Published online Feb 21, 2022. doi: 10.3748/wjg.v28.i7.745
Peer-review started: September 13, 2021
First decision: October 16, 2021
Revised: November 11, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: February 21, 2022
Processing time: 157 Days and 1.1 Hours
Pancreatic cancer is a malignancy with one of the poorest prognoses amongst all cancers. Patients with unresectable tumours either receive palliative care or undergo various chemoradiotherapy regimens. Conventional techniques are often associated with acute gastrointestinal toxicities, as adjacent critical structures such as the duodenum ultimately limits delivered doses. Stereotactic body radiotherapy (SBRT) is an advanced radiation technique that delivers highly ablative radiation split into several fractions, with a steep dose fall-off outside target volumes.
To discuss the latest data on SBRT and whether there is a role for magnetic resonance-guided techniques in multimodal management of locally advanced, unresectable pancreatic cancer.
We conducted a search on multiple large databases to collate the latest records on radiotherapy techniques used to treat pancreatic cancer. Out of 1229 total records retrieved from our search, 36 studies were included in this review.
Studies indicate that SBRT is associated with improved clinical efficacy and toxicity profiles compared to conventional radiotherapy techniques. Further dose escalation to the tumour with SBRT is limited by the poor soft-tissue visualisation of computed tomography imaging during radiation planning and treatment delivery. Magnetic resonance-guided techniques have been introduced to improve imaging quality, enabling treatment plan adaptation and re-optimisation before delivering each fraction.
Therefore, SBRT may lead to improved survival outcomes and safer toxicity profiles compared to conventional techniques, and the addition of magnetic resonance-guided techniques potentially allows dose escalation and conversion of unresectable tumours to operable cases.
Core Tip: Locally advanced pancreatic cancer has very poor outcomes. These cases are treated with chemoradiotherapy regimens, but conventional radiotherapy techniques often yield minimal survival benefit while accruing significant toxicities. Stereotactic body radiotherapy (SBRT) is an advanced technique that is associated with improved survival outcomes and reduced toxicities compared to its predecessors. The addition of Magnetic resonance-guided techniques to SBRT provides excellent imaging that enables intra-treatment plan adaptations. This provides the possibility of dose escalation, which may be the key to achieving surgical resectability and thus potentially increasing the chances of cure.
- Citation: Ermongkonchai T, Khor R, Muralidharan V, Tebbutt N, Lim K, Kutaiba N, Ng SP. Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer. World J Gastroenterol 2022; 28(7): 745-754
- URL: https://www.wjgnet.com/1007-9327/full/v28/i7/745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i7.745
Pancreatic cancer is one of the leading causes of cancer deaths, with a 5-year overall survival (OS) rate of less than 10%[1]. Surgical resection is the only curative option, but is seldom feasible due to a lack of early detection markers, late presentation with locally advanced disease or the lesion being medically inoperable[2-6]. In the cohort of patients who received surgery in the PREOPANC-1 randomised trial, a subgroup analysis in patients with borderline resectable disease demonstrated a survival advantage in those receiving preoperative chemoradiotherapy compared to those receiving immediate surgery[7]. However, the data for definitive radiotherapy (RT) in unresectable pancreatic cancers is conflicting. Traditionally, locally advanced pancreatic cancers (LAPC) unsuitable for curative surgery are treated with chemotherapy regimens or conventionally fractionated radiotherapy (CFRT), or both[8]. However, the role of RT is controversial as radiation-induced toxicities remain a concern. Conventional radiotherapy is often associated with significant grade ≥ 3 toxicities while achieving a median OS of only 5 to 15 mo[2]. The LAP-07 trial demonstrated that the survival outcomes of those who received conventionally fractionated chemoradiotherapy is not superior to chemotherapy alone. However, despite its known caveats, the trial indicated that there is a benefit from RT in multimodal regimens in achieving improved local control (LC), which approached 70% at 12 mo[9].
Stereotactic body radiotherapy (SBRT) is an emerging RT technique due to its ability to deliver highly ablative radiation doses in several fractions[10]. A study by Park et al[4] found that the use of a five-fraction SBRT regimen achieved improved quality-of-life scores and tolerable acute toxicities, with comparable late grade ≥ 3 toxicities to intensity-modulated radiotherapy (IMRT) (15.9% SBRT vs 13.7% IMRT)[4]. But while SBRT strives for more accuracy and precision, there are some obstacles that prevent further dose escalation without compromising safety. First is the susceptibility of the pancreas to intra-fractional movement during respiratory cycles and digestion. Secondly, the adjacent surrounding organs-at-risk (OAR) which comprises of the stomach, duodenum and small intestine are highly radiosensitive, therefore care needs to be taken to limit doses to these structures to avoid significant treatment-related toxicity. And finally, the current imaging modalities and fiducial markers provide poor visualisation of targets during treatment planning[11].
The recent development of magnetic resonance-guided RT (MRgRT) provides potential to circumvent these challenges, as magnetic-resonance imaging (MRI) offers excellent soft-tissue contrast that can guide dosimetric adjustments to the target volume and limit OAR exposure. This review will evaluate the role of SBRT in the treatment of LAPC, its shortcomings, and present the potential use of MR-guided adaptive techniques to mitigate those caveats.
Searches were conducted in the online databases PubMed and Ovid (Medline) from August to September 2020, using Medical Subject Headings (MeSH) terms/keywords of pancreatic cancer, stereotactic, radiotherapy and magnetic-resonance. Records were included if it studied the treatment outcomes of SBRT and/or MRgRT in unresectable pancreatic cancers. The excluded literature were review articles or studies done on metastatic disease. Studies that involved resectable tumours or used chemoradiotherapy as adjuvant treatment post-surgery were also omitted. Only results in the English language were included. Additional literature was also sought from references of included studies. A final shortlist of studies was selected based on relevance. A study was considered as relevant if it investigated the effect of SBRT and/or MRgRT on any survival metric in patients with inoperable LAPC.
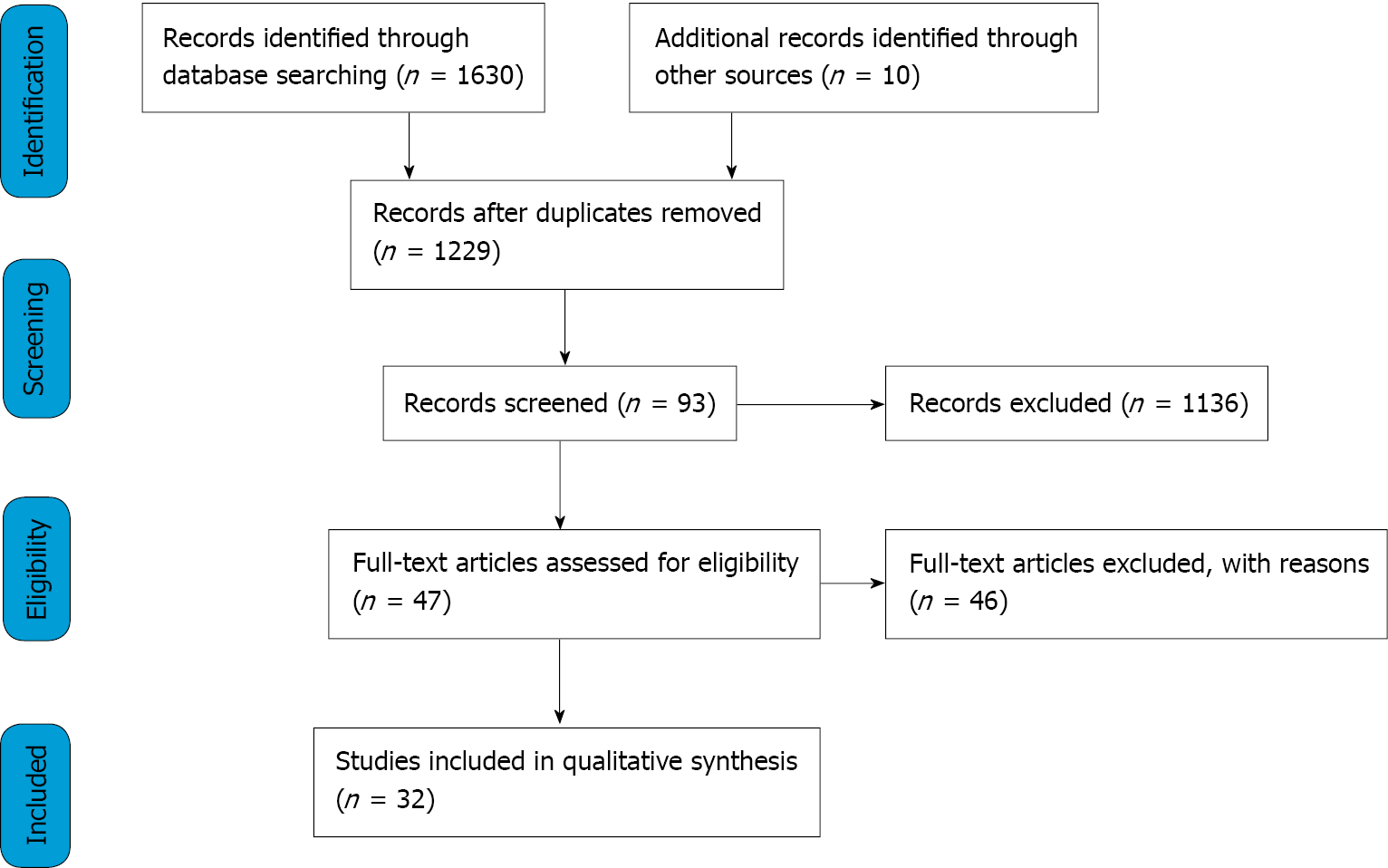
Figure 1 illustrates the search and screening processes done to assess the eligibility of studies. A total of 1630 records were found from the databases using the search strategy, with an additional 10 retrieved from references of included studies. There was a total of 411 duplicates, and after removal of these we resulted with 1229 records. Screening was conducted by the primary author. The first screening phase was done by screening the titles and abstracts of the 1229 records, which resulted in 93 potential studies. The second screening phase assessed full texts, and 46 further studies were excluded for reasons such as use of novel therapies, investigating metrics not relevant to survival outcomes in pancreatic cancer, or using in-vivo animal models. This resulted in 47 eligible texts and out of those, 36 were used to synthesise the discussion. The final 36 texts chosen represented the latest seminal work pertaining to SBRT and MRgRT in treatment of pancreatic cancer.
For patients with unresectable LAPC, chemotherapy has been the mainstay of treatment. Early radiation techniques such as CFRT and IMRT have called into question the value of irradiation in LAPC management due to their considerable toxicity profiles[4,9], with minimal to no impact on survival outcomes[9]. However, SBRT is an advanced radiation technique which can be delivered on the same linear accelerator at most centres. It has gained attraction due to three main reasons: Firstly, it allows delivery of high biologically effective doses (BED) split into several fractions (typically 3-5). Secondly, the technique allows a sharp radiation dose falloff at the edge of target volumes, thereby reducing doses to OARs[12]. Thirdly, it offers an overall shorter treatment time, as SBRT is normally delivered in 1-3 wk, compared to CFRT which takes 5-6 wk[13,14]. Hence, SBRT ensures there is minimal interruption to chemotherapy, which is important given that the main pattern of failure in this disease is distant metastasis (DM)[3]. In addition to this, patients with limited prognoses will be able to complete RT courses in 3 wk instead of 6 wk (which may account for a quarter of their remaining lifespan). This greatly improves quality-of-life, as it requires less commuting and reduces associated costs on patients and families[10,13].
Table 1 summarises the studies of SBRT in unresectable pancreatic cancer. The majority of studies demonstrated an OS of 10-16 mo, freedom from local disease progression (FFLP) rates of approximately 80% and progression-free survival (PFS) of 8-10 mo with SBRT[2-4,13,15-17]. A systematic review by Petrelli et al[18] assessed prospective trials and retrospective studies of SBRT use in LAPC, with the pooled results showing a median OS of 17 mo[18]. Other studies compared SBRT’s efficacy compared to other RT techniques. A retrospective review by Zhong et al[13] showed that patients who received SBRT had improved median OS times and 2-year OS rates relative to CFRT[13]. Similar results were found by Dohopolski et al[3] who also demonstrated a higher median OS for the SBRT group (12.6 mo) compared to its counterpart (11.2 mo)[3]. Other studies also showed that patients who had SBRT achieved at least similar outcomes as those who had IMRT[4,19]. Park et al[4] demonstrated no significant difference in median OS between those who had SBRT vs IMRT[4]. However, Shaib et al[19] found that SBRT achieves at least a month longer median OS (8.6 mo vs 6.7 mo; P < 0.001) and more than double compared to supportive care alone (8.6 mo vs 3.4 mo; P < 0.001)[19]. However, large prospective trials are needed to definitively conclude SBRT’s efficacy compared to conventional techniques, but the evidence so far suggests that SBRT is associated with better survival outcomes.
| Ref. | Participants | Dosimetry | Outcome | Toxicity | Resectability post-treatment |
| Herman et al[15], 2015, Phase 2 Trial | 49 LAPC | 33 Gy/5 fractions | (1) Median OS 13.9 mo; (2) 59% 1-yr OS; and (3) 18% 2-yr OS | (1) 1 patient acute grade 4 duodenal ulcer; (2) 10% acute grade ≥ 3; (3) 11% late grade ≥ 2; and (4) 6% serious late GI toxicity | 10% resectable after treatment |
| Comito et al[2], 2017, Phase 2 trial | 43 LAPC | 45 Gy/6 fractions | Median OS 13 mo | (1) 49% acute grade 1 or 2; (2) 0 acute grade ≥ 3; (3) 2 patients late G2 gastritis; and (4) 0 late grade ≥ 3 | 7% resectable after treatment |
| Dohopolski et al[3], 2017, Retrospective | 696 LAPC | 24-40 Gy/3-5 fractions | Median OS 12.6 mo (compared to 11.2 mo for CFRT) | Not recorded | Not recorded |
| Park et al[4], 2017, Retrospective | 44 unresectable | 30-33 Gy/5 fractions | (1) 56% 1-yr OS; (2) 26% 2-yr OS; and (3) Median OS 15.7 mo (no significant difference from IMRT) | (1) 7% acute grade ≥ 2 GI toxicity (24% for IMRT); (2) 5% grade ≥ 3 haematological toxicity (26% for IMRT); and (3) 9% late GI bleed | 7% resectable after treatment (no significant difference from IMRT) |
| Yechieli et al[10], 2017, Retrospective | 18 unresectable | 30-36 Gy/3-5 fractions | (1) Median recurrence-free survival 6.8 mo; and (2) Median OS 6.4 mo | (1) 50% no toxicity; (2) 15% grade ≥ 3; and (3) 10% GI bleed | Not recorded |
| Zhong et al[13], 2017, Retrospective | 631 LAPC | Median 40 Gy/5 fractions | (1) 22% 2-yr OS (17% for CFRT); and (2) Median OS 13.9 mo (11.6 mo for CFRT) | 0 grade ≥ 3 | 11% resectable after treatment (9% for CFRT) |
| Mazzola et al[14], 2018, Retrospective | 33 LAPC | 36-45 Gy/6 fractions | (1) 81% 1-yr LC; and (2) 75% 1-yr OS | (1) 15% acute grade 1; (2) 9% acute grade 2; (3) 0 acute grade ≥ 3; and (4) No late toxicity | 18% resectable after treatment |
| Jung et al[16], 2019, Retrospective | 95 LAPC | 24-36 Gy/4-5 fractions | (1) Median OS 16.7 mo; and (2) 67% 1-yr OS | (1) 3% acute grade 3 GI; and (2) 3% late grade 3 | 7% resectable after treatment |
| Shaib et al[19], 2020, Retrospective | 6950 LAPC (64 received SBRT) | Median 30 Gy | (1) Median OS 8.6 mo (6.7 mo for IMRT, 3.4 mo for no RT); (2) 32% 1-yr OS (22% for IMRT, 15% for no RT); and (3) 9% 2-yr OS (7% for IMRT, 5% for no RT) | Not recorded | Not recorded |
| Toesca et al[17], 2020, Retrospective | 149 unresectable | 20-45 Gy/3-6 fractions (high-dose group ≥ 40 Gy, standard-dose group < 40 Gy) | (1) Median OS 16 mo both groups; (2) Median OS 23 mo for high-dose group (14 mo for standard-dose group); and (3) 82% 1-yr OS for high-dose group (57% for standard-dose group) | (1) 10% grade ≥ 2 for high-dose group (15% for low-dose group); and (2) 6% grade ≥ 3 for high-dose group (7% for low-dose group) | 5% resectable after treatment |
Another advantage of SBRT is its favourable toxicity profiles. Studies in Table 1 report no more than 15% and 10% of patients receiving SBRT suffering from acute grade ≥ 3 toxicities and late side effects (such as duodenal bleeding and gastric ulcer perforation), respectively. Compared to IMRT, SBRT had significantly lower acute grade ≥ 2 gastrointestinal toxicity rates (7% vs 24%)[4]. Petrelli et al[18]’s systematic review found late grade 3 to 4 toxicity rates of up to 11% in their studies, with only 3 of their included studies reporting > 10% risk of severe gastrointestinal ulceration[18]. The patients of those studies all received higher doses per fraction due to previously failed RT[18], suggesting that a relationship exists between delivered doses and toxicity severity in SBRT treatment. The lower toxicity rates may be attributed to SBRT’s rapid dose falloffs and the utilisation of motion mitigation methods. The pancreas is a retroperitoneal organ embedded around gastrointestinal structures, hence it undergoes significant motion during respiratory cycles and physiological processes such as digestion. The two commonly used motion mitigation methods during SBRT are respiratory gating and abdominal compression. Respiratory gating uses an external surrogate marker that represents the internal tumour position, where the radiation beam is only delivered when this marker correlates to a certain phase of the respiratory cycle. Abdominal compression requires applying pressure onto the abdomen to suppress diaphragmatic movements, but is less preferred due to patient discomfort and the occasional displacement of OARs closer to the radiation volume[11]. A prospective study by Campbell et al[11] confirmed that both methods reduce motion and OAR exposure compared to no mitigation, however respiratory gating achieves greater motion reduction than abdominal compression by more than 20%[11].
Interestingly, while the studies only included unresectable patients, a small proportion were able to receive surgical resection after their SBRT course. As shown in Table 1, the rate of conversion to surgical resectability by SBRT was 5%-18%. In these studies, surgical resectability was decided upon multidisciplinary review including operating surgeons. This is important because if SBRT can induce local tumour regression and subsequently convert the tumour from unresectable to resectable, then it can possibly improve the chances of cure. The study by Mazzola et al[14] yielded the highest rates of resectability at 18%, all of which were participants that received higher doses of SBRT at 42-45 Gy in 6 fractions[14]. Meanwhile, Petrelli et al[18] found that higher total doses and number of fractions are significantly associated with 1-year locoregional control[18]. These results suggest that dose escalation may be the key determinant in achieving LC and thus conversion to surgical resectability. Currently, for five-fraction regimens, dose escalations of up to 60 Gy is feasible without compromising adequate target coverage and OAR constraints[20,21].
Recent evidence indicates that patients may benefit from alternative fractionation regimens, especially for those with gross tumour abutment into surrounding structures or invasion into peripancreatic nodes. The rationale is to prolong the treatment regime (≥ 10 fractions) such that higher overall BEDs can be delivered while still accounting for OAR toxicity. Reyngold et al[22] studied ablative schemes of 75 Gy in 25 fractions (BED = 97.5 Gy) and 67.5 Gy in 15 fractions (BED = 97.88 Gy) for patients with significant tumour abutment to the stomach/intestines, demonstrating a median OS of 18.2 mo and a 2-year OS of 38%[22]. This is an improvement from standard 1-5 fraction regimens, as the reported 2-year OS from those studies ranged from 9%–26%[4,13,15,19].
Despite the advances of SBRT, its overall management of LAPC is limited by its imaging modalities. SBRT utilises computed tomography (CT)-based techniques such as 4-Dimensional CT (4DCT) and Cone Beam CT to assess tumour movement and carry-out the motion mitigation techniques[23]. This is a limitation because CT has poor soft-tissue contrast and is unable to accurately determine the appropriate therapy volumes. Furthermore, CT often involves larger planning target volumes (PTV) or use of an internal target volume (ITV) to account for tumour motion, thus putting the surrounding OARs at increased toxicity risk and ultimately preventing any possibility of dose escalation[24]. Furthermore, 4DCT only provides the average of motion amplitude over several respiratory cycles. Since the fourth dimension represents “phase” of respiration rather than being real-time, tumour motion might even be underestimated[25]. This explains why despite SBRT’s evidence in reducing acute toxicity, there are still significant concerns with late toxicity as previous published studies report rates of up to 47% of late grade ≥ 2 toxicity[2]. Therefore, SBRT is constrained by dose-limitations placed on the surrounding OARs. Another concern is its steep dose gradient and the marginal misses that may result[26]. This is made more challenging given that conventional CT tends to underestimate the true pathologic size of the pancreatic tumour[27]. To optimise SBRT’s therapy volumes and dose distribution, a better imaging modality needs to be incorporated.
MRgRT has been proposed as the solution to the inconsistencies of onboard imaging with RT. MRI provides superior soft-tissue visualisation compared to CT and thus allows better delineation of the target tumour from surrounding OARs. Its real-time feedback also tracks inter-fractional and intra-fractional organ changes[8,28,29]. Another benefit of MRI is its exploration of multiple breathing cycles over different days to quantify daily changes[25]. Therefore, MRgRT can be used to guide treatment plan adaptations, such that therapy volumes account for intra-treatment tissue changes[30]. This led to the advent of Stereotactic MR-guided Adaptive Radiotherapy (SMART), which is the application of the principles of MRgRT combined with SBRT. A non-randomised trial by Heerkens et al[25] assessing the feasibility of MRgRT with SBRT showed that it is safe with dosimetric plans of at least 24 Gy, with no cases of acute or late grade ≥ 3 toxicity. They were also able to deliver higher doses under free-breathing conditions while ensuring adequate target coverage and OAR sparing[25]. SMART has become a promising technique in LAPC by possibly enabling SBRT dose escalation without exposing OARs to higher toxicity risk[8,25].
Table 2 summarises recent studies of MRgRT use in LAPC. Rudra et al[31] investigated the use of MRgRT with standard-dose and high-dose SBRT plans, and were able to demonstrate that dose escalation is possible. Patients in the high-dose group (receiving 40-52 Gy) achieved significantly higher survival rates compared to those in the standard-dose group (receiving 30-35 Gy), despite the former cohort having worse prognostic factors such as older age and higher Carbohydrate Antigen 19-9 biomarker levels[31]. There was no incidence of severe toxicity amongst the higher dose group, with all cases of grade ≥ 3 gastrointestinal toxicities reported from the standard-dose cohort[31]. A study by Luterstein et al[8] on a patient case yielded similar results. The patient with clinical stage III (T4N1M0) LAPC was given a high BED of 72 Gy via SMART after chemotherapy and achieved LC at 16 mo post-radiation (21 mo since diagnosis) with no significant side effects or toxicities[8]. Furthermore, a multi-institutional study at the American Society for Radiation Oncology suggested that adaptive plans that allow safe delivery of BED > 70 Gy can achieve higher OS rates than BED < 70 Gy without impacting surrounding OARs[8]. These results indicate that MRgRT’s precision can potentially address prior issues with RT. And since previous studies recommend that five-fraction regimens should use a dose prescription of 40 Gy to cover the gross tumour[32], the advances of MRgRT provides potential to maximise this limit in the future without compromising safety.
| Ref. | Participants | Dosimetry | Outcome | Toxicity |
| Heerkens et al[25], 2018, Trial | 20 (18 LAPC, 2 unresectable) | 24 Gy/3 fractions | (1) Median OS 8.5 mo; (2) 69% improved QOL compared to baseline at 1 mo; and (3) 33% improved QOL compared to baseline at 12 mo | No grade ≥ 3 acute or late toxicity |
| Luterstein et al[8], 2018, Case Report | 1 LAPC | 40 Gy/5 fractions | LC at 16 mo | None |
| Rudra et al[31], 2019, Retrospective | 44 unresectable (22 received SBRT) | 30-35 Gy/5 fractions (standard-dose group, n = 6); 40-52 Gy/5 fractions (high-dose group, n = 16) | (1) 49% 2-yr OS (high-dose group); (2) 30% 2-yr OS (standard-dose group); (3) 77% 2-yr FFDF (high-dose group); and (4) 57% 2-yr FFDF (standard-dose group) | Acute: (1) 7% grade ≥ 3 GI (all in standard-dose group); and (2) 2% grade 4 |
With the implementation of MRgRT in its early stages, some caveats have emerged such as workflow disruptions. Utilising MRI to guide therapy also poses new challenges unique to the MRI magnet, including but not limited to patient selection and MRI safety. This needs particular consideration as the MRI magnet is now being used outside of a radiology department where MRI safety protocols are firmly embedded into work practices. As with any novel modality or technological advancement, there will be a learning curve and an initial period to bolster awareness of safety requirements.
Concomitantly, adaptive techniques also require increased time investment as plans need to be re-optimised between fractions. Hence, MRgRT is costly and resource-intensive because it involves multidisciplinary teams to re-contour images, review and re-approve the adapted plans daily[28,30]. There is now an emerging interest to use artificial intelligence tools such as auto-contouring methods and radiomics to increase the workflow efficiency of treatment planning.
Our review methodology covers a wide range of literature, but it comes with limitations. The review mostly sought evidence from large retrospective studies without individual data for each patient. Hence, it was difficult to identify confounding factors that may exist due to the variability of patient characteristics. The review also excluded studies on patients with DM as it aimed to investigate SBRT’s effect locally. This may artificially elevate survival rates as those without DM will naturally have better outcomes.
The included evidence came with strengths and limitations. Firstly, SBRT has mature follow up data from several large retrospective analyses, with evidence dating back over a decade. This provided ample evidence to suggest that SBRT is a safe and beneficial technique for multimodal management of LAPC. However, the heterogeneity in study designs contributes to a large variability in the data. Since LAPC management differs on a case-by-case basis according to tumour staging and the physician’s clinical judgement, many of these studies include patient cohorts that received different chemotherapy regimens from each other. As a result, it is unsure how much survival benefit can be attributed to SBRT. It is also noteworthy that many of these studies could involve selection bias, since the most unwell patients often received no treatment and went into palliative care. This led to “healthier” subjects chosen for SBRT and thus better OS rates. Many of the included studies are retrospective analyses of database records, presenting another source of selection bias. Meanwhile, there is limited research on MRgRT so far, thus definitive conclusions about this technique cannot be made. There is a need for large prospective trials on SBRT and MRgRT, with comparisons to other treatment modalities to validate the results of previous retrospective studies. However, given LAPC’s generally poor outcomes, long-term prospective studies will be challenging.
SBRT is an advanced radiation technique that allows delivery of ablative doses in several fractions. It is highly precise, time-efficient and can limit OAR exposure when combined with motion mitigation techniques. SBRT is associated with improved treatment outcomes and safer toxicity profiles compared to other conventional RT techniques. And by implementing MR-guided imaging techniques with SBRT, the excellent soft-tissue contrast of MRI enables the physician to make daily plan adaptations such that target volumes are optimised according to intra- and inter-fractional tissue changes. This enables the possibility of dose escalation, which may be the key in achieving long-term LC and converting unresectable LAPC into operable cases. The current evidence on MR-guided SBRT is still limited, but early protocols have suggested its promise. Further research should focus on validating the feasibility, safety and efficacy of MRgRT with comparison to other treatment modalities.
Pancreatic cancer is associated with significant mortality, and unresectable tumours are commonly treated with chemoradiotherapy regimens. Conventional radiotherapy (RT) techniques have minimal impact on survival and often cause considerable toxicities. Stereotactic body radiotherapy (SBRT) is an advanced radiotherapy technique that delivers highly ablative doses in several fractions, with a steep dose fall-off outside target volumes.
Previous studies have supported the benefit of radiotherapy in multi-modal management of unresectable pancreatic cancers. However, there is no consensus of which RT technique yields the best survival outcomes. There is also a need for research to explore onboard imaging such as magnetic resonance-guided radiotherapy (MRgRT), which will enable treatment plans to be optimised according to intra-treatment tissue changes.
We aim to collate the latest data on SBRT and evaluate its survival outcomes and toxicity profiles, with comparison to conventional RT techniques. Our review will also cover the safety and efficacy of MRgRT.
Searches were conducted on PubMed and Ovid (Medline), resulting in 1229 records. After multiple rounds of screening, 36 texts were chosen to synthesise the discussion. Records were included if they studied SBRT or MRgRT in unresectable cancers, and excluded if they involved metastatic disease, resectable tumours or used chemoradiotherapy as adjuvant to surgery.
SBRT is associated with improved survival outcomes and toxicity profiles compared to conventional RT techniques. A small proportion of unresectable patients were able to undergo surgical resection after their SBRT course. Conversion to resectability was associated with higher doses. However, dose escalation in SBRT is limited by the onboard computed tomography (CT) imaging due to its poor soft-tissue contrast. MRgRT may address these issues as magnetic resonance imaging (MRI) provides excellent tissue visualisation and is appropriate for real-time scanning. Early data indicates MRgRT as a safe and efficacious technique.
SBRT may lead to improved survival outcomes and safer toxicity profiles compared to conventional RT, but is ultimately limited by onboard CT imaging. The addition of MRI-guided techniques allows the potential for dose escalation, which may be the key to achieving surgical resectability and possibly increasing the chances of cure.
There is a need for large prospective trials to definitively conclude if SBRT is superior to other RT techniques. Large studies are also required to validate the safety, feasibility and efficacy of MRgRT with comparison to other RT techniques.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li CG, Ryckman JM S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15475] [Article Influence: 2579.2] [Reference Citation Analysis (2)] |
| 2. | Comito T, Cozzi L, Clerici E, Franzese C, Tozzi A, Iftode C, Navarria P, D'Agostino G, Rimassa L, Carnaghi C, Personeni N, Tronconi MC, De Rose F, Franceschini D, Ascolese AM, Fogliata A, Tomatis S, Santoro A, Zerbi A, Scorsetti M. Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Technol Cancer Res Treat. 2017;16:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Dohopolski MJ, Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stereotactic body radiotherapy for locally-advanced unresectable pancreatic cancer-patterns of care and overall survival. J Gastrointest Oncol. 2017;8:766-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Park JJ, Hajj C, Reyngold M, Shi W, Zhang Z, Cuaron JJ, Crane CH, O'Reilly EM, Lowery MA, Yu KH, Goodman KA, Wu AJ. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017;56:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Qing SW, Ju XP, Cao YS, Zhang HJ. Dose escalation of Stereotactic Body Radiotherapy (SBRT) for locally advanced unresectable pancreatic cancer patients with CyberKnife: protocol of a phase I study. Radiat Oncol. 2017;12:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhu X, Li F, Ju X, Cao F, Cao Y, Fang F, Qing S, Shen Y, Jia Z, Zhang H. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017;6:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 723] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 8. | Luterstein E, Cao M, Lamb J, Raldow AC, Low DA, Steinberg ML, Lee P. Stereotactic MRI-guided Adaptive Radiation Therapy (SMART) for Locally Advanced Pancreatic Cancer: A Promising Approach. Cureus. 2018;10:e2324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouché O, Shannon J, André T, Mineur L, Chibaudel B, Bonnetain F, Louvet C; LAP07 Trial Group. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 764] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 10. | Yechieli RL, Robbins JR, Mahan M, Siddiqui F, Ajlouni M. Stereotactic Body Radiotherapy for Elderly Patients With Medically Inoperable Pancreatic Cancer. Am J Clin Oncol. 2017;40:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Campbell WG, Jones BL, Schefter T, Goodman KA, Miften M. An evaluation of motion mitigation techniques for pancreatic SBRT. Radiother Oncol. 2017;124:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Goldsmith C, Plowman PN, Green MM, Dale RG, Price PM. Stereotactic ablative radiotherapy (SABR) as primary, adjuvant, consolidation and re-treatment option in pancreatic cancer: scope for dose escalation and lessons for toxicity. Radiat Oncol. 2018;13:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Zhong J, Patel K, Switchenko J, Cassidy RJ, Hall WA, Gillespie T, Patel PR, Kooby D, Landry J. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123:3486-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Mazzola R, Fersino S, Aiello D, Gregucci F, Tebano U, Corradini S, Di Paola G, Cirillo M, Tondulli L, Ruffo G, Ruggieri R, Alongi F. Linac-based stereotactic body radiation therapy for unresectable locally advanced pancreatic cancer: risk-adapted dose prescription and image-guided delivery. Strahlenther Onkol. 2018;194:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, Iacobuzio-Donahue CA, Griffith ME, Pawlik TM, Pai JS, O'Reilly E, Fisher GA, Wild AT, Rosati LM, Zheng L, Wolfgang CL, Laheru DA, Columbo LA, Sugar EA, Koong AC. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 16. | Jung J, Yoon SM, Park JH, Seo DW, Lee SS, Kim MH, Lee SK, Park DH, Song TJ, Ryoo BY, Chang HM, Kim KP, Yoo C, Jeong JH, Kim SC, Hwang DW, Lee JH, Song KB, Jo YY, Park J, Kim JH. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One. 2019;14:e0214970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Toesca DAS, Ahmed F, Kashyap M, Baclay JRM, von Eyben R, Pollom EL, Koong AC, Chang DT. Intensified systemic therapy and stereotactic ablative radiotherapy dose for patients with unresectable pancreatic adenocarcinoma. Radiother Oncol. 2020;152:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, Barni S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int J Radiat Oncol Biol Phys. 2017;97:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Shaib WL, Zakka K, Shahin AA, Yared F, Switchenko JM, Wu C, Akce M, Alese OB, Patel PR, Mcdonald M, El-Rayes BF. Radiation as a Single-Modality Treatment in Localized Pancreatic Cancer. Pancreas. 2020;49:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Colbert LE, Rebueno N, Moningi S, Beddar S, Sawakuchi GO, Herman JM, Koong AC, Das P, Holliday EB, Koay EJ, Taniguchi CM. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv Radiat Oncol. 2018;3:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Mazzarotto R, Simoni N, Guariglia S, Rossi G, Micera R, De Robertis R, Pierelli A, Zivelonghi E, Malleo G, Paiella S, Salvia R, Cavedon C, Milella M, Bassi C. Dosimetric Feasibility Study of Dose Escalated Stereotactic Body Radiation Therapy (SBRT) in Locally Advanced Pancreatic Cancer (LAPC) Patients: It Is Time to Raise the Bar. Front Oncol. 2020;10:600940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Reyngold M, O'Reilly EM, Varghese AM, Fiasconaro M, Zinovoy M, Romesser PB, Wu A, Hajj C, Cuaron JJ, Tuli R, Hilal L, Khalil D, Park W, Yorke ED, Zhang Z, Yu KH, Crane CH. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. 2021;7:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 23. | Vinogradskiy Y, Goodman KA, Schefter T, Miften M, Jones BL. The Clinical and Dosimetric Impact of Real-Time Target Tracking in Pancreatic SBRT. Int J Radiat Oncol Biol Phys. 2019;103:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Placidi L, Romano A, Chiloiro G, Cusumano D, Boldrini L, Cellini F, Mattiucci GC, Valentini V. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Tech Innov Patient Support Radiat Oncol. 2020;15:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Heerkens HD, van Vulpen M, Erickson B, Reerink O, Intven MP, van den Berg CA, Molenaar IQ, Vleggaar FP, Meijer GJ. MRI guided stereotactic radiotherapy for locally advanced pancreatic cancer. Br J Radiol. 2018;91:20170563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Ryckman JM, Reames BN, Klute KA, Hall WA, Baine MJ, Abdel-Wahab M, Lin C. The timing and design of stereotactic radiotherapy approaches as a part of neoadjuvant therapy in pancreatic cancer: Is it time for change? Clin Transl Radiat Oncol. 2021;28:124-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Arvold ND, Niemierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Olberg S, Green O, Cai B, Yang D, Rodriguez V, Zhang H, Kim JS, Parikh PJ, Mutic S, Park JC. Optimization of treatment planning workflow and tumor coverage during daily adaptive magnetic resonance image guided radiation therapy (MR-IGRT) of pancreatic cancer. Radiat Oncol. 2018;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | El-Bared N, Portelance L, Spieler BO, Kwon D, Padgett KR, Brown KM, Mellon EA. Dosimetric Benefits and Practical Pitfalls of Daily Online Adaptive MRI-Guided Stereotactic Radiation Therapy for Pancreatic Cancer. Pract Radiat Oncol. 2019;9:e46-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Bohoudi O, Bruynzeel AME, Meijerink MR, Senan S, Slotman BJ, Palacios MA, Lagerwaard FJ. Identification of patients with locally advanced pancreatic cancer benefitting from plan adaptation in MR-guided radiation therapy. Radiother Oncol. 2019;132:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, Wan L, Portelance L, Mellon EA, Bruynzeel A, Lagerwaard F, Bassetti MF, Parikh PJ, Lee PP. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 32. | Koay EJ, Hanania AN, Hall WA, Taniguchi CM, Rebueno N, Myrehaug S, Aitken KL, Dawson LA, Crane CH, Herman JM, Erickson B. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract Radiat Oncol. 2020;10:e495-e507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |