Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1779
Peer-review started: October 29, 2022
First decision: January 3, 2023
Revised: January 13, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: March 28, 2023
Processing time: 150 Days and 2.3 Hours
Obesity is prevalent within the inflammatory bowel disease (IBD) population, particularly in newly developed countries. Several epidemiological studies have suggested that 15%-40% of IBD patients are obese, and there is a potential role of obesity in the pathogenesis of IBD. The dysfunction of mesenteric fat worsens the inflammatory course of Crohn’s disease and may induce formation of strictures or fistulas. Furthermore, obesity may affect the disease course or treatment response of IBD. Given the increasing data supporting the pathophysiologic and epidemiologic relationship between obesity and IBD, obesity control is being suggested as a novel management for IBD. Therefore, this review aimed to describe the influence of obesity on the outcomes of IBD treatment and to present the current status of pharmacologic or surgical anti-obesity treatments in IBD patients.
Core Tip: Obesity is prevalent within the inflammatory bowel disease (IBD) population, particularly in newly developed countries. The dysfunction of mesenteric fat worsens the inflammatory course of Crohn’s disease and may induce formation of strictures or fistulas. Furthermore, obesity may affect the disease course or treatment response of IBD. Along with the increasing data that support pathophysiologic and epidemiologic relationship between obesity and IBD, attention is being focused on obesity control as a novel management of IBD. The main purpose of this review is to describe the influence of obesity on the outcomes of IBD treatment and to present the current status of pharmacologic or surgical anti-obesity treatments in IBD patients.
- Citation: Kim JH, Oh CM, Yoo JH. Obesity and novel management of inflammatory bowel disease. World J Gastroenterol 2023; 29(12): 1779-1794
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1779
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory condition with an unclear etiology and pathophysiology that remain to be fully elucidated[1,2]. Obesity is a pathological condition in which there is an abnormal or excessive accumulation of body fat resulting from an imbalance between energy intake and consumption. In Western populations, a body mass index (BMI) exceeding 25 kg/m2 is commonly classified as an overweight condition, and a BMI over 30 kg/m2 is regarded as an obese condition. In Asian populations, the diagnostic thresholds for obesity and overweight have been established as BMIs of 25 kg/m2 and 23 kg/m2, respectively[3]. Obesity is a clear risk factor for a spectrum of chronic diseases, including type 2 diabetes, cardiovascular disease, respiratory problems, and cancer. Moreover, obesity is also associated with the occurrence of autoimmune diseases such as rheumatoid arthritis, psoriasis, and systemic lupus erythematosus[4].
The worldwide prevalence of obesity has nearly tripled since 1975[5], and IBD has also shown a similar trend[6]. This analogous increase is perhaps related to lifestyle changes caused by westernization and urbanization, including the lack of exercise and a westernized diet, which are common risk factors for obesity and IBD. Historically, it has been common for clinicians to associate IBD patients with a low or normal BMI due to the complications of IBD such as decreased food intake, malabsorption, weight loss, and nutritional deficiencies. However, obesity is increasingly being associated with IBD due to its overall pro-inflammatory effect. Several epidemiological studies have suggested that 15%-40% of IBD patients are obese[7-11], and hypothesize that obesity contributes to the development of IBD. Furthermore, obesity may affect the disease course or treatment response of IBD. With the increasing data supporting pathophysiologic and epidemiologic relationship between obesity and IBD, research interest on interventions for obesity as a novel management of IBD is also increasing.
The present review aims to provide a comprehensive summary of the pathophysiology of obesity in IBD, the influence of obesity on IBD outcomes, the effect of obesity on IBD management, and the potential impact of obesity treatment on IBD outcomes. Further, this review aimed to present the current status of anti-obesity treatments in IBD patients.
Previous studies have reported that 15%-40% of IBD patients are obese and 20%-40% are overweight[7-11]. Additionally, severe obesity (BMI ≥ 40 kg/m2) is also reported in 2%-3.2% of IBD patients[7]. The prevalence of obesity among IBD patients has been observed to increase over time, which appears to correspond with the global trend of rising obesity rates. A single-center study in France confirmed that the proportion of obesity among CD patients from 1974 to 2000 increased from 1.7% before 1981 to 4% after 1990[12]. A single-center study in Korea, utilizing data from the Asan IBD Registry from 1989 to 2016, reported that out of 6803 patients diagnosed with IBD, 16 with CD and 27 with UC were classified as obese (BMI ≥ 30 kg/m2). The study did not reveal any clinically meaningful differences between the obese and non-obese patient groups[13]. An analysis of 10282 CD patients enrolled in a randomized controlled clinical trial revealed a significant increase in mean BMI at enrollment, which rose from 20.8 kg/m2 in 1991 to 27.0 kg/m2 in 2008[14].
Obesity may be a potential risk factor for developing IBD, particularly CD (Table 1). In the Unite States Nurses’ Health Study, which followed around 110000 women, found that obesity at age 18 was a significant predictor for the development of CD but not UC, when compared to individuals with a normal BMI [adjusted hazard ratio (aHR) = 2.33; 95% confidence interval (CI): 1.15-4.69][15]. This study exhibited that a higher degree of weight gain (between the ages of 18 and enrolment) was linked with elevated risk of developing CD (weight gain > 13.6 kg vs < 2.3 kg; HR = 1.52; 95%CI: 0.87-2.65). The Copenhagen School Health Records Register cohort study investigated the potential association between BMI during the ages of 7 to 13 years and the onset of adult-onset IBD[16]. Obesity in early adolescence has been shown to increase the risk of CD before the age of 30 years (HR = 1.2; 95%CI: 1.1-1.3) while decreasing the risk of UC (HR = 0.9; 95%CI: 0.9-1.0). In a recent meta-analysis of five prospective cohort studies, obesity was found to be associated with an elevated risk of developing older-onset CD, while no significant association was found with UC[17]. The analysis showed that obese patients had an increased risk of developing CD compared to those with a normal BMI (aHR = 1.34; 95%CI: 1.05-1.71; I2 = 0%). Moreover, every 5 kg/m2 increase in baseline BMI was found to correspond to a 16% increase in the risk of CD (aHR = 1.16; 95%CI: 1.05-1.22; I2 = 0%). However, the European Prospective Investigation into Cancer and Nutrition-IBD study, which involved a large sample size, concluded no significant correlation between BMI and the incidence of IBD, showing contrasting results to previous studies[18].
| Ref. | Study design and study population | Key findings |
| Khalili et al[15], 2015 | United States Nurses’ Health Study cohort study: Prospective cohort study of United States women (n = 111498 women); BMI at age 18, baseline, and every 2 yr since baseline was obtained; 2028769 person-years of follow up. CD (n = 153); UC (n = 229) | Obesity at age 18 was an independent risk factor for the development of CD compared to normal BMI (aHR = 2.33, 95%CI: 1.15-4.69). No association between BMI at age 18, baseline BMI, and updated BMI and risk of UC. Higher weight gain was associated with increased risk of CD (Ptrend = 0.04). A greater magnitude of weight gain (from age 18 to age at enrolment) associated with increased risk of developing CD (weight gain > 13.6 kg vs < 2.3 kg, HR = 1.52, 95%CI: 0.87-2.65). No association between weight change (from age 18 to baseline) and risk of UC (Ptrend = 0.17) (weight gain > 13.6 kg vs < 2.3 kg, HR = 0.92, 95%CI: 0.60-1.40) |
| Harpsøe et al[101],2014 | Danish National Birth Cohort study: A large population-based cohort study (n = 75008 women); BMI: Obtained at study baseline (based on prepregnancy body weigh); median 11.4 yr of follow-up. CD (n = 138); UC (n = 394) | An increased risk of developing fetal CD in both underweight (HR = 2.57, 95%CI: 1.30-5.06) and obese women (HR = 1.88, 95%CI: 1.02-3.47) compared with normal-weight women, pointing to a U-shaped association. No association between pregnancy obesity and risk of developing UC (HR = 0.77, 95%CI: 0.48-1.25) |
| Jensen et al[16], 2018 | Copenhagen School Health Records Register cohort study: Cohort from the Copenhagen School Health Records Register (n = 316799); relationship between BMI in the ages of 7 to 13 yr and adult-onset IBD; BMI: Obtained at ages 7 through 13 yr; approximately 10 million person-years of follow-up. CD (n = 1500); UC (n = 2732) | Obesity in early adolescence (at each age from 7 to 13 yr) increased the risk of CD diagnosed before age 30 yr (HR = 1.2, 95%CI: 1.1-1.3) while decreasing the risk of UC (HR = 0.9, 95%CI: 0.9-1.0). No associations between changes in BMI between 7 and 13 yr and later risk of CD or UC |
| Chan et al[17], 2022 | Pooled analysis of 5 prospective cohort studies from the Dietary and Environmental Factors IN-IBD study (n = 601009): BMI: Obtained at study baseline and during follow-up period; 10110018 person-years of follow-up. CD (n = 563); UC (n = 1047) | Obesity was associated with an increased risk of older-onset CD but not UC. The risk of developing CD increased in obese patients compared against those with a normal BMI (aHR = 1.34, 95%CI: 1.05-1.7, I2 = 0%). Each 5 kg/m2 increment in baseline BMI was associated with a 16% increase in risk of CD (aHR = 1.16, 95%CI: 1.05-1.22; I2 = 0%). With each 5 kg/m2 increment in early adulthood BMI (age 18-20 years), there was a 22% increase in risk of CD (pooled aHR = 1.22, 95%CI: 1.05-1.40, I2 = 13.6%). An increase in waist-hip ratio was associated with an increased risk of CD that did not reach statistical significance (pooled aHR across quartiles = 1.08, 95%CI: 0.97-1.19, I2 = 0%). No associations were observed between measures of obesity and risk of UC. For every 5 kg/m2 increase in BMI, the multivariable-adjusted HR was 1.00 (95%CI: 0.90-1.05). For every 5 kg/m2 increase in early adulthood BMI, the multivariable-aHR for UC was 1.05 (95%CI: 0.90-1.22, I2 = 0%) |
| Chan et al[18], 2013 | European Prospective Investigation into Cancer and Nutrition-IBD study (n = 300724): BMI: Obtained at study baseline and during follow-up period. CD (n = 75); UC (n = 177) | No associations with the four higher categories of BMI compared with a normal BMI for UC (Ptrend = 0.36) or CD (Ptrend = 0.83). The lack of associations was consistent when BMI was analyzed as a continuous or binary variable (BMI 18.5 < 25.0 vs ≥ 25 kg/m2). Physical activity and total energy intake, factors that influence BMI, did not show any association with UC (physical activity, Ptrend = 0.79; total energy intake, Ptrend = 0.18) or CD (physical activity, Ptrend = 0.42; total energy, Ptrend = 0.11) |
Weight gain may occur during the treatment of IBD. Several preclinical data suggest that, in IBD patients, imbalance of gut microbiota and altered metabolic intestinal signaling mediated by hormones, bile acids, and satiety-related peptides have been implicated in the development of obesity and dysmetabolism[19,20]. Smoking cessation as a lifestyle modification and utilization of corticosteroids could affect the weight gain in IBD patients[21,22]. Additionally, there are a few studies investigating weight gain among IBD patients treated with biologic therapies, especially anti-tumor necrosis factor alpha (TNF)-α therapy. In 21 CD patients, total abdominal fat increased by 18% after 8 wk of infliximab induction therapy (P = 0.027)[23]. A recent retrospective study examined the longitudinal changes in weight among patients with IBD receiving different biologic drugs including infliximab, adalimumab, vedolizumab and ustekinumab[24]. The study revealed a statistically significant increase in body weight over time among patients receiving infliximab and vedolizumab, with the infliximab/vedolizumab group experiencing a greater degree of weight gain as compared to the adalimumab group. Moreover, observed weight gain among IBD patients undergoing biologic therapy was found to be linked with other clinical factors, such as male gender, high levels of C-reactive protein (CRP), and low serum albumin.
Obesity induces a chronic, low-grade inflammatory state, characterized by the release of multiple pro-inflammatory signaling molecules from hypertrophic adipocytes in mesenteric visceral adipose tissue (VAT)[25-27]. Mesenteric VAT is characterized by the presence of M1 macrophages that secrete a variety of inflammatory cytokines and it has the potential to affect intestinal barrier function[28,29]. Several studies have established a relationship between adipocyte mass and the degree of cytokine expression[30]. Obesity has been shown to be positively associated with visceral adiposity, as determined by volumetric cross-sectional imaging analysis. Furthermore, in obese individuals, there is a direct correlation between visceral adiposity and circulating levels of interleukin-6 (IL-6). Additionally, BMI has been found to be linked with increased levels of CRP in this population[31].
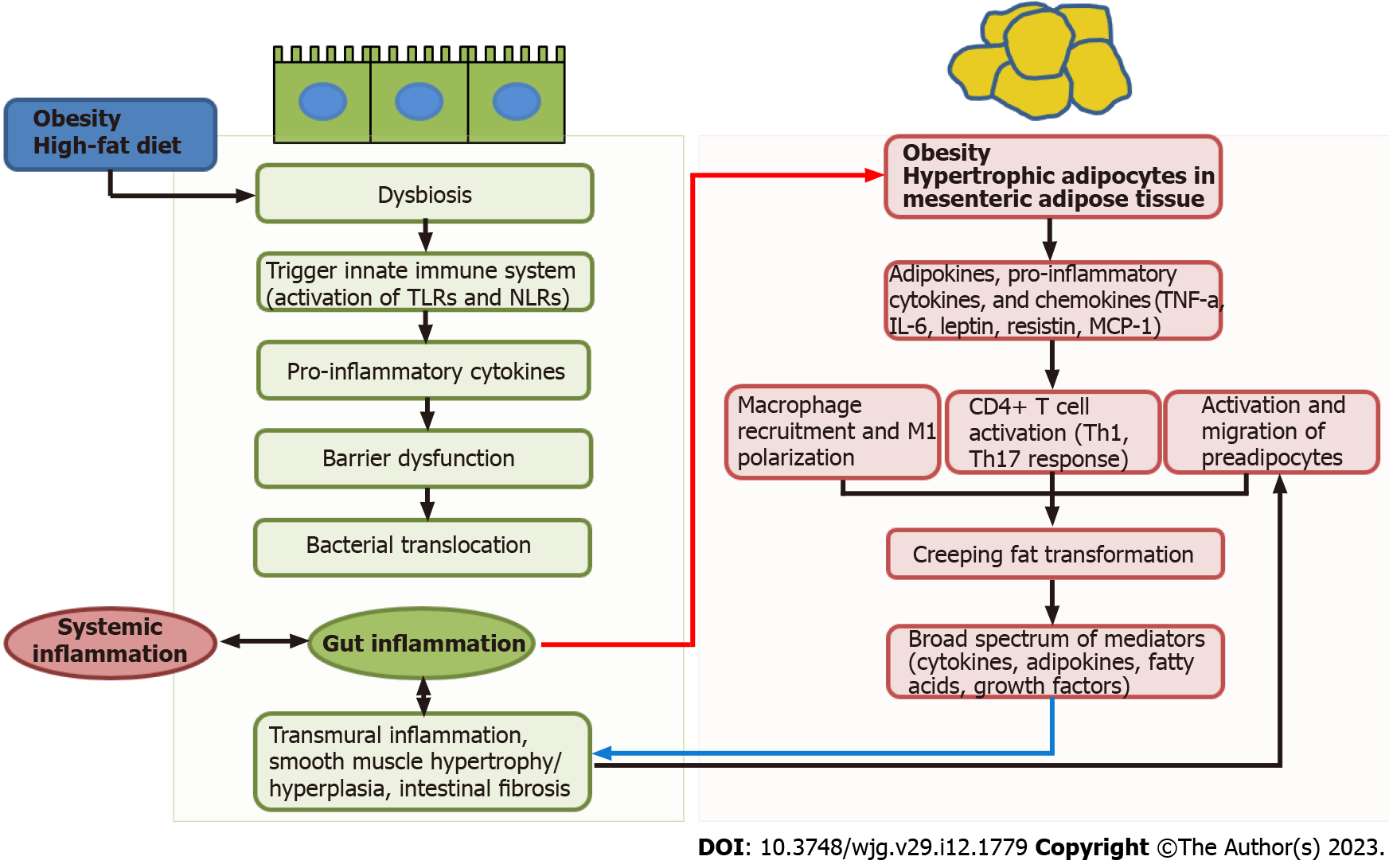
Other mechanisms of obesity in IBD include intestinal barrier dysfunction and alterations in the intestinal microbiota (Figure 1). Obesity and IBD are associated with dysbiosis along with a reduction in bacterial diversity[32]. High fat diet during obesity decreases the diversity of the gut microbiota (dysbiosis), which, in turn, leads to the reduction of anti-inflammatory bacterial species or metabolites. These changes can trigger innate immune system by activation of pattern recognition receptors, including Nod-like receptors or Toll-like receptors, which are present on intestinal epithelial cells or dendritic cells. Pro-inflammatory cytokines secreted from innate immune system can also contribute to increased intestinal permeability, which may increase the risk of bacterial translocation[17,25]. Numerous studies have demonstrated that impaired gut barrier function is a characteristic feature of obesity. This dysfunction permit increased bacterial translocation, leading to the subsequent initiation of systemic inflammation[33,34].
Accumulating evidence suggests a connection between changes in the mesenteric fat and IBD, in particular CD[27]. Intestinal barrier dysfunction and transmural inflammation may induce bacterial translocation to the surrounding mesenteric adipose tissue, which subsequently leads to the adipocyte hypertrophy (red arrow, Figure 1). The hypertrophic adipocytes releases pro-inflammatory cytokines, adipokines, and chemokines such as TNF-α, IL-6, leptin, resistin, and monocyte chemoattractant protein-1). These pro-inflammatory mediators can induce the infiltration of M1 macrophages and CD4+ T cell activation into T helper (Th) 1 and Th17 cells[35]. This process may contribute to the development of hypertrophic mesenteric fat wrapping around the inflamed intestine, known as “creeping fat” that is pathognomonic of CD[27]. Creeping fat itself is thought to be an intrinsic component of inflammatory dysregulation in the pathogenesis of CD. Compared to other VAT, creeping fat demonstrates greater immunological activity. Additionally, there is a significant correlation between the extent of creeping fat and the degree of histological inflammation, as well as the level of infiltration by lymphocytes or macrophages[36]. In addition, the creeping fat formation may be induced by migration of preadipocytes from mesenteric VAT in response to the increased fibronectin signal released from activated muscularis propria smooth muscle cells in inflamed gut[37]. The presence of creeping fat in CD patients has been associated with various pathological changes, including muscularis propria hyperplasia, transmural inflammation, and intestinal fibrosis. These alterations may contribute to the stricturing form of the disease[37,38]. Notably, creeping fat has been found to contain higher levels of fibrotic tissue and T cells in the ileum, as compared to colonic fat from patients with either CD or UC[39]. Creeping fat secretes a broad spectrum of pro-inflammatory and pro-fibrotic mediators such as adipokines, cytokines, fatty acids, and growth factors[40]. Moreover, expression of leptin and adiponectin is elevated in the creeping fat of CD patients. The severity and activity of CD have been correlate with the levels of other adipokines, including resistin[41]. A case-control study showed that the gene expression profile of creeping fat in CD patients was similar to that of VAT from obese patients, indicating a greater inflammatory state as compared to VAT obtained from non-obese individuals[42].
The effect of obesity on the disease phenotype of IBD remains uncertain. While a retrospective review has indicated that obesity may be a risk factor for perineal disease in IBD[12], other studies have not found any differences in disease distribution or behavior between patients with UC or CD who are obese compared to those who are not[8,43]. Obesity has been linked to increased disease activity and unfavorable clinical outcomes in several chronic inflammatory diseases, such as psoriasis and rheumatoid arthritis[44,45]. However, evidence regarding the effect of obesity on outcomes in IBD is limited and inconclusive. A study of 581 IBD patients showed that obese individuals had significantly lower frequencies of hospitalization (42.1% vs 66.0%, P < 0.001) and a reduced likelihood of requiring surgical intervention (41.1% vs 61.1%, P = 0.02), when compared to those patients with normal BMI[8]. However, in other population-based study for 143190 IBD patients, it was demonstrated that obesity was an independent predictor of higher rates of all-cause re-admission at both 30 d [18% vs 13%; adjusted odds ratio (aOR) = 1.16; P = 0.005] and 90 d (29% vs 21%; aOR = 1.27; P < 0.0001), in com-parison with non-obese patients[46]. A systematic review reported that there is no significant difference in the rates of corticosteroid usage, hospitalization, surgical intervention, or emergency room visits between obese and normal-weight individuals with IBD[7]. This finding has been supported by a recent retrospective study conducted in South Korea, which found no significant differences in clinical features or treatment outcomes between obese and non-obese patients with IBD, as measured by the cumulative probabilities of receiving various treatments[13].
In CD, many studies show that obesity may indeed have a beneficial effect. A previous report supports this hypothesis by showing that the small VAT adipocytes in CD patients exhibit higher anti-inflammatory genes, which implies a potential protective role of VAT in CD[42]. A retrospective analysis of 221 patients with CD found that an increase of 1 kg/m2 in BMI at diagnosis led to a 5% reduction in the risk of requiring surgical intervention in the future (HR = 0.95; 95%CI: 0.91-0.99). Whereas, the risk of corticosteroid use or future hospitalization did not differ[47]. The results of a retrospective study showed that obese patients with CD had lower frequencies of corticosteroid usage, anti-TNF treatment, hospitalization, and surgery in contrast to those with normal weight[8]. However, there were also contrasting results. For instance, one study reported that obese patients with CD were more at risk of active disease (OR = 1.50; 95%CI: 1.07-2.11) or hospitalization (OR = 2.35; 95%CI: 1.56-3.52)[12]. Alternatively, a prospective case-control study revealed no correlation between BMI and the risk of undergoing surgery or using corticosteroids in the future[9].
In UC, several studies suggest that obesity may have a worse prognosis. In one population-based cohort study that included 267 patients with UC, each incremental increase in BMI by 1 kg/m2 was associated with a 3.4% increase in the risk of hospitalization (P = 0.052) and a 6% increase in the risk of surgery (P = 0.01)[47]. A recent population-based cohort of 417 UC patients, of whom 20.6% were obese, confirmed that obesity among individuals with UC may have unfavorable prognostic implication, particularly with regards to the subsequent hospitalization (HR = 1.72; 95%CI: 1.10-2.71; P = 0.018) and corticosteroid usage (HR = 1.026; 95%CI: 1.00-1.05; P = 0.05) in comparison to patients with normal weight[48]. Moreover, an increase of 1 kg/m2 in BMI was associated with a 5% escalation in the probability of subsequent hospitalization (HR = 1.05; 95%CI: 1.01-1.08; P = 0.008) and a 2.6% increase in the chance of requiring corticosteroid usage (HR = 1.026; 95%CI: 1.00-1.05; P = 0.05). However, the retrospective analysis of 284 UC patients showed that obese people displayed a reduced risk of developing complications[8].
There is evidence to suggest that obesity may be an adverse prognostic factor in a patient’s response to drug therapy. The reason being obesity is considered a chronic inflammatory state and obesity can affect the pharmacokinetics of drugs. Obesity may decrease drug half-life and trough levels due to an increase in drug clearance and volume of distribution.
Immunomodulators: Despite extensive clinical experience with thiopurine, few studies have evaluated clinical predictors of response to thiopurine in IBD. A retrospective study of 1176 IBD patients evaluating azathioprine responsiveness based on BMI found that a reciprocal association between BMI and outcomes in patients with UC and CD. More specifically, favorable outcomes were observed in UC patients with a BMI below 25 kg/m2 and in CD patients with a BMI exceeding 25 kg/m2[49]. In a cohort of 132 patients with IBD treated with thiopurines dosed according to BMI, a study found that patients with obesity were less likely to reaching optimal therapeutic concentrations of the thiopurine metabolite, 6-thioguanine, which has been shown to correlate with remission of IBD[50].
Anti TNF-α therapy: Several data have suggested that obesity may induce a reduced response to anti-TNF-α agents[51]. The augmented clearance and expanded volumes of distribution associated with obesity can lead to lower trough levels, resulting in either a diminished response or a heightened risk of response loss to anti-TNF-α agents. For adalimumab, body weight has been shown to be the most significant predictor of drug clearance and volume of distribution in psoriasis patients[52]. In contrast to other rheumatoid conditions, studies examining the impact of obesity on anti-TNF therapy in IBD have produced inconsistent findings.
Obesity has been linked to an increased risk of failure of anti-TNF therapy, irrespective of the method of administration (subcutaneous vs intravenous) and dosing regimens (weight-based vs fixed-dose regimens). These results could imply that there is an intrinsic factor to obesity that reduces treatment response, regardless of drug level and mode of exposure. A recent meta-analysis found that obese patients with UC were at a greater risk of anti-TNF-α treatment failure (OR = 1.41; 95%CI: 1.01-1.98; P = 0.045) than non-obese patients, irrespective of whether they received fixed-dose or weight-based treatment regimens[53]. Furthermore, in another study that evaluated 160 UC patients treated with various biologic agents, both weight-based (infliximab) and fixed-dose regimens (adalimumab, certolizumab, vedolizumab, and golimumab), regardless of the type of biologic used, an increase in BMI of 1 was associated with a 4% greater risk of treatment failure (aHR = 1.04; 95%CI: 1.00-1.08) for all patients[54]. However, a pooled data analysis of over 1200 infliximab-treated IBD patients reported that BMI did not have a significant effect on clinical remission rate[55]. A metaanalysis for multiple immune-mediated diseases showed that obesity was associated with anti-TNF therapy failure in several rheumatic diseases, but no such association was observed in IBD[56]. However, in a recent meta-analysis including the newer 6 articles into the meta-analysis, obesity was associated with higher risk of failing anti-TNF therapy in UC patients, but not in CD patients[53]. Although there are insufficient studies on the impact of obesity on safety of biologic agents, a cohort study of over 6000 biologic-treated IBD patients reported no association between obesity and an increased risk of serious infections[57].
Vedolizumab: There is insufficient data regarding the potential impact of obesity on the therapeutic efficacy of vedolizumab in the management of IBD. Vedolizumab trough drug levels are known to be inversely proportional to body weight[58]. A recent retrospective study showed that obesity was not significantly related to increased rates of dose escalation for vedolizumab therapy[59]. However, a higher BMI was linked to reduced rates of vedolizumab discontinuation and CRP normalization, although not with endoscopic remission.
Ustekinumab: In chronic inflammatory conditions other than IBD, such as psoriasis and rheumatoid arthritis, obesity may negatively affect the clinical response to biological drugs. Of note, anti-interleukin drugs appear to be more strongly affected by BMI than anti-TNF-α drugs. However, in IBD, there is very little research on the impact of obesity on treatment response with this drug. A post hoc analysis of IM-UNITI study, which evaluated the efficacy of ustekinumab for maintenance of CD, revealed a statistically significant decrease in ustekinumab trough levels among obese patients (median 2.98 mcg/mL) compared to patients with overweight (4.84 mcg/mL; P = 0.021) and underweight or normal weight (4.43 mcg/mL; P = 0.014)[60]. While BMI appeared to have an impact on ustekinumab drug levels, it was not found to be a significant predictor of clinical remission.
Tofacitinib: A pharmacokinetic profile of tofacitinib revealed that increased body weight had some effect on the drug’s plasma concentrations, with higher body weight associated with lower peak and higher trough concentrations. However, these differences were considered to be not clinically significant[61]. The evidence suggests that psoriatic arthritis patients with a baseline BMI over 35 kg/m2 have lower rates of response to tofacitinib than those with lower baseline BMI[62]. However, in a post hoc analysis of the OCTAVE studies, effectiveness and safety profile of tofacitinib in UC patients is not impacted by obesity[63]. In the UC population, BMI was not identified as a significant predictor of outcomes, and no clear relationship between BMI and adverse events was observed.
It has been well established that obesity increase operative time and the risk for conversion to laparotomy[64]. Obesity also may heighten the risk of short-term perioperative complications, such as surgical site infections and wound complications after abdominal surgery[65]. Obesity also makes surgery for IBD more challenging, particularly those requiring pelvic exposure. A study involving 382637 inpatient hospitalizations for surgery in IBD patients revealed that obese patients had significantly higher rates of postoperative complications. Specifically, the study found that obese patients had increased odds of postoperative wound complications (OR = 1.35, P = 0.01), pulmonary complications (OR = 1.21, P = 0.02), infections (OR = 1.16, P = 0.02), and shock (OR = 1.30, P = 0.02)[66]. A recent meta-analysis strongly supported the association between obesity and postoperative complications in IBD[67].
Stoma creation and ileal pouch-anal anastomosis (IPAA) in obese patients are more difficult to perform and also appear to increase the risk of complications in IBD patients. Obesity has been identified as a risk factor for stoma-related complications[68]. Additionally, it may increase the likelihood of postoperative complications, including pelvic sepsis, which has been shown to have a negative impact on long-term pouch function[69]. However, several studies have indicated that overweight and obese patients who undergo a three-stage IPAA procedure with the utilization of a diverting stoma may achieve comparable long-term outcomes to non-obese patients[64].
Given the rising prevalence of obesity within the IBD population, there is a growing need to explore the effects of weight loss interventions on outcomes of IBD. It is widely recognized that weight loss can have a favorable influences on the outcome of numerous chronic diseases[70]. Despite considerable evidence that obesity has a negative influence on the response to therapies for IBD, there is currently a lack of data regarding whether interventions aimed at treating obesity can improve outcomes in individuals with IBD. To date, no interventional studies have been conducted specifically to investigate the impact of intentional weight loss on IBD.
Current therapeutic strategies to obesity includes lifestyle modifications, pharmacologic treatment, bariatric surgery, and bariatric endoscopic applications, of which the intragastric balloon is the most widely used. The European Society for Clinical Nutrition and Metabolism (ESPEN)/United European Gastroenterology (UEG) guidelines suggest that the management of obesity in patients with IBD should involve a stepwise approach, starting with dietary and lifestyle interventions. If necessary, anti-obesity drugs or bariatric surgery can be considered as options (Table 2). However, the guidelines do not provide a one-size-fits-all approach and individualized care is recommended[71].
| Interventions | Study design | Key finding | Ref. | |
| Lifestyle and dietary interventions | Diet: No data on the effects of overall calorie intake or supervised dietary weight loss on outcomes in IBD patients | Retrospective study: (1) Impact of mediterranean diet on the liver steatosis, clinical disease activity, and QoL in IBD patients (n = 142); (2) 84 UC, 58 CD; and (3) BMI: Collected at study baseline and after 6 mo | Diet-adherent CD and UC improved BMI (UC: -0.42, P = 0.002; CD: -0.48, P = 0.032) and waist circumference (UC: -1.25 cm, P = 0.037; CD: -1.37 cm, P = 0.041). The number of patients affected by liver steatosis of any grade was significantly reduced in both groups after mediterranean diet intervention (UC: 36.9% vs 21.4%, P = 0.0016; CD: 46.6% vs 31.0%, P < 0.001). Mediterranean diet improved QoL in both UC and CD | Chiccoet al[72], 2021 |
| Exercise: (1) Anti-inflammatory effects through a variety of mechanisms, including reducing visceral fat, reducing the secretion of inflammatory adipokines, and reducing stress-induced intestinal barrier dysfunction; and (2) Experts have recommended a prescription of exercise for IBD patients that consists of walking 20-30 min at 60% of maximal heart rate 3 d per week along with resistance training 2-3 times per week for its impact on bone mineral density[102], however this has not been tested prospectively | Prospective study: IBD patients with mild active disease or in remission (n = 32) | IBD patients performed low-intensity walking at an interval of 3 times per week for a duration of 3 mo. IBD patients who exercise have improved sense of well-being and QoL | Ng et al[103], 2007 | |
| 30 patients with moderate-to-mild CD. Randomized to moderate-intensity running 3 × weekly for 10 wk vs usual care | No significant difference in total IBDQ scores, IBDQ social subscores did improve in intervention group (P = 0.023). No disease exacerbation | Klare et al[104], 2015 | ||
| Prospective study: Using the Crohn’s and Colitis Foundation of America Partners Internet-based cohort of IBD patients (n = 1857); 549 UC, 1308 CD | Reduced risk of CD exacerbation (RR = 0.72, 95%CI: 0.55-0.94), reduced risk of UC exacerbation (RR = 0.78, 95%CI: 0.54-1.13), with higher levels of exercise | Jones et al[74], 2015 | ||
| Pharmacologic treatment: BMI of 30 kg/m2 or a BMI of 27 kg/m2 with obesity-related diseases (e.g., hypertension, type 2 diabetes mellitus, and sleep apnea) | Orlistat: (1) By inhibiting gastric and pancreatic lipases, reducing absorption of monoaclglycerides and free fatty acids; and (2) Should be avoided in IBD patients because of the mechanism of action and common side effect | No data on the effect of Orlistat on outcomes in IBD patients | ||
| Liraglutide: Glucagon-like peptide-1 receptor agonist also known as incretin mimetics | Case report: CD patient with type 2 diabetes and active CD | Switching from insulin to liraglutide improved glycemic control and the QoL scores | Kuwata et al[76], 2014 | |
| A nationwide cohort study using Danish registries: Patients with IBD and type 2 diabetes (n = 3751) | A lower risk of adverse clinical events (a composite of the need for oral corticosteroid treatment, need for TNF-α-inhibitor treatment, IBD-related hospitalization, or IBD-related major surgery) amongst patients treated with GLP-1 based therapies compared with treatment with other antidiabetic therapies (adjusted IRR = 0.52, 95%CI: 0.42-0.65) | Villumsen et al[77], 2021 | ||
| Naltrexone/bupropion: Naltrexone and bupropion alone may have anti-inflammatory properties | Uncontrolled studies of IBD patients not in remission (n = 47): Low-dose naltrexone for 12 wk | Low dose naltrexone induced clinical improvement in 74.5%, and remission in 25.5% of patients | Lie et al[78], 2018 | |
| Retrospective study of IBD patients who had received low-dose naltrexone (n = 582) | Initiation of low-dose naltrexone in IBD was followed by reduced dispensing of several drugs considered essential in the treatment of IBD | Raknes et al[79], 2018 | ||
| Phentermine/topiramate: (1) A highly efficacious oral weight-loss agent, which acts centrally to suppress appetite and increase satiety; and (2) Early experimental data on topiramate suggested that it could significantly reduce colonic tissue damage in animal models of IBD | Large retrospective cohort study using United States administrative claims data (n = 1731): Compared new users of topiramate with users of other anticonvulsant/anti-migraine medications | Topiramate use was not associated with markers of IBD flares including steroid prescriptions (HR = 1.14, 95%CI: 0.74-1.73), initiation of biologic agents (HR = 0.93, 95%CI: 0.39-2.19), abdominal surgery (HR = 1.04, 95%CI: 0.17-6.41), or hospitalization (HR = 0.86, 95%CI: 0.62-1.19) | Crocket et al[83], 2014 | |
| Bariatric endoscopic applications | Intragastric balloon: Weight loss achieved through endoscopic bariatric interventions might achieve the same effect on outcomes in IBD as in other autoimmune diseases, but has not been studied | Case report of UC patient | UC worsened after insertion of an intragastric balloon for the treatment of obesity | Manguso et al[88], 2008 |
| Bariatric surgery: BMI ≥ 40 kg/m2 or 35-39.9 kg/m2 with obesity-related comorbidities and previously failed to achieve adequate weight reduction with non-surgical interventions | Bariatric surgery: (1) Several studies have demonstrated that bariatric surgery is likely feasible, safe, and effective weight loss stratege, that may lead to improved outcomes of IBD patients; and (2) No RCTs or prospective studies were found that compared the different bariatric procedures in patients with IBD | Case-control study of 85 IBD patients, matched to non-IBD patients with BS (n = 85): (1) 20 UC, 64 CD, 1 unclassified IBD; (2) BMI 41.6 ± 5.9 kg/m2; and (3) 3 RYGB/73 SG/12 LAGB | Bariatric surgery is a safe and effective procedure in obese IBD patients: (1) At a mean follow-up of 34 mo, mean weight was 88.6 ± 22.4 kg; (2) Complications: 8 (9%); and (3) No difference was observed between cases and controls for postoperative complications (P = 0.31), proportion of weight loss (P = 0.27), or postoperative deficiencies (P = 0.99) | Reenaers et al[93], 2022 |
| Case-control study of 25 IBD patients who underwent BS, matched to IBD patients who did not undergo BS (n = 47) | IBD patients with weight loss after BS had fewer IBD-related complications compared with matched controls: (1) Median decrease in body mass index after bariatric surgery was 12.2; and (2) Rescue corticosteroid usage and IBD-related surgeries were numerically less common in cases than controls (24% vs 52%, OR = 0.36, 95%CI: 0.08-1.23; 12% vs 28%, OR = 0.2, 95%CI: 0.004-1.79) | Braga Neto et al[95], 2020 | ||
| Retrospective review (n = 20): (1) 13 UC, 7 CD; (2) BMI 50.1 ± 9 kg/m2; and (3) 9 SG/7 RYGB/3 AGB/ 1 AGB to RYGB | BS is safe and mitigate IBD: (1) Weight loss: 14.3 ± 5.7 kg/m2 or 58.9% ± 21.1%; (2) Complications: Early 7 (5 Dr, 1 PE, 1 WI), late 5 (2 Pnt, 2 VH, 1 MU), mortality 1 (unrelated); and (3) IBD status after BS: Remit 9, exacerbate 2, no change 9 | Aminian et al[91], 2016 | ||
| Prospective case-control study (n = 6/101): (1) 1 UC, 5 CD; (2) BMI 40.6 ± 3.74 kg/m2; and (3) 1 Maclean gastroplasty/1 SG + end colostomy/2 SG/2 SG + ileocecal resection | BS is safe and effective and IBD Rx decreasing: (1) Weight loss: 11.45 ± 2.8 kg/m2 or 28.14% ± 6.6%; (2) Complications: Late 1 (1 vomiting/dysphagia); and (3) IBD status after BS: Remit 5, exacerbate 1 | Colombo et al[105], 2015 | ||
| Prospective study (n = 10): (1) 2 UC, 8 CD; (2) BMI 42.6 ± 5.6 kg/m2; and (3) 9 LSG/1 LAGB | BS is effective and safe: (1) Weight loss: 71.4 ± 5.9 EWL%; (2) Complications: Early 1 (1 SLL) late 4 (4 VitD); and (3) IBD status after BS: Remit 2, exacerbate 3, no change 3, improved 1 | Keidar et al[106], 2015 | ||
| Retrospective case-control (n = 4): (1) 4 CD; (2) BMI 45 ± 5.3 (40-51) kg/m2; and (3) 4 LSG | SG is safe in CD: (1) Weight loss: 32.8 ± 4.3 kg/m2 or 60.2% ± 13.7% EWL; (2) Complications: Early 1 (1 SLB); and (3) IBD status after BS: Remit 4 | Ungar et al[107], 2013 | ||
| Retrospective inpatient study (n = 493/15319): (1) 245 UC, 248 CD; (2) BMI 40.6 ± 3.74 kg/m2; and (3) 48% SG, 35% RYGB, 17% LAGB | Complications: 0.4% malnutrition, 0.2% thromboembolism, 12% strictures, 0.6% renal failure; prior-bariatric surgery was associated with decreased IRR for renal failure, under-nutrition, and fistulae formation in morbidly obese IBD patients [(IRR = 0.1; 95%CI: 0.02-0.3; P < 0.001), (IRR = 0.2; 95%CI: 0.05-0.8; P = 0.03), and (IRR = 0.1; 95%CI: 0.2-08; P = 0.03), respectively] | Sharma et al[108], 2018 | ||
A combination of dietary adjustments, physical exercise, and behavioral modifications as part of a lifestyle intervention is generally considered the preferred approach for achieving weight loss in the general population. Of course, IBD patients should also consider this approach, but there are special considerations in this cohort that require the advice of a certified dietitian. Specifically, individuals with active IBD may find it challenging to tolerate certain “healthy” foods, such as fruits and vegetables, which are commonly recommended as part of a healthy diet. Additionally, IBD patients, despite being overweight, may be vulnerable to the deficiency of various vitamins and micronutrients, and thus careful consideration of a balanced diet is essential. One study involving IBD patients showed improvements in body weight, waist circumference, and steatosis when a mediterranean diet was prescribed[72]. There is no available data to provide evidence on the impact of overall calorie intake or supervised dietary weight loss on outcomes in patients with IBD.
Moreover, exercise may have a positive effect on regulating IBD activity. Moderate-intensity exercise has been shown to have anti-inflammatory effects through a variety of mechanisms, such as reducing visceral fat, decreasing the secretion of inflammatory adipokines, and reducing stress-induced dysfunction of the intestinal barrier[73]. A prospective study suggested that high levels of physical exercise may be associated with a lower risk of active disease in IBD patients[74]. However, it should be noted that these studies have primarily relied on subjective measures of well-being and have not utilized specific objective indicators of disease activity.
Currently, anti-obesity treatment is often recommended for patients with a BMI of 30 kg/m2 or above, or a BMI of 27 kg/m2 or higher in the presence of obesity-related comorbidities[75]. There are several prescription drugs (orlistat, liraglutide, phentermine-topiramate and naltrexone-bupropion) available for weight loss, but there are no randomized controlled trials of these anti-obesity drugs in patients with IBD. The ESPEN/UEG guideline recommends that anti-obesity drugs may be utilized in patients with IBD, provided they are indicated for such use, with the exception of orlistat. Orlistat should be avoided due to its mechanism of action and associated adverse effects. Notably, a case report demonstrated the use of liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist, in a patient with active CD and type 2 diabetes resulted in improved glycemic control and quality of life scores following a switch from insulin therapy[76]. A recent cohort study of patients with both IBD and type 2 diabetes showed that treatment with GLP-1-based therapies was linked with a lower risk of adverse clinical events, defined as a composite of the need for oral corticosteroid treatment, TNF-α-inhibitor treatment, IBD-related hospitalization, or IBD-related major surgery, as compared to treatment with other antidiabetic therapies [adjusted incidence rate ratio (IRR) = 0.52, 95%CI: 0.42-0.65)[77]. These observations suggest that GLP-1 based therapies may be a novel treatment option for IBD. Naltrexone and bupropion individually may have anti-inflammatory properties. Two small uncontrolled studies of IBD patients showed that naltrexone alone reduced disease activity and induced endoscopic response[78]. The initiation of low-dose naltrexone in IBD patients has been found to result in a decrease in the prescription of several medications that are typically deemed crucial for the treatment of IBD[79]. Bupropion has been associated with clinical improvement in case reports and case series in IBD patients[80,81]. Phentermine-topiramate is a weight-loss drug that has demonstrated highly efficacy in promoting weight loss in obese individuals. While the precise mechanisms are still being investigated, preclinical studies have provided evidence supporting the anti-inflammatory properties of both topiramate and phentermine. Early experimental evidence indicated that topiramate could significantly reduce colonic tissue injury in animal models of IBD. However, these findings were not corroborated in a subsequent retrospective cohort study conducted in human subjects[82,83]. A phase 2 clinical trial is currently in progress to investigate the effectiveness and safety of phentermine-topiramate in obese biologic-treated patients with UC (NCT04721873). Sodium-glucose linked transporter 2 inhibitor is the new glucose lowering drug, which has shown beneficial effects on the obesity and heart failure as well as type 2 diabetes[84]. This drug significantly improved acetic acid-induced IBD in animal models by activating autophagy signaling, inhibiting apoptosis and pro-inflammatory cytokines, lowering oxidative stress, and increasing wound healing[85-87].
Intragastric balloon therapies are a minimally invasive and temporary treatment for inducing weight loss in obese patients. While this approach has been used successfully in the management of obesity, there is limited research on the effectiveness and safety of intragastric balloon therapy in patients with IBD. Although a small series has examined the application of intragastric ballooning in patients with IBD, reports that detail the long-term outcomes associated with weight loss and complications are lacking. The use of intragastric balloon is currently contraindicated for IBD patients due to concerns about the potential for exacerbation of symptoms and complications. A case report demonstrated that the placement of an intragastric balloon for weight loss may exacerbate symptoms of UC[88]. Further, there is currently a scarcity of high-quality evidence regarding the efficacy of other endoscopic procedures for weight loss in patients with IBD.
For the general population, in obese patients with BMI ≥ 40 kg/m2 or 35-39.9 kg/m2 with obesity-related comorbidities and previously failed to achieve adequate weight reduction with non-surgical interventions, bariatric surgery is superior to lifestyle and diet interventions and reduces mortality[89]. There are several different techniques for bariatric surgery, the most common being a Roux-en-Y gastric bypass (RYGB) and a sleeve gastrostomy (SG). These are now mainly performed laparoscopically.
Although data are sparse, bariatric surgery may be a viable, effective, and safe weight loss approach that could potentially result in improved outcomes for patients with IBD[90-93]. In most cases revised from two systematic reviews, weight loss resulting from bariatric surgery can be beneficial in achieving remission, reducing disease activity, and decreasing medication dependence in patients with IBD[92,94]. Bariatric surgery resulted in similar weight loss and risk of complications in IBD patients as in non-IBD patients. A case-control study that included 88 bariatric procedures performed on 85 IBD patients, matched 1:2 for age, sex, BMI, hospital of surgery, and type of bariatric surgery with non-IBD patients who underwent bariatric surgery, found that bariatric surgery was a safe and effective intervention for weight loss, producing similar results to those observed in the control group over a 2-year period[93]. A case-control study involving 25 patients with IBD who received bariatric surgery, and matched with IBD patients who did not undergo bariatric surgery, found that the usage of rescue corticosteroids and the requirement for IBD-related surgery were less prevalent in the former group than in the controls[95]. However, long-term effects of bariatric surgery in IBD patients are poorly understood.
Although conclusive findings have yet to be derived from randomized trials, current evidence suggests that SG may confer an advantage over RYGB in patients with IBD, as SG solely involves the stomach and thus may minimize the risk of small intestinal bacterial overgrowth[90,96]. By avoiding anatomical alterations in the small intestine, the risk of complications such as strictures, abscesses, and fistulas may be reduced and future IBD-related surgeries may be simplified.
The development of de-novo IBD subsequent to bariatric surgery has been reported in several studies. The potential mechanisms underlying this association include exposure to viable toxins as a result of anatomical alterations, increased release of cytokines due to changes in adipose tissue, and alterations in the gut microbiome[95,97]. A Danish nationwide population-based cohort study has reported that bariatric surgery is related to an increased risk of developing new-onset CD, but not UC[98]. After bariatric surgery, the onset of IBD symptoms varied from 1 mo to 16 years[97]. In a case series, it was found that 44 patients who had previously received bariatric surgery, with RYGB being the most common procedure, developed de-novo IBD after a median latency period of 7 years[99]. Recent metal-analysis including 149385 patients reported that the pooled odds ratio for the de-novo IBD following bariatric surgery is 1.17[100]. Thus, the possibility of de-novo IBD should be taken into account as a potential cause of symptoms such as abdominal pain and diarrhea in patients who have undergone bariatric surgery.
Both obesity and IBD are rapidly increasing in modern society, and the proportion of obesity among IBD patients is also reported to be higher now than that in the past. There are claims that obesity contributes to the pathogenesis of IBD or that there are common factors contributing to both diseases, such as dysbiosis, but it is still insufficient to know the causal relationship or direction between them. Although data to assess the effect of obesity on outcomes in IBD are sparse and inconclusive, obesity may have a protective effect in CD and a poorer prognosis in UC. Obesity can clearly affect the treatment of IBD or surgery. Therefore, clinicians need to be aware that obesity can affect the treatment response to drugs and increase surgical complications. Although there are few studies, obesity treatment appears to have the potential to have a relatively favorable effect on IBD outcomes. Bariatric surgery appears to be relatively safe and effective in obese IBD patients. Therefore, it is necessary to compare the benefits and side effects of bariatric surgery for IBD patients. Some patients develop new onset IBD, especially CD, after bariatric surgery, so clinicians are advised to keep this in mind.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu D, China; Nambi G, Saudi Arabia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Kaibullayeva J, Ualiyeva A, Oshibayeva A, Dushpanova A, Marshall JK. Prevalence and patient awareness of inflammatory bowel disease in Kazakhstan: a cross-sectional study. Intest Res. 2020;18:430-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Keller R, Mazurak N, Fantasia L, Fusco S, Malek NP, Wehkamp J, Enck P, Klag T. Quality of life in inflammatory bowel diseases: it is not all about the bowel. Intest Res. 2021;19:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Nam GE, Park HS. Perspective on Diagnostic Criteria for Obesity and Abdominal Obesity in Korean Adults. J Obes Metab Syndr. 2018;27:134-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM. 2018;111:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Fact sheet: obesity and overweight. [cited 14 September 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 6. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1874] [Article Influence: 187.4] [Reference Citation Analysis (1)] |
| 7. | Seminerio JL, Koutroubakis IE, Ramos-Rivers C, Hashash JG, Dudekula A, Regueiro M, Baidoo L, Barrie A, Swoger J, Schwartz M, Weyant K, Dunn MA, Binion DG. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2857-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 8. | Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (4)] |
| 9. | Pringle PL, Stewart KO, Peloquin JM, Sturgeon HC, Nguyen D, Sauk J, Garber JJ, Yajnik V, Ananthakrishnan AN, Chan AT, Xavier RJ, Khalili H. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn's Disease. Inflamm Bowel Dis. 2015;21:2304-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lynn AM, Harmsen WS, Tremaine WJ, Loftus EV. Su1872-Trends in the Prevalence of Overweight and Obesity at the Time of Inflammatory Bowel Disease Diagnosis: A Population-Based Study. Gastroenterology. 2018;154:S-614. [DOI] [Full Text] |
| 11. | Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 12. | Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 13. | Kim SK, Lee HS, Kim BJ, Park JH, Hwang SW, Yang DH, Ye BD, Byeon JS, Myung SJ, Yang SK, Park SH. The Clinical Features of Inflammatory Bowel Disease in Patients with Obesity. Can J Gastroenterol Hepatol. 2021;2021:9981482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Moran GW, Dubeau MF, Kaplan GG, Panaccione R, Ghosh S. The increasing weight of Crohn's disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis. 2013;19:2949-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, Chan AT. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Jensen CB, Ängquist LH, Mendall MA, Sørensen TIA, Baker JL, Jess T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: a population-based cohort study. Am J Gastroenterol. 2018;113:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Chan SSM, Chen Y, Casey K, Olen O, Ludvigsson JF, Carbonnel F, Oldenburg B, Gunter MJ, Tjønneland A, Grip O; DEFINe-IBD Investigators, Lochhead P, Chan AT, Wolk A, Khalili H. Obesity is Associated With Increased Risk of Crohn's disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin Gastroenterol Hepatol. 2022;20:1048-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Chan SS, Luben R, Olsen A, Tjonneland A, Kaaks R, Teucher B, Lindgren S, Grip O, Key T, Crowe FL, Bergmann MM, Boeing H, Hallmans G, Karling P, Overvad K, Palli D, Masala G, Kennedy H, vanSchaik F, Bueno-de-Mesquita B, Oldenburg B, Khaw KT, Riboli E, Hart AR. Body mass index and the risk for Crohn's disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study). Am J Gastroenterol. 2013;108:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Zietek T, Rath E. Inflammation Meets Metabolic Disease: Gut Feeling Mediated by GLP-1. Front Immunol. 2016;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes Rev. 2016;17:1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr Res. 2014;34:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Parmentier-Decrucq E, Duhamel A, Ernst O, Fermont C, Louvet A, Vernier-Massouille G, Cortot A, Colombel JF, Desreumaux P, Peyrin-Biroulet L. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009;15:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Kaazan P, Tan Z, Maiyani P, Mickenbecker M, Edwards S, McIvor C, Andrews JM. Weight and BMI Patterns in a Biologicals-Treated IBD Cohort. Dig Dis Sci. 2022;67:5628-5636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 26. | Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 27. | Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Drouet M, Dubuquoy L, Desreumaux P, Bertin B. Visceral fat and gut inflammation. Nutrition. 2012;28:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 547] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 31. | Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn's disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015;49 Suppl 1:S20-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 34. | Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 35. | Suau R, Pardina E, Domènech E, Lorén V, Manyé J. The Complex Relationship Between Microbiota, Immune Response and Creeping Fat in Crohn's Disease. J Crohns Colitis. 2022;16:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 36. | Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Mao R, Doyon G, Gordon IO, Li J, Lin S, Wang J, Le THN, Elias M, Kurada S, Southern B, Olman M, Chen M, Zhao S, Dejanovic D, Chandra J, Mukherjee PK, West G, Van Wagoner DR, Fiocchi C, Rieder F. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn's disease. Gut. 2022;71:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, Rieder F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn's Disease. Inflamm Bowel Dis. 2019;25:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 39. | Kredel LI, Jödicke LJ, Scheffold A, Gröne J, Glauben R, Erben U, Kühl AA, Siegmund B. T-cell Composition in Ileal and Colonic Creeping Fat - Separating Ileal from Colonic Crohn's Disease. J Crohns Colitis. 2019;13:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 41. | Batra A, Zeitz M, Siegmund B. Adipokine signaling in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1897-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 43. | Barroso T, Conway F, Emel S, McMillan D, Young D, Karteszi H, Gaya DR, Gerasimidis K. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann Gastroenterol. 2018;31:566-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Iannone F, Lopalco G, Rigante D, Orlando I, Cantarini L, Lapadula G. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev. 2016;15:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 46. | Weissman S, Patel K, Kolli S, Lipcsey M, Qureshi N, Elias S, Walfish A, Swaminath A, Feuerstein JD. Obesity in Inflammatory Bowel Disease Is Associated with Early Readmissions Characterised by an Increased Systems and Patient-level Burden. J Crohns Colitis. 2021;15:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Lynn AM, Harmsen WS, Tremaine WJ, Bazerbachi F, Dayyeh BKA, Loftus EVJG. Su1887-Impact of Obesity on Future IBD-Related Complications in a Population-Based Cohort of Crohn's Disease (CD) and Ulcerative Colitis (UC) Patients. Gastroenterology. 2018;154:S-620. [DOI] [Full Text] |
| 48. | Johnson AM, Harmsen WS, Aniwan S, Tremaine WJ, Abu Dayyeh BK, Loftus EV. Prevalence and Impact of Obesity on Disease-specific Outcomes in a Population-based Cohort of Patients with Ulcerative Colitis. J Crohns Colitis. 2021;15:1816-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 49. | Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, Vogel I, Böcker U, Böhm S, Büning C, Duchmann R, Gerken G, Herfarth H, Lügering N, Kruis W, Reinshagen M, Schmidt J, Stallmach A, Stein J, Sturm A, Galle PR, Hommes DW, D'Haens G, Rutgeerts P, Neurath MF. Significant differences between Crohn's disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: results from a European multicenter study in 1,176 patients. Dig Dis Sci. 2010;55:1066-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Poon SS, Asher R, Jackson R, Kneebone A, Collins P, Probert C, Dibb M, Subramanian S. Body Mass Index and Smoking Affect Thioguanine Nucleotide Levels in Inflammatory Bowel Disease. J Crohns Colitis. 2015;9:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 52. | Mostafa NM, Nader AM, Noertersheuser P, Okun M, Awni WM. Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Dai ZH, Xu XT, Ran ZH. Associations Between Obesity and the Effectiveness of Anti-Tumor Necrosis Factor-α Agents in Inflammatory Bowel Disease Patients: A Literature Review and Meta-analysis. Ann Pharmacother. 2020;54:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, Evans E, Grunvald EL, Zarrinpar A, Sandborn WJ, Singh S. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Singh S, Proudfoot J, Xu R, Sandborn WJ. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am J Gastroenterol. 2018;113:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, Grunvald EL, Curtis JR, Sandborn WJ. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One. 2018;13:e0195123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 57. | Singh S, Heien HC, Sangaralingham L, Shah ND, Sandborn WJ. Obesity Is Not Associated With an Increased Risk of Serious Infections in Biologic-Treated Patients With Inflammatory Bowel Diseases. Clin Transl Gastroenterol. 2021;12:e00380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Al-Bawardy B, Ramos GP, Willrich MAV, Jenkins SM, Park SH, Aniwan S, Schoenoff SA, Bruining DH, Papadakis KA, Raffals L, Tremaine WJ, Loftus EV. Vedolizumab Drug Level Correlation With Clinical Remission, Biomarker Normalization, and Mucosal Healing in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Levine LJ, Gaidos JKJ, Proctor DD, Viana AV, Al-Bawardy B. Effect of obesity on vedolizumab response in inflammatory bowel disease. Ann Gastroenterol. 2022;35:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Wong ECL, Marshall JK, Reinisch W, Narula N. Body Mass Index Does Not Impact Clinical Efficacy of Ustekinumab in Crohn's Disease: A Post Hoc Analysis of the IM-UNITI Trial. Inflamm Bowel Dis. 2021;27:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. 2021;44:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Giles JT, Ogdie A, Gomez Reino JJ, Helliwell P, Germino R, Stockert L, Young P, Joseph W, Mundayat R, Graham D, Ritchlin C. Impact of baseline body mass index on the efficacy and safety of tofacitinib in patients with psoriatic arthritis. RMD Open. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Farraye FA, Qazi T, Kotze PG, Moore GT, Mundayat R, Lawendy N, Sharma PP, Judd DT. The impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis clinical programme. Aliment Pharmacol Ther. 2021;54:429-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | McKenna NP, Mathis KL, Khasawneh MA, Dozois EJ, Larson DW, Pemberton JH, Lightner AL. Obese Patients Undergoing Ileal Pouch-Anal Anastomosis: Short-and Long-term Surgical Outcomes. Inflamm Bowel Dis. 2017;23:2142-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Wahl TS, Patel FC, Goss LE, Chu DI, Grams J, Morris MS. The Obese Colorectal Surgery Patient: Surgical Site Infection and Outcomes. Dis Colon Rectum. 2018;61:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 66. | Jain A, Limketkai BN, Hutfless SJG. Mo1243 The effect of obesity on post-surgical complications during hospitalizations for inflammatory bowel disease: a nationwide analysis. Gastroenterology. 2014;146:S-595. [DOI] [Full Text] |
| 67. | Jiang K, Chen B, Lou D, Zhang M, Shi Y, Dai W, Shen J, Zhou B, Hu J. Systematic review and meta-analysis: association between obesity/overweight and surgical complications in IBD. Int J Colorectal Dis. 2022;37:1485-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 68. | Beck SJ. Stoma issues in the obese patient. Clin Colon Rectal Surg. 2011;24:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Efron JE, Uriburu JP, Wexner SD, Pikarsky A, Hamel C, Weiss EG, Nogueras JJ. Restorative proctocolectomy with ileal pouch anal anastomosis in obese patients. Obes Surg. 2001;11:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 71. | Bischoff SC, Barazzoni R, Busetto L, Campmans-Kuijpers M, Cardinale V, Chermesh I, Eshraghian A, Kani HT, Khannoussi W, Lacaze L, Léon-Sanz M, Mendive JM, Müller MW, Ockenga J, Tacke F, Thorell A, Vranesic Bender D, Weimann A, Cuerda C. European guideline on obesity care in patients with gastrointestinal and liver diseases - Joint European Society for Clinical Nutrition and Metabolism / United European Gastroenterology guideline. United European Gastroenterol J. 2022;10:663-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Chicco F, Magrì S, Cingolani A, Paduano D, Pesenti M, Zara F, Tumbarello F, Urru E, Melis A, Casula L, Fantini MC, Usai P. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm Bowel Dis. 2021;27:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 73. | Saxena A, Fletcher E, Larsen B, Baliga MS, Durstine JL, Fayad R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J Inflamm (Lond). 2012;9:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Jones PD, Kappelman MD, Martin CF, Chen W, Sandler RS, Long MD. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis. 2015;21:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 75. | Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes Facts. 2015;8:402-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 862] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 76. | Kuwata H, Tsujii S, Fujita N, Okamura S, Iburi T, Mashitani T, Kitatani M, Furuya M, Hayashino Y, Ishii H. Switching from insulin to liraglutide improved glycemic control and the quality of life scores in a case of type 2 diabetes and active Crohn's disease. Intern Med. 2014;53:1637-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Villumsen M, Schelde AB, Jimenez-Solem E, Jess T, Allin KH. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine. 2021;37:100979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Lie MRKL, van der Giessen J, Fuhler GM, de Lima A, Peppelenbosch MP, van der Ent C, van der Woude CJ. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J Transl Med. 2018;16:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 79. | Raknes G, Simonsen P, Småbrekke L. The Effect of Low-Dose Naltrexone on Medication in Inflammatory Bowel Disease: A Quasi Experimental Before-and-After Prescription Database Study. J Crohns Colitis. 2018;12:677-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Kast RE, Altschuler EL. Remission of Crohn's disease on bupropion. Gastroenterology. 2001;121:1260-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Kane S, Altschuler EL, Kast RE. Crohn's disease remission on bupropion. Gastroenterology. 2003;125:1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Dudley JT, Sirota M, Shenoy M, Pai RK, Roedder S, Chiang AP, Morgan AA, Sarwal MM, Pasricha PJ, Butte AJ. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. 2011;3:96ra76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 83. | Crockett SD, Schectman R, Stürmer T, Kappelman MD. Topiramate use does not reduce flares of inflammatory bowel disease. Dig Dis Sci. 2014;59:1535-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. 2021;107:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 85. | Arab HH, Al-Shorbagy MY, Saad MA. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. 2021;335:109368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 86. | ElMahdy MK, Antar SA, Elmahallawy EK, Abdo W, Hijazy HHA, Albrakati A, Khodir AE. A Novel Role of Dapagliflozin in Mitigation of Acetic Acid-Induced Ulcerative Colitis by Modulation of Monocyte Chemoattractant Protein 1 (MCP-1)/Nuclear Factor-Kappa B (NF-κB)/Interleukin-18 (IL-18). Biomedicines. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Morsy MA, Khalaf HM, Rifaai RA, Bayoumi AMA, Khalifa EMMA, Ibrahim YF. Canagliflozin, an SGLT-2 inhibitor, ameliorates acetic acid-induced colitis in rats through targeting glucose metabolism and inhibiting NOX2. Biomed Pharmacother. 2021;141:111902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Manguso F, Picascia S, Balzano A. Ulcerative colitis exacerbating after placement of intragastric balloon for the treatment of obesity. Inflamm Bowel Dis. 2008;14:872-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1180] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 90. | Bazerbachi F, Sawas T, Vargas EJ, Haffar S, Deepak P, Kisiel JB, Loftus EV Jr, Abu Dayyeh BK. Bariatric Surgery Is Acceptably Safe in Obese Inflammatory Bowel Disease Patients: Analysis of the Nationwide Inpatient Sample. Obes Surg. 2018;28:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Aminian A, Andalib A, Ver MR, Corcelles R, Schauer PR, Brethauer SA. Outcomes of Bariatric Surgery in Patients with Inflammatory Bowel Disease. Obes Surg. 2016;26:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 92. | Shoar S, Shahabuddin Hoseini S, Naderan M, Mahmoodzadeh H, Ying Man F, Shoar N, Hosseini M, Bagheri-Hariri S. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: A systematic review. Surg Obes Relat Dis. 2017;13:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Reenaers C, de Roover A, Kohnen L, Nachury M, Simon M, Pourcher G, Trang-Poisson C, Rajca S, Msika S, Viennot S, Alttwegg R, Serrero M, Seksik P, Peyrin-Biroulet L, Picon L, Bourbao Tournois C, Gontier R, Gilletta C, Stefanescu C, Laharie D, Roblin X, Nahon S, Bouguen G, Carbonnel F, Attar A, Louis E, Coffin B. Bariatric Surgery in Patients With Inflammatory Bowel Disease: A Case-Control Study from the GETAID. Inflamm Bowel Dis. 2022;28:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Hudson JL, Barnes EL, Herfarth HH, Isaacs KL, Jain A. Bariatric Surgery Is a Safe and Effective Option for Patients with Inflammatory Bowel Diseases: A Case Series and Systematic Review of the Literature. Inflamm Intest Dis. 2019;3:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Braga Neto MB, Gregory MH, Ramos GP, Bazerbachi F, Bruining DH, Abu Dayyeh BK, Kushnir VM, Raffals LE, Ciorba MA, Loftus EV, Deepak P. Impact of Bariatric Surgery on the Long-term Disease Course of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 97. | Cañete F, Mañosa M, Clos A, Cabré E, Domènech E. Review article: the relationship between obesity, bariatric surgery, and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Allin KH, Jacobsen RK, Ungaro RC, Colombel JF, Egeberg A, Villumsen M, Jess T. Bariatric Surgery and Risk of New-onset Inflammatory Bowel Disease: A Nationwide Cohort Study. J Crohns Colitis. 2021;15:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Braga Neto MB, Gregory M, Ramos GP, Loftus EV Jr, Ciorba MA, Bruining DH, Bazerbachi F, Abu Dayyeh BK, Kushnir VM, Shah M, Collazo-Clavell ML, Raffals LE, Deepak P. De-novo Inflammatory Bowel Disease After Bariatric Surgery: A Large Case Series. J Crohns Colitis. 2018;12:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Kermansaravi M, Valizadeh R, Farazmand B, Mousavimaleki A, Taherzadeh M, Wiggins T, Singhal R. De Novo Inflammatory Bowel Disease Following Bariatric Surgery: a Systematic Review and Meta-analysis. Obes Surg. 2022;32:3426-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, Nohr EA, Linneberg A, Jess T. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 102. | Pérez CA. Prescription of physical exercise in Crohn's disease. J Crohns Colitis. 2009;3:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with Crohn's disease. Clin J Sport Med. 2007;17:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Klare P, Nigg J, Nold J, Haller B, Krug AB, Mair S, Thoeringer CK, Christle JW, Schmid RM, Halle M, Huber W. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: a prospective randomized controlled trial. Digestion. 2015;91:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | Colombo F, Rizzi A, Ferrari C, Frontali A, Casiraghi S, Corsi F, Sampietro GM, Foschi D. Bariatric surgery in patients with inflammatory bowel disease: an accessible path? J Crohns Colitis. 2015;9:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 106. | Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015;11:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Ungar B, Kopylov U, Goitein D, Lahat A, Bardan E, Avidan B, Lang A, Maor Y, Eliakim R, Ben-Horin S. Severe and morbid obesity in Crohn's disease patients: prevalence and disease associations. Digestion. 2013;88:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Sharma P, McCarty TR, Njei B. Impact of Bariatric Surgery on Outcomes of Patients with Inflammatory Bowel Disease: a Nationwide Inpatient Sample Analysis, 2004-2014. Obes Surg. 2018;28:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |