Published online Dec 7, 2022. doi: 10.3748/wjg.v28.i45.6345
Peer-review started: July 15, 2022
First decision: September 1, 2022
Revised: October 10, 2022
Accepted: November 16, 2022
Article in press: November 16, 2022
Published online: December 7, 2022
Processing time: 140 Days and 6.6 Hours
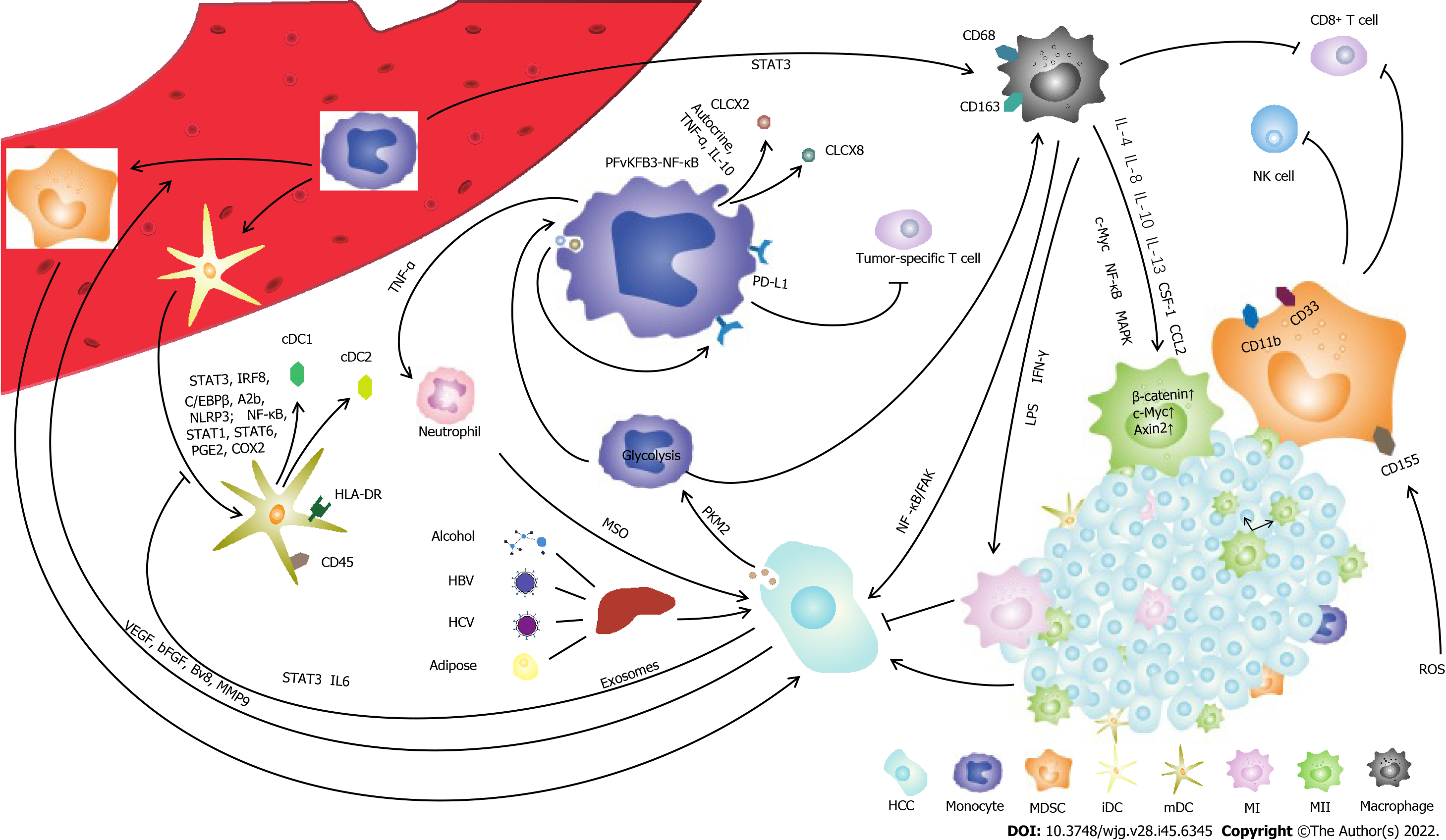
The mononuclear phagocyte system (MPS) consists of monocytes, dendritic cells and macrophages, which play vital roles in innate immune defense against cancer. Hepatocellular carcinoma (HCC) is a complex disease that is affected or initiated by many factors, including chronic hepatitis B virus infection, hepatitis C virus infection, metabolic disorders or alcohol consumption. Liver function, tumor stage and the performance status of patients affect HCC clinical outcomes. Studies have shown that targeted treatment of tumor microenvironment disorders may improve the efficacy of HCC treatments. Cytokines derived from the innate immune response can regulate T-cell differentiation, thereby shaping adaptive immunity, which is associated with the prognosis of HCC. Therefore, it is important to elucidate the function of the MPS in the progression of HCC. In this review, we outline the impact of HCC on the MPS. We illustrate how HCC reshapes MPS cell phenotype remodeling and the production of associated cytokines and characterize the function and impairment of the MPS in HCC.
Core Tip: The hepatocellular carcinoma (HCC) is a complex disease affected or initiated by many factors, including chronic hepatitis B virus infection, hepatitis C virus infection, metabolic disorders or alcohol consumption. Innate immune system can shape the acquired immune response, which can surveillance HCC directly. As the main component of innate immunity, the mononuclear phagocyte system (MPS) plays a vital role in HCC. In this review, we outline the impact of HCC on MPS. We illustrate how HCC reshapes MPS cell phenotype remodeling and producing the associated cytokines, and characterize the function and impairment of MPS in HCC.
- Citation: Qiao DR, Shan GY, Wang S, Cheng JY, Yan WQ, Li HJ. The mononuclear phagocyte system in hepatocellular carcinoma. World J Gastroenterol 2022; 28(45): 6345-6355
- URL: https://www.wjgnet.com/1007-9327/full/v28/i45/6345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i45.6345
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is predicted to be the sixth most-diagnosed cancer and the fourth-leading cause of death among all types of cancers[1-3]. HCC is influenced or initiated by many factors, including chronic hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, metabolic disorder or chronic alcohol consumption[4-7]. More than 85% of HCC cases are accompanied by HBV infection in China[8-9]. Evidence has shown that HCC is a type of tumor with low or moderate immunogenicity[10]. Chronic inflammation creates an immunosuppressive microenvironment in the liver, facilitating HCC tumorigenesis and progression[11]. Although great progress has been made in the treatment of HCC in recent decades, the long-term survival rate of HCC is still poor[12]. Many factors, such as liver function, tumor stage and the performance status of patients, affect HCC clinical outcomes. According to previous studies, targeted treatment of dysregulated tumor microenvironments may improve the efficacy of HCC treatments[11,12].
The mononuclear phagocyte system (MPS) includes monocytes, dendritic cells (DCs) and macro
In addition to its role in tissue development, homeostasis, inflammation and innate immune defense against pathogens, the MPS also plays a vital role in cancer[17,18]. Innate immune cell-derived cytokines can regulate T-cell differentiation, thereby shaping adaptive immunity, which is associated with the prognosis of HCC. It is important to illustrate the critical role of the MPS in the progression of HCC. In this review, we discuss how the HCC microenvironment remodels MPS cell phenotypes and cytokine production and describe the function and impairment of MPS components in HCC.
Monocytes originate in the bone marrow and spleen and account for approximately 5%-10% of human peripheral blood mononuclear cells[13]. Monocytes transform into macrophages and DCs during inflammation[19-23]. It has been reported that the increase in activated monocytes [human leukocyte antigen (HLA)-DRhighCD68+ cells] in the liver is related to disease progression[24]. Chemokine (C-C motif) ligand 15 (CCL15) recruits CCR1+CD14+ monocytes to the edge of HCC tissue. High expression of CCL15 is associated with poor clinical prognosis. CCR1+CD14+ monocytes suppress antitumor immunity, facilitate tumor metastasis and promote tumor cell proliferation and invasion[25]. Blocking CCL2/CCR2-mediated macrophage accumulation has been proposed as a treatment strategy for HCC[26-29].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid progenitors and immature myeloid cells[30]. MDSCs in the tumor microenvironment, which are essential for tumor progression and are effective inhibitors of natural killer (NK) cells in HCC patients, play an important role in immunosuppression[31-33]. In humans, MDSCs usually express CD11b and CD33 and have low or no expression of HLA-DR. Monocyte-derived MDSCs (mMDSCs) express CD14, while granulocyte-derived MDSCs named gMDSCs express CD15 and CD66b[34]. MDSCs inhibit T and NK-cell proliferation and have an inhibitory effect on conventional NK cells in a TIGIT-dependent manner. Compared with conventional NK cells, adaptive NK cells express lower levels of TIGIT and resist the inhibitory effect of MDSCs[35]. MDSCs express CD155, which is induced by reactive oxygen species, and inhibit NK-cell function via the CD155-TIGIT interaction[35]. Compared with normal liver tissues, hepatoma tissues are rich in granulocytes and mononuclear cells. Mononuclear cells and MDSCs suppress the CD8+ T-cell response[36].
S100A9 is a marker that can be used to distinguish MDSCs from monocytes[37]. Studies have iden
DCs are a class of bone marrow-derived cells in the blood, epithelia and lymphoid tissues and are the most powerful professional APCs[39]. DCs participate in the regulation of innate and adaptive immunity[40]. DCs have antigen delivery capabilities, which make them attractive carriers for therapeutic tumor vaccines and platforms for vaccine development[41]. Human DCs are usually induced from monocytes isolated from peripheral blood mononuclear cells by M-CSF and IL-4 stimulation and develop into mature DCs (mDCs) after being loaded with antigens[42].
Human DCs express CD45 and HLA-DR and are divided into different populations according to CD11c, CD123 and IL-3Rα expression. Myeloid DCs (mDCs) are important antigen-presenting cells and express high levels of CD11c but low levels of CD123. Plasmacytoid DCs (pDCs) express high levels of CD11c, CD123, Toll-like receptor (TLR)-7 and TLR-9[43,44]. In normal liver tissues, CD141+ hepatic DCs express CLEC9A, ILT3 and ILT4, but cell percentages and functions are damaged in hepatocellular carcinoma[45-47]. Evidence indicates that DCs have high antitumor and cytotoxic activity against HCC cells in vitro and in vivo[48]. DCs inhibit the growth of HCC cells by loading LCSC antigens[49].
Peptide-pulsed DCs present antigens to naive T cells, thereby activating and inducing naive T cells to become antigen-specific cytotoxic T lymphocytes (CTLs) to kill tumor cells[50]. DCs incubated with epithelial adhesion molecule peptides have a significant inhibitory effect on tumor growth[51]. DC precursors that are sensitized by dexamethasone can effectively trigger the major histocompatibility complex class I (MHC I)-restricted CTL response, allowing DCs to make full use of secondary antigen peptides, thereby maximizing the specificity of the HCC immune response[52].
Macrophages originating in the liver are generally described as Kupffer cells (KCs)[53]. KCs express MHC class II and have varying levels of costimulatory markers, such as CD40, CD80, and CD86, and express the inhibitory marker Z39Ig[20,54]. The activation of STAT3 is involved in the differentiation of monocytes into macrophages[55]. Although tumor-infiltrating macrophages are generally believed to be derived from circulating monocytes, emerging evidence suggests that tissue macrophages can be maintained through self-renewal[56]. Liver resident macrophages are established by progenitor cells derived from the fetal liver and are maintained by self-proliferation and monocyte migration[57,58]. KCs in the liver are negatively correlated with patient prognosis by inhibiting T-cell antitumor functions[59].
Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment and play a key role in immunosuppression[60]. TAMs are important in tumor-related inflammation and can be polarized into M1 or M2 phenotypes[61]. M1 macrophages are induced by LPS and IFN-γ; M2 macrophages are induced by IL-4 and IL-13[62]. It is generally believed that M1 TAMs have antitumor functions, while M2 TAMs promote tumor proliferation[63]. Studies have shown that IL-10 is involved in the polarization of TAMs to the M2 phenotype[64]. CD68 and CD163 are two popular TAM cell surface markers[65,66]. It has been reported that CD68+HLA-DR+ TAMs enhance HCC proliferation through the NFκB/FAK pathway[67].
TAMs induce CD8+ T-cell exhaustion via the PD-1/PD-L1 and Tim3 signaling pathways in the tumor microenvironment[68,69]. CCR2+ TAMs accumulate and express the inflammatory marker S100A9 at the border of highly vascularized HCC, while CD163+ TAMs accumulate in the center area of HCC tissue[70]. Silencing SIRT4 in TAMs regulates macrophage activation and significantly promotes HCC cell growth[71]. Downregulation of SIRT4 expression is related to an increase in macrophage infiltration in HCC tissues and a high ratio of M2/M1 macrophages[72]. The miR-148B/CSF1 pathway regulates the entry of TAMs into HCC tissue. A lack of miR-148B induces CSF1 expression and macrophage infiltration, thereby promoting liver cancer metastasis and poor prognosis[73].
Studies have shown that the tumor environment can trigger early activation of monocytes in the surrounding tumor tissues[74,75]. Tumor environmental factors induce monocytes to express PD-L1 transiently in the early stage, activate monocytes, and induce PD-L1 expression in an autocrine cytokine-dependent manner[75]. HCC cells promote monocyte-to-macrophage differentiation via the PKM2 pathway[76]. In addition, monocytes in human peripheral blood differentiate into immature dendritic cells[77]. Monocyte-derived TNF-α works synergistically with tumor-derived soluble factors to induce neutrophils to produce the metastasis-promoting factor OSM. Tumor-infiltrating monocytes mediate the production of CXCL2 and CXCL8 through the PFKFB3/NFκB signaling pathway. The level of PFKFB3 and the production of CXCL2/CXCL8 in monocytes are positively correlated with the infiltration of OSM-producing neutrophils in human HCC tissues[78].
The tumor microenvironment inhibits DC maturation and activation, leading to an immunosuppressive phenotype and function[79]. In addition, hepatoma cells recruit immunosuppressive DCs and MDSCs to suppress CD8+ T cells, leading to tumor escape from immune surveillance[80]. Tumor-derived exosomes impair the differentiation and maturation of DCs through the IL-6/STAT3 signaling pathway, which prevents myeloid precursor cells from differentiating into CD11c+ DCs and induces apoptosis, thereby reducing T-cell activation and mediating immunosuppression[81,82].
The proliferation of tumor-infiltrated macrophages is more powerful than that of macrophages in nontumor tissues. Increased levels of macrophage proliferation are positively correlated with the density of macrophages in the tumor and the poor prognosis of HCC patients. Proliferating macrophages can be induced by small soluble adenosine, which is derived from tumor cells. GM-CSF released by tumors stimulates macrophages to express the A2A receptor, which then coordinates with adenosine to cause HCC-infiltrating macrophage proliferation[83]. Mitochondrial nucleoid structural changes lead to the release of mitochondrial DNA (mtDNA) into the cytoplasm, which is considered to be mtDNA stress, thereby regulating innate immunity[84].
CSF-1 and CCL2 in the tumor microenvironment polarize macrophages to the M2 phenotype[85,86]. Crosstalk between macrophages and liver tumor cells can also affect the polarization of TAMs through the Wnt/β-catenin signaling pathway[87]. Compared to those in M1 cells, β-catenin, c-Myc and Axin2 are more enriched in M2 TAMs. It is believed that the c-Myc signaling pathway promotes the polarization of TAMs toward the M2 phenotype[88]. Neurotensin-induced IL-8 polarizes TAMs to the M2 phenotype and promotes epithelial-mesenchymal (EMT) in HCC through the MAPK and NFκB pathways[89]. In addition, hypoxia-inducible factors 1α (HIF-1α) and HIF-2α cause TAM polarization through hypoxia in the tumor microenvironment, leading to HCC malignancy[90,91]. Hypoxia-induced high mobility group protein box 1 may affect tumor progression by regulating TAMs, leading to poor clinical prognosis[92].
Components of the MPS have important but distinct roles in HCC. The MPS is associated with specific cell phenotypes and functions in the HCC microenvironment. Monocytes are progenitors of DCs and macrophages, which play an important role in inhibiting the immune response. In addition, MDSCs are a heterogeneous population composed of immature myeloid cells and myeloid progenitor cells. Increasing evidence suggests that tumor progression is associated with the accumulation of MDSCs, which cause local and systemic immunosuppression[93]. Furthermore, increased accumulation of MDSCs is associated with early tumor recurrence and is considered to be one of the predictors of poor prognosis in HCC patients who undergo clinical treatment[94]. Cytokine-induced killer (CIK) cells include a wide variety of T-cell receptor-specific effector cells, which are a mixed cell population. CIK cells have cytotoxic activity against tumor cells, which is not MHC-restricted. Studies have shown that adjuvant immunotherapy for HCC using CIK cells can reduce tumor recurrence and improve overall survival[95]. MDSCs inhibit CIK cell lysis via ARG1 and iNOS.
Human DCs are usually induced from monocytes isolated from peripheral blood. DCs have antigen delivery abilities, which make them attractive carriers for therapeutic tumor vaccines and platforms for vaccine development. In normal liver tissues, hepatic DCs have powerful antigen presentation functions and immunomodulatory abilities, but their percentages and functions are damaged in hepatocellular carcinoma[45]. Monocytes act as precursor cells that differentiate into DCs and macrophages under normal physiological conditions. The following are questions that remain to be investigated. Will monocyte impairments be inherited by DCs and macrophages? Will DCs and macrophages acquire immunosuppressive phenotypes and functions in HCC after differentiation?
Although the majority of previous studies have focused on individual MPS components, interactions and cooperation among various MPS components are common[96-98]. For instance, KCs are the main immune cells in liver tissues and play a key role in DC recruitment to the liver[97]. pDC survival and IFN-α production are affected by IL-10 and TNF-α secretion by monocytes. MDSCs produce increased levels of IL-10, which leads to pDC apoptosis. How do MDSCs interact with pDCs in the HCC microenvironment? Additionally, monocyte- or macrophage-derived IL-15 and pDC-derived IFN-α synergistically stimulate IFN-γ production in NK cells during HCV infection[96]. Does this interaction occur in HCC? Other forms of cooperation between different MPS components remain to be analyzed.
In this review, we described the impact of HCC on each component of the MPS and the immune function of the MPS in HCC development (Figure 1). Monocytes are precursors of DCs and macrophages, which play an important role in the regulation of immune function in HCC. Through crosstalk with hepatoma cells or regulation of the tumor microenvironment, monocytes are capable of producing high levels of IL-10, resulting in an inhibitory microenvironment. DCs can suppress the stimulation of T cells and inhibit efficient antitumor T-cell functions. TAMs are the most abundant immune cells in the tumor microenvironment and play a key role in immunosuppression. The tumor microenvironment inhibits DC maturation and activation, leading to an immunosuppressive phenotype and function. The HCC microenvironment remodels MPS cell phenotypes and cytokine production and impairs MPS components in HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M, India; Wang GX, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21352] [Article Influence: 2135.2] [Reference Citation Analysis (3)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55666] [Article Influence: 7952.3] [Reference Citation Analysis (132)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12982] [Article Influence: 1442.4] [Reference Citation Analysis (2)] |
| 4. | Rilling WS, Drooz A. Multidisciplinary management of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S259-S263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 732] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 7. | Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunology. 2017;6:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2081] [Article Influence: 130.1] [Reference Citation Analysis (1)] |
| 9. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1970] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 10. | Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, Zhang C, Lunceford JK, Joe A, Cheng J, Webber AL, Ibrahim N, Plimack ER, Ott PA, Seiwert TY, Ribas A, McClanahan TK, Tomassini JE, Loboda A, Kaufman D. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1644] [Article Influence: 234.9] [Reference Citation Analysis (0)] |
| 11. | Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Liu GM, Li XG, Zhang YM. Prognostic role of PD-L1 for HCC patients after potentially curative resection: a meta-analysis. Cancer Cell Int. 2019;19:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol. 2014;35:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Tu ZK, Liu XK, Zhang P. Mononuclear phagocyte system in hepatitis C virus infection. World J Gastroenterol. 2018;24:4962-4973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Liu EG, Yin X, Swaminathan A, Eisenbarth SC. Antigen-Presenting Cells in Food Tolerance and Allergy. Front Immunol. 2020;11:616020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 17. | Strauss O, Dunbar PR, Bartlett A, Phillips A. The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver--a systematic review. J Hepatol. 2015;62:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1822] [Article Influence: 121.5] [Reference Citation Analysis (0)] |
| 19. | Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2014] [Cited by in RCA: 2410] [Article Influence: 200.8] [Reference Citation Analysis (0)] |
| 20. | Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 21. | Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1142] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 22. | Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 580] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 691] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 24. | Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3531] [Cited by in RCA: 3811] [Article Influence: 200.6] [Reference Citation Analysis (0)] |
| 25. | Liu LZ, Zhang Z, Zheng BH, Shi Y, Duan M, Ma LJ, Wang ZC, Dong LQ, Dong PP, Shi JY, Zhang S, Ding ZB, Ke AW, Cao Y, Zhang XM, Xi R, Zhou J, Fan J, Wang XY, Gao Q. CCL15 Recruits Suppressive Monocytes to Facilitate Immune Escape and Disease Progression in Hepatocellular Carcinoma. Hepatology. 2019;69:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, Cheung OK, Sun H, Zeng X, Tang W, Mok MTS, Wong J, Yeung PC, Lai PBS, Chen Z, Jin H, Chen J, Chan SL, Chan AWH, To KF, Sung JJY, Chen M, Cheng AS. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020;69:365-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 27. | Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 538] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 28. | Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, Jacob ST, Yu J, Ghoshal K. Blocking the CCL2-CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. Mol Cancer Ther. 2017;16:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, Heikenwalder M, Wang XW, Zender L, Greten TF. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell. 2016;30:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 30. | Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2369] [Cited by in RCA: 2858] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 31. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5311] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 32. | Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015;128:95-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 33. | Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 635] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 34. | Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 825] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 35. | Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res. 2016;76:5696-5706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 36. | Liu YT, Tseng TC, Soong RS, Peng CY, Cheng YH, Huang SF, Chuang TH, Kao JH, Huang LR. A novel spontaneous hepatocellular carcinoma mouse model for studying T-cell exhaustion in the tumor microenvironment. J Immunother Cancer. 2018;6:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF, Korangy F. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology. 2012;136:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183:4119-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 897] [Article Influence: 128.1] [Reference Citation Analysis (0)] |
| 40. | Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2370] [Cited by in RCA: 2337] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 41. | Peng W, Zhao G, Ma Y, Yu H, Wang X. Dendritic cells transfected with PEG10 recombinant adenovirus elicit anti-tumor immune response in vitro and in vivo. Vaccine. 2011;29:3501-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, Teijeira A, Kandalaft LE, Romero P, Coukos G, Neyns B, Sancho D, Melero I, de Vries IJM. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 43. | MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512-4520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 44. | Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 1622] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 45. | Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, Wu L, Shortman K, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703-2717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 46. | Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 47. | Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Yang T, Zhang W, Wang L, Xiao C, Gong Y, Huang D, Guo B, Li Q, Xiang Y, Nan Y. Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer. 2018;18:984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Wierecky J, Mueller M, Brossart P. Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol Immunother. 2006;55:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Choi YJ, Park SJ, Park YS, Park HS, Yang KM, Heo K. EpCAM peptide-primed dendritic cell vaccination confers significant anti-tumor immunity in hepatocellular carcinoma cells. PLoS One. 2018;13:e0190638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Li J, Huang S, Zhou Z, Lin W, Chen S, Chen M, Ye Y. Exosomes derived from rAAV/AFP-transfected dendritic cells elicit specific T cell-mediated immune responses against hepatocellular carcinoma. Cancer Manag Res. 2018;10:4945-4957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Wake K. Karl Wilhelm Kupffer And His Contributions To Modern Hepatology. Comp Hepatol. 2004;3 Suppl 1:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Kwekkeboom J, Kuijpers MA, Bruyneel B, Mancham S, De Baar-Heesakkers E, Ijzermans JN, Bouma GJ, Zondervan PE, Tilanus HW, Metselaar HJ. Expression of CD80 on Kupffer cells is enhanced in cadaveric liver transplants. Clin Exp Immunol. 2003;132:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Guo S, Yang C, Mei F, Wu S, Luo N, Fei L, Chen Y, Wu Y. Down-regulation of Z39Ig on macrophages by IFN-gamma in patients with chronic HBV infection. Clin Immunol. 2010;136:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64:2028-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 57. | van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, Guilliams M. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity. 2016;44:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 58. | Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 59. | Sheng J, Zhang J, Wang L, Tano V, Tang J, Wang X, Wu J, Song J, Zhao Y, Rong J, Cheng F, Wang J, Shen Y, Wen L, He J, Zhang H, Li T, Zhang Q, Bai X, Lu Z, Liang T. Topological analysis of hepatocellular carcinoma tumour microenvironment based on imaging mass cytometry reveals cellular neighbourhood regulated reversely by macrophages with different ontogeny. Gut. 2022;71:1176-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 60. | Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1778] [Cited by in RCA: 2822] [Article Influence: 352.8] [Reference Citation Analysis (0)] |
| 61. | Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019;458:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2054] [Article Influence: 228.2] [Reference Citation Analysis (0)] |
| 63. | Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1122] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 64. | Li L, Sun P, Zhang C, Li Z, Cui K, Zhou W. MiR-98 modulates macrophage polarization and suppresses the effects of tumor-associated macrophages on promoting invasion and epithelial-mesenchymal transition of hepatocellular carcinoma. Cancer Cell Int. 2018;18:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, Steidl C, Gordon LI, Horning SJ, Gascoyne RD. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 66. | Kridel R, Xerri L, Gelas-Dore B, Tan K, Feugier P, Vawda A, Canioni D, Farinha P, Boussetta S, Moccia AA, Brice P, Chavez EA, Kyle AH, Scott DW, Sanders AD, Fabiani B, Slack GW, Minchinton AI, Haioun C, Connors JM, Sehn LH, Steidl C, Gascoyne RD, Salles G. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin Cancer Res. 2015;21:3428-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 67. | Wang H, Wang X, Li X, Fan Y, Li G, Guo C, Zhu F, Zhang L, Shi Y. CD68(+)HLA-DR(+) M1-like macrophages promote motility of HCC cells via NF-κB/FAK pathway. Cancer Lett. 2014;345:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 68. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 69. | Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 70. | Bartneck M, Schrammen PL, Möckel D, Govaere O, Liepelt A, Krenkel O, Ergen C, McCain MV, Eulberg D, Luedde T, Trautwein C, Kiessling F, Reeves H, Lammers T, Tacke F. The CCR2+ Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol Gastroenterol Hepatol. 2019;7:371-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu XW, Gong JP. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38:469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Ke M, Zhang Z, Cong L, Zhao S, Li Y, Wang X, Lv Y, Zhu Y, Dong J. MicroRNA-148b-colony-stimulating factor-1 signaling-induced tumor-associated macrophage infiltration promotes hepatocellular carcinoma metastasis. Biomed Pharmacother. 2019;120:109523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 74. | Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1659] [Article Influence: 87.3] [Reference Citation Analysis (2)] |
| 75. | Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 723] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 76. | Hou PP, Luo LJ, Chen HZ, Chen QT, Bian XL, Wu SF, Zhou JX, Zhao WX, Liu JM, Wang XM, Zhang ZY, Yao LM, Chen Q, Zhou D, Wu Q. Ectosomal PKM2 Promotes HCC by Inducing Macrophage Differentiation and Remodeling the Tumor Microenvironment. Mol Cell. 2020;78:1192-1206.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 77. | Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 78. | Peng ZP, Jiang ZZ, Guo HF, Zhou MM, Huang YF, Ning WR, Huang JH, Zheng L, Wu Y. Glycolytic activation of monocytes regulates the accumulation and function of neutrophils in human hepatocellular carcinoma. J Hepatol. 2020;73:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 79. | Motta JM, Rumjanek VM. Sensitivity of Dendritic Cells to Microenvironment Signals. J Immunol Res. 2016;2016:4753607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867-6875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 83. | Wang J, Wang Y, Chu Y, Li Z, Yu X, Huang Z, Xu J, Zheng L. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 2021;74:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 84. | Bao D, Zhao J, Zhou X, Yang Q, Chen Y, Zhu J, Yuan P, Yang J, Qin T, Wan S, Xing J. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene. 2019;38:5007-5020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 85. | Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4053] [Cited by in RCA: 3854] [Article Influence: 275.3] [Reference Citation Analysis (0)] |
| 86. | Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 684] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 87. | Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC, Han H, Liu WC, Qin HY. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 88. | Mao J, Wang D, Wang Z, Tian W, Li X, Duan J, Wang Y, Yang H, You L, Cheng Y, Bian J, Chen Z, Yang Y. Combretastatin A-1 phosphate, a microtubule inhibitor, acts on both hepatocellular carcinoma cells and tumor-associated macrophages by inhibiting the Wnt/β-catenin pathway. Cancer Lett. 2016;380:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Xiao P, Long X, Zhang L, Ye Y, Guo J, Liu P, Zhang R, Ning J, Yu W, Wei F, Yu J. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology. 2018;7:e1440166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 90. | Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 372] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 91. | Jiang J, Wang GZ, Wang Y, Huang HZ, Li WT, Qu XD. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6. Exp Cell Res. 2018;367:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Zhang QB, Jia QA, Wang H, Hu CX, Sun D, Jiang RD, Zhang ZL. High-mobility group protein box1 expression correlates with peritumoral macrophage infiltration and unfavorable prognosis in patients with hepatocellular carcinoma and cirrhosis. BMC Cancer. 2016;16:880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 587] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 94. | Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 95. | Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, Fu Q, Agdashian D, Rosato U, Korangy F, Greten TF. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol. 2019;70:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 96. | Zhang S, Saha B, Kodys K, Szabo G. IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J Hepatol. 2013;59:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Uwatoku R, Suematsu M, Ezaki T, Saiki T, Tsuiji M, Irimura T, Kawada N, Suganuma T, Naito M, Ando M, Matsuno K. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology. 2001;121:1460-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |