Published online Jan 7, 2023. doi: 10.3748/wjg.v29.i1.75
Peer-review started: October 4, 2022
First decision: November 15, 2022
Revised: November 29, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 7, 2023
Processing time: 91 Days and 20.5 Hours
Nonalcoholic fatty liver disease (NAFLD), a leading chronic disease worldwide, affects approximately a quarter of the global population. Nonalcoholic steatohepatitis (NASH) is an advanced form of NAFLD and is more likely to progress to liver fibrosis than simple steatosis. NASH is also identified as the most rapidly growing cause of hepatocellular carcinoma. Although in the past decade, several phase II/III clinical trials have shown promising results in the use of novel drugs targeting lipid synthase, farnesoid X receptor signaling, peroxisome proliferator-activated receptor signaling, hepatocellular injury, and inflammatory signaling, proven pharmaceutical agents to treat NASH are still lacking. Thus, continuous exploration of the mechanism underlying the pathogenesis of NAFLD and the identification of novel therapeutic targets remain urgent tasks in the field. In the current review, we summarize studies reported in recent years that not only provide new insights into the mechanisms of NAFLD development but also explore the possibility of treating NAFLD by targeting newly identified signaling pathways. We also discuss evidence focusing on the intrahepatic targets involved in the pathogenesis of NAFLD as well as extrahepatic targets affecting liver metabolism and function.
Core Tip: Because of the urgent need to develop therapeutic approaches to treat nonalcoholic fatty liver disease (NAFLD), a large body of basic research has focused on the mechanisms of NAFLD to explore the possibility of new approaches to treat the disease. The current review summarizes studies reported in recent years that not only provide new insights into the mechanisms of NAFLD development but also explore the possibility of treating NAFLD by targeting newly identified signaling pathways. Evidence focusing on the intrahepatic targets involved in the pathogenesis of NAFLD as well as extrahepatic targets affecting liver metabolism and function are discussed.
- Citation: Wang GY, Zhang XY, Wang CJ, Guan YF. Emerging novel targets for nonalcoholic fatty liver disease treatment: Evidence from recent basic studies. World J Gastroenterol 2023; 29(1): 75-95
- URL: https://www.wjgnet.com/1007-9327/full/v29/i1/75.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i1.75
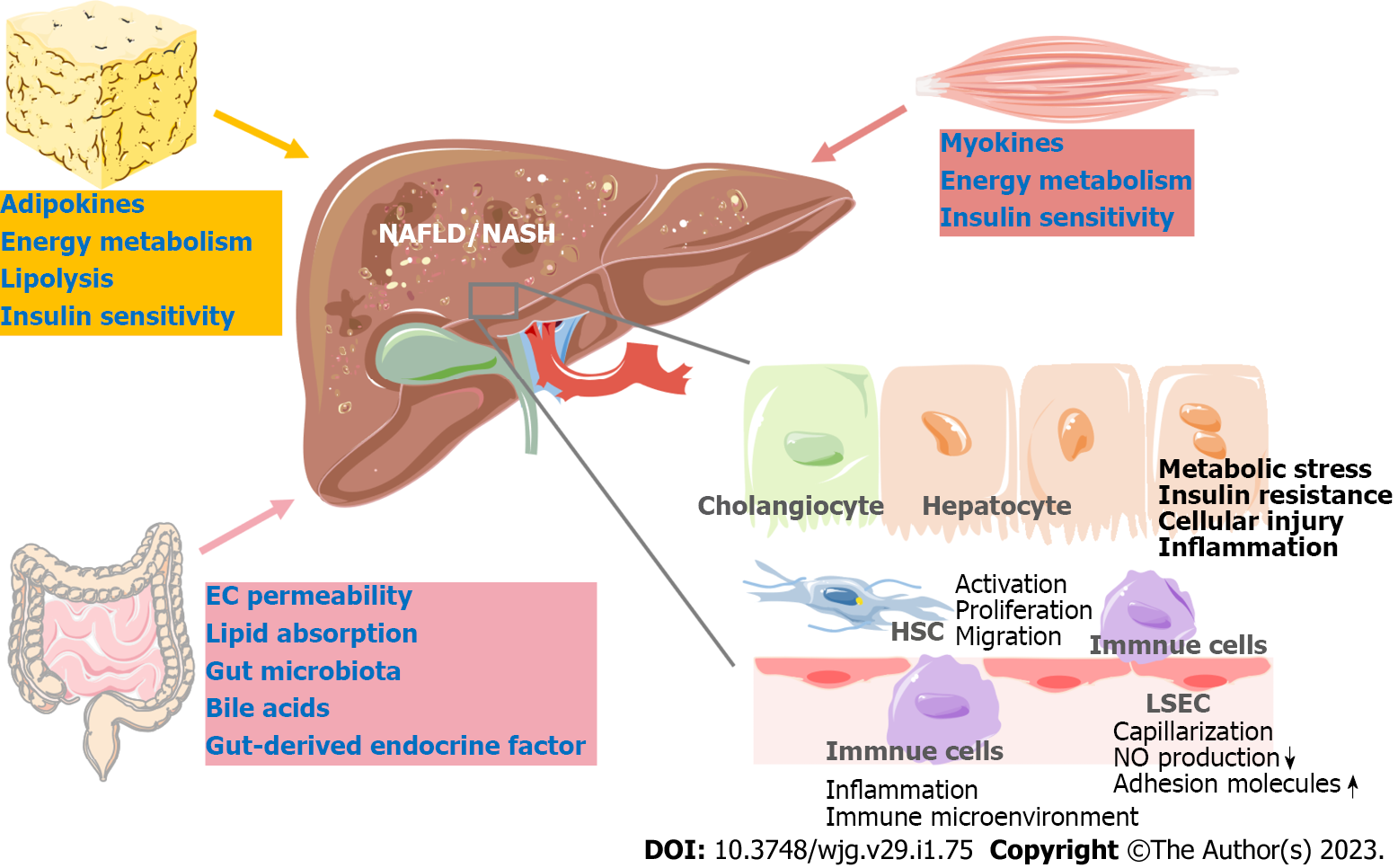
Nonalcoholic fatty liver disease (NAFLD) has become a leading chronic disease worldwide, affecting approximately a quarter of the global population. Nonalcoholic steatohepatitis (NASH), the advanced form of NAFLD, is closely related to liver fibrosis and even cirrhosis[1]. NASH has also been identified as the most rapidly growing cause of hepatocellular carcinoma (HCC) in liver transplant candidates in the United States[2]. Multiple factors, including disturbed lipid homeostasis, insulin resistance, and inflammation, lead to metabolic stress in hepatocytes and subsequent hepatocyte injury in NAFLD. Hepatocyte injury further triggers the wound-healing response, which involves immune cells, liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs) and cholangiocytes, eventually leading to liver inflammation and fibrosis[3].
Despite the increasing prevalence of NASH, the United States Food and Drug Administration (FDA) has not yet approved any pharmaceuticals to treat patients with NASH. The mainstay of the current clinical recommendation for patients with NASH is lifestyle modification, with optimization of dietary structure, control of excessive consumption of fructose and fats and increased physical exercise, which may alleviate the progression of NASH to a certain extent[4]. In addition, bariatric surgery may be indicated for some patients with NASH suffering from severe obesity[5]. Regarding the pharmacotherapy for NASH, several biological pathways critical for glycolipid and bile acid metabolism, inflammation, hepatocellular damage (oxidative stress) and liver fibrosis have been explored as drug targets. Pharmaceutical agents, including modulators of farnesoid X receptor, peroxisome proliferator-activated receptors (PPARs), fibroblast growth factor, acetyl-CoA carboxylase (ACC), and apoptosis signal-regulating kinase 1 (ASK1), have been shown to exhibit some positive effects against NAFLD/NASH with various limitations in multiple clinical trials[6]. Thus, there is still an unmet clinical need to identify and validate novel targets for the treatment of NAFLD/NASH.
Recently, numerous novel therapeutic targets for NAFLD have been explored through basic research. These studies have focused on different pathophysiologic processes in NAFLD, including metabolic stress, liver inflammation, liver fibrosis and NASH-associated HCC. Diverse liver cells have been studied, such as hepatocytes, liver immune cells, HSCs and LSECs (Figure 1). As a metabolic disorder, crosstalk between the liver and extrahepatic organs, including the gut, adipose tissue, and skeletal muscle, largely contributes to the pathogenesis of NAFLD[7,8]. Thus, targets outside the liver broaden potential approaches to ameliorate NAFLD (Figure 1). The drugs in phase II/III clinical trials were extensively discussed in a recently published review[6]. In the present review, we summarize recent findings that not only provide new insights into the mechanisms of NAFLD development but also explore the possibility of treating NAFLD by targeting novel signaling pathways.
Lipid accumulation in hepatocytes is a major feature of NAFLD. Disturbance of lipid and glucose metabolism causes metabolic stress in the liver. Therapeutic candidates directly regulating fatty acid metabolism that are currently in clinical trials include ACC, fatty acid synthase and stearoyl-CoA desaturase-1 inhibitors[6,9]. In addition to the targets acting on these lipid metabolic enzymes, in-depth basic research has uncovered multiple mechanisms underlying the metabolic disturbance during NAFLD, including but not limited to lipid, glucose, lactate and fructose metabolism, and has provided novel targets for NAFLD treatment.
Sterol regulatory element-binding proteins (SREBPs), including SREBP1a, SREBP1c and SREBP2, are key regulators mediating free fatty acid, triglyceride (TG) and cholesterol synthesis. 25-Hydroxanoanosterol (25-HL) is a newly identified SREBP inhibitor that induces the SCAP-INSIG interaction, which retains SREBPs in the endoplasmic reticulum (ER). 25-HL reduces hepatic TG and cholesterol levels and improves liver inflammation and fibrosis in western diet-fed mice[10]. Orosomucoid (ORM) 2, a secreted protein, has been shown to inhibit SREBP1c by activating AMP-activated protein kinase (AMPK). Recombinant ORM2 protein or stabilized ORM2–FC fusion protein inhibits lipogenesis and improves steatohepatitis[11].
Citrate regulates lipid metabolism as the substrate for lipogenesis. In the cytoplasm, citrate is catabolized by ATP-citrate lyase (ACLY) to generate acetyl-CoA and oxaloacetate. Acetyl-CoA is then converted to malonyl-CoA by ACC to fuel lipogenesis. The mitochondrial citrate carrier Slc25al plays an important role in regulating cytoplasmic and mitochondrial citrate pools. Tan and colleagues revealed that an inhibitor of Slc25a1, CTPI-2, inhibits the lipogenic pathway and prevents hepatic lipid accumulation in high-fat diet (HFD)-fed mice. Additionally, CTPI-2 also showed beneficial effects on liver inflammation[12]. ACLY, the enzyme mediating the production of acetyl-CoA from citrate, has been identified to be upregulated in the transition from simple steatosis to NASH[13]. Hepatocyte-specific deletion of ACLY reduces liver fatty acid and sterol synthesis, increases fatty acid oxidation, and attenuates glucose intolerance, liver steatosis, and ballooning[14]. Consistent with the observations following genetic inhibition, bempedoic acid, a pharmacological inhibitor of ACLY, also reduces fibrosis[14]. During the de novo lipogenesis process, nicotinamide adenine dinucleotide phosphate (NADPH), as an electron donor, aids in the reduction of acetyl-CoA. Thus, fine-tuning the NADPH pool can regulate lipogenesis. One-carbon units come largely from serine catabolism by the enzyme serine hydroxymethyltransferase (SHMT)[15]. Zhang et al[16] demonstrated that SHMT1-driven serine catabolism is a substantial NADPH source in the liver. They further found that inhibition of serine catabolism by Shmt1 gene knockout or pharmacological inhibition of SHMT1/2 enzymes significantly decreased hepatic lipogenesis[16]. Moreover, in sucrose-induced fatty liver, lipogenesis also requires NADPH from serine catabolism, which can be inhibited by the SHMT1/2 inhibitor SHIN2 IV. Therefore, SHMT1/2 inhibition has the potential to treat NAFLD by decreasing de novo lipogenesis.
As an energy source and an important glycolysis product, lactate is increased in the plasma and liver of NAFLD individuals. A recent study showed that the acetylation of lactate dehydrogenase B (LDHB) is markedly increased in the livers of mice with NAFLD, which leads to decreased LDHB activity and increased hepatic lactate levels. The authors further found that P300/CBP-associated factor (PCAF)-mediated LDHB acetylation at K82 exacerbates lipid accumulation and inflammatory responses in HFD-fed mice. Consistently, embelin, an inhibitor of PCAF, significantly improves hepatic steatosis and inflammation in NASH mice[17]. In addition, increasing evidence has demonstrated that overconsumption of fructose is associated with NAFLD. Fructose is metabolized to fructose-1-phosphate by ketohexokinase. PF-06835919 is a ketohexokinase inhibitor that showed protective effects against fructose-induced liver steatosis. Moreover, the safety of PF-06835919 has been verified in a phase I clinical trial[18].
Mitochondrial and ER functions are crucial in lipid and glucose metabolism, and mitochondrial dysfunction and ER stress are closely related to NAFLD[19]. Methylation-controlled J (MCJ) protein is located at the inner mitochondrial membrane and restrains mitochondrial respiration. Its expression level is found to be increased in NAFLD patients. MCJ deficiency reduces hepatic steatosis and improves liver fibrosis in methionine- and choline-deficient (MCD) diet-fed mice. In a recent study, lipid-nanoparticle-encapsulated (LNP) siRNA was employed to target MCJ in vivo. LNP-siMCJ increased the β-oxidation of fatty acids and ameliorated lipid accumulation and liver fibrosis in MCD diet- or high-fat/high-fructose diet-induced NASH mice. To further specifically target MCJ in hepatocytes, N-acetylgalactosamine (GalNAc)-modified siMCJ was used to treat NASH mice. Consistent with LNP-siMCJ, GalNAc-siMCJ also reduced liver steatosis and fibrosis[20]. In addition, low-dose sorafenib, the first small-molecule multi-kinase inhibitor, was reported to induce mitochondrial uncoupling and subsequently suppress free fatty acid-induced lipid accumulation and inflammation by activating AMPK in hepatocytes. Low-dose sorafenib protects high-fat/high-cholesterol diet-fed mice against liver steatosis, inflammation and fibrosis and prevents the onset of NASH-associated HCC in mice. Notably, the beneficial effects of low-dose sorafenib were also confirmed in NASH monkeys[21]. Moreover, cyclophilin D is located in the mitochondrial matrix and mediates mitochondrial permeability transition pore opening. Cyclophilin D is upregulated in HFD-fed mice, which induces mitochondrial stress and hepatic steatosis. Cyclosporine A, an inhibitor of cyclophilin D, decreased liver TG levels in HFD-fed mice by decreasing SREBP1c expression[22]. ER stress also plays important roles in NAFLD. The expression of forkhead box A3 (FOXA3) is induced by ER stress, and FOXA3 mediates ER stress-induced liver steatosis. As expected, targeting FOXA3 via a siRNA-based approach attenuates liver steatosis in HFD-fed mice[23].
It has been previously reported that the expression of DEAD-box protein 5 (DDX5), an ATP-dependent RNA helicase, is reduced in the livers of patients and mouse models with NASH, as well as in rodent models with NASH-HCC[24]. Hepatic DDX5 improves lipid metabolism by inhibiting mammalian target of rapamycin complex 1 (mTORC1) activation via recruitment of the tuberous sclerosis complex (TSC)1/2 complex to mTOR[24]. Through screening a natural compound library, hyperforcinol K was found to upregulate DDX5 expression by blocking tripartite motif protein 5-mediated ubiquitinated degradation of DDX5. In an animal study, hyperforcinol K was found to be effective in attenuating lipid accumulation in the livers of NASH mice[24].
Sterile 20-type kinase serine/threonine kinase 25 (STK25) has been demonstrated to play important roles in liver lipid partitioning[25,26] and systemic glucose and insulin homeostasis[27]. The level of STK25 protein is positively correlated with the development of NASH in patients[28]. Moreover, STK25 deficiency can protect against liver steatosis, inflammation, and fibrosis in mouse models of NASH-HCC[29]. STK25 antisense oligonucleotides (ASOs) significantly improved the NASH phenotype, and this preclinical validation of the effective metabolic efficacy of pharmacological inhibition of STK25 merits recognition[28].
Many nuclear receptors play critical roles in metabolic regulation. In current phase II and III trials, PPARs, farnesoid X receptor, and thyroid hormone receptor-β are promising candidate targets for NASH treatment[6]. Based on recent basic studies, modulation of retinoic acid receptor-related orphan receptor alpha (RORa) provides a novel target to treat NASH. RS-2982, a newly identified agonist of RORa, exhibited beneficial effects on reducing body weight and hepatic steatosis in HFD-fed mice. Furthermore, RS-2982 decreased alanine aminotransferase (ALT) and aspartate aminotransferase levels and reduced liver inflammation and fibrosis in mice fed an atherogenic diet. Mechanistically, an increase in miR-122 expression may account for these beneficial effects of RS-2982 on obesity and NAFLD/NASH[30].
Arachidonic acid (ARA) is an ω-6 long-chain polyunsaturated fatty acid (PUFA) that can be catalyzed into a series of bioactive eicosanoids via cycloxygenases (COX-1 and COX-2) and microsomal prostaglandin E synthases (mPGES-1 and mPGES-2). It has been recently reported that mPGES-2 deficiency exhibits significant protective effects against diet-induced NASH-associated phenotypes, including hepatic steatosis, inflammation and fibrosis. However, the beneficial effect of mPGES-2 deficiency against NAFLD is dependent on decreased cytochrome P450 4A14 (CYP4A14) and increased acyl-CoA thioesterase 4 levels but not PGE2[31]. Mechanistically, mPGES-2 binds with heme, which is released after mPGES2 inhibition and in turn activates the heme receptor nuclear receptor subfamily 1 group D member 1 (NR1D1) to upregulate CYP4A14 and acyl-CoA thioesterase 4 expression[31]. CYP4A14 catalyzes omega-hydroxylation of medium-chain fatty acids and ARA in mice. Its expression was previously found to be significantly increased in the livers of patients and mice with NAFLD. Loss of CYP4A14 function also markedly attenuated liver damage, inflammation, and fibrosis in MCD diet-induced NASH[32]. Moreover, both the mPGES-2 inhibitor SZ0232 and the CYP4A inhibitor TS-011 are capable of ameliorating the NASH phenotype in MCD diet-fed mice[31-33]. Taken together, these findings demonstrate that ARA metabolic enzymes play critical roles in liver lipid homeostasis and represent attractive targets for developing therapeutic drugs for NAFLD/NASH.
Emerging evidence suggests that novel therapeutic targets can be developed based on the discovery of genetic variants related to liver lipid metabolism, such as PNPLA3, TM6SF2, MBOAT7 and HSD17B13[34-37]. It has been reported that phosphorylation of HSD17B13 at serine 33 by PKA promotes lipolysis. Reproterol, a β2 agonist used for treating asthma, protects against the NASH phenotype via PKA-mediated Ser33 phosphorylation of 17β-HSD13[38]. Most recently, a variant in the pleckstrin and Sec7 domain-containing 3 (PSD3) gene, rs71519934, was reported to reduce susceptibility to fatty liver disease, consistent with the finding that the expression level of PSD3 is increased in patients with NAFLD and that knockdown of PSD3 by siRNA decreases TG synthesis in hepatocytes. Furthermore, specific downregulation of PSD3 in hepatocytes by GalNAc-conjugated ASOs resulted in decreased hepatic lipid content and plasma ALT levels and improved liver fibrosis in NASH mice[39], suggesting that PSD3 may be a potential therapeutic target for the treatment of NAFLD/NASH.
Lipid accumulation in hepatocytes can cause cytotoxicity (lipotoxicity) in NAFLD[40,41]. Hepatocyte injury is a key event during the development of NASH, and ballooned hepatocytes are an essential feature to diagnose NASH. In phase II/III clinical trials, pancaspase inhibitors and ASK1 inhibitors are currently under evaluation as therapeutic agents directly targeting apoptosis[6]. Studies focused on developing targets to prevent hepatocyte injury have also shown therapeutic effects in NASH.
Mitochondrial stress is a major factor driving hepatocyte injury and promotes the progression from simple steatosis to NASH. Mitochondrial matrix caseinolytic protease P (ClpP) is a protease component of the caseinolytic protease complex that maintains protein homeostasis in mitochondria. Its expression level is frequently downregulated in NASH, which induces mitochondrial stress and inflammation in hepatocytes. The ClpP activator A54556A is capable of greatly ameliorating the NASH phenotype in high-fat/high-fructose diet-fed mice[42]. SH3 homology-associated BTK binding protein (SAB) was originally identified as a phosphorylated c-Jun N-terminal kinase (p-JNK) docking protein and a substrate of JNK in the mitochondrial outer membrane. The interaction of JNK with SAB leads to increased mitochondrial reactive oxygen species (ROS) production and promotes liver injury. In established NASH, hepatocyte-targeted GalNAc-Sab-ASO treatment can reverse steatohepatitis and fibrosis by abrogating the adverse effects of the JNK-SAB-ROS activation loop[43]. Sirtuin activation plays an important role in maintaining mitochondrial homeostasis and shows protective effects on NAFLD[44]. Increased NAD+ levels can activate sirtuin. The enzyme α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase controls NAD+ levels, and its inhibitor TES-991 boosts de novo NAD+ synthesis and protects mice against liver injury and steatosis by improving mitochondrial function[45]. In addition, the serine/threonine protein phosphatase 2A inhibitors LB100[46], allyl isothiocyanate[47], celastrol[48] and the heme oxygenase inducer cobalt protoporphyrin[49] have all been found to significantly ameliorate NASH via the Sirt1 pathway. Nuclear factor erythroid 2–related factor 2 (Nrf2) is a well-known important transcription factor involved in the expression of antioxidant genes during oxidative stress. Targeted deletion of Nrf2 results in enhanced susceptibility to a variety of oxidative stress-induced liver injuries, including NASH. In contrast, pharmacological or genetic activation of hepatic Nrf2 leads to hepatic protection against oxidative damage. Studies have shown beneficial effects of hepatocyte Nrf2 activation in NASH[50]. Pharmacological activation of Nrf2 by the acetylenic tricyclic bis (cyano enone) compounds TBE-31[51], NK-252[52], and dimethyl fumarate[53] suppresses NASH. These findings demonstrate that strategies that increase Nrf2 expression and activity may be attractive strategies to limit the development of NAFLD/NASH by attenuating hepatocyte lipotoxicity and injury.
As mentioned above, apoptosis plays an important role in hepatocyte injury or loss during the development of NAFLD/NASH. BCL-2 belongs to the BCL-2 family, which is antiapoptotic. Through screening a small molecule library, acridone derivative A22 was found to increase BCL-2 expression by stabilizing the BCL-2 promoter i-motif. As expected, A22 can attenuate HFD-induced hepatocyte injury, hepatic steatosis and liver fibrosis[54]. In addition, necroptosis, another form of programmed cell death, was found to be increased in NASH mouse models and the livers of NAFLD patients. Receptor-interacting protein 3 (RIP3) and receptor-interacting protein kinase (RIPK) 1 are key mediators of necroptosis. Liver RIP3 deficiency can attenuate MCD diet-induced liver injury, steatosis, inflammation and fibrosis[55]. Similarly, the RIPK1 inhibitor RIPA-56 can reduce liver inflammation and fibrosis in HFD-fed mice by abrogating necroptosis. In addition, RIPA-56 is able to ameliorate hepatic steatosis by suppressing mixed lineage kinase domain-like protein levels[56].
Iron overload leads to hepatic oxidative stress and hepatocellular ballooning injury and plays a multifactorial role in the pathogenesis of NASH[57]. Approximately one-third of patients with NAFLD show interrupted iron homeostasis[58]. Ferroptosis is a nonapoptotic programmed cell death process characterized by iron-dependent and lipid peroxidation-associated cell death[59]. The ferroptosis inhibitors Trolox and deferiprone can protect hepatocytes from cell death and suppress the subsequent initiation of inflammation in fatty liver[60].
In contrast to hepatocytes, agents that induce HSC apoptosis show beneficial effects against NASH-related liver fibrosis[61]. Thus, when using pharmacological agents to prevent hepatocyte injury, strategies more accurately targeting hepatocytes may strengthen the therapeutic effects of these agents against NASH with limited side effects.
Inflammatory responses mediated by various immune cells and hepatocytes promote the onset of NASH and liver fibrosis. Infiltration of neutrophils and macrophages is the main pathological feature of NASH. Neutrophil depletion attenuates diet-induced NASH[62]. CXC chemokine receptor 2 (CXCR2) signaling is considered to play a pivotal role in the entry of neutrophils into peripheral tissues. Leslie and colleagues demonstrated that human and mouse livers with NASH-HCC have more CXCR2+ neutrophils[63]. The expression of CXCR2 in neutrophils is induced in NASH in an autocrine manner involving the upregulation of neutrophil-derived lipocalin 2[64]. AZD5069 is a small-molecule inhibitor of CXCR2. AZD5069 can significantly improve liver pathology in NAFLD, with reduced lipid content and hepatic neutrophil accumulation and improved insulin sensitivity[65]. In addition, AZD5069-mediated CXCR2 inhibition induces reprogramming of the tumor immune microenvironment, which promotes immune checkpoint inhibition in NASH-HCC[63], suggesting that blocking infiltrating neutrophil CXCR2 can restore sensitivity to immunotherapy in the NASH liver[66].
It is well known that the expression of macrophage scavenger receptor 1 (MSR1) mediates lipid uptake in macrophages and subsequently induces inflammatory activation via the JNK pathway. MSR1 was found to be correlated with liver inflammation in NAFLD patients[67]. Notably, therapeutic inhibition of MSR1 with an anti-MSR1 antibody improved hepatic steatosis in a mouse model of NASH. The number of F4/80-positive cells and the expression level of tumor necrosis factor alpha (TNF-α) were also found to be reduced by the anti-MSR1 antibody therapy[67].
It has long been recognized that the NOD-like receptor protein 3 (NLRP3) inflammasome is activated in NASH livers, which leads to increased inflammation and programmed cell death. MCC950, an NLRP3 inhibitor, can improve NAFLD pathology and fibrosis in obese diabetic mice[68]. X-box binding protein-1 (XBP1) is a key factor regulating the unfolded protein response. Macrophage XBP1 is upregulated in NASH livers, which activates macrophage NLRP3 signaling and promotes hepatocyte steatosis and HSC activation. As expected, the XBP1 inhibitor toyocamycin exhibits protective effects against NASH[69].
Nuclear factor-kappaB (NF-κB) is an essential transcription factor that mediates the inflammatory response and proinflammatory cytokine production in NASH[70]. miR-378 markedly facilitates the NF-κB-TNF-α axis in NASH development by directly targeting the Prkag2 gene, which encodes AMPK-γ2. Similarly, downregulation of miR-378 by ASO is also capable of alleviating NASH development[71].
In addition to neutrophils and macrophages, other immune cells, including dendritic cells (DCs) and lymphocytes, play pivotal roles in NASH[8]. DC recruitment to the liver promotes the inflammatory response in NAFLD progression. Reportedly, delivery of liposomal curcumin or calcitriol to lipid-rich inflammatory DCs shifted their inflammatory profile toward a regulatory phenotype and improved hepatic steatosis, inflammation and fibrosis in a NASH mouse model[72]. Inflammatory CX3CR1+ monocyte-derived inflammatory DCs (moDCs) are found to contribute to sustained inflammation and liver injury during NASH. Treating MCD-fed mice with the hydrogen sulfide donor i.e., sodium hydrosulphide, prevented the accumulation of CX3CR1+ moDCs and ameliorated parenchymal injury[73]. Moreover, Toll-like receptor 7 (TLR7) signaling can induce proinflammatory cytokine production in Kupffer cells and DCs, subsequently suppressing regulatory T cells (Tregs) and leading to steatohepatitis. Notably, treatment with IRS-661, a TLR7 antagonist, could ameliorate NASH development[74]. It has been reported that α4β7-mediated homing of CD4 T cells to the intestine and liver facilitates NASH development, and α4β7 blockade by neutralizing monoclonal antibody can attenuate hepatic inflammation and fibrosis and improve metabolic dysfunction associated with NASH[75]. In addition, a high level of Tregs reportedly promotes the initiation and progression of cancer in NASH livers. In a NASH-HCC model, anti-CD25 antibodies, capable of alternatively depleting Tregs, decreases the tumor burden and increased survival time[76]. The interaction of B2 Lymphocytes with T cells contributes to NASH progression, B-cell activating factor-neutralizing monoclonal antibody Sandy-2 prevented hepatic B2 cell response and ameliorated the evolution of NASH in mice[77].
Bioactive lipids are important nodes in lipid metabolism and tissue homeostasis networks[78]. An imbalance between protective and deteriorative bioactive lipids contributes to NASH progression[79]. Pharmacological targeting of the synthesizing enzymes or receptors of these bioactive lipids has shown beneficial effects in NASH. Leukotriene B4 (LTB4), a proinflammatory metabolite derived from the ω-6 PUFA ARA, is significantly increased in patients with NAFLD[80]. Both in vivo and in vitro experiments have shown that LTB4 can promote hepatocyte lipogenesis, which is dependent on the RNase activity of IRE1α through leukotriene B4 receptor 1 (Ltb4r1). Furthermore, LTB4/Ltb4r1 stimulation increases intracellular cAMP and then promotes IRE1α Ser724 phosphorylation by PKA[80]. The Ltb4r1 inhibitor CP-105696 significantly alleviated ER stress and dyslipidemia in NAFLD mice[80]. Unlike ω-6 PUFAs, ω-3 PUFAs and their metabolites show anti-inflammatory effects. Fat-1 mice, a transgenic animal model in which tissues are endogenously enriched with ω-3 PUFAs[81], are protected from diet-induced metabolic dysfunction and fibrosis[82]. However, they are vulnerable to lipid peroxidation, which limits their clinical applications[83]. Fraser et al[84] developed a structurally modified ω-3 fatty acid, icosabutate, which was designed to resist oxidation and incorporation into hepatocytes and possesses the potential to activate free fatty acid receptor 4. They found that icosabutate, but not the ω-3 PUFA eicosapentaenoic acid, can ameliorate liver inflammation and fibrosis in NASH rats. Moreover, icosabutate treatment decreases liver injury in patients at high risk of NASH and cardiovascular disease[84].
In clinical trials for NASH drug development, improvement of liver fibrosis has been frequently employed as a primary or secondary endpoint. Given the central role of HSCs in liver fibrosis, targeting HSC activation has long been proposed as a therapeutic strategy to prevent NASH-related fibrosis progression.
The Notch, Hedgehog, Hippo and WNT/β-catenin signaling pathways in hepatocytes play important roles in NASH-related liver fibrosis by modulating the hepatic microenvironment. The production of osteopontin (OPN), as a paracrine factor, from hepatocytes or cholangiocytes increases in NASH, which leads to the activation of HSCs[85-87]. Increased Notch signaling activity has been found to be responsible for the induction of OPN[85]. Thus, several studies have targeted Notch signaling to treat NASH, including studies on Nicastrin ASO and nanoparticle-mediated delivery systems targeting Notch antagonism[85,88]. In addition, nuclear factor of activated T-cell 4 (NFATc4) was also reported to induce OPN expression by negatively regulating the transcriptional activity of PPARα. As expected, inhibition of NFATc4 decreased lipid content and improved inflammation and fibrosis in NASH mice[89]. Hepatocyte transcriptional co-activator with PDZ-binding motif (TAZ) was found to promote NASH-related liver fibrosis by increasing Indian hedgehog, which activates HSCs[90]. A recent study reported that stabilized GalNAc-siRNAs targeting hepatocyte TAZ can significantly ameliorate liver inflammation and fibrosis in mice with established NASH[91]. Furthermore, WNT1-inducible signaling pathway protein 1 (WISP1), a member of the cellular communication network family, has recently been identified as an extracellular activator of the myocardin-related transcription factor-cytoskeleton pathway in HSCs. Activation of the WISP1-MRIF signaling pathway induces HSC migration and promotes liver fibrosis progression. Notably, an anti-WISP1 antibody significantly ameliorated liver fibrosis in NASH mice[92].
Lnterleukin-11 (IL-11) has been demonstrated to activate HSCs. Deletion of IL-11 receptor subunit alpha (IL-11RA) protects mice from NASH diet-induced hepatocyte death, liver inflammation and fibrosis. Widjaja et al[93] developed a neutralizing anti-IL-11 antibody and neutralizing anti-IL-11RA antibody and found that both of them greatly decreased ALT levels and significantly improved hepatic steatosis and liver fibrosis in mouse models of NASH[93].
Activation of the JAK/signal transducer and activator of transcription (STAT) pathway in HSCs promotes liver fibrosis. Ruxolitinib, an effective small-molecule JAK1/2 selective inhibitor, has been approved by the FDA for myelofibrosis treatment. Recently, it has been reported that ruxolitinib can block HSC activation and attenuate liver fibrosis progression[94]. In addition, the JAK2 inhibitor pacritinib affords protection against NAFLD-related liver fibrosis by inhibiting HSC activation[95]. Rilpivirine, a nonnucleoside reverse transcriptase inhibitor, is widely used to treat HIV infection. Rilpivirine can also ameliorate liver fibrosis, possibly through selective STAT1-dependent induction of apoptosis in HSCs, and suppress HFD- and CCl4-induced liver fibrosis. In addition, rilpivirine enhances STAT3-dependent proliferation in hepatocytes as an effect secondary to its pro-apoptotic effect in HSCs[96].
Protease-activated receptor-2 (PAR2) is an emerging new target for NASH that regulates liver injury, inflammation and fibrosis and plays a critical role in regulating hepatic cholesterol and glucolipid metabolism[97-99]. Pharmacological inhibition of PAR2 with pepducin PZ-235, a full antagonist of PAR2, not only protects against the activation of HSCs and fibrosis but also promotes hepatocellular viability by inhibiting mitochondrial ROS production induced by PAR2 stimulation[97].
LSECs are highly specialized endothelial cells with fenestrae and lack a basement membrane. LSECs interact with hepatocytes, liver immune cells and HSCs and play important roles in regulating liver function. LSEC dysfunction, including LSEC capillarization, disturbed nitric oxide release from LSECs, and increased expression of adhesion molecules, all contribute to NAFLD pathogenesis[100]. Vascular cell adhesion molecule 1 (VCAM-1) is an adhesion molecule upregulated in LSECs in NASH mouse livers. Anti-VCAM1 antibody attenuates hepatic inflammation in NASH mice[101]. Endothelial nitric oxide synthase (eNOS) expression in LSECs is decreased in NASH mice, which is likely the result of Notch activation. Pharmacological inhibition of Notch signaling using DAPT and LY3039478, as well as the eNOS activator YC-1, improved the NASH phenotype in MCD diet-fed mice[102]. Although the role of LESCs in NAFLD has been increasingly emphasized, the underlying mechanisms and therapeutic approaches targeting LESCs need more exploration.
Cholangiocytes also play an important role in NASH development. In addition to hepatocytes, OPN is strongly expressed in cholangiocytes in NASH livers. Thus, it is highly possible that cholangiocyte-derived OPN can promote liver fibrosis by activating HSCs[87]. Cardiotrophin-like cytokine factor 1 is another cholangiocyte-derived paracrine factor that exerts beneficial effects in NASH. Downregulation of a subunit of its receptor complex leukemia inhibitory factor receptor contributes to the progression of NASH[103]. Compared with other nonparenchymal cells, there are fewer studies addressing the effects of cholangiocytes on NASH, and a related therapeutic approach is still lacking.
It is well-established that multiple organs form a network to regulate whole-body energy metabolism. Extrahepatic organs influence liver function in an endocrine manner by releasing hormones or inflammatory factors or by regulating systemic metabolic homeostasis such as energy expenditure and insulin sensitivity. Thus, targeting these extrahepatic organs provides additional options for treating NAFLD/NASH.
Diets with reduced contents of sugars, refined carbohydrates and saturated fat are recommended to treat NAFLD[104]. Moreover, exercise is considered as an effective strategy for preventing and treating NAFLD via metabolic pathways and interorgan crosstalk[105]. Glucagon-like peptide (GLP)-1 receptor agonists, which enhance insulin secretion and reduce food intake, show beneficial effects against NASH in clinical trials[6]. In addition, fibroblast growth factor 21 analogs improved systemic energy metabolism and demonstrated potential in NASH therapy[6]. In a recent basic study, a novel Zn supplement, ulvan oligosaccharide (UO)-Zn, can activate AMPK, leading to improved metabolic pathways in HFD-fed mice[106]. The increased hepatic expression of branched chain ketoacid dehydrogenase kinase (BDK) partly induced high levels of branched-chain amino acids (BCAA) in metabolic diseases[107-109]. Moreover, 3,6-dichlorobrenzo(b)thiophene-2-carboxylic acid, a small-molecule allosteric inhibitor of BDK, lowered levels of circulating BCAAs, reduced hepatic steatosis, and improved glucose tolerance in rodent models[107,108].
The crosstalk between the gut and liver is regarded as an important pathway in regulating hepatic functions. Intestinal inflammation and dysfunction of intestinal lipid and bile acid absorption, epithelial cell permeability and the microbiome affect liver function and contribute to NAFLD progression. The relationship between gut microbiota and potential therapeutic targets has been recently discussed in depth elsewhere[110,111]; thus, we only focus on other aspects of the gut-liver axis.
Serum ceramide levels are positively related to NAFLD, and intestine-derived ceramide has been demonstrated to promote NAFLD[112,113]. Intestinal hypoxia-inducible factor 2α (HIF-2α) and myelocytomatosis oncogene (MYC) affect NAFLD by regulating ceramide metabolism. Intestinal HIF-2α, but not HIF-1α, is activated in obese patients and mice. Intestine-specific HIF-2α knockout improves hepatic steatosis by decreasing intestine-derived ceramide levels. Mechanistically, HIF-2α transcriptionally upregulates the expression of Neu3, a key enzyme in the ceramide salvage pathway. Targeting HIF-2α with its inhibitor PT2385 significantly attenuated hepatic lipid accumulation and decreased plasma ALT levels in HFD-fed mice[112]. Moreover, oral administration of PT2385 contributes to reduced body weight and improved insulin sensitivity[112]. Intestinal MYC expression is positively related to body mass index and serum ALT levels in humans. Genetic ablation of MYC in the intestine suppressed obesity and hepatic steatosis and led to decreased ceramide levels in HFD-fed mice. Ceramide synthase 4 has been further demonstrated to be a target gene of MYC. In line with this finding, pharmacological inhibition of MYC with 10058-F4 was found to ameliorate the metabolic disorders and liver fibrosis induced by HFD feeding[114]. In addition, increased intestinal ceramide also meditates the acceleration of NASH induced by nicotine[115].
Gut-derived 5-hydroxytryptamine (5-HT) has been reported to promote lipogenesis in the liver. Moreover, liver-specific knockout of the 5-HT receptor 2α (Htr2α) gene significantly lowered liver lipid content in HFD-fed mice and decreased the expression of genes involved in lipogenesis. To evaluate the potential of targeting the gut-5-HT-liver Htr2α pathway as a therapeutic strategy for NAFLD, the 5-HT receptor HTR2A antagonist sarpogrelate was used to treat HFD-fed mice. The results showed that sarpogrelate greatly ameliorates hepatic steatosis in NAFLD mice[116].
Yan et al[117] recently clarified that gut PPARα activation promotes NASH development by inducing fatty acid binding protein 1 expression, thereby aggravating fatty acid absorption in the small intestine[117]. GW6471, a PPARα-specific antagonist, was found to accumulate in the small intestine at much higher levels than in the liver. Intestine-specific PPARα deficiency and gut PPARα antagonism by GW6471 both improve NASH phenotypes[117]. The pleotropic roles of PPARα in different tissues in modulating NASH are worth discussing. Hepatocyte PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD[118]. Therapeutic strategies to prevent or treat NAFLD by activating hepatocyte PPARα and/or inhibiting gut PPARα may help guide drug delivery. Monoacylglycerol acyltransferase 2 (MGAT2) mediates the conversion of monoacylglycerol to diacylglycerol and is critical for dietary fat absorption. In the liver, MGAT2 regulates TG synthesis. BMS-963272, a potent MGAT2 inhibitor, improves the NAFLD activity score and liver fibrosis in NAFLD/NASH mice[119]. Of note, its safety has been confirmed in phase 1 clinical trials[119].
In addition, a long-acting dual agonist of GLP-1 and GLP-2 receptors, GLP1/2-Fc, has been found to greatly alter the microbiome composition and exhibits positive effects on gastrointestinal volume and the intestinal barrier. More importantly, GLP1/2-Fc can significantly ameliorate NASH phenotypes, including hepatic fat accumulation, inflammation, fibrosis and insulin tolerance[120], and its effects are superior to those of liraglutide, which has minimal efficacy on inflammation or fibrosis[121].
Recently, several novel adipokines have been identified and verified to play important roles in NASH development and progression by mediating the crosstalk between adipose tissue and the liver. Isthmin-1 (ISM1) was identified as an adipokine that can activate phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-protein kinase B (Akt) signaling independent of insulin. ISM1 gene expression is positively correlated with body mass index, and its deficiency leads to glucose intolerance via inhibition of PI3K-Akt signaling. In addition, ISM1 suppressed de novo lipogenesis in the liver. Recombinant ISM1 protein exhibited beneficial effects on both glucose tolerance and hepatic steatosis in NAFLD mice[122]. Recently, Liu et al[123] demonstrated that secreted protein acidic and rich in cysteine-like protein 1 (Sparcl1), which is a white adipose tissue-secreted protein, is correlated with hepatic pathological features in NASH patients. The authors further found that sparcl1 can induce liver inflammation by increasing C-C motif chemokine ligand 2 expression in the liver. To further determine the role of sparcl1 in the pathogenesis of NASH, the authors developed a neutralizing antibody against sparcl1 and found that the sparcl1-neutralizing antibody markedly ameliorated liver inflammation and liver fibrosis in NASH mice[123]. Gremlin 1, a newly identified adipokine, is positively related to insulin resistance and NAFLD/NASH. Treatment with recombinant Gremlin 1 protein impairs insulin sensitivity in various cell types, including human primary adipocytes, skeletal muscle cells, and liver cells. The insulin-sensitizing effect of the neutralizing anti–Gremlin 1 antibody indicates its beneficial effects on insulin resistance and NAFLD/NASH[124]. Neuregulin 4 (NRG4) is an adipose tissue-derived endocrine factor that has been demonstrated to suppress NASH-associated HCC by restraining the tumor-prone liver immune microenvironment. A recombinant fusion protein comprising amino acids 1–55 of human NRG4 and the Fc domain of immunoglobulin G 1 was found to suppress HCC induced by a NASH diet plus oncogene overexpression[125].
SWELL1/LRRC8a, a leucine-rich repeat-containing transmembrane protein, functionally encodes an ion channel signaling complex on the adipocyte plasma membrane[126]. Adipocyte SWELL1 is dispensable for adipose development[127]. SWELL1 protein is reduced in the adipocytes of type 2 diabetic animals. The small molecule SN-401, which binds at a constriction point within the SWELL hexamer, can increase adipocyte SWELL1 protein expression and SWELL1-dependent insulin signaling. Similarly, SN-401 can normalize glucose tolerance by augmenting tissue glucose uptake, suppressing hepatic glucose production, increasing serum fibroblast growth factor 21 levels, and reducing hepatic steatosis and hepatocyte ballooning in obese type 2 diabetic mice[128]. Taken together, these findings demonstrate that targeting the adipose tissue-liver axis may be an attractive strategy to treat NAFLD/NASH.
The crosstalk between skeletal muscle and the liver also impacts NAFLD progression. As one of the energy metabolism organs, skeletal muscle can affect liver lipid and glucose metabolism by regulating systemic metabolic homeostasis. In addition, decreased skeletal muscle mass and altered myokine secretion are associated with exacerbation of NAFLD progression[129,130]. Myostatin signaling negatively regulates muscle mass and is positively related to liver fibrosis[131]. Irisin (encoded by fibronectin type III domain containing 5, Fndc5), a myokine induced by exercise, is also expressed in adipose tissue and the liver. Serum irisin levels are inversely associated with TG content in the livers of obese adults[132]. Recombinant Fndc5/irisin significantly improved the NASH phenotype in HFD-fed mice[133]. The administration of nicotinamide riboside can alleviate obesity and steatosis by increasing irisin levels in HFD-fed mice[133]. Collectively, therapeutic strategies to improve skeletal muscle quantity and quality or target myokine signaling may provide promising options to treat NASH, but basic studies to collect more evidence are warranted.
Because of the urgent need to develop therapeutic approaches to treat NAFLD/NASH and the complexity of the pathophysiologic process of NAFLD, a large body of basic research has focused on the mechanisms of NAFLD to explore the possibility of applying new approaches to treat the disease (Tables 1 and 2). Metabolic stress-induced hepatocyte injury is an initial factor driving NASH development. Thus, in hepatocytes, strategies for treating NASH aim to improve metabolic disturbance and protect hepatocytes from injury. Alteration of the liver microenvironment and the interaction between diverse liver cells promote liver inflammation and fibrosis. Therefore, the inflammatory response of hepatocytes and immune cells in the liver is also an important target for NASH treatment. Strategies targeting HSC activation, proliferation and migration show beneficial effects on liver fibrosis and may become a novel way to attenuate NASH outcomes (Figure 1). As an indispensable part of hepatic metabolic regulation, extrahepatic organs play an essential role in liver lipid homeostasis, and their dysfunction contributes to the development and progression of NAFLD/NASH. The gut, adipose tissue and skeletal muscle can functionally affect liver or whole-body energy metabolic homeostasis via various endocrine factors (Figure 1 and Table 2).
| Molecular target | Potential agent | Classification | Function | Effects on NAFLD | Ref. |
| SREBPs | 25-HL | Inhibitor | Decrease TG and cholesterol synthesis | -Hepatic steatosis | [10] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| ORM2 | Recombinant ORM2; ORM2-FC fusion protein | Recombinant protein | Inhibit lipogenesis | -Hepatic steatosis | [11] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| Slc25al | CTPI-2 | Inhibitor | Regulating cytoplasmic and mitochondrial citrate pool | -Hepatic steatosis | [12] |
| -Liver inflammation | |||||
| ACLY | Bempedoic acid | Inhibitor | Inhibit ACLY activity | -Hepatic steatosis | [14] |
| -Liver injury | |||||
| SHMT1/2 | SHIN2 IV | Inhibitor | Decrease serine catabolism derived NADPH | -Hepatic lipogenesis | [16] |
| PCAF | Embelin | Inhibitor | Inhibit acetylation of LDHB | -Hepatic steatosis | [17] |
| -Liver inflammation | |||||
| Ketohexokinase | PF-06835919 | Inhibitor | Regulating fructose metabolism | -Fructose-induced liver steatosis | [18] |
| Mitochondrial uncoupling | Sorafenib | Small molecule | Induce mitochondrial uncoupling and activate AMPK | -Hepatic steatosis | [21] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| -NASH-associated HCC | |||||
| MCJ | LNP-siMCJ; GalNAc-siMCJ | Nucleic acid-based therapy | Maintain mitochondrial function | +β-oxidation | [20] |
| -Hepatic steatosis | |||||
| -Liver fibrosis | |||||
| Cyclophilin D | CsA | Inhibitor | -Hepatic steatosis | [22] | |
| ClpP | A54556A | Activator | -Hepatic steatosis | [42] | |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| SAB | GalNAc-Sab ASO | Nucleic acid-based therapy | -Hepatic steatosis | [43] | |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| ACMSD | TES-991 | Inhibitor | -Hepatic steatosis | [45] | |
| -Liver inflammation | |||||
| FOXA3 | FOXA3 siRNA | Nucleic acid-based therapy | Attenuate ER stress induced liver steatosis | -Hepatic steatosis | [23] |
| TRIM5-DDX5 | Hyperforcinol K | N/A | Inhibit ubiquitinated degradation of DDX5 | -Hepatic steatosis | [24] |
| -Liver inflammation | |||||
| STK25 | STK25 ASO | Nucleic acid-based therapy | Regulating energy homeostasis | -Hepatic steatosis | [28] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| RORa | RS-2982 | Agonist | Increase miR-122 level | -Hepatic steatosis | [30] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| HSD17B13 | Reproterol | Small molecule | Induce the Ser33 phosphorylation of 17β-HSD13 protein | -Hepatic steatosis | [38] |
| -Liver fibrosis | |||||
| PSD3 | GalNAc-Psd3 ASO | Nucleic acid-based therapy | Decrease TG synthesis | -Hepatic steatosis | [39] |
| -Liver injury | |||||
| -Liver fibrosis | |||||
| Nrf2 | TBE-31; NK-252; Dimethyl fumarate | Activator | Reduce oxidative stress | -Hepatic steatosis | [51-53] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| BCL-2 | A22 | Small molecule | Anti-apoptosis | -Liver injury | [54] |
| -Liver fibrosis | |||||
| RIPK1 | RIPA-56 | Inhibitor | Abrogating necroptosis | -Hepatic steatosis | [56] |
| -Liver inflammation | |||||
| CXCR2 | AZD5069 | Inhibitor | Inhibit neutrophil infiltration | -Hepatic steatosis | [63,65] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| -NASH-associated HCC | |||||
| MSR1 | Anti-MSR1 antibody | Neutralizing antibody | Inhibit inflammatory response | -Hepatic steatosis | [67] |
| -Liver inflammation | |||||
| NLRP3 | MCC950 | Inhibitor | Inhibit NLRP3 activation | -Liver inflammation | [68] |
| -Liver fibrosis | |||||
| XBP1 | Toyocamycin | Inhibitor | -Hepatic steatosis | [69] | |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| DCs | Curcumin; calcitriol | Small molecule | Shift hepatic DC inflammatory profile toward a regulatory phenotype | -Hepatic steatosis | [72] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| Hydrogen sulfide | NaHS | Hydrogen sulfide donor | Prevent the accumulation of TNF-α-producing CX3CR1+ moDCs | -Liver injury | [73] |
| TLR7 | IRS-661 | Antagonist | Decrease proinflammatory cytokine production in Kupffer cells and DCs | -Hepatic steatosis | [74] |
| -Liver inflammation | |||||
| Integrin | Anti-α4β7 antibody | Neutralizing antibody | Decrease α4β7+ CD4 T-cell recruitment | -Hepatic steatosis | [75] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| Tregs | Anti-CD25 antibodies | Neutralizing antibody | Deplete Tregs | -NASH-associated HCC | [76] |
| BAFF | Sandy-2 | Neutralizing antibody | Prevent hepatic B2-cell responses | -Hepatic steatosis | [77] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| Notch | Nicastrin ASO | Nucleic acid-based therapy | Inhibit Notch signaling | -Liver fibrosis | [85] |
| NP- dibenzazepine | Inhibitor with target delivery system | -Liver fibrosis | [88] | ||
| TAZ | GalNAc-TAZ siRNA | Nucleic acid-based therapy | Inhibit TAZ in hepatocyte and inhibit HSC activation | -Liver inflammation | [91] |
| -Liver fibrosis | |||||
| WISP1 | Anti-WISP1 antibody | Neutralizing antibody | Inhibit HSC migration | -Liver fibrosis | [92] |
| IL-11 | Anti-IL-11 antibody | Neutralizing antibody | Inhibit HSC activation; inhibit hepatocyte injury | -Liver injury | [93] |
| -Liver fibrosis | |||||
| JAK1/2 | Ruxolitinib | Inhibitor | Inhibit HSC activation | -Liver fibrosis | [94] |
| JAK2 | Pacritinib | Inhibitor | Inhibit HSC activation | -Liver fibrosis | [95] |
| STAT1 | Rilpivirine | Inhibitor | Induce HSC apoptosis and promote hepatocyte proliferation | -Liver fibrosis | [96] |
| PAR2 | Pepducin PZ-235 | Antagonist | Inhibit HSC activation | -Liver injury | [97] |
| -Liver fibrosis | |||||
| VCAM-1 | Anti-VCAM-1 antibody | Neutralizing antibody | Suppress monocyte adhesion to LSEC | -Liver inflammation | [101] |
| -Liver fibrosis | |||||
| eNOS | YC-1 | Activator | Increase NO production from LSEC | -Hepatic steatosis | [102] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| Ltb4r1 | CP-105696 | Inhibitor | Inhibit the effects of LTB4 | -Hepatic steatosis | [80] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| mPGES-2 | SZ0232 | Inhibitor | Regulating ARA metabolism | -Hepatic steatosis | [31] |
| CYP4A | TS-011 | Inhibitor | -Hepatic steatosis | [32] | |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| N/A | Icosabutate | Structurally modified ω-3 fatty acid | Resist oxidation and activate free fatty acid receptor 4 | -Liver inflammation | [84] |
| -Liver fibrosis |
| Molecular target | Potential agent | Classification | Function | Effect | Ref. |
| AMPK | UO-Zn | Small molecule | Improve lipid metabolism | -Lipid accumulation in liver and circulation | [106] |
| BDK | BT2 | Inhibitor | Regulating BCAA metabolism | -Hepatic steatosis | [107,108] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| HIF-2α (gut) | PT2385 | Inhibitor | Decrease intestine-derived ceramide | -Hepatic steatosis | [112] |
| -Liver injury | |||||
| MYC (gut) | 10058-F4 | Inhibitor | Decrease intestine-derived ceramide | -Hepatic steatosis | [114] |
| -Liver injury | |||||
| -Liver fibrosis | |||||
| 5-HT(gut)/HTR2A | Sarpogrelate | Antagonist | Inhibit gut-5-HT-liver Htr2α pathway | -Hepatic steatosis | [116] |
| PPARα (gut) | GW6471 | Antagonist | Inhibit fatty acid absorption by intestine | -Hepatic steatosis | [117] |
| -Liver inflammation | |||||
| -Liver fibrosis | |||||
| MGAT2 | BMS-963272 | Inhibitor | Regulating fat absorption and liver TG synthesis | -Liver inflammation | [119] |
| -Liver fibrosis | |||||
| ISM1 (AT) | Recombinant Ism1 | Recombinant protein | Suppress lipogenesis in liver | -Hepatic steatosis | [122] |
| +Glucose tolerance | |||||
| Sparcl1 (AT) | Anti-Sparcl1 antibody | Neutralizing antibody | Decrease CCL2 expression in liver | -Liver inflammation | [123] |
| -Liver fibrosis | |||||
| Gremlin 1 (AT) | Anti-Gremlin 1 antibody | Neutralizing antibody | Increase insulin sensitivity | -Insulin resistance | [124] |
| NRG4 | hNRG4-Fc | Recombinant protein | Restrain the tumor-prone liver immune microenvironment | -NASH-associated HCC | [125] |
| SWELL1/LRRC8a (AT) | SN-401 | Small molecule | Enhance insulin sensitivity and secretion | +Insulin sensitivity | [128] |
| -Hepatic steatosis | |||||
| -Hepatocyte damage |
With the rapid development of research techniques, single-cell sequencing and spatial transcriptomics, single-cell proteomics and metabolomics have been widely used and will undoubtedly accelerate the discovery of novel NAFLD/NASH mechanisms and therapeutic targets. Drug libraries enable us to quickly find effective small molecules to target specific pathways identified in the basic research field. Endocrine factors that mediate the crosstalk between the liver and extrahepatic tissues provide an ideal target for developing neutralization antibodies or recombinant proteins for the treatment of NAFLD/NASH. In addition to traditional small molecules, neutralizing antibodies and recombinant proteins, nucleic acid-based therapy has become an attractive approach for the development of drugs for NAFLD/NASH patients. Nucleic acid-based therapeutics include ASOs, siRNAs, miRNAs and mRNAs with modifications to improve their stability, immunogenicity, and diversity[134]. Through nucleic acid-based approaches, therapeutic target genes can be specifically silenced or overexpressed in the liver. As an example, the safety and efficiency of HSD17B13 RNAi[36] and DGAT2 ASOs have been examined in a few clinical trials, which showed a positive impact on liver function and stiffness in patients with NASH[135].
To avoid side effects, efforts have been made to better target the liver or more accurately target specific hepatic cell types by exploring novel drug delivery systems. Based on the hepatocyte-specific expression of asialoglycoprotein receptor, conjugation of GalNAc with nucleic acids or nanoparticles can provide better targeting to hepatocytes. To specifically target HSCs, nanoparticles integrated with vitamin A, cyclic peptides, mannose 6-phosphate, and antibodies against synaptophysin have shown high affinity for HSCs[136,137]. By using these approaches, drugs can be more precisely delivered to HSCs to alleviate NASH-related liver fibrosis. For hepatic macrophages, nanoparticles modified with the phospholipid serine to mimic apoptotic cells can enhance uptake of the nanoparticles by hepatic macrophages[137]. However, challenges remain in targeting other cell types.
Finally, novel preclinical and in vitro models are required for drug screening for NASH. A 3D human liver model constructed via coculture of hepatocytes with non-parenchymal cells in a 3D collagen matrix has been shown to mimic NASH features when treated with free fatty acids and TNF-α[138]. Liver organoids from pluripotent stem cells or induced pluripotent stem cells also provide unique preclinical platforms to fill the gap between animal studies and clinical trials for therapeutic target validation and drug development[139].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katada K, Japan; Savari F, Iran S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 664] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 2. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 575] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 3. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 919] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 4. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 998] [Article Influence: 199.6] [Reference Citation Analysis (0)] |
| 5. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Yang YY, Xie L, Zhang NP, Zhou D, Liu TT, Wu J. Updates on novel pharmacotherapeutics for the treatment of nonalcoholic steatohepatitis. Acta Pharmacol Sin. 2022;43:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Arab JP, Arrese M, Trauner M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu Rev Pathol. 2018;13:321-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 8. | Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 295] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 9. | Loomba R, Mohseni R, Lucas KJ, Gutierrez JA, Perry RG, Trotter JF, Rahimi RS, Harrison SA, Ajmera V, Wayne JD, O'Farrell M, McCulloch W, Grimmer K, Rinella M, Wai-Sun Wong V, Ratziu V, Gores GJ, Neuschwander-Tetri BA, Kemble G. TVB-2640 (FASN Inhibitor) for the Treatment of Nonalcoholic Steatohepatitis: FASCINATE-1, a Randomized, Placebo-Controlled Phase 2a Trial. Gastroenterology. 2021;161:1475-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 10. | Jiang SY, Yang X, Yang Z, Li JW, Xu MQ, Qu YX, Tang JJ, Li YF, Wang L, Shao YW, Meng XY, Hu H, Song BL, Rao Y, Qi W. Discovery of an insulin-induced gene binding compound that ameliorates nonalcoholic steatohepatitis by inhibiting sterol regulatory element-binding protein-mediated lipogenesis. Hepatology. 2022;76:1466-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Zhou B, Luo Y, Ji N, Hu C, Lu Y. Orosomucoid 2 maintains hepatic lipid homeostasis through suppression of de novo lipogenesis. Nat Metab. 2022;4:1185-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Tan M, Mosaoa R, Graham GT, Kasprzyk-Pawelec A, Gadre S, Parasido E, Catalina-Rodriguez O, Foley P, Giaccone G, Cheema A, Kallakury B, Albanese C, Yi C, Avantaggiati ML. Inhibition of the mitochondrial citrate carrier, Slc25a1, reverts steatosis, glucose intolerance, and inflammation in preclinical models of NAFLD/NASH. Cell Death Differ. 2020;27:2143-2157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Ryaboshapkina M, Hammar M. Human hepatic gene expression signature of non-alcoholic fatty liver disease progression, a meta-analysis. Sci Rep. 2017;7:12361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Morrow MR, Batchuluun B, Wu J, Ahmadi E, Leroux JM, Mohammadi-Shemirani P, Desjardins EM, Wang Z, Tsakiridis EE, Lavoie DCT, Reihani A, Smith BK, Kwiecien JM, Lally JSV, Nero TL, Parker MW, Ask K, Scott JW, Jiang L, Paré G, Pinkosky SL, Steinberg GR. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab. 2022;34:919-936.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 15. | García-Cañaveras JC, Lancho O, Ducker GS, Ghergurovich JM, Xu X, da Silva-Diz V, Minuzzo S, Indraccolo S, Kim H, Herranz D, Rabinowitz JD. SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia. Leukemia. 2021;35:377-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 16. | Zhang Z, TeSlaa T, Xu X, Zeng X, Yang L, Xing G, Tesz GJ, Clasquin MF, Rabinowitz JD. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat Metab. 2021;3:1608-1620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Wang T, Chen K, Yao W, Zheng R, He Q, Xia J, Li J, Shao Y, Zhang L, Huang L, Qin L, Xu M, Zhang Z, Pan D, Li Z, Huang F. Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J Hepatol. 2021;74:1038-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Gutierrez JA, Liu W, Perez S, Xing G, Sonnenberg G, Kou K, Blatnik M, Allen R, Weng Y, Vera NB, Chidsey K, Bergman A, Somayaji V, Crowley C, Clasquin MF, Nigam A, Fulham MA, Erion DM, Ross TT, Esler WP, Magee TV, Pfefferkorn JA, Bence KK, Birnbaum MJ, Tesz GJ. Pharmacologic inhibition of ketohexokinase prevents fructose-induced metabolic dysfunction. Mol Metab. 2021;48:101196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Di Ciaula A, Passarella S, Shanmugam H, Noviello M, Bonfrate L, Wang DQ, Portincasa P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 20. | Barbier-Torres L, Fortner KA, Iruzubieta P, Delgado TC, Giddings E, Chen Y, Champagne D, Fernández-Ramos D, Mestre D, Gomez-Santos B, Varela-Rey M, de Juan VG, Fernández-Tussy P, Zubiete-Franco I, García-Monzón C, González-Rodríguez Á, Oza D, Valença-Pereira F, Fang Q, Crespo J, Aspichueta P, Tremblay F, Christensen BC, Anguita J, Martínez-Chantar ML, Rincón M. Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat Commun. 2020;11:3360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Jian C, Fu J, Cheng X, Shen LJ, Ji YX, Wang X, Pan S, Tian H, Tian S, Liao R, Song K, Wang HP, Zhang X, Wang Y, Huang Z, She ZG, Zhang XJ, Zhu L, Li H. Low-Dose Sorafenib Acts as a Mitochondrial Uncoupler and Ameliorates Nonalcoholic Steatohepatitis. Cell Metab. 2020;31:892-908.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 22. | Wang X, Du H, Shao S, Bo T, Yu C, Chen W, Zhao L, Li Q, Wang L, Liu X, Su X, Sun M, Song Y, Gao L, Zhao J. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology. 2018;68:62-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Liu C, Zhou B, Meng M, Zhao W, Wang D, Yuan Y, Zheng Y, Qiu J, Li Y, Li G, Xiong X, Bian H, Zhang H, Wang H, Ma X, Hu C, Xu L, Lu Y. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J Hepatol. 2021;75:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Ye S, Lu W, Zhong J, Leng Y, Yang T, Luo J, Xu W, Zhang H, Kong L. RNA helicase DEAD-box protein 5 alleviates nonalcoholic steatohepatitis progression via tethering TSC complex and suppressing mTORC1 signaling. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Amrutkar M, Kern M, Nuñez-Durán E, Ståhlman M, Cansby E, Chursa U, Stenfeldt E, Borén J, Blüher M, Mahlapuu M. Protein kinase STK25 controls lipid partitioning in hepatocytes and correlates with liver fat content in humans. Diabetologia. 2016;59:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Amrutkar M, Cansby E, Nuñez-Durán E, Pirazzi C, Ståhlman M, Stenfeldt E, Smith U, Borén J, Mahlapuu M. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J. 2015;29:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Amrutkar M, Chursa U, Kern M, Nuñez-Durán E, Ståhlman M, Sütt S, Borén J, Johansson BR, Marschall HU, Blüher M, Mahlapuu M. STK25 is a critical determinant in nonalcoholic steatohepatitis. FASEB J. 2016;30:3628-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Nuñez-Durán E, Aghajan M, Amrutkar M, Sütt S, Cansby E, Booten SL, Watt A, Ståhlman M, Stefan N, Häring HU, Staiger H, Borén J, Marschall HU, Mahlapuu M. Serine/threonine protein kinase 25 antisense oligonucleotide treatment reverses glucose intolerance, insulin resistance, and nonalcoholic fatty liver disease in mice. Hepatol Commun. 2018;2:69-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Kurhe Y, Caputo M, Cansby E, Xia Y, Kumari S, Anand SK, Howell BW, Marschall HU, Mahlapuu M. Antagonizing STK25 Signaling Suppresses the Development of Hepatocellular Carcinoma Through Targeting Metabolic, Inflammatory, and Pro-Oncogenic Pathways. Cell Mol Gastroenterol Hepatol. 2022;13:405-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Chai C, Cox B, Yaish D, Gross D, Rosenberg N, Amblard F, Shemuelian Z, Gefen M, Korach A, Tirosh O, Lanton T, Link H, Tam J, Permyakova A, Ozhan G, Citrin J, Liao H, Tannous M, Hahn M, Axelrod J, Arretxe E, Alonso C, Martinez-Arranz I, Betés PO, Safadi R, Salhab A, Amer J, Tber Z, Mengshetti S, Giladi H, Schinazi RF, Galun E. Agonist of RORA Attenuates Nonalcoholic Fatty Liver Progression in Mice via Up-regulation of MicroRNA 122. Gastroenterology. 2020;159:999-1014.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Zhong D, Cai J, Hu C, Chen J, Zhang R, Fan C, Li S, Zhang H, Xu Z, Jia Z, Guo D, Sun Y. Inhibition of mPGES-2 ameliorates NASH by activating NR1D1 via heme. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Zhang X, Li S, Zhou Y, Su W, Ruan X, Wang B, Zheng F, Warner M, Gustafsson JÅ, Guan Y. Ablation of cytochrome P450 omega-hydroxylase 4A14 gene attenuates hepatic steatosis and fibrosis. Proc Natl Acad Sci U S A. 2017;114:3181-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Li S, Su W, Zhang XY, Guan YF. [Arachidonic acid metabolism in liver glucose and lipid homeostasis]. Sheng Li Xue Bao. 2021;73:657-664. [PubMed] |
| 34. | Su W, Wang Y, Jia X, Wu W, Li L, Tian X, Li S, Wang C, Xu H, Cao J, Han Q, Xu S, Chen Y, Zhong Y, Zhang X, Liu P, Gustafsson JÅ, Guan Y. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2014;111:11437-11442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 35. | Kozlitina J. Genetic Risk Factors and Disease Modifiers of Nonalcoholic Steatohepatitis. Gastroenterol Clin North Am. 2020;49:25-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Zhang HB, Su W, Xu H, Zhang XY, Guan YF. HSD17B13: A Potential Therapeutic Target for NAFLD. Front Mol Biosci. 2021;8:824776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Su W, Mao Z, Liu Y, Zhang X, Zhang W, Gustafsson JA, Guan Y. Role of HSD17B13 in the liver physiology and pathophysiology. Mol Cell Endocrinol. 2019;489:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Su W, Wu S, Yang Y, Guo Y, Zhang H, Su J, Chen L, Mao Z, Lan R, Cao R, Wang C, Xu H, Zhang C, Li S, Gao M, Chen X, Zheng Z, Wang B, Liu Y, Liu Z, Wang Z, Liu B, Fan X, Zhang X, Guan Y. Phosphorylation of 17β-hydroxysteroid dehydrogenase 13 at serine 33 attenuates nonalcoholic fatty liver disease in mice. Nat Commun. 2022;13:6577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 39. | Mancina RM, Sasidharan K, Lindblom A, Wei Y, Ciociola E, Jamialahmadi O, Pingitore P, Andréasson AC, Pellegrini G, Baselli G, Männistö V, Pihlajamäki J, Kärjä V, Grimaudo S, Marini I, Maggioni M, Becattini B, Tavaglione F, Dix C, Castaldo M, Klein S, Perelis M, Pattou F, Thuillier D, Raverdy V, Dongiovanni P, Fracanzani AL, Stickel F, Hampe J, Buch S, Luukkonen PK, Prati D, Yki-Järvinen H, Petta S, Xing C, Schafmayer C, Aigner E, Datz C, Lee RG, Valenti L, Lindén D, Romeo S. PSD3 downregulation confers protection against fatty liver disease. Nat Metab. 2022;4:60-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537-2564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1300] [Cited by in RCA: 1198] [Article Influence: 299.5] [Reference Citation Analysis (36)] |
| 41. | Ioannou GN. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol Metab. 2016;27:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 42. | Choi SE, Hwang Y, Lee SJ, Jung H, Shin TH, Son Y, Park S, Han SJ, Kim HJ, Lee KW, Lee G, Kemper JK, Song HK, Kang Y. Mitochondrial protease ClpP supplementation ameliorates diet-induced NASH in mice. J Hepatol. 2022;77:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Win S, Min RWM, Zhang J, Kanel G, Wanken B, Chen Y, Li M, Wang Y, Suzuki A, Aung FWM, Murray SF, Aghajan M, Than TA, Kaplowitz N. Hepatic Mitochondrial SAB Deletion or Knockdown Alleviates Diet-Induced Metabolic Syndrome, Steatohepatitis, and Hepatic Fibrosis. Hepatology. 2021;74:3127-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Tang BL. Sirt1 and the Mitochondria. Mol Cells. 2016;39:87-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 45. | Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P, Giacchè N, Stokar-Regenscheit N, Legouis D, de Seigneux S, Ivanisevic J, Raffaelli N, Schoonjans K, Pellicciari R, Auwerx J. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 46. | Chen XY, Cai CZ, Yu ML, Feng ZM, Zhang YW, Liu PH, Zeng H, Yu CH. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol. 2019;25:6607-6618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Li CX, Gao JG, Wan XY, Chen Y, Xu CF, Feng ZM, Zeng H, Lin YM, Ma H, Xu P, Yu CH, Li YM. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J Gastroenterol. 2019;25:5120-5133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Zhang Y, Geng C, Liu X, Li M, Gao M, Fang F, Chang Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol Metab. 2017;6:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Liu X, Gao Y, Li M, Geng C, Xu H, Yang Y, Guo Y, Jiao T, Fang F, Chang Y. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. J Hepatol. 2015;63:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Mohs A, Otto T, Schneider KM, Peltzer M, Boekschoten M, Holland CH, Hudert CA, Kalveram L, Wiegand S, Saez-Rodriguez J, Longerich T, Hengstler JG, Trautwein C. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis. J Hepatol. 2021;74:638-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 51. | Sharma RS, Harrison DJ, Kisielewski D, Cassidy DM, McNeilly AD, Gallagher JR, Walsh SV, Honda T, McCrimmon RJ, Dinkova-Kostova AT, Ashford MLJ, Dillon JF, Hayes JD. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell Mol Gastroenterol Hepatol. 2018;5:367-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 52. | Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M, Mochizuki H. Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol. 2013;84:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Sano A, Kakazu E, Hamada S, Inoue J, Ninomiya M, Iwata T, Tsuruoka M, Sato K, Masamune A. Steatotic Hepatocytes Release Mature VLDL Through Methionine and Tyrosine Metabolism in a Keap1-Nrf2-Dependent Manner. Hepatology. 2021;74:1271-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Li X, Wang J, Gong X, Zhang M, Kang S, Shu B, Wei Z, Huang ZS, Li D. Upregulation of BCL-2 by acridone derivative through gene promoter i-motif for alleviating liver damage of NAFLD/NASH. Nucleic Acids Res. 2020;48:8255-8268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, Rodrigues CM. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci (Lond). 2015;129:721-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 56. | Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, Mestiri T, Lagouge M, Foufelle F, Ballenghien F, Ledent T, Moldes M, Cadoret A, Fouassier L, Delaunay JL, Aït-Slimane T, Courtois G, Fève B, Scatton O, Prip-Buus C, Rodrigues CMP, Housset C, Gautheron J. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J Hepatol. 2020;72:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 57. | Handa P, Morgan-Stevenson V, Maliken BD, Nelson JE, Washington S, Westerman M, Yeh MM, Kowdley KV. Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G117-G127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | Datz C, Müller E, Aigner E. Iron overload and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Wang S, Liu Z, Geng J, Li L, Feng X. An overview of ferroptosis in non-alcoholic fatty liver disease. Biomed Pharmacother. 2022;153:113374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 60. | Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, Imai H, Yuet-Yin Kok C, Okochi H, Nakano H, Miyajima A, Tanaka M. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 368] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 61. | Kawahara A, Kanno K, Yonezawa S, Otani Y, Kobayashi T, Tazuma S, Ito M. Depletion of hepatic stellate cells inhibits hepatic steatosis in mice. J Gastroenterol Hepatol. 2022;37:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Ou R, Liu J, Lv M, Wang J, Zhu L, Zhao L, Xu Y. Neutrophil depletion improves diet-induced non-alcoholic fatty liver disease in mice. Endocrine. 2017;57:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Leslie J, Mackey JBG, Jamieson T, Ramon-Gil E, Drake TM, Fercoq F, Clark W, Gilroy K, Hedley A, Nixon C, Luli S, Laszczewska M, Pinyol R, Esteban-Fabró R, Willoughby CE, Haber PK, Andreu-Oller C, Rahbari M, Fan C, Pfister D, Raman S, Wilson N, Müller M, Collins A, Geh D, Fuller A, McDonald D, Hulme G, Filby A, Cortes-Lavaud X, Mohamed NE, Ford CA, Raffo Iraolagoitia XL, McFarlane AJ, McCain MV, Ridgway RA, Roberts EW, Barry ST, Graham GJ, Heikenwälder M, Reeves HL, Llovet JM, Carlin LM, Bird TG, Sansom OJ, Mann DA. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. 2022;71:2093-2106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 64. | Ye D, Yang K, Zang S, Lin Z, Chau HT, Wang Y, Zhang J, Shi J, Xu A, Lin S. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol. 2016;65:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 65. | Phillips BE, Lantier L, Engman C, Garciafigueroa Y, Singhi A, Trucco M, Mantzoros C, Wasserman D, Giannoukakis N. Improvement in insulin sensitivity and prevention of high fat diet-induced liver pathology using a CXCR2 antagonist. Cardiovasc Diabetol. 2022;21:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 845] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 67. | Govaere O, Petersen SK, Martinez-Lopez N, Wouters J, Van Haele M, Mancina RM, Jamialahmadi O, Bilkei-Gorzo O, Lassen PB, Darlay R, Peltier J, Palmer JM, Younes R, Tiniakos D, Aithal GP, Allison M, Vacca M, Göransson M, Berlinguer-Palmini R, Clark JE, Drinnan MJ, Yki-Järvinen H, Dufour JF, Ekstedt M, Francque S, Petta S, Bugianesi E, Schattenberg JM, Day CP, Cordell HJ, Topal B, Clément K, Romeo S, Ratziu V, Roskams T, Daly AK, Anstee QM, Trost M, Härtlova A. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 68. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 829] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 69. | Wang Q, Zhou H, Bu Q, Wei S, Li L, Zhou J, Zhou S, Su W, Liu M, Liu Z, Wang M, Lu L. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J Hepatol. 2022;77:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 70. | Franceschetti L, Bonomini F, Rodella LF, Rezzani R. Critical Role of NFκB in the Pathogenesis of Non-alcoholic Fatty Liver Disease: A Widespread Key Regulator. Curr Mol Med. 2021;21:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Zhang T, Hu J, Wang X, Zhao X, Li Z, Niu J, Steer CJ, Zheng G, Song G. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J Hepatol. 2019;70:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 72. | Maradana MR, Yekollu SK, Zeng B, Ellis J, Clouston A, Miller G, Talekar M, Bhuyan ZA, Mahadevaiah S, Powell EE, Irvine KM, Thomas R, O'Sullivan BJ. Immunomodulatory liposomes targeting liver macrophages arrest progression of nonalcoholic steatohepatitis. Metabolism. 2018;78:80-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Sutti S, Locatelli I, Bruzzì S, Jindal A, Vacchiano M, Bozzola C, Albano E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin Sci (Lond). 2015;129:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Roh YS, Kim JW, Park S, Shon C, Kim S, Eo SK, Kwon JK, Lim CW, Kim B. Toll-Like Receptor-7 Signaling Promotes Nonalcoholic Steatohepatitis by Inhibiting Regulatory T Cells in Mice. Am J Pathol. 2018;188:2574-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, Kumar P, Smith T, Singhi AD, Nusrat A, Parkos CA, Monga SP, Czaja MJ, Anania FA, Raeman R. Blocking integrin α(4)β(7)-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020;73:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 76. | Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, Shen C, Hu Z, Beane J, Ansa-Addo EA, Huang H, Tian D, Tsung A. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 77. | Bruzzì S, Sutti S, Giudici G, Burlone ME, Ramavath NN, Toscani A, Bozzola C, Schneider P, Morello E, Parola M, Pirisi M, Albano E. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic Biol Med. 2018;124:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 78. | Wang C, Yang J, Zhang X. Editorial: The Role of Bioactive Lipids in Homeostasis and Pathology. Front Physiol. 2021;12:773632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 79. | Musso G, Cassader M, Paschetta E, Gambino R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:282-302.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 80. | Liu X, Wang K, Wang L, Kong L, Hou S, Wan Y, Ma C, Chen J, Xing X, Xing C, Jiang Q, Zhao Q, Cui B, Huang Z, Li P. Hepatocyte leukotriene B4 receptor 1 promotes NAFLD development in obesity. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Kaliannan K, Li XY, Wang B, Pan Q, Chen CY, Hao L, Xie S, Kang JX. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun Biol. 2019;2:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | López-Vicario C, Sebastián D, Casulleras M, Duran-Güell M, Flores-Costa R, Aguilar F, Lozano JJ, Zhang IW, Titos E, Kang JX, Zorzano A, Arita M, Clària J. Essential lipid autacoids rewire mitochondrial energy efficiency in metabolic dysfunction-associated fatty liver disease. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Duan J, Song Y, Zhang X, Wang C. Effect of ω-3 Polyunsaturated Fatty Acids-Derived Bioactive Lipids on Metabolic Disorders. Front Physiol. 2021;12:646491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 84. | Fraser DA, Wang X, Lund J, Nikolić N, Iruarrizaga-Lejarreta M, Skjaeret T, Alonso C, Kastelein JJP, Rustan AC, Kim YO, Schuppan D. A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH. J Hepatol. 2022;76:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Zhu C, Kim K, Wang X, Bartolome A, Salomao M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, Schwabe RF, Tabas I, Valenti L, Lavine JE, Pajvani UB. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 86. | Wang G, Duan J, Pu G, Ye C, Li Y, Xiu W, Xu J, Liu B, Zhu Y, Wang C. The Annexin A2-Notch regulatory loop in hepatocytes promotes liver fibrosis in NAFLD by increasing osteopontin expression. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 87. | Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 88. | Richter LR, Wan Q, Wen D, Zhang Y, Yu J, Kang JK, Zhu C, McKinnon EL, Gu Z, Qiang L, Pajvani UB. Targeted Delivery of Notch Inhibitor Attenuates Obesity-Induced Glucose Intolerance and Liver Fibrosis. ACS Nano. 2020;14:6878-6886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 89. | Du M, Wang X, Yuan L, Liu B, Mao X, Huang D, Yang L, Huang K, Zhang F, Wang Y, Luo X, Wang C, Peng J, Liang M. Targeting NFATc4 attenuates non-alcoholic steatohepatitis in mice. J Hepatol. 2020;73:1333-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, Tabas I. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016;24:848-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 320] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 91. | Wang X, Sommerfeld MR, Jahn-Hofmann K, Cai B, Filliol A, Remotti HE, Schwabe RF, Kannt A, Tabas I. A Therapeutic Silencing RNA Targeting Hepatocyte TAZ Prevents and Reverses Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol Commun. 2019;3:1221-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 92. | Xi Y, LaCanna R, Ma HY, N'Diaye EN, Gierke S, Caplazi P, Sagolla M, Huang Z, Lucio L, Arlantico A, Jeet S, Brightbill H, Emson C, Wong A, Morshead KB, DePianto DJ, Roose-Girma M, Yu C, Tam L, Jia G, Ramalingam TR, Marsters S, Ashkenazi A, Kim SH, Kelly R, Wu S, Wolters PJ, Feldstein AE, Vander Heiden JA, Ding N. A WISP1 antibody inhibits MRTF signaling to prevent the progression of established liver fibrosis. Cell Metab. 2022;34:1377-1393.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D'Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, Tripathi M, Lim SY, Shekeran SG, Chothani SP, Rabes A, Sombetzki M, Bruinstroop E, Min LP, Sinha RA, Albani S, Yen PM, Schafer S, Cook SA. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology. 2019;157:777-792.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 94. | Song Z, Liu X, Zhang W, Luo Y, Xiao H, Liu Y, Dai G, Hong J, Li A. Ruxolitinib suppresses liver fibrosis progression and accelerates fibrosis reversal via selectively targeting Janus kinase 1/2. J Transl Med. 2022;20:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 95. | Torres S, Ortiz C, Bachtler N, Gu W, Grünewald LD, Kraus N, Schierwagen R, Hieber C, Meier C, Tyc O, Joseph Brol M, Uschner FE, Nijmeijer B, Welsch C, Berres ML, Garcia-Ruiz C, Fernandez-Checa JC, Trautwein C, Vogl TJ, Zeuzem S, Trebicka J, Klein S. Janus kinase 2 inhibition by pacritinib as potential therapeutic target for liver fibrosis. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Martí-Rodrigo A, Alegre F, Moragrega ÁB, García-García F, Martí-Rodrigo P, Fernández-Iglesias A, Gracia-Sancho J, Apostolova N, Esplugues JV, Blas-García A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut. 2020;69:920-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 97. | Shearer AM, Rana R, Austin K, Baleja JD, Nguyen N, Bohm A, Covic L, Kuliopulos A. Targeting Liver Fibrosis with a Cell-penetrating Protease-activated Receptor-2 (PAR2) Pepducin. J Biol Chem. 2016;291:23188-23198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Shearer AM, Wang Y, Fletcher EK, Rana R, Michael ES, Nguyen N, Abdelmalek MF, Covic L, Kuliopulos A. PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and Akt interference. Hepatology. 2022;76:1778-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 99. | Rana R, Shearer AM, Fletcher EK, Nguyen N, Guha S, Cox DH, Abdelmalek M, Wang Y, Baleja JD, Covic L, Kuliopulos A. PAR2 controls cholesterol homeostasis and lipid metabolism in nonalcoholic fatty liver disease. Mol Metab. 2019;29:99-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Hammoutene A, Rautou PE. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70:1278-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 101. | Furuta K, Guo Q, Pavelko KD, Lee JH, Robertson KD, Nakao Y, Melek J, Shah VH, Hirsova P, Ibrahim SH. Lipid-induced endothelial vascular cell adhesion molecule 1 promotes nonalcoholic steatohepatitis pathogenesis. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 102. | Fang ZQ, Ruan B, Liu JJ, Duan JL, Yue ZS, Song P, Xu H, Ding J, Xu C, Dou GR, Wang L. Notch-triggered maladaptation of liver sinusoidal endothelium aggravates nonalcoholic steatohepatitis through endothelial nitric oxide synthase. Hepatology. 2022;76:742-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 103. | Liu T, Wang Q, Zhou L, Zhang P, Mi L, Qiu X, Chen Z, Kuang H, Li S, Lin JD. Intrahepatic paracrine signaling by cardiotrophin-like cytokine factor 1 ameliorates diet-induced NASH in mice. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Yki-Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18:770-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 105. | Gonzalez-Gil AM, Elizondo-Montemayor L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 106. | Chi Y, Wu Z, Du C, Zhang M, Wang X, Xie A, Wang P, Li R. Regulatory effects mediated by ulvan oligosaccharide and its zinc complex on lipid metabolism in high-fat diet-fed mice. Carbohydr Polym. 2023;300:120249. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, Grenier-Larouche T, An J, Lapworth AL, Astapova I, Hannou SA, George T, Arlotto M, Olson LB, Lai M, Zhang GF, Ilkayeva O, Herman MA, Wynn RM, Chuang DT, Newgard CB. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018;27:1281-1293.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 108. | Bollinger E, Peloquin M, Libera J, Albuquerque B, Pashos E, Shipstone A, Hadjipanayis A, Sun Z, Xing G, Clasquin M, Stansfield JC, Tierney B, Gernhardt S, Siddall CP, Greizer T, Geoly FJ, Vargas SR, Gao LC, Williams G, Marshall M, Rosado A, Steppan C, Filipski KJ, Zhang BB, Miller RA, Roth Flach RJ. BDK inhibition acts as a catabolic switch to mimic fasting and improve metabolism in mice. Mol Metab. 2022;66:101611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 109. | White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019;363:582-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 110. | Forlano R, Sivakumar M, Mullish BH, Manousou P. Gut Microbiota-A Future Therapeutic Target for People with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 111. | Liu Q, Liu S, Chen L, Zhao Z, Du S, Dong Q, Xin Y, Xuan S. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Exp Ther Med. 2019;18:1935-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Xie C, Yagai T, Luo Y, Liang X, Chen T, Wang Q, Sun D, Zhao J, Ramakrishnan SK, Sun L, Jiang C, Xue X, Tian Y, Krausz KW, Patterson AD, Shah YM, Wu Y, Gonzalez FJ. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat Med. 2017;23:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 113. | Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, Duan J, Sullards MC, Leiker TJ, Barkley RM, Quehenberger O, Armando AM, Milne SB, Mathews TP, Armstrong MD, Li C, Melvin WV, Clements RH, Washington MK, Mendonsa AM, Witztum JL, Guan Z, Glass CK, Murphy RC, Dennis EA, Merrill AH Jr, Russell DW, Subramaniam S, Brown HA. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 114. | Luo Y, Yang S, Wu X, Takahashi S, Sun L, Cai J, Krausz KW, Guo X, Dias HB, Gavrilova O, Xie C, Jiang C, Liu W, Gonzalez FJ. Intestinal MYC modulates obesity-related metabolic dysfunction. Nat Metab. 2021;3:923-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 115. | Chen B, Sun L, Zeng G, Shen Z, Wang K, Yin L, Xu F, Wang P, Ding Y, Nie Q, Wu Q, Zhang Z, Xia J, Lin J, Luo Y, Cai J, Krausz KW, Zheng R, Xue Y, Zheng MH, Li Y, Yu C, Gonzalez FJ, Jiang C. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature. 2022;610:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (1)] |
| 116. | Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, Lee HW, Han KH, Park S, Jeong JS, Bang G, Kim YH, Yadav VK, Karsenty G, Ju YS, Choi C, Suh JM, Park JY. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018;9:4824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 117. | Yan T, Luo Y, Yan N, Hamada K, Zhao N, Xia Y, Wang P, Zhao C, Qi D, Yang S, Sun L, Cai J, Wang Q, Jiang C, Gavrilova O, Krausz KW, Patel DP, Yu X, Wu X, Hao H, Liu W, Qu A, Gonzalez FJ. Intestinal peroxisome proliferator-activated receptor α-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 118. | Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C, Benhamed F, Iroz A, Bertrand-Michel J, Al Saati T, Cano P, Mselli-Lakhal L, Mithieux G, Rajas F, Lagarrigue S, Pineau T, Loiseau N, Postic C, Langin D, Wahli W, Guillou H. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 564] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 119. | Cheng D, Zinker BA, Luo Y, Shipkova P, De Oliveira CH, Krishna G, Brown EA, Boehm SL, Tirucherai GS, Gu H, Ma Z, Chu CH, Onorato JM, Kopcho LM, Ammar R, Smith J, Devasthale P, Lawrence RM, Stryker SA, Dierks EA, Azzara AV, Carayannopoulos L, Charles ED, Lentz KA, Gordon DA. MGAT2 inhibitor decreases liver fibrosis and inflammation in murine NASH models and reduces body weight in human adults with obesity. Cell Metab. 2022;34:1732-1748.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 120. | Kim ER, Park JS, Kim JH, Oh JY, Oh IJ, Choi DH, Lee YS, Park IS, Kim S, Lee DH, Cheon JH, Bae JW, Lee M, Cho JW, An IB, Nam EJ, Yang SI, Lee MS, Bae SH, Lee YH. A GLP-1/GLP-2 receptor dual agonist to treat NASH: Targeting the gut-liver axis and microbiome. Hepatology. 2022;75:1523-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 121. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1470] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 122. | Jiang Z, Zhao M, Voilquin L, Jung Y, Aikio MA, Sahai T, Dou FY, Roche AM, Carcamo-Orive I, Knowles JW, Wabitsch M, Appel EA, Maikawa CL, Camporez JP, Shulman GI, Tsai L, Rosen ED, Gardner CD, Spiegelman BM, Svensson KJ. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and hepatic steatosis. Cell Metab. 2021;33:1836-1852.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 123. | Liu B, Xiang L, Ji J, Liu W, Chen Y, Xia M, Liu Y, Zhu P, Jin Y, Han Y, Lu J, Li X, Zheng M, Lu Y. Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 124. | Hedjazifar S, Khatib Shahidi R, Hammarstedt A, Bonnet L, Church C, Boucher J, Blüher M, Smith U. The Novel Adipokine Gremlin 1 Antagonizes Insulin Action and Is Increased in Type 2 Diabetes and NAFLD/NASH. Diabetes. 2020;69:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 125. | Zhang P, Chen Z, Kuang H, Liu T, Zhu J, Zhou L, Wang Q, Xiong X, Meng Z, Qiu X, Jacks R, Liu L, Li S, Lumeng CN, Li Q, Zhou X, Lin JD. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab. 2022;34:1359-1376.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 126. | Gunasekar SK, Xie L, Sah R. SWELL signalling in adipocytes: can fat 'feel' fat? Adipocyte. 2019;8:223-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 127. | Zhang Y, Xie L, Gunasekar SK, Tong D, Mishra A, Gibson WJ, Wang C, Fidler T, Marthaler B, Klingelhutz A, Abel ED, Samuel I, Smith JK, Cao L, Sah R. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat Cell Biol. 2017;19:504-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 128. | Gunasekar SK, Xie L, Kumar A, Hong J, Chheda PR, Kang C, Kern DM, My-Ta C, Maurer J, Heebink J, Gerber EE, Grzesik WJ, Elliot-Hudson M, Zhang Y, Key P, Kulkarni CA, Beals JW, Smith GI, Samuel I, Smith JK, Nau P, Imai Y, Sheldon RD, Taylor EB, Lerner DJ, Norris AW, Klein S, Brohawn SG, Kerns R, Sah R. Small molecule SWELL1 complex induction improves glycemic control and nonalcoholic fatty liver disease in murine Type 2 diabetes. Nat Commun. 2022;13:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Chakravarthy MV, Siddiqui MS, Forsgren MF, Sanyal AJ. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne). 2020;11:592373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 130. | Kuchay MS, Martínez-Montoro JI, Kaur P, Fernández-García JC, Ramos-Molina B. Non-alcoholic fatty liver disease-related fibrosis and sarcopenia: An altered liver-muscle crosstalk leading to increased mortality risk. Ageing Res Rev. 2022;80:101696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 131. | García PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 132. | Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, Han CK, Zhuang XJ, Lu Y, Li XJ, Yang SY, Li XY. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 133. | Li DJ, Sun SJ, Fu JT, Ouyang SX, Zhao QJ, Su L, Ji QX, Sun DY, Zhu JH, Zhang GY, Ma JW, Lan XT, Zhao Y, Tong J, Li GQ, Shen FM, Wang P. NAD(+)-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics. 2021;11:4381-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 134. | Gupta A, Andresen JL, Manan RS, Langer R. Nucleic acid delivery for therapeutic applications. Adv Drug Deliv Rev. 2021;178:113834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 135. | Loomba R, Morgan E, Watts L, Xia S, Hannan LA, Geary RS, Baker BF, Bhanot S. Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 136. | Li Y, Pu S, Liu Q, Li R, Zhang J, Wu T, Chen L, Li H, Yang X, Zou M, Xiao J, Xie W, He J. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. J Control Release. 2019;303:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 137. | Kumar V, Xin X, Ma J, Tan C, Osna N, Mahato RI. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv Drug Deliv Rev. 2021;176:113888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 138. | Duriez M, Jacquet A, Hoet L, Roche S, Bock MD, Rocher C, Haussy G, Vigé X, Bocskei Z, Slavnic T, Martin V, Guillemot JC, Didier M, Kannt A, Orsini C, Mikol V, Fèvre AL. A 3D Human Liver Model of Nonalcoholic Steatohepatitis. J Clin Transl Hepatol. 2020;8:359-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 139. | Lee J, Mun SJ, Shin Y, Lee S, Son MJ. Advances in liver organoids: model systems for liver disease. Arch Pharm Res. 2022;45:390-400. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |