Published online Feb 14, 2022. doi: 10.3748/wjg.v28.i6.624
Peer-review started: May 29, 2021
First decision: June 22, 2021
Revised: July 30, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 14, 2022
Processing time: 256 Days and 6.6 Hours
Pancreatic cystic lesions (PCLs) are becoming more prevalent due to more frequent abdominal imaging and the increasing age of the general population. It has become crucial to identify these PCLs and subsequently risk stratify them to guide management. Given the high morbidity associated with pancreatic surgery, only those PCLs at high risk for malignancy should undergo such treatment. However, current diagnostic testing is suboptimal at accurately diagnosing and risk stratifying PCLs. Therefore, research has focused on developing new techniques for differentiating mucinous from non-mucinous PCLs and identifying high risk lesions for malignancy. Cross sectional imaging radiomics can potentially improve the predictive accuracy of primary risk stratification of PCLs at the time of detection to guide invasive testing. While cyst fluid glucose has reemerged as a potential biomarker, cyst fluid molecular markers have improved accuracy for identifying specific types of PCLs. Endoscopic ultrasound guided approaches such as confocal laser endomicroscopy and through the needle microforceps biopsy have shown a good correlation with histopathological findings and are evolving techniques for identifying and risk stratifying PCLs. While most of these recent diagnostics are only practiced at selective tertiary care centers, they hold a promise that management of PCLs will only get better in the future.
Core Tip: Pancreatic cystic lesions (PCLs) are highly prevalent. It is critical to accurately diagnose PCLs and risk stratify them to guide management. Current diagnostic techniques are suboptimal; hence, recent investigations have focused on developing, refining, and validating novel technologies for accurately diagnosing specific cyst type and ascertaining high-risk lesions for malignancy. Radiomics, cyst-fluid biomarkers, confocal laser endomicroscopy and microforceps biopsy hold the promise of accurately diagnosing PCLs and improving their management.
- Citation: Ardeshna DR, Cao T, Rodgers B, Onongaya C, Jones D, Chen W, Koay EJ, Krishna SG. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J Gastroenterol 2022; 28(6): 624-634
- URL: https://www.wjgnet.com/1007-9327/full/v28/i6/624.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i6.624
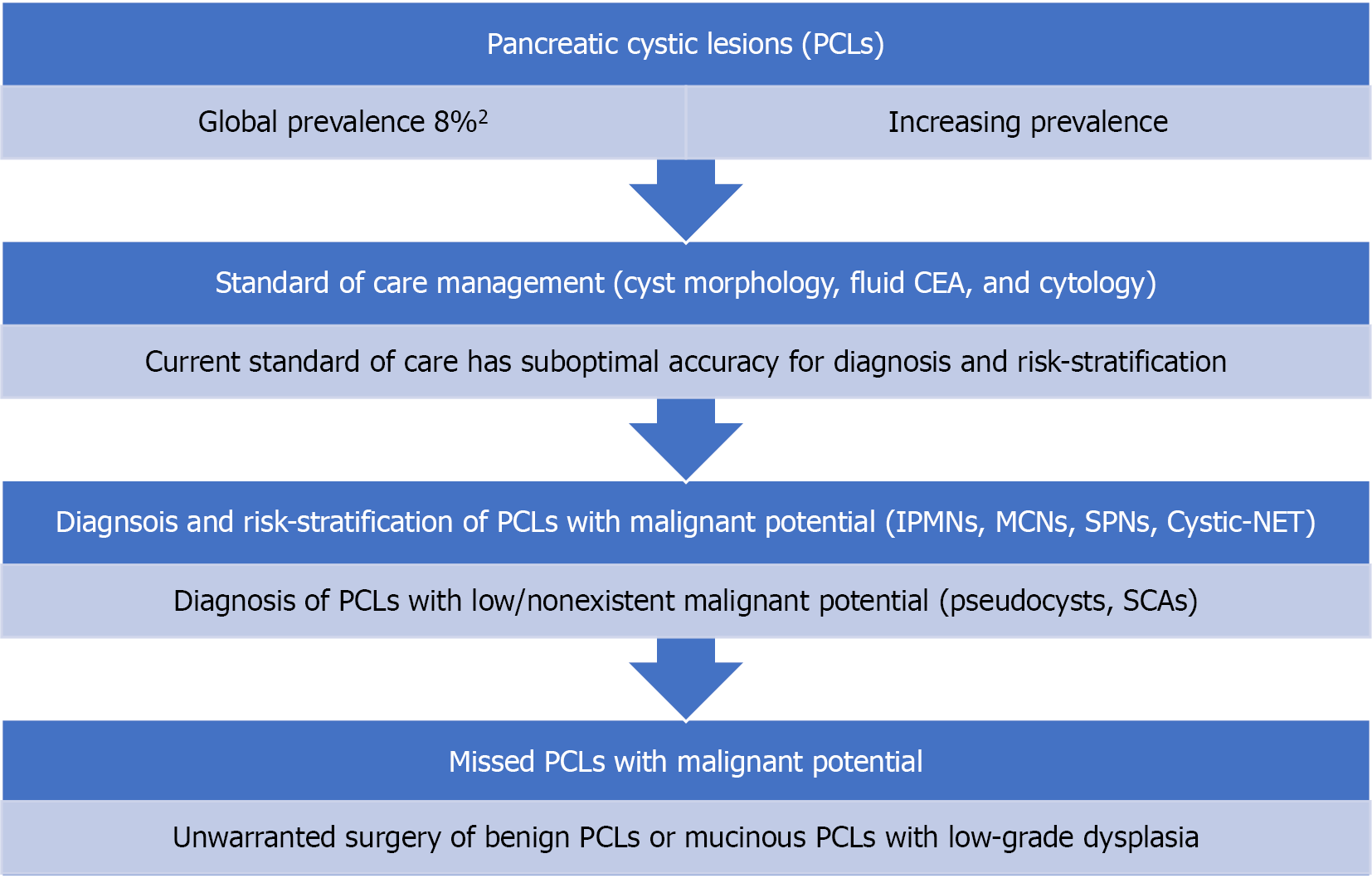
Pancreatic cystic lesions (PCLs) are increasingly detected, largely due to advances in imaging techniques and the increasing age of the general population[1]. With prevalence estimated in the range of 4%-14% in the general population and increasing constantly, it has become essential to characterize and risk stratify these cysts to guide management[2]. Current guidelines for evaluating PCLs are limited to less than optimal diagnostic techniques, resulting in either missed detection of early cancer or surgical over-treatment (see Figure 1). Resection of PCLs should be extremely selective since pancreatic surgery generally has a 20%-40% morbidity rate and an approximate 2% mortality rate[3-5]. Therefore, research and utilization of safe and effective diagnostic modalities with high accuracy are needed to evaluate cysts and introduce properly timed interventions.
Addressing this issue is especially relevant for intraductal papillary mucinous neoplasms (IPMNs), a type of PCL with one of the highest risks for malignancy. Of the two IPMN subtypes, main duct IPMNs are reported to have a risk from 38% to 68% and branch duct IPMNs from 12% to 47%[6]. Substantial research has addressed the use of consensus guidelines for evaluating IPMNs, but all mention that significant areas of improvement is imperative[7-9].
Current standards for the evaluation of cyst morphology include computed tomography (CT) scan, magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS). Fine needle aspiration (FNA) of cyst fluid for carcinoembryonic antigen (CEA) and cytology is performed during EUS. Considerable heterogeneity exists among the five widely used guidelines, which indicate a lack of standardization in diagnostic workups[7-12]. In terms of the target population, American College of Gastroenterology and European guidelines include all PCLs, American College of Radiology guidelines focus on incidental PCLs, Fukuoka guidelines only focuses on IPMNs and American Gastroenterological Association (AGA) includes all PCLs except main-duct IPMNs. Guidelines differ in recommending evaluation with EUS and EUS-FNA, and surgical resection. Multiple studies have compared some of these guidelines for identifying high-risk PCLs. Amongst patients who underwent surgery, the current guidelines directed clinical decision with an accuracy, sensitivity and specificity of 49.6%, 23.5%, 84.3% for 2015 AGA guidelines, 41.2%, 39.7%, 43.1% for revised Fukuoka guidelines and 58%, 67.7%, 45.1% for 2018 European guidelines[13].
A better understanding of investigational characteristics that lead to malignancy is necessary to improve existing criteria and accurately determine associated risks (Figure 1). While cyst fluid glucose has reemerged as a potential biomarker, novel techniques such as cyst fluid molecular analysis, EUS-guided needle-based confocal laser endomicroscopy (EUS-nCLE) and microforceps biopsy (EUS-MFB) have been introduced. The aim of this review is to provide an update of the recent literature in the management of PCLs with an emphasis on novel diagnostic methods.
Increasing prevalence of incidental PCLs has placed significant pressure on the necessity of discerning low-risk and high-risk lesions identified in radiological images. Radiomics is the analysis of mathematically derived textural features from cross-sectional imaging studies. The features are generally beyond human visual perception. Using radiometric feature extraction tool, radiomics can quantify individual pixels and their associated gray-scale value from cross-sectional imaging in a temporal and spatial plane to create a cyst impression. While studies have varied in the extraction of radiometric data, these features can potentially risk stratify PCLs. Hence, radiomics can guide downstream invasive diagnostics.
Studies in radiomics can be classified into two broad categories: (1) Differentiating types of PCLs, and (2) Risk stratification of IPMNs. Investigators have applied machine learning algorithms to radiomic features for automatic classification of PCLs. Some recent studies have evaluated nomograms and algorithms combining radiomics, cyst morphology, and clinical features. For differentiating PCLs, several investigations have demonstrated promising results. One of the first studies by Dmitriev et al[14], achieved a reasonable accuracy of 83.6% in discriminating PCL types into IPMNs, mucinous cystic neoplasms (MCNs), serous cystadenoma (SCAs) and solid neoplasms[14]. Their model had 93.2%-95.9% accuracy at predicting IPMNs. Subsequently, three investigations utilized CT-Scan radiomics to differentiate serous cystadenomas from other PCLs (area under the curve (AUC) 0.77-0.99, sensitivity 69%-95%, specificity 71%-96%)[15-17]. In one of these studies, radiomics outperformed radiologic characteristics in differentiating MCNs and macrocystic SCAs; comparative diagnostic parameters included sensitivity (93.6% vs 74.2%), specificity (96.2% vs 80.8%) and accuracy (94.7% vs 77.2%), respectively[16]. Combining radiomics with radiological findings or clinical parameters significantly improved the accuracy to distinguish cyst types in comparison to radiomics alone (P < 0.05) [16,18].
Only a few studies have evaluated the role of radiomics in differentiating IPMNs with advanced neoplasia, from indolent lesions with low-grade dysplasia (Table 1)[19-23]. Most of the studies evaluating radiomics in IPMNs have used CT scans, and included patients with confirmed surgical histopathology as ground truth. A recent study by Cui et al[24], presents the first publication where a radiomic signature incorporating 9 features was combined with clinical variables to predict high-grade dysplasia or adenocarcinoma (advanced neoplasia) in branch duct-IPMNs. Their predictive nomogram diagnosed advanced neoplasia with AUC values of 0.903 (training cohort; sensitivity 95%, specificity 73%), and 0.884 (one of two external validation cohorts; sensitivity 79%, specificity 90%)[24].
| Ref. | n | Image type | No. of radiomic features | Best model | Performance training set |
| Hanania et al[19], United States, 2016 | 53 | CECT | 360 | 10 radiomic features | AUC: 0.82 |
| SP: 85%, SP: 68% | |||||
| Permuth et al[20], United States, 2016 | 38 | CECT | 112 | 14 radiomic features +blood 5 mi-RNAs | AUC: 0.92 |
| SN: 83%, SP: 89% | |||||
| Attiyeh et al[21], United States, 2019 | 103 | CECT | 255 | Radiomic + clinical features | AUC: 0.79 |
| SN: 71%, SP: 82% | |||||
| Williams et al[22], United States, 2020 | 33 | CECT | 12 | Radiomic features + cyst fluid protein markers | AUC: 0.88 |
| SN: 71%, SP: 92% | |||||
| Hoffman et al[23], United States, 2017 | 18 | MRI w/ DWI | N/A | Entropy | AUC: 0.86 |
| SN: 100%; SP: 70% |
Thus, radiomics represents a promising non-invasive approach for the classification and risk stratification of PCLs and will favorably impact patient management. However, radiomics continues to be a novel concept and has been largely used to date in clinical trials at academic centers. While radiomics has demonstrated an immense potential for diagnosis, prognosis, and risk assessment in PCLs; there is a need for standardized protocols for image acquisition, segmentation, feature extraction, and analysis.
CEA and amylase: Traditionally pancreatic cyst fluid is aspirated using EUS-FNA for biomarker and cytologic analysis. In early studies, cyst fluid CEA levels above 192 ng/mL was shown to correlate with mucinous PCL with 79% (88/111) accuracy (P < 0.0001)[25]. However more recent studies, have estimated CEA sensitivity and specificity at 63% and 88%, respectively in differentiating mucinous from non-mucinous cysts[26]. This level of accuracy would result in misdiagnosis of 35%-39% of mucinous cysts. Additionally, CEA levels across sites are difficult to compare and levels have not been shown to correlate with PCL malignant potential[25,27-29]. Regarding amylase levels, a low cyst fluid amylase level has very high specificity for excluding pseudocyst. However, high amylase levels have been shown to have no diagnostic utility[26,30]. As a result, measuring amylase has fallen out of favor for the diagnosis of PCLs.
Cytology: Cyst fluid analysis by cytology has been shown to lack sensitivity for the diagnosis of PCLs. A meta-analysis with 937 patients demonstrated cyst fluid cytology to have 63% sensitivity and 88% specificity for the diagnosis of PCL[31]. Another meta-analysis calculated cytology to have 51% and 94% sensitivity and specificity, respectively[32]. This lack of sensitivity results from cytology evaluations usually detecting only intact exfoliated cells that are typically few in number[25,33].
Glucose: Intracystic glucose has good accuracy at differentiating mucinous and non-mucinous cysts but this economical diagnostic tool has not been used in routine clinical practice. However, recent prospective studies have provided improved and sustained evidence that cyst fluid glucose should be considered for standard of care evaluation of PCLs. Low intra-cystic glucose concentration is predictive of a mucinous cyst while high concentrations are consistent with non-mucinous cysts. In 2020, Ribaldone et al[34] reported from 56 patients that intra-cystic glucose concentration < 50 mg/dL had significantly better sensitivity than a CEA level > 192 ng/mL for diagnosing mucinous cysts (93.6% vs 54.8%; P = 0.003). Both CEA and intra-cystic glucose had high specificity for diagnosing mucinous cysts (96% vs 100%; P = 1). They reported that intra-cystic glucose concentration of more than 50 mg/dL had higher sensitivity than CEA values of less than 5 ng/mL for diagnosing non-mucinous cysts (96% vs 72%, P = 0.07).
A meta-analysis of 7 studies encompassing 566 patients reported that lower (cut-off < 50 mg/dL) intra-cystic glucose concentration had a pooled sensitivity of 90.1% (95%CI: 87.2-92.5) and pooled specificity of 85.3% (95%CI: 76.8-91.1) when differentiating mucinous from non-mucinous cysts[35]. In a subset analysis, point-of-care glucometer measurements for intra-cystic glucose (3 studies) also revealed comparable pooled sensitivity of 89.5% (95%CI: 85.5-92.5; I2 = 0) and pooled specificity of 83.9% (95%CI: 68.5-92.6; I2 = 43) for the differentiation of PCLs[35]. A more recent (2021) meta-analysis that included 8 studies with 609 PCLs showed pooled sensitivities for glucose vs CEA of 91% (95%CI: 88-94) vs 56% (95%CI 46-66) (comparative P value < 0.001), pooled specificities were 86% (95%CI: 81-90) vs 96% (95%CI: 90-99), P > 0.05, respectively[36].
Estimation of glucose levels is a low-cost diagnostic test that has repeatedly demonstrated better accuracy at differentiating mucinous and non-mucinous cyst. While not being definitive, cyst fluid glucose is a practical and economical diagnostic tool that can help in the differentiation of PCLs.
Molecular markers: With the introduction of next-generation sequencing (NGS), diagnosis of PCLs with either small gene panels and whole exome NGS have been employed. This method allows assessment of intact cell and cell-free nucleic acid that has been shed into the cyst fluid. DNA mutations that are commonly associated with pancreatic adenocarcinoma (KRAS, CDKN2A, SMAD4, PTEN, PIK3CA, and TP53) may also be present in precursor PCLs, with the latter five associated with advanced neoplasia.
Similar to radiomics, molecular analysis of cyst fluid can contribute to the classification of PCLs, and risk stratification of IPMNs. In a meta-analysis (6 studies, 785 PCLs), McCarty et al[37] reported that the dual presence of KRAS and GNAS mutations detected mucinous PCLs with a sensitivity of 75% (95%CI: 58-87%), specificity of 99% (95%CI: 67-100%), and diagnostic accuracy of 97% (95%CI: 95-98%), respectively. For specifically diagnosing IPMNs, dual KRAS/GNAS mutation had 94% (95%CI: 72-99%) sensitivity, 91% (95%CI: 72-98; I2 = 89.83%) specificity and 97% (95%CI: 95-98%) accuracy, respectively. Recently, our group identified, for the first time, that uncommon BRAF mutations (and occasional MAP2K1 mutations) characterize a significant subset of IPMNs that lack KRAS mutations, indicating that RAS-MAPK dysregulation is ubiquitous in these tumors[12]. In the same study, we showed 88.5% sensitivity, 100% specificity, and 90.3% accuracy for NGS differentiation of PCLs[12].
For the risk stratification of IPMNs, Singhi et al[38] used next-generation sequencing to evaluate DNA mutations associated with advanced neoplasia. In a subgroup analysis of 102 patients with histopathologic diagnosis, they reported that the presence of TP53, PIK3CA and/or PTEN mutation had 88% (95%CI: 62-98%) sensitivity and 95% (95%CI: 88-98%) specificity, respectively for diagnosing IPMNs with advanced neoplasia.
Cyst fluid molecular analysis by next generation sequencing is superior to measuring cyst CEA levels with superior accuracy and the ability to provide risk stratification for IPMNs. However, it is selectively available and represents a logistical and financial barrier for universal adaptation.
EUS-guided needle confocal laser endomicroscopy (nCLE) permits real-time microscopic imaging of intra-cystic epithelium within a single plane. It allows for in vivo pathological analysis of PCLs. Early studies have established the characteristic features for IPMNs. Investigations by Napoleon et al[40] in the CONTACT study established defining criteria for MCNs, SCAs, and cystic neuroendocrine tumors[39,40]. In 2020, the INDEX study provided further support for nCLE as a viable diagnostic tool by demonstrating high performance in differentiating PCLs amongst the highest number (n = 65) of patients with surgical histopathology[41]. For the differentiation of PCLs into mucinous and non-mucinous lesions, a recent meta-analysis with 7 studies and 324 patients reported a pooled sensitivity, specificity and accuracy of 85% (95%CI: 71-93%), 99% (95%CI: 90-100%) and 99% (95%CI: 98-100%), respectively. The pooled risk of post-procedure acute pancreatitis was 1% (95%CI: 0-3%)[42]. Another recent meta-analysis (10 studies, 536 patients) reported a pooled sensitivity, specificity, and accuracy of 82.4% (95%CI: 74.7-90.1%), 96.6% (95%CI: 94.3-99%), and 88.6% (95%CI: 83.7-93.4%), respectively, for the differentiation of mucinous from non-mucinous PCLs[43].
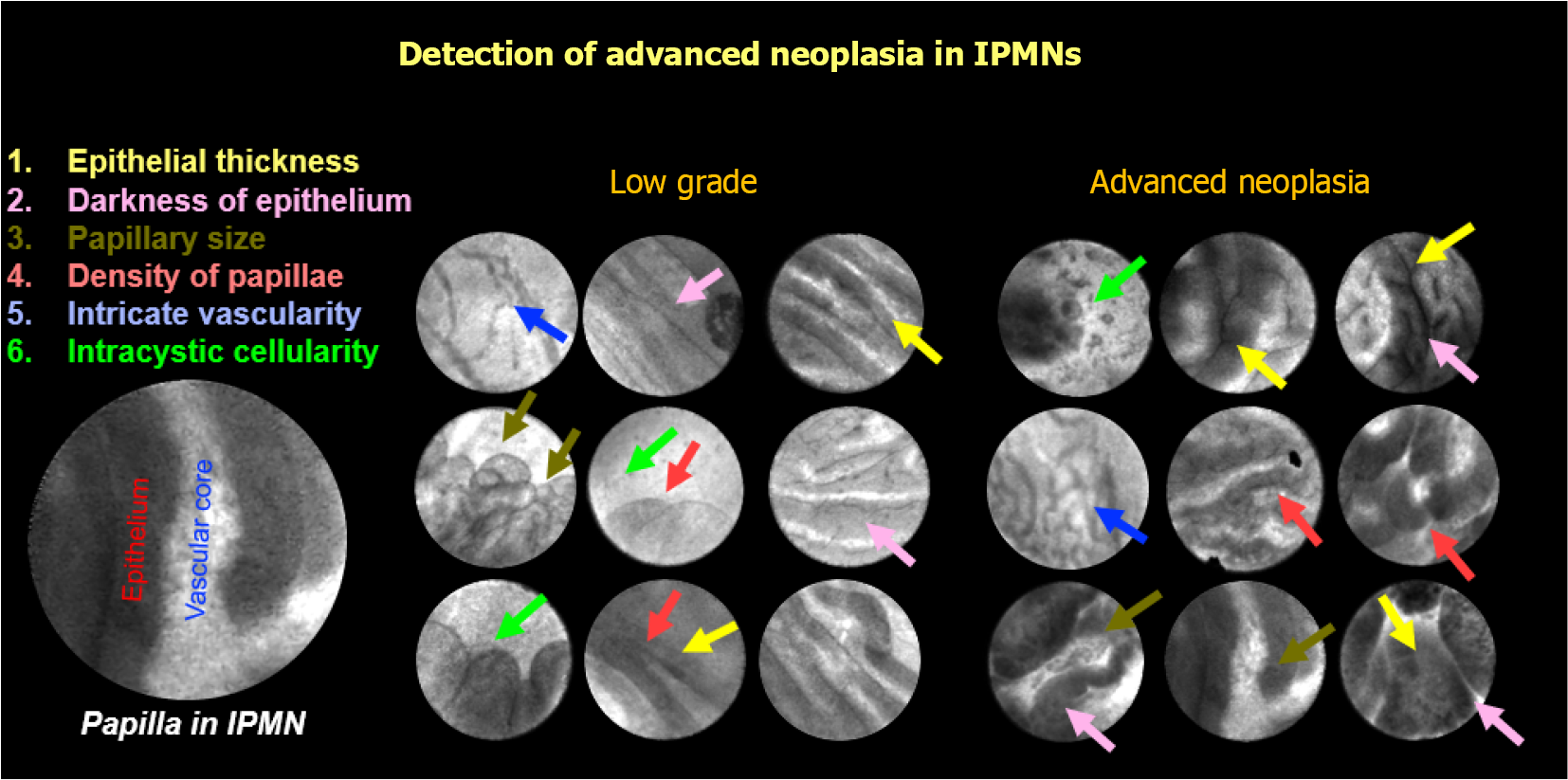
In addition to the high accuracy of diagnosing IPMNs and other cysts, nCLE can potentially determine the risk for advanced neoplasia in PCLs. To detect advanced neoplasia in IPMNs, multiple nCLE imaging variables were identified in a post-hoc analysis of the INDEX study[44], Figure 2. This study identified that the variables with the highest interobserver agreement were papillary epithelial thickness and darkness. Specifically, nCLE visualized papillary epithelial thickness (width ≥ 50 μm) had a sensitivity, specificity, and AUC of 87.5% (95%CI: 62%-99%), 100% (95%CI: 69%-100%), and 0.95, respectively for the detection of advanced neoplasia. Also, estimation of the papillary epithelial darkness (cut-off ≤ 90 pixel intensity) revealed a sensitivity, specificity, and AUC of 87.5% (95%CI: 62%-99%), 100% (95%CI: 69%-100%), and 0.90, respectively[44]. Analogously for mucinous cysts, Feng et al[45] reported that nCLE pattern of “dark aggregates of neoplastic cells” correlated with the morphologic features of "irregular branching and budding" and was diagnostic of malignancy, with 75% sensitivity, 100% specificity and 94% accuracy, respectively.
However, potential limitations of nCLE include differences in interobserver interpretation of images and the tedious nature of manually determining papillary epithelial thickness and darkness. Both of these issues were addressed with the development of a machine learning artificial intelligence model that identified advanced neoplasia in IPMNs with a sensitivity (83%) and specificity (88%) well above the Fukuoka or AGA guidelines[46].
Despite the growing evidence of nCLE as a viable diagnostic technique, its incorporation into standard clinical evaluation is lacking. The primary challenges include equipment costs, optimal training in image acquisition and interpretation, and prevention of adverse events higher than the standard EUS-FNA process.
This technique utilizes an EUS guided approach to pass a specialized device, the Moray micro forceps (Moray micro forceps, US Endoscopy, Mentor, Ohio, United States) through the 19-gauge EUS needle to collect tissue sample from PCLs. Multiple recent studies have demonstrated an improved diagnostic yield and accuracy in the diagnosis of specific types of PCLs[47,48].
Multiple meta-analyses have been published and the most recent studies include the following. Tacelli et al[49] (2020) included 9 studies with 454 patients and pooled technical success, histological accuracy and diagnostic yield for specific types of PCLs were 98.5% (95%CI: 97.3%-99.6%), 86.7% (95%CI: 80.1-93.4) and 69.5% (95%CI: 59.2-79.7%), respectively. Additionally sensitivity and specificity for diagnosis of mucinous PCLs were 88.6% and 94.7%, respectively. However, the overall complication rate was 8.6% (95%CI: 4.0-13.1%) with studies reporting rates ranging from 1%-23%. Of the reported complications, 57.1% had self-limiting bleedings (most commonly intra-cystic bleeding), 24.5% had mild pancreatitis, 6.1% had infections and 14.3% had abdominal pain. Westerveld et al[50] analyzed 8 studies with 426 patients reporting similar results. The MFB approach had significantly higher diagnostic yield for specific cyst type compared to cytology (72.5%, 95%CI: 60.6-83.0% vs 38.1%, 95%CI: 18.0-60.5%). Additionally, MFB had significantly higher diagnostic yield for mucinous cyst compared to cytology (OR: 3.86; 95%CI: 2.0-7.44, I2 = 72%). Overall MFB procedures had a 7% complication rate with 5% incidence of intra-cystic hemorrhage and 2.3% risk of acute pancreatitis. More importantly, in a subgroup analysis of 92 patients who had surgical resection of their PCLs, MFB findings had concordance of 82.3% (95%CI: 71.9-90.7%) for specific cyst diagnosis. MFB findings for mucinous cysts had a sensitivity of 90.1% (95%CI: 78.4-97.6%) and specificity of 94% (95%CI: 81.5-99.7%). Additionally, the concordance rate for histological grade of dysplasia was 75.6% (95%CI: 62.3-86.8).
In another meta-analysis that included patients with surgical histopathology as reference diagnosis, the pooled sensitivity and specificity for diagnosing mucinous PCL was 86% (95 %CI: 62-96%) and specificity 95% (95%CI: 79-99%) respectively[51]. For diagnosis of specific cyst type, the pooled sensitivity and specificity were 69% (95%CI: 50-83%) and specificity 47% (95%CI: 28-68%), respectively. The authors also grouped IPMNs and MCNs with advanced neoplasia, SPNs, and cystic neuroendocrine tumors as high-risk cysts. MFB demonstrated a pooled sensitivity and specificity of 78% (95%CI: 61-89%) and 99% (95%CI: 90-99%) respectively for diagnosis of a high-risk cyst.
While MFB represents an excellent technique for acquisition of tissue and accurately diagnosing PCLs, the high rates of adverse events including acute pancreatitis and intra-cystic bleeding may deter clinicians from using this technique.
EUS when combined with contrast enhancers allows detection of vascularity within PCLs. This allowed contrast-enhanced EUS (CE-EUS) to differentiate pseudocysts from true PCLs and identify mural nodules within PCLs. Despite early studies reporting no improvement over traditional EUS at differentiating PCLs[52], recent studies have reported higher diagnostic yield for PCLs using CE-EUS (96% compared to 71% for traditional EUS)[53]. CE-EUS detected small lesions initially missed on contrast-enhanced CT or EUS-FNA[54]. Recent literature on CE-EUS has reported higher accuracy at diagnosing PCLs compared to CT, MRI and traditional EUS[55]. Despite these encouraging results, CE-EUS has not gained traction in clinical management of PCLs.
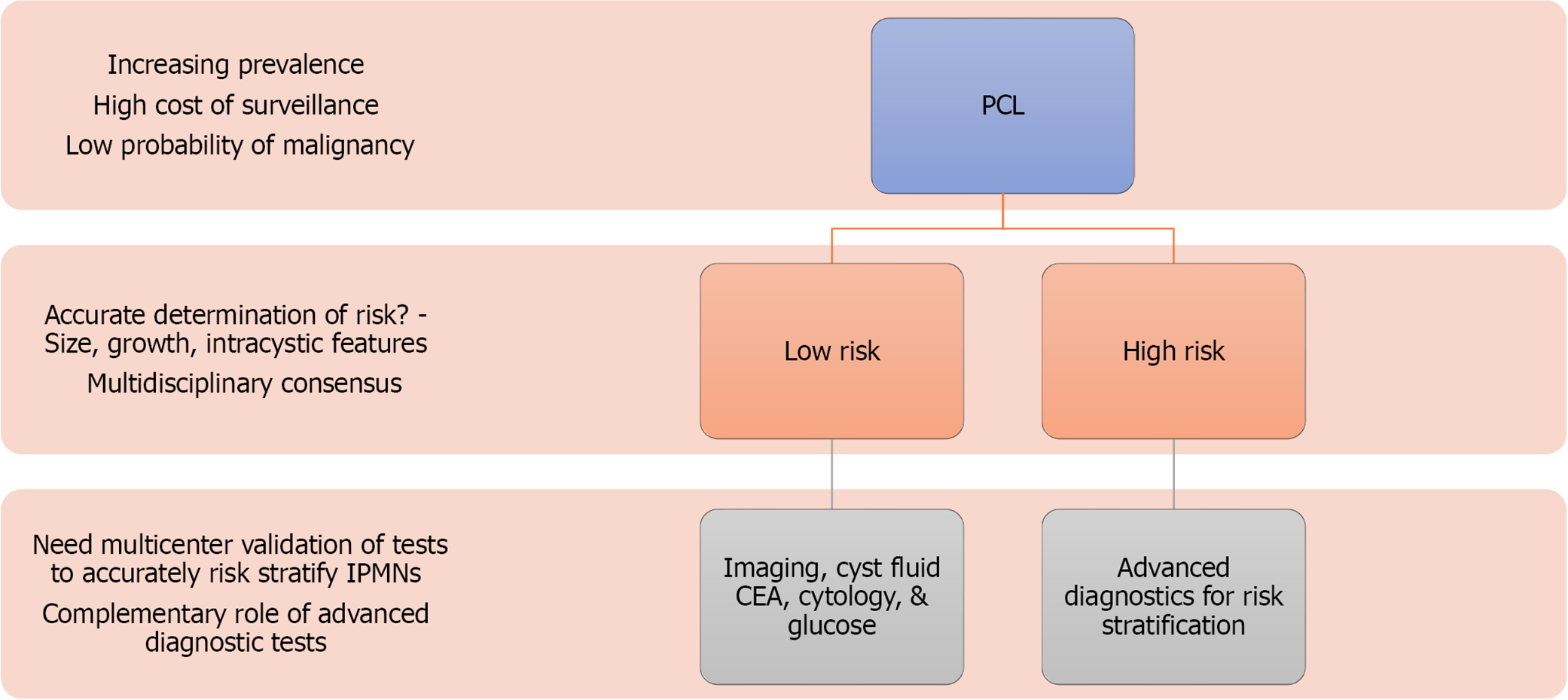
Reliable and accurate diagnosis of PCLs is a bottleneck for appropriate management of these lesions. Although, novel diagnostics have improved the diagnostic accuracy, there is still a dearth of prospective multicenter studies and a need to understand the complementary role of these tests. Radiomics, as a non-invasive tool has the potential for preliminary risk stratification of PCLs into low-and-high risk lesions (Figure 3). The technique holds a potential to allow clinicians to skip expensive and invasive diagnostic techniques on certain low risk PCLs.
For low-risk PCLs, and when EUS-FNA is indicated, low-cost cyst fluid analysis with glucose, CEA, and cytology can guide management (Figure 3). If radiomics and EUS cyst morphology are indicative of a high-risk PCL, advanced diagnostics with cyst fluid molecular analysis, nCLE, or microforceps biopsy can be considered based on the center and endoscopists’ expertise. The rate of adverse events with microforceps biopsy needs to be considered when considering this test.
Despite the availability of multiple diagnostic methods, the diagnosis and management of PCLs continues to be challenging. The more recent diagnostic modalities lack supportive larger multicenter data and there is need to demonstrate cost-effectiveness when compared to using suboptimal techniques and resultant unwarranted resection of otherwise benign or indolent PCLs. Apart from diagnosis, surveillance methods for low-risk lesions needs innovation as current tools are resource-consumptive.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Okasha HH S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, Völzke H, Mayerle J, Kühn JP. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 2. | Zerboni G, Signoretti M, Crippa S, Falconi M, Arcidiacono PG, Capurso G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Oh HC, Seo DW, Song TJ. Resolution of a septated pancreatic cyst by booster endoscopic ultrasonography-guided ablation. J Dig Dis. 2011;12:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 4. | Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-48.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 8. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 9. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 10. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 11. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | Ren R, Krishna SG, Chen W, Frankel WL, Shen R, Zhao W, Avenarius MR, Garee J, Caruthers S, Jones D. Activation of the RAS pathway through uncommon BRAF mutations in mucinous pancreatic cysts without KRAS mutation. Mod Pathol. 2021;34:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Nijhuis J, Bosscher F, Liem M. Accuracy of treatment decision-making in pancreatic cystic neoplasms based on available guidelines. European Journal of Surgical Oncology 2020; 46(2): e26-e27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Dmitriev K, Kaufman AE, Javed AA, Hruban RH, Fishman EK, Lennon AM, Saltz JH. Classification of Pancreatic Cysts in Computed Tomography Images Using a Random Forest and Convolutional Neural Network Ensemble. Med Image Comput Comput Assist Interv. 2017;10435:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Yang J, Guo X, Zhang H, Zhang W, Song J, Xu H, Ma X. Differential diagnosis of pancreatic serous cystadenoma and mucinous cystadenoma: utility of textural features in combination with morphological characteristics. BMC Cancer. 2019;19:1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Xie H, Ma S, Guo X, Zhang X, Wang X. Preoperative differentiation of pancreatic mucinous cystic neoplasm from macrocystic serous cystic adenoma using radiomics: Preliminary findings and comparison with radiological model. Eur J Radiol. 2020;122:108747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Wei R, Lin K, Yan W, Guo Y, Wang Y, Li J, Zhu J. Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol Cancer Res Treat. 2019;18:1533033818824339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Shen X, Yang F, Yang P, Yang M, Xu L, Zhuo J, Wang J, Lu D, Liu Z, Zheng SS, Niu T, Xu X. A Contrast-Enhanced Computed Tomography Based Radiomics Approach for Preoperative Differentiation of Pancreatic Cystic Neoplasm Subtypes: A Feasibility Study. Front Oncol. 2020;10:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Hanania AN, Bantis LE, Feng Z, Wang H, Tamm EP, Katz MH, Maitra A, Koay EJ. Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget. 2016;7:85776-85784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Permuth JB, Choi J, Balarunathan Y, Kim J, Chen DT, Chen L, Orcutt S, Doepker MP, Gage K, Zhang G, Latifi K, Hoffe S, Jiang K, Coppola D, Centeno BA, Magliocco A, Li Q, Trevino J, Merchant N, Gillies R, Malafa M; Florida Pancreas Collaborative. Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget. 2016;7:85785-85797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Attiyeh MA, Chakraborty J, Gazit L, Langdon-Embry L, Gonen M, Balachandran VP, D'Angelica MI, DeMatteo RP, Jarnagin WR, Kingham TP, Allen PJ, Do RK, Simpson AL. Preoperative risk prediction for intraductal papillary mucinous neoplasms by quantitative CT image analysis. HPB (Oxford). 2019;21:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Williams TL, Harrington KA, Lawrence SA, Chakraborty J, Al Efishat MA, Attiyeh MA, Askan G, Chou Y, Pulvirenti A, McIntyre CA. A combined radiomics and cyst fluid inflammatory markers model to predict preoperative risk in pancreatic cystic lesions. Proceedings of the Medical Imaging 2020: Image-Guided Procedures, Robotic Interventions, and Modeling; 2020. International Society for Optics and Photonics: 113151Q. [DOI] [Full Text] |
| 23. | Hoffman DH, Ream JM, Hajdu CH, Rosenkrantz AB. Utility of whole-lesion ADC histogram metrics for assessing the malignant potential of pancreatic intraductal papillary mucinous neoplasms (IPMNs). Abdom Radiol (NY). 2017;42:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Cui S, Tang T, Su Q, Wang Y, Shu Z, Yang W, Gong X. Radiomic nomogram based on MRI to predict grade of branching type intraductal papillary mucinous neoplasms of the pancreas: a multicenter study. Cancer Imaging. 2021;21:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 26. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Park WG, Mascarenhas R, Palaez-Luna M, Smyrk TC, O'Kane D, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, Chari ST. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas. 2011;40:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Ngamruengphong S, Lennon AM. Analysis of Pancreatic Cyst Fluid. Surg Pathol Clin. 2016;9:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756-2766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Wang QX, Xiao J, Orange M, Zhang H, Zhu YQ. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: a Meta-Analysis. Cell Physiol Biochem. 2015;36:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Stelow EB, Stanley MW, Bardales RH, Mallery S, Lai R, Linzie BM, Pambuccian SE. Intraductal papillary-mucinous neoplasm of the pancreas. The findings and limitations of cytologic samples obtained by endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol. 2003;120:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Ribaldone DG, Bruno M, Gaia S, Cantamessa A, Bragoni A, Caropreso P, Sacco M, Fagoonee S, Saracco GM, De Angelis C. Differential diagnosis of pancreatic cysts: A prospective study on the role of intra-cystic glucose concentration. Dig Liver Dis. 2020;52:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Mohan BP, Madhu D, Khan SR, Kassab LL, Ponnada S, Chandan S, Facciorusso A, Crino SF, Barresi L, McDonough S, Adler DG. Intracystic Glucose Levels in Differentiating Mucinous From Nonmucinous Pancreatic Cysts: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | McCarty TR, Garg R, Rustagi R. Pancreatic cyst fluid glucose in differentiating mucinous from non-mucinous pancreatic cysts: a systemic review and meta-analysis. Gastrointest Endosc. 2021;94: 698-712.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | McCarty TR, Paleti S, Rustagi T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:1019-1033.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 39. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Krishna SG, Hart PA, Malli A, Kruger AJ, McCarthy ST, El-Dika S, Walker JP, Dillhoff ME, Manilchuk A, Schmidt CR, Pawlik TM, Porter K, Arnold CA, Cruz-Monserrate Z, Conwell DL. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin Gastroenterol Hepatol. 2020;18:432-440.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 42. | Konjeti VR, McCarty TR, Rustagi T. Needle-based Confocal Laser Endomicroscopy (nCLE) for Evaluation of Pancreatic Cystic Lesions: A Systematic Review and Meta-analysis. J Clin Gastroenterol 2020. [DOI] [Full Text] |
| 43. | Facciorusso A, Buccino VR, Sacco R. Needle-based confocal laser endomicroscopy in pancreatic cysts: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Krishna SG, Hart PA, DeWitt JM, DiMaio CJ, Kongkam P, Napoleon B, Othman MO, Yew Tan DM, Strobel SG, Stanich PP, Patel A, Luthra AK, Chan MQ, Blaszczak AM, Lee D, El-Dika S, McCarthy ST, Walker JP, Arnold CA, Porter K, Conwell DL. EUS-guided confocal laser endomicroscopy: prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest Endosc. 2020;91:551-563.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Feng Y, Chang X, Zhao Y, Wu D, Meng Z, Wu X, Guo T, Jiang Q, Zhang S, Wang Q, Yang A. A new needle-based confocal laser endomicroscopy pattern of malignant pancreatic mucinous cystic lesions (with video). Endosc Ultrasound 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 46. | Machicado JD, Chao W-L, Carlyn DE, Pan T-Y, Poland S, Alexander VL, Maloof TG, Dubay K, Ueltschi O, Middendorf DM, Jajeh MO, Vishwanath AB, Porter K, Hart PA, Papachristou GI, Cruz-Monserrate Z, Conwell DL, Krishna SG. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointestinal Endoscopy 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 47. | Barresi L, Tacelli M, Ligresti D, Traina M, Tarantino I. Tissue acquisition in pancreatic cystic lesions. Dig Liver Dis. 2019;51:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Huelsen A, Cooper C, Saad N, Gupta S. Endoscopic ultrasound-guided, through-the-needle forceps biopsy in the assessment of an incidental large pancreatic cystic lesion with prior inconclusive fine-needle aspiration. Endoscopy. 2017;49:E109-E110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Tacelli M, Celsa C, Magro B, Barchiesi M, Barresi L, Capurso G, Arcidiacono PG, Cammà C, Crinò SF. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;32:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Westerveld DR, Ponniah SA, Draganov PV, Yang D. Diagnostic yield of EUS-guided through-the-needle microforceps biopsy vs EUS-FNA of pancreatic cystic lesions: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E656-E667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Rift CV, Scheie D, Toxværd A, Kovacevic B, Klausen P, Vilmann P, Hansen CP, Lund EL, Hasselby JP. Diagnostic accuracy of EUS-guided through-the-needle-biopsies and simultaneously obtained fine needle aspiration for cytology from pancreatic cysts: A systematic review and meta-analysis. Pathol Res Pract. 2021;220:153368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, Imai H, Sakamoto H, Harwani Y, Chikugo T, Chiba Y, Matsumoto I, Takeyama Y, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Buxbaum J, Ko C, Varghese N, Lee A, Sahakian A, King K, Serna J, Lee H, Tchelepi H, Van Dam J, Duddalwar V. Qualitative and Quantitative Contrast-enhanced Endoscopic Ultrasound Improves Evaluation of Focal Pancreatic Lesions. Clin Gastroenterol Hepatol. 2020;18:917-925.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Kamata K, Takenaka M, Minaga K, Omoto S, Miyata T, Yamao K, Imai H, Nakai A, Tanaka H, Chiba Y, Watanabe T, Sakurai T, Nishida N, Chikugo T, Matsumoto I, Takeyama Y, Kitano M, Kudo M. Value of additional endoscopic ultrasonography for surveillance after surgical removal of intraductal papillary mucinous neoplasms. Dig Endosc. 2018;30:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Zhong L, Chai N, Linghu E, Li H, Yang J, Tang P. A Prospective Study on Contrast-Enhanced Endoscopic Ultrasound for Differential Diagnosis of Pancreatic Cystic Neoplasms. Dig Dis Sci. 2019;64:3616-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |