| Arabic | 1 (3) |
| Unspecified | 25 (62) |
| Predisposing conditions, n (%) | |
| History of liver disease | 6 (15) |
| History of autoimmune disease | 11 (28) |
| Medications, n (%) | |
| Acetaminophen | 3 (8) |
| Other hepatotoxic drugs (statins, peg-IFN, AZT) | 5 (13) |
| Vaccine, n (%) | |
| Pfizer-BioNTech | 17 (43) |
| Moderna | 11 (28) |
| AstraZeneca | 10 (25) |
| Coronavac | 2 (5) |
| Symptom onset (days after a dose, mean ± SD) | 16.6 ± 12.8 |
| Symptoms, n (%) | |
| Jaundice | 24 (60) |
| Astenia/Malaise/Fatigue | 13 (33) |
| Choluria | 10 (25) |
| Pruritus | 8 (20) |
| Abdominal pain | 4 (10) |
| Fever | 5 (13) |
| Loss of appetite | 4 (10) |
| Nausea/vomiting | 5 (13) |
| Diagnostics | |
| Peak laboratory values (mean ± SD) | |
| AST (IU/l) | 936 ± 523 |
| ALT (IU/l) | 1060 ± 567 |
| GGT (IU/l) | 312 ± 220 |
| ALP (IU/l) | 209 ± 95 |
| Bilirubin (mg/dL) | 9.8 ± 8.5 |
| IgG (g/L) | 21.7 ± 6.7 |
| Antibodies, n (%) | |
| ANA | 33 (83) |
| ASMA | 7 (18) |
| SLA/LP | 2 (5) |
| AMA | 5 (13) |
| Others (ANCA, DS-DNA) | 3 (8) |
| Liver biopsy | |
| Performed | 39 |
| Not performed | 1 |
| Morphologic characteristics, n (%) | |
| Interface hepatitis | 31 (78) |
| Lymphoplasmacellular infiltrates | 36 (90) |
| Rosette formation | 5 (13) |
| Treatment | |
| First agent | |
| Budesonide | 1 |
| Prednisone | 15 |
| Prednisolone | 17 |
| Methylprednisolone | 3 |
| Hydrocortisone | 1 |
| Steroids | 3 |
| No treatment | 2 |
| Second agent | |
| Azathioprine | 8 |
| Prednisolone | 2 |
| Clinical outcomes | |
| Time to the first improvement, days (mean ± SD) | 4.6 ± 2.9 |
| Time to resolution, days (mean ± SD) | 44.6 ± 37 |
| Alive | 37 |
| Dead | 3 |
Most patients (83%) were female, with a mean age of 58.7 years (range 27-82). In 25 patients, race and ethnicity were not specified, whereas, among the remaining cases, 11 patients were Caucasian, three were Asian, and 1 was Arabic. Seventeen patients (42.5%) had a history of either liver (6) or auto-immune disease (11). One patient was three months postpartum[45].
Although five patients were taking potentially hepatotoxic medication, including substitutive hormonal therapy[62], statin[49,58,76,77], azathioprine[51,76], pegylated interferon for polycythaemia vera[53] and nitrofurantoin[73], each patient had been taking their medications for a long time without recent regimens changes. The patient who reported nitrofurantoin completed a three-day course nearly 90 d before the vaccine, and thus nitrofurantoin-induced liver injury was considered highly improbable. Two patients[63,73] took acetaminophen soon after receiving the first dose, whereas one patient[78] was on chronic treatment. The trigger vaccine was Pfizer-BioNTech in 17 cases (43%), Moderna in 11 (28%), Oxford AstraZeneca in 10 (20%), while CoronaVac, an inactivated whole-virion vaccine, was the trigger of AIH in two cases (5%).
Symptomatic AIH was observed in most cases (78%), with a latency time after receiving the COVID-19 vaccine of about two weeks (16.6 ± 12.8 d, range 2-60). The most common symptoms were jaundice (60%), asthenia/fatigue (33%), choluria (25%) and pruritus (20%), whereas other symptoms such as fever, abdominal pain, nausea, and vomiting were variably reported. Interestingly, two patients presented worsening symptoms after receiving the second dose[62,68]. At the laboratory, most cases showed a hepatocellular pattern of liver injury, with markedly elevated transaminases (mean values of AST and ALT of 936 ± 521 U/L and 1060 ± 567 U/L, respectively). Mean GGT, ALP, and bilirubin were 312 ± 220 U/L, 209 ± 95 U/L, and 9.8 ± 8.5 mg/dL, respectively. Immunoglobulin G was > 20 g/L in 13 patients (46.5%) with a mean value of 21.7 g/L. Thirty-seven patients (93%) had at least one positive autoantibody, and ANA was positive in 33 (83%). The scoring systems, such as the Simplified AIH score[79] and the Revised Original Score[80], guided the diagnosis of AIH in 18 cases. A liver biopsy was performed in almost all cases (98%). Findings were typical with AIH in 3 patients and compatible with AIH in 31 cases.
Steroids were used as a first-line treatment in 38 patients (95%), and azathioprine was used as a second agent in 8 patients. The mean time to the first improvement and disease resolution were 4.6 and 45.9 d, respectively. Improvement in laboratory data or complete resolution was seen in 37 out of 40 patients (93%). Three patients died of AIH triggered by viral vector vaccine (AstraZeneca), one after refusing liver transplantation[66], while the other two died of liver failure and sepsis[52,76].
Notably, vaccine-induced AIH has been previously described following other vaccinations than that against SARS-CoV-2, such as hepatitis A[81,82] or influenza virus[83,84].
Although the precise mechanisms have not been elucidated, molecular mimicry is one of the major explanations of autoimmunity after vaccination. Significant homology of amino acid sequences between determinants of vaccines and self-antigen may result in the synthesis of anti-spike antibodies that cross-react with structurally similar host peptide proteins[85]. Other proposed mechanisms include bystander activation (defined as an intense stimulation of innate immunity after administration of adjuvants) and epitope spreading (immune responses to endogenous epitopes resulting from the release of self-antigens during chronic autoimmune/inflammatory processes)[86].
Cases of AIH reactivation after vaccines underline the necessity for close follow-up in individuals with a pre-existing diagnosis of AILDs.
Current knowledge does not allow us to clearly define whether these cases represent a true AIH triggered by vaccine or transient vaccine-induced liver injury, which could have similar clinical, laboratory and histological characteristics.
A longer follow-up could be helpful to rule out vaccine-induced liver injury since biochemical and histological resolution can spontaneously occur, and immunosuppressive treatment can be withdrawn without the risk of relapse.
In summary, although a causative link between AIH and SARS-CoV-2 vaccination cannot be confirmed, emerging case reports suggest this association could be more than coincidental.
Since the initial stage of the COVID-19 pandemic, concerns about the risk of poorer outcomes following SARS-CoV-2 infection have been raised in patients with pre-existing chronic liver disease. We found that people with AILDs are not at increased risk of being infected by SARS-CoV-2, despite immunosuppressive treatment that, however, may predispose them to develop a more severe course of the disease.
Furthermore, increasing evidence highlighted the intrinsic capability of the virus to hyper-stimulate immune response leading to the development of autoimmune disease. However, only two reports identify SARS-CoV-2 as a trigger instrument for developing AILDs, making any correlation not feasible.
Post-vaccine AIH-like syndrome raises worries about a risk of unprecedented immunological side effects, especially in individuals predisposed to autoinflammatory disorders. However, COVID-19 vaccine-induced AIH is extremely uncommon and has an exceptional prognosis. Hepatologists should consider AIH in patients with jaundice or elevated liver enzymes following COVID-19 vaccination to readily initiate further work-up and treatment with steroids.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen S, China; Popovic DD, Serbia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14100] [Article Influence: 2820.0] [Reference Citation Analysis (1)] |
| 2. | WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020-World Health Organization. World Heal Organ 2020. [cited 10 November 2022]. Available from: https://www.who.int/. |
| 3. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18852] [Article Influence: 3770.4] [Reference Citation Analysis (7)] |
| 4. | Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, Viazis N, Liatsos C, Mantzaris GJ. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579-4588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (5)] |
| 5. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 7. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5513] [Article Influence: 1102.6] [Reference Citation Analysis (1)] |
| 8. | Qi X, Liu C, Jiang Z, Gu Y, Zhang G, Shao C, Yue H, Chen Z, Ma B, Liu D, Zhang L, Wang J, Xu D, Lei J, Li X, Huang H, Wang Y, Liu H, Yang J, Pan H, Liu W, Wang W, Li F, Zou S, Zhang H, Dong J. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 10. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14238] [Article Influence: 2847.6] [Reference Citation Analysis (0)] |
| 11. | Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68:310-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Verhelst X, Somers N, Geerts A, Degroote H, Van Vlierberghe H. Health status of patients with autoimmune hepatitis is not affected by the SARS-CoV-2 outbreak in Flanders, Belgium. J Hepatol. 2021;74:240-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zecher BF, Buescher G, Willemse J, Walmsley M, Taylor A, Leburgue A, Schramm C, Lohse AW, Sebode M. Prevalence of COVID-19 in patients with autoimmune liver disease in Europe: A patient-oriented online survey. United European Gastroenterol J. 2021;9:797-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Murtas R, Andreano A, Gervasi F, Guido D, Consolazio D, Tunesi S, Andreoni L, Greco MT, Gattoni ME, Sandrini M, Riussi A, Russo AG. Association between autoimmune diseases and COVID-19 as assessed in both a test-negative case-control and population case-control design. Auto Immun Highlights. 2020;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Rigamonti C, Cittone MG, De Benedittis C, Rizzi E, Casciaro GF, Bellan M, Sainaghi PP, Pirisi M. Rates of Symptomatic SARS-CoV-2 Infection in Patients With Autoimmune Liver Diseases in Northern Italy: A Telemedicine Study. Clin Gastroenterol Hepatol. 2020;18:2369-2371.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, Pozzoni P, Claar E, Pasulo L, Fagiuoli S, Cristoferi L, Carbone M, Invernizzi P. Coronavirus Disease 2019 in Autoimmune Hepatitis: A Lesson From Immunosuppressed Patients. Hepatol Commun. 2020;4:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, Rıza Calışkan A, Peralta M, Gerussi A, Massoumi H, Catana AM, Torgutalp M, Purnak T, Rigamonti C, Gomez Aldana AJ, Khakoo N, Kacmaz H, Nazal L, Frager S, Demir N, Irak K, Ellik ZM, Balaban Y, Atay K, Eren F, Cristoferi L, Batıbay E, Urzua Á, Snijders R, Kıyıcı M, Akyıldız M, Ekin N, Carr RM, Harputluoğlu M, Hatemi I, Mendizabal M, Silva M, Idilman R, Silveira M, Drenth JPH, Assis DN, Björnsson E, Boyer JL, Invernizzi P, Levy C, Schiano TD, Ridruejo E, Wahlin S. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73:2099-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 20. | Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, Calışkan AR, Peralta M, Gerussi A, Massoumi H, Catana AM, Purnak T, Rigamonti C, Aldana AJG, Khakoo N, Nazal L, Frager S, Demir N, Irak K, Melekoğlu-Ellik Z, Kacmaz H, Balaban Y, Atay K, Eren F, Alvares-da-Silva MR, Cristoferi L, Urzua Á, Eşkazan T, Magro B, Snijders R, Barutçu S, Lytvyak E, Zazueta GM, Demirezer-Bolat A, Aydın M, Heurgue-Berlot A, De Martin E, Ekin N, Yıldırım S, Yavuz A, Bıyık M, Narro GC, Kıyıcı M, Akyıldız M, Kahramanoğlu-Aksoy E, Vincent M, Carr RM, Günşar F, Reyes EC, Harputluoğlu M, Aloman C, Gatselis NK, Üstündağ Y, Brahm J, Vargas NCE, Güzelbulut F, Garcia SR, Aguirre J, Anders M, Ratusnu N, Hatemi I, Mendizabal M, Floreani A, Fagiuoli S, Silva M, Idilman R, Satapathy SK, Silveira M, Drenth JPH, Dalekos GN, N Assis D, Björnsson E, Boyer JL, Yoshida EM, Invernizzi P, Levy C, Montano-Loza AJ, Schiano TD, Ridruejo E, Wahlin S. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 22. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 23. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 24. | Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19:102695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3333] [Article Influence: 666.6] [Reference Citation Analysis (0)] |
| 26. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 27. | Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, Sule G, Gockman K, Madison JA, Zuo M, Yadav V, Wang J, Woodard W, Lezak SP, Lugogo NL, Smith SA, Morrissey JH, Kanthi Y, Knight JS. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 28. | Vlachoyiannopoulos PG, Magira E, Alexopoulos H, Jahaj E, Theophilopoulou K, Kotanidou A, Tzioufas AG. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79:1661-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 29. | Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 268] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Bartoli A, Gitto S, Sighinolfi P, Cursaro C, Andreone P. Primary biliary cholangitis associated with SARS-CoV-2 infection. J Hepatol. 2021;74:1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Singh B, Kaur P, Maroules M. Autoimmune Hepatitis-Primary Biliary Cholangitis Overlap Syndrome Triggered by COVID-19. Eur J Case Rep Intern Med. 2021;8:002264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Muratori P, Granito A, Pappas G, Pendino GM, Quarneti C, Cicola R, Menichella R, Ferri S, Cassani F, Bianchi FB, Lenzi M, Muratori L. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am J Gastroenterol. 2009;104:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Chai XQ, Hu LF, Zhang Y, Han WY, Lu Z, Ke AW, Zhou J, Shi GM, Fang N, Fan J, Cai JB, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766. [DOI] [Full Text] |
| 35. | Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (2)] |
| 37. | Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, Mo X, Wang J, Wang Y, Peng P, Chen X, Hong W, Xiao G, Liu J, Zhang L, Hu F, Li F, Zhang F, Deng X, Li L. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med (N Y). 2020;1:105-113.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 38. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10666] [Article Influence: 2133.2] [Reference Citation Analysis (1)] |
| 40. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7534] [Article Influence: 1883.5] [Reference Citation Analysis (1)] |
| 41. | Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, McEvoy C, DeJesus E, Hassman M, Little SJ, Pahud BA, Durbin A, Pickrell P, Daar ES, Bush L, Solis J, Carr QO, Oyedele T, Buchbinder S, Cowden J, Vargas SL, Guerreros Benavides A, Call R, Keefer MC, Kirkpatrick BD, Pullman J, Tong T, Brewinski Isaacs M, Benkeser D, Janes HE, Nason MC, Green JA, Kelly EJ, Maaske J, Mueller N, Shoemaker K, Takas T, Marshall RP, Pangalos MN, Villafana T, Gonzalez-Lopez A; AstraZeneca AZD1222 Clinical Study Group. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385:2348-2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 42. | Our World in Data. Coronavirus (COVID-19) Vaccinations [cited 10 November 2022]. Available from: https://ourworldindata.org/covid-vaccinations. |
| 43. | Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 530] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 44. | Akinosoglou K, Tzivaki I, Marangos M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol. 2021;226:108721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 45. | Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75:222-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 46. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 846] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 47. | Lee SK, Kwon JH, Yoon N, Lee SH, Sung PS. Immune-mediated liver injury represented as overlap syndrome after SARS-CoV-2 vaccination. J Hepatol. 2022;77:1209-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 49. | Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, Cerny A, Dayer E, Vergani D, Terziroli Beretta-Piccoli B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J Autoimmun. 2021;123:102706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 50. | Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J Autoimmun. 2021;125:102745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 51. | Mahalingham A, Duckworth A, Griffiths WJH. First report of post-transplant autoimmune hepatitis recurrence following SARS-CoV-2 mRNA vaccination. Transpl Immunol. 2022;72:101600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 52. | Erard D, Villeret F, Lavrut PM, Dumortier J. Autoimmune hepatitis developing after COVID 19 vaccine: Presumed guilty? Clin Res Hepatol Gastroenterol. 2022;46:101841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun. 2021;125:102741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 54. | Goulas A, Kafiri G, Kranidioti H, Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following Covid-19 mRNA vaccination. More than a coincidence? Liver Int. 2022;42:254-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2022;75:757-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 56. | Vuille-Lessard É, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123:102710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Suzuki Y, Kakisaka K, Takikawa Y. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: Need for population-based epidemiological study. Hepatology. 2022;75:759-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75:1250-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 59. | Zhou T, Fronhoffs F, Dold L, Strassburg CP, Weismüller TJ. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis - should we be more vigilant? J Hepatol. 2022;76:218-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Kang SH, Kim MY, Cho MY, Baik SK. Autoimmune Hepatitis Following Vaccination for SARS-CoV-2 in Korea: Coincidence or Autoimmunity? J Korean Med Sci. 2022;37:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casuality. J Hepatol. 2021;75:728-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 62. | Londoño MC, Gratacós-Ginès J, Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J Hepatol. 2021;75:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 63. | McShane C, Kiat C, Rigby J, Crosbie Ó. The mRNA COVID-19 vaccine - A rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 64. | Clayton-Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75:1249-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 65. | Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology. 2022;75:489-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 66. | Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123:102688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 67. | Camacho-Domínguez L, Rodríguez Y, Polo F, Restrepo Gutierrez JC, Zapata E, Rojas M, Anaya JM. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022;5:100140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 68. | Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 69. | Torrente S, Castiella A, Garmendia M, Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol Hepatol. 2022;45 Suppl 1:115-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Fimiano F, D'Amato D, Gambella A, Marzano A, Saracco GM, Morgando A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following Covid-19 vaccination? Liver Int. 2022;42:1204-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Izagirre A, Arzallus T, Garmendia M, Torrente S, Castiella A, Zapata EM. Autoimmune hepatitis following COVID-19 vaccination. J Autoimmun. 2022;132:102874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Hasegawa N, Matsuoka R, Ishikawa N, Endo M, Terasaki M, Seo E, Tsuchiya K. Autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination: case report and literature review. Clin J Gastroenterol. 2022;15:791-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76:211-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 74. | Efe C, Harputluoğlu M, Soylu NK, Yilmaz S. Letter to the editor: Liver transplantation following severe acute respiratory syndrome-coronavirus-2 vaccination-induced liver failure. Hepatology. 2022;75:1669-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Shahrani S, Sooi CY, Hilmi IN, Mahadeva S. Autoimmune hepatitis (AIH) following coronavirus (COVID-19) vaccine-No longer exclusive to mRNA vaccine? Liver Int. 2022;42:2344-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Nik Muhamad Affendi NA, Ravindran S, Siam TS, Leow AH, Hilmi I. Jaundice in a Primary Sclerosing Cholangitis Patient: A New Cause in a New Era. Inflamm Bowel Dis. 2022;28:e29-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Mekritthikrai K, Jaru-Ampornpan P, Komolmit P, Thanapirom K. Autoimmune Hepatitis Triggered by COVID-19 Vaccine: The First Case From Inactivated Vaccine. ACG Case Rep J. 2022;9:e00811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Mathew M, John SB, Sebastian J, Ravi MD. COVID-19 vaccine triggered autoimmune hepatitis: case report. Eur J Hosp Pharm. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 80. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1985] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 81. | Berry PA, Smith-Laing G. Hepatitis A vaccine associated with autoimmune hepatitis. World J Gastroenterol. 2007;13:2238-2239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | van Gemeren MA, van Wijngaarden P, Doukas M, de Man RA. Vaccine-related autoimmune hepatitis: the same disease as idiopathic autoimmune hepatitis? Scand J Gastroenterol. 2017;52:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Muratori P, Serio I, Lalanne C, Lenzi M. Development of autoimmune hepatitis after influenza vaccination; trigger or killer? Clin Res Hepatol Gastroenterol. 2019;43:e95-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Sasaki T, Suzuki Y, Ishida K, Kakisaka K, Abe H, Sugai T, Takikawa Y. Autoimmune hepatitis following influenza virus vaccination: Two case reports. Medicine (Baltimore). 2018;97:e11621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? Clin Immunol. 2021;224:108665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 86. | Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |