Published online Nov 14, 2022. doi: 10.3748/wjg.v28.i42.6068
Peer-review started: August 7, 2022
First decision: August 31, 2022
Revised: October 14, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 14, 2022
Processing time: 95 Days and 7.1 Hours
Hepatic venous pressure gradient (HVPG) is the gold standard for diagnosis of portal hypertension (PH). However, its use can be limited because it is an invasive procedure. Therefore, it is necessary to explore a non-invasive method to assess PH.
To investigate the correlation of computed tomography (CT) perfusion of the liver with HVPG and Child-Pugh score in hepatitis B virus (HBV)-related PH.
Twenty-eight patients (4 female, 24 male) with gastroesophageal variceal bleeding induced by HBV-related PH were recruited in our study. All patients received CT perfusion of the liver before transjugular intrahepatic portosystemic stent-shunt (TIPS) therapy. Quantitative parameters of CT perfusion of the liver, including liver blood flow (LBF), liver blood volume (LBV), hepatic artery fraction, splenic blood flow and splenic blood volume were measured. HVPG was recorded during TIPS therapy. Correlation of liver perfusion with Child-Pugh score and HVPG were analyzed, and the receiver operating characteristic curve was analyzed. Based on HVPG (> 12 mmHg vs ≤ 12 mmHg), patients were divided into moderate and severe groups, and all parameters were compared.
Based on HVPG, 18 patients were classified into the moderate group and 10 patients were classified into the severe group. The Child-Pugh score, HVPG, LBF and LBV were significantly higher in the moderate group compared to the severe group (all P < 0.05). LBF and LBV were negatively associated with HVPG (r = -0.473, P < 0.05 and r = -0.503, P < 0.01, respectively), whereas splenic blood flow was positively associated with hepatic artery fraction (r = 0.434, P < 0.05). LBV was negatively correlated with Child-Pugh score. Child-Pugh score was not related to HVPG. Using a cutoff value of 17.85 mL/min/100 g for LBV, the sensitivity and specificity of HVPG ≥ 12 mmHg for diagnosis were 80% and 89%, respectively.
LBV and LBF were negatively correlated with HVPG and Child-Pugh scores. CT perfusion imaging is a potential non-invasive quantitative predictor for PH in HBV-related liver cirrhosis.
Core Tip: Hepatic venous pressure gradient (HVPG) is the gold standard for the diagnosis of portal hypertension (PH), but its use is limited because it is an invasive procedure. Non-invasive assessment of HVPG requires further research. Computed tomography perfusion of the liver may be a useful tool for the evaluation of HVPG. Our results showed that a cutoff of 17.85 mL/min/100 g for liver blood volume yielded an 80% sensitivity and 89% specificity for severe PH. Therefore, computed tomography perfusion of the liver has the potential as a non-invasive quantitative predictor for PH in hepatitis B virus-related liver cirrhosis.
- Citation: Wang L, Zhang Y, Wu YF, Yue ZD, Fan ZH, Zhang CY, Liu FQ, Dong J. Computed tomography perfusion in liver and spleen for hepatitis B virus-related portal hypertension: A correlation study with hepatic venous pressure gradient. World J Gastroenterol 2022; 28(42): 6068-6077
- URL: https://www.wjgnet.com/1007-9327/full/v28/i42/6068.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i42.6068
Gastroesophageal variceal bleeding is a common complication of portal hypertension (PH) in decompensated liver cirrhosis. There is a 60% recurrence rate and 20% mortality rate in the 1st year, and it is the leading cause of liver transplantation and mortality[1-4]. The diagnostic criteria for PH include hepatic venous pressure gradient (HVPG) ≥ 5 mmHg. Notably, when HVPG is higher than 12 mmHg, patients have a significantly increased risk of gastroesophageal bleeding. It was reported that HVPG was positively associated with individual risk of gastroesophageal variceal bleeding, and the incidence of variceal bleeding increased proportionally with an increase in HVPG[1,5-8]. In addition, HVPG can be applied clinically for risk stratification, therapeutic adoption, drug efficacy and adverse events for PH[4,9-12]. However, HVPG is an invasive procedure, which has limited its wide application for the evaluation of therapeutic effects or long-term follow-up. Therefore, studies continue to focus on non-invasive evaluation of HVPG, including anatomy (e.g., liver volume, maximal diameter of spleen), lab results (e.g., platelet level, coagulation function), liver function (e.g., Child-Pugh score, model for end-stage liver disease [commonly known as MELD] score), liver and spleen stiffness (e.g., FibroScan, FibroTouch, magnetic resonance elastography), and even computation simulation modeling. However, none of these methods has demonstrated satisfactory accuracy and reproducibility.
Computed tomography (CT) perfusion of the liver is traditionally utilized to evaluate liver cancer, metastatic tumors, and liver cirrhosis. Decreased blood flow perfusion from the portal vein system and increased blood flow perfusion from the hepatic artery system can be identified with CT perfusion of the liver[13-16]. Furthermore, liver blood perfusion after transjugular intrahepatic portosystemic stent-shunt (TIPS) can be quantitatively assessed with CT perfusion[17]. However, very few reports have focused on the correlation between HVPG and CT perfusion in gastroesophageal bleeding. Talakić et al[13] reported that HVPG had no correlation with CT perfusion in end-stage cirrhosis. Therefore, we aimed to explore the relationship between quantitative indices of CT perfusion with HVPG and the Child-Pugh score and to investigate the feasibility of CT perfusion as a non-invasive imaging tool for HVPG in gastroesophageal variceal bleeding induced by hepatitis B virus (HBV)-related PH.
This prospective study was approved by the Institutional Ethics Committee at our hospital, and all written informed consents were obtained from each participant. Patients with recurrent gastroesophageal variceal bleeding resulting from HBV-related PH were randomly recruited from January 1, 2019 to June 30, 2019. All patients previously underwent drug and/or endoscopic therapy and were prepared for the TIPS procedure. The inclusion criteria were as follows: (1) Gastroesophageal bleeding as a consequence of HBV-related PH; (2) CT perfusion and Child-Pugh score available 1 wk before TIPS surgery; and (3) HVPG measured during TIPS and HVPG ≥ 5 mmHg. The exclusion criteria were as follows: (1) Gastroesophageal bleeding caused by any other etiology; (2) liver tumors, including primary and metastatic; (3) any other conditions leading to hemodynamic changes in the liver, such as partial hepatectomy, splenectomy, hepatic tumor surgery, TIPS, etc; (4) any factors affecting liver blood perfusion, such as portal vein thrombosis, cavernous transformation, Budd-Chiari syndrome, etc; (5) dysfunction in vital organs, such as cardiac, renal or respiratory damage/failure; and (6) any factors that reduced the quality of CT images, such as motion and metal artifacts.
CT perfusion was performed by a Revolution CT scanner (GE Healthcare, Chicago, IL, United States) with 16 cm Z-axis coverage axial scanning mode to cover most parts of the liver. Scanning parameters were set as tube voltage 100 kVp, automatic tube current from 50 mA to 200 mA with noise index as 14, slice thickness of 5 mm, rotation speed of 1.0 sec, helical pitch of 0.992:1.000 and 80% adaptive statistical iterative reconstruction (commonly known as ASIR). Initially, 50 mL nonionic contrast media (Omnipaque 350; GE Healthcare) followed by a 50-mL saline chaser were injected through the antecubital vein at a rate of 5 mL/sec, using a dual-head pump injector (Stellant; Medtron, Saarbrucken, Germany). The scanning was fixed with a 9-sec time delay after injection. Then, CT perfusion was performed. The CT perfusion was compromised of 26 pass acquisitions and 25 interscan gap without table movement, including 10 early acquisitions with an interscan gap of 1 sec, 12 acquisitions with an interscan gap of 2 sec, and 4 acquisitions with an interscan gap of 4 sec. Thus, total scanning time was 80 sec. All patients were instructed to avoid deep and irregular breathing during the procedure. A band compressing the upper abdomen was used to reduce respiratory motion artifacts.
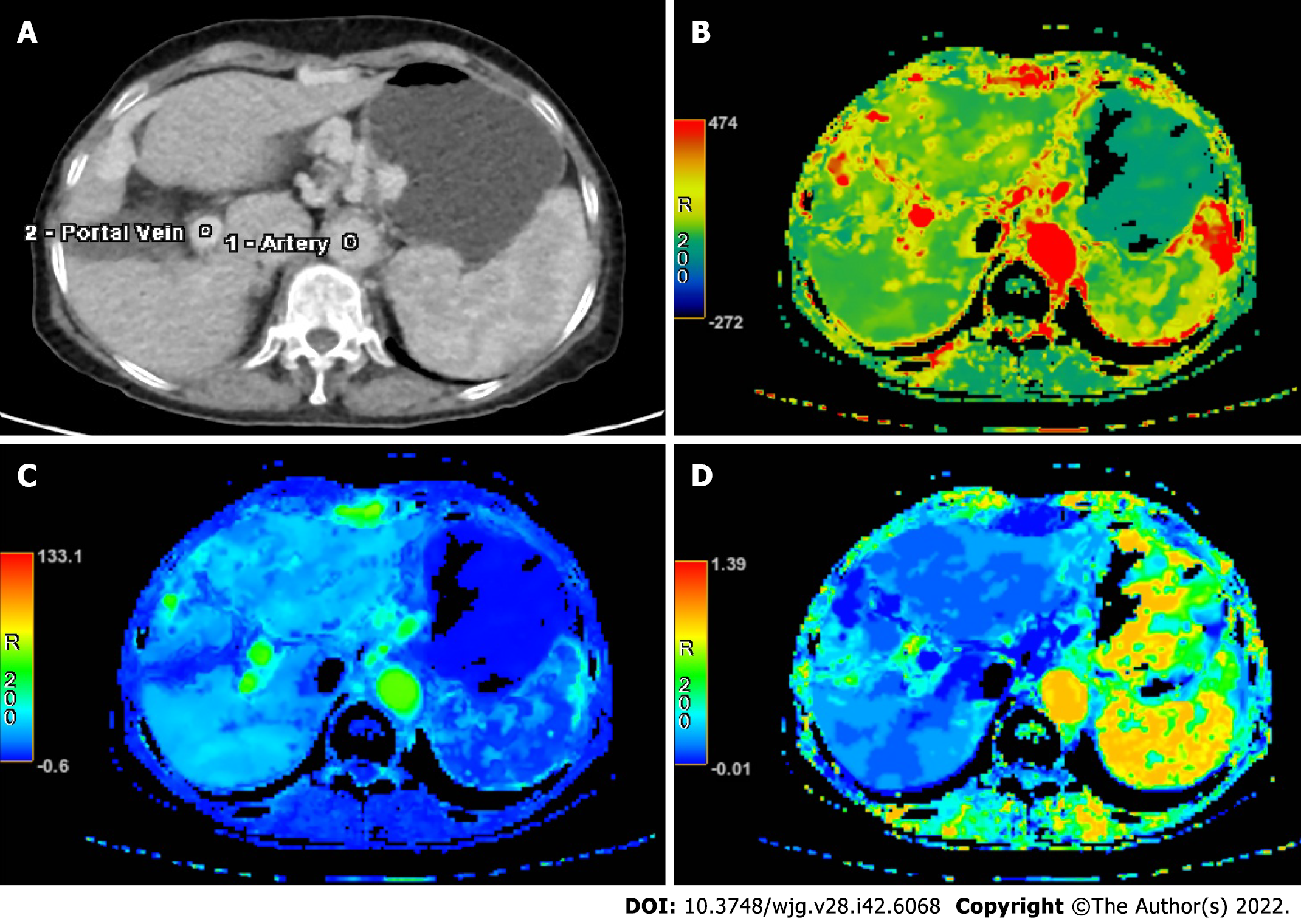
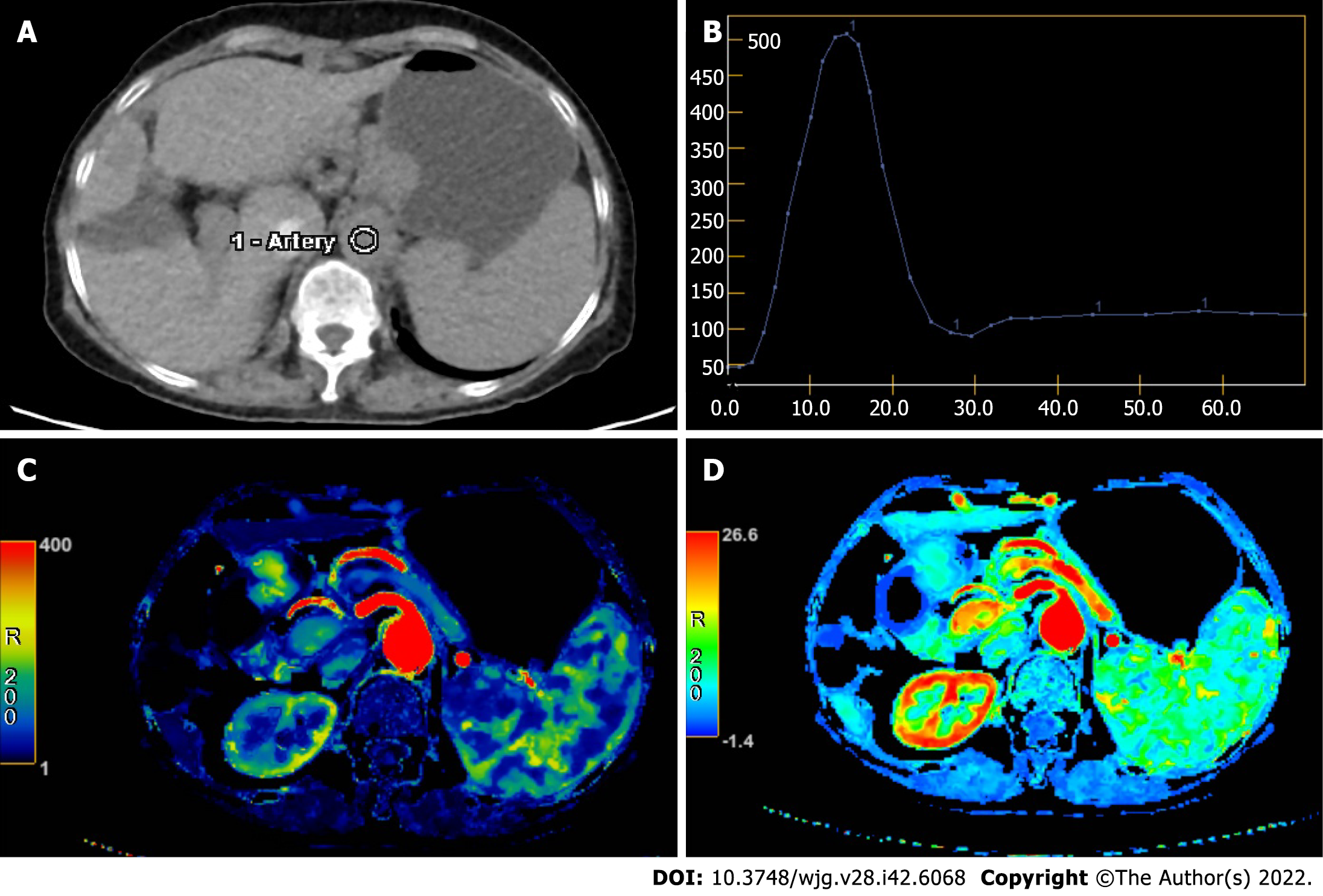
Raw data generated by CT perfusion were reconstructed with a thickness of 2.5 mm. Post-processing was performed separately by two radiologists with 11 years and 7 years respectively of experience in the CT perfusion procedure. First, iterative registration reconstruction was performed to correct respiratory motion between each dynamic acquisition. Second, corrected data were post-processed with a commercial software (CT Perfusion 4D AW 4.7; GE Healthcare). Third, regions of interest were placed in the abdominal aorta and portal vein separately for liver perfusion (Figure 1). The region of interest was placed in the abdominal aorta only for splenic perfusion (Figure 2). Then, the perfusion map would be generated automatically for the liver and spleen (Figures 1 and 2). Finally, three volumes-of-interest would be selected in the left and right liver parenchyma without any hepatic vessels. By contrast, three volumes-of-interest were also selected in the superior, medial and inferior splenic parenchyma. Then, average values of perfusion parameters, including liver blood volume (LBV) (mL/100 mL), liver blood flow (LBF) (mL/100 mL/min), hepatic arterial fraction (HAF) (%), splenic blood volume (SBV) (mL/100 mL/min) and splenic blood flow (SBF) (mL/100 mL/min) were calculated and recorded.
HVPG was measured according to established standards[18,19] during the TIPS procedure. After fasting for more than 8 h, all patients underwent local anesthesia. The right internal jugular vein was cannulated using the Seldinger technique, and a 5-French balloon catheter (Edwards Lifesciences LLC, Irvine, CA, United States) was placed into the right hepatic vein, and the wedged and free hepatic venous pressure was measured three times in each patient. Then, HVPG was calculated as the difference between average wedged and free hepatic venous pressure.
Statistical analysis was performed with SPSS 24.0 software (IBM Corp., Armonk, NY, United States). All data were described as mean ± SD or range [95% confidence interval (CI)]. Kolmogorov-Smirnov was performed for the normal distribution test. Pearson or Spearman was used to evaluate the relationship among quantitative indices. Kappa was applied to analyze the agreement between observers. Patients were classified into two groups according to the HVPG value [> 12 mmHg (moderate) vs ≤ 12 mmHg (severe)]. Quantitative indices, including LBV, HAF, LBF, and SBV, were compared between the two groups. Receiver operating characteristic (ROC) was performed to calculate a cutoff value for differentiation between moderate and severe PH. A P value of less than 0.05 was considered significant.
Initially, 35 patients had portal vein thrombosis. Then, 13 patients with splenectomy, 3 patients with liver tumors and 2 patients with motion artifacts (leading to unavailable CT perfusion) were excluded. Finally, 28 patients (4 female and 24 male) were included in our study, with an age range of 28-years-old to 68-years-old and an average age of 53.7 years ± 10.4 years. Patient characteristics are summarized in Table 1, including demographics, medical history, Child-Pugh class, and HVPG.
| Characteristic | Value |
| Sex as female/male, n | 4/24 |
| Age, yr, mean ± SD | 53.7 ± 10.4 |
| Height, cm, mean ± SD | 169.4 ± 5.8 |
| Weight, kg, mean ± SD | 62.9 ± 11.6 |
| Previous episodes of variceal bleeding, mean ± SD | 3 ± 2 |
| Treatment history, n (%) | |
| β blockade only | 3 (10.7) |
| Sclera therapy only | 4 (14.3) |
| β blockade and sclerotherapy | 21 (75.0) |
| Child-Pugh stage, n (%) | |
| A | 11 (39.3) |
| B | 16 (57.1) |
| C | 1 (3.6) |
| Ascites, n (%) | |
| None | 17 (60.7) |
| Mild | 2 (7.1) |
| Severe | 9 (32.1) |
| HVPG, mmHg, n (%) | |
| < 12 | 10 (35.7) |
| ≥ 12 | 18 (64.3) |
Ten patients had moderate PH (HVPG < 12 mmHg), and the remaining eighteen patients had severe PH (HVPG ≥ 12 mmHg). The median HVPG was 10 mmHg (interquartile range: 9.0 mmHg; range: 8.0-11.0 mmHg) in the moderate PH group and 21 mmHg (interquartile range: 17.5 mmHg; range: 14.0-31.0 mmHg) in the severe PH group. In the moderate PH group, 6 patients were Child-Pugh A and 4 patients were Child-Pugh B. In the severe PH group, 5 patients were Child-Pugh A, 12 patients were Child-Pugh B, and 1 patient was Child-Pugh C. For the moderate PH group, HVPG and Child-Pugh scores were lower than those in the severe PH group (9.6 mmHg ± 1.3 mmHg vs 18.9 mmHg ± 4.4 mmHg, P < 0.001) (Table 2).
| Index | Moderate PH | Severe PH | P value |
| Sex as female/male | 2/8 | 2/16 | 0.520 |
| Age, yr | 54.2 ± 10.9 | 53.4 ± 10.5 | 0.848 |
| Height, cm | 168.0 ± 6.0 | 170.1 ± 5.6 | 0.362 |
| Weight, kg | 64.8 ± 12.3 | 61.8 ± 11.4 | 0.528 |
| Child-Pugh score | 7.1 ± 1.9 | 7.8 ± 1.8 | 0.023 |
| HVPG | 9.6 ± 1.3 | 18.9 ± 4.4 | 0.000 |
| Perfusion CT | |||
| LBF | 114.6 ± 36.0 | 87.9 ± 24.8 | 0.029 |
| LBV | 19.7 ± 3.0 | 15.5 ± 2.2 | 0.000 |
| HAF as × 10-2 | 8.2 ± 2.3 | 8.7 ± 4.7 | 0.731 |
| SBF | 96.0 ± 30.0 | 108.7 ± 31.4 | 0.308 |
| SBV | 13.9 ± 2.9 | 11.9 ± 2.5 | 0.084 |
The two radiologists demonstrated good agreement (Kappa = 0.821, P < 0.01) in the evaluation of the CT perfusion parameters. Quantitative parameters of CT perfusion of the liver are summarized in Table 2. Both LBF and LBV in moderate PH were higher than in severe PH (114.6 ± 36.0 vs 87.9 ± 24.8 and 19.7 ± 3.0 vs 15.5 ± 2.2, respectively, all P < 0.05). No significant difference was observed between the two groups for the other indices (Table 2).
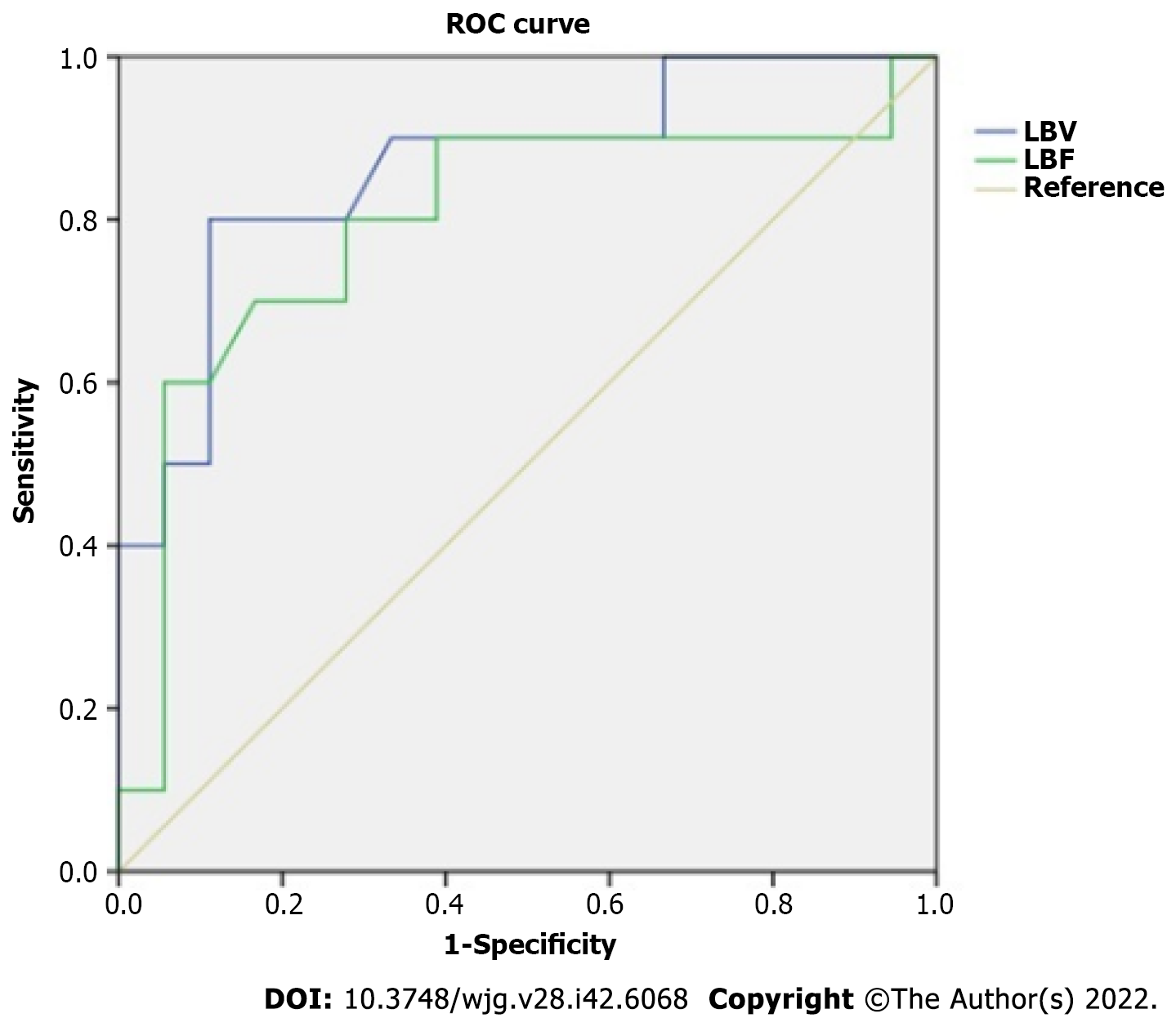
LBF was negatively associated with HVPG (r = -0.398, P < 0.05). LBV was negatively related to HVPG (r = -0.504, P < 0.01) and Child-Pugh (r = -0.563, P < 0.01). SBF was positively related to HAF (r = 0.498, P < 0.01). No association was observed among HAF, SBV, SBF, Child-Pugh score and HVPG. The ROC of LBV for differentiation between moderate and severe PH resulted in an area under the curve of 0.864 with a standard error of 0.075 (95%CI: 0.72-1.00) (Figure 3). Using a cutoff value of 17.85 mL/min/100 mL for LBV, the sensitivity and specificity for detection of severe PH was 80% and 89%, respectively. ROC of LBF resulted in an area under the curve of 0.797 with a standard error of 0.100 (95%CI: 0.60-1.00) (Figure 3). Using a cutoff value of 111.3 mL/min/100 mL for LBF, the sensitivity and specificity for detection of severe PH was 60% and 94%, respectively.
HVPG is the gold standard for diagnosis of liver cirrhosis-induced PH and is an independent risk factor for evaluating the prognosis of decompensated liver cirrhosis[5,19,20]. However, as an invasive measurement requiring a complex operation, wide clinical application of HVPG has been limited. It was reported that quantitative parameters (e.g., LBF, LBV) from CT perfusion of the liver can be used to evaluate the blood supply changes in the liver and spleen with good sensitivity and specificity[13,21,22]. Therefore, our study investigated the correlation of CT perfusion for quantitative assessment of PH in HBV-related PH. Our results suggested that LBV and LBF were negatively correlated with HVPG and Child-Pugh scores, and CT perfusion imaging is a potential non-invasive quantitative predictor for PH in HBV-related liver cirrhosis.
In our study, LBV and LBF were negatively correlated with HVPG. This was explained by a significant decrease in hepatic flow[20-22] after hepatitis B infection when patients were suffering from cirrhosis-induced PH. A decrease in hepatic flow results from hepatocyte damage caused by HBV, deconstruction in normal liver structure, deposition of collagen fibers in the perisinusoidal space and formation of pseudo-lobules and fibroses, which together remarkably increases the resistance of the portal vein blood flow into the liver[1,4]. In this study, LBV and LBF were negatively related to HVPG. It is possible that the decrease of LBV and LBF is the consequence of the increase of HVPG, suggesting significantly reduced blood perfusion in the liver as PH increases. Therefore, CT perfusion is potentially feasible for the non-invasive evaluation of HVPG using LBV and LBF in patients with HBV-related PH.
In this study, liver blood perfusion parameters (e.g., LBV and LBF) in the moderate PH group were significantly higher than those in the severe PH group. For distinguishing moderate PH from severe PH, LBV had a ROC curve with a sensitivity and specificity of 80% and 89%, respectively. LBF had a sensitivity and specificity of 60% and 94%, respectively. Therefore, CT perfusion parameters (LBV and LBF) can be used to distinguish moderate PH and severe PH in PH-induced gastroesophageal variceal bleeding in patients with HBV-related PH.
LBV was negatively correlated with Child-Pugh score, suggesting that liver reserve function decreases with reduced LBV. Moreover, the Child-Pugh score in the moderate PH group was significantly lower than that in the severe PH group. Similarly, liver reserve function was better in the moderate PH group than the severe PH group. This was related to pathophysiological mechanisms underlying hepatitis B cirrhosis and PH. In addition, HVPG in the severe PH group was significantly higher than the moderate PH group. The intrahepatic portal venous system pressure in severe PH may increase, leading to progressively decreased blood flow and gradually weakening the reserve capacity of liver function. However, in this study, the Child-Pugh score was not associated with HVPG, which was consistent with previous studies[3,7,10,23]. The Child-Pugh score is mainly used to evaluate liver reserve function, which can only provide a crude evaluation of PH.
HAF was not related to HVPG, suggesting no correlation between the hepatic artery perfusion ratio and PH in liver cirrhosis. HAF mainly indicates the proportion of hepatic artery blood supply to the total liver blood supply in cirrhosis. When cirrhosis occurs due to damage in the liver sinusoid and liver lobule structure, the blood in the portal vein meets increasing resistance against its return to the liver. When portal vein pressure increases, the blood supply flowing to the liver is reduced. Likewise, compensatory hepatic artery blood perfusion can increase. However, the portal vein blood supply accounts for about three-quarters of the total liver blood supply[24]. The compensatory increase in hepatic artery blood supply could not compensate for a substantial decrease in blood flow in the liver caused by reduced portal vein blood supply. This buffering effect is not enough to maintain the hepatic blood supply[22-24]. In addition, HAF is affected by various factors, such as blood pressure, blood volume and cardiac function. This might explain why HAF was not correlated with HVPG.
The perfusion parameters of the spleen (e.g., SBF, SBV) were not related to HVPG and Child-Pugh classification. This was consistent with a previous study. However, in that cohort, blood flow and blood volume of the liver were not associated with HVPG[13]. This may be related to different samples included in our study, where patients suffering from liver cirrhosis caused by hepatitis B were classified as relatively moderate cases. Among them, according to the Child-Pugh classification, 11 cases were defined as grade A, 16 cases as grade B, and 1 case as grade C. By contrast, patients included in the previous study were primarily suffering from alcoholic cirrhosis with Child-Pugh grade B and C. Furthermore, in the previous study, all patients were suffering from more severe diseases and were planning for liver transplantation as treatment. Moreover, our study excluded factors that may affect portal vein hemodynamics (such as splenic resection, portal vein thrombosis), which may explain the differences between the two studies.
Limitations existed in our study. First, our study only included cases of HBV-related PH, with a remarkable disproportion in patient sex. The majority of patients were Child-Pugh A and Child-Pugh B. A larger sample size is required to identify the clinical application of CT perfusion in patients with different causes of cirrhosis and higher Child-Pugh scores, including alcoholic cirrhosis, drug-induced metabolic liver disease and autoimmune liver disease. Second, our study primarily targeted patients who were suffering from gastric fundus esophageal variceal bleeding as a consequence of PH and excluded other factors like thrombosis, cavernous transformation and splenectomy that could affect liver hemodynamics. Nonetheless, further research is required to determine its application in PH with multiple complications. Finally, our study did not focus on pathology, laboratory and comparative imaging evaluation (such as volume and elasticity of the liver and spleen). Thus, further research is required.
Quantitative parameters of CT perfusion imaging, in particular LBV and LBF, were negatively correlated with HVPG and Child-Pugh scores. Therefore, CT perfusion imaging is a potential application for non-invasive quantitative evaluation of HVPG in patients with HBV-related PH.
Hepatic venous pressure gradient (HVPG) is the gold standard for diagnosis of portal hypertension (PH), but the measurement of HVPG is an invasive procedure, which has limited its widespread use. Therefore, we aimed to investigate the feasibility of computed tomography (CT) perfusion as a non-invasive imaging tool for HVPG in PH.
To date, no satisfactory non-invasive method has been proposed as an alternative for HVPG. Determining the feasibility of CT perfusion indices as a non-invasive tool to assess HVPG would be beneficial to patients.
To investigate the correlation of CT perfusion of the liver with HVPG and Child-Pugh score in hepatitis B virus (HBV)-related PH.
We prospectively selected 28 HBV-related PH patients in our hospital from January 2019 to June 2019. CT perfusion was performed in all patients, and quantitative parameters of CT perfusion were applied to evaluate HVPG non-invasively. Quantitative indices, including liver blood volume (LBV), liver blood flow (LBF), hepatic artery fraction, splenic blood volume and splenic blood flow, were calculated. The correlation analysis was calculated, and receiver operating characteristic curve analysis was performed.
Quantitative parameters of CT perfusion imaging, in particular LBV and LBF, were negatively correlated with HVPG and Child-Pugh scores.
Our findings showed that CT perfusion parameters, LBV and LBF, were negatively correlated with HVPG and Child-Pugh scores. CT perfusion imaging showed potential as a non-invasive quantitative method for the evaluation of HVPG in HBV-related PH.
Non-invasive assessment of HVPG has been an area of interest for decades, and multi-modality research should be explored in the future, including CT perfusion, anatomical information, lab results, liver and spleen stiffness and computation simulation modeling.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fakhreddine AY; Kim E, United States; van Kester MS, The Netherlands S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 2. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 3. | Ripoll C. Hepatic venous pressure gradient and outcomes in cirrhosis. J ClinGastroenterol. 2007;41 Suppl 3:S330-S335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 5. | La Mura V, Garcia-Guix M, Berzigotti A, Abraldes JG, García-Pagán JC, Villanueva C, Bosch J. A Prognostic Strategy Based on Stage of Cirrhosis and HVPG to Improve Risk Stratification After Variceal Bleeding. Hepatology. 2020;72:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Villanueva C, Graupera I, Aracil C, Alvarado E, Miñana J, Puente Á, Hernandez-Gea V, Ardevol A, Pavel O, Colomo A, Concepción M, Poca M, Torras X, Reñe JM, Guarner C. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology. 2017;65:1693-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, Bosch J; Spanish Cooperative Group for Portal Hypertension and Variceal Bleeding. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Vorobioff J, Groszmann RJ, Picabea E, Gamen M, Villavicencio R, Bordato J, Morel I, Audano M, Tanno H, Lerner E, Passamonti M. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology. 1996;111:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 812] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 11. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Villanueva C, Albillos A, Genescà J, Abraldes JG, Calleja JL, Aracil C, Bañares R, Morillas R, Poca M, Peñas B, Augustin S, Garcia-Pagan JC, Pavel O, Bosch J. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Talakić E, Schaffellner S, Kniepeiss D, Mueller H, Stauber R, Quehenberger F, Schoellnast H. CT perfusion imaging of the liver and the spleen in patients with cirrhosis: Is there a correlation between perfusion and portal venous hypertension? EurRadiol. 2017;27:4173-4180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Weidekamm C, Cejna M, Kramer L, Peck-Radosavljevic M, Bader TR. Effects of TIPS on liver perfusion measured by dynamic CT. AJR Am J Roentgenol. 2005;184:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 21. | Preibsch H, Spira D, Thaiss WM, Syha R, Nikolaou K, Ketelsen D, Lauer UM, Horger M. Impact of transjugular intrahepatic portosystemic shunt implantation on liver perfusion measured by volume perfusion CT. ActaRadiol. 2017;58:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 24. | Bhardwaj A, Kedarisetty CK, Vashishtha C, Bhadoria AS, Jindal A, Kumar G, Choudhary A, Shasthry SM, Maiwall R, Kumar M, Bhatia V, Sarin SK. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017;66:1838-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |