Published online Nov 21, 2022. doi: 10.3748/wjg.v28.i43.6078
Peer-review started: September 14, 2022
First decision: October 3, 2022
Revised: October 6, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: November 21, 2022
Processing time: 62 Days and 21.7 Hours
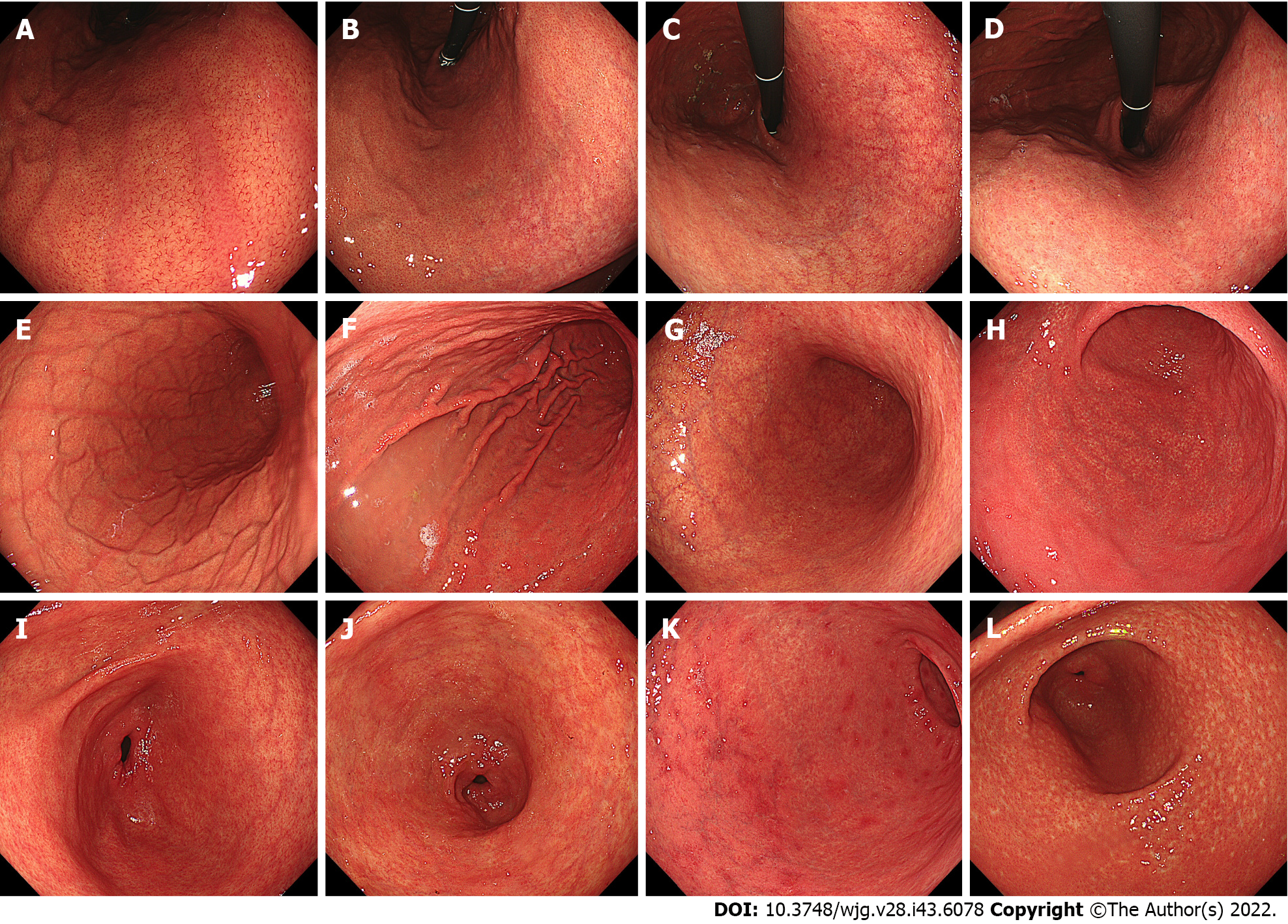
This editorial provides an update of the recent evidence on the endoscopy-based Kyoto classification of gastritis, clarifying the shortcomings of the Kyoto classification, and providing prospects for future research, with particular focus on the histological subtypes of gastric cancer (GC) and Helicobacter pylori (H. pylori) infection status. The total Kyoto score is designed to express GC risk on a score ranging from 0 to 8, based on the following five endoscopic findings: Atrophy, intestinal metaplasia (IM), enlarged folds (EF), nodularity, and diffuse redness (DR). The total Kyoto score reflects H. pylori status as follows: 0, ≥ 2, and ≥ 4 indicate a normal stomach, H. pylori-infected gastritis, and gastritis at risk for GC, respectively. Regular arrangement of collecting venules (RAC) predicts non-infection; EF, nodularity, and DR predict current infection; map-like redness (MLR) predicts past infection; and atrophy and IM predict current or past in
Core Tip: Endoscopy-based Kyoto classification of gastritis assesses gastric cancer (GC) risk and Helicobacter pylori (H. pylori) infection status. Total Kyoto scores of 0, ≥ 2, and ≥ 4 indicate a normal stomach, H. pylori-infected gastritis, and gastritis at risk for GC, respectively. Atrophy, intestinal metaplasia (IM), and enlarged folds (EF) increase H. pylori-infected GC incidence. Map-like redness is a specific risk factor for H. pylori-eradicated GC, while regular arrangement of collecting venules result in less GC risk. Diffuse-type GC is induced by active inflammation, depicting EF, nodularity, and atrophy. Intestinal-type GC develops through atrophy and IM; however, the GC risk-scoring design still needs to be improved.
- Citation: Toyoshima O, Nishizawa T. Kyoto classification of gastritis: Advances and future perspectives in endoscopic diagnosis of gastritis. World J Gastroenterol 2022; 28(43): 6078-6089
- URL: https://www.wjgnet.com/1007-9327/full/v28/i43/6078.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i43.6078
The Kyoto classification of gastritis aims to match the endoscopic and histopathological findings of gastritis. It further aims to evaluate gastric cancer (GC) risk and Helicobacter pylori (H. pylori) infection of gastritis. The Kyoto classification was first advocated by the Japan Gastroenterological Endoscopy Society in 2013 and is widely used in recent clinical practice worldwide[1]. Technological advances in endoscopy have significantly improved the accuracy of identifying premalignant mucosal changes[2]. This editorial provides an update of the recent evidence on the Kyoto classification, clarifying the shortcomings of the Kyoto classification, and providing prospects for future research. This article is divided into the following four chapters: (1) H. pylori infection according to the Kyoto classification; (2) The histological consistency of the Kyoto classification; (3) Risk of GC according to the Kyoto classification; and (4) Future prospects in the Kyoto classification.
In the Kyoto classification, the total Kyoto score has been developed as a GC risk score. The total Kyoto score is calculated as the sum of the following 5 endoscopic findings: Atrophy, intestinal meta
| Endoscopic findings | Kyoto score | ||
| 0 | 1 | 2 | |
| Atrophy1 | None, C1 | C2, C3 | O1-O3 |
| Intestinal metaplasia | None | Antrum | Corpus and antrum |
| Enlarged folds | Absence | Presence | - |
| Nodularity | Absence | Presence | - |
| Diffuse redness | None | Mild with RAC | Severe without RAC |
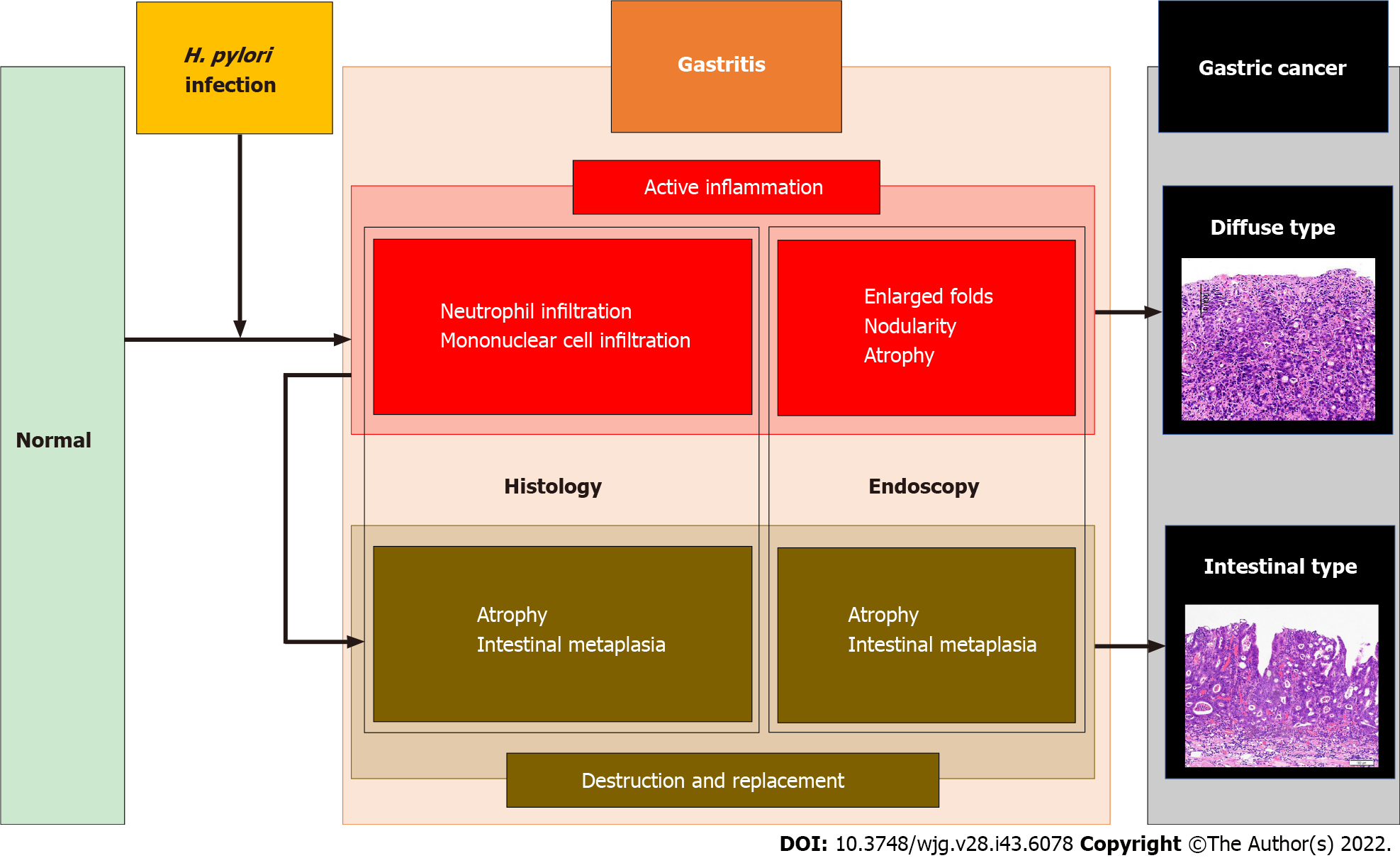
GCs consist of two distinct histological subtypes: Lauren’s diffuse and intestinal GC[4]. Diffuse-type GC develops directly from highly active inflammation, whereas intestinal-type GC develops through destruction and replacement of tissues, such as atrophy and IM, and is termed Correa’s cascade[5-7]. GCs can also be described according to the different rates of incidence[8,9], lesion characteristics[10-12], and prognoses[13-16] as per the corresponding H. pylori infection status. In this editorial, we specifically describe the histological subtypes of GC and H. pylori infection status.
Evidence of RAC as an indicator of non-infection has been reported in both in Japan[17,18] and several other countries[19-21], including in the west[22-24], as shown in Table 2. Two recent meta-analyses reported that the sensitivity and specificity of RAC for predicting non-infection were 78%-80% and 94%-97%, respectively[25,26]. The high reliability of RAC for non-infectious cases has also been verified.
| Ref. | Country | No. of patients | Sensitivity | Specificity | Accuracy | |
| Non-infection | ||||||
| RAC | Garcés-Durán et al[22], 2019 | Spain | 140 | 100 | 49.0 | 65.0 |
| Yoshii et al[17], 2020 | Japan | 485 | 89.1 | 79.8 | 85.6 | |
| Zhao et al[19], 2020 | China | 583 | 62.4 | 73.7 | 69.3 | |
| Ebigbo et al[23], 2021 | Germany | 200 | 80.8 | 57.4 | - | |
| Fiuza et al[20], 2021 | Brazil | 187 | 70.7 | 87.2 | 74.9 | |
| Glover et al[24], 2021 | UK | 153 | 78.4 | 64.3 | 75.8 | |
| Yuan et al[21], 2021 | China | 165 | 51.4 | 96.7 | 76.4 | |
| Hirai et al[18], 2021 | Japan | 1761 | 93.2 | 83.2 | 90.6 | |
| Current infection | ||||||
| Atrophy | Toyoshima et al[27], 2018 | Japan | 136 | 82.6 | 85.8 | 85.3 |
| Zhao et al[19], 2020 | China | 583 | 54.9 | 61.1 | 58.5 | |
| Ebigbo et al[23], 2021 | Germany | 200 | 80.4 | 69.7 | - | |
| Intestinal metaplasia | Toyoshima et al[27], 2018 | Japan | 136 | 39.1 | 95.6 | 86.0 |
| Enlarged folds | Toyoshima et al[27], 2018 | Japan | 136 | 17.4 | 99.1 | 85.3 |
| Yoshii et al[17], 2020 | Japan | 494 | 23.1 | 96.6 | 85.0 | |
| Nodularity | Toyoshima et al[27], 2018 | Japan | 136 | 8.7 | 100 | 84.6 |
| Yoshii et al[17], 2020 | Japan | 494 | 6.4 | 98.3 | 83.8 | |
| Toyoshima et al[28], 2020 | Japan | 265 | 33.3 | 99.6 | 89.1 | |
| Fiuza et al[20], 2021 | Brazil | 187 | 10.6 | 98.6 | 76.5 | |
| Diffuse redness | Toyoshima et al[27], 2018 | Japan | 136 | 52.2 | 93.8 | 86.8 |
| Yoshii et al[17], 2020 | Japan | 485 | 60.0 | 94.7 | 89.7 | |
| Zhao et al[19], 2020 | China | 583 | 20.3 | 97.6 | 65.0 | |
| Fiuza et al[20], 2021 | Brazil | 187 | 80.9 | 73.6 | 75.4 | |
| Total Kyoto score | Toyoshima et al[27], 20181 | Japan | 136 | 78.3 | 92.0 | 89.7 |
| Sumi et al[30], 20222 | Japan | 561 | 98.7 | 98.4 | 98.6 | |
All five Kyoto scores, atrophy (61.1%-85.8% and 58.5%-85.3%)[19,23,27], IM (95.6% and 86.0%)[27], EF (96.6%-99.1% and 85.0%-85.3%)[17,27], nodularity (98.3%-100% and 76.5%-89.1%)[17,20,27,28], and DR (73.6%-97.6% and 65.0%-89.7%)[17,19,20,27], commonly offer high specificity and accuracy for categorizing current infections (Table 2).
Three studies have previously compared patients with non-infectious, current, and past infections, all of which reported that RAC was strongly correlated with non-infection [odds ratios (ORs) = 4.6-55.0]; MLR was a highly specific finding indicative of past infection (ORs = 7.8-12.9), and DR, EF, and nodularity provided high ORs of 10.5-26.4, 6.0-8.6, and 4.0-22.5, respectively, for current infection. Atrophy and IM were associated with both current (ORs = 1.9-21.6 and 4.3) and past infections (ORs = 1.9-22.8 and 4.4), respectively[17,19,25]. A previous study reported an algorithm with an accuracy of 80.0% for defining the presence of RAC as non-infection, DR and mucosal edema as current infection, and MLR as post-eradication[24]. H. pylori eradication decreases the Kyoto EF, nodularity, and DR scores, but does not improve the Kyoto atrophy and IM scores[29]. These results indicate that the presence of RAC predicts non-infection; EF, nodularity, and DR predict current infection; MLR predicts past infection; and atrophy and IM predict current or past infection.
Several studies have previously focused on the association between the total Kyoto score and H. pylori infection. The sensitivity and specificity of the total Kyoto score for current infection were good at 78.3%-98.7% and 92.0%-98.4%, respectively (Table 2)[27,30]. The area under the curve (AUC) of the total Kyoto score for predicting current infection was 0.85, with a cutoff value of 2[27]. Current infection rates increased stepwise, with total Kyoto scores of 0-1, 2-3, and ≥ 4 (8.6%, 61.4%, and 85.7%, respectively)[31]. The mean total Kyoto scores differed among patients with current, past, and non-infection (3.4, 1.1, and 0.0, respectively)[32]. A combination of the total Kyoto score and serum H. pylori antibody titer allows for the accurate diagnosis of current infection[33]. The total Kyoto score decreases from 3.9 to 2.8 following H. pylori eradication[29]. In summary, total Kyoto scores of 0 and ≥ 2 express non-infection and current infection, respectively.
The purpose of the Kyoto classification is to match endoscopic and histological findings of gastritis. Regarding atrophy and IM, considerable evidence exists to indicate the consistency between endoscopy and histology. In recent studies, a high Kyoto atrophy score and severe endoscopic IM are associated with histologically advanced stages of operative link for gastritis assessment and operative link for gastric IM assessment, respectively[34,35].
Consistency between the endoscopic findings of the Kyoto scores and histological grading of the updated Sydney system (USS) scores has been examined individually. All five Kyoto scores were associated with histological inflammation, namely the USS score for neutrophil and mononuclear cell infiltration, which is an indicator of H. pylori infection. The Kyoto atrophy and IM scores correlated with both histological atrophy and IM in the corpus[34,36]. Among H. pylori-infected patients, the Kyoto EF, nodularity, and DR scores indicated histologically high inflammation in the corpus[36-38]. In summary, the Kyoto atrophy and IM scores were concordant with histological corpus atrophy and IM scores. The Kyoto EF and nodularity scores were associated with the histological corpus inflammation.
Significant evidence to indicate endoscopic atrophy as a risk factor for GC has been accumulated. The incidence of GC based on atrophy is summarized in Table 3. GC incidences for mild, moderate, and severe atrophy are 0.06%-0.15%, 0.12%-0.34%, 0.31%-1.60%, respectively, indicating the severity of atrophy as a risk factor for GC development, even after H. pylori eradication[39-41]. A recent study from Western countries also showed that a Kyoto atrophy score of 2 was associated with GC development with a hazard ratio of 6.4 in patients with baseline IM[42].
| Ref. | Population | No. of subjects | No. of cancers | Duration, yr | Gastric cancer incidence, %/yr | ||
| Atrophy (mild) | Atrophy (moderate) | Atrophy (severe) | |||||
| Shichijo et al[39], 20161,2 | Post eradication | 573 | 21 | 6.2 ± 4.8 | 0.07 | 0.34 | 1.60 |
| Kaji et al[40], 20193 | Screening | 12941 | 63 | 3.7 ± 0.8 | 0.10 | 0.16 | 0.31 |
| Post eradication | 2571 | 20 | 3.7 ± 0.8 | 0.06 | 0.12 | 0.42 | |
| Take et al[41], 20201 | Post eradication | 2737 | 68 | 7.1 ± 5.4 | 0.15 | 0.29 | 0.67 |
The ORs for the histological subtypes of GC based on the Kyoto classification are summarized in Table 4. The Kyoto atrophy score is a predictor of GC with ORs of 2.5-7.4[43-45]. Two recent meta-analyses showed that a Kyoto atrophy score of 2 had high risk ratios (2.8-8.0 for developing GC)[46,47]. In an examination based on histological subtypes, a high Kyoto atrophy score was found to be associated with both diffuse-type and intestinal-type GCs with ORs of 2.3 and 6.2, respectively[44].
| Ref. | H. pylori status | No. of subjects | No. of GC | No. of diffuse-type GC | No. of intestinal-type GC | OR for GC | OR for diffuse-type GC | OR for intestinal-type GC | |
| Atrophy | Sekikawa et al[43], 2016 | Current, past, and no infection | 1823 | 29 | 3 | 26 | 7.41 | ||
| Toyoshima et al[44], 2021 | Current infection | 499 | 132 | 39 | 93 | 2.8 | 2.3 | 6.2 | |
| Kawamura et al[45], 2022 | Current, past, and no infection | 380 | 115 | 19 | 96 | 2.51 | |||
| Intestinal metaplasia | Shichijo et al[48], 2017 | Current, past, and no infection | 3392 | 107 | 22 | 85 | 0.22 | ||
| Toyoshima et al[44], 2021 | Current infection | 499 | 132 | 39 | 93 | 1.6 | 1.7 | ||
| Enlarged folds | Nishibayashi et al[81], 2003 | Current infection | 276 | 135 | 69 | 66 | 5.0 | ||
| Toyoshima et al[44], 2021 | Current infection | 499 | 132 | 39 | 93 | 0.5 | |||
| Nodularity | Nishikawa et al[53], 2018 | Current infection | 674 | 25 | 9 | 16 | 10.0 | ||
| Toyoshima et al[44], 2021 | Current infection | 499 | 132 | 39 | 93 | 0.5 | 0.3 | ||
| RAC | Kawamura et al[45], 2022 | Current, past, and no infection | 380 | 115 | 19 | 96 | 0.23 | ||
| Total Kyoto score | Toyoshima et al[44], 2021 | Current infection | 499 | 132 | 39 | 93 | 1.6 | 1.3 | 1.7 |
| Lin et al[61], 2022 | Current, past, and no infection | 1848 | 37 | - | - | 1.5 |
A high Kyoto IM score indicates a high risk for GC (OR = 1.6), especially intestinal-type GC (OR = 1.7), but a low risk for diffuse-type GC (OR = 0.2)[44,45,48,49]. In a direct comparison of diffuse-type and intestinal-type GCs, a high Kyoto IM score was associated with intestinal-type GC (ORs = 1.7-2.1)[44,49]. Furthermore, a high Kyoto IM score was associated with multiple GCs[50].
In a study on asymptomatic H. pylori-infected patients, the hazard ratio of patients with EF for GC development during the 5 years was high at 43.3[51]. In contrast, EF was associated with a low risk of intestinal-type GC (OR = 0.5)[44]. Furthermore, a direct comparison between diffuse-type and intestinal-type GCs indicated EF as a risk factor for diffuse-type GC (OR = 1.3)[44]. EF is reported to be an indicator of submucosal invasion in patients with GC (OR = 3.4; submucosal invasion vs intramucosal depth)[52].
The risk of nodularity is controversial. Previous studies found that nodularity was associated with a high risk for diffuse-type GC (OR = 10.0)[53], notably in young H. pylori-infected patients (OR = 64.2)[54]. In contrast, nodularity was described as a low risk factor for GC (OR = 0.5), especially intestinal-type GC (OR = 0.3)[44]. Nodularity decreases with age and the risk of intestinal-type GC increases with age[28]. Therefore, the risk of nodularity in GC should be stratified according to age.
Previously, RAC has been revealed as a predictor of non-GC[45]. Collectively, the Kyoto atrophy, EF, and nodularity scores were associated with diffuse-type GC, whereas the Kyoto atrophy and IM scores were related to intestinal-type GC, as shown in Figure 2.
Recently, the risk of GC after H. pylori eradication has been intensively investigated. Table 5 shows the risk of GC following H. pylori eradication. A Kyoto atrophy score of 2 and MLR are both indicators of GC after eradication, with ORs of 8.1 and 1.8-5.3, respectively[55-57]. Additionally, RAC was inversely associated with eradicated GC (ORs = 0.3-0.4)[56,58]. Studies have further revealed that the hazard ratios of Kyoto atrophy 2 and MLR for GC development were 4.9 and 3.6, respectively[55,59]. Take et al[41] previously reported the long-term incidence of GC after eradication based on endoscopic atrophy. The incidence of diffuse-type GC was higher in the second decade of follow-up than in the first decade. This increase was only observed in patients with mild-to-moderate gastric atrophy, indicating that even if atrophy is not severe, the risk of GC can persist long after eradication.
The total Kyoto scores of patients with GC, H. pylori-infected GC, and H. pylori-eradicated GC were 4.0-4.6, 4.8-5.6, and 4.2, respectively[44,50,58,60]. A high total Kyoto score was associated not only with GC (ORs = 1.5-1.6), but also with both diffuse-type and intestinal-type GCs (ORs = 1.3 and 1.7, respectively, Table 4)[44,61]. Additionally, some investigators showed that the incidence of GC increased stepwise with the total Kyoto scores of 0-1, 2-3, ≥ 4, and that the AUC of the nomogram to predict GC using the total Kyoto score was 0.79[31,61]. Taken together, a total Kyoto score of 4 or more is useful for de
The total Kyoto score was developed to evaluate GC risk, with a score ≥ 4 indicating risk. However, designing a method to simply add each component of the Kyoto score is problematic. First, the GC risks of the diffuse and intestinal types were distinctly different. For example, IM is associated with a high risk of intestinal-type GC but a low risk of diffuse-type GC. Conversely, EF and nodularity are high risk factors for diffuse-type GC, but indicate a low risk of intestinal-type GC (Table 4 and Figure 2). The majority of GC cases are classified as intestinal type, which indicates that the intestinal-type GC risk may be overestimated, whereas the diffuse-type GC risk may be underestimated. Second, two points were assigned to Kyoto atrophy, IM, and DR scores in the total Kyoto score. The verification of the weighting of the total Kyoto score is a future task. Therefore, this scoring method should be revised in the future. A modified Kyoto score has been suggested as the sum of the following points: 2 points for invisible RAC, and 1 point each for Kyoto atrophy score 2, Kyoto IM score 2, and corpus MLR. Compared with the scores of 0-1, the ORs of the GC morbidity for the modified Kyoto scores of 2-3 and 4-5 were higher, at 8.6 and 28.0, respectively. Although statistical significance was not reached, the AUC of the modified Kyoto score had a higher predictive ability than that of the original total Kyoto score (0.75 vs 0.71, respectively)[45]. Furthermore, a scoring system specific to histological GC subtypes is needed. Third, MLR has been shown to predict GC after H. pylori eradication. Since IM manifests as MLR after H. pylori eradication[3], MLR may be more suitable than IM to assess the risk of eradicated GC.
In Western countries, RAC and endoscopic IM have been extensively studied; however, other endoscopic findings, such as atrophy, EF, nodularity, DR, and MLR, have been less extensively explored, and further studies in more varied populations are required. This article does not mention a variety of important endoscopic findings, including spotty redness as a predictor of H. pylori infection[62]; xanthoma[57,63,64], foveolar hyperplastic polyp[65], refluxed bile[66], and a lack of fundic gland polyp[64] to predict GC; and depressive erosion and fundic gland polyp as indicators of functional dyspepsia[32,67]. Further research is required to confirm these findings. RAC provides high kappa values of intra-observer and inter-observer agreements of 0.88-0.91 and 0.74-0.79, respectively[21,68]; however, agreement between the other endoscopic findings needs to be clarified.
Autoimmune gastritis (AIG) is gaining attention as an important factor owing to the decrease in H. pylori infection[69]. Both severe endoscopic atrophy and a Kyoto IM score of 2 have been reported as AIG features[70,71]. Further steps should be taken to elucidate the differential diagnosis between AIG and H. pylori-associated gastritis using the Kyoto classification.
Recently, image-enhanced endoscopy (IEE) has been widely used in clinical practice. Two meta-analyses previously reported the utility of narrow-band imaging (NBI) for the diagnosis of IM[72,73]. Additionally, an improved diagnostic accuracy on using NBI, blue laser imaging, and linked color imaging have been reported[74-77]. In the future, research on endoscopic assessment using IEE, including texture and color enhancement imaging[78,79] will be required.
In conclusion, the total Kyoto score and individual Kyoto score, including atrophy, IM, EF, nodularity, and DR, can predict GC risk and H. pylori infection. Total Kyoto scores of 0, ≥ 2, and ≥ 4 indicate a normal stomach, H. pylori-infected gastritis, and gastritis at risk for GC, respectively; RAC predicts non-infection; EF, nodularity, and DR predict current infection; MLR predicts past infection; and atrophy and IM predict current or past infection. Atrophy, IM, and EF all increase in H. pylori-infected GC, MLR is a specific risk factor for H. pylori-eradicated GC, while RAC indicates a lesser GC risk. Diffuse-type GC can be induced by active inflammation, which presents as EF, nodularity, and atrophy on endoscopy, and neutrophil and mononuclear cell infiltration on histological examination. In contrast, intestinal-type GC develops via atrophy and IM, and is consistent on endoscopy and histology. However, the GC risk-scoring design still needs to be improved.
We would like to thank Dr. Hidenobu Watanabe to provide histological gastric cancer images.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jia J, China; Zhang J, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Kato M, Kamada T. Endoscopic Findings for Risk Stratification of Gastric Cancer. In: Haruma K, Kato M, Inoue K, Murakami K, Kamada T. Kyoto Classification of Gastritis. 1st ed. Tokyo: Nihon Medical Center, 2017: 97-110. |
| 2. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 3. | Nagata N, Shimbo T, Akiyama J, Nakashima R, Kim HH, Yoshida T, Hoshimoto K, Uemura N. Predictability of Gastric Intestinal Metaplasia by Mottled Patchy Erythema Seen on Endoscopy. Gastroenterology Res. 2011;4:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4322] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 5. | Dixon MF. Pathology of Gastritis and Peptic Ulceration. In: Helicobacter pylori: Physiology and Genetics. 2001;. [PubMed] |
| 6. | Nardone G, Rocco A, Malfertheiner P. Review article: helicobacter pylori and molecular events in precancerous gastric lesions. Aliment Pharmacol Ther. 2004;20:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527-536.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 9. | Kawai S, Wang C, Lin Y, Sasakabe T, Okuda M, Kikuchi S. Lifetime incidence risk for gastric cancer in the Helicobacter pylori-infected and uninfected population in Japan: A Monte Carlo simulation study. Int J Cancer. 2022;150:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 10. | Tahara S, Tahara T, Horiguchi N, Kato T, Shinkai Y, Yamashita H, Yamada H, Kawamura T, Terada T, Okubo M, Nagasaka M, Nakagawa Y, Shibata T, Yamada S, Urano M, Tsukamoto T, Kurahashi H, Kuroda M, Ohmiya N. DNA methylation accumulation in gastric mucosa adjacent to cancer after Helicobacter pylori eradication. Int J Cancer. 2019;144:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Okada K, Suzuki S, Naito S, Yamada Y, Haruki S, Kubota M, Nakajima Y, Shimizu T, Ando K, Uchida Y, Hirasawa T, Fujisaki J, Tsuchida T. Incidence of metachronous gastric cancer in patients whose primary gastric neoplasms were discovered after Helicobacter pylori eradication. Gastrointest Endosc. 2019;89:1152-1159.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Miyaoka M, Yao K, Tanabe H, Kanemitsu T, Imamura K, Ono Y, Ohtsu K, Ishikawa S, Kojima T, Hasegawa R, Hirano A, Ikezono G, Hisabe T, Ueki T, Ota A, Haraoka S, Iwashita A. Usefulness of vessel plus surface classification system for the diagnosis of early gastric cancer after Helicobacter pylori eradication. Ann Gastroenterol. 2021;34:354-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H, Kanda M, Hirahashi M, Matsumoto T. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M, Ushijima T, Oda I. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Kim YJ, Seo SI, Shin WG, Park CH. Impact of the timing of Helicobacter pylori eradication on the risk of development of metachronous lesions after treatment of early gastric cancer: a population-based cohort study. Gastrointest Endosc. 2020;92:613-622.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Nakata R, Nagami Y, Hashimoto A, Sakai T, Ominami M, Fukunaga S, Otani K, Hosomi S, Tanaka F, Ohira M, Taira K, Yamagami H, Tanigawa T, Watanabe T, Fujiwara Y. Successful Eradication of Helicobacter pylori Could Prevent Metachronous Gastric Cancer: A Propensity Matching Analysis. Digestion. 2021;102:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 18. | Hirai R, Hirai M, Shimodate Y, Minami M, Ishikawa S, Kanadani T, Takezawa R, Doi A, Nishimura N, Mouri H, Matsueda K, Yamamoto H, Mizuno M. Feasibility of endoscopic evaluation of Helicobacter pylori infection status by using the Kyoto classification of gastritis in the population-based gastric cancer screening program: A prospective cohort study. Health Sci Rep. 2021;4:e325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zhao J, Xu S, Gao Y, Lei Y, Zou B, Zhou M, Chang D, Dong L, Qin B. Accuracy of Endoscopic Diagnosis of Helicobacter pylori Based on the Kyoto Classification of Gastritis: A Multicenter Study. Front Oncol. 2020;10:599218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Fiuza F, Maluf-Filho F, Ide E, Furuya CK Jr, Fylyk SN, Ruas JN, Stabach L, Araujo GA, Matuguma SE, Uemura RS, Sakai CM, Yamazaki K, Ueda SS, Sakai P, Martins BC. Association between mucosal surface pattern under near focus technology and Helicobacter pylori infection. World J Gastrointest Endosc. 2021;13:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 21. | Yuan C, Lin XM, Ou Y, Cai L, Cheng Q, Zhou P, Liao J. Association between regular arrangement of collecting venules and Helicobacter pylori status in routine endoscopy. BMC Gastroenterol. 2021;21:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Garcés-Durán R, García-Rodríguez A, Córdova H, Cuatrecasas M, Ginès À, González-Suárez B, Araujo I, Llach J, Fernández-Esparrach G. Association between a regular arrangement of collecting venules and absence of Helicobacter pylori infection in a European population. Gastrointest Endosc. 2019;90:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Ebigbo A, Marienhagen J, Messmann H. Regular arrangement of collecting venules and the Kimura-Takemoto classification for the endoscopic diagnosis of Helicobacter pylori infection: Evaluation in a Western setting. Dig Endosc. 2021;33:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Glover B, Teare J, Patel N. Assessment of Helicobacter pylori status by examination of gastric mucosal patterns: diagnostic accuracy of white-light endoscopy and narrow-band imaging. BMJ Open Gastroenterol. 2021;8:e000608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Glover B, Teare J, Ashrafian H, Patel N. The endoscopic predictors of Helicobacter pylori status: a meta-analysis of diagnostic performance. Ther Adv Gastrointest Endosc. 2020;13:2631774520950840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Li L, Jing J, Gao H, Zhang C, Lou H, Pan W. Regular arrangement of collecting venules under endoscopy for predicting a Helicobacter pylori-negative stomach: A systematic review and meta-analysis. Gastroenterol Hepatol. 2021;44:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Toyoshima O, Nishizawa T, Arita M, Kataoka Y, Sakitani K, Yoshida S, Yamashita H, Hata K, Watanabe H, Suzuki H. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol. 2018;24:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 28. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Watanabe H, Yoshida S, Nakai Y, Hata K, Ebinuma H, Suzuki H, Koike K. Nodularity-like appearance in the cardia: novel endoscopic findings for Helicobacter pylori infection. Endosc Int Open. 2020;8:E770-E774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Kinoshita K, Torii A, Yamada A, Suzuki H, Koike K. Helicobacter pylori eradication improved the Kyoto classification score on endoscopy. JGH Open. 2020;4:909-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Sumi N, Haruma K, Kamada T, Suehiro M, Manabe N, Akiyama T, Shiotani A, Yamanaka Y, Fujimoto S, Takao T. Diagnosis of histological gastritis based on the Kyoto classification of gastritis in Japanese subjects - including evaluation of aging and sex difference of histological gastritis. Scand J Gastroenterol. 2022;57:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Liu XM, Ma XY, Liu F, Liu ZL, Tang XY, Ji MZ, Zheng JX. Gastric Cancer Screening Methods: A Comparative Study of the Chinese New Gastric Cancer Screening Score and Kyoto Classification of Gastritis. Gastroenterol Res Pract. 2022;2022:7639968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Takahashi K, Sugimoto M, Kawai Y, Hamada M, Iwata E, Niikura R, Nagata N, Fukuzawa M, Itoi T, Ohtsubo T, Kawai T. Association between dyspeptic symptoms and endoscopic findings based on the Kyoto classification of gastritis in Japanese male. J Clin Biochem Nutr. 2022;70:79-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Nishizawa T, Sakitani K, Suzuki H, Yamakawa T, Takahashi Y, Yamamichi N, Watanabe H, Seto Y, Koike K, Toyoshima O. A combination of serum anti-Helicobacter pylori antibody titer and Kyoto classification score could provide a more accurate diagnosis of H pylori. United European Gastroenterol J. 2019;7:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Quach DT, Hiyama T, Le HM, Nguyen TS, Gotoda T. Use of endoscopic assessment of gastric atrophy for gastric cancer risk stratification to reduce the need for gastric mapping. Scand J Gastroenterol. 2020;55:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Na HK, Choi KD, Park YS, Kim HJ, Ahn JY, Lee JH, Jung KW, Kim DH, Song HJ, Lee GH, Jung HY. Endoscopic scoring system for gastric atrophy and intestinal metaplasia: correlation with OLGA and OLGIM staging: a single-center prospective pilot study in Korea. Scand J Gastroenterol. 2022;57:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 36. | Toyoshima O, Nishizawa T, Yoshida S, Matsuno T, Odawara N, Toyoshima A, Sakitani K, Watanabe H, Fujishiro M, Suzuki H. Consistency between the endoscopic Kyoto classification and pathological updated Sydney system for gastritis: A cross-sectional study. J Gastroenterol Hepatol. 2022;37:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Kako S, Iwaya Y, Nagaya T, Hara D, Okamura T, Iwaya M, Kurasawa S, Kato S, Nakayama Y, Akamatsu T, Umemura T. Clinicopathological features of nodular gastritis in three classes of age. Helicobacter. 2021;26:e12845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Okamoto K, Kodama M, Mizukami K, Okimoto T, Abe H, Ogawa R, Fukuda K, Matsunari O, Hirashita Y, Wada Y, Fukuda M, Murakami K. Immunohistochemical differences in gastric mucosal damage between nodular and non-nodular gastritis caused by Helicobacter pylori infection. J Clin Biochem Nutr. 2021;69:216-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 40. | Kaji K, Hashiba A, Uotani C, Yamaguchi Y, Ueno T, Ohno K, Takabatake I, Wakabayashi T, Doyama H, Ninomiya I, Kiriyama M, Ohyama S, Yoneshima M, Koyama N, Takeda Y, Yasuda K. Grading of Atrophic Gastritis is Useful for Risk Stratification in Endoscopic Screening for Gastric Cancer. Am J Gastroenterol. 2019;114:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, Yoshida T, Yokota K, Mitsuhashi T, Okada H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 42. | Maric L, Castaneda D, Singh H, Bejarano P, Jimenez Cantisano B, Castro FJ. Kimura-Takemoto Classification: A Tool to Predict Gastric Intestinal Metaplasia Progression to Advanced Gastric Neoplasia. Dig Dis Sci. 2022;67:4092-4099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Sekikawa A, Fukui H, Sada R, Fukuhara M, Marui S, Tanke G, Endo M, Ohara Y, Matsuda F, Nakajima J, Henmi S, Saito S, Tsumura T, Maruo T, Kimura T, Osaki Y. Gastric atrophy and xanthelasma are markers for predicting the development of early gastric cancer. J Gastroenterol. 2016;51:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Toyoshima O, Nishizawa T, Yoshida S, Aoki T, Nagura F, Sakitani K, Tsuji Y, Nakagawa H, Suzuki H, Koike K. Comparison of endoscopic gastritis based on Kyoto classification between diffuse and intestinal gastric cancer. World J Gastrointest Endosc. 2021;13:125-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Kawamura M, Uedo N, Koike T, Kanesaka T, Hatta W, Ogata Y, Oikawa T, Iwai W, Yokosawa S, Honda J, Asonuma S, Okata H, Ohyauchi M, Ito H, Abe Y, Ara N, Kayaba S, Shinkai H, Shimokawa T. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig Endosc. 2022;34:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 46. | Sui Z, Chen J, Li P, Shao L, Ye J, Lu X, Cai J. Risk for gastric cancer in patients with gastric atrophy: a systematic review and meta-analysis. Transl Cancer Res. 2020;9:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 47. | Xiao S, Fan Y, Yin Z, Zhou L. Endoscopic grading of gastric atrophy on risk assessment of gastric neoplasia: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Shin SY, Kim JH, Chun J, Yoon YH, Park H. Chronic atrophic gastritis and intestinal metaplasia surrounding diffuse-type gastric cancer: Are they just bystanders in the process of carcinogenesis? PLoS One. 2019;14:e0226427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Sakitani K, Nishizawa T, Toyoshima A, Yoshida S, Matsuno T, Yamada T, Irokawa M, Takahashi Y, Nakai Y, Toyoshima O, Koike K. Kyoto classification in patients who developed multiple gastric carcinomas after Helicobacter pylori eradication. World J Gastrointest Endosc. 2020;12:276-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 51. | Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T, Iguchi M, Tamai H, Utsunomiya H, Yamamichi N, Fujishiro M, Iwane M, Tekeshita T, Mohara O, Ushijima T, Ichinose M. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Toyoshima O, Yoshida S, Nishizawa T, Toyoshima A, Sakitani K, Matsuno T, Yamada T, Matsuo T, Nakagawa H, Koike K. Enlarged folds on endoscopic gastritis as a predictor for submucosal invasion of gastric cancers. World J Gastrointest Endosc. 2021;13:426-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Nishikawa I, Kato J, Terasoma S, Matsutani H, Tamaki H, Tamaki T, Kuwashima F, Nakata H, Tomeki T, Matsunaka H, Ibata Y, Yamashita Y, Maekita T, Higashi K, Ichinose M. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open. 2018;2:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Kamada T, Tanaka A, Yamanaka Y, Manabe N, Kusunoki H, Miyamoto M, Tanaka S, Hata J, Chayama K, Haruma K. Nodular gastritis with helicobacter pylori infection is strongly associated with diffuse-type gastric cancer in young patients. Digest Endosc. 2007;19:180-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Moribata K, Iguchi JK, Nakachi K, Maeda Y, Shingaki N, Niwa T, Deguchi H, Inoue I, Maekita T, Tamai H, Ichinose M. Endoscopic features associated with development of metachronous gastric cancer in patients who underwent endoscopic resection followed by Helicobacter pylori eradication. Dig Endosc. 2016;28:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Majima A, Dohi O, Takayama S, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest Endosc. 2019;90:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Yan X, Hu X, Duan B, Zhang X, Pan J, Fu J, Xu M, Xu Q. Exploration of endoscopic findings and risk factors of early gastric cancer after eradication of Helicobacter pylori. Scand J Gastroenterol. 2021;56:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ohno A, Miyoshi J, Kato A, Miyamoto N, Yatagai T, Hada Y, Kusuhara M, Jimbo Y, Ida Y, Tokunaga K, Okamoto S, Hisamatsu T. Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication -analysis based on the Kyoto classification. BMC Gastroenterol. 2020;20:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Hanaoka N, Uedo N, Shiotani A, Inoue T, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Haruma K, Tatsuta M. Autofluorescence imaging for predicting development of metachronous gastric cancer after Helicobacter pylori eradication. J Gastroenterol Hepatol. 2010;25:1844-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Lin J, Su H, Zhou Q, Pan J, Zhou L. Predictive value of nomogram based on Kyoto classification of gastritis to diagnosis of gastric cancer. Scand J Gastroenterol. 2022;57:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Cho JH, Jeon SR, Jin SY, Park S. Standard vs magnifying narrow-band imaging endoscopy for diagnosis of Helicobacter pylori infection and gastric precancerous conditions. World J Gastroenterol. 2021;27:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 63. | Shibukawa N, Ouchi S, Wakamatsu S, Wakahara Y, Kaneko A. Gastric Xanthoma Is a Predictive Marker for Early Gastric Cancer Detected after Helicobacter pylori Eradication. Intern Med. 2019;58:779-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Yamashita K, Suzuki R, Kubo T, Onodera K, Iida T, Saito M, Arimura Y, Endo T, Nojima M, Nakase H. Gastric Xanthomas and Fundic Gland Polyps as Endoscopic Risk Indicators of Gastric Cancer. Gut Liver. 2019;13:409-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Hu H, Zhang Q, Chen G, Pritchard DM, Zhang S. Risk factors and clinical correlates of neoplastic transformation in gastric hyperplastic polyps in Chinese patients. Sci Rep. 2020;10:2582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Li D, Zhang J, Yao WZ, Zhang DL, Feng CC, He Q, Lv HH, Cao YP, Wang J, Qi Y, Wu SR, Wang N, Zhao J, Shi YQ. The relationship between gastric cancer, its precancerous lesions and bile reflux: A retrospective study. J Dig Dis. 2020;21:222-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Tanaka F, Tominaga K, Fujikawa Y, Morisaki T, Otani K, Hosomi S, Nagami Y, Kamata N, Taira K, Nakano A, Kimura T, Yamagami H, Tanigawa T, Morikawa H, Fukumoto S, Watanabe T, Kawada N, Hirata K, Fujiwara Y. Association between Functional Dyspepsia and Gastric Depressive Erosions in Japanese Subjects. Intern Med. 2019;58:321-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Garcés-Durán R, Galdín-Ferreyra M, Delgado-Guillena PG, Cuatrecasas M, Córdova H, García-Rodríguez A, Rodrigo-Calvo MT, Jimeno-Ramiro M, Araujo IK, Ginès A, Llach J, Fernandez-Esparrach G. Diagnosis of Helicobacter pylori Infection by the Arrangement of Collecting Venules Using White Light Endoscopy: Evaluation of Interobserver Agreement. Dig Dis. 2022;40:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Lenti MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, De Block C, Hershko C, Di Sabatino A. Autoimmune gastritis. Nat Rev Dis Primers. 2020;6:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 70. | Kishikawa H, Nakamura K, Ojiro K, Katayama T, Arahata K, Takarabe S, Sasaki A, Miura S, Hayashi Y, Hoshi H, Kanai T, Nishida J. Relevance of pepsinogen, gastrin, and endoscopic atrophy in the diagnosis of autoimmune gastritis. Sci Rep. 2022;12:4202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Dilaghi E, Esposito G, Pivetta G, Galli G, Pilozzi E, Annibale B, Lahner E. Endoscopic diagnosis of gastric intestinal metaplasia in patients with autoimmune gastritis using narrow-band imaging: does pseudopyloric metaplasia muddy the waters? Endosc Int Open. 2022;10:E434-E440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Rodríguez-Carrasco M, Esposito G, Libânio D, Pimentel-Nunes P, Dinis-Ribeiro M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: a systematic review and meta-analysis. Endoscopy. 2020;52:1048-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 73. | Desai M, Boregowda U, Srinivasan S, Kohli DR, Al Awadhi S, Murino A, Yu LHK, Dinis-Ribeiro DM, Sharma P. Narrow band imaging for detection of gastric intestinal metaplasia and dysplasia: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:2038-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Chen H, Liu Y, Lu Y, Lin X, Wu Q, Sun J, Li C. Ability of blue laser imaging with magnifying endoscopy for the diagnosis of gastric intestinal metaplasia. Lasers Med Sci. 2018;33:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Min M, Dong TH, Liu Y, Bi YL, Ma CY. Novel endoscopic findings as visualized by non-magnification endoscopy with linked color imaging are indicative of gastric intestinal metaplasia. Chin Med J (Engl). 2019;132:782-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Matsumura S, Dohi O, Yamada N, Harusato A, Yasuda T, Yoshida T, Ishida T, Azuma Y, Kitae H, Doi T, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Takagi T, Ishikawa T, Konishi H, Morinaga Y, Kishimoto M, Yagi N, Naito Y, Itoh Y. Improved Visibility of Early Gastric Cancer after Successful Helicobacter pylori Eradication with Image-Enhanced Endoscopy: A Multi-Institutional Study Using Video Clips. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 78. | Ishikawa T, Matsumura T, Okimoto K, Nagashima A, Shiratori W, Kaneko T, Oura H, Tokunaga M, Akizue N, Ohta Y, Saito K, Arai M, Kato J, Kato N. Efficacy of Texture and Color Enhancement Imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci Rep. 2021;11:6910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 79. | Abe S, Yamazaki T, Hisada IT, Makiguchi ME, Yoshinaga S, Sato T, Nonaka S, Suzuki H, Oda I, Saito Y. Visibility of early gastric cancer in texture and color enhancement imaging. DEN Open. 2022;2:e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 80. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (3)] |
| 81. | Nishibayashi H, Kanayama S, Kiyohara T, Yamamoto K, Miyazaki Y, Yasunaga Y, Shinomura Y, Takeshita T, Takeuchi T, Morimoto K, Matsuzawa Y. Helicobacter pylori-induced enlarged-fold gastritis is associated with increased mutagenicity of gastric juice, increased oxidative DNA damage, and an increased risk of gastric carcinoma. J Gastroenterol Hepatol. 2003;18:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |