Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6589
Peer-review started: May 23, 2022
First decision: June 19, 2022
Revised: July 3, 2022
Accepted: November 2, 2022
Article in press: November 2, 2022
Published online: December 14, 2022
Processing time: 198 Days and 17.2 Hours
Functional gastrointestinal disorders (FGIDs) are common during the pediatric age. FGIDs are not related to biochemical or structural abnormalities. However, since they have a high prevalence, several studies have evaluated an overlap between FGIDs and organic diseases. Individuals with celiac disease (CD) have been shown to be at an increased risk for functional abdominal pain, even if they adhere well to a gluten-free diet (GFD). Little information is available for the pediatric age group. The aims of our study were to evaluate the prevalence of FGIDS in CD children 1 year after diagnosis and to compare the prevalence of FGIDs in CD children on a GFD with processed foods compared with those on a GFD with natural products.
To assess the prevalence of FGIDs in children with CD after 1 year of follow-up and to compare the prevalence of FGIDs in children with CD on a GFD with processed foods and in children on a GFD with natural products.
We recruited pediatric patients aged 1-18 years with a new CD diagnosis. Participants were randomized to two groups: Group A on a GFD with processed foods (diet 1); and group B on a GFD with natural products (diet 2). Clinical monitoring, diet assessment and the questionnaire on pediatric gastrointestinal symptoms-Rome IV version were performed at diagnosis (T0) and after 12 mo of follow-up (T1). Dietary intake was assessed using a 3-d food diary record. Data from the diaries were evaluated using WinFood nutrient analysis software. We assessed the prevalence of FGIDs at T1 and the correlation with the type of GFD.
We registered 104 CD children, with 55 patients in group A (53.0%) and 49 patients in group B (47.0%). Initially, 30 of the 55 (54.5%) CD children were symptomatic in group A, while 25 of 49 (51.0%) were symptomatic in group B. At T1, in spite of a low or negative serology for CD, FGIDs prevalence was 10/55 (18.0%) in group A and 8/49 (16.3%) in group B, with no statistically significant difference between the two groups (P = 0.780). At T1 the macro- and micronutrient intake was similar across the two groups with no significant differences in nutrient analysis. However, in both groups at T1 we found that a lower prevalence of FGIDs (P = 0.055) was associated with an inferior caloric (odds ratio = 0.99, 95% confidence interval: 0.99-1.00) and fat (odds ratio = 0.33, 95% confidence interval: 0.65-0.95) intake.
Our results showed that CD children on a GFD have gastrointestinal symptoms with an elevated prevalence of FGIDs. Our study suggests that developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products. However, long-term monitoring is required to evaluate a correlation between FGIDs and various types of GFDs.
Core Tip: In spite of a strict adhesion to a classic gluten-free diet, patients with celiac disease are more likely to suffer from functional abdominal pain disorders. Our findings suggest that the prevalence of functional gastrointestinal disorders may be linked to the caloric intake and fat content in the diet. However, it does not differ between a processed gluten-free diet with commercial products or a gluten free diet with natural products.
- Citation: Fiori Nastro F, Serra MR, Cenni S, Pacella D, Martinelli M, Miele E, Staiano A, Tolone C, Auricchio R, Strisciuglio C. Prevalence of functional gastrointestinal disorders in children with celiac disease on different types of gluten-free diets. World J Gastroenterol 2022; 28(46): 6589-6598
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6589
Celiac disease (CD) is a chronic immune-mediated enteropathy characterized by mucosal inflammation and villous atrophy[1] and triggered by the ingestion of gluten in genetically predisposed individuals. CD is now recognized as a global disease with a prevalence of about 1% of the world’s population[2]. The clinical presentation ranges from features of malabsorption such as abdominal pain, diarrhea, steatorrhea and weight loss or growth failure to atypical forms of CD with more subtle gastrointestinal (GI) manifestations similar to functional GI disorders (FGIDs) or asymptomatic individuals diagnosed by screening high-risk groups[3].
The only available treatment for CD is lifetime adherence to a gluten-free diet (GFD)[4]. Just over a decade ago, Turco et al[5] showed that subjects with CD have a higher risk of developing FGIDs, fulfilling the Rome III diagnostic criteria, despite strict adherence to a classic GFD. However, concordant data has not been produced in this area in recent years[6,7]. When it is present an organic abnormality FGID cannot be diagnosed. However, these disorders have a high prevalence. Therefore, numerous studies have assessed the potential for overlap between FGIDs and organic diseases[8]. A great innovation introduced in the new Rome IV criteria is the specification that the diagnosis can only be made ‘after appropriate medical evaluation, the symptoms cannot be attributed to another medical condition’. This wording replaces the previous statement that there should be an ‘absence of inflammatory, anatomic, metabolic, or neoplastic process that explains the subject’s symptoms’. This change makes it possible for a patient suffering from another organic disease, like CD or inflammatory bowel disease, to also have a functional disorder (common event) and should reduce the amount of tests necessary to diagnose FGIDs[9].
However, only little and contrasting data are available in children regarding the prevalence of symptoms of FGID in children with CD or the effects of a GFD on those symptoms. The objectives of this study were to evaluate the prevalence of FGIDs among children with CD at the moment of diagnosis and after 1 year of follow-up comparing a conventional GFD with commercially available products and a GFD based mainly on natural products.
We prospectively followed up for 1 year two groups of CD children who received two different GFDs to evaluate if specific dietary factors were involved in the development of FGIDs at 1 year of follow-up. This group consisted of 104 consecutive children [34 male children, 69 female children; mean age 7.2 (4.1) years; range: 4-17 years] who received a diagnosis of CD from December 2017 to January 2019 at the Department of Pediatrics, University ‘Federico II’ in Naples, Italy and the Department of Pediatrics, University of Campania, “Luigi Vanvitelli” in Naples, Italy. All children received a GFD and were followed up for 1 year. CD diagnosis, according to the ESPGHAN criteria[10], was based on the serum concentration of anti-transglutaminase-immunoglobulin A (IgA) determined by an indirect solid-phase enzyme immunoassay test and serum concentration of endomysial antibodies determined by indirect immunofluorescence using monkey’s esophagus sections as substrate. To exclude the presence of selective IgA deficiency, serum IgA levels were assayed by nephelometry. According to the ESPGHAN guidelines[11], in children and adolescents with signs or symptoms suggestive of CD, high anti-transglutaminase titers (> 10 times), positivity of endomysial antibodies and human leukocyte antigen DQ2/8, biopsies were omitted. In all other cases, patients underwent upper endoscopy with multiple duodenal biopsies. Class II antigen human leukocyte antigen typing was also performed by polymerase chain reaction sequence-specific oligonucleotide using DQ-CD Typing Plus[12]. We excluded from the study patients with systemic or GI infection, patients with other known GI, renal, cardiac, pulmonary, hematological, neurological and cerebral pathologies and patients with an inability or unwillingness to give informed consent.
The patients were randomly divided into two groups: Group A (n = 55) received a controlled GFD with processed foods (diet 1); and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). All registered patients and/or their parents underwent validated questionnaires for GI symptoms according to the Rome IV criteria. The pediatric GI symptoms Questionnaire-Rome IV version was used to diagnose FGIDs. Subjects were classified as having an FGIDs through their questionnaire responses and were able to meet criteria for multiple disorders.
A clinical follow-up and symptom questionnaire based on the Rome IV criteria were carried out for each child at two different times: At diagnosis (T0) and at the 12-mo (T2) follow-up. At 12 mo of follow-up, children were considered in clinical remission from CD if they reverted positive serological tests to negative following treatment with a strict GFD, despite having GI symptoms defined as functional according to the Rome IV criteria. The potential consumption of gluten-containing products was assessed using a combination of CD serology, self-reported adherence questions and an interview with an experienced dietician. Dietary intake was assessed on the basis of compiling a 3-d food diary and a dietary interview by an expert nutritionist. The nutrients analysis was conducted using the WinFood software.
Data were presented as frequency (percent) for categorical variables and as mean (standard deviation) for continuous variables. Differences in nutrient intake between patients in group A and group B were computed with the student’s t test or Mann-Whitney U as appropriate. Associations between nutrient intake and FGIDs prevalence for the two groups were computed with logistic regression. Comparison between the prevalence of FGIDs at T0 or T1 in patients were computed with the McNemar test. For all analyses, a P value of 0.05 was considered significant. All statistical analyses were performed using the R statistical environment, version 4.0.3.
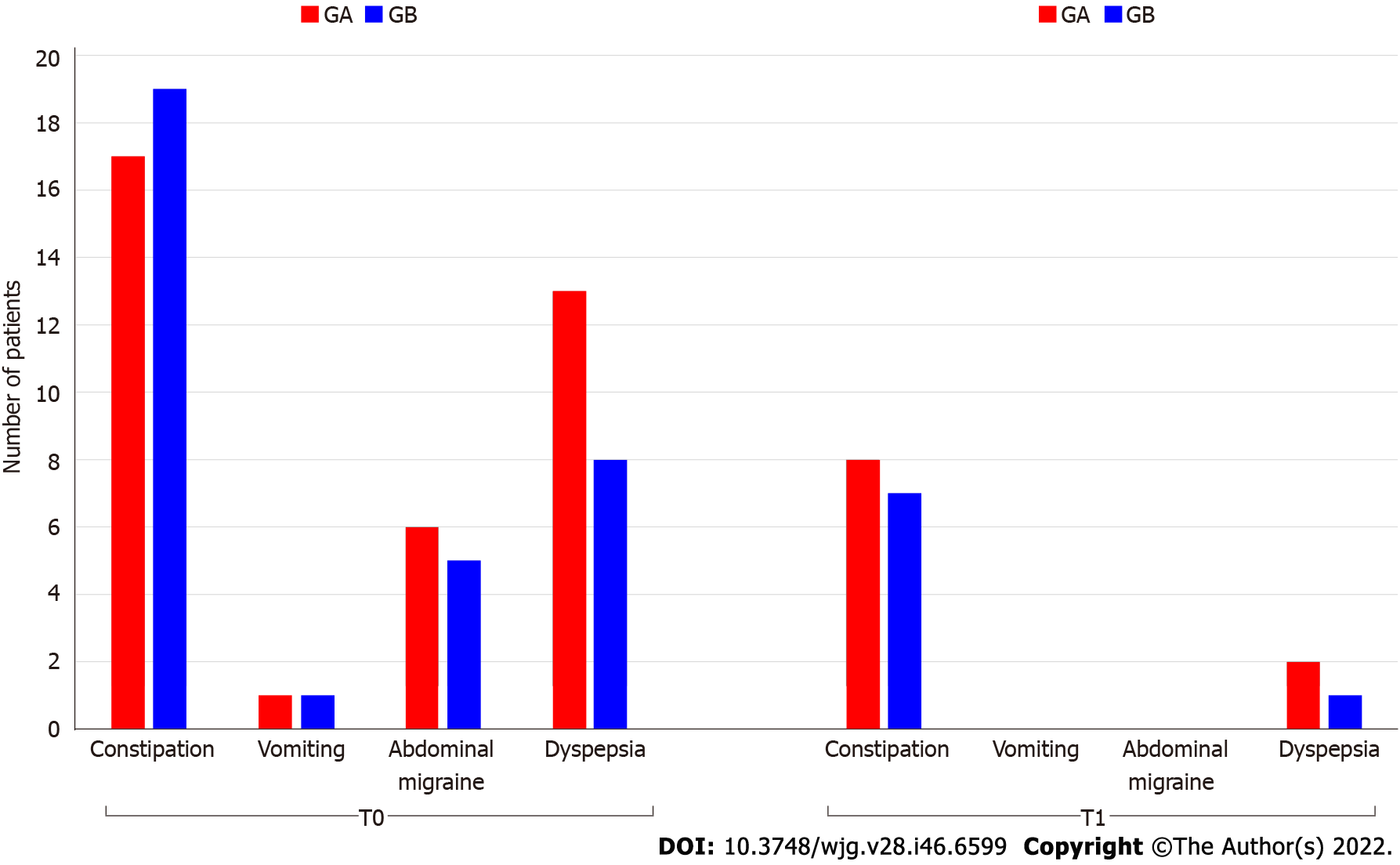
The symptomatic children at enrollment were 30 of 55 (54.5%) in group A and 25 of 49 (51.0%) in group B. Constipation (28.8%) was the most prevalent symptom, followed by abdominal pain (24.0%) and vomiting (4.8%). As expected after 1 year of GFD the frequency of GI symptoms significantly decreased in both groups (Table 1). At 1 year from CD diagnosis children were investigated for repeated serology for CD (endomysial and anti-transglutaminase antibodies), of which 99/104 (95.2%) were negative, 2 (1.9%) were positive, and 3 (2.9%) were borderline.
Despite negative serology for CD, the prevalence of FGIDs, classified according to the Rome IV criteria, was 10/55 (18.0%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1 (P = 0.780) (Figure 1). Among CD children with FGIDs, functional constipation (FC) (12.5%) was the most prevalent disorder (7.7% in group A and 6.7% in group B) followed by postprandial distress syndrome (1.9% in group A and 1.0% in group B) at T1.
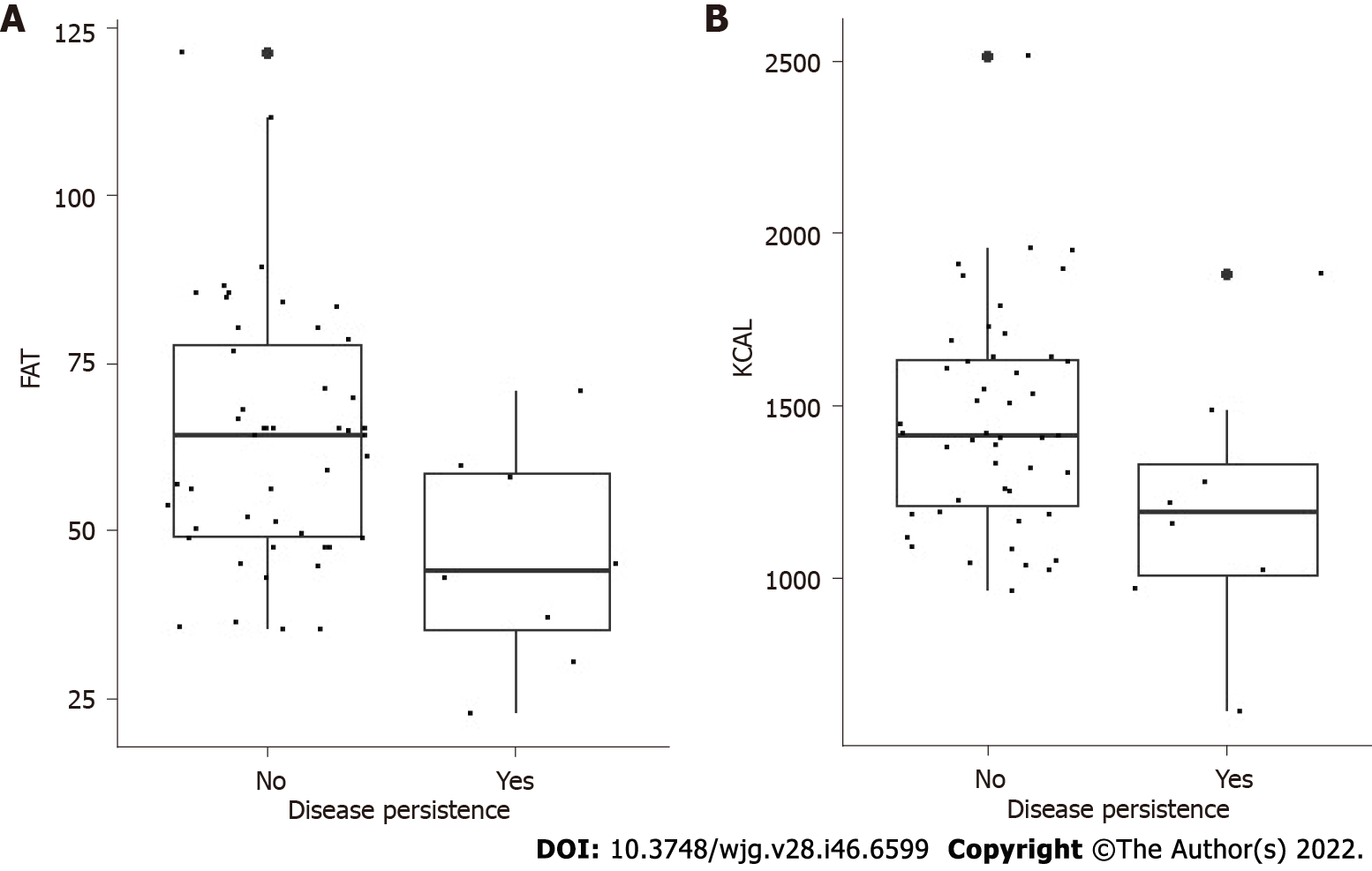
We observed that children who presented with symptoms at diagnosis, more frequently developed a FGID at 1 year than patients who were asymptomatic at the time of diagnosis. The individual analysis of the intake of macro- and micronutrients at T1 showed that there were no important differences in nutrient intake between group A and group B (Table 2). However, in both groups, we found a relationship between reduced caloric and fat intake (odds ratio = 0.99, 95% confidence interval: 0.99-1.00 and odds ratio = 0.33, 95% confidence interval: 0.65-0.95, respectively) and a decreased prevalence of FGIDs after 1 year of a GFD (P = 0.05) (Figure 2). No statistically significant difference was found in the subcategories of fat analyzed (saturated, polyunsaturated and monounsaturated fatty acids).
| Variable, mean (SD) | Group A, n = 55 | Group B, n = 49 | P value |
| Energy intake, kcal/d | 1417 (338) | 1376 (367) | 0.558 |
| Total protein, g/d | 52 (14) | 50 (15) | 0.509 |
| Lipids, g/d | 63 (19) | 59 (20) | 0.324 |
| Carbohydrates, g/d | 189 (137) | 167 (51) | 0.277 |
| Oligosaccharides, g/d | 40 (20) | 34 (17) | 0.119 |
| Starch, g/d | 41 (33) | 33 (33) | 0.250 |
| Cholesterol, mg/d | 135 (60) | 155 (109) | 0.251 |
| Saturated fatty acids, g/d | 15.1 (7.0) | 13.7 (6.0) | 0.277 |
| Poly-unsaturated fatty acids, g/d | 4.99 (1.83) | 4.02 (2.02) | 0.012 |
| Mono-unsaturated fatty acids, g/d | |||
| Fiber, g/die | 25 (10) | 22 (11) | 0.089 |
| Calcium, mg/d | |||
| Sodium, mg/d | 8.1 (5.5) | 7.1 (5.2) | 0.382 |
| Potassium, mg/d | 412 (190) | 421 (219) | 0.819 |
| Phosphorus, mg/d | 881 (565) | 780 (669) | 0.410 |
| Iron, mg/d | 1.386 (592) | 1.300 (550) | 0.433 |
| Zinc, mg/d | 672 (229) | 653 (259) | 0.685 |
| Folic acid, mcg/d | 4.68 (2.70) | 4.47 (2.29) | 0.644 |
| Niacin, mg/d | 5.36 (2.38) | 5.04 (2.16) | 0.482 |
| Riboflavin, mg/d | 100 (74) | 104 (75) | 0.793 |
| Thiamine, mg/d | 8.2 (4.4) | 7.5 (4.2) | 0.433 |
| Vitamin A, mcg/d | 0.82 (0.33) | 0.90 (0.64) | 0.466 |
| Vitamin B6, mg/d | 0.53 (0.23) | 0.49 (0.21) | 0.373 |
| Vitamin C, mg/d | 333 (201) | 382 (214) | 0.234 |
| Vitamin D, mcg/d | 0.87 (0.51) | 0.82 (0.48) | 0.577 |
| Vitamin E, mg/d | 71 (87) | 57 (49) | 0.341 |
| 1.53 (1.73) | 1.47 (1.43) | 0.826 | |
| 7.1 (3.2) | 6.4 (3.8) | 0.308 |
To the best of our knowledge this is the first pediatric study that investigated the prevalence of FGIDs in children with CD on a GFD with processed products vs a GFD with natural products. We found that there was no difference in the prevalence of FGIDs according to the type of diet (18.4% vs 16.3%), and the most frequent FGID was FC in both groups. Interestingly, in both groups we found an association between lower caloric and fat intake and lower prevalence of FGIDs after 1 year of a GFD.
In a previous work we found a higher prevalence of CD patients who continued having GI symptoms and fulfilled Rome III criteria for FGIDs despite the GFD compared to control (28.0% vs 8.9%, respectively). FC was the most frequent disorder. The likely cause for the difference in the current study is related to the application of the Rome IV criteria, which resulted in a lower prevalence of FGIDs as previously described[13,14]. Indeed, our findings are in accordance with the results of a recent large study conducted in Italy from Cristofori et al[6] that found a prevalence of functional abdominal pain disorders of 11.5% according to Rome IV criteria among patients with celiac disease compared to 6.7% of the control. FC and irritable bowel syndrome (IBS) were the most frequent disorders found in CD patients. We also found that FC was the most common disorder. However, we noted an increase of functional dyspepsia and a decrease of IBS compared to Cristofori et al[6]. Two large American reports and our recent European study conducted on healthy subjects also reported an increased level of functional dyspepsia (3.0%-7.6%), with postprandial distress syndrome being the most common subtype (2.7%-7.2%), as in our population[13-15]. It is interesting to note that in these studies, the prevalence of functional dyspepsia exceeded that of IBS, which was previously the most prevalent functional abdominal pain disorder[16].
Patients with CD and FGIDs can be categorized as “atypical forms” of CD. Although, the term “atypical” CD is primarily used for patients who present with extraintestinal symptoms like IgA-nephropathy, hemosiderosis of the lungs and a variety of neurological diseases. Moreover, another difference is that the atypical forms generally respond to the GFD with a disappearance of symptoms[17].
Although numerous studies have been carried out to study the pathogenesis of both FGIDs and CD, there are still many unanswered questions. Possible explanations behind the persistence of GI symptoms could be the presence of another unrecognized GI disease, altered bowel motility due to the persistence of low-grade inflammation despite a GFD, microbiota alteration or a continuous intentional or inadvertent intake of gluten[6]. Only a few studies have been reported on the overlap between CD and functional abdominal pain disorders in children, but it seems likely that intestinal inflammation (infectious and non-infectious) predisposes children to develop visceral hypersensitivity that can manifest as functional abdominal pain disorders[18].
O’Leary et al[19] hypothesized that despite the GFD, particularly in treated, occasionally noncompliant celiac patients, low-grade of inflammation that induced sensory or motor dysfunction and IBS-type symptoms persisted and precipitated a motility disturbance in many patients. Another theory is persistent intestinal inflammation could be due to a short follow-up on a GFD. Moreover, gut microbiota of CD patients is characterized by increased Bacteroides spp, Escherichia coli, Proteobacteria and Staphylococcus and decreased Bifidobacterium spp and Lactobacillus[20]. Multiple studies reported similar changes in the microbiota of IBS patients[21,22]. According to the most recent data, intestinal dysbiosis might be responsible for the persistence of symptoms, even in patients on a GFD. In fact, a GFD, though capable of improving the nutritional status of CD patients without causing nutritional problems, is only partially effective in restoring microbiota and may be partly responsible for intestinal dysbiosis due to the reduction in the intake of polysaccharides (fructans), which has a prebiotic action on Bifidobacterium spp.
Moreover, the reduced amount of fiber in a GFD may be considered as one of the reasons why CD patients often suffer from FC[5]. The new appearance of constipation after the introduction of a GFD likely reflects a decrease in fiber intake, and many of these patients may react to the addition of dietary fiber. Constipation can also reflect a return to a predisposition for constipation after resolution of malabsorption. Another possible explanation could be that functional abdominal pain can be triggered not only by gluten but also by other components of wheat including α-amylase/trypsin inhibitors, wheat, lectin, agglutinin and fructans as described in the study by Llanos-Chea and Fasano[23]. They showed that the consumption of wheat and other cereal grains can contribute to the manifestation of chronic inflammation and autoimmune diseases by increasing intestinal permeability and initiating a proinflammatory immune response.
Chronically increased intestinal permeability allows for the increased translocation of both microbial and dietary antigens to the periphery, which can then interact with cells of the immune system and stimulate pathways of innate immunity. According to Barone et al[24], CD patients compared to healthy individuals eat significantly higher amounts of fat and sugar and small amounts of fiber on a GFD[20-23]. Numerous studies have demonstrated that the total fat content of gluten-free foods is at least double that of gluten-containing foods, which helps to improve the taste of these products.
In our study we found that there was no significant difference in the prevalence of GFIDs between children on an industrial manufactured GFD and children who followed a GFD using natural products. However, in both groups we found an association between lower calorie and fat intake and a lower prevalence of FGIDs after 1 year of a GFD.
Dietary fat has been associated with the onset of symptoms after a meal challenge or reported as inducing symptoms of dyspepsia in some studies[25-27], specifically dyspeptic symptoms of nausea[25,26], bloating[25,26], post-prandial fullness/discomfort[25,27] and epigastric pain[26,27]. It is not entirely clear how food factors cause dyspeptic symptoms. Studies have shown that sensitivity to stomach distention and chemical stimuli like nutrients (fat), acid and GI hormones (CCK) and interactions between them have a central role inducing symptomatic response[28]. Zito et al[29] showed that some alimentary regimens or even some meals are able to trigger GI symptoms in predisposed individuals. Fats, in particular, seem to influence gastric activity by delaying gastric emptying and promoting relaxation of the fundus. These mechanisms are closely related to the onset of GI symptoms. Functional dyspeptic patients, for example, usually report that meal size, eating patterns, caloric intake as well as nutrient composition (lipid content in particular) strongly influences the onset of dyspeptic symptoms.
Our study has several strengths including a large sample size representative of the various age groups and well-defined CD and FGIDs diagnoses. The main limitations of our study was the short follow-up associated with the absence of a follow-up endoscopy, which could underestimate the persistence of a slight degree of inflammation. The self-compilation of food diaries associated with an autonomous choice of foods to be consumed during the duration of the study was another limitation. This may have led to bias in the comparison of the macro- and micronutrients of the two groups, possibly leading to a difference in the persistence of symptoms. However, the presence of an experienced dietician who performed a diet check with the patient at each visit coupled with a negative serological test ensured us that the patient was strictly adhering to the GFD. Therefore, accidental and/or occasional consumption of gluten is unlikely to explain GI symptoms. However, this approach limits our ability to evaluate whether the lack of compliance could be responsible for the appearance of symptoms.
In conclusion, this is the first study to show that the presence of functional GI symptoms in children with CD on a GFD are possibly related to higher caloric and fat intake. It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.
When an organic disorder, like celiac disease (CD), is present, functional gastrointestinal disorders (FGIDs) cannot be diagnosed. However, these disorders have a high prevalence. Therefore, a number of studies have assessed the possibility of overlap between FGIDs with organic disorders.
Few data are available regarding the risk of FGIDs in children strictly adhering to a conventional gluten free diet (GFD).
The objectives of this study were to estimate the prevalence of FGIDs in patients affected by CD at the moment of the diagnosis (T0) and after 1 year of follow-up (T1) comparing two different types of GFD.
This study involved 104 celiac pediatric patients (aged 1 year to 18 years) randomized to: Group A, on a GFD with processed foods; and group B, on a GFD with natural products. Clinical follow-up, a 3-d dietary diary evaluation and a questionnaire based on the Rome IV criteria were completed for each child at T0 and T1. We examined the FGIDs after 12 mo and the relationship to the type of GFD.
At the time of enrollment, 54.5% of CD children had symptoms in group A, and 51.0% of CD children had symptoms in group B. At T1, in spite of low or negative CD serology, the prevalence of FGIDs was 18.0% in group A and 16.3% in group B (P = 0.780). In both groups after 12 mo of a GFD an intraindividual analysis showed a significantly lower prevalence of FGIDs (P = 0.055) in patients lower calorie and fat intake.
Many children still have gastrointestinal symptoms and FGIDs despite a strict GFD, and it could be linked to the caloric intake and the amount of fat in the diet. However, it does not seem affected by a GFD with commercial or natural products.
To evaluate the correlation between FGIDs and different types of GFDs, long-term monitoring is necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang XJ, China; Makovicky P, Slovakia; Rostami K, New Zealand S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1160] [Article Influence: 96.7] [Reference Citation Analysis (1)] |
| 2. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 944] [Article Influence: 134.9] [Reference Citation Analysis (1)] |
| 3. | Hujoel IA, Reilly NR, Rubio-Tapia A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol Clin North Am. 2019;48:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Bascuñán KA, Vespa MC, Araya M. Celiac disease: understanding the gluten-free diet. Eur J Nutr. 2017;56:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Turco R, Boccia G, Miele E, Giannetti E, Buonavolontà R, Quitadamo P, Auricchio R, Staiano A. The association of coeliac disease in childhood with functional gastrointestinal disorders: a prospective study in patients fulfilling Rome III criteria. Aliment Pharmacol Ther. 2011;34:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Cristofori F, Tripaldi M, Lorusso G, Indrio F, Rutigliano V, Piscitelli D, Castellaneta S, Bentivoglio V, Francavilla R. Functional Abdominal Pain Disorders and Constipation in Children on Gluten-Free Diet. Clin Gastroenterol Hepatol. 2021;19:2551-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Saps M, Sansotta N, Bingham S, Magazzu G, Grosso C, Romano S, Pusatcioglu C, Guandalini S. Abdominal Pain-Associated Functional Gastrointestinal Disorder Prevalence in Children and Adolescents with Celiac Disease on Gluten-Free Diet: A Multinational Study. J Pediatr. 2017;182:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Lewis ML, Palsson OS, Whitehead WE, van Tilburg MAL. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents. J Pediatr. 2016;177:39-43.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 9. | Koppen IJ, Nurko S, Saps M, Di Lorenzo C, Benninga MA. The pediatric Rome IV criteria: what's new? Expert Rev Gastroenterol Hepatol. 2017;11:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (22)] |
| 10. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Mäki M, Ribes-Koninckx C, Ventura A, Zimmer KP; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1837] [Article Influence: 141.3] [Reference Citation Analysis (1)] |
| 11. | Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, Shamir R, Troncone R, Auricchio R, Castillejo G, Christensen R, Dolinsek J, Gillett P, Hróbjartsson A, Koltai T, Maki M, Nielsen SM, Popp A, Størdal K, Werkstetter K, Wessels M. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 690] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 12. | Buyse I, Decorte R, Baens M, Cuppens H, Semana G, Emonds MP, Marynen P, Cassiman JJ. Rapid DNA typing of class II HLA antigens using the polymerase chain reaction and reverse dot blot hybridization. Tissue Antigens. 1993;41:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Robin SG, Keller C, Zwiener R, Hyman PE, Nurko S, Saps M, Di Lorenzo C, Shulman RJ, Hyams JS, Palsson O, van Tilburg MAL. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J Pediatr. 2018;195:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 14. | Saps M, Nichols-Vinueza DX, Rosen JM, Velasco-Benítez CA. Prevalence of functional gastrointestinal disorders in Colombian school children. J Pediatr. 2014;164:542-5.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Strisciuglio C, Cenni S, Serra MR, Dolce P, Kolacek S, Sila S, Trivic I, Lev MRB, Shamir R, Kostovski A, Papadopoulou A, Roma E, Katsagoni C, Jojkic-Pavkov D, Salvatore S, Pensabene L, Scarpato E, Miele E, Staiano A; Collaborators: Angelo Campanozzi, and Maria Fotoulaki. Functional Gastrointestinal Disorders in Mediterranean Countries According to Rome IV Criteria. J Pediatr Gastroenterol Nutr. 2022;74:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10:e0126982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 17. | Holtmeier W, Caspary WF. Celiac disease. Orphanet J Rare Dis. 2006;1:3. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Langshaw AH, Rosen JM, Pensabene L, Borrelli O, Salvatore S, Thapar N, Concolino D, Saps M. Overlap between functional abdominal pain disorders and organic diseases in children. Rev Gastroenterol Mex (Engl Ed). 2018;83:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | O'Leary C, Wieneke P, Buckley S, O'Regan P, Cronin CC, Quigley EM, Shanahan F. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol. 2002;97:1463-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 21. | Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 812] [Article Influence: 90.2] [Reference Citation Analysis (5)] |
| 22. | Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Llanos-Chea A, Fasano A. Gluten and Functional Abdominal Pain Disorders in Children. Nutrients. 2018;10:1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Barone M, Della Valle N, Rosania R, Facciorusso A, Trotta A, Cantatore FP, Falco S, Pignatiello S, Viggiani MT, Amoruso A, De Filippis R, Di Leo A, Francavilla R. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur J Clin Nutr. 2016;70:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Khodarahmi M, Azadbakht L. Dietary fat intake and functional dyspepsia. Adv Biomed Res. 2016;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Feinle-Bisset C, Meier B, Fried M, Beglinger C. Role of cognitive factors in symptom induction following high and low fat meals in patients with functional dyspepsia. Gut. 2003;52:1414-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Houghton LA, Mangall YF, Dwivedi A, Read NW. Sensitivity to nutrients in patients with non-ulcer dyspepsia. Eur J Gastro Hepatol. 1993;5:109-113. |
| 28. | Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, Talley NJ, Feinle-Bisset C. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103:2613-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Zito FP, Polese B, Vozzella L, Gala A, Genovese D, Verlezza V, Medugno F, Santini A, Barrea L, Cargiolli M, Andreozzi P, Sarnelli G, Cuomo R. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J Gastrointest Pharmacol Ther. 2016;7:564-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |