Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1811
Peer-review started: November 15, 2022
First decision: December 11, 2022
Revised: December 23, 2022
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: March 28, 2023
Processing time: 133 Days and 6 Hours
Pancreatic cancer (PC) has a low incidence rate but a high mortality, with patients often in the advanced stage of the disease at the time of the first diagnosis. If detected, early neoplastic lesions are ideal for surgery, offering the best prognosis. Preneoplastic lesions of the pancreas include pancreatic intraepithelial neoplasia and mucinous cystic neoplasms, with intraductal papillary mucinous neoplasms being the most commonly diagnosed. Our study focused on predicting PC by identifying early signs using noninvasive techniques and artificial intelligence (AI). A systematic English literature search was conducted on the PubMed electronic database and other sources. We obtained a total of 97 studies on the subject of pancreatic neoplasms. The final number of articles included in our study was 44, 34 of which focused on the use of AI algorithms in the early diagnosis and prediction of pancreatic lesions. AI algorithms can facilitate diagnosis by analyzing massive amounts of data in a short period of time. Correlations can be made through AI algorithms by expanding image and electronic medical records databases, which can later be used as part of a screening program for the general population. AI-based screening models should involve a combination of biomarkers and medical and imaging data from different sources. This requires large numbers of resources, collaboration between medical practitioners, and investment in medical infrastructures.
Core Tip: To improve the clinical management and prognosis for patients with pancreatic cancer (PC), new diagnostic methods should be developed to identify precursor lesions. Artificial intelligence (AI) is a tool that can offer a personalized approach in this regard by analyzing a large quantity of heterogeneous data and can also help in decision-making, increasing the prediction accuracy for an early diagnosis. The aim of this study was to provide a comprehensive overview of the advances in detecting PC noninvasively with an emphasis on early lesions and AI.
- Citation: Faur AC, Lazar DC, Ghenciu LA. Artificial intelligence as a noninvasive tool for pancreatic cancer prediction and diagnosis. World J Gastroenterol 2023; 29(12): 1811-1823
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1811
The World Health Organization estimates that early cancer detection will result in 30% greater cure rates for most cancer types[1]. Pancreatic cancer (PC) has a low incidence but is currently the 4th leading cause of all cancer deaths. The 5-year survival rate for PC is between 7% and 20%, with the best prognosis obtained for pancreatic neoplasms diagnosed in early stages[2-4]. Studies have reported that the 5-year survival rate can be as high as 39.9% in localized pancreatic tumors, but in patients who develop metastatic disease, the rate drops significantly to 2.9%[2-4].
There are two main types of PC, exocrine carcinomas and neuroendocrine neoplasms, with the former accounting for more than 95% of pancreatic tumors[5]. Patients with exocrine tumors compared with those with endocrine ones have a median survival of approximately 4 mo vs 27 mo[6]. Approximately 90% of exocrine PC cases are pancreatic ductal adenocarcinoma (PDAC); 80% of PDAC patients are in the advanced stage of the disease at the time of the first diagnosis[4,7]. A total of 49.6% of PDAC patients are diagnosed with distant metastases, and only approximately 15% have a surgically resectable tumor[1,2,4,7]. By 2030, PDAC is expected to become the second most common cause of cancer-related death in Western countries[8]. An important aspect in the diagnosis of PC is the size of the tumor, which correlates with outcome. For early-stage PDAC with node-negative, small tumors (< 2 cm), the survival rate can be as high as 80%[8]. Less than 2% of PCs are pancreatic neuroendocrine tumors (PNT). Current data have shown that an earlier diagnosis of PNTs is not associated with an improved survival rate. Compared with PDACs, PNTs have a better prognosis[9,10].
Preneoplastic lesions that frequently arise in the exocrine pancreas include pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasms (MCN), and intraductal papillary mucinous neoplasms (IPMNs)[11]. Low-grade PanIN is quite common (present in 40%-75% of adults), especially after the age of 40[12]. The progression from low-grade PanIN to invasive tumors is unknown, and currently, identifying this type of lesion is not sufficient to justify surgical intervention. However, if high-grade PanIN is detected, surgery is highly indicated[1]. MCNs of the pancreas rarely progress to PC[11], while IPMNs are fairly common and, if left untreated, can progress to cancer[13]. An association between PDAC and IPMN has been reported in 11%-80% of cases[14]. Identifying high-risk IPMNs before surgical intervention enables patients in the low-risk category to avoid unnecessary surgery for benign disease. At this time, there are no accurate methods for the preoperative discrimination between high- and low-risk IPMNs[3,15].
Pancreatitis and cystic lesions of the pancreas are considered risk factors for developing PDAC. Chronic pancreatitis was identified in patients with PDAC 10 to 20 years earlier and acute pancreatitis 1 to 2 years before tumor diagnosis. Studies have shown that less than 5% of patients with chronic pancreatitis also develop PDAC. Cystic precursor lesions of PC include IPMN and MCNs, with IPMN being the most commonly diagnosed[1,14]. Pancreatic cyst fluid cytologic analysis is a candidate method for identifying progression to high-grade PanIN or cancer with a 25%-88% sensitivity in detecting pancreatic malignancy[1,16].
A challenge in the early diagnosis PC is the absence of specific symptoms or clinical data for patients with pancreatic tumors. The symptoms of PC, if present, are not specific and manifest approximately 6 mo after the identification of PDAC. Patients with symptoms from PC complain of abdominal pain and unexplained weight loss and are often found to have jaundice[4]. The majority of patients are asymptomatic, however[14].
Initial tests for evaluating patients suspected of having PC include serological investigation and abdominal imaging[5]. The latter is often performed using magnetic resonance imaging (MRI), MRI cholangiopancreatography, computed tomography (CT), endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), and positron emission tomography[5,14,17]. One of the newest techniques in MRI involves diffusion-weighted imaging, which is capable of discriminating between PC and pancreatitis[17]. Certain features can be indicative of malignancy on imaging studies of early lesions for PC, including internal septation, mural nodularity, solid masses, duct dilatation, and vascular invasion. In 36%-70% of IPMN-associated cancers, mural nodules are diagnosed[14]. CT and MRI have a 56%-88% accuracy in detecting IPMN, but EUS has a higher resolution[14]. Pancreatic cysts are found in 3% of CT scans and 20% of MRI scans and can reach a 40% incidence in patients over 80 years old[14]. The most common imaging methods used, however, are EUS and CT. New EUS techniques (contrast-enhanced EUS and elastography) and the use of two or more imaging methods can enhance the diagnostic accuracy[7].
Additional methods implemented for PC include the analysis of personal data, biomarkers, and genomic features. Conditions such as family history of PC, body mass index, smoking, and alcohol abuse are epidemiologically associated with PC[18]. A total of 75%-80% of PCs are found in the head of the pancreas[14]. Diabetes is identified in 10%-40% of cases with IPMN, and a strong association between insulin and the risk of IPMN has been reported[14]. Patients with Peuts-Jegher syndrome, McCum-Albright syndrome, and familial adenomatous polyposis have also been diagnosed with IPMN[12,14]. MicroRNAs are biomarkers that have shown potential as a diagnostic tool for PDAC because of their high expression in the plasma of these patients[2]. MUC4, MUC16, differentially methylated DNA, telomerase protease expression, and overexpression of Das-1 biomarkers are associated with PDACs and are currently being validated for clinical use[1,14]. Elevated levels of carbohydrate antigen (CA)19-9 and carcinoembryonic antigen (CEA) and the identification of a new protein (cell migration-inducing hyaluronan-binding protein) are considered useful in PC prediction[14,19]. High concentrations of CEA are reported in mucinous pancreatic cysts but lack the potential to differentiate between IPMNs and PDACs[14]. The only biomarker for PDAC commonly considered reliable is CA19-9, but in the early stage disease, it has a sensitivity between 25% and 50% and is present in 51.1% of patients with PC stage I and 44.1% of patients with stage II[1,5].
Specific genomic features have been described for PDAC[1]. The top genes targeted during pancreatic carcinogenesis include KRAS, CDKN2A, TP53, and SMAD4. In patients with hereditary pancreatitis, mutation of the PRSS1 gene is associated with a higher risk of PC[12]. In early lesions of low-grade PanIN, KRAS mutations have identified, while inactivation of CDK2A and alterations in TP53, SMDA4, and BRCA2 occur in high-grade PanIN. Patients with these gene alterations can be classified as high-risk PDAC cohorts and considered for PC screening. These cohorts can also include patients with pancreatic cysts, pancreatitis, and new-onset diabetes who are 60 to 75 years of age[1]. KRAS, GNAS, TP53, SMAD4, PIK3CR, PTEN, and AKT1 mutations have been reported in IPMN-associated neoplasms with a 32%-89% sensitivity and 96%-100% specificity[14]. However, screening for PC asymptomatic subjects without a well-defined high-risk group is currently considered cost prohibitive[1].
Surgery is seen as a potentially curative treatment for PC if the patient is diagnosed in the early stage. For these patients, the pancreatic tumor can be completely resected, and by doing so, the survival rate significantly increases to 50%[4]. Treatment for the advanced state of PC typically includes partial resection, which is associated with tumor recurrence and a 5-year survival rate of less than 10%[4]. Unfortunately, pancreatic resection for PC has a high morbidity rate of up to 60%. Postoperative pancreatic fistula, stroke, cardiac arrest, wound dehiscence, infection, hemorrhage, and renal failure have been reported in patients the first month after pancreatic surgery[20]. Considering these factors, predicting PC by diagnosing early lesions and implementation of a screening program for PC becomes a matter of paramount importance. Artificial intelligence (AI) models integrating multisource risk factors are the future of early PC diagnosis.
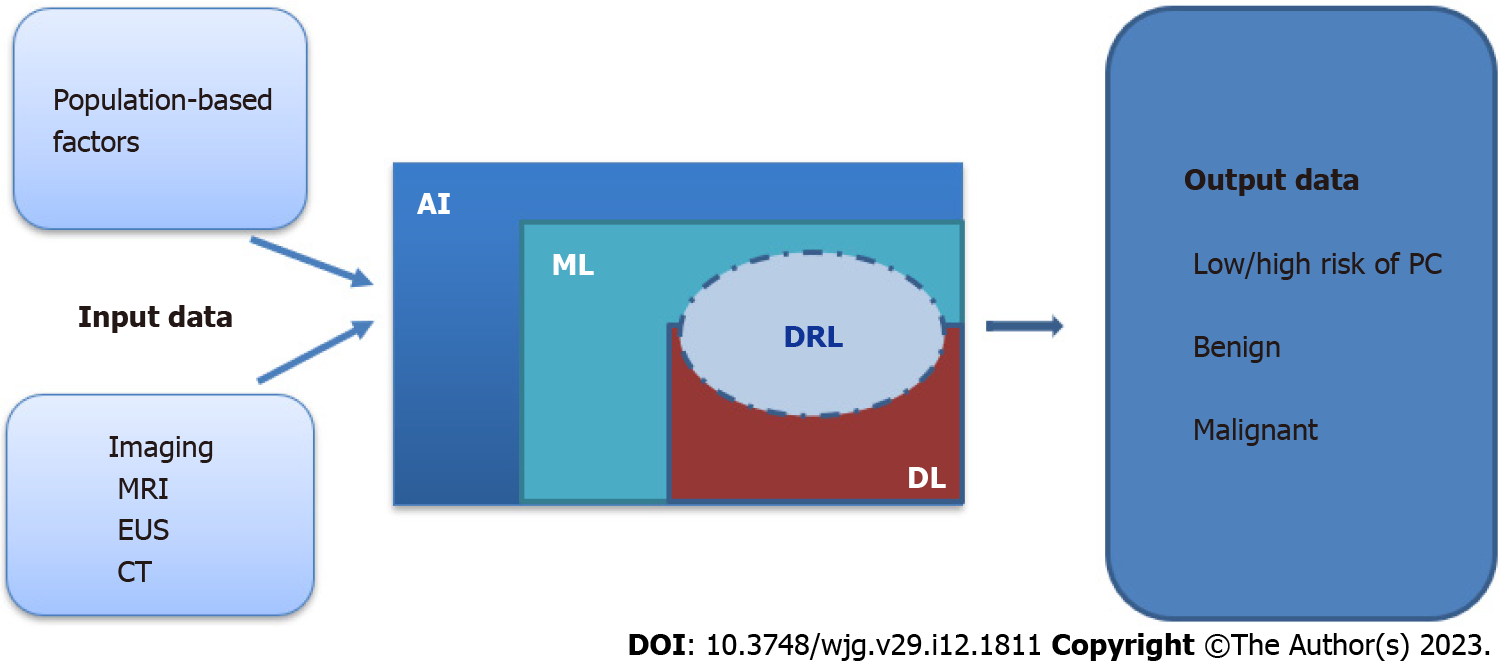
Machine learning (ML), deep learning (DL) and the ability of computers to independently solve problems by using specific algorithms, is known today as AI. Although the terms ML and AI are commonly used interchangeably, ML is a subfield of AI. ML is based on developing mathematical algorithms for the analysis of data with the goal of creating a prediction model by recognizing patterns in these data. The algorithms can produce a machine-based diagnostic outcome for a specific disease. ML algorithms can be supervised or unsupervised and represented by support vector machines (SVMs), Bayesian interference, regressions, ensemble methods, decision trees, k-nearest neighbors, linear discriminants, and neural networks[16,21]. Supervised algorithms are based on data previously known by the computer, meaning that the computer is first trained with datasets from the domain of interest to later accurately analyze new datasets with the same subject and produce the desired mathematical outcome. In unsupervised algorithms, the computer sorts the input data and identifies features that are grouped into clusters or associations and analyzes them to reach a desired outcome[22]. Reinforcement learning is an advanced type of ML that is used to develop models that will help in the prediction of cancer but also facilitate the decision-making process[23]. Deep reinforcement learning is the combined process of reinforcement learning with DL. DL methods are trained to analyze large datasets through multilayer neural networks[24]. Developing algorithms by using AI methods helps in analyzing large amounts of data at the same time and integrating data obtained from different sources. Therefore, the results of clinical tests, imaging, personal data, biomarkers, and genomic features can be quickly analyzed, compared with existing knowledge, and used to reach an initial machine-made diagnosis. The purpose of our study was to identify the current diagnostic methods for detecting PC by using noninvasive techniques with an emphasis on early lesions and AI.
English-language literature published in the last 10 years covering diagnosing PC was searched by accessing the PubMed electronic database and other sources. The following key words were used for the search: “Pancreatic cancer”, “early pancreatic lesions”, “predicting pancreatic neoplasia”, “artificial intelligence”, “deep learning”, “machine learning”, “radiomics”, “diagnosis”, and “pancreas”. We obtained a database of 97 studies on pancreatic neoplasms, including both single studies and reviews. We then removed duplicates, resulting in 86 papers. We excluded abstracts, reviews with insufficiently explained results, and articles not in the field of interest/irrelevant topics or with repetitive information. The final number of articles included was 44. The article selection algorithm for the study is depicted in Figure 1. We divided the remaining articles into two datasets. One contained the newest review articles and studies on obtaining information about current knowledge in assessing PC (10), and the other included studies that explored the use of AI algorithms in the diagnosis of pancreatic lesions (34). This second dataset included 24 studies on the current status of AI diagnostic methods for PC and 10 studies discussing the implications for early PC and prediction of the use of AI algorithms. The obtained data from the two datasets are presented narratively. The results of our analysis on the PC prediction methods for diagnosing early lesions are summarized in Table 1 and Figure 2.
| Ref. | Purpose of the model | Type of study | Type of model | Input data | Type of validation | No. cases |
| Qureshi et al[19], 2022 | Identifying predictive features on prediagnostic CT scans for PDAC | Retrospective | NB | 4000 radiomics from CT | External | 72 (36 with PC) |
| Sekaran et al[22], 2020 | Predicting PC | Retrospective | CNN | 19000 images from CT | Internal | 80 (NS) |
| Chen et al[36], 2018 | Identification and classification methods for PC on MRI | Retrospective | CNN | 863 images from MRI | Internal | 40 (20 with PC) |
| Muhammad et al[18], 2019 | Prediction of PC risk | Retrospective | CNN | 18 features of epidemiologic and clinical data | External | 800144 (898 with PC) |
| Alves et al[8], 2022 | Detection and localization of small PDAC lesions on contrast-enhanced CT | Retrospective | DL | 242 images from CT-CE | External | 242 (119 with PC) |
| Kuwahara et al[35], 2019 | Investigate the value of EUS in predicting malignancy in IPMN | Retrospective | CNN | 3970 radiomics from EUS | Internal | 50 (23 malignant) |
| Hussein et al[3], 2019 | Identification of IPMN | Retrospective | CAD | 171 MRI images | Internal | 171 (133 IMPN) |
| Chakraborty et al[15], 2018 | Identification of high-risk IPMN | Retrospective | SVM | 103 CT images | Internal | 103 (27 high-risk IMPN) |
| Liu et al[28], 2020 | Classifying images as cancerous or noncancerous PC | Retrospective | CNN | 21105 CT images | Internal and external | 1242 (752 with PC) |
| Lee et al[44], 2022 | Prediction of risk for PC | Retrospective | DNN | 9 factors | Internal and external | 2952 (738 with PC) |
ML in medicine is based on analyzing digital medical images from CT, MRI, and EUS and extracting image features in a process called radiomics. These radiomics features are converted into data that can be subjected to statistical analysis. In supervised methods, a human operator selects and labels the lesion in an image that will constitute the input data before an ML algorithm analyses these data. Unsupervised algorithms use a large amount of data for training and learn how to extract the region that will be later subjected to analysis. These mathematical algorithms will then examine the input data through a network similar to the neuronal network of the human brain. The human central nervous system receives input through various receptors, and then sends this information to the brain via the ascending pathway, where the data is then examined before a specific response is delivered through descending pathways to the intended receiver organs. There are similarities between the AI network and the human model of neural processing. We can consider humans as receptors of patient information, the input data as the ascending pathways, computers organized in layers as the brain, and output data as the descending pathways reaching the desired outcome as the receiver. Deep neural network or DL models are composed of sets of so-called hidden layers capable of studying a large amount of data. Each of the layers selects and amplifies certain features of the input information. For example, the first layer identifies a specific region and its length, the next layer analyzes the depth, another layer detects the pattern, and the subsequent layers compare the input with known or trained patterns so that when the information is clear, it is transformed into the output represented by the feature that best characterizes a specific type of lesion. In this way, AI is similar to humans but with significantly reduced human intervention and fewer human errors[25].
The interest in AI in healthcare is growing. The SVM algorithm has been used to classify breast cancer and has achieved a lower error rate than K-nearest neighbor, naïve Bayes, and C4.5 algorithms[22,26,27]. Electronic phenotyping algorithms have been built by researchers for predicting a disease correctly by using the electronic health records of the patients[22]. A convolutional neural network (CNN) has been used to prognosticate rectum toxicity in cervical cancer subjects with data provided from beam radiotherapy and brachytherapy. An alternative to surgery for the treatment of PC is radiation therapy. Given the challenging anatomy of the pancreas, sometimes the target area for treatment is difficult to identify. For these patients, metallic fiducials are implanted into the tumor target. These fiducials, however, can cause metal artifacts, obscure the tumor target, or be a source of complications such as pancreatitis or infections, all leading to delayed treatment. Therefore, developing mathematical algorithms for PC radiation therapy is a current subject of research. AI models using CT image data to localize the target PC for radiation therapy and calculate the dose of stereotactic body radiation have been reported with encouraging results[16,25]. DL algorithms have analyzed the mitotic rate of neoplastic cells to classify tumors. DL involves up to 150 hidden layers in the neural network, while traditional neural networks contain 2-3 layers. Some authors have suggested that tumors can be characterized by using computer aided design tools with supervised and unsupervised DL algorithms using CNN and transfer learning techniques. CNNs can be trained to automatically extract relevant features from images and classify the objects in the images with acceptable accuracy[22]. For AI-driven technologies to see further development in healthcare, ethical concerns must be addressed. Because AI algorithms require large datasets for training and for validation, data collection, storage, processing, confidentiality, and sharing must be considered[23].
The potential of CNNs has been explored in the diagnosis of skin neoplasms, diabetic retinopathy, and liver tumors, but its utility in the detection of PC remains to be fully determined. CNN algorithms have been used for segmentation of the pancreas, risk stratification for IPMN, assessment of the grade (G) of pancreatic neuroendocrine neoplasm, targeting PC for radiotherapy, and classification of the pancreatic cysts[28].
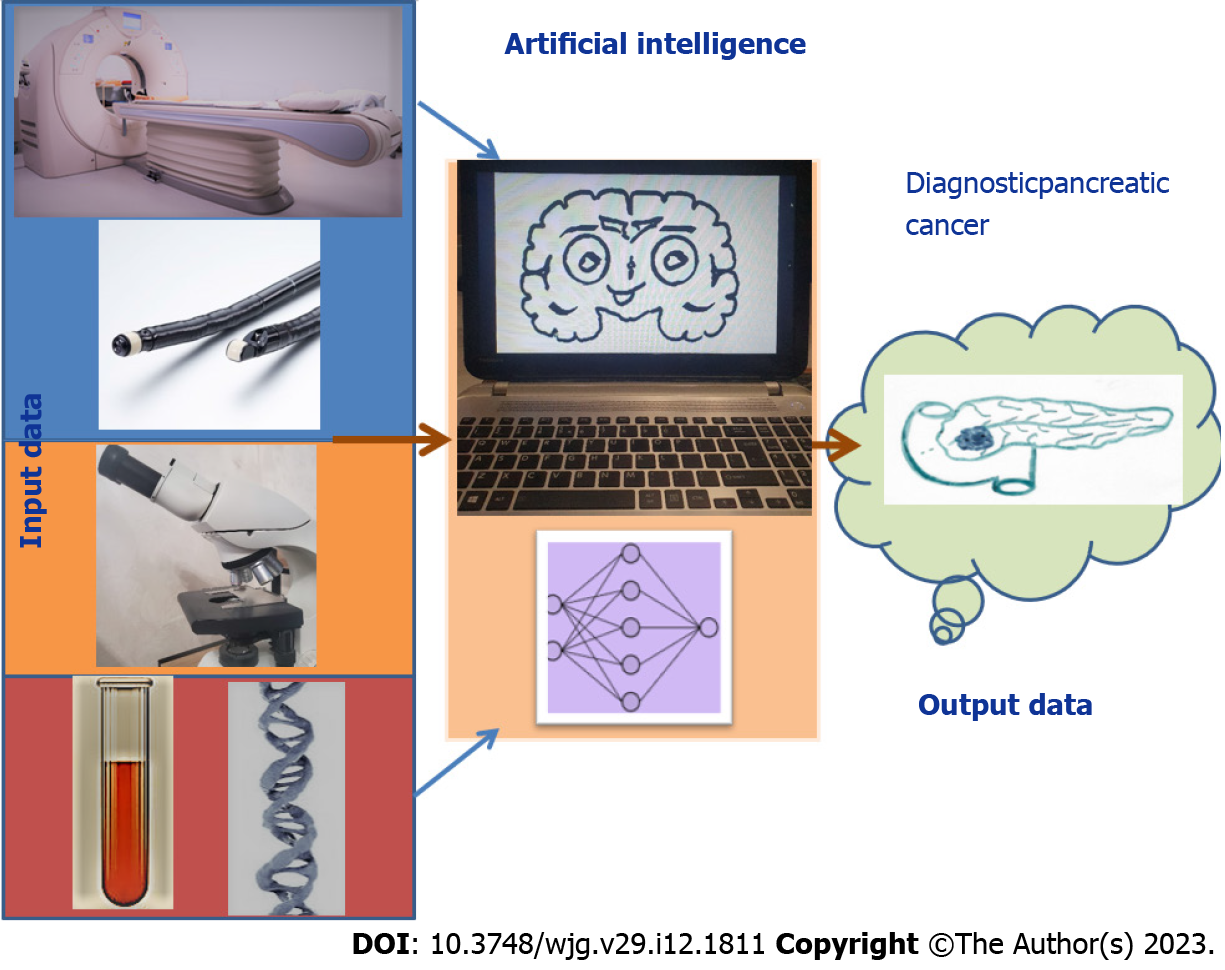
In the diagnosis of PCs, AI is used for the analysis of radiomics features from CT, MRI, and EUS, of images from histopathological slides, and of tumor markers[29] (Figure 3). Because CT is commonly performed when investigating patients, it is currently the most explored imaging modality with AI. In the United States, approximately 7 million patients per year present to the emergency room with abdominal pain, for which a CT scan analysis is required by hospital protocols. These protocols offer a database that can be consulted for patients who later develop PC[1,19]. In identifying PC, contrast-enhanced CT has a sensitivity of 70%-100%, and the usual CT scan has an accuracy of 83.3%, sensitivity of 81.4%, and specificity of 43%. In the detection of PDAC, CT has a sensitivity of 76%-96% and offers information about the location, size, and morphology of the pancreatic mass[1,2,25]. According to Qureshi et al[19], healthy cells transforming into neoplastic cells appear darker on a CT scan. Even with these results, CT scan-based analysis is not taken into consideration as a screening method for early pancreatic lesions because of the constant exposure of the patient to radiation[1,2,25]. MRI techniques identify early modifications in the pancreatic parenchyma, such as fibrosis and inflammation, with enhanced resolution. New developments in these techniques have allowed the evaluation of tissue vascularization[3]. PDAC can be diagnosed on MRI with an accuracy of 89.1%, sensitivity of 89.5%, and specificity of 63.4%. EUS has demonstrated the highest precision in tumor detection for pancreatic lesions, with a sensitivity as high as 74%-94%[1,25]; in the detection of PCs, an endoscopist analyzing EUS images can reach a sensitivity of 94%[1,29-31]. Some studies have shown that even though PDAC is the most commonly identified histopathological type of PC, PNTs have a higher predominance in small pancreatic lesions. The differential diagnosis between PNTs and other types of PC can be made using EUS because of the better contrast offered by their rich vascularization[30]. In neuroendocrine tumors, EUS has a 75%-97% detection rate for PNTs but gives the best results when combined with MRI studies in the initial assessment[30]. By using AI algorithms in EUS for detecting PC, the sensitivity increases to 83%-100% with an overall accuracy of 80%-97%[1,21]. However, there are limitations to using EUS as a common analytical method for clinical diagnosis; although it is more cost-effective than MRI, EUS is limited by: (1) The need for patient sedation; (2) The need for a technician with suitable knowledge and experience in performing the method; and (3) The narrow field investigated, as EUS cannot visualize extrapancreatic organs[1]. The sensitivity of EUS in diagnosing PC ranges from 54%-98%; hence, new techniques, such as ML algorithms, are needed[16,25].
Diagnosing PC is often difficult due to the lack of well-defined radiographic images and symptoms of the disease in early lesions as well as mimickers of neoplasia[4]. In performing CT segmentation, a radiologist must analyze approximately 300 images containing visual information for each patient, so a mathematical algorithm developed for the analysis of data extracted from a CT scan of the patients listed as high-risk in a screening program would increase efficiency[1]. Even though the image of a neoplasm can be identified months before the time of diagnosis, the sensitivity for PC is low, so a screening method involving AI algorithms could help predict and identify early stages of PC. On CT scans, PC presents with irregular contours and poorly defined margins. Early lesion studies have shown that on CT, alterations are present in the pancreas 18 mo before the diagnosis of PDAC but with a sensitivity of about 15%[1,2,25]. In early disease stages, when the lesions are small, these masses can be overlooked by radiologists[31]. Typically, pancreatic tumors smaller than 2 cm are difficult to identify from the surrounding tissue, and the gland itself represents approximately 1.3% of a CT image, offering only a small amount of data[1,2,31]. Lesions that can alter the depiction of the pancreas on a CT scan by causing shape and size deformations and that appear similar to PC include parenchymal atrophy, IPMN, lithiasis, pancreatitis, and pancreatic duct ectasia. These lesions increase the tissue heterogeneity of the pancreas on CT, making the diagnosis of PC difficult[4]. One report showed that in 90% of cases, the misdiagnosis of PC on imaging was due to inflammation that obscured the underlying tumor mass[23]. Studies of PC with AI have been conducted to help classify PNTs by analyzing preoperative CT and MRI accurately and showed that these AI algorithms can be useful in differentiating exocrine from endocrine PC in complicated cases. Commonly, there are different patterns in the vascularization of PNT and pancreatic adenocarcinomas, but there are small proportions of cases with atypical findings that make the differential diagnosis difficult, and for these situations, a histogram analysis on CT can be used[31]. Studies have assessed tumor grade, with results showing that DL methods applied to MRI can differentiate between histological aspects of PNT by classifying G1 from G2 and G3 neoplasms and G1 and G2 from G3[31,32].
EUS has an elevated detection rate for IPMN and in situ PC (stage 0)[33]. Digital image analysis of EUS images using computers with SVM- and artificial neural network (ANN)-based methods has demonstrated high accuracy[25,34]. The overall accuracy in using ANNs on EUS for the diagnosis of PC ranges between 89%-94%. Elastography performed during EUS is reported to have an accuracy of 83%-90%; this imaging technique can differentiate the consistency of the issue on an EUS image by color coding, with red indicating softer tissue and blue indicating harder areas[25]. Better results of EUS analysis with AI techniques have been obtained by studying an extended neural network of EUS elastography images with an accuracy of 89.7% in differentiating between malignant and benign pancreatic lesions. Additionally, contrast-enhanced EUS and contrast-enhanced harmonic EUS have been shown to be useful in identifying focal pancreatic masses together with a diagnosis of a benign or malignant lesion. In contrast-enhanced EUS, contrast agents are intravenously injected for better identification of focal pancreatic lesions[21]. Preoperative AI sorting of EUS images of IPMNs presented a higher accuracy in predicting the malignant component than a human technician (94% vs 56%, respectively). A study performed with a DL back propagation master for EUS training and quality control, which had both internal and external validation, achieved an accuracy ranging from 82.4% to 94.2% in detecting PC. The decision tree method, implemented for patients who underwent ERCP, precisely identified PC with an 87%-91% sensitivity and 80% specificity[16].
A meta-analysis of published data aiming to investigate the diagnostic value of AI EUS in PC showed a very good diagnostic accuracy. The meta-analysis extracted data from 10 studies that included 1871 patients with AI algorithms based on a CNN, ANN, and SVM. The ANN model had the best accuracy in detecting PC, with a sensitivity in detecting small pancreatic tumors of 93% for EUS, 53% for CT, and 67% for MRI. However, the authors stated that the results of these 10 studies did not have a general utility because the data were evaluated only with internal validation, and thus the results could have been overestimated. To be relevant for patients from different populations, the sources used for validation must be diverse, external, and from more than one center[24]. Studies have reported that ML models such as ANNs and CNNs can be trained to extract quantitative data that can be correlated with the histological type of PC but also with the survival rates and the response to chemotherapy[11,25,31].
A retrospective study was performed by Kuwahara et al[35] on 50 patients diagnosed surgically with IPMN. A total of 3970 radiomics features from EUS were investigated with a CNN algorithm. After augmenting the data, the sample fully investigated was composed of 508160 images. The author’s goal was then to classify IPMNs as benign or malignant. For this, the ability to predict early lesions was investigated by comparing AI methods with physicians. The AI methods demonstrated an accuracy of 94.0% in predicting malignancy, while the humans achieved an accuracy of 56.0%. This study had a number of limitations; however, it was retrospective, had a small sample of cases, only performed internal validation, and used data from only one center. Additionally, all the patients included in the study were surgically investigated, and for IPMNs, the recommendation was surveillance[35].
Chen et al[36] implemented a number of 3D CNNs, including ResNet18, ResNet34, ResNet52, and Inception-ResNet, to classify pancreatic tumors from MRI images. These authors used the MRI images of 20 normal patients and 20 patients with tumors, from whom 77 benign MRI images and 38 malignant MRI images were obtained, and through data augmentation, 442 benign MRI images and 421 malignant MRI images were finally analyzed[36]. Data augmentation oversamples images to generate synthetic data in cases with small sample sizes and limited availability of image data, such as rare cancers and PCs[31]. The ResNet18 method achieved an accuracy of 91% in classifying benign and malignant lesions. Other studies have reported analyses with 3 DCNN using MRI for Alzheimer’s disease diagnosis and the classification of lung, brain, and prostate lesions[25,31,36].
Hussein et al[3] used the MRI data from 171 subjects to identify IPMN, including 38 normal subjects and 133 diagnosed with IPMN. Two-dimensional axial slices were used to generate regions of interest, and supervised and unsupervised learning was used in computer-aided diagnosis. The authors used unsupervised DL features for image classification with a CNN, and the highest accuracy in classifying the IPMN was obtained with nonlinearity clustering and implementation of a VGG-fc7 layer. In supervised learning, the deep network associated with adaptive synthetic sampling performed better on small datasets[3].
Chakraborty et al[15] studied CT images from 103 patients confirmed with IPMN using SVM and random forest methods. The random forest algorithm had better results in extracting the features of the IPMN lesions. These authors experimentally developed an architecture designed for predicting high-risk patients with IPMN, but their method lacked independent validation. Additional limitations include its retrospective nature, the need for manual segmentation of the lesion and the pancreas, and the use of small datasets. Pancreatic cystic lesions were identified on 2.6% of the abdominal CT scans, with 25% of all lesions being diagnosed as IPMN and approximately one-third being associated with invasive carcinoma. The criteria for establishing whether IPMNs are at low or high risk of harboring malignancy are limited. Distinguishing between these two lesions is important, as low-risk patients are recommended to undergo surveillance, while high-risk patients should undergo surgical resection[1,16].
Alves et al[8] performed a study on the contrast-enhanced CT scans from 119 PDAC patients and 123 patients without PDAC. PDAC was detected using a self-configuring framework for medical segmentation, NNU net, focusing on small lesions and assigning a label of tumor or nontumor. Their results showed that DL models can diagnose early PDAC lesions, but the study included only tumors of the pancreatic head and required resources for the manual labeling of PDAC images[8].
In an attempt to use AI as a prediction model for early PDAC diagnosis, Qureshi et al[4] used three types of radiomics features from CT scans. Two types belonged to the same patients but were executed (one before and one after the diagnosis was histopathologically confirmed) and another type was extracted from normal subjects. The prediagnostic CT scans of the patients were obtained 6 mo to 3 years before the PC was identified. In their study, Qureshi et al[4] analyzed 108 CT scans from 72 patients classified into two datasets, an internal dataset consisting of 66 contrast-enhanced abdominal CT scans for building the model, and an external dataset consisting of 42 scans for validation. A naïve Bayes classifier was trained to perform automatic classification of the CT scans of two groups, one representing the healthy control group and the other the prediagnostic group. A total of 4000 radiomics features were extracted from each of the 66 scans with the aim of identifying patterns in the images obtained before and after the PC diagnosis. The study concluded that prediagnostic CT images provided sufficient information to validate the contribution of AI in predicting patients at risk of PDAC, but further studies are needed to address the potential overfitting due to the limited dataset used[4].
A retrospective study on 6084 contrast-enhanced CT images in patients with histopathologically confirmed PDAC used a Faster R-CNN model to develop an automatic system for automatically diagnosing PC. The AI produced its diagnoses in 3 s, shorter than the 8 min required by the image specialist. The limitations of this study were as follows: It was a retrospective study of patients with PC diagnosis pathologically confirmed from a single center, normal patients and those with benign lesions were excluded, and it did not have external validation[37].
Sekaran et al[22] proposed a DL network by analyzing a dataset consisting of 19000 radiomics features from 82 abdominal CT images. The method involved a Gaussian mixture model with an EM algorithm and a DL CNN. They proposed a lump recognition algorithm, with the input or region of interest being the area in which the growth of a lump is detected. Each layer of the CNN extracts features of this region to build a model for assessing the features of the lump in terms of size, shape, and weight. Their results were able to help the patients by identifying the rate of spread of the tumor in the head of the pancreas after diagnosis and treatment. This study was limited by the fact that the tumors were analyzed in only one part of the pancreas, the head.
Liu et al[28] used two datasets, one consisting of 471 patients, 355 with histopathologically diagnosed or cytologically confirmed PC, and the other for external and internal validation. The control set participants had no pancreatic lesions at the time when the CT scan was performed. The study was conducted in the patch and patient levels. In the patch-based analysis, the region of interest was processed into patches, and the patients were classified as having or not having PC using a modified CNN model from the Visual Geometry Group. For the patient-based approach, age, sex, and tumor stage and size were taken into consideration. This study designed a CNN-based algorithm for classifying patients with or without PC by using CT scans, with an accuracy of 99%. Additionally, their method ensured avoidance of overfitting by using training and validation datasets that were sufficiently different but included patients of different races and ethnicities, preprocessing CT images into patches, and using data augmentation methods such as moving windows and flipping. Radiologists were provided with the clinical data of the PC patients, and the CNN had no information. The patches used for training the CNN were segmented by the radiologists. The limitations of these results were manual labeling of pancreatic images with a modest sample size and only Asian participants from a single institution.
Cardobi et al[38] found that a CNN-based analysis on CT images for identifying malignancies in IPMNs showed a sensitivity, specificity, and accuracy in the classification of tumors of 95.7%, 92.6%, and 94.0%, respectively. Additionally, AI algorithm models built for the segmentation of the pancreas and used to determine the pancreas volume in autoimmune pancreatitis were 2.38 times faster than manual approaches[38].
Studies using neural networks for the analysis of tumor markers for PC diagnosis have shown that the diagnostic performance of a single marker is lower than that of the AI model multiple for tumor marker analysis. Detecting the values of only one of CA19-9, CEA, and CA125 for PC diagnosis with AI has a low sensitivity[39]. In a mouse model, Serrao et al[39] measured the concentrations of alanine and lactate and the activities of lactate dehydrogenase and alanine aminotransferase in the pancreas of animals with different lesions ranging from pancreatitis to tumors. Their method used metabolic magnetic resonance spectroscopic imaging with hyperpolarized [1-13C] pyruvate for detecting and monitoring the progression of PC precursor lesions. Their results were able to distinguish pancreases with predominantly low-grade PanIN from tissue with high-grade PanIN and tumors. However, the studied animals had a small pancreas, and the high-grade PanINs occupied approximately 40% of the tissue. The human pancreas is larger than that of mice, and these lesions are usually small, so additional data are needed before this method can be used for human patients[18,32]. A new field of liquid biopsies offers data available from biomarkers such as exosomes, proteomes, proteins, cell-free DNA, and circulating microRNA that can be analyzed using AI methods for the early diagnosis of PC[24].
With the development of ML, digital medical imaging can play a role in assisting with diagnosis based on histopathological images. Today, it is possible to digitize glass slides using whole-slide imaging. AI techniques have been applied in pathology slide analysis for prostate and breast cancers. For these methods, a high-resolution digital image of the tissue from the glass slide is obtained by using a specialized scanner; the images are then analyzed by AI methods. Currently, however, only a few studies have investigated the use of AI on physical PC specimens. With an ANN algorithm, an overall accuracy of 77% was obtained in the reclassification of specimens as malignant or benign[25]. A study using a deep CNN on histopathological images of PDAC achieved a 95.3% classification accuracy by examining a total of 231 tissue samples from 171 PC and 60 normal pancreas slides using whole-slide imaging and image augmentation techniques[40]. Sehmi et al[41] developed DL models for grading PC from pathology slides that had a 95.61% classification accuracy. Naito et al[42] trained a DL method to assess PDAC on endoscopic ultrasonography-guided fine-needle biopsies and obtained a model that was able to detect small amounts of cancer cells in difficult cases. Cutting-edge digital pathology tools that can scan, analyze, and store data from an entire tissue sample continue to be developed[23].
Si et al[43] proposed a fully end-to-end deep-learning-based (FEE-DL) algorithm for the automatic diagnosis of different types of PCs. A total of 284 PCs, such as IPMN, PNET, serous cystic neoplasms, and a group labeled “other”-represented by gallbladder tumors, cholangiocarcinoma, ampullary carcinomas, duodenal cancer and metastasis-was investigated. The dataset was augmented using random elastic brightness, random contrast, random elastic transformation, and random cropping. The FEE-DL consisted of a tree connected subnetwork: ResNet18 for recognizing images containing the pancreas, U-Net-32 for making predictions on each image for pancreas segmentation, and ResNet34 for diagnosing pancreatic tumors. This model had an average accuracy of 82.7% for all tumor types, with a 100% accuracy in identifying IPMN and 87.6% for PDAC on a dataset that included 347 patients following four stages: Image screening, pancreas location, pancreas segmentation, and PC diagnosis. The FEE-DL algorithm independently identified different histologic types of PC with precise results in detecting PDAC and IPMN.
Muhammad et al[18] designed an ANN method to predict PC based on a study of a total of 18 personal health features (age, diabetes, smoking, exercise status, alcohol consumption, and family history of PC) from 800114 participants, including 898 with PC. Their results showed that the risk of PC can be predicted and stratified using an ANN that analyzes easily obtainable personal health features with an 80.7% sensitivity and 80.7% specificity. This study used two broad data sources: The National Health Interview Survey, established in the United States to monitor the general health status of the population with 131 PC patients from 645217 respondents, and the Prostate, Lung, Colorectal and Ovarian screening program, including 797 PC patients from 154897 participants[18].
Lee et al[44] developed a CNN predictive model for PC using data from the Taiwan Health Insurance database, which covers 99.98% of the population of the region. A total of 3690 subjects were selected, 2952 of whom presented with risk factors associated with PC, including pancreatitis, diabetes, peptic ulcer, cholangitis, hepatitis, periodontal disease, sleep disorders, and fasciitis, but factors obtained from the subjects’ medical history were further added. Finally, 74 candidate factors were included in this study; 738 patients had PC, and 2214 subjects representing the control group were cancer-free. This study constructed a PC prediction model with an accuracy higher than that of previous studies using nine key independent predictors: Abdominal pain, peptic ulcer, flatulence, gastritis, abnormal gastric function, hepatitis, sleep disorders, cholangitis, and pancreatitis.
For PC, a cost-effective screening method for early lesions is needed. AI models developed for a diverse group of patients from high-risk PDAC cohorts belonging to broad datasets from different sources can be of great use for improving PDAC treatment outcomes and enhancing diagnostic precision. AI methods represent a needed step to reach a standardized interpretation of patient data and investigations while reducing human bias or error. For this, a wide range of data from different sources is necessary. All of these data have a central role in training AI methods in diagnosing PC and establishing the most accurate algorithm to be used for such a diagnosis. Correlations can be made through AI algorithms by expanding the image and electronic medical record databases, which will significantly improve the diagnostic accuracy and provide an early diagnosis for PC patients, thereby producing a better prognosis and more effective therapy. AI creates a common ground in diagnosing PC that can be standardized and used for the general population. Such an AI model should contain a combination of biomarkers, medical data, and imaging data obtained by alternating CT, EUS, and MRI testing so that maximized accuracy and early diagnosis with noninvasive techniques can be achieved.
The coronavirus disease 2019 pandemic has produced a model for forming an international infrastructure that can be studied with AI algorithms and even used for predicting and early diagnosing cancers. The collaboration between scientists and academic centers has shown that humans from different countries and continents can work together to share information in a common attempt to save lives while creating a vast database. This will require resources, global solidarity, and support investment in medical infrastructures worldwide. However, the need to develop health infrastructure and multidisciplinary research studies is crucial, as the pandemic situation has already shown.
Our analysis of the current diagnostic methods for detecting PC noninvasively with an emphasis on early lesions has revealed a series of common features and limitations: Published studies about predicting early lesions in PC are lacking. For PC diagnosis, noninvasive tests such as imaging, tumor marker analysis, and population-based studies can be used to train AI algorithms to identify features, patterns, and subtle changes that can help classify pancreatic lesions and build predictive models that can improve the awareness of the risk of pancreatic neoplasia. Published studies typically included only small samples from a limited number of cases. The analysis of these studies focused mostly on just one type of investigation (radiomics features, personal data, biomarkers, or genomic features without making correlations between all the data that a physician analyses for a diagnosis of PC in a specific patient). A high percentage of the studies only performed internal validation, so bias and overfitting can constitute a problem when generalizing their conclusions. Commonly, for machine diagnosis, the mechanism that generates the output is not clearly explained, so assessing how a specific AI algorithm makes its final diagnosis remains controversial. AI algorithms for assessing pancreatic volumes with and without tumors are time- and cost-consuming that are usually run manually and in direct relation with the radiologist’s experience. Finding an AI-driven automated volumetric segmentation algorithm is difficult because of the variations in shape, the shallowness of the boundaries, and the small size of the pancreas. Pancreatic tumors smaller than 2 cm frequently have inconspicuous borders with high similarity to the surrounding tissues on radiomics analysis, making early lesion diagnosis challenging. However, AI methods have the potential to improve accuracy and support clinical decisions for the preoperative diagnosis of pancreatic lesions and to aid in the surgical management and prognosis of PC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Charalampopoulou A, Italy; Chen G, China; Yang Y, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Kenner B, Chari ST, Kelsen D, Klimstra DS, Pandol SJ, Rosenthal M, Rustgi AK, Taylor JA, Yala A, Abul-Husn N, Andersen DK, Bernstein D, Brunak S, Canto MI, Eldar YC, Fishman EK, Fleshman J, Go VLW, Holt JM, Field B, Goldberg A, Hoos W, Iacobuzio-Donahue C, Li D, Lidgard G, Maitra A, Matrisian LM, Poblete S, Rothschild L, Sander C, Schwartz LH, Shalit U, Srivastava S, Wolpin B. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas. 2021;50:251-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Hayashi H, Uemura N, Matsumura K, Zhao L, Sato H, Shiraishi Y, Yamashita YI, Baba H. Recent advances in artificial intelligence for pancreatic ductal adenocarcinoma. World J Gastroenterol. 2021;27:7480-7496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Hussein S, Kandel P, Bolan CW, Wallace MB, Bagci U. Lung and Pancreatic Tumor Characterization in the Deep Learning Era: Novel Supervised and Unsupervised Learning Approaches. IEEE Trans Med Imaging. 2019;38:1777-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Qureshi TA, Gaddam S, Wachsman AM, Wang L, Azab L, Asadpour V, Chen W, Xie Y, Wu B, Pandol SJ, Li D. Predicting pancreatic ductal adenocarcinoma using artificial intelligence analysis of pre-diagnostic computed tomography images. Cancer Biomark. 2022;33:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Gheorghe G, Bungau S, Ilie M, Behl T, Vesa CM, Brisc C, Bacalbasa N, Turi V, Costache RS, Diaconu CC. Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Casà C, Piras A, D'Aviero A, Preziosi F, Mariani S, Cusumano D, Romano A, Boskoski I, Lenkowicz J, Dinapoli N, Cellini F, Gambacorta MA, Valentini V, Mattiucci GC, Boldrini L. The impact of radiomics in diagnosis and staging of pancreatic cancer. Ther Adv Gastrointest Endosc. 2022;15:26317745221081596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Mahmoudi T, Kouzahkanan ZM, Radmard AR, Kafieh R, Salehnia A, Davarpanah AH, Arabalibeik H, Ahmadian A. Segmentation of pancreatic ductal adenocarcinoma (PDAC) and surrounding vessels in CT images using deep convolutional neural networks and texture descriptors. Sci Rep. 2022;12:3092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Alves N, Schuurmans M, Litjens G, Bosma JS, Hermans J, Huisman H. Fully Automatic Deep Learning Framework for Pancreatic Ductal Adenocarcinoma Detection on Computed Tomography. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Philips S, Shah SN, Vikram R, Verma S, Shanbhogue AK, Prasad SR. Pancreatic endocrine neoplasms: a current update on genetics and imaging. Br J Radiol. 2012;85:682-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 620] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 11. | Chidambaram S, Kawka M, Gall TM, Cunningham D, Jiao LR. Can we predict the progression of premalignant pancreatic cystic tumors to ductal adenocarcinoma? Future Oncol. 2022;18:2605-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Del Chiaro M, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol. 2014;20:12118-12131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Legrand T, Salleron J, Conroy T, Marchal F, Thomas J, Monard L, Biagi JJ, Lambert A. Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (1)] |
| 15. | Chakraborty J, Midya A, Gazit L, Attiyeh M, Langdon-Embry L, Allen PJ, Do RKG, Simpson AL. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys. 2018;45:5019-5029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Goyal H, Mann R, Gandhi Z, Perisetti A, Zhang Z, Sharma N, Saligram S, Inamdar S, Tharian B. Application of artificial intelligence in pancreaticobiliary diseases. Ther Adv Gastrointest Endosc. 2021;14:2631774521993059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Chen W, Butler RK, Zhou Y, Parker RA, Jeon CY, Wu BU. Prediction of Pancreatic Cancer Based on Imaging Features in Patients With Duct Abnormalities. Pancreas. 2020;49:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Muhammad W, Hart GR, Nartowt B, Farrell JJ, Johung K, Liang Y, Deng J. Pancreatic Cancer Prediction Through an Artificial Neural Network. Front Artif Intell. 2019;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Qureshi TA, Javed S, Sarmadi T, Pandol SJ, Li D. Artificial intelligence and imaging for risk prediction of pancreatic cancer: a narrative review. Chin Clin Oncol. 2022;11:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Schlanger D, Graur F, Popa C, Moiș E, Al Hajjar N. The role of artificial intelligence in pancreatic surgery: a systematic review. Updates Surg. 2022;74:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Laoveeravat P, Abhyankar PR, Brenner AR, Gabr MM, Habr FG, Atsawarungruangkit A. Artificial intelligence for pancreatic cancer detection: Recent development and future direction. Artif Intell Gastroenterol. 2021;2:56-68. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (3)] |
| 22. | Sekaran K, Chandana P, Krishna MN, Kadry S. Deep learning convolutional neural network (CNN) with Gaussian mixture model for predicting pancreatic cancer. Multimed Tools Appl. 2020;79:10233-10247. [DOI] [Full Text] |
| 23. | Hameed BS, Krishnan UM. Artificial Intelligence-Driven Diagnosis of Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Huang B, Huang H, Zhang S, Zhang D, Shi Q, Liu J, Guo J. Artificial intelligence in pancreatic cancer. Theranostics. 2022;12:6931-6954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 25. | Mendoza Ladd A, Diehl DL. Artificial intelligence for early detection of pancreatic adenocarcinoma: The future is promising. World J Gastroenterol. 2021;27:1283-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 26. | Asri H, Mousannif H, AI Moatassime H, Noel T. Using machine learning algorithms for breast cancer risk prediction and diagnosis. Proc Com Sci. 2016;83:1064-1069. [RCA] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 27. | Bakasa W, Viriri S. Pancreatic Cancer Survival Prediction: A Survey of the State-of-the-Art. Comput Math Methods Med. 2021;2021:1188414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Liu KL, Wu T, Chen PT, Tsai YM, Roth H, Wu MS, Liao WC, Wang W. Deep learning to distinguish pancreatic cancer tissue from non-cancerous pancreatic tissue: a retrospective study with cross-racial external validation. Lancet Digit Health. 2020;2:e303-e313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 29. | Dumitrescu EA, Ungureanu BS, Cazacu IM, Florescu LM, Streba L, Croitoru VM, Sur D, Croitoru A, Turcu-Stiolica A, Lungulescu CV. Diagnostic Value of Artificial Intelligence-Assisted Endoscopic Ultrasound for Pancreatic Cancer: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Deguelte S, de Mestier L, Hentic O, Cros J, Lebtahi R, Hammel P, Kianmanesh R. Preoperative imaging and pathologic classification for pancreatic neuroendocrine tumors. J Visc Surg. 2018;155:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Thomasian NM, Kamel IR, Bai HX. Machine intelligence in non-invasive endocrine cancer diagnostics. Nat Rev Endocrinol. 2022;18:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Gao X, Wang X. Deep learning for World Health Organization grades of pancreatic neuroendocrine tumors on contrast-enhanced magnetic resonance images: a preliminary study. Int J Comput Assist Radiol Surg. 2019;14:1981-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Kurihara K, Hanada K, Shimizu A. Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Arulmozhi S, Shankar R, Duraisamy S. A review: deep learning techniques for image classification of pancreatic tumor. Int J Video Proc. 2020;11:2217-2223. |
| 35. | Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Kurita Y, Koda H, Toriyama K, Onishi S, Ishihara M, Tanaka T, Tajika M, Niwa Y. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Transl Gastroenterol. 2019;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 36. | Chen X, Chen Y, Ma C, Liu X, Tang X. Classification of pancreatic tumors based on MRI images using 3D convolutional neural networks. Proceedings of the 2nd International Symposium on Image and Computing and Digital Medicine; 2018 Oct 13-14; Chengdu, China. New York: Association for Computing Machinery, 2018: 92-96. |
| 37. | Liu SL, Li S, Guo YT, Zhou YP, Zhang ZD, Lu Y. Establishment and application of an artificial intelligence diagnosis system for pancreatic cancer with a faster region-based convolutional neural network. Chin Med J (Engl). 2019;132:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Cardobi N, Dal Palù A, Pedrini F, Beleù A, Nocini R, De Robertis R, Ruzzenente A, Salvia R, Montemezzi S, D'Onofrio M. An Overview of Artificial Intelligence Applications in Liver and Pancreatic Imaging. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Serrao EM, Kettunen MI, Rodrigues TB, Dzien P, Wright AJ, Gopinathan A, Gallagher FA, Lewis DY, Frese KK, Almeida J, Howat WJ, Tuveson DA, Brindle KM. MRI with hyperpolarised [1-13C]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut. 2016;65:465-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Fu H, Mi W, Pan B, Guo Y, Li J, Xu R, Zheng J, Zou C, Zhang T, Liang Z, Zou J, Zou H. Automatic Pancreatic Ductal Adenocarcinoma Detection in Whole Slide Images Using Deep Convolutional Neural Networks. Front Oncol. 2021;11:665929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Sehmi MNM, Fauzi MFA, Ahmad WSHMW, Chang EWL. Pancreatic cancer grading in pathological images using deep learning convolutional neural network. F1000 Res. 2021;10:1057. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Naito Y, Tsuneki M, Fukushima N, Koga Y, Higashi M, Notohara K, Aishima S, Ohike N, Tajiri T, Yamaguchi H, Fukumura Y, Kojima M, Hirabayashi K, Hamada Y, Norose T, Kai K, Omori Y, Sukeda A, Noguchi H, Uchino K, Itakura J, Okabe Y, Yamada Y, Akiba J, Kanavati F, Oda Y, Furukawa T, Yano H. A deep learning model to detect pancreatic ductal adenocarcinoma on endoscopic ultrasound-guided fine-needle biopsy. Sci Rep. 2021;11:8454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Si K, Xue Y, Yu X, Zhu X, Li Q, Gong W, Liang T, Duan S. Fully end-to-end deep-learning-based diagnosis of pancreatic tumors. Theranostics. 2021;11:1982-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 44. | Lee HA, Chen KW, Hsu CY. Prediction Model for Pancreatic Cancer-A Population-Based Study from NHIRD. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |