Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6599
Peer-review started: September 11, 2022
First decision: October 19, 2022
Revised: October 29, 2022
Accepted: November 19, 2022
Article in press: November 19, 2022
Published online: December 14, 2022
Processing time: 88 Days and 3.8 Hours
There is growing evidence that patients with coronavirus disease 2019 (COVID-19) frequently present with liver impairment. Hepatitis B virus (HBV) remains a major public health threat in current society. Both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and HBV can cause liver damage, and current findings on whether HBV infection increases disease severity in COVID-19 patients are inconsistent, and whether SARS-CoV-2 infection accelerates hepatitis B progression or leads to a worse prognosis in hepatitis B patients has not been adequately elucidated.
To explore the complex relationship between COVID-19 and hepatitis B in order to inform the research and management of patients co-infected with SARS-CoV-2 and HBV.
An experienced information specialist searched the literature in the following online databases: PubMed, China National Knowledge Infrastructure, Google Scholar, Scopus, Wiley, Web of Science, Cochrane, and ScienceDirect. The literature published from December 2019 to September 1, 2022 was included in the search. We also searched medRxiv and bioRxiv for gray literature and manually scanned references of included articles. Articles reporting studies conducted in humans discussing hepatitis B and COVID-19 were included. We excluded duplicate publications. News reports, reports, and other gray literature were included if they contained quantifiable evidence (case reports, findings, and qualitative analysis). Some topics that included HBV or COVID-19 samples but did not have quantitative evidence were excluded from the review.
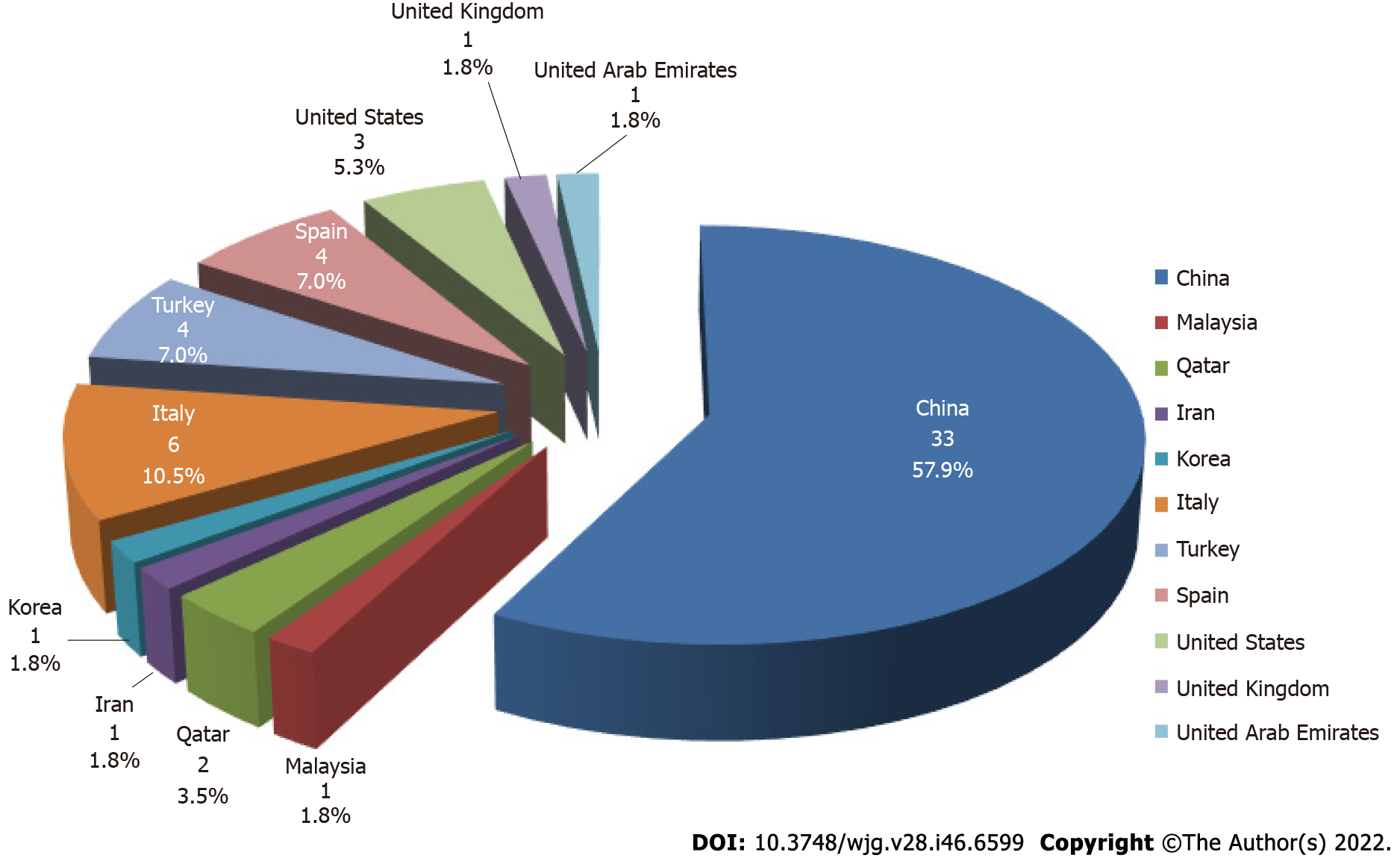
A total of 57 studies were eligible and included in this review. They were from 11 countries, of which 33 (57.9%) were from China. Forty-two of the 57 studies reported abnormalities in liver enzymes, three mainly reported abnormalities in blood parameters, four indicated no significant liver function alterations, and another eight studies did not provide data on changes in liver function. Fifty-seven studies were retrospective and the total number of co-infections was 1932, the largest sample size was 7723, and the largest number of co-infections was 353. Most of the studies suggested an interaction between hepatitis B and COVID-19, while 12 studies clearly indicated no interaction between hepatitis B and COVID-19. Six of the 57 studies clearly reported HBV activation. Six studies were related to liver transplant patients.
There is some association between COVID-19 and hepatitis B. Future high-quality randomized trials are needed to further elucidate the interaction between COVID-19 and hepatitis B.
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hepatitis B virus (HBV) infections are two major current global public health crises. Both infection with SARS-CoV-2 and infection with HBV can cause liver damage. There are conflicting views on whether HBV infection aggravates the prognosis of patients with coronavirus disease 2019 (COVID-19). There is a potential association between COVID-19 and hepatitis B. Clarification of this association could benefit these special patients with SARS-CoV-2 and HBV co-infection.
- Citation: He YF, Jiang ZG, Wu N, Bian N, Ren JL. Correlation between COVID-19 and hepatitis B: A systematic review. World J Gastroenterol 2022; 28(46): 6599-6618
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6599.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6599
Since the outbreak of coronavirus disease 2019(COVID-19), there has been increasing evidence that patients with COVID-19 frequently present with hepatic impairment[1-3]. This may be caused by pre-existing liver disease, viral infection of hepatocytes, and certain medications. Hepatitis B virus (HBV) remains a major current public health threat to society, with approximately 300 million people chronically infected worldwide[4]. Since both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and HBV can cause liver damage, current findings on whether HBV infection increases disease severity in patients with COVID-19 are inconsistent, with some reports suggesting that co-infection with SARS-CoV-2 and HBV has no effect on the course and prognosis of COVID-19[5,6], while several studies have shown that patients with SARS-CoV-2 and HBV co-infection are more likely to have serious outcomes[7-9]. Does pre-existing HBV infection increase susceptibility to SARS-CoV-2 infection, leading to more severe disease and a worse prognosis? Conversely, does SARS-CoV-2 infection accelerate the progression of hepatitis B or lead to worse outcomes? The potential association between hepatitis B and COVID-19 has not been fully elucidated, so it is important and interesting to understand how COVID-19 and hepatitis B interact with each other. In order to elucidate this complexity, we summarize almost all current clinical studies to provide a detailed description of the interaction between COVID-19 and hepatitis B, possible mechanisms, and clinical interventions to provide a reference for the clinical management of these special patients.
Does SARS-CoV-2 and HBV co-infection have any effect on the course and prognosis of COVID-19? And vice versa, does COVID-19 accelerate the progression of hepatitis B and lead to a severe prognosis?
The literature published from December 2019 to September 1, 2022 was included in the search. To avoid missing any relevant and important literature, we used an inclusive search strategy. An experienced information specialist searched the literature in the following online databases: PubMed, China National Knowledge Infrastructure, Google Scholar, Scopus, Wiley, Web of Science, Cochrane, and ScienceDirect. We also searched medRxiv and bioRxiv for gray literature and manually searched the references of the included studies to further ensure the comprehensiveness of the search. Articles that met the criteria in the Reference Citation Analysis (https://www.referencecitationanalysis.com) were also included in this review. Articles performed in humans and discussing SARS-CoV-2 in patients with hepatitis B and HBV in COVID-19 patients were included. Detailed search strategies are shown in Supplementary material.
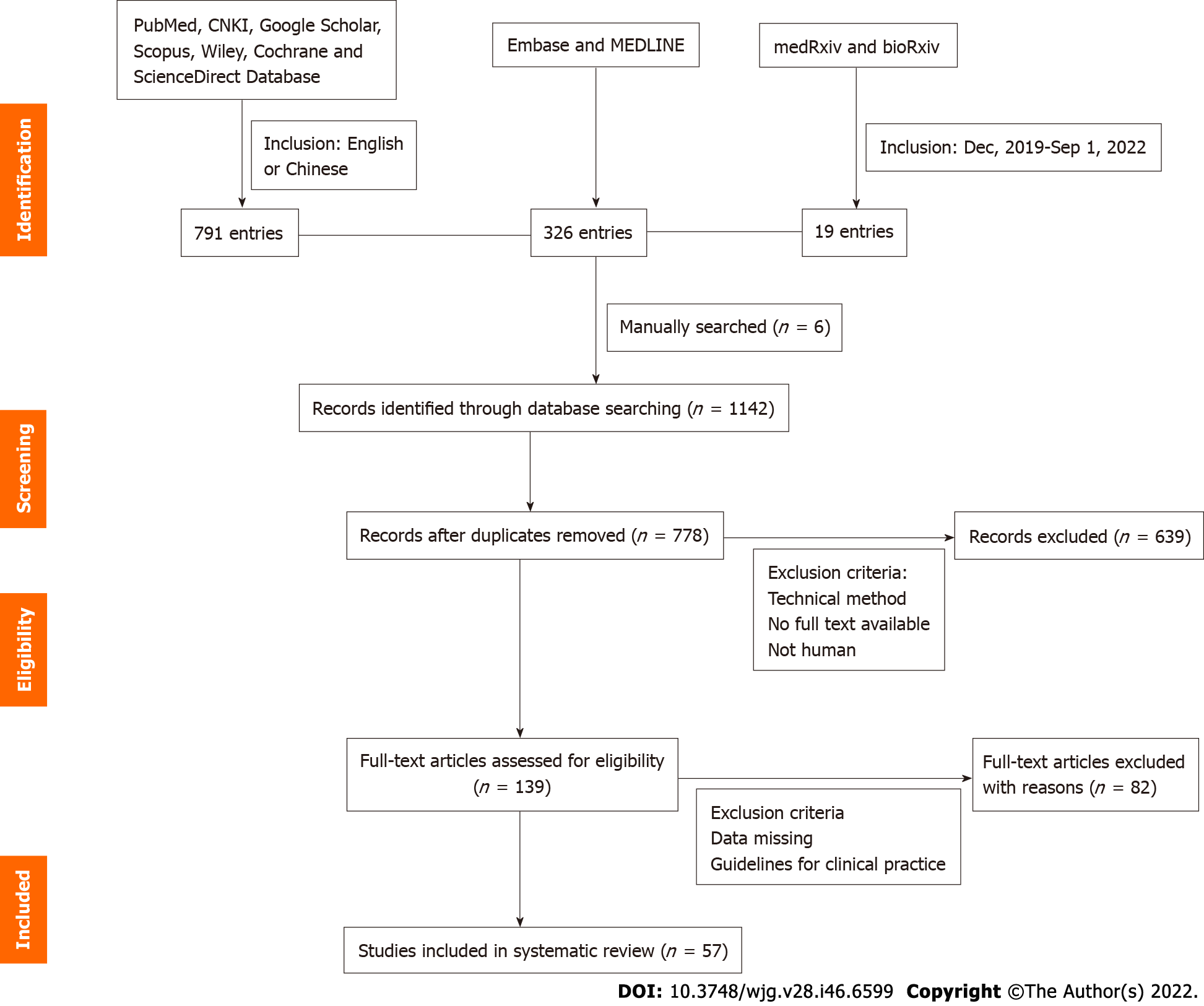
Several types of articles were included after our initial search, including case reports, case series, qualitative studies, and systematic and scoping reviews. We excluded repetitive publications. Articles containing more complete descriptions of the data were used for data charting. News articles, case reports, survey results, qualitative analyses, and other gray literature were also included in our review if they contained quantifiable evidence. We read the full text, synthesized the relevant evidence, and organized the literature thematically. All five authors participated in the discussion and decided on a theme. Some topics that included HBV or COVID-19 samples but did not have quantitative evidence were also excluded from the review. Figure 1 shows a visual representation of inclusion workflow.
As of September 1, 2022, we retrieved 1136 publications, adding six manually retrieved papers for a total of 1142. We tested 139 full-text articles for eligibility after being screened by the above inclusion criteria, and 57 were finally included in this review (Figure 1). Two independent reviewers extracted data from the full-text papers of eligible studies, including the name of the first author, publication month and year, country of the study, number of included patients, number of SARS-CoV-2 and HBV co-infections, main biochemical characteristics of co-infected patients, their clinical outcome (death or survival), and main conclusion of each study. A summary of information on the included studies is presented in Table 1[9-60], and details of important parameters are presented in Supplementary Table 1. This systematic review has been registered on the PROSPERO platform: https://www.crd.york.ac.uk/prospero/#recordDetails.
| Ref. | Site | Sample size (n) | No. of patients with HBV infection, n (%) | Major serum biochemical characteristics of co-infected patients | Outcomes | |
| Death, n (%) | Survival, n (%) | |||||
| Guan et al[53] | China | 1590 | 28 (1.8) | NR | 1 (3.6) | 27 (96.4) |
| Zou et al[2] | China | 93 | 93 (100.0) | Abnormal liver function | 7 (7.5) | 86 (92.5) |
| Song et al[47] | China | 4 | 2 (50.0) | Abnormal blood parameters | 0 (0.0) | 2 (100.0) |
| Qi et al[10] | China | 3 | 1 (33.3) | Abnormal liver enzyme | 1 (100) | 0 (0.0) |
| Hambali et al[11] | Malaysia | 1 | 1 (100.0) | Abnormal liver enzyme | 0 (0.0) | 1 (100.0) |
| Ali et al[12] | Qatar | 1 | 1 (100.0) | Elevated liver enzymes | 1 (100) | 0 (0.0) |
| Zha et al[13] | China | 31 | 2 (6.5) | Elevated liver enzymes | 0 (0.0) | 2 (100.0) |
| Cai et al[14] | China | 298 | 5 (1.7) | Elevated liver enzymes | NR | NR |
| Naderi et al[15] | Iran | 931 | 13 (13.8)2 | Elevated liver enzymes | 0 (0.0) | 13 (100.0) |
| Yip et al[16] | Hong Kong SAR, China | 5639 | 353 (6.3)3 | ALT abnormality | 8 (2.3) | 345 (97.7) |
| Richardson et al[17] | United States | 5700 | 8 (0.1) | Elevated liver enzymes | NA | NA |
| Kang et al[54] | Korea | 7723 | 267 (3.5) | NR | 12 (5.1) | 255 (94.9) |
| Li et al[18] | China | 7 | 7 (100.0) | Elevated liver enzymes | 0 (0.0) | 7 (100.0) |
| Chen et al[9] | China | 123 | 15 (12.2) | Abnormal liver enzyme | 2 (13.3) | 13 (86.7) |
| Bongiovanni et al[19] | Italy | 1 | 1 (100.0) | Liver enzymes increased | 0 (0.0) | 1 (100.0) |
| He et al[20] | China | 571 | 15 (2.6) | Liver enzymes increased | 0 (0.0) | 15 (100.0) |
| Wen et al[21] | China | 110 | 5 (4.5) | Abnormal liver enzyme | NA | NA |
| Zou et al[7] | China | 105 | 105 (100.0) | Liver enzymes increased | 98 (93.3) | 7 (6.7) |
| Wang et al[8] | China | 436 | 109 (25.0) | Abnormal liver enzyme system | 13 (11.93) | 96 (88.1) |
| Chen et al[5] | China | 326 | 20 (6.1) | Lower level of prealbumin | 0 (0.0) | 20 (100.0) |
| Zhang et al[22] | China | 23 | 23 (100.0) | Liver enzymes increased | 0 (0.0) | 23 (100.0) |
| Liu et al[48] | China | 220 | 50 (22.7) | Abnormal blood parameters | 4 (8) | 46 (92.0) |
| Yu et al[6] | China | 67 | 7 (10.4) | No significant change | 0 (0.0) | 7 (100.0) |
| Liu et al[23] | China | 714 | 20 (28.2)4 | Abnormal liver enzyme system | 0 (0.0) | 20 (100.0) |
| Lin et al[24] | China | 133 | 17 (12.8) | Liver enzymes increased | NR | NR |
| Bekçibaşı and Arslan[25] | Turkey | 156 | 20 (12.8) | Liver enzymes increased | 0 (0.0) | 20 (100.0) |
| Colaneri et al[26] | Italy | 1 | 1 (100.0) | Liver enzymes increased | 0 (0.0) | 1 (100.0) |
| Ma et al[50] | China | 109 | 1 (0.9) | Normal liver enzymes | NA | NA |
| Chen et al[27] | China | 274 | 11 (4.0) | Abnormal liver function | 5 (45.5) | 6 (54.5) |
| Ding et al[28] | China | 2073 | 134 (6.5) | Liver enzymes increased | 8 (6.0) | 126 (94.0) |
| Rodríguez-Tajes et al[29] | Spain | 484 | 72 (14.9) | Liver enzymes increased | 8 (11.1) | 64 (88.9) |
| Parlar et al[51] | Turkey | 4795 | 43 (9.0)2 | No significant change | 0 (0.0) | 43 (100.0) |
| Yang et al[30] | China | 2899 | 105 (3.6) | Liver enzymes increased | 18 (17.1) | 87 (82.9) |
| Yigit et al[31] | Qatar | 1 | 1 (100.0) | Liver enzymes increased | 1 (100) | 0 (0.0) |
| Aldhaleei et al[32] | United Arab Emirates | 1 | 1 (100.0) | Liver enzymes increased | 0 (0.0) | 1 (100.0) |
| Ji et al[33] | China | 140 | 7 (5.0) | Liver enzymes increased | 0 (0.0) | 7 (100.0) |
| Kim et al[34] | United States | 867 | 62 (7.2) | Liver enzymes increased | 5 (8.1) | 57 (91.9) |
| Wang et al[35] | China | 1 | 1 (100.0) | Abnormal liver enzyme system | 0 (0.0) | 1 (100.0) |
| Zhong et al[36] | China | 2 | 1 (50.0) | Liver enzymes increased | 0 (0.0) | 1 (100.0) |
| Fernández-Ruiz et al[49] | Spain | 18 | 2 (11.1) | Decreased white blood cells | 1 (50.0) | 1 (50.0) |
| Huang et al[37] | China | 1 | 1 (100.0) | Elevated total bilirubin | 1 (100) | 0 (0.0) |
| Patrono et al[57] | Italy | 10 | 2 (20.0) | NR | 0 (0.0) | 2 (100.0) |
| Qin et al[38] | China | 1 | 1 (100.0) | Abnormal liver enzyme system | 0 (0.0) | 1 (100.0) |
| Liu et al[58] | China | 1 | 1 (100.0) | NR | 0 (0.0) | 1 (100.0) |
| Loinaz et al[39] | Spain | 19 | 4 (21.1) | Abnormal liver enzyme system | 1 (25.0) | 3 (75.0) |
| Adali et al[40] | Turkey | 231 | 77 (33.3) | Abnormal liver enzyme system | 6 (7.8) | 71 (92.2) |
| Oruç et al[55] | Turkey | 92 | 4 (4.3) | NR | 0 (0.0) | 4 (100.0) |
| Guardigni et al[56] | Italy | 606 | 12 (2.0) | NR | NA | NA |
| Sagnelli et al[41] | Italy | 1 | 1 (100.0) | Liver enzymes increased | 1 (100) | 0 (0.0) |
| Lens et al[59] | Spain | 17645 | 9 (0.5)2 | NR | 0 (0.0) | 9 (100.0) |
| Phipps et al[42] | United States | 2273 | 15 (0.7) | Abnormal liver enzyme system | NA | NA |
| Li et al[52] | China | 85 | 2 (2.4) | Normal liver enzymes | 0 (0.0) | 2 (100.0) |
| Wu et al[43] | China | 1 | 1 (100.0) | Elevated liver enzyme | 0 (0.0) | 1 (100.0) |
| Wu et al[44] | China | 620 | 70 (11.3) | Elevated liver enzymes | 0 (0.0) | 70 (100.0) |
| Iavarone et al[45] | Italy | 50 | 5 (10.0) | Elevated liver enzymes | NA | NA |
| Marjot et al[60] | United Kingdom | 745 | 96 (12.9) | NR | 23 (24.0) | 73 (76.0) |
| Huang et al[46] | China | 1 | 1 (100.0) | Elevated liver enzymes | 0 (0.0) | 1 (100.0) |
| Total | 57 | 37375 | 1932 | |||
A total of 57 studies were eligible and included in this review. They were from 11 countries, of which 33 (57.9%) were from China (Figure 2). Forty-two of the 57 studies reported abnormalities in liver enzymes[2,5,7-46], three mainly reported abnormalities in blood parameters[47-49], four indicated no significant liver function alterations[6,50-52], and another eight studies did not provide data on changes in liver function[53-60]. Fifty-seven studies were retrospective, and the total number of co-infections was 1932, the largest sample size was 7723[54], and the largest number of co-infections was 353[16]. Most of the studies suggested an interaction between hepatitis B and COVID-19, while 12 studies clearly indicated no interaction between hepatitis B and COVID-19[5,11,16,18,21,25,28,50,51,54-56]. Six of the 57 studies clearly reported HBV activation[23,29,41,43,46,52]. Six studies were related to liver transplant patients[36,38,39,49,57,58].
As previously mentioned, the possible reasons why HBV infection was not associated with clinical outcomes of COVID-19, despite the fact that some patients had high liver enzyme levels, are as follows: (1) The infection rate is not affected. The main consideration is the positive effect of nucleoside analogues on resistance to SARS-CoV-2[59]. In the included studies, some hepatitis B patients received long-term treatment with nucleoside analogues, such as tenofovir, which binds tightly to RNA-dependent RNA polymerase and terminates SARS-CoV-2 RNA synthesis[61,62], resulting in a low rate of SARS-CoV-2 infection in chronic hepatitis B (CHB) patients treated with anti-HBV drugs; (2) The severity of the disease is not affected. The main consideration is the key role played by immune dysfunction in SARS-CoV-2 and HBV co-infected patients. The cytokine storm caused by SARS-CoV-2 infection leads to overproduction of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-2, while HBV infection with persistent viral antigens leads to virus-specific CD4+ and CD8+ T cell depletion, resulting in impaired cytokine secretion, especially IL-2 and TNF-α, in which the depleted and immunosuppressed state of HBV-specific T lymphocytes may attenuate or avoid the excessive host immune response to SARS-CoV-2 and reduce the cytokine storm, resulting in a less severe disease[63-65]; and (3) Prognosis is not affected. Previous studies have demonstrated that hepatitis C virus infection can limit the replication of HBV[66], a phenomenon called viral interference, in which one virus in the host prevents the replication of another co-infected virus by competitive inhibition rather than by the action of immune antibodies. Impaired type I interferon activity has been found to be a major feature of patients with severe COVID-19[67-69]. When co-infected, HBV can inhibit the replication and proliferation of co-infected SARS-CoV-2 by enhancing type I interferon signaling, thereby reducing viral load and prolonging survival. In addition to this, the small number of HBV co-infected patients in the 57 studies that we included may have influenced the results.
Explanation of enzyme system abnormalities: Many studies have suggested that elevated liver enzyme systems are associated with drug therapy (Supplementary Table 1). Corticosteroids have been widely used to treat and benefit COVID-19[70]. Systemic corticosteroids are also associated with an increased risk of aspartate aminotransferase (AST) elevation[71]. Several studies have reported that elevated liver enzyme systems are associated with antiviral therapy medication[72-74]. The results of these studies showed that liver enzyme levels in patients dropped to near normal within a few days after discontinuation of medication. In addition, a study by Lei et al[71] showed that elevated AST and alkaline phosphatase levels were associated with the use of antifungal drugs. Another explanation is that some enzymes reflecting liver function, such as glutamic aminotransferase, are nonspecific and are expressed in a wide range of tissues, including heart, skeletal muscle, red blood cells, and liver, and can originate from other sites[75,76].
Explanation for the decrease in albumin: Patients with SARS-CoV-2 and HBV co-infection show a decrease in albumin, especially in heavy and critically ill patients, which may be due to the high catabolic state of the patient’s body, poor nutritional status, negative nitrogen balance, and insufficient raw materials for synthesizing albumin, as well as impaired hepatic synthesis of albumin due to hepatocyte damage. Corticosteroid treatment can also cause a decrease in albumin during the treatment process[21]. COVID-19 patients co-infected with HBV have lower prealbumin levels due to weaker hepatic reserve capacity, which also contributes to the decrease in albumin. With the resolution of the disease, most of the patients’ liver function will return to normal.
Explanation for lymphocytopenia and thrombocytopenia: Patients with SARS-CoV-2 and HBV co-infection have significantly lower white blood cell counts, mainly due to reduced lymphocyte counts and significantly lower monocyte levels. The mechanism leading to lymphocytopenia remains unclear. Since low lymphocyte counts are associated with increased disease severity and mortality in COVID-19[77,78], patients with SARS-CoV-2 and HBV co-infection may be in greater need of increased clinical surveillance. Thrombocytopenia is common in liver disease caused by HBV infection. The mechanism of thrombocytopenia in COVID-19 may be that SARS-CoV-2 reduces platelet production, increases platelet destruction, or increases platelet consumption[79]. Specifically, the lung is one of the organs where megakaryocytes dynamically release platelets[80], and SARS-CoV-2 damages the lungs of COVID-19 patients through angiotensin-converting enzyme 2 (ACE2), leading to increased destruction of megakaryocytes in the lung and resulting in decreased platelet production. In addition, SARS-CoV-2 may directly invade hematopoietic cells or infect bone marrow stromal cells by binding to CD13 or CD66a receptors, etc., damaging megakaryocytes and platelets and exacerbating apoptosis[81]. Cytokine storm leads to immune hyperactivation, causing cellular damage and increased platelet destruction through autoantibody or immune complex activation of complement; at the same time, immune hyperactivation results in the release of large amounts of inflammatory factors that promote excessive platelet activation and platelet-monocyte aggregation formation[82], forming thrombi at the site of injury and further leading to increased platelet depletion and destruction.
We mainly describe the effects of COVID-19 on hepatitis B from the aspects of HBV reactivation, abnormal liver enzyme system, mortality, and liver histopathological changes.
HBV reactivation: Six of the 57 included studies reported COVID-19-induced HBV reactivation (Supplementary Table 1), which primarily refers to the re-detection of HBV DNA in patients who previously had HBV DNA below the lower limit of detection or a sudden and substantial increase in HBV DNA levels in individuals with detectable HBV DNA[83]. In some cases, it may also evolve into fulminant liver failure. The mechanism of HBV reactivation after SARS-CoV-2 infection is mainly due to a disruption of the balance between the immune status of the host and viral replication. The intensity of glucocorticoid or immunosuppressive therapy is a major risk factor for HBV reactivation during COVID-19 treatment[29,41,84]. Age, male sex, and severe comorbidities (such as hypertension, diabetes, hypercholesterolemia, and chronic kidney disease) are considered risk factors for HBV reactivation[29]. In addition, SARS-CoV-2 infection causes significant lymphopenia, which may increase the likelihood of HBV reactivation.
Although there is a risk of HBV reactivation with SARS-CoV-2 infection, studies have shown that the overall risk is low. In a study by Rodríguez-Tajes et al[29], 61 patients with severe COVID-19 on immunosuppressive therapy were followed up for at least one month and no cases of hepatitis B surface antigen (HBsAg) reversal were found, with only two (3%) patients having detectable serum HBV-DNA.
Considering the risk of reactivation, it is recommended that HBV patients should start or continue anti-HBV therapy once COVID-19 is diagnosed[85], and some scholars recommend treatment with the nucleoside analogs entecavir and tenofovir[83], and closely monitoring HBV virological indicators and indicators related to liver injury. It has been suggested that the use of IL-6 receptor antagonists can reduce the risk of HBV reactivation, and in Watanabe et al[86]’s study, 152 patients who recovered from HBV infection treated with disease-modifying antirheumatic drugs had a very low risk of HBV reactivation (< 5%).
Abnormalities in liver enzymes: Most studies have evaluated the effects of COVID-19 in HBV infected patients mainly manifesting as abnormalities in liver enzymes, dominated by elevated levels of alanine aminotransferase (ALT), AST, and total bilirubin (TBIL), especially in severely ill patients[2,5,7-46] (Supplementary Table 1). Several studies have shown a predominance of AST elevations in COVID-19 patients[14,17,71,87], reflecting the severity of the disease. A large study including 5700 subjects showed elevated levels of AST (58%) and ALT (39%) in hospitalized COVID-19 patients[17]. Sixty-two percent of critically ill patients had elevated AST levels[88]. Abnormal liver function tests ranged from 37% to 69%, ALT and AST increased by 18.2% to 31.6% and 14.8% to 35.4%, respectively[14,52,89,90], and several studies have reported elevated TBIL elevation (5.1% to 11.5%)[89,91]. It is worth noting that AST alone does not prove with certainty that more liver damage occurred, as other tissues, including heart or muscle tissue, can also release glutathione[75,76].
Zhao et al[92] also noted that COVID-19 patients had significantly higher levels of γ-glutamyl transpeptidase, lactate dehydrogenase (LDH), and α-hydroxybutyric dehydrogenase than non-COVID-19 patients. A study by Chen et al[27] including 113 deceased patients (five of whom were co-infected with SARS-CoV-2 and HBV) showed that the deceased patients had creatinine, creatine kinase (CK), LDH, cardiac troponin I, N-terminal pro-brain natriuretic peptide (NT-proBNP), D-dimer, IL-2 receptor (IL2R), IL-6, IL-8, IL-10, and TNF-α concentrations that were significantly higher compared to those in recovered patients. Among these, several studies have confirmed that NT-proBNP is strongly and independently associated with mortality in COVID-19 patients[93-95]. In addition, serum albumin was below the normal range, and prothrombin time was prolonged due to the compromised synthetic function of the liver.
Mortality: Several reports have suggested that pre-existing liver disease may be associated with high mortality in patients with COVID-19[96-99]. Studies have noted that the risk of mortality is associated with pre-existing liver disease status and not with age, race, body mass index, hypertension, or diabetes[96]. Several studies have evaluated the relationship between the severity of abnormal liver tests and mortality in patients with COVID-19[1,14,96,97]. A study from the United States showed that among 2780 patients with COVID-19, the mortality rate was 12% in patients with chronic liver disease (10 out of 250 patients with chronic liver disease have CHB), compared to 4% in patients without liver disease[99]. Another study of 152 consecutively submitted COVID-19 cases in patients with chronic liver disease found a higher mortality rate in COVID-19 patients with combined chronic liver disease, 11.8% of whom were hepatitis B patients[96]. These studies support that SARS-CoV-2 affects the immune response, exacerbating HBV replication during the acute and chronic phases of hepatitis B infection[12]. Iavarone et al[45] also concluded that COVID-19 is associated with increased mortality in patients with deteriorating liver function. The cause of death may be related to the failure of patients to receive timely specialist care, inadequate hepatoprotective therapy, and hepatitis B infection complicated by SARS-CoV-2 leading to fulminant hepatitis and multi-organ failure.
Histopathological changes of the liver: Histopathology-specific manifestations of the liver in patients with COVID-19 include binucleated or multinucleated hepatocytes, swollen mitochondria, and reduced glycogen granules[47]. Macrovesicular steatosis is one of the common nonspecific manifestations[72], in addition to mild portal lymphocytic infiltration, mild sinusoidal dilatation, inflammatory cells in the sinusoids, and portal vein inflammation, with electron microscopy also showing the presence of viral particles[100-103].
We will describe the effect of hepatitis B on COVID-19 in terms of the rate of infection in patients with hepatitis B, changes in biochemical indicators and cytokines, and the prognostic effect of hepatitis B on COVID-19 (including mortality, complications, and severe COVID-19).
Rate of infection: An important indicator of the impact of hepatitis B on COVID-19 is the rate of infection. It has been shown that hepatitis virus infection reduces the chance of COVID-19 infection to some extent in people with hepatitis B[15]. Antiviral drugs also reduce susceptibility to COVID-19 infection. Data from Naderi et al[15] showed that the risk of COVID-19 in healthy individuals was 2.3 times higher than that in patients with CHB. Antiviral therapy in patients with CHB, including tenofovir and entecavir, resulted in a decreased incidence of SARS-CoV-2 positivity. Kang et al[54]reported that the occurrence of CHB resulted in a lower rate of SARS-CoV-2 positivity. A recently published survey of a larger cohort in Spain showed a reduced incidence of COVID-19 infection in CHB patients given tenofovir, suggesting a positive effect of tenofovir on SARS-CoV-2[59]. A large study in the United States including 5700 inpatients with COVID-19 showed that 14% (13/93) of CHB patients and 32.25% (20/62) of controls were infected with SARS-CoV-2, respectively, and the risk of developing COVID-19 was 2.3 times higher in healthy controls than in CHB patients, suggesting that antiviral drugs also reduced the susceptibility to SARS-CoV-2 infection[17].
Cytokines and biochemical indicators: As with the effects of COVID-19 on hepatitis B, SARS-CoV-2 and HBV co-infection aggravates liver injury in COVID-19 patients[5,24], leading to abnormalities in the liver enzyme system. In addition to ALT, AST, alkaline phosphatase, and TBIL described previously[30,32,37], IL-6, LDH, D-dimer TNF-α, CK, and other inflammatory factors levels are elevated[14,27,104,105].
Among the cytokines in co-infected patients, IL-6 was most significantly elevated. IL-6 is the result of SARS-CoV-2 activation of the immune system leading to massive cytokine release[106]. IL-6 plays a role as a pro-inflammatory factor in the regulation of vascular leakage, complement activation, and coagulation pathways. The coagulation biomarker D-dimer is derived from the formation and cleavage of cross-linked fibrin, and the systemic pro-inflammatory cytokine response following viral infection may induce endothelial cell dysfunction, leading to overproduction of thrombin, which activates platelets and stimulates fibrinolysis, resulting in elevated D-dimer[107,108]. D-dimer has also been found in COVID-19 patients to be associated with poor outcome and abnormally dysregulated in CHB patients[109]. LDH not only plays an important role in glucose metabolism by catalyzing the conversion of pyruvate to lactate, but also regulates the immune response by inducing T cell activation and enhancing immunosuppressive cells through lactate production[110]. Serum CK levels are the most sensitive indicator of muscle damage[111]. Hypovolemia occurring in dehydrated patients with severe COVID-19 may lead to renal failure, which may increase CK levels[25]. Two recent studies suggest that high CK, IL-6, and D-dimer levels may be early warning indicators of serious disease and monitoring these indicators may help in the early detection of adverse events[25,112].
Mortality, complications, or serious prognosis: Patients with SARS-CoV-2 and HBV co-infection are more likely to have severe disease and a worse prognosis, including higher mortality and complications[44,55,60]. Two recent studies have shown that patients with COVID-19 who have pre-existing liver disease have a higher mortality rate compared to patients without liver disease[96,97]. Mirzaie et al[113] found that of the reported SARS-CoV-2-HBV co-infection cases, the mortality rate was 4.7%, 14.1% were transferred to the intensive care unit (ICU), and 38.8% reported severe COVID-19. In a study by Chen et al[9], seven of 15 HBV-infected patients (46.7%) developed severe disease, compared with 24.1% of severe cases in COVID-19 patients without HBV infection. In a study of 436 patients with COVID-19, more patients with CHB developed a severe status compared to non-CHB patients [30/109 (27.52% vs 5.20%) 17/327], with mortality rates of 13/109 (11.93%) and 8/327 (2.45%), respectively[8]. Another recent study by Wu et al[44] involving 70 co-infections showed a higher proportion of severe patients and critical patients in the COVID-19 and HBV co-infected group than in the non-HBV infected group (32.86% vs 15.27%). Data from a study by Yang et al[30] showed that patients with COVID-19 combined with CHB had a higher risk of death and ICU admission than patients without CHB. These data support the hypothesis that patients with liver disease may be associated with serious outcomes in patients with COVID-19. However, it is unclear whether these results reflect the severity of the patient’s pre-existing liver disease or the severe prognosis caused by SARS-CoV-2 itself. However, it has also been reported to the contrary, that is, the incidence of ICU admission or death may be lower in patients with observed pre-existing HBV infection [none (0%) vs 36 (6.47%)] compared to 556 of 571 COVID-19 cases without HBV infection[20]. These findings suggest that the immune status of the host is influenced to some extent by CHB, which may affect the consequences of infection by SARS-CoV-2.
Yu et al[6] included 7 HBsAg(+) and 60 HBsAg(-) (a total of 67) COVID-19 patients and found no extensive fluctuations in markers of HBV replication during the acute course of SARS-CoV-2 infection, indicating that SARS-CoV-2 had no effect on HBV kinetics, and that co-infection did not prolong viral shedding or latency cycles in COVID-19 patients, and it did not trigger reactivation or seroconversion of CHB. Therefore, they concluded that SARS-CoV-2 infection would not be a source of HBV reactivation. Ding et al[28] conducted a study in which 204 of 2073 patients with COVID-19 had pre-existing liver disease, including 134 patients with hepatitis B and five patients with CHB cirrhosis, and after adjusting for baseline characteristics by matching propensity scores between groups, the in-hospital mortality rate in patients with hepatitis B was similar to that in patients without liver disease. These results suggest that SARS-CoV-2 infection may not increase the risk of severe liver injury in patients with hepatitis B and in patients with compensated liver function.
In a study by Wen et al[21], patients with COVID-19 combined with hepatitis B had no significant increase in the probability of abnormal ALT, AST, and albumin compared to patients without combined hepatitis B. No differences were found in various adverse clinical outcomes in patients with CHB compared to HBsAg negative patients. Chen et al[5] divided 326 COVID-19 confirmed cases into 20 patients with co-infection with HBV and 306 patients without HBV infection for observation and found no significant differences in the levels of liver function parameters, discharge rates, length of stay, or mortality between the two groups. Similarly, a study by Yu et al[6] showed that co-infection with HBV did not increase the severity of COVID-19 or prolong the length of hospital stay. There was also no significant difference between the two groups in terms of disease recovery at discharge. Zou et al[7] and Zhang et al[22] showed that the proportion of organ damage in patients co-infected with HBV was not significantly different from that in patients infected with SARS-CoV-2 alone. Liu et al[23] matched 21 patients with COVID-19 and HBV co-infection to 51 patients with COVID-19 without HBV, and found that HBV did not prolong the shedding of SARS-CoV-2, nor did it add to the progression of COVID-19 or increase the risk of poor prognosis. Similarly, Li et al[18] and He et al[20] had similar conclusions. Hepatic biochemistry (ALT, AST, and TBIL) was similarly dynamic and insignificantly different between the two groups of patients at each time point. In addition, HBV antiviral therapy has been reported to have a limited impact on the incidence and outcome of COVID-19[114]. The analysis by Zhu et al[115] also supports the view that HBV is not a significant risk factor for serious adverse outcomes among hospitalized patients with COVID-19.
Data from several studies have shown that co-infection did not negatively affect the course of COVID-19, although the SARS-CoV-2/HBV co-infection group had higher results in terms of liver function index levels[16,25,28]. Guardigni et al[56] in a study of 606 subjects found no statistical difference in mortality between the two groups, despite the higher rate of admission to the ICU in HBV-positive individuals. Pre-existing viral liver infections had no impact on the clinical and virological evolution of COVID-19. A study by Liu et al[48] found that although patients co-infected with SARS-CoV-2 and HBV exhibited more severe monocytopenia, thrombocytopenia, and hepatic dysfunction in albumin production and lipid metabolism, no differences in inflammatory cytokine levels were observed between patients co-infected with SARS-CoV-2 and HBV and those infected with SARS-CoV-2 alone. Most of the disturbances could be reversed with recovery from COVID-19, and HBV co-infection had no significant effect on the outcome of COVID-19.
The mechanisms underlying liver dysfunction in patients with SARS-CoV-2 and HBV co-infection are not fully understood and may include direct injury, immune injury, drug injury, ischemia and hypoxia, and reactivation of HBV with immunosuppressive drugs[8-11], or may be the result of a combination of factors.
Direct injury: SARS-CoV-2 replication directly causes liver damage. There is increasing evidence that SARS-CoV-2 can directly cause liver damage in patients with COVID-19. ACE2 is widely present in human organ tissues, with the highest expression in the small intestine and also in the spleen, brain, muscle, heart, and liver[116]. It has been shown that SARS-CoV-2 can use ACE2 as a receptor for cell entry[117,118]. Dipeptidyl peptidase 4 and transmembrane serine protease 2 can also act as receptors to mediate SARS-CoV-2 virus entry into host cells[119,120]. Pre-existing liver disease is associated with increased expression of ACE2 in hepatocytes, thereby increasing the likelihood of SARS-CoV-2 entry into hepatocytes[121,122]. Recent data have shown higher ACE2 expression in bile duct cells than in hepatocytes, suggesting that the virus may bind directly to ACE2 expressed on bile duct cells, leading to hepatic impairment[117,123]. These findings suggest that abnormal liver function may be caused by the preferential binding of SARS-CoV-2 to bile duct cells[124]. SARS-CoV-2 granules in the cytoplasm of COVID-19 patients’ hepatocytes, mitochondrial swelling and structural damage, and massive apoptosis of hepatocytes were shown by electron microscopy, strongly suggesting direct cytopathy of SARS-CoV-2 in hepatocytes[125,126].
Immune impairment: Currently, the most discussed immune injury is the ‘cytokine storm’. During the immune activation phase, the replication and proliferation of SARS-CoV-2 lead to cellular inflammatory necrosis and release of pro-inflammatory cytokines[127], activating T and B cells and recruiting macrophages[128-130], which in turn produce additional inflammatory factors, such as C-reactive protein, IL-6, IL-1, IL-8, and IL2R, leading to a cytokine storm that causes severe immune damage to the lung as well as the liver[131,132]. IL-6 is a potential risk factor for severe liver injury in patients with COVID-19[133,134]. IL-6 is produced via classical CIS-signaling or TRANS-signaling, two different pathways that lead to a systemic cytokine storm[135].
Drug-induced liver injury: The liver is an important organ for the metabolism of drugs, and antivirals are one of the main measures for the treatment of COVID-19. The drugs currently used for treatment, including antibacterial drugs, nonsteroidal anti-inflammatory drugs, traditional Chinese medicine, antiviral drugs, and other drugs used in clinical trials, such as chloroquine, hydroxychloroquine, and tocilizumab, may produce hepatotoxicity[72,96]. Cai et al[72] observed that lopinavir and ritonavir contributed significantly to liver injury in patients with COVID-19. Antifungal drugs and systemic corticosteroids have also shown a positive association with liver injury[71]. Traditional Chinese medicine plays a crucial role in the treatment of COVID-19, but it can also contribute to liver injury[136]. The mechanisms of liver injury are variable and it is generally believed that the pathogenesis of liver injury with antiviral drugs is related to mitochondrial toxicity, hypersensitivity/induced autoimmune hepatitis, and secondary bacterial infections[124,137]. In addition to this, HBV reactivation is also considered to be one of the manifestations of drug-induced liver injury, as HBV reactivation occurs at an increased rate in patients treated with high doses of long-term corticosteroids or immunomodulators[43].
Ischemia and hypoxia in the tissues and organs of COVID-19 patients after SARS-CoV-2 infection are common pathophysiological phenomena[78]. The consequences of progressive hypoxia may include viral proliferation, cytokine release, inflammation, and intravascular coagulation[78], leading to hypoxic injury in several organs, including the liver, and in severe cases, hypoxic hepatitis[138]. Cellular damage caused by hepatic ischemia and inflammatory response caused by reperfusion both lead to hepatocyte apoptosis and elevation of liver enzymes[139,140]. In addition, patients’ prior underlying diseases, such as chronic lung disease[91], diabetes[105], and gastrointestinal disease[141], have been predicted as possible mechanisms of liver injury in the context of viral infection. Interestingly, being male has also been reported to be associated with a high risk of liver injury[71,142].
COVID-19 combined with chronic liver disease has become a very prominent clinical problem, and the treatment and prevention of COVID-19 combined with liver injury are particularly important. In the treatment of COVID-19 combined with hepatitis B, the first priority is to treat the cause, followed by a comprehensive treatment plan of antiviral therapy, use of antibiotics, liver protection, and nutritional support, as well as aggressive treatment of comorbidities, such as hypertension and diabetes. Corticosteroids are now widely used to treat COVID-19, antiviral therapy is a basic treatment, and in China, traditional Chinese medicine is also used to treat SARS-CoV-2 infection[143]. Nucleoside (acid) analogues are used in the treatment of CHB. These drugs are effective in inhibiting HBV replication, reducing viral load, and improving liver histological lesions. Some studies have shown that in patients with SARS-CoV-2 and HBV co-infection, the use of nucleoside analogs is safe and does not lead to exacerbation of the disease due to drug administration. However, antiviral drugs may also cause liver damage, and corticosteroids may increase the risk of hepatitis exacerbation in patients with chronic HBV infection, especially when combined with other immunosuppressive drugs[144]. Therefore, the use of hepatoprotective drugs and close monitoring of changes in liver function are recommended in clinical work to minimize the risk of liver injury and HBV reactivation.
Interestingly, some authors have suggested that hemoperfusion can delay the progression of the hyperinflammatory process of COVID-19 and remove the toxins involved in acute liver failure, and could be an alternative option to mitigate disease progression in patients with COVID-19[26]. In addition, plasma therapy in recovering patients has been reported to be very effective in patients who still have the virus in their system[145,146].
During the COVID-19 pandemic, delays in the care of patients other than those with COVID-19 may result in negative outcomes for CHB patients due to discontinuation of antiviral therapy. Patients with hepatitis B, especially those in the decompensated phase of cirrhosis, many of whom have a decline in blood cells associated with hypersplenism, will frequently present with pulmonary and abdominal infections due to their reduced immune function, and this is a particularly important situation to screen and identify. For patients with HBV combined with SARS-CoV-2 infection, it is recommended not to discontinue anti-HBV drugs and to strengthen the dynamic monitoring of the patient’s liver function. The diagnosis and treatment of chronic diseases are long-term, and a variety of problems may still be faced in the post-pandemic era. Therefore, it is important to emphasize the importance of network hospitals, strengthen the use of network cloud platforms, increase network consultation and follow-up, provide online assistance to these patients, and reduce unnecessary hospital visits. It is also important to avoid large gatherings during a pandemic, and be careful to wear masks and wash your hands more often in public places.
The already licensed COVID-19 vaccine is immunogenic and has a good short-term safety record[147-151]. Given the potential for SARS-CoV-2 infection to have serious health consequences in patients with hepatitis B, patients with liver disease should be considered a priority population for receiving the vaccine. The potential interactions between the COVID-19 vaccine and other vaccines have not been well studied, and the few data available is limited to the concurrent use of the COVID-19 vaccine and influenza vaccine, with studies showing that antibody responses to both vaccines were maintained, and no safety issues were reported[137]. Future clinical studies on the interaction between the COVID-19 vaccine and hepatitis B vaccine are urgently needed.
The present review has several limitations. First, most of the current research papers on SARS-CoV-2 and HBV co-infection were retrospectively designed, and no randomized controlled studies were found, which means that a selection bias must be acknowledged. Second, some studies do not distinguish the clinical stage of HBV infection and do not clarify the immune stage of the patient. Clinical outcomes may be different in patients with chronic HBV infection or acute HBV infection, and the prognosis of COVID-19 may be influenced by the status of the patient’s immune system, which may affect the interpretation of the results. Third, the results related to hepatitis B were derived from a limited sample size of observational and case studies, some of which were not classical studies on hepatitis B. The participation of patients with hepatitis B may have partially contributed to these studies, and thus the overall analysis may have overstated its role in COVID-19. Finally, because different countries have different institutions, policies, and economic conditions regarding COVID-19, and the 57 studies extracted for our review were distributed across 11 countries, it is necessary to carefully interpret whether there is regional or population bias in these results (Figure 2) and whether they are representative of the entire population.
In conclusion, both SARS-CoV-2 and HBV are globally pathogenic viruses, and there are currently few and conflicting data on the potential association between COVID-19 and hepatitis B. In the future, high-quality randomized trials are needed to further elucidate the interaction between COVID-19 and hepatitis B. To our knowledge, our review is the most comprehensive report to date describing the association between COVID-19 and hepatitis B. By reviewing these studies, we provide direct evidence of some association between COVID-19 and hepatitis B, explain the clinical phenomena that they exhibit, explore the possible mechanisms of the interaction between COVID-19 and hepatitis B, and provide management measures for co-infected patients, and therefore, our review can provide a reference for future scientific studies and clinical management.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hepatitis B virus (HBV) infections are two major current global public health crises. Both SARS-CoV-2 and HBV infections can cause liver damage. It is unclear whether HBV itself makes patients more susceptible to coronavirus disease 2019 (COVID-19), or whether COVID-19 leads to worse outcomes in patients with HBV infection. There are few and conflicting data on the association between COVID-19 and hepatitis B.
All current studies on patients with both COVID-19 and hepatitis B will be searched to explore the complex relationship between COVID-19 and hepatitis B in order to inform the research and management of patients co-infected with SARS-CoV-2 and HBV.
We searched almost all current clinical studies on COVID-19 combined with hepatitis B to describe their interaction, possible mechanisms, and clinical interventions to inform the clinical management of this special population with SARS-CoV-2 and HBV co-infection.
We used an inclusive search strategy and searched the literature in the following online databases: PubMed, China National Knowledge Infrastructure, Google Scholar, Scopus, Wiley, Web of Science, Cochrane, and ScienceDirect, as well as medRxiv and bioRxiv, and also manually searched references of the included studies. Articles reporting studies conducted in humans and discussing hepatitis B and COVID-19 were included. We extracted the relevant data from the full text of eligible studies for the table after excluding duplicative publications. Some topics that included HBV or COVID-19 samples but did not have quantitative evidence were excluded from the review.
After excluding duplications and publications without quantitative evidence, a total of 57 studies were eligible and included in this review; all were retrospective, and they were from 11 countries. Most of the studies suggested an interaction between hepatitis B and COVID-19, mainly in the form of abnormal liver enzymes, abnormal blood parameters, and HBV reactivation; however, 12 of these studies clearly indicated no effect between hepatitis B and COVID-19.
Both SARS-CoV-2 and HBV are both globally pathogenic viruses, and there are few and conflicting data on the potential association between COVID-19 and hepatitis B. Our review provides direct evidence for some associations between COVID-19 and hepatitis B, explains the clinical phenomena that they exhibit, explores the possible mechanisms of the interaction between COVID-19 and hepatitis B, and provides management measures for co-infected patients.
To our knowledge, our review is the most comprehensive report to date describing the association between COVID-19 and hepatitis B. In the future, high-quality randomized trials are needed to further elucidate the interaction between COVID-19 and hepatitis B.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cozma CT, Romania; Samadder S, India S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12969] [Article Influence: 2593.8] [Reference Citation Analysis (1)] |
| 2. | Zou XJ, Wu L, Zhou W, Fang MH. Characteristics of liver function of patients with chronic hepatitis B and coronavirus disease. J Mil Med Sci. 2020;44:370-373. |
| 3. | Zhong ZF, Huang J, Yang X, Peng JL, Zhang XY, Hu Y, Fu N, Lin HL, Jiang B, Tian YY, Yao HY, Deng LP, Tang XQ, Zhou JC, Tang J, Xie X, Liu Q, Liu J, Dou CY, Dai RJ, Yan B, Yang XF. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020;8:2554-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Xia Y, Liang TJ. Development of Direct-acting Antiviral and Host-targeting Agents for Treatment of Hepatitis B Virus Infection. Gastroenterology. 2019;156:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Yu R, Tan S, Dan Y, Lu Y, Zhang J, Tan Z, He X, Xiang X, Zhou Y, Guo Y, Deng G, Chen Y, Tan W. Effect of SARS-CoV-2 coinfection was not apparent on the dynamics of chronic hepatitis B infection. Virology. 2021;553:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 7. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Qi X, Wang J, Li X, Wang Z, Liu Y, Yang H, Shi J, Xiang H, Liu T, Kawada N, Maruyama H, Jiang Z, Wang F, Takehara T, Rockey DC, Sarin SK; COVID-Cirrhosis-CHESS Group. Clinical course of COVID-19 in patients with pre-existing decompensated cirrhosis: initial report from China. Hepatol Int. 2020;14:478-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Hambali NL, Mohd Noh M, Paramasivam S, Chua TH, Hayati F, Payus AO, Tee TY, Rosli KT, Abd Rachman Isnadi MF, Manin BO. A Non-severe Coronavirus Disease 2019 Patient With Persistently High Interleukin-6 Level. Front Public Health. 2020;8:584552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Ali E, Ziglam H, Kohla S, Ahmed M, Yassin M. A Case of Fulminant Liver Failure in a 24-Year-Old Man with Coinfection with Hepatitis B Virus and SARS-CoV-2. Am J Case Rep. 2020;21:e925932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zha L, Li S, Pan L, Tefsen B, Li Y, French N, Chen L, Yang G, Villanueva EV. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust. 2020;212:416-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 14. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 15. | Naderi M, Hosseini S, Behnampour N, Shahramian I, Moradi A. Impact of COVID-19 in Chronic Viral Hepatitis B Patients on Virological, Clinical, and Paraclinical Aspects. Jundishapur J Microb. 2022;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6515] [Article Influence: 1303.0] [Reference Citation Analysis (0)] |
| 18. | Li Y, Li C, Wang J, Zhu C, Zhu L, Ji F, Liu L, Xu T, Zhang B, Xue L, Yan X, Huang R, Wu C. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92:2785-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Bongiovanni M, Zago T. Acute hepatitis caused by asymptomatic COVID-19 infection. J Infect. 2021;82:e25-e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | He Q, Zhang G, Gu Y, Wang J, Tang Q, Jiang Z, Shao C, Zhang H, Chen Z, Ma B, Liu D, Xie G, Xu D, Huang Y, Liang M, Huang H, Wang Y, Liu H, Yang J, Pan H, Zou S, Li F, Wang F, Liu C, Wang W, Xiong B, Li X, Liu L, Qi X. Clinical Characteristics of COVID-19 Patients With Pre-existing Hepatitis B Virus Infection: A Multicenter Report. Am J Gastroenterol. 2021;116:420-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Wen M, Lu J, Xie Y. Clinical characteristics of coronavirus disease 2019 patients complicated with liver injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Zhang B, Huang W, Zhang S. Clinical Features and Outcomes of Coronavirus Disease 2019 (COVID-19) Patients With Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2020;18:2633-2637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2021;8:484-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Bekçibaşı M, Arslan E. Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) /Hepatitis B virus (HBV) Co-infected Patients: A case series and review of the literature. Int J Clin Pract. 2021;75:e14412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Colaneri M, Valsecchi P, Perotti L, Ludovisi S, Seminari E, Pieri TC, Sacchi P, Bruno R. Running out of bullets: The challenging management of acute hepatitis and SARS-COV-2 from the SMatteo COvid19 Registry (SMACORE). Liver Int. 2020;40:2655-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2548] [Article Influence: 509.6] [Reference Citation Analysis (2)] |
| 28. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, Lens S, Mariño Z, Forns X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Yang S, Wang S, Du M, Liu M, Liu Y, He Y. Patients with COVID-19 and HBV Coinfection are at Risk of Poor Prognosis. Infect Dis Ther. 2022;11:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Yigit Y, Haddad M, Elmoheen A, Shogaa MR, Tawel R, Mohamed YK, Salem W, Fawzy Eltawagny M. Can COVID-19 Cause Flare-Ups of Acute Hepatitis B? Case Rep Infect Dis. 2021;2021:8818678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID-19 Induced Hepatitis B Virus Reactivation: A Novel Case From the United Arab Emirates. Cureus. 2020;12:e8645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 35. | Wang H, Liu Y, Gao XL, Lei XY. Severe Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection: a case report and literature review. J Community Med. 2021;19:588-590. |
| 36. | Zhong Z, Zhang Q, Xia H, Wang A, Liang W, Zhou W, Zhou L, Liu X, Rao L, Li Z, Peng Z, Mo P, Xiong Y, Ye S, Wang Y, Ye Q. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20:1916-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 37. | Huang JF, Zheng KI, George J, Gao HN, Wei RN, Yan HD, Zheng MH. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20:1907-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, Yuan Y, Li H. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology. 2020;72:1491-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 39. | Loinaz C, Marcacuzco A, Fernández-Ruiz M, Caso O, Cambra F, San Juan R, Justo I, Calvo J, García-Sesma A, Manrique A, Pérez-Jacoiste Asín MA, Folgueira MD, Aguado JM, Lumbreras C. Varied clinical presentation and outcome of SARS-CoV-2 infection in liver transplant recipients: Initial experience at a single center in Madrid, Spain. Transpl Infect Dis. 2020;22:e13372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Adali G, Gokcen P, Guzelbulut F, Gokcen Degirmenci Salturk A, Bugra Agaoglu N, Unal B, Doganay L, Ozdil K. Are nucleos(t)ide analogues effective against severe outcomes in COVID-19 and hepatitis B virus coinfection? Hepatol Forum. 2021;2:91-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 41. | Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, Sagnelli E, Coppola N. COVID-19 as Another Trigger for HBV Reactivation: Clinical Case and Review of Literature. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 43. | Wu YF, Yu WJ, Jiang YH, Chen Y, Zhang B, Zhen RB, Zhang JT, Wang YP, Li Q, Xu F, Shi YJ, Li XP. COVID-19 or treatment associated immunosuppression may trigger hepatitis B virus reactivation: A case report. World J Clin Cases. 2021;9:5266-5269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Wu J, Yu J, Shi X, Li W, Song S, Zhao L, Zhao X, Liu J, Wang D, Liu C, Huang B, Meng Y, Jiang B, Deng Y, Cao H, Li L. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: A multicentre descriptive study. J Viral Hepat. 2021;28:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 46. | Huang SP, Xu CT, Liu M, Shang ZY, Lu HZ. A case of HBV reactivation induced by novel coronavirus pneumonia. Chinese Hepatology. 2020;25:467-468. |
| 47. | Song SH, Chen TL, Deng LP, Zhang YX, Mo PZ, Gao SC, Hu WJ, Xiong Y, Ma ZY. Clinical characteristics of four cancer patients with SARS-CoV-2 infection in Wuhan, China. Infect Dis Poverty. 2020;9:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Liu R, Zhao L, Cheng X, Han H, Li C, Li D, Liu A, Gao G, Zhou F, Liu F, Jiang Y, Zhu C, Xia Y. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2021;41:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 49. | Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C, Aguado JM. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020;20:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 50. | Ma GG, Shen YX, Wu L, Luo Z, Zhu CW, Chen SY, Yu KH, Li F. Effect of liver injury on prognosis and treatment of hospitalized patients with COVID-19 pneumonia. Ann Transl Med. 2021;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Parlar Y, Keskin O, Kirmizigul B, Gencdal G, Zeybel M, Gumussoy M, Idilman R, Yurdaydin C. The course of COVID-19 infection in patients with chronic hepatitis B and Delta. J Hepatol. 2022;77:S293. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Li L, Li S, Xu MM, Yu PF, Zheng SJ, Duan ZP, Liu J, Chen Y, Li JF. Risk factors related to hepatic injury in patients with corona virus disease 2019. 2020 Preprint. Available from: medRxiv:2020.02.28.20028514. [DOI] [Full Text] |
| 53. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18866] [Article Influence: 3773.2] [Reference Citation Analysis (7)] |
| 54. | Kang SH, Cho DH, Choi J, Baik SK, Gwon JG, Kim MY. Association between chronic hepatitis B infection and COVID-19 outcomes: A Korean nationwide cohort study. PLoS One. 2021;16:e0258229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 55. | Oruç Z, Ebinç S, Kalkan Z, Kaplan M, Küçüköner M, Urakçı Z, Oruç İ, Işıkdoğan A. COVID-19 infection in cancer patients: the effect of Hepatitis B immunization. Med Res J. 2022;6:86-93. [DOI] [Full Text] |
| 56. | Guardigni V, Rosselli Del Turco E, Badia L, Galli S, Scolz K, Viale P, Verucchi G. Pre-Existing HBV and HCV Infections Do Not Affect COVID-19-Related Outcomes: An Observational Retrospective Study. Hepat Mon. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Patrono D, Lupo F, Canta F, Mazza E, Mirabella S, Corcione S, Tandoi F, De Rosa FG, Romagnoli R. Outcome of COVID-19 in liver transplant recipients: A preliminary report from Northwestern Italy. Transpl Infect Dis. 2020;22:e13353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Liu B, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20:1891-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 59. | Lens S, Miquel M, Mateos-Muñoz B, García-Samaniego J, Forns X. SARS-CoV-2 in patients on antiviral HBV and HCV therapy in Spain. J Hepatol. 2020;73:1262-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 61. | Jockusch S, Tao C, Li X, Anderson TK, Chien M, Kumar S, Russo JJ, Kirchdoerfer RN, Ju J. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 2020;180:104857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 62. | Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 640] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 63. | Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 64. | Anugwom CM, Aby ES, Debes JD. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin Infect Dis. 2021;72:180-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514-10527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 66. | Li N, Ma WT, Pang M, Fan QL, Hua JL. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front Immunol. 2019;10:1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 67. | Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2291] [Cited by in RCA: 2166] [Article Influence: 433.2] [Reference Citation Analysis (0)] |
| 68. | Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, Manry J, Shaw E, Haljasmägi L, Peterson P, Lorenzo L, Bizien L, Trouillet-Assant S, Dobbs K, de Jesus AA, Belot A, Kallaste A, Catherinot E, Tandjaoui-Lambiotte Y, Le Pen J, Kerner G, Bigio B, Seeleuthner Y, Yang R, Bolze A, Spaan AN, Delmonte OM, Abers MS, Aiuti A, Casari G, Lampasona V, Piemonti L, Ciceri F, Bilguvar K, Lifton RP, Vasse M, Smadja DM, Migaud M, Hadjadj J, Terrier B, Duffy D, Quintana-Murci L, van de Beek D, Roussel L, Vinh DC, Tangye SG, Haerynck F, Dalmau D, Martinez-Picado J, Brodin P, Nussenzweig MC, Boisson-Dupuis S, Rodríguez-Gallego C, Vogt G, Mogensen TH, Oler AJ, Gu J, Burbelo PD, Cohen JI, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Rossignol P, Mayaux J, Rieux-Laucat F, Husebye ES, Fusco F, Ursini MV, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Castagnoli R, Montagna D, Licari A, Marseglia GL, Duval X, Ghosn J; HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort, Tsang JS, Goldbach-Mansky R, Kisand K, Lionakis MS, Puel A, Zhang SY, Holland SM, Gorochov G, Jouanguy E, Rice CM, Cobat A, Notarangelo LD, Abel L, Su HC, Casanova JL. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1932] [Cited by in RCA: 1915] [Article Influence: 383.0] [Reference Citation Analysis (0)] |
| 69. | Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, Rosain J, Bilguvar K, Ye J, Bolze A, Bigio B, Yang R, Arias AA, Zhou Q, Zhang Y, Onodi F, Korniotis S, Karpf L, Philippot Q, Chbihi M, Bonnet-Madin L, Dorgham K, Smith N, Schneider WM, Razooky BS, Hoffmann HH, Michailidis E, Moens L, Han JE, Lorenzo L, Bizien L, Meade P, Neehus AL, Ugurbil AC, Corneau A, Kerner G, Zhang P, Rapaport F, Seeleuthner Y, Manry J, Masson C, Schmitt Y, Schlüter A, Le Voyer T, Khan T, Li J, Fellay J, Roussel L, Shahrooei M, Alosaimi MF, Mansouri D, Al-Saud H, Al-Mulla F, Almourfi F, Al-Muhsen SZ, Alsohime F, Al Turki S, Hasanato R, van de Beek D, Biondi A, Bettini LR, D'Angio' M, Bonfanti P, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Oler AJ, Tompkins MF, Alba C, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F, Soler-Palacin P, Martin-Nalda A, Colobran R, Morange PE, Keles S, Çölkesen F, Ozcelik T, Yasar KK, Senoglu S, Karabela ŞN, Rodríguez-Gallego C, Novelli G, Hraiech S, Tandjaoui-Lambiotte Y, Duval X, Laouénan C; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group, Snow AL, Dalgard CL, Milner JD, Vinh DC, Mogensen TH, Marr N, Spaan AN, Boisson B, Boisson-Dupuis S, Bustamante J, Puel A, Ciancanelli MJ, Meyts I, Maniatis T, Soumelis V, Amara A, Nussenzweig M, García-Sastre A, Krammer F, Pujol A, Duffy D, Lifton RP, Zhang SY, Gorochov G, Béziat V, Jouanguy E, Sancho-Shimizu V, Rice CM, Abel L, Notarangelo LD, Cobat A, Su HC, Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1632] [Article Influence: 326.4] [Reference Citation Analysis (0)] |
| 70. | World Health Organization. Corticosteroids for COVID-19. [cited 11 August 2022]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. |
| 71. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 72. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 73. | Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 74. | Falcão MB, Pamplona de Góes Cavalcanti L, Filgueiras Filho NM, Antunes de Brito CA. Case Report: Hepatotoxicity Associated with the Use of Hydroxychloroquine in a Patient with COVID-19. Am J Trop Med Hyg. 2020;102:1214-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Malakouti M, Kataria A, Ali SK, Schenker S. Elevated Liver Enzymes in Asymptomatic Patients - What Should I Do? J Clin Transl Hepatol. 2017;5:394-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Oh RC, Hustead TR, Ali SM, Pantsari MW. Mildly Elevated Liver Transaminase Levels: Causes and Evaluation. Am Fam Physician. 2017;96:709-715. [PubMed] |
| 77. | Zhang N, Hartig H, Dzhagalov I, Draper D, He YW. The role of apoptosis in the development and function of T lymphocytes. Cell Res. 2005;15:749-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6656] [Article Influence: 1331.2] [Reference Citation Analysis (0)] |
| 79. | Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 80. | Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 752] [Cited by in RCA: 805] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 81. | Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. J Thromb Haemost. 2020;18:1514-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 82. | Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 577] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 83. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 442] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 84. | Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 85. | Reddy KR. SARS-CoV-2 and the Liver: Considerations in Hepatitis B and Hepatitis C Infections. Clin Liver Dis (Hoboken). 2020;15:191-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Watanabe T, Fukae J, Fukaya S, Sawamukai N, Isobe M, Matsuhashi M, Shimizu M, Akikawa K, Tanimura K, Atsumi T, Koike T. Incidence and risk factors for reactivation from resolved hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis. 2019;22:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 88. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 89. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 90. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 91. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 92. | Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, Guo F, Zhao H, Gao R. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) Pneumonia With Other Pneumonias. Clin Infect Dis. 2020;71:756-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 93. | Caro-Codón J, Rey JR, Buño A, Iniesta AM, Rosillo SO, Castrejon-Castrejon S, Rodriguez-Sotelo L, Martinez LA, Marco I, Merino C, Martin-Polo L, Garcia-Veas JM, Martinez-Cossiani M, Gonzalez-Valle L, Herrero A, López-de-Sa E, Merino JL; CARD-COVID Investigators. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur J Heart Fail. 2021;23:456-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 94. | Belarte-Tornero LC, Valdivielso-Moré S, Vicente Elcano M, Solé-González E, Ruíz-Bustillo S, Calvo-Fernández A, Subinara I, Cabero P, Soler C, Cubero-Gallego H, Vaquerizo B, Farré N. Prognostic Implications of Chronic Heart Failure and Utility of NT-proBNP Levels in Heart Failure Patients with SARS-CoV-2 Infection. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Weng H, Yang F, Zhang L, Jin H, Liu S, Fan F, Liu Z, Zheng X, Yang H, Li Y, Yi T, Li H, Zhang Y, Li J. Joint Predictive Value of cTnI and NT-proBNP on Mortality in Patients with Coronavirus Disease 2019: A Retrospective Research in Wuhan, China. J Transl Int Med. 2021;9:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 97. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 98. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 99. | The OpenSAFELY Collaborative, Williamson E, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong A, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans S, Smeeth L, Goldacre, B. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. 2020 Preprint. Available from: medRxiv:2020.05.06.20092999. [DOI] [Full Text] |
| 100. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 102. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 103. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 104. | Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 105. | Fu L, Fei J, Xu S, Xiang HX, Xiang Y, Tan ZX, Li MD, Liu FF, Li Y, Han MF, Li XY, Zhao H, Xu DX. Acute liver injury and its association with death risk of patients with COVID-19: a hospital-based prospective case-cohort study. 2020 Preprint. Available from: medRxiv:2020.04.02.20050997. [DOI] [Full Text] |
| 106. | Patra T, Meyer K, Geerling L, Isbell TS, Hoft DF, Brien J, Pinto AK, Ray RB, Ray R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020;16:e1009128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 107. | Chen Z, Xu W, Ma W, Shi X, Li S, Hao M, Fang Y, Zhang L. Clinical laboratory evaluation of COVID-19. Clin Chim Acta. 2021;519:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 108. | Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1709] [Article Influence: 341.8] [Reference Citation Analysis (0)] |
| 109. | Qi T, Zhu C, Lu G, Hao J, He Q, Chen Y, Zhou F, Chen J, Hou J. Elevated D-dimer is associated with increased 28-day mortality in acute-on-chronic liver failure in China: a retrospective study. BMC Gastroenterol. 2019;19:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 111. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3415] [Article Influence: 683.0] [Reference Citation Analysis (0)] |
| 112. | Wang Y, Hu Z, Luo J, Zhang F, Huang L, Li H, Wen X, Pan Y, Chen M, Ying R, Jiang H, Chen S, Pan Z, Chen H, Xu H, Lei C, Han Y. Clinical Characteristics and Abnormal Parameters Evolution in Patients With Novel Coronavirus Infection: A Case Series of 272 Cases in Guangzhou. Disaster Med Public Health Prep. 2021;1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Mirzaie H, Vahidi M, Shokoohi M, Darvishian M, Sharifi H, Sharafi H, Karamouzian M. COVID-19 among patients with hepatitis b or hepatitis C: A systematic review. 2020 Preprint. Available from: medRxiv:2020.10.22.20216317. [DOI] [Full Text] |
| 114. | Yousefifard M, Zali A, Mohamed Ali K, Madani Neishaboori A, Zarghi A, Hosseini M, Safari S. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8:e45. [PubMed] |
| 115. | Zhu JH, Peltekian KM. HBV coinfection and in-hospital outcomes for COVID-19: a systematic review and meta-analysis. Can Liver J. 2021;4:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Han T, Kang J, Li G, Ge J, Gu J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med. 2020;8:1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 117. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766v1. [DOI] [Full Text] |
| 118. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14118] [Article Influence: 2823.6] [Reference Citation Analysis (1)] |
| 119. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14253] [Article Influence: 2850.6] [Reference Citation Analysis (0)] |
| 120. | Strollo R, Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020;36:e3330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 121. | Meijnikman AS, Bruin S, Groen AK, Nieuwdorp M, Herrema H. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J Hepatol. 2021;74:748-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 122. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 123. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (1)] |
| 124. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 125. | Elghannam MT, Hassanien MH, Ameen YA, ELattar GM, ELRay AA, Turky EA, ELTalkawy MD. COVID-19 and liver diseases. Egypt Liver J. 2022;12:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 126. | Bangash MN, Patel JM, Parekh D, Murphy N, Brown RM, Elsharkawy AM, Mehta G, Armstrong MJ, Neil D. SARS-CoV-2: Is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 127. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1805] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 128. | Shimizu Y. Understanding the immunopathogenesis of COVID-19: Its implication for therapeutic strategy. World J Clin Cases. 2020;8:5835-5843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang GX, Rostami A. Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy. J Immunol. 2015;194:5085-5093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 130. | Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1373] [Article Influence: 274.6] [Reference Citation Analysis (0)] |
| 131. | Crayne CB, Albeituni S, Nichols KE, Cron RQ. The Immunology of Macrophage Activation Syndrome. Front Immunol. 2019;10:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 132. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 133. | Zhan K, Liao S, Li J, Bai Y, Lv L, Yu K, Qiu L, Li C, Yuan G, Zhang A, Mei Z. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut. 2021;70:628-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 134. | Eljilany I, Elzouki AN. D-Dimer, Fibrinogen, and IL-6 in COVID-19 Patients with Suspected Venous Thromboembolism: A Narrative Review. Vasc Health Risk Manag. 2020;16:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 135. | Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1743] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 136. | Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 137. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Zhao XY, Xu XX, Yin HS, Hu QM, Xiong T, Tang YY, Yang AY, Yu BP, Huang ZP. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 139. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 140. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 141. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 869] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 142. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group*. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159:1137-1140.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 143. | Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. [cited 11 August 2022]. Available online: http://www.nhc.gov.cn/jkj/s3577/202009/318683cbfaee4191aee29cd774b19d8d/files/f9ea38ce2c2d4352bf61ab0feada439f.pdf. |
| 144. | Hatano M, Mimura T, Shimada A, Noda M, Katayama S. Hepatitis B virus reactivation with corticosteroid therapy in patients with adrenal insufficiency. Endocrinol Diabetes Metab. 2019;2:e00071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Gul MH, Htun ZM, Shaukat N, Imran M, Khan A. Potential specific therapies in COVID-19. Ther Adv Respir Dis. 2020;14:1753466620926853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 146. | Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S, Percivalle E, Glingani C, Musella V, Belliato M, Garuti M, Meloni F, Frigato M, Di Sabatino A, Klersy C, De Donno G, Franchini M, Covid-Plasma Task Force. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105:2834-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 147. | Alrashdan MS, El-Kishawi M, Al Kawas S. The Co-Administration of COVID-19 and Hepatitis B Vaccines, Should Safety Be a Concern? Infect Chemother. 2022;54:542-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 148. | Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 176] [Reference Citation Analysis (0)] |
| 149. | Mahallawi WH, Mumena WA. Reactogenicity and Immunogenicity of the Pfizer and AstraZeneca COVID-19 Vaccines. Front Immunol. 2021;12:794642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 150. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10685] [Article Influence: 2137.0] [Reference Citation Analysis (1)] |
| 151. | Lazarus R, Baos S, Cappel-Porter H, Carson-Stevens A, Clout M, Culliford L, Emmett SR, Garstang J, Gbadamoshi L, Hallis B, Harris RA, Hutton D, Jacobsen N, Joyce K, Kaminski R, Libri V, Middleditch A, McCullagh L, Moran E, Phillipson A, Price E, Ryan J, Thirard R, Todd R, Snape MD, Tucker D, Williams RL, Nguyen-Van-Tam JS, Finn A, Rogers CA; ComfluCOV Trial Group. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398:2277-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |