Published online Mar 14, 2023. doi: 10.3748/wjg.v29.i10.1589
Peer-review started: December 28, 2022
First decision: January 10, 2023
Revised: January 23, 2023
Accepted: March 6, 2023
Article in press: March 6, 2023
Published online: March 14, 2023
Processing time: 71 Days and 16 Hours
Cholelithiasis is a common digestive disease affecting 10% to 15% of adults. It imposes significant global health and financial burdens. However, the patho
Core Tip: Cholelithiasis is a common digestive disease that imposes significant global health and financial burdens. High-throughput screening demonstrated the relationship between bile, gallstones, and the fecal microbiome in cholelithiasis and provided evidence that gastrointestinal (GI) microbiota dysbiosis is associated with gallstone formation. We summarize the current literature, pool the available cholelithiasis-related studies about the GI microbiome, discuss the underlying mechanisms by which the GI microbiome modulates cholelithiasis, and suggest potential microbiome-targeting therapeutics for cholelithiasis prevention.
- Citation: Dan WY, Yang YS, Peng LH, Sun G, Wang ZK. Gastrointestinal microbiome and cholelithiasis: Current status and perspectives. World J Gastroenterol 2023; 29(10): 1589-1601
- URL: https://www.wjgnet.com/1007-9327/full/v29/i10/1589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i10.1589
Cholelithiasis or gallstones is a common digestive disease with a high incidence and relatively low mortality. With an overall prevalence of 11.0% in China[1], cholelithiasis is an important public health problem. Gallstone disease’s prevalence ranges from 0.60 to 1.39% per year in European population surveys[2]. In the United States, 20 to 25 million adults have cholelithiasis, costing more than $6 billion annually[3,4]. Although many cholelithiasis patients are asymptomatic, approximately one-third develop biliary-pancreatic diseases, including acute or chronic cholecystitis, cholangitis, pancreatitis, and even biliopancreatic cancerous lesions. These diseases impose significant global health and financial burdens.
The study of the human microbiome, particularly the gastrointestinal (GI) microbiome, has rapidly evolved in recent decades, primarily due to the new generation of sequencing technology. The composition, diversity, and richness of microbial communities in the GI tract change during disease states. In addition to definite intestinal dysbiosis-related digestive diseases, the GI microbiome is altered in many biliary disorders, which are rarely traditionally considered microbial in etiology. A predictive model including the genera Burkholderia, Caballeronia, and Paraburkholderia was better able to predict cholangiocarcinoma than the tumor marker carbohydrate antigen 19-9[5]. The proportion of Streptococcus is proportionate to the severity of primary sclerosing cholangitis[6].
Investigations of the GI microbiome have extended to cholelithiasis. In the early 20th century, studies supported the existence of interactions between gallstones and bacteria such as Helicobacter. Although it has been recognized that specific bacteria contribute to gallstone formation, studies have recently shown that a complex GI microbiome rich in Desulfovibrionales promotes gallstone formation by regulating bile metabolism. This review examines the literature implicating the role of the GI microbiome in cholelithiasis, including the biliary, gallstone, and fecal microbiomes (Table 1). We will summarize the literature and discuss mechanisms by which the GI microbiome modulates cholelithiasis; finally, we discuss advances in microbiome-based therapeutics.
| Microbial changes | Samples | Disease types vs control | Methods | Ref. |
| ↑Desulfovibrionales | Feces | Cholesterol gallstone vs gallstone-free | 16S sequencing | Hu et al[51] |
| ↑Megamonas, Comamonas, Ruminococcaceae_UCG-014, Coprobacillus, Adlercreutzia, unclassified_p_Firmicutes, Morganella, CHKCI002, and Tyzzerella_4; ↓Ruminococcaceae_UCG-008, Sutterella, GCA-900066755, Butyricicoccus, unclassified_o_Lactobacillales, and Lachnospiraceae_ND3007_group | Feces | Asymptomatic gallstone vs gallstone-free | 16S sequencing | Song et al[33] |
| ↓Akkermansia muciniphila, Prevotella spp., Bifidobacterium adolescentis, Alistipes spp., Bacteroides spp., Dorea spp., Methanobacteria, Methanobrevibacter smithii, Ruminococcus spp., and Faecalibacterium prausnitzii | Feces | Cholesterol gallstone vs pigment gallstone | 16S sequencing | Georgescu et al[37] |
| ↑7α-dehydroxylating bacteria | Feces | Gallstone vs gallstone-free | Culture | Wells et al[49] |
| ↑Proteobacteria; ↓Faecalibacterium, Lachnospira, and Roseburia | Feces | Gallstone vs controls | 16S sequencing | Wu et al[9] |
| ↑Aeromonas, Enterococcus, Unclassified_Enterobacteriaceae, and Citrobacter↓Prevotella, Alloprevotella, Nesterenkonia, and Pyramidobacter | Bile | Recurrent CBD stone vs new-onset CBD stone | 16S sequencing | Chen et al[19] |
| ↑Synergistetes; ↓Bacteroidetes, and Actinobacteria | Bile | Recurrent CBD stone vs primary CBD stone | 16S sequencing | Tan et al[20] |
| ↑Bacteroidaceae, Prevotellaceae, Porphyromonadaceae, and Veillonellaceae | Bile | Gallstone vs controls | 16S sequencing | Molinero et al[8] |
| ↑Brevundimonas and Prevotella_1; ↓Actinomyces, Proteus, Clostridiumsensu_stricto, Klebsiella, Actinobacillus, Lachnospiraceae_UCG-008, Butyrivibrio, Roseburia, Porphyromonas, Streptococcus, Helicobacter, Enterobacter | Bile | Primary CBD stone vs controls | 16S sequencing | Lyu et al[12] |
| ↑Alcaligenaceae | Bile | Primary bile duct stone vs secondary bile duct stone | 16S sequencing | Feng et al[18] |
Studies suggest that the healthy biliary tract is a sterile environment. With the development of sequencing technology, studies revealed the existence of a biliary microbiome. Stewart et al[7] detected bacteria in bile samples in nearly a third of gallstone patients. The first study to identify the existence of human biliary microbiota in the gallbladder described the composition of human biliary microbiota using 16S ribosomal RNA (rRNA) gene sequencing. The healthy biliary microbiota is dominated by Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia at the phylum level[8]. In addition to bacteria, Cyanobacteria and Spirochaetes were also present in low amounts.
There are some resemblances and dissimilarities between the biliary and GI microbiomes. The comparative metagenomic analysis demonstrated no significant differences between the GI tract and bile in the predominant phylum Firmicutes and the rare phylum Fusobacteria. However, the microbial diversity in the biliary tract is more diverse than in the GI tract[9]. The bile duct and duodenum share the core microbiota with the genus Escherichia–Shigella, Fusobacterium, and Enterococcus[10]. Given that the bile duct is anatomically connected to the GI tract via the duodenal papilla, it was hypothesized that the biliary microbiota originates from intestinal bacteria and migrates retrograde into the biliary tract. Consistent with the hypothesis, studies demonstrated that the biliary microbiota shared a compositional similarity to duodenal microbiota, and all bacteria in bile were detected in the upper GI using 16S sequencing[11,12]. It is noteworthy that the intestinal microbiome contributes to the heterogeneity of the biliary microbiome in cholelithiasis patients, despite the high prevalence of oral cavity and respiratory tract inhabitants to intestinal inhabitants[13].
Because of interactions between the biliary microbiome and metabolic disease, investigators analyzed the bacterial composition associated with cholelithiasis. A Colombian study demonstrated a predominance of Pseudomonas spp. in gallbladder tissue and bile[14]. To compare the difference in bile microbiome between gallbladder stone patients and healthy individuals, Molinero et al[8] measured bile samples using 16S rRNA sequencing. They demonstrated that the relative abundance of the family Propionibacteriaceae in patients with gallbladder stones was lower than in healthy controls. In contrast, the relative abundance of the family Bacteroidaceae, Prevotellaceae, Porphyromonadaceae, and Veillonellaceae was higher.
Short-chain fatty acids (SCFAs) consist of butyrate, propionate, and acetate; these are associated with inflammatory diseases and cancers and may serve as nutritional and therapeutic agents in diseases[15,16]. Evidence suggests that low levels of expression of bacteria-producing SCFAs harm the microbial balance of the biliary tract, affecting gallstone formation. There is a significantly decreased abundance of Clostridiumsensu_stricto, Lachnospiraceae _UCG-008, Butyrivibrio, and Roseburia (which produce SCFAs) at the genera level in patients with primary choledocholithiasis. Lyu et al[12] found that specific bacteria-producing SCFAs might participate in generating common bile duct stones.
In recent years, several groups interrogated the human biliary microbiome in recurrent bile duct stones patients, albeit in different disease states. The biliary microbiome in the recurrence of choledocholithiasis showed a significantly low diversity[17]. The composition of biliary bacteria differed significantly between primary bile duct stone patients and those with secondary bile duct stones. By contrast, several families, such as Propionibacteriaceae, Sphingomonadaceae, and Lactobacillaceae, were enriched in the recurrent cholelithiasis group[18]. Chen et al[19] analyzed the biliary microbiota of 16 patients with recurrent choledocholithiasis and 44 patients with primary choledocholithiasis. The 16S rRNA sequencing revealed a prevalence of the phyla Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria (all of which are intestinal bacteria) in the primary choledocholithiasis group. These data suggest that the biliary microbiota might originate from gut microbiota and access the biliary tract through the duodenal papilla. Furthermore, there was substantial enrichment of the genera Prevotella, Alloprevotella, Nesterenkonia, and Pyramidobacter (without significant alterations in microbial diversity) compared with recurrent choledocholithiasis patients[19]. One study described the biliary microbiome in recurrent cholelithiasis[20]. Investigators compared the biliary microbiomes of patients with primary choledocholithiasis with those with recurrent choledocholithiasis. Consistently, the phyla Proteobacteria and Firmicutes predominated in both groups. Patients with recurrent choledocholithiasis showed less microbial biliary diversity (with reduced Bacteroidetes and Actinobacteria and enrichment of Synergistetes at the phylum level) than controls. A study considered 11 adults with recurrent choledocholithiasis and nine post-endoscopic removal patients to assess the characteristics of the biliary microbiome. The richness and diversity decreased in the recurrence group. The recurrence group had a higher relative abundance of phylum Actinobacteria and Firmicutes and a lower relative abundance of Bacteroidetes than the non-recurrence group[21].
These association studies demonstrate a correlation between the biliary microbiome and cholelithiasis; however, these studies sampled the microbiome from bile but not the bile duct epithelium.
Awareness of the gallstone microbiome arose via a circuitous route. The first microbial study of gallstone formation dates back decades; the authors found a lower prevalence of gallstones in germ-free mice[22]. In the 1990s, bacterial DNA was detected using PCR in all common bile duct stones, mixed cholesterol stones, and brown pigment stones[23], demonstrating the existence of bacteria in gallstone formation. In the 21st century, researchers found that 42% of patients had bacteria gallstone samples[7], and over 80% of the stone cores contained bacteria, primarily from the intestine[24]. These results were vital for assessing the connections between the microbiota and cholelithiasis.
According to scanning electron microscopy studies, there are bacterial biofilms on gallstone surfaces[25]. Bacterial biofilms are microbial communities attached to the surface or embedded in the matrix[26]. The typical surface-related biofilm originates from single planktonic cells attached to the surface. These cells divide and produce extracellular polymeric substances. Microbial communities then form the three-dimensional structure attached to the surface. Biofilms are environmental reservoirs for pathogens that correlate with infectious kidney stones, bacterial endocarditis, and airway infections in cystic fibrosis patients[27]. Studies found that biofilm formed by Salmonellae aggregates on the surface of human gallstones[28]. The appearance of gallstones led to a 4.5-fold increase in hyperbiofilm isolates. Salmonella spp. increased in persistence in bile via genetic alterations[29].
The gut microbiome in human health and diseases has received considerable attention. The intestinal microbiota is dominated by Bacteroidetes, followed by Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia[30]. Investigations of the microbial composition of cholelithiasis patients using 16S rRNA profiling identified a predominance of Firmicutes and, to a lesser extent, Bacteroidetes, Actino
The gut microbiome of cholelithiasis patients is maladjusted. Most studies showed reduced gut microbiota diversity in cholelithiasis patients[5,32]. There are alterations of the gut microbiome in cholelithiasis patients of various statuses. Song and colleagues demonstrated that Klebsiella, Roseburia, Collinsella, Dialister, and Enterobacerin at the genus level were significantly decreased, whereas Streptococcus, Lactobacillus, Dorea, Romboutsia, Fusobacterium, and Megamonas were over-represented in asymptomatic gallstone patients relative to controls[33]. Wu et al[9] compared the gut microbiota of twenty-nine patients with gallbladder stones and thirty-eight healthy controls. They found increased abundances of Proteobacteria and decreased abundances of Faecalibacterium, Lachnospira, and Roseburia; this was the first study to characterize gut microbiota dysbiosis in gallstone patients. A genome-wide search suggested an underlying link between the biliary tract core microbiome and the formation of cholesterol gallstones.
There have been attempts to utilize dysbiosis as a predictive and diagnostic tool or biomarker. Keren et al[34] described a significantly increased abundance of Oscillospira and a reduced abundance of Roseburia at the genus level in cholelithiasis patients compared with healthy controls. They suggested that the genera Oscillospira and Roseburia may be used in the early prediction or diagnosis of cholelithiasis as microbial biomarkers[34]. The relative abundance of the genus Eubacterium, which metabolizes and removes cholesterol, was lower in cholelithiasis patients. Line discriminant analysis effect size analysis showed that Ruminococcus gnavus predicted cholelithiasis[32]. These findings suggest that the gut microbiome is altered during cholelithiasis and that these changes may be valuable for diagnosis.
Gallstones are organic matrixes of cholesterol crystals, calcium bilirubinate, mucin, and proteins in the gallbladder or biliary tract[35]. Based on the major constituents, gallstones are classified as cholesterol gallstones (> 90%) or pigment gallstones (< 10%)[36]. Cholesterol gallstones form mixed or pure cholesterol gallstones. More than 80% of gallbladder stones are composed of pure cholesterol. Cholecystolithiasis originates primarily from the gallbladder and is composed of cholesterol or mixed gallstones, primarily cholesterol or black pigment stones. Several groups independently analyzed the human microbiome of cholelithiasis patients with different components. Bacterial diversity and several functional bacterial species of cholesterol-rich gallstones significantly decreased compared to those of pigment gallstones. These species include Akkermansia muciniphila, Prevotella spp., Bifidobacterium adolescentis, Alistipes spp., Bacteroides spp., Dorea spp., Methanobacteria, Methanobrevibacter smithii, Ruminococcus spp., and Faecalibacterium prausnitzii[37]. There were various compositions of the gut microbiome in the mice in which a lithogenic diet-induced cholesterol gallstones. There was reduced richness, α diversity, the proportion of Firmicutes, and the ratio of Firmicutes to Bacteroidetes in the lithogenic diet group[38]. These findings suggest that alterations in the gut microbiome may play an essential role in forming cholesterol gallstones.
Helicobacter spp. were reported to participate in the formation of murine cholesterol gallstones. Mice fed a lithogenic diet and infected with various enterohepatic Helicobacter spp. showed a significantly higher prevalence of cholesterol gallstones than uninfected controls[39]. In humans, a retrospective cohort study demonstrated that patients with gallstone disease were at increased risk of Helicobacter pylori (H. pylori) infection[40].
Pigment gallstones include brown pigment stones or black pigment stones. Brown pigment stones are associated with biliary tract infections. By contrast, black pigment stones are common in patients with hemolytic anemia, cirrhosis, and cardiac valve replacements. These findings were supported by human and animal research, which found changes in human microbiota in cholesterol gallstones. Nevertheless, there has been little attention paid to pigment gallstones. Notably, a pilot study indicated that the gallstone microbiome might participate in developing pigment stones[41]. The genera Klebsiella and Enterococcus (involved in bacterial biofilm formation) were dominant in pigment stones. These results suggest the participation of a gallstone microbiome in cholelithiasis. Kim and colleagues compared the biliary microbiota in patients with pigment common bile duct stones to other causes of biliary obstruction; there was enrichment of the genus Enterococcus in patients with common pigment bile duct stones[42]. These findings suggest a possible association between Enterococcus and pigment stone formation.
Gallstones are formed when there is an imbalance of biliary cholesterol homeostasis. The primary pathophysiological defect in gallstones is the supersaturation of cholesterol in bile. Other factors include genetic factors (particularly lithogenic gene 1 and mitochondrial DNA variant), hepatic hypersecretion, gallbladder motility function obstacle, cholesteric phase transition, excessive secretion and accumulation of mucin, and excessive cholesterol[43,44]. Neutrophil extracellular DNA traps are involved in human gallstone formation and growth[45]. This evidence suggests the involvement of the GI microbiome in cholelithiasis. The alteration of the host GI microbiome modulates gallbladder motility and inflammation (especially mucin content), inhibiting cholesterol cholelithogenesis. The prevalence of gallstones in germ-free mice was higher than in specific pathogen-free mice[46], suggesting an underlying role of the GI microbiome in cholesterol cholelithogenesis.
Like gastric secretions and hydrochloric acid, bile is a bactericidal agent in the GI system[47]. The human gut microbiome is highly capable of transforming BAs. The oxidation of 3α-, 7α-, or 12α-hydroxyl groups on the steroid core, catalyzed by hydroxysteroid dehydrogenases, were the most prevalent BAs transformations[48]. A study found that 43 isolates of 41 species can modify human unconjugated BAs in vitro[48]. The gut microbiome chemically modifies primary BAs (e.g., dehy
The genus Clostridium appears to be active in 7α-dehydroxylation. A study identified other strains, including Bacteroides vulgatus, Bifidobacterium adolescentis, and Roseburia intestinalis at the phylum level[48]. The abundance of 7α-dehydroxylating bacteria significantly increased[32] and was 42-fold higher in gallstone subjects than in gallstone-free contrlos[49]. Antibiotic treatment significantly reduced 7α-dehydroxylation activity and cholesterol saturation[50], suggesting a possible pathogenic role for 7α-dehydroxylating bacteria. Hu et al[51] found that patients with cholesterol gallstone disease showed significant enrichment of Desulfovibrionales at the order level compared with gallstone-free controls. Following fecal transplantation from gallstone patients into mice, the mice showed a higher prevalence of gallstones; this study suggested Desulfovibrionales as a microbial trigger contributing to gallstone formation.
These studies elucidated the underlying mechanisms of the biliary microbiome on gallstone formation. Desulfovibrionales were enriched in cholelithiasis patients. The bacterial overgrowth shifts the biliary microbiome to a cholelithogenesis phenotype. The GI microbiome, rich in Desulfovibrionales, induces the formation of cholesterol gallstones by regulating hepatic BA metabolism in several ways[51]. First, the secondary BAs in the cecum increase with the number of 7α-dehydroxylating bacteria. Second, the biliary microbiome regulates the expression of hepatic farnesoid X receptor (FXR)-CYP7A, inhibiting synthesis. Third, a specific microbiome promotes intestinal cholesterol absorption and secretion of canalicular cholesterol into bile[51].
Mucin is a glycoprotein with high molecular weight. It protects and lubricates the ducts and lumens in vivo. Mucins are classified according to their structural characteristics as secreted gel-forming mucins, soluble mucins, and trans-membrane mucins. The gel-forming mucin known as MUC5AC and the trans-membrane mucin known as the multifunctional protein MUC4 participate in cell signaling due to differential expression in normal and pathophysiological conditions. For example, the abnormal expression of MUC4 and MUC5AC was detected in biliary tract cancer, whereas it is rarely detected in healthy biliary tract[52]. Yoo et al[53] reported that concentrations and gene expression of MUC3 and MUC5B were significantly overexpressed in a cholesterol stone group than in normal controls. Patients with gallbladder stones were subdivided according to the density of gallstones into an isopycnic group and a calcified group. The enriched expression of MUC4 was detected, and the proportion of bacteria (especially gram-positive bacteria) was positively associated with the expression of MUC4 in the calcification group[54]. Because of the function of MUC4 in adhesion, a high concentration of MUC4 may be beneficial for bacterial growth and subsequently modulate gallstone formation and calcification.
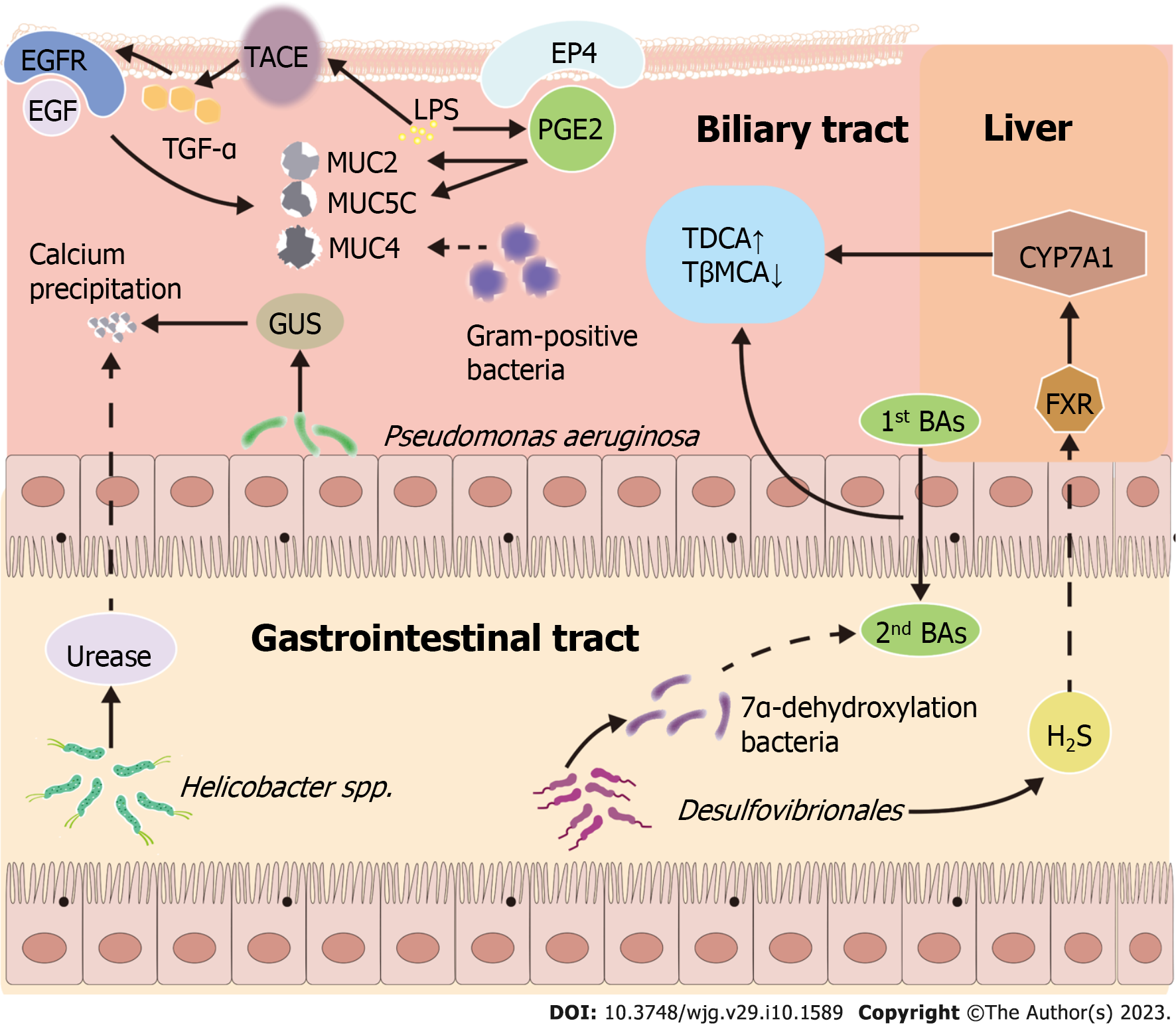
Mucin hypersecretion should be considered a requirement in gallstone formation[43]. Studies found that MUC5AC plays an essential role in hepatolithiasis formation and recurrence; there was increased expression of MUC5AC and MUC2 in hepatolithiasis patients. In another investigation, the effect of MUC5AC on hepatolithiasis formation was elucidated. MUC5AC was upregulated through several pathways. Lipopolysaccharide (LPS), a major surface component of the gram-negative bacteria, upregulated MUC5AC expression in biliary epithelial cells. LPS significantly upregulated the expression of prostaglandin E2 (PGE2). The expression of MUC5AC and MUC2 mRNA was induced by exogenous PGE2. The agonist of EP4, a G-protein coupled receptor, significantly increased MUC2 and MUC5AC expression. P38MAPK mediates PGE2/EP4-induced MUC2 and MUC5AC upregulation. PGE2 induces MUC2 and MUC5AC expression through the EP4/p38MAPK pathway[55]. LPS promoted epidermal growth factor receptor activation by increasing the secretion of transforming growth factor-α, resulting in overexpression of MUC5AC. By contrast, the LPS-induced MUC5AC overexpression was abolished by inhibiting tumor necrosis factor-α converting enzyme activity[56]. Mucin hypersecretion in bile may result from the upregulation of MUC genes and result in a higher bile viscosity, retaining cholesterol crystals in the biliary tract[53].
Bacterial enzymes, including β-glucuronidase (GUS) and phospholipase (PL), which are related to bacterial proliferation and severe infections[7], contribute to cholelithogenesis. Some bacteria produce exogenous GUS, including Escherichia coli and Salmonella enterica. GUS induces the hydrolysis of bilirubin diglucuronides to produce unconjugated bilirubin, resulting in the precipitation of calcium bilirubinate[57]. PL hydrolyzes lecithin to water-insoluble free fatty acids and lysophospholipids, enhancing the precipitation of calcium salts and mucin secretion from the biliary epithelium[58]. Nearly one-third of the cultured strains of cholesterol gallstone could secrete GUS and PLA2[59]. GUS and PLA2 levels in Pseudomonas aeruginosa (P. aeruginosa) strains were highest in culturable strains, suggesting that P. aeruginosa was involved in gallstone pathogenesis.
In addition to Desulfovibrionales, Helicobacter spp. are essential mediators during gallstone formation. Helicobacter infections (especially with H. pylori) positively correlate with the prevalence of chronic cholecystitis and cholelithiasis[60]. H. pylori is the primary pathogenic agent of chronic gastritis, gastric ulcer, and gastric cancer.
Helicobacter spp. play a unique role in the formation of murine cholesterol gallstones. Belzer et al[61] identified an underlying mechanism of gallstones by testing the ability of different Helicobacter spp. to precipitate calcium. Urease-positive Helicobacter spp. precipitate calcium, while urease-negative Helicobacter species cannot[61]. This finding suggests that gallstone formation may be induced by Helicobacter spp., which precipitate calcium directly via urease activity. Nevertheless, there is scant evidence to support causality in mechanisms for the GI microbiome contributing to cholelithogenesis as described above (Figure 1), and the mechanistic links between pathobionts and cholelithiasis formation require further exploration.
The interactions and mutual influences between diet and GI microbiota are well known. Nevertheless, there is little information about the interactions between diet and biliary microbiota. The relationship between diet, biliary microbiota, and cholelithiasis is intricate. A case-control study compared the diet and biliary microbiota of patients and healthy people with cholelithiasis to identify potential associations. The authors found that the intake of dairy products was inversely associated with the relative abundance of the phylum Bacteroidetes, the family Bacteroidaceae, and the genus Bacteroides in bile. In contrast, seafood and meats were positively associated with the relative abundance of the family Pasteurellaceae[62].
Epidemiological studies indicated that age, gender, pregnancy, rapid weight loss, excessive obesity, and diabetes are the primary risk factors for gallstones[63]. The prevalence of gallstone disease progressively increases with age[35]. Age impacts the composition of the human microbiome, potentially via the influence of health conditions, medication use, and lifestyle factors[64]. A retrospective study investigated positive bile samples from patients with biliopancreatic system diseases and found that age was positively associated with gram-negative bacterial infections and negatively related to gram-positive bacterial infections in bile[65].
Guidelines recommend endoscopic sphincterotomy (EST) and stone extraction for treating bile duct stones patients and cholecystectomy as the first-line treatment of symptomatic cholelithiasis[66,67]. Cholecystectomy alters the communication between the bile and intestine, altering the BA metabolism pathway and the intestinal microbiota. Several studies explored the effect of cholecystectomy on the GI microbiota. One study compared the composition of gut microbiota before and after cholecystectomy. Post-cholecystectomy patients showed significant enrichment in the phylum Bacteroidetes[34] and a reduction in the genus Faecalibacterium[68]. Other studies compared the gut microbiota of volunteers with and without cholecystectomy. Compared to controls, post-cholecystectomy patients had a lower relative abundance of the genera Prevotella, Desulfovibrio, Barnesiella, Paludibacter, and Alistipes[69] and a higher relative abundance of the Blautia obeum and Veillonella parvula, which are members of the phylum Firmicutes[70]. Reductions of Candida albicans and enrichments of Candida glabrata and Aspergillus unassigned were also reported[71]. These findings suggest that cholecystectomy affects the GI microbiota.
EST is an invasive procedure recommended for treating bile duct stones. When comparing the biliary microbial composition of choledocholithiasis patients with or without a history of EST, significant differences were found between these groups; the genus Pyramidobacter showed positive associations with previous EST[72]. After EST, sphincter of Oddi laxity (SOL) resulted in duodenal content flow into the bile duct, altering the biliary microbiome. In two case-control studies, cholelithiasis patients with and without SOL significantly differed in the biliary microbiome[73,74]. Compared with those without SOL, cholangiolithiasis patients with SOL had increased phylotypes of family, including Desulfovibrionaceae and Shewanellaceae, and a larger abundance of Bilophila and Shewanella algae[73]. Compared with those without SOL, there was an enrichment of Rhizobiaceae in choledocholithiasis patients with SOL[74]. These findings suggest that SOL after EST plays a pivotal role in the bile duct microenvironment of cholelithiasis patients.
Management of cholelithiasis depends on the gallstone location. Patients with gallbladder stones are usually treated with cholecystectomy and medical dissolution, whereas patients with extrahepatic bile duct stones are usually treated with EST or endoscopic papillary balloon dilation. Despite many strategies for cholelithiasis, efficacious methods of prevention are still needed. Oral administration of ursodeoxycholic acid, statins, and ezetimibe can prevent gallstones[75]. Given the cost-benefit ratios, oral administration of ursodeoxycholic acid or statins is not recommended for cholelithiasis prevention[36]. In recent years, the GI microbiome has emerged as one of the critical regulators of gallstone formation. Therefore, regulation of the host GI microbiome might be a method to prevent cholelithiasis. Some prevention strategies might include Lactobacilli, nanoscale iron sulfide (nFeS), and Astragalus polysaccharide.
Lactobacillus species are among the most common probiotics and are associated with health benefits, including antimicrobial activity and tumor suppression[76]. Indeed, many intervention studies using Lactobacillus showed promising results[77], including on cholesterol, triglyceride, and low-density lipoprotein levels[78]. Investigators demonstrated the preventive effects of Lactobacillus on gallstone formation in a murine model based on the probiotic’s hypocholesterolemic properties. The mechanism by which Lactobacillus targets the GI microbiome and attenuates cholesterol gallstones was elucidated. Oh and colleagues found that Lactobacillus acidophilus ATCC 43121 had a hypocholesterolemic effect by decreasing the expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the liver, which in turn reduces the expression of MUC5AC and MUC5B in the gallbladder[79]. Ye and colleagues found that Lactobacillus reuteri CGMCC 17942 and Lactobacillus plantarum CGMCC 14407 contribute to BA redistribution through the activation of the FXR pathway[80]. These findings suggest that supplementation with Lactobacilli might prevent cholesterol gallstone formation. However, further clinical trials are needed to develop this approach as a probiotic supplement to prevent human cholelithiasis.
Antimicrobial clinical management with bacteria or bacterial biofilms may benefit patients with gallbladder stones; nFeS is a nanomaterial with high levels of antibacterial efficacy. The evidence suggests that oral administration of nFeS supernatants significantly reduces bacterial activity and disrupts biofilm structure, inhibiting gallstone formation[81]. In a mouse model of cholelithiasis, oral administration of nFeS supernatants resulted in approximately twice the antibacterial efficacy of oral ciprofloxacin. Moreover, nFeS significantly cleared gallbladder stones compared with controls.
Astragalus polysaccharide is a natural macromolecule extracted from a standard traditional Chinese medicine known as the “Astragalus,” which has immunomodulatory, anti-inflammatory, and anti-cancer effects in several diseases, including kidney stones[82], ulcerative colitis[83], constipation[84], and lung adenocarcinoma[85]. A recent study reported that Astragalus polysaccharide had beneficial effects on ameliorating the formation of cholesterol gallstones and reversing GI dysbiosis in mice[84]. Gallstone mice with Astragalus polysaccharide supplementation had a higher relative abundance of Bacteroidota and a lower relative abundance of Vemucomicrobiota at the phylum level compared to controls. These results suggest that Astragalus inhibits gallstone formation by improving intestinal microbial diversity.
These findings suggest a significant alteration of the GI microbiome in cholelithiasis patients. The GI microbiome is involved in the pathogenesis of cholelithiasis through several pathways: Biliary microbiome induces gallstone formation by regulating BA metabolism; Helicobacter species induce gallstone formation by precipitating calcium; LPS upregulates mucins via the tumor necrosis factor-α converting enzyme/transforming growth factor-α/epidermal growth factor receptor pathway and the EP4/p38MAPK pathway; GUS and PL accelerate precipitation of calcium bilirubinate. Nevertheless, the mechanisms for the GI microbiome contributing to cholelithogenesis lack evidence to support causality. The composition of the GI microbiome could be regulated in individuals with cholelithiasis by surgery, SOL, age, diet, and lifestyle. These modifiable factors for cholelithiasis may be crucial to prevent cholelithiasis. Given the regulability of the GI microbiota, studies should explore microbiome-targeting interventions for preventing cholelithiasis, including Lactobacilli, nFeS, and Astragalus polysaccharide.
Studies on cholelithiasis in the GI microbiome are mostly single-omics types, whereas multi-omics studies, including genome, epigenome, transcriptome, proteomics, and metabolomics, are limited. The field of the human microbiome in cholelithiasis is relatively young and limited to the bacterial microbiome (i.e., there is no study of the mycobiome and virome). Existing studies are heterogeneous, possibly due to the influence of disease states, disease types, sample types and sites, gallstone components, and medical intervention. They fail accurately to characterize the structural, functional, and metabolic features of the GI microbiomes in cholelithiasis, and most lack validation cohorts. Moreover, these results do not test the specific strains, functional genes, and metabolites identified by screening validation in vitro, preventing in-depth studies on the pathogenesis of cholelithiasis. Consequently, longitudinal human intervention and in-depth analysis of the mechanism are needed to address the critical question of causality. If host-microbiome interactions are to be targeted as pathogenesis of cholelithiasis, well-designed mechanistic studies of the interactions between the GI microbiome and host are required. Such studies might identify causality between the GI microbiome and gallstone formation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isogai M, Japan; Kitamura K, Japan; Lee SH, South Korea S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Su Z, Gong Y, Liang Z. Prevalence of gallstone in Mainland China: A meta-analysis of cross-sectional studies. Clin Res Hepatol Gastroenterol. 2020;44:e69-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol. 2018;34:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 488] [Article Influence: 25.7] [Reference Citation Analysis (6)] |
| 5. | Zhang T, Zhang S, Jin C, Lin Z, Deng T, Xie X, Deng L, Li X, Ma J, Ding X, Liu Y, Shan Y, Yu Z, Wang Y, Chen G, Li J. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients With Cholangiocarcinoma. Front Cell Infect Microbiol. 2021;11:751795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Pereira P, Aho V, Arola J, Boyd S, Jokelainen K, Paulin L, Auvinen P, Färkkilä M. Bile microbiota in primary sclerosing cholangitis: Impact on disease progression and development of biliary dysplasia. PLoS One. 2017;12:e0182924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Stewart L, Griffiss JM, Jarvis GA, Way LW. Gallstones containing bacteria are biofilms: bacterial slime production and ability to form pigment solids determines infection severity and bacteremia. J Gastrointest Surg. 2007;11:977-83; discussion 983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Molinero N, Ruiz L, Milani C, Gutiérrez-Díaz I, Sánchez B, Mangifesta M, Segura J, Cambero I, Campelo AB, García-Bernardo CM, Cabrera A, Rodríguez JI, González S, Rodríguez JM, Ventura M, Delgado S, Margolles A. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome. 2019;7:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, Shi P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Han J, Wu S, Fan Y, Tian Y, Kong J. Biliary Microbiota in Choledocholithiasis and Correlation With Duodenal Microbiota. Front Cell Infect Microbiol. 2021;11:625589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Ye F, Shen H, Li Z, Meng F, Li L, Yang J, Chen Y, Bo X, Zhang X, Ni M. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS One. 2016;11:e0150519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Lyu Z, Yu T, Zhang L, Xu X, Zhang Y, Li J, Li Z, Zhang W, Hou S. Analysis of the relationship between bile duct and duodenal microbiota reveals that potential dysbacteriosis is the main cause of primary common bile duct stones. Synth Syst Biotechnol. 2021;6:414-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Shen H, Ye F, Xie L, Yang J, Li Z, Xu P, Meng F, Li L, Chen Y, Bo X, Ni M, Zhang X. Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Sci Rep. 2015;5:17450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Arteta AA, Carvajal-Restrepo H, Sánchez-Jiménez MM, Diaz-Rodriguez S, Cardona-Castro N. Gallbladder microbiota variability in Colombian gallstones patients. J Infect Dev Ctries. 2017;11:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | González-Bosch C, Boorman E, Zunszain PA, Mann GE. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021;47:102165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 16. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1846] [Article Influence: 307.7] [Reference Citation Analysis (1)] |
| 17. | Ye C, Zhou W, Zhang H, Miao L, Lv G. Alterations of the Bile Microbiome in Recurrent Common Bile Duct Stone. Biomed Res Int. 2020;2020:4637560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Feng R, Zhang T, Kayani MUR, Wang Z, Shen Y, Su KL, Bielike K, Chen L. Patients with Primary and Secondary Bile Duct Stones Harbor Distinct Biliary Microbial Composition and Metabolic Potential. Front Cell Infect Microbiol. 2022;12:881489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Chen B, Fu SW, Lu L, Zhao H. A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma. Biomed Res Int. 2019;2019:1092563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Tan W, Chen R, Song J, He D, Wu J, Chen X, Yang X, Ye L. Microbiota analysis with next-generation 16S rDNA gene sequencing in recurrent common bile duct stones. Ann Transl Med. 2022;10:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Choe JW, Lee JM, Hyun JJ, Lee HS. Analysis on Microbial Profiles & Components of Bile in Patients with Recurrent CBD Stones after Endoscopic CBD Stone Removal: A Preliminary Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Frey C, Thorpe C, Abrams G. Gallstone formation in the germ-free mouse. Am J Surg. 1968;115:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Lee DK, Tarr PI, Haigh WG, Lee SP. Bacterial DNA in mixed cholesterol gallstones. Am J Gastroenterol. 1999;94:3502-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Hazrah P, Oahn KT, Tewari M, Pandey AK, Kumar K, Mohapatra TM, Shukla HS. The frequency of live bacteria in gallstones. HPB (Oxford). 2004;6:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Cheng CL, Chang HH, Chen TH, Tsai PJ, Huang YT, Huang PJ, Lin SY. Spectral and morphological classification of different chronic and acute Taiwanese gallstones via FTIR, SEM and ESEM-EDX microanalyses. Dig Liver Dis. 2016;48:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Sauer K, Stoodley P, Goeres DM, Hall-Stoodley L, Burmølle M, Stewart PS, Bjarnsholt T. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol. 2022;20:608-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 557] [Article Influence: 185.7] [Reference Citation Analysis (0)] |
| 27. | Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1081] [Cited by in RCA: 1057] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 28. | Crawford RW, Rosales-Reyes R, Ramírez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 2010;107:4353-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 29. | Neiger MR, González JF, Gonzalez-Escobedo G, Kuck H, White P, Gunn JS. Pathoadaptive Alteration of Salmonella Biofilm Formation in Response to the Gallbladder Environment. J Bacteriol. 2019;201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 31. | Grigor'eva I, Romanova T, Naumova N, Alikina T, Kuznetsov A, Kabilov M. Gut Microbiome in a Russian Cohort of Pre- and Post-Cholecystectomy Female Patients. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Wang Q, Hao C, Yao W, Zhu D, Lu H, Li L, Ma B, Sun B, Xue D, Zhang W. Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 2020;20:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Song ST, Cai LY, Zeng X, Xie WF. Gut Microbial Profile in Asymptomatic Gallstones. Front Microbiol. 2022;13:882265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Keren N, Konikoff FM, Paitan Y, Gabay G, Reshef L, Naftali T, Gophna U. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 35. | Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 490] [Article Influence: 25.8] [Reference Citation Analysis (2)] |
| 36. | Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 37. | Georgescu D, Ionita I, Lascu A, Hut EF, Dragan S, Ancusa OE, Ionita M, Calamar-Popovici D, Georgescu LA, Lighezan DF. Gallstone Disease and Bacterial Metabolic Performance of Gut Microbiota in Middle-Aged and Older Patients. Int J Gen Med. 2022;15:5513-5531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Wang Q, Jiao L, He C, Sun H, Cai Q, Han T, Hu H. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017;17:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 39. | Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, Carey MC, Fox JG. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Cen L, Wu J, Zhu S, Pan J, Zhou T, Yan T, Shen Z, Yu C. The potential bidirectional association between Helicobacter pylori infection and gallstone disease in adults: A two-cohort study. Eur J Clin Invest. 2023;53:e13879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Kose SH, Grice K, Orsi WD, Ballal M, Coolen MJL. Metagenomics of pigmented and cholesterol gallstones: the putative role of bacteria. Sci Rep. 2018;8:11218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Kim B, Park JS, Bae J, Hwang N. Bile Microbiota in Patients with Pigment Common Bile Duct Stones. J Korean Med Sci. 2021;36:e94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am. 2010;39:185-207, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Sun D, Niu Z, Zheng HX, Wu F, Jiang L, Han TQ, Wei Y, Wang J, Jin L. A Mitochondrial DNA Variant Elevates the Risk of Gallstone Disease by Altering Mitochondrial Function. Cell Mol Gastroenterol Hepatol. 2021;11:1211-1226.e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Crunkhorn S. Targeting NETs to treat gallstones. Nat Rev Drug Discov. 2019;18:748. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Fremont-Rahl JJ, Ge Z, Umana C, Whary MT, Taylor NS, Muthupalani S, Carey MC, Fox JG, Maurer KJ. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS One. 2013;8:e70657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 47. | Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009;58:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Lucas LN, Barrett K, Kerby RL, Zhang Q, Cattaneo LE, Stevenson D, Rey FE, Amador-Noguez D. Dominant Bacterial Phyla from the Human Gut Show Widespread Ability To Transform and Conjugate Bile Acids. mSystems. 2021;e0080521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 49. | Wells JE, Berr F, Thomas LA, Dowling RH, Hylemon PB. Isolation and characterization of cholic acid 7alpha-dehydroxylating fecal bacteria from cholesterol gallstone patients. J Hepatol. 2000;32:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Berr F, Kullak-Ublick GA, Paumgartner G, Münzing W, Hylemon PB. 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology. 1996;111:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, Zhang X, Weng Z, Lu Q, Jiao L, Chen C, Sun H, Jiang Z, Gu A. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun. 2022;13:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (3)] |
| 52. | Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 53. | Yoo KS, Choi HS, Jun DW, Lee HL, Lee OY, Yoon BC, Lee KG, Paik SS, Kim YS, Lee J. MUC Expression in Gallbladder Epithelial Tissues in Cholesterol-Associated Gallbladder Disease. Gut Liver. 2016;10:851-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Hu FL, Chen HT, Guo FF, Yang M, Jiang X, Yu JH, Zhang FM, Xu GQ. Biliary microbiota and mucin 4 impact the calcification of cholesterol gallstones. Hepatobiliary Pancreat Dis Int. 2021;20:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Yang L, Junmin S, Hong Y, Shuodong W. PGE(2) induces MUC2 and MUC5AC expression in human intrahepatic biliary epithelial cells via EP4/p38MAPK activation. Ann Hepatol. 2013;12:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Liu Z, Tian F, Feng X, He Y, Jiang P, Li J, Guo F, Zhao X, Chang H, Wang S. LPS increases MUC5AC by TACE/TGF-α/EGFR pathway in human intrahepatic biliary epithelial cell. Biomed Res Int. 2013;2013:165715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Osnes T, Sandstad O, Skar V, Osnes M. Lipopolysaccharides and beta-glucuronidase activity in choledochal bile in relation to choledocholithiasis. Digestion. 1997;58:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 59. | Peng Y, Yang Y, Liu Y, Nie Y, Xu P, Xia B, Tian F, Sun Q. Cholesterol gallstones and bile host diverse bacterial communities with potential to promote the formation of gallstones. Microb Pathog. 2015;83-84:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Wang L, Chen J, Jiang W, Cen L, Pan J, Yu C, Li Y, Chen W, Chen C, Shen Z. The Relationship between Helicobacter pylori Infection of the Gallbladder and Chronic Cholecystitis and Cholelithiasis: A Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol. 2021;2021:8886085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Belzer C, Kusters JG, Kuipers EJ, van Vliet AH. Urease induced calcium precipitation by Helicobacter species may initiate gallstone formation. Gut. 2006;55:1678-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Gutiérrez-Díaz I, Molinero N, Cabrera A, Rodríguez JI, Margolles A, Delgado S, González S. Diet: Cause or Consequence of the Microbial Profile of Cholelithiasis Disease? Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Sanders G, Kingsnorth AN. Gallstones. BMJ. 2007;335:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut. 2018;67:2213-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 65. | Serra N, Di Carlo P, D'Arpa F, Battaglia E, Fasciana T, Gulotta G, Maida CM, Rodolico V, Giammanco A, Sergi C. Human bile microbiota: A retrospective study focusing on age and gender. J Infect Public Health. 2021;14:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (2)] |
| 67. | Tazuma S, Unno M, Igarashi Y, Inui K, Uchiyama K, Kai M, Tsuyuguchi T, Maguchi H, Mori T, Yamaguchi K, Ryozawa S, Nimura Y, Fujita N, Kubota K, Shoda J, Tabata M, Mine T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol. 2017;52:276-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 68. | Frost F, Kacprowski T, Rühlemann M, Weiss S, Bang C, Franke A, Pietzner M, Aghdassi AA, Sendler M, Völker U, Völzke H, Mayerle J, Weiss FU, Homuth G, Lerch MM. Carrying asymptomatic gallstones is not associated with changes in intestinal microbiota composition and diversity but cholecystectomy with significant dysbiosis. Sci Rep. 2021;11:6677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Wang W, Wang J, Li J, Yan P, Jin Y, Zhang R, Yue W, Guo Q, Geng J. Cholecystectomy Damages Aging-Associated Intestinal Microbiota Construction. Front Microbiol. 2018;9:1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Yoon WJ, Kim HN, Park E, Ryu S, Chang Y, Shin H, Kim HL, Yi SY. The Impact of Cholecystectomy on the Gut Microbiota: A Case-Control Study. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Xu J, Ren X, Liu Y, Zhang Y, Chen G, Huang Q, Liu Q, Zhou J. Alterations of Fungal Microbiota in Patients With Cholecystectomy. Front Microbiol. 2022;13:831947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Shen H, Zhu J, Ye F, Xu D, Fang L, Yang J, Lv H, Lou Q, Jin H, Ni M, Zhang X. Biliary Microbial Structure of Gallstone Patients With a History of Endoscopic Sphincterotomy Surgery. Front Cell Infect Microbiol. 2020;10:594778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Liang T, Su W, Zhang Q, Li G, Gao S, Lou J, Zhang Y, Ma T, Bai X. Roles of Sphincter of Oddi Laxity in Bile Duct Microenvironment in Patients with Cholangiolithiasis: From the Perspective of the Microbiome and Metabolome. J Am Coll Surg. 2016;222:269-280.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Zhang Q, Ye M, Su W, Chen Y, Lou Y, Yang J, Ma T, Chen W, Gao S, Que R, Zhang B, Li H, Bai X, Liang T. Sphincter of Oddi laxity alters bile duct microbiota and contributes to the recurrence of choledocholithiasis. Ann Transl Med. 2020;8:1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Wang HH, Portincasa P, de Bari O, Liu KJ, Garruti G, Neuschwander-Tetri BA, Wang DQ. Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest. 2013;43:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Slattery C, Cotter PD, O'Toole PW. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 77. | Mandal H, Bagchi T. In Vitro Screening of Indigenous Lactobacillus Isolates for Selecting Organisms with Better Health-Promoting Attributes. Appl Biochem Biotechnol. 2018;185:1060-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | El-Dein AN, Nour El-Deen AM, El-Shatoury EH, Awad GA, Ibrahim MK, Awad HM, Farid MA. Assessment of exopolysaccharides, bacteriocins and in vitro and in vivo hypocholesterolemic potential of some Egyptian Lactobacillus spp. Int J Biol Macromol. 2021;173:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Oh JK, Kim YR, Lee B, Choi YM, Kim SH. Prevention of Cholesterol Gallstone Formation by Lactobacillus acidophilus ATCC 43121 and Lactobacillus fermentum MF27 in Lithogenic Diet-Induced Mice. Food Sci Anim Resour. 2021;41:343-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 80. | Ye X, Huang D, Dong Z, Wang X, Ning M, Xia J, Shen S, Wu S, Shi Y, Wang J, Wan X. FXR Signaling-Mediated Bile Acid Metabolism Is Critical for Alleviation of Cholesterol Gallstones by Lactobacillus Strains. Microbiol Spectr. 2022;10:e0051822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 81. | Ding L, Jiang J, Cheng L, Wang Y, Zhang W, Li D, Xu Z, Gao L, Li Z. Oral Administration of Nanoiron Sulfide Supernatant for the Treatment of Gallbladder Stones with Chronic Cholecystitis. ACS Appl Bio Mater. 2021;4:3773-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Huang F, Sun XY, Ouyang JM. Preparation and characterization of selenized Astragalus polysaccharide and its inhibitory effect on kidney stones. Mater Sci Eng C Mater Biol Appl. 2020;110:110732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Zhong Y, Xiao Q, Kang Z, Huang J, Ge W, Wan Q, Wang H, Zhou W, Zhao H, Liu D. Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int Immunopharmacol. 2022;111:109108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Zhuang Q, Ye X, Shen S, Cheng J, Shi Y, Wu S, Xia J, Ning M, Dong Z, Wan X. Astragalus Polysaccharides Ameliorate Diet-Induced Gallstone Formation by Modulating Synthesis of Bile Acids and the Gut Microbiota. Front Pharmacol. 2021;12:701003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Liao CH, Yong CY, Lai GM, Chow JM, Cheng CF, Fang CL, Lin PC, Chang CL, Zheng YM, Chuang SE, Whang-Peng J, Yao CJ. Astragalus Polysaccharide (PG2) Suppresses Macrophage Migration Inhibitory Factor and Aggressiveness of Lung Adenocarcinoma Cells. Am J Chin Med. 2020;48:1491-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |