Published online Oct 28, 2022. doi: 10.3748/wjg.v28.i40.5865
Peer-review started: July 25, 2022
First decision: August 19, 2022
Revised: August 20, 2022
Accepted: October 13, 2022
Article in press: October 13, 2022
Published online: October 28, 2022
Processing time: 95 Days and 4.9 Hours
Immune dysfunction is the crucial cause in the pathogenesis of inflammatory bowel disease (IBD), which is mainly related to lymphocytes (T or B cells, incl-uding memory B cells), mast cells, activated neutrophils, and macrophages. As the precursor of B cells, the activation of memory B cells can trigger and differentiate B cells to produce a giant variety of inducible B cells and tolerant B cells, whose dysfunction can easily lead to autoimmune diseases, including IBD.
To investigate whether or not curcumin (Cur) can alleviate experimental colitis by regulating memory B cells and Bcl-6-Syk-BLNK signaling.
Colitis was induced in mice with a dextran sulphate sodium (DSS) solution in drinking water. Colitis mice were given Cur (100 mg/kg/d) orally for 14 con-secutive days. The colonic weight, colonic length, intestinal weight index, occult blood scores, and histological scores of mice were examined to evaluate the curative effect. The levels of memory B cells in peripheral blood of mice were measured by flow cytometry, and IL-1β, IL-6, IL-10, IL-7A, and TNF-α expression in colonic tissue homogenates were analyzed by enzyme-linked immunosorbent assay. Western blot was used to measure the expression of Bcl-6, BLNK, Syk, and other signaling pathway related proteins.
After Cur treatment for 14 d, the body weight, colonic weight, colonic length, colonic weight index, and colonic pathological injury of mice with colitis were ameliorated. The secretion of IL-1β, IL-6, TNF-α, and IL-7A was statistically decreased, while the IL-35 and IL-10 levels were considerably increased. Activation of memory B cell subsets in colitis mice was confirmed by a remarkable reduction in the expression of IgM, IgG, IgA, FCRL5, CD103, FasL, PD-1, CD38, and CXCR3 on the surface of CD19+ CD27+ B cells, while the number of CD19+ CD27+ IL-10+ and CD19+ CD27+ Tim-3+ B cells increased significantly. In addition, Cur significantly inhibited the protein levels of Syk, p-Syk, Bcl-6, and CIN85, and increased BLNK and p-BLNK expression in colitis mice.
Cur could effectively alleviate DSS-induced colitis in mice by regulating memory B cells and the Bcl-6-Syk-BLNK signaling pathway.
Core Tip: As the precursor of B cells, the activation of memory B cells can trigger the activation of B cells, thus producing numerous inducible B cells and tolerant B cells, whose dysfunction can easily result in inflammatory bowel disease (IBD). Also, the disorder of humoral immune function mediated by memory B cells has a critical position in the pathogenesis of IBD. As an immune suppressant, curcumin (Cur) has a therapeutic role in treating many immune diseases, such as IBD and rheumatoid arthritis. Meanwhile, it has proven efficacy in experimental colitis mice and patients with ulcerative colitis (UC), while the disturbances in memory B cells have been observed in IBD. However, few studies have explored whether Cur treatment of colitis is closely related to memory B cells. In the present research, our results indicated that Cur effectively alleviated DSS-induced UC in mice via a potential mechanism involving memory B cells and the Bcl-6-Syk-BLNK signaling pathway.
- Citation: Wei SY, Wu TT, Huang JQ, Kang ZP, Wang MX, Zhong YB, Ge W, Zhou BG, Zhao HM, Wang HY, Liu DY. Curcumin alleviates experimental colitis via a potential mechanism involving memory B cells and Bcl-6-Syk-BLNK signaling. World J Gastroenterol 2022; 28(40): 5865-5880
- URL: https://www.wjgnet.com/1007-9327/full/v28/i40/5865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i40.5865
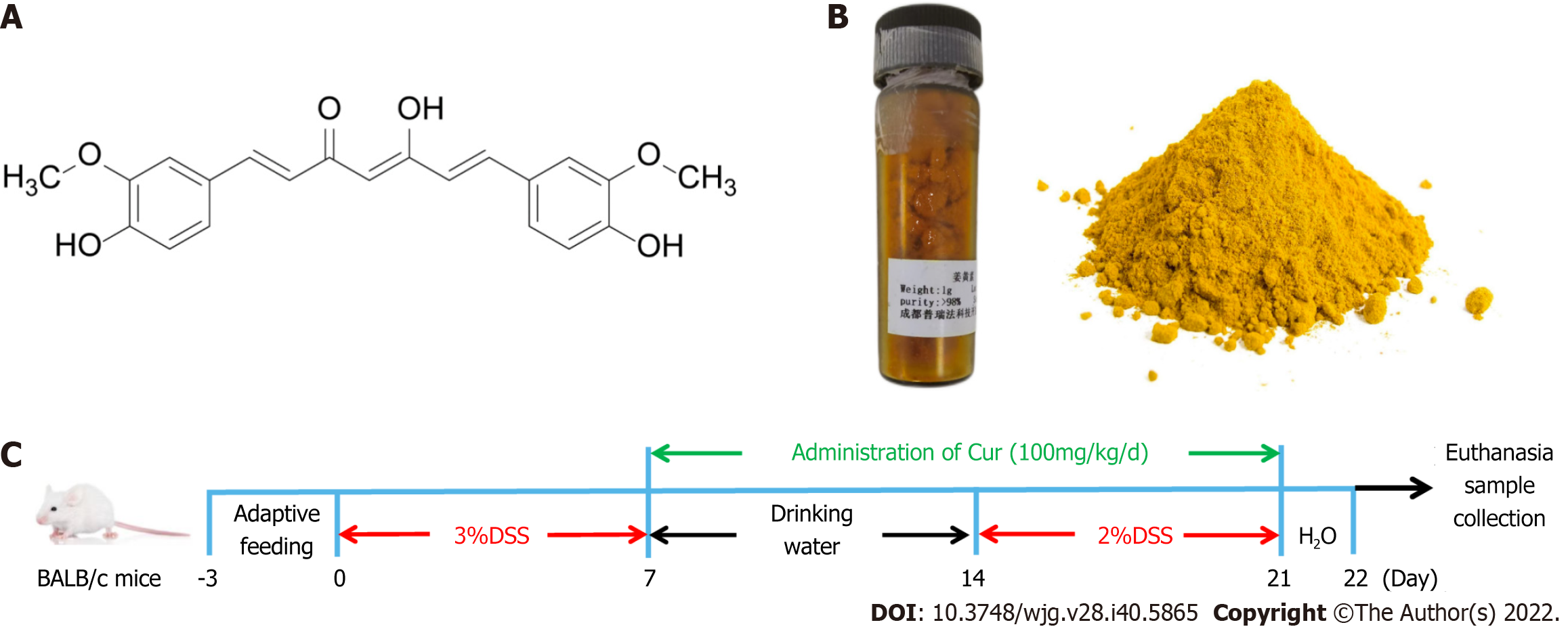
Curcuma longa L, also named turmeric, is a perennial herb pertaining to the genus Curcuma in the Zingiberaceae of the Scitamineae, native to China, Indonesia, and India. According to the existing literature, Curcuma longa L can promote Qi, break blood stasis, facilitate menstruation, and relieve pain[1]. The bioactive natural polyphenols extracted from the rhizome of Zingiberaceae are called curcumin (Cur) [1,7-bis(4-hydroxy-3-methoxyphenyl)- 1,6-heptadiene-3,5-dione]. They are also known as diferuloylmethane, and their molecular formula C21H20O6 accounts for 75% to 80% of curcumin compounds[2] (a structure is shown in Figure 1A)[3]. According to recent carefully designed clinical studies, with an excellent safety profile, Cur has the potential effect of prevention and/or management of various diseases including inflammatory bowel disease, dysentery, chronic enteritis, gastrointestinal syndrome, and so on, due to its anti-oxidant, anti-apoptotic, and anti-inflammatory properties[4]. In addition, many preclinical studies have indicated that Cur has perfect anti-cancer properties to inhibit carcinogenesis and the proliferation of various cancer cells[5], including prostate, oral epithelial leukemia, hepatic, breast, and colon cancers in human and animals, and the probable mechanisms are closely related to modulating the activation of a variety of cellular signals as apoptosis and angiogenesis[6].
As a chronic nonspecific inflammatory disorder, inflammatory bowel disease (IBD), classified into ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by recurrent abdominal pain, diarrhea, anemia, bleeding, and weight loss. However, the pathogenesis of IBD is still unclear, and is closely associated with heredity, environment, infection, immunity, and so on[7]. Among these, immune system dysfunction has been regard as the main cause of IBD, which is mainly involving abnormal lymphocytes (including T and B cells, including memory B cells), macrophages, mast cells, and activated neutrophils[8]. As a precursor to B cells, the activation of memory B cells can trigger the activation of B cells, thus producing numerous inducible B cells and tolerant B cells, whose dysfunction predisposes to autoimmune diseases, including IBD[9]. Pararasa et al[10] reported a decrease in CD27-IgD- memory B cells in the blood of patients with IBD, but a concomitant increase in CD27-IgD- memory B cells in gut-associated lymphoid tissue, which suggests that the disorder of humoral immune function mediated by memory B cells has a very important role in the pathogenesis of IBD.
Many previous studies have demonstrated that Cur can be used to effectively treat UC patients and animals. An endoscopic study revealed that NCB-02 enema (containing 140 mg Cur) used once per day for 8 wk could ameliorate the disease symptoms in patients with UC[11]. In an animal study, Cur administration effectively alleviated the colonic mucosal inflammation, restored colonic length, and reduced colonic weight and colonic damage[10]. Potential mechanisms of Cur for treating UC are diverse, including regulating immune function (such as regulating the function and level of T cells, regulatory T cells, memory T cells, macrophages, and dendritic cells), inhibiting the secretion of IL-2, IL-6, IL-12, IL-17, and TNF-α[12], downregulating the expression of costimulatory molecules[13], inhibiting the chemotaxis of chemokines and neutrophils, and inducing antioxidant effect[14]. Cur may induce apoptosis in colorectal cancer as it induces reactive oxygen species production, suppressing NF-κB signaling activation and cyclooxygenase-2 expression, activating caspase 3 and c-Jun N-terminal kinases (JNK), and inducing the release of cytochrome C. It is known that Cur can promote the activation of caspase 3, caspase 8, caspase 9, Bax, and poly (ADP-ribose) polymerase (PARP) to induce apoptosis in HCT-116 colon cancer cells[15]. Furthermore, it also exerts its effect on other autoimmune diseases by effectively regulating memory B cells. Elham and colleagues[16] found that Cur achieved effective results for autoimmune diseases such as RA and systemic lupus erythematosus (SLE) by targeting B lymphocyte stimulating factor (BLYS), which is an important cytokine for memory B cell proliferation. However, it is unclear whether Cur exerts a therapeutic effect on IBD by regulating the subsets or function of memory B cells. Therefore, in this study, DSS-induced colitis model, a classic animal model replicating human UC, was used to evaluate and explore the mechanism and therapeutic effect of Cur on IBD. Flow cytometry and other experiments were performed to explore the possible mechanisms and the changes in memory B cell subsets after Cur treatment.
BALB/c male mice, aged 8-9 wk and weighing 20-22 g, were purchased from the Hunan Silaike Jingda Experimental Animal Co. Ltd. (Changsha, China; Animal Certificate No. SCXK 2016-0002). Mice were provided with standard food and water ad libitum, and lived under specific pathogen-free (SPF) conditions at the temperature of 23 °C ± 2 °C and relative humidity of 55% ± 10% with alternating light/darkness for 12 h. They were acclimatized for 3 d prior to the start of the experiment. All animal experiments were conducted and performed in line with the regulations and guidelines of the Jiangxi University of Chinese Medicine Animal Care and Use Committee and the AAALAC and the IACUC (Permit No. JZLLSC2021-196).
All experimental mice were randomly divided into the following four groups: Control group, in which mice were fed normal food without DSS or drug treatment; Control + Cur group, in which normal mice were given Cur treatment; DSS group, in which mice with DSS-induced colitis were administrated with Cur; DSS + Cur group, in which mice with DSS-induced colitis were treated with Cur.
As previously described, mice with colitis were freely given a 3% (w/v) solution of DSS (Batch No. 160110) in drinking water for 7 d (Figure 1C), then with sterile drinking water for 7 d, and finally with 2% (w/v) DSS for 7 d. Meanwhile, mice in the control group were given tap water. Cur was provided by Chengdu Purifa Technology Development Co., LTD (Chengdu, China; Batch No. PRF10052344, purity > 98%), as shown in Figure 1B. In order to better dissolve the drug, Cur was dissolved in 1.5% sodium carboxymethylcellulose. According to our previous study[17], mice in the DSS + Cur group and Control + Cur group were administrated with Cur (100 mg/kg/d) by gavage for 14 consecutive days, while mice in the Control and DSS groups were administrated with an equal volume of saline starting from day 8. The mice were weighed daily to evaluate body weight changes, and their fecal character, stool bleeding, and live state were monitored[18].
After the last administration, the mice were fasted for 12 h. All mice were weighed before anesthesia with sodium pentobarbital (20 mg/kg i.p.). After euthanasia, the whole colons were rapidly separated on the ice box, and their length and weight were measured to calculate the colon weight index (CWI) as [colon weight/body weight] × 100%, and the colon length index (CLI) as (colon weight/colon length) × 100%. The distal colon tissue was fixed in 4% paraformaldehyde for 72 h. After embedding in paraffin to cut 4 μm thick sections, the sections were dehydrated in an ethanol gradient series and stained with hematoxylin-eosin for histopathological analysis. Finally, the slices were sealed with neutral gum. And then the colonic pathological changes were observed and double-blindly scored by two pathologists under a microscope (Leica, Wetzlar, Germany, Product model: DM2500). According to the previous standards, a composite score was computed and totalized with inflammatory cell infiltration (scores 0-3), mucosal damage (scores 0-3), crypt damage (scores 0-4), and regeneration (scores 0-4)[19].
In order to isolate peripheral lymphocytes, 500 μL of peripheral blood from each mouse was collected and treated with 1 mL of lysing buffer (BD Biosciences, Franklin Lakes, NJ, United States) and incubated for 15 min in the darkness to remove red blood cells. The cell suspension was combined with an Fcγ receptor-blocking mAb (CD16/32; BioLegend, San Diego, CA, United States) for 15 min at 4 °C. Later on, the cells were detected for surface antigens by labelling with BV510 rat anti-mouse CD19 (Lot No. BD562956), AF488 rat anti-mouse CD27 (Lot No. BD124222), PE-A rat anti-mouse IgM (Lot No. BD553409), BV421 rat anti-mouse IgG (Lot No. BD742475), APC rat anti-mouse IL-10 (Lot No. BD505010), BV421 rat anti-mouse IgA (Lot No. BD743293), APC rat anti-mouse FCRL5 (Lot No. BD340305), PE-A rat anti-mouse CD103 (Lot No. BD562772), BV421 rat anti-mouse FasL (Lot No. BD740054), APC rat anti-mouse PD-1 (Lot No. BD562671), BV421 rat anti-mouse CXCR3 (Lot No. BD126529), and PE-A rat anti-mouse Tim-3 (Lot No. BD119704), which were purchased from BD Bioscience (San Jose, CA, United States). Finally, the single-cell suspensions were analyzed with a FACS Canto II flow cytometer (BD Biosciences, Franklin Lakes, NJ, United States). Gates were set for the quadrant markers based on negative populations and isotype controls. FlowJo VX software (TreeStar, San Carlos, CA, United States) was used to analyze all data to differentiate the memory B cell subgroups.
One hundred micrograms of mouse colon tissue was homogenized by adding 1000 μL of RIPA lysis buffer (Cell Signaling Technology, Danvers, MA, United States). The samples were incubated at 4 °C for 1 h, ultrasonically homogenized, and centrifuged at 13000 rpm for 10 min, and the supernatant was taken to obtain colonic tissue homogenate. The total protein in colonic tissue homogenate was quantified with a total protein detection kit (Aidlab Biotechnologies Co., Ltd., Beijing, China; Lot No. PP0102). According to the manufacturer’s instructions, commercial ELISA kits (Thermo Fisher Scientific, Waltham, MA, United States) were used to detect the secretion of IL-1β (Lot No. BMS6002TEN), IL-6 (Lot No. BMS603-2), IL-10 (Lot No. 88-7105-88), IL-35 (Lot No. BMS616), IL-17A (Lot No. BMS6001), and TNF-α (Lot No. BMS607-3).
Colon tissue homogenate was prepared and protein concentration determination was performed according to the above methods. First, equal amounts of colonic mucosa protein were resolved by polyacrylamid gel electrophoresis and transferred onto PVDF membranes, which were then blocked with 3% bovine serum albumin (BSA) solution for 1 h at room temperature and then incubated with the following primary antibodies overnight at 4 °C: BLNK (Abcam, ab32418, 1:500), p-BLNK (Abcam, ab174837, 1:1000), Syk (Abcam, ab40781, 1:1000), p-Syk (Abcam, ab300410, 1:1000), CIN85 (1:1000), Bcl-6 (Abcam, ab19011, 1:1000), and anti-GAPDH (Abcam, ab181602, 1:1000) antibodies. Next, the corresponding HRP-coupled secondary antibody (Abcam, ab205718, 1:5000) was added and incubated for 2 h at room temperature, and the ECL hypersensitive solution (Thermo, Rockford, IL, United States) was used to visualize the protein blotting. Photographs were taken by using the Highly Sensitive Chemiluminescence Imaging system (UVP ChemStudio 515; Analytik Jena, Jena, Germany). Image-Pro Plus 6.0 software (Media Cybernetic, Bethesda, MD, United States) was used to quantify the images, and the gray value of the band and the ratio to that of the internal reference GAPDH was used for statistical analysis.
GraphPad Prism 7.0 software (San Diego, CA, United States) was used to analyze the difference between groups by performing independent samples t-test, one-way analysis of variance, least-significant difference test, and Turkey test for multiple comparisons. All data are presented as the mean ± SE. P < 0.05 was considered statistically significant.
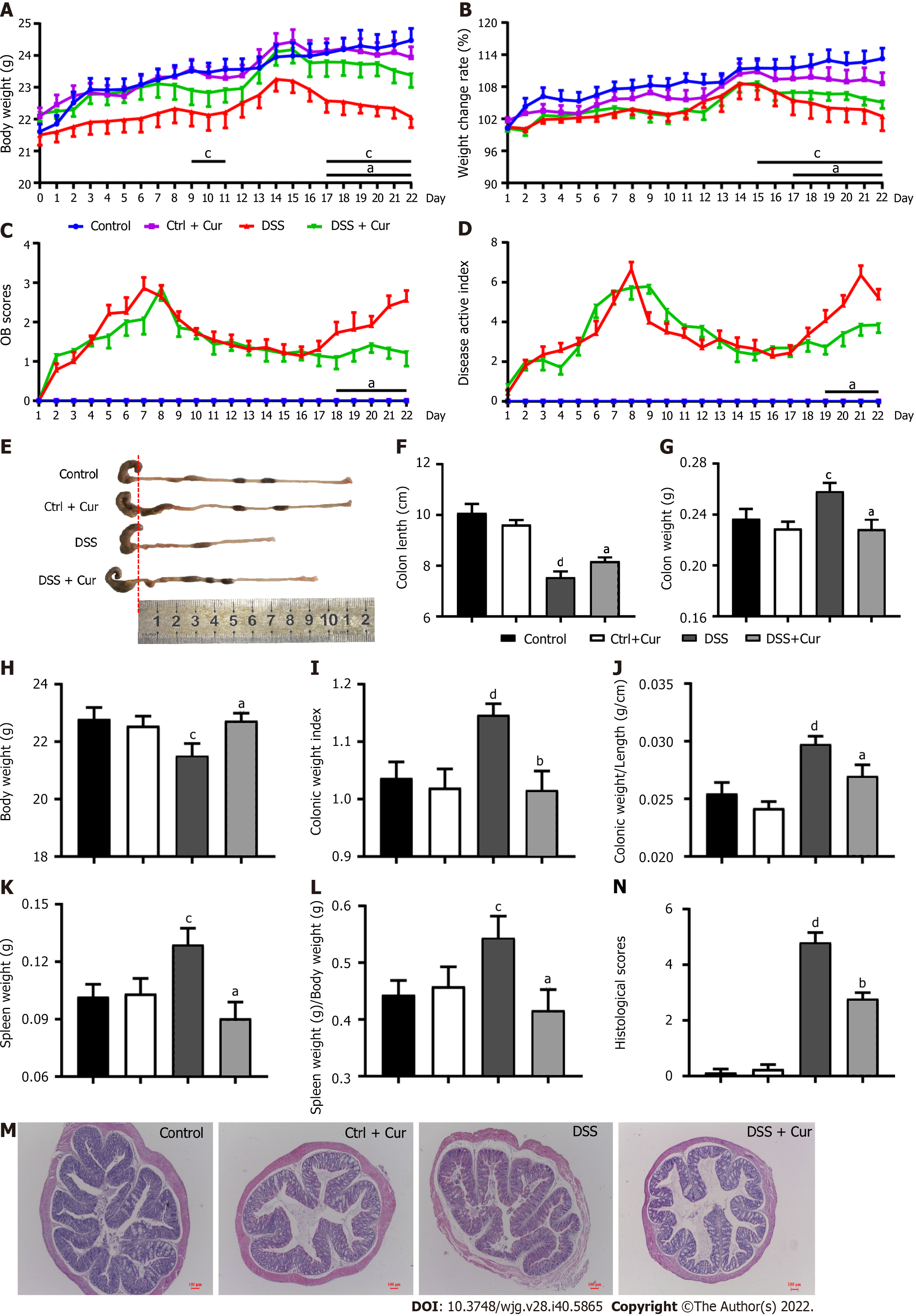
Many studies have shown that mice with DSS-induced colitis may present diarrhea, abdominal pain, bloody stool, weight loss, shortened colon length, increased colon weight, and other symptoms[20]. Under the microscope, altered colonic gland structure, a decrease in the number of goblet cells, massive infiltration of inflammatory cells in the mucosa and submucosa, and obvious erosive ulcer in DSS-induced colitis can be observed[21]. In our pilot study, DSS-induced colitis mice exhibited significant weight loss from day 4 to day 21 of the experiment (Figure 2A), and the changes in body weight (Figure 2B) were significantly lower in contrast with those of the Control group. From days 1 to 22, the occult blood (OB) test score (Figure 2C) gradually started to be different from that of the Control group, while disease activity index (DAI) scores (Figure 2D), which were significantly higher than those of the Control group, exhibited a similar trend to OB scores. In addition, the colonic weight (Figure 2G), index of colon length (Figure 2I), index of colonic weight (Figure 2J), spleen weight (Figure 2K), and the spleen weight index (Figure 2L) of mice in the DSS group statistically increased, whereas the body weight in the last day (Figure 2H) and colonic length (Figure 2E and F) decreased significantly. Meanwhile, the histopathological analysis (Figure 2M) showed that the colonic mucosa of mice was intact in the control group, with no obvious ulceration or inflammatory cell infiltration, neat arrangement of cup-shaped cells, and no congestion or edema, while the colitis mice showed exudation of the colonic mucosal epithelium, changes in crypt structure, disordered arrangement, local ulcer formation, hyperemia and edema in the colon mucosa, and infiltration of inflammatory cells in the lamina propria and submucosa, and the pathological injury scores were significantly increased (Figure 2N), which was consistent with our previous study. The above findings indicated that the chronic colitis model has been successfully constructed.
After Cur gavage administration, significant changes in body weight (Figure 2A), weight change rate (Figure 2B), OB scores (Figure 2C), and DAI scores (Figure 2D) were effectively reversed in colitis mice. Also, colon weight (Figure 2G), colon mass per unit length (Figure 2J), index of colonic weight (Figure 2I), spleen weight (Figure 2K), and spleen weight index (Figure 2L) were significantly decreased when compared with those in the DSS group, while the body weight in the last day (Figure 2H) and colonic length (Figure 2E and F) were obviously higher in the DSS + Cur group than in the DSS group. Meanwhile, ulceration and inflammatory infiltration in the colitis mice treated with Cur were distinctly improved (Figure 2M), and the pathological injury scores (Figure 2N) were observably decreased compared to those of colitis mice without treatment. Moreover, the above data were not evidently different between the Control and Control + Cur groups. Therefore, these data suggest that Cur can effectively alleviate DSS-induced colonic injury.
UC is a form of IBD characterized by rising levels of pro-inflammatory factors and declining levels of anti-inflammatory factors[22]. Disruption of the balance between pro-inflammatory and anti-inflammatory factors is one of the important characteristics of IBD[23].
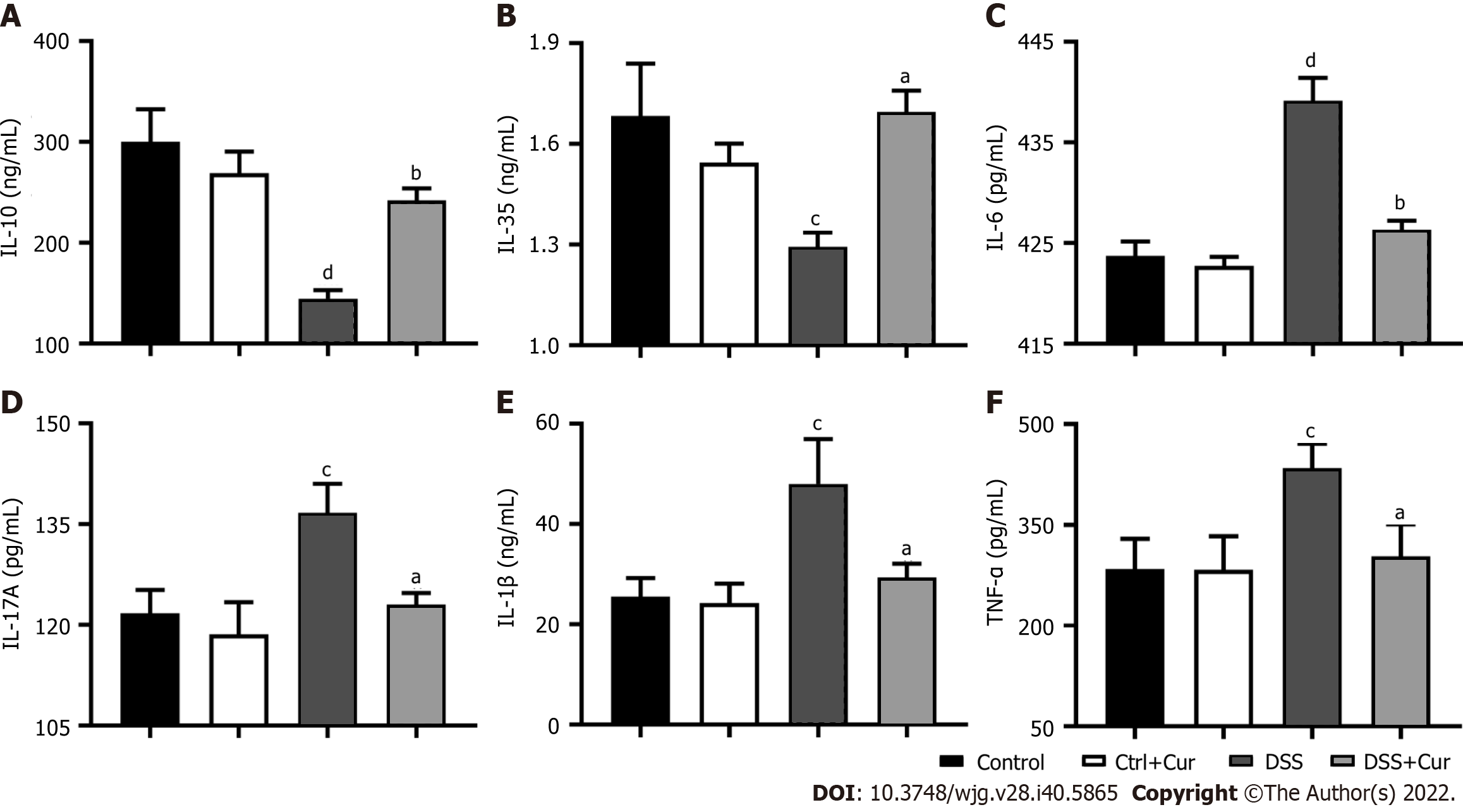
In our experiment, we found that the levels of IL-6 (Figure 3C), IL-17A (Figure 3D), IL-1β (Figure 3E), and TNF-α (Figure 3F) were remarkably increased in the DSS group compared to the Control group, while the IL-10 (Figure 3A) and IL-35 levels (Figure 3B) in the DSS group were dramatically decreased, suggesting that the imbalance of pro-inflammatory and anti-inflammatory factors was one of the important characters in DSS-induced colitis. More importantly, the IL-6 (Figure 3C), IL-17A (Figure 3D), IL-1β (Figure 3E), and TNF-α (Figure 3F) expression was significantly downregulated, while IL-10 (Figure 3A) and IL-35 (Figure 3B) expression was observably upregulated in colitis mice after Cur treatment. Altogether, Cur could effectively decrease IL-1β, IL-6, IL-17A and TNF-α production and increase IL-10 level, thus reestablishing the equilibrium between anti-inflammatory and pro-inflammatory factors in colitis.
In the humoral immune response, there are two layers of memory cells, namely, long-lived B memory cells and B effector cells (plasma cells). The production of long-lived cells depends on the antigenic activation of B cells in the presence of T helper cells to induce germinal centers[24]. When the body comes in second contact with an antigen, memory B cells are quickly activated, differentiate into new tolerant or inducible plasma cells, secrete high-quality antibodies, and produce more efficient, faster, and more specific humoral immunity[9]. The tolerant type generally expresses factors such as IL-10 and TIM-3, while the induced type usually expresses molecules such as IgG, IgM, IgA, PD-1, CD38, CXCR3, FCRL5, CD103, and FasL, thus having opposite roles in the process of disease development[25]. Their balance determines the development, genesis, and development of memory B cells, while their dysfunction is one of the pathogenesis of IBD.
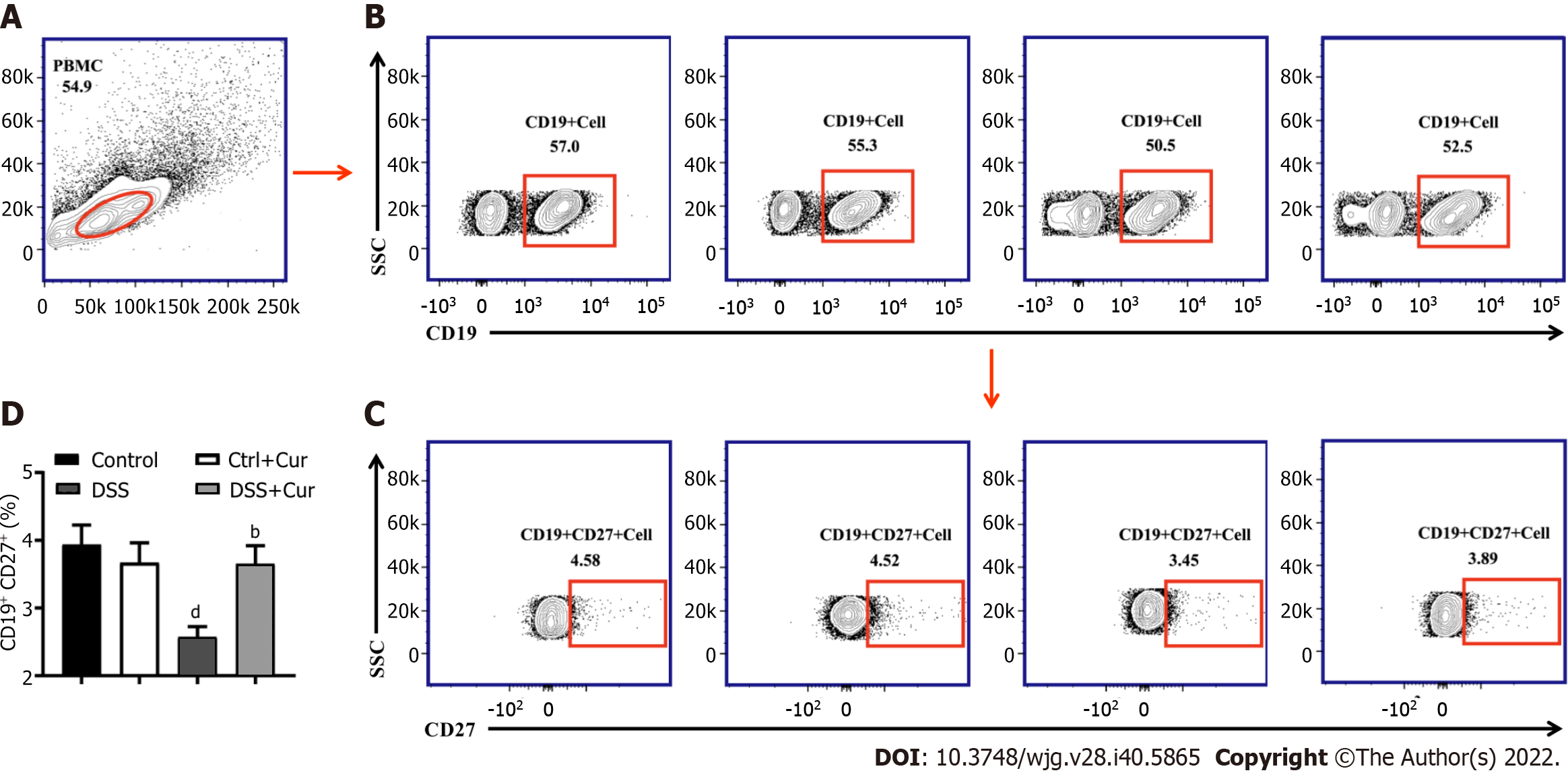
Recent studies have revealed that CD27 is expressed at higher levels in memory B cells than in plasma blasts and can be used as a biological biomarker of memory B cells. The CD19-positive cells were selected as the gate of B cells. Memory B cells (CD19+ CD27+) could be marked and separated according to the expression of CD27 and CD19, which could then be divided into many subgroups in accordance with the expression of different cytokines on the surface of memory B cells[26,27].
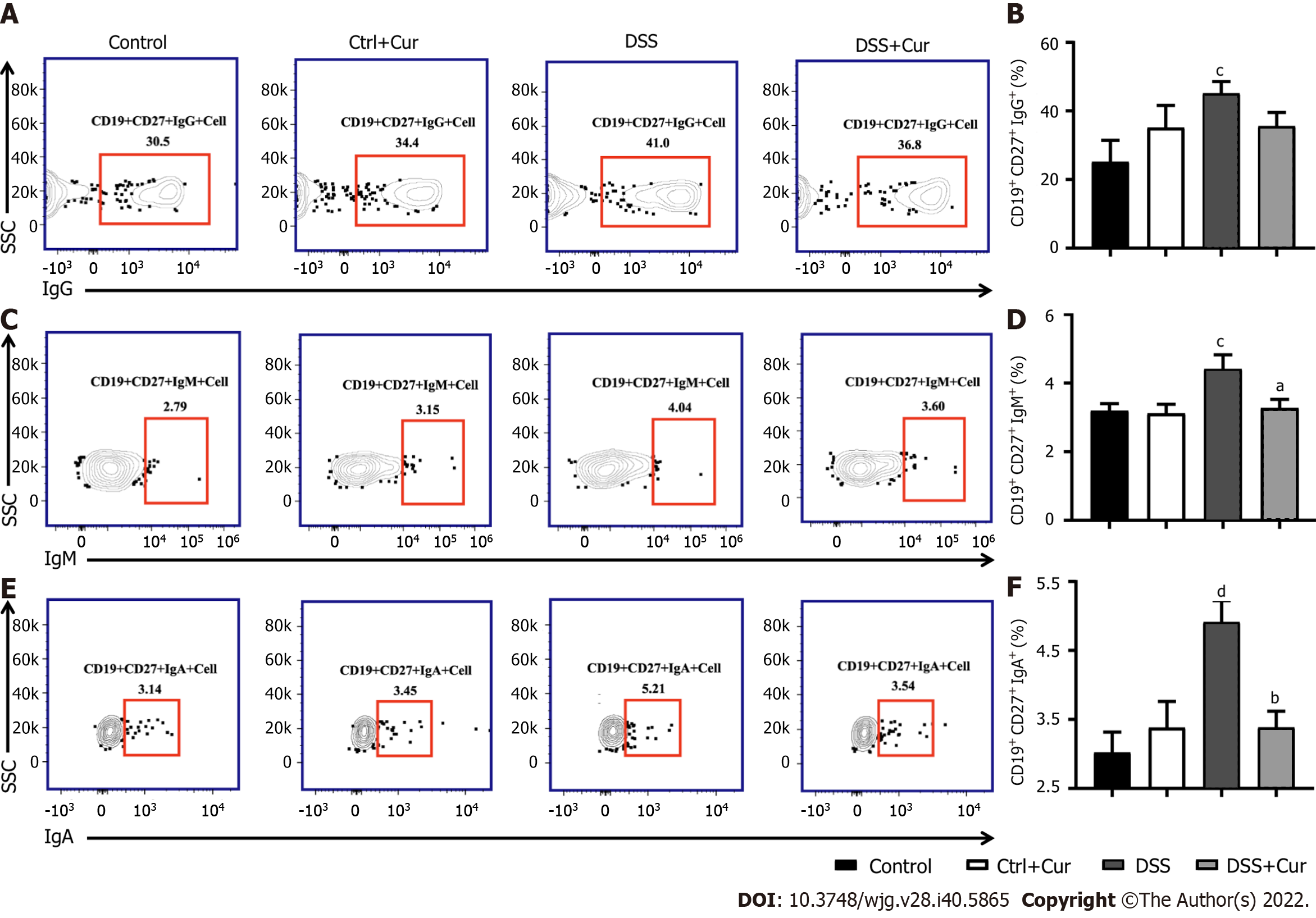
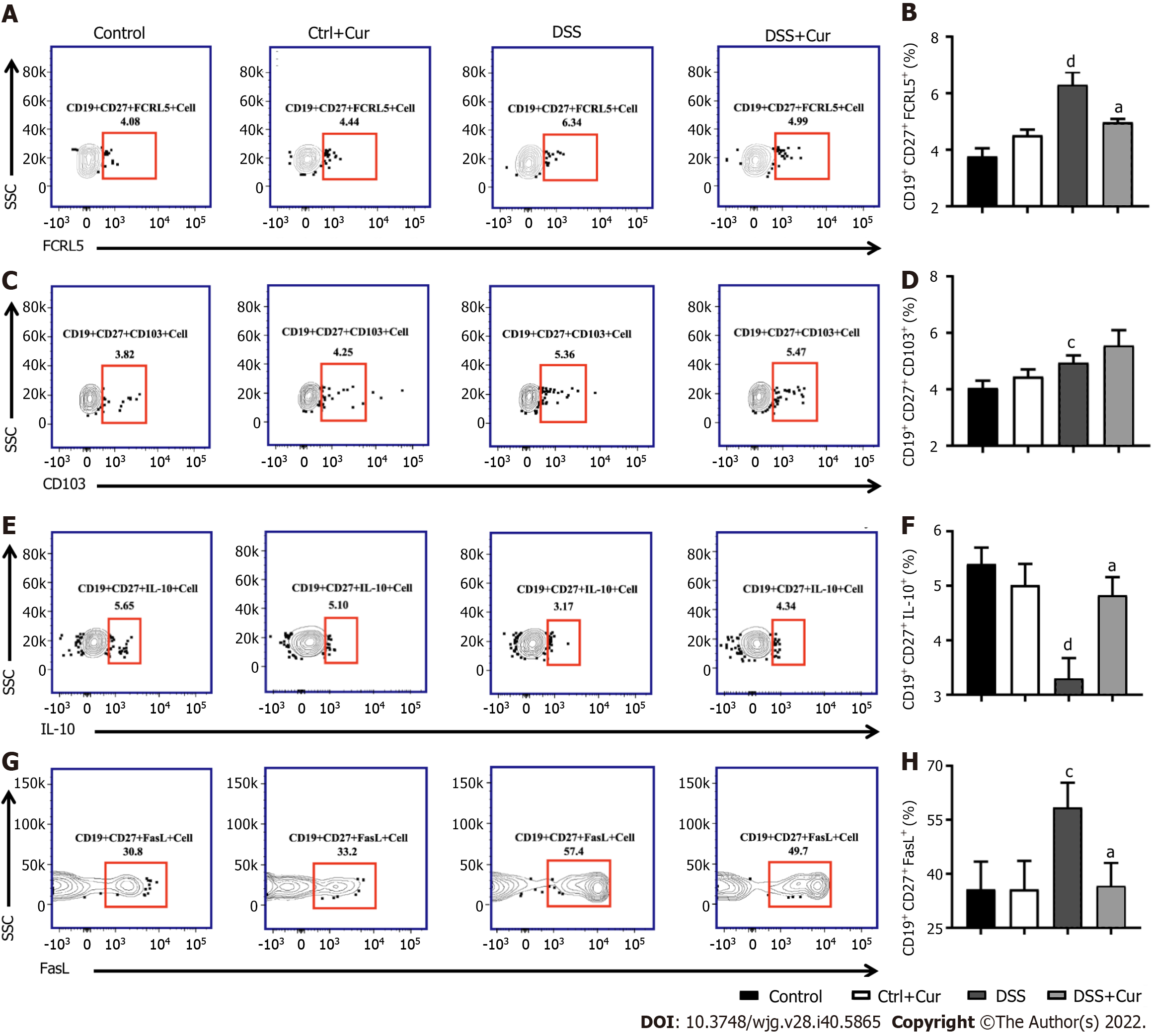
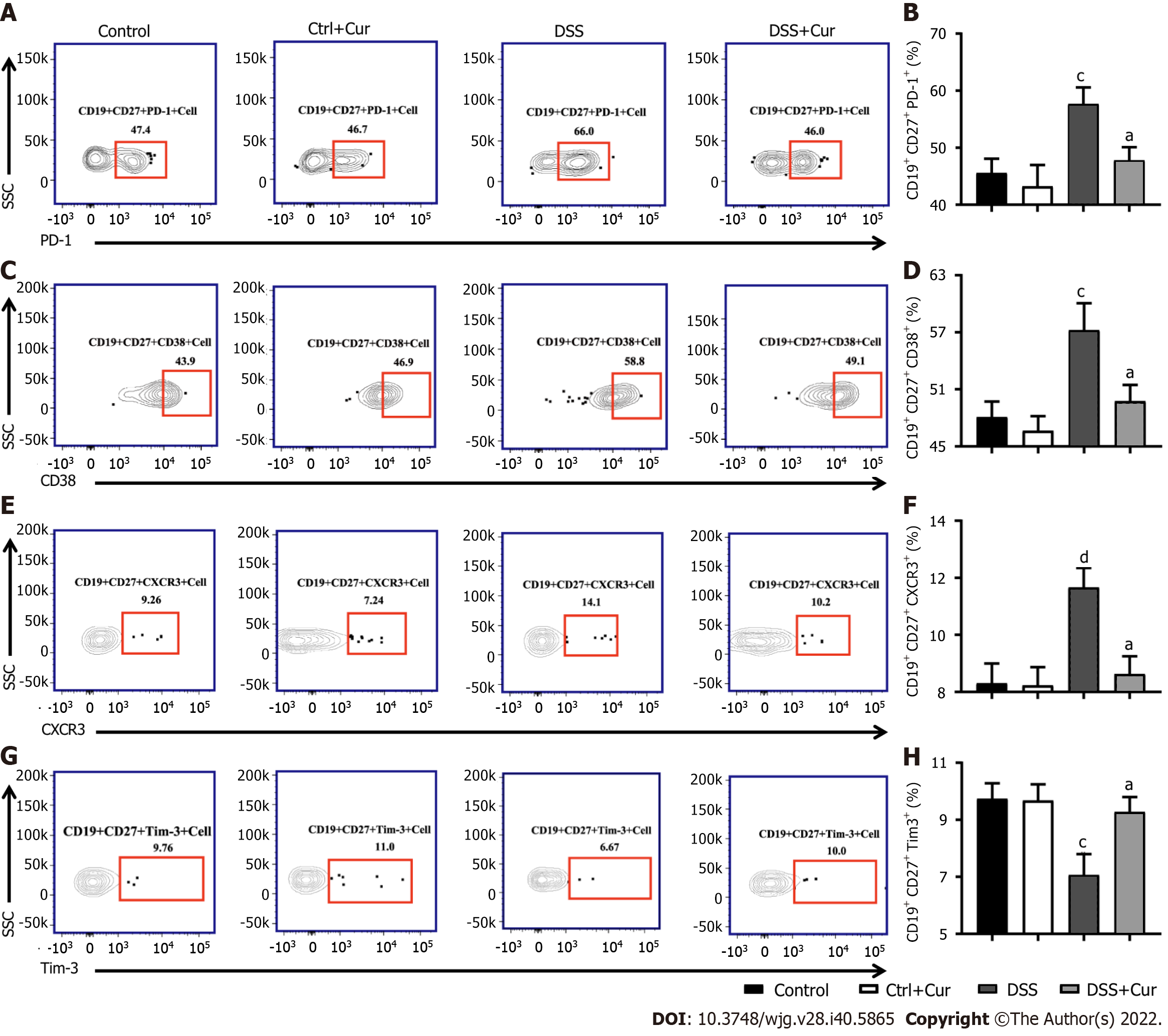
In this study, compared with mice in the Control group, the level of CD19+ CD27+ memory B cells in peripheral blood of colitis mice was significantly decreased (Figure 4A-D), the percentages of CD19+ CD27+ IgM+ (Figure 5A and B), CD19+ CD27+ IgG+ (Figure 5C and D), and CD19 + CD27+ IgA+ (Figure 5E and F) cells were obviously increased, and the percentages of CD19+ CD27+ FCRL5+ (Figure 6A and B), CD19+ CD27+ CD103+ (Figure 6C and D), CD19 + CD27 + FasL+ (Figure 6G and H), CD19 + CD27 + PD-1 + (Figure 7A and B), CD19+ CD27+ CD38+ (Figure 7C and D), and CD19+ CD27+ CXCR3+ (Figure 7E and F) cells were significantly increased. These percentages were significantly decreased after DSS-induced colitis mice were treated with Cur for 14 d. Meanwhile, the percentages of CD19+ CD27+ IL-10+ (Figure 6E and F) and CD19+ CD27+ Tim-3+ (Figure 7G and H) cells were remarkably decreased in mice of the DSS group, whereas the percentages of CD19+ CD27+ Tim-3+ and CD19+ CD27+ IL-10+ cells were markedly increased after experimental colitis mice were treated with Cur. These results suggested that Cur could effectively regulate memory B cell subsets in mice with colitis.
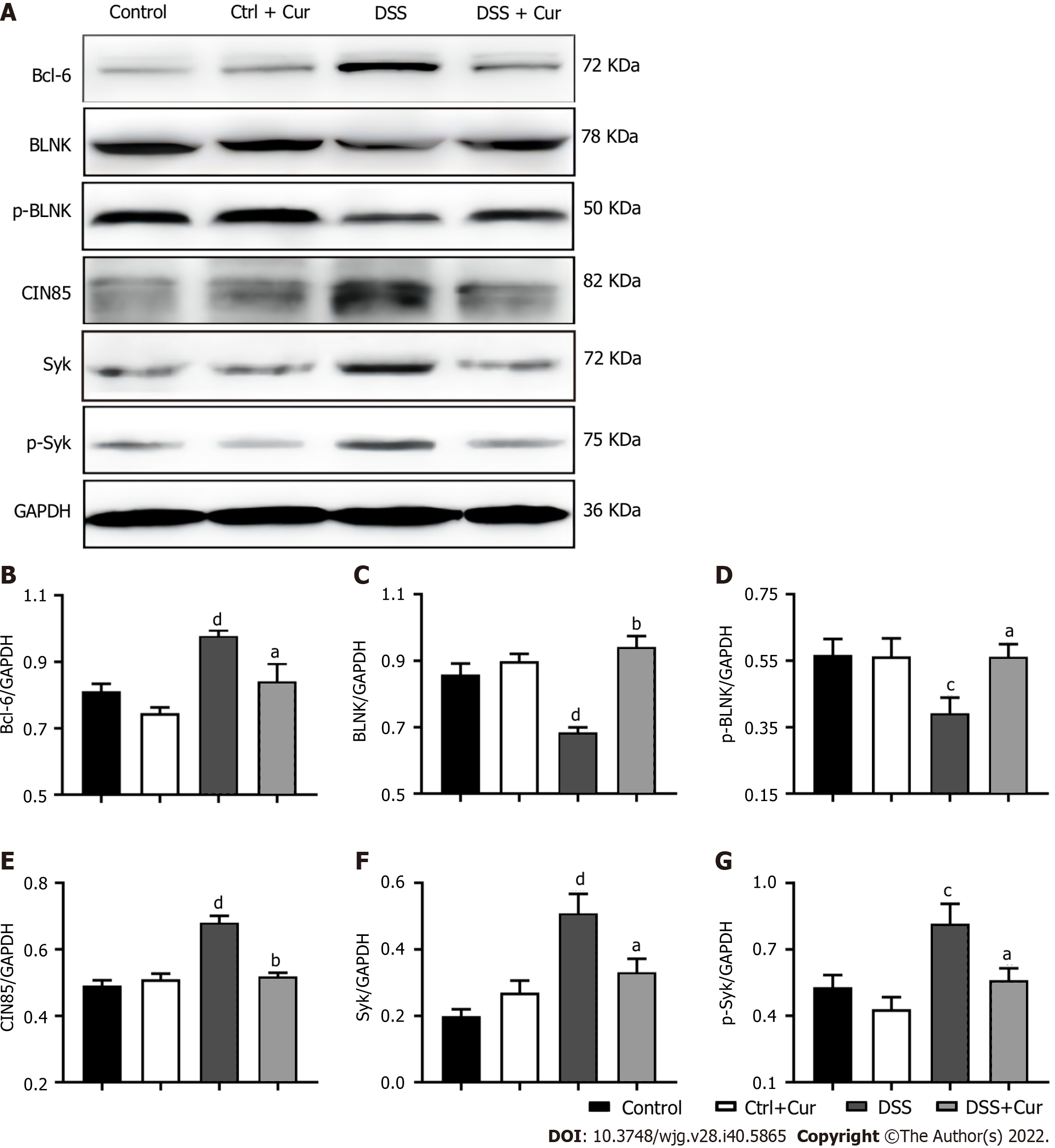
Next, we used Western blot analysis to explore the effect of Cur on the regulation of memory B-cell-related signaling pathways in mice with colitis. The Bcl-6-Syk-BLNK signaling pathway is a crucial signaling pathway involved in the activation, differentiation, proliferation, and functional display of immune cells, including B cells and memory B cells. In our experiments, the expression levels of Bcl-6 (Figure 8A and B), CIN85 (Figure 8A and E), Syk (Figure 8A and F), and p-Syk (Figure 8A and G) were higher in the DSS group than in the Control group. After 14 d of Cur treatment, Bcl-6, CIN85, Syk, and p-Syk expression in the colonic tissues of colitis mice was significantly inhibited. In addition, the expression levels of BLNK (Figure 8A and C) and p-BLNK (Figure 8A and D) were obviously lower in the DSS group than in the Control group, and the expression levels of BLNK and p-BLNK in the treatment group were evidently increased compared with the DSS group. Thus, these results suggested that Cur effectively regulates the activation of the Bcl-6-Syk-BLNK signaling pathway in colitic mice.
The incidence of UC has been gradually increasing worldwide, especially in adults between 30 and 40 years old. Patients with UC have mucosal inflammation, which starts in the rectum but continues into the proximal colon, usually presenting with bloody stool. Its pathogenesis is multifactorial, involving destroyed epithelial barrier, dysregulated immune responses, genetic predisposition, and imbalance of the gut microbiota. The dysfunction of immune cells and the destruction of immune homeostasis are deemed as the direct causes of UC[28,29].
B cells have an important role in adaptive immunity, and can differentiate into plasma cells and participate in inflammatory responses by producing a large number of antibodies and suppressing immune responses by releasing anti-inflammatory cytokines[30]. Previous studies on the role of B cells in immune diseases have indicated that the disruption of B cell subsets was a common characteristic in various autoimmune diseases. The variations in B cell frequency, abnormal molecular expression, key pathways, and the imbalance in subgroup distribution are closely related to the immunological pathogenesis of many diseases. Different B cell subsets can promote and inhibit inflammation. Over-activation of B cell responses, abnormal expression of signaling factors and cytokines, and imbalance in subpopulations, including memory B cells, are involved in the pathogenesis of many immune diseases. Meanwhile, memory B cells were considered as the source of several pro-inflammatory cytokines, which are involved in the pathogenesis of many autoimmune diseases[31]. In recent years, domestic and foreign scholars have extensively focused on this special class of B cells and their relationship with autoimmune diseases, such as myasthenia grais, Gillan barre syndrome, and other immune system diseases[32]. Dysregulation of memory B cells was found in several autoimmune diseases. Previous studies[17] have found that the B cell immune response, namely, the antibody response, is abnormally activated, and the memory B cells are significantly reduced in UC patients.
It is known that the body’s immune system generates memory during the body’s first antigen-antagonism and antibody response to specific antigens. The initial B cells can transform into memory B cells to complete the rapid category conversion in the next immunization[33]. These plasma cells migrate to bone marrow and lymphoid tissue, secrete specific antibodies such as immunoglobulins, inflammatory cytokines, and chemokines, mediate rapid and effective secondary immune response, and have an important role in humoral immunity[34].
When memory B cells are stimulated again by the same antigen, they are rapidly activated to produce IgG, IgM, IgA, FCRL5, CD103, IL-10, FasL, PD-1, CD38, CXCR3, Tim3, and so on. It is known that the destruction of the intestinal mucosal barrier during the onset of IBD causes abnormal expression of immunoglobulins, which are a major participant in humoral immunity, including IgG, IgA, IgM, etc[35]. IgG has the function of opsonizing phagocytes and neutralizing toxins, IgA has an antibacterial and antiviral function, while IgM has the function of lysing pathogens and binding antigens. Previous studies have shown that the increased number of endoplasmic cells in the intestinal mucosa of IBD patients leads to an increase in the secretion of IgG, IgA, IgM, and other immunoglobulins, which further participate in the formation of local immune complexes in the intestinal mucosa and trigger intestinal inflammation[36]. “FCR-like” (FCRL) is an evolutionarily conserved gene family associated with IgG and IgE Fc receptors (FCRS). FCRL5 is preferentially expressed in B cells and encodes transmembrane proteins with tyrosine-based immunoregulatory motifs[37]. CD103 (human mucosal lymphocyte antigen-1) is highly expressed at mucosal sites, and CD103-positive lymphocytes have a remarkable ability to move directly toward the intestinal epithelial cell model and immobilize mucosal lymphocytes with E-cadherin at the basolateral side of the intestinal epithelium[38]. IL-10 has anti-inflammatory effects by inhibiting inflammation and cellular immune response, improving B cell survival rate, B cell proliferation, MHC II antigen expression, and immunoglobulin secretion, and inhibiting the production of NK cytokines, monocytes, and macrophage pro-inflammatory factors[39]. In the gastrointestinal tract, IL-10 is secreted by T cells, B cells, macrophages, neutrophils, and natural killer cells, and maintains mucosal homeostasis and immune tolerance during inflammation[40]. FasL is a membrane receptor protein that belongs to the superfamily of nerve growth factor receptors. It has a signal transduction role in cell apoptosis, and can induce colon epithelial cells and protect Th2 cells from apoptosis or even directly kill Th2 cells. In colonic epithelial cells, FasL can mediate the apoptosis of colonic epithelial cells through caspase-mediated apoptosis, which ultimately leads to the destruction of the colonic mucosal barrier and the formation of ulcers[41]. Indeed, expressed of Fas L in B cells is upregulated by IL-10 in some autoimmune diseases and GVHD models. CD5 is expressed in both FasL+ B cells and IL-10-producing B10 cells, suggesting the possibility of a feedforward cycle in which B cells can express IL-10 to enhance their other immunomodulatory mechanisms[42]. Programmed death receptor 1 (PD-1) is a coinhibitory receptor expressed on T cells, B cells, natural killer cells, and monocytes. It has been confirmed that PD-1 is highly expressed in intestinal mucosal cells. Its ligand binding can participate in the occurrence of intestinal mucositis by activating initial T lymphocytes, inhibiting activated effector T lymphocytes, and regulating the secretion of cytokines[43]. Studies have shown that in patients with UC, the expression level of PD-1 in inflammatory sites is significantly increased, while intestinal mucositis is alleviated when the PD-1 pathway is blocked[44]. CD38 is expressed on intestinal inflammatory cells, which has been reported to promote intestinal inflammation. Therefore, CD38 indirectly promotes intestinal inflammation[45]. CXCR3 is a chemokine receptor of the CXC family, expressed in epithelial and endothelial cells, as well as various lymphocytes such as NK cells, B cells, memory T cells, monocytes, and neutrophils. In IBD patients, CXCR3 and its corresponding ligands are strongly expressed in the intestinal mucosa. They can also recruit pro-inflammatory cells into the colon during colitis, leading to IBD[46]. As an important immune checkpoint molecule, Tim-3 regulates immune response. According to previous studies, Tim-3 is also expressed on the surface of B cells, while B cell activation affects the expression of Tim-3. After knocking out Tim-3, its protective effect disappeared, and the protective effect of B cells depended on Tim-3. Wang et al[47] found that during the development of acute enteritis, the expression of Tim-3 on the surface of infiltrated immune cells, especially B cells in the lamina propria of the colon, significantly decreased. After knocking out Tim-3, enteritis was significantly aggravated, and the protective effect of B cells on enteritis potentially depended on Tim-3. When the humoral immune balance related to memory B cells is disrupted, the expression of proinflammatory factors is induced, and the expression of anti-inflammatory factors is inhibited, leading to a variety of autoimmune diseases, including IBD[48].
Our experimental results revealed that the level of memory B cells in the peripheral blood of DSS-induced colitis mice was obviously abnormal, which was consistent with other literature reports. In our experiment, induced memory B cells (IgG+, IgM+, IgA+, FCRL5+, CD103+, FasL+, PD-1+, CD38+, and CXCR3+ memory B cells) were over-expressed and tolerant memory B cells (IL-10+ and Tim-3+ memory B cell) were under-expressed. After Cur administration, the levels of induced memory B cells with pro-inflammatory factor expression were significantly inhibited, while the levels of tolerant memory B cells and anti-inflammatory factors were increased. These phenomena were accompanied by Cur’s effective inhibition of pathological damage in colitis mice, which indicated that Cur could effectively regulate the balance of memory B cells in colitis mice, so as to restore the balance of pro-inflammatory factors and anti-inflammatory factors and alleviate the symptoms of DSS-induced colitis. These findings suggest that the therapeutic effect of Cur on UC in mice might be closely related to the balance regulation of memory B cells.
In order to explore the mechanisms by which Cur regulates the balance of memory B cells, we examined the activation of the Bcl-6-Syk-BLNK signaling pathway in colon tissue. Bcl-6 protein is highly expressed in germinal B cells, and the germinal response is the maturation of antigen-specific B cells into memory B cells, which constitute the cellular components of immune memory of the B cell lines[49]. Previous studies have suggested that Bcl-6 expression is necessary to enable germinal center B cells to differentiate into memory B cells. Bcl-6 expression was also found to enhance B cell survival and thereby regulate self-renewal of memory B cell. High expression of Bcl-6 sustains a germinal center B cell phenotype and inhibits the differentiation of memory B cells in vitro. Furthermore, it has been established that Syk/p-Syk is a key signal pathway, which is required to couple the BCR to all downstream signaling pathways in mature B cells. Also, the B cells with Syk deficiency could not differentiate into the germinal center or plasma cells, suggesting that Syk is required for the survival of memory B cells[50,51]. When BLNK/p-BLNK is deficient, the transformation of primordial B cells into memory B cells is disrupted, and the level of memory B cells is significantly reduced[52]. CIN85 is widely involved in various physiological activities in the body, especially vesicle transport, membrane transport, and invasion and migration of various cancer cells. It also participates in cell differentiation, cell cycle, and apoptosis[53,54]. According to the above analysis, the Bcl-6-Syk-BLNK signaling pathway plays a crucial role in the development, activation, and differentiation of B cells and memory B cells.
In our experiment, after Cur administration, the levels of other proteins were increased, but BLNK and p-BLNK protein expression was decreased. This result indicated that Cur could significantly inhibit the activation of the Bcl-6-Syk-BLNK signaling pathway, which is consistent with its regulatory effect on memory B-cell balance.
As a new member of regulatory immunity, the biological characteristics and functions of memory B cells have been studied extensively, providing a new perspective for studying immune regulation mode and a new method for treating IBD. It has been proven that Cur is effective against DSS-induced micron colitis. In this experiment, we found that Cur could effectively improve the mucosal damage of DSS-induced colitis mice and significantly regulate the balance of induced type and tolerance memory B cells in colitis mice. As a result, the Bcl-6-Syk-BLNK signaling pathway was significantly inhibited. The effectiveness of Cur for murine UC was closely related to the regulation of memory B cell balance. Cur regulated the balance of memory B cell subgroups in mice with colitis, which was realized by activating the Bcl-6-Syk-BLNK signaling pathway. These results suggest that through the activation of the Bcl-6-Syk-BLNK signaling pathway, Cur harmonizes the differentiation and activation of inducible and inhibitory memory B cells, promotes the balance between them, reduces the expression of chemokines related to the inhibition of inducible memory B cells, and prevents excessive accumulation of inflammatory cells. It can also prevent overproduction of pro-inflammatory factors, increase the expression of tolerant memory B cells and anti-inflammatory factors to alleviate the immune damage by correcting memory B cell disorder, and maintain the humoral immune homeostasis mediated by memory B cells.
Cur could effectively alleviate DSS-induced colitis in mice by regulating memory B cells and the Bcl-6-Syk-BLNK signaling pathway.
Curcumin (Cur) has shown promising efficacy in experimental colitis mice and ulcerative colitis patients. Disturbance in memory B cells has been observed in various autoimmune diseases, including inflammatory bowel disease. However, few studies have explored whether Cur treatment of colitis is associated with memory B cells.
To the best of our knowledge, this is the first study to explore the mechanisms by which Cur regulates memory B cells in the treatment of experimental colitis.
To investigate whether Cur can alleviate experimental colitis induced by DSS through regulating memory B cells and Bcl-6-Syk-BLNK signaling.
Cur (100 mg/kg/d) was intragastrically administered in mice with colitis induced by DSS for 14 consecutive days. The effect of Cur was evaluated by macroscopic and histological observation. The levels of memory B cell subgroups in mouse peripheral blood were detected by flow cytometry, and the levels of cytokines in colonic tissue homogenates were measured by using ELISA. The expression of Bcl-6, BLNK, and Syk was measured by Western blot analysis.
After mice were treated with Cur for 14 d, the body weight, colonic weight and length, colonic weight index, and histopathological injury were ameliorated. In colitis mice, the concentrations of IL-1β, IL-6, TNF-α, and IL-7A were significantly decreased, while the concentrations of anti-inflammatory cytokines IL-35 and IL-10 were obviously increased. Activation of memory B cell subsets in colitis mice was confirmed by a remarkable reduction in the expression of IgM+, IgG+, IgA+, FCRL5+, CD103+, FasL+, PD-1+, CD38+, and CXCR3+ on the surface of CD19+CD27+B cells, while the number of CD19+ CD27+ IL-10+ and CD19+CD27+ Tim-3+ B cells increased significantly. In addition, Cur observably decreased the protein levels of Syk, p-Syk, Bcl-6, and CIN85, and increased BLNK and p-BLNK expression in colitis mice.
Cur could effectively alleviate DSS-induced colitis in mice, which is realized via a potential mechanism involving memory B cells and the Bcl-6-Syk-BLNK signaling pathway.
In the present study, Cur effectively ameliorated the pathological colonic injury induced by DSS, which was achieved through a potential mechanism involving regulating the balance of memory B cells and activating the Bcl-6-Syk-BLNK signaling pathway.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Leo A, Italy; Losurdo G, Italy; Zhao G, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, DE Lillo A, Laino L, Lo Muzio L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med. 2015;10:1615-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and Health. Molecules. 2016;21:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 3. | Rao EV, Sudheer P. Revisiting curcumin chemistry part I: a new strategy for the synthesis of curcuminoids. Indian J Pharm Sci. 2011;73:262-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 4. | Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother Res. 2018;32:957-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7:52517-52529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 6. | Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363-398. [PubMed] |
| 7. | Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 458] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 8. | Yu Y, Sitaraman S, Gewirtz AT. Intestinal epithelial cell regulation of mucosal inflammation. Immunol Res. 2004;29:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Cancro MP, Tomayko MM. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol Rev. 2021;303:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 10. | Pararasa C, Zhang N, Tull TJ, Chong MHA, Siu JHY, Guesdon W, Chavele KM, Sanderson JD, Langmead L, Kok K, Spencer J, Vossenkamper A. Reduced CD27-IgD- B Cells in Blood and Raised CD27-IgD- B Cells in Gut-Associated Lymphoid Tissue in Inflammatory Bowel Disease. Front Immunol. 2019;10:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, Deb R, Tiwari V, Rohatgi S, Dhingra R, Kedia S, Sharma PK, Makharia G, Ahuja V. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis-a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (2)] |
| 12. | Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr. 2020;60:887-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J, Sun Z, Qiao S, Song Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol Rep. 2016;35:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Wan P, Chen H, Guo Y, Bai AP. Advances in treatment of ulcerative colitis with herbs: from bench to bedside. World J Gastroenterol. 2014;20:14099-14104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Ismail NI, Othman I, Abas F, H Lajis N, Naidu R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J Cell Physiol. 2018;233:830-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Zhong YB, Kang ZP, Zhou BG, Wang HY, Long J, Zhou W, Zhao HM, Liu DY. Curcumin Regulated the Homeostasis of Memory T Cell and Ameliorated Dextran Sulfate Sodium-Induced Experimental Colitis. Front Pharmacol. 2020;11:630244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Sann H, Erichsen Jv, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci. 2013;92:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1-15.25.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1307] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 20. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 443] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 21. | Das S, Batra SK, Rachagani S. Mouse Model of Dextran Sodium Sulfate (DSS)-induced Colitis. Bio Protoc. 2017;7:e2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Sarlos P, Kovesdi E, Magyari L, Banfai Z, Szabo A, Javorhazy A, Melegh B. Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J Gastrointest Pathophysiol. 2014;5:304-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 23. | Kiernan MG, Coffey JC, Sahebally SM, Tibbitts P, Lyons EM, O'leary E, Owolabi F, Dunne CP. Systemic Molecular Mediators of Inflammation Differentiate Between Crohn's Disease and Ulcerative Colitis, Implicating Threshold Levels of IL-10 and Relative Ratios of Pro-inflammatory Cytokines in Therapy. J Crohns Colitis. 2020;14:118-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Sabatino JJ Jr, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20:728-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 25. | Good-Jacobson KL. Strength in diversity: Phenotypic, functional, and molecular heterogeneity within the memory B cell repertoire. Immunol Rev. 2018;284:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Potter KN, Mockridge CI, Rahman A, Buchan S, Hamblin T, Davidson B, Isenberg DA, Stevenson FK. Disturbances in peripheral blood B cell subpopulations in autoimmune patients. Lupus. 2002;11:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004;50:1918-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2449] [Article Influence: 306.1] [Reference Citation Analysis (2)] |
| 29. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 964] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 30. | Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092-6099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 32. | Dizon BLP, Pierce SK. The tangled web of autoreactive B cells in malaria immunity and autoimmune disease. Trends Parasitol. 2022;38:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 34. | Jin W, Luo Z, Yang H. Peripheral B Cell Subsets in Autoimmune Diseases: Clinical Implications and Effects of B Cell-Targeted Therapies. J Immunol Res. 2020;2020:9518137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Rai T, Wu X, Shen B. Frequency and risk factors of low immunoglobulin levels in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015;3:115-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Gouni-Berthold I, Baumeister B, Berthold HK, Schmidt C. Immunoglobulins and IgG subclasses in patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:1720-1723. [PubMed] |
| 37. | Zhu Z, Li R, Li H, Zhou T, Davis RS. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Natl Acad Sci U S A. 2013;110:E1282-E1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Oshitani N, Watanabe K, Maeda K, Fujiwara Y, Higuchi K, Matsumoto T, Arakawa T. Differential expression of homing receptor CD103 on lamina propria lymphocytes and association of CD103 with epithelial adhesion molecules in inflammatory bowel disease. Int J Mol Med. 2003;12:715-719. [PubMed] |
| 39. | Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, Nguyen DD, Samsom JN, Escher JC, Somech R, Weiss B, Beier R, Conklin LS, Ebens CL, Santos FG, Ferreira AR, Sherlock M, Bhan AK, Müller W, Mora JR, Quintana FJ, Klein C, Muise AM, Horwitz BH, Snapper SB. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 40. | Morhardt TL, Hayashi A, Ochi T, Quirós M, Kitamoto S, Nagao-Kitamoto H, Kuffa P, Atarashi K, Honda K, Kao JY, Nusrat A, Kamada N. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci Rep. 2019;9:1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Liu D, Huang X, Cheng S, Tong W, Wan P, Guan Y, Zhao H. [Regulation of sishen wan on Bax/Bcl-2 mRNA, Fas/FasL in colonic tissue from rats with colitis]. Zhongguo Zhong Yao Za Zhi. 2011;36:3484-3488. [PubMed] |
| 42. | Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int Immunol. 2015;27:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Collins M, Michot JM, Danlos FX, Mussini C, Soularue E, Mateus C, Loirat D, Buisson A, Rosa I, Lambotte O, Laghouati S, Chaput N, Coutzac C, Voisin AL, Soria JC, Marabelle A, Champiat S, Robert C, Carbonnel F. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 44. | Roosenboom B, Horjus Talabur Horje CS, Smids C, Leeuwis JW, van Koolwijk E, Groenen MJM, Wahab PJ, van Lochem EG. Distribution of mucosal PD-1 expressing T cells in patients with colitis of different etiologies. Scand J Gastroenterol. 2021;56:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Ning L, Shan G, Sun Z, Zhang F, Xu C, Lou X, Li S, Du H, Chen H, Xu G. Quantitative Proteomic Analysis Reveals the Deregulation of Nicotinamide Adenine Dinucleotide Metabolism and CD38 in Inflammatory Bowel Disease. Biomed Res Int. 2019;2019:3950628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Yuan YH, ten Hove T, The FO, Slors JF, van Deventer SJ, te Velde AA. Chemokine receptor CXCR3 expression in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Wang S, Liu Y, Yuan X, Yao L, Yang S, Ma R. Fecal bacteria from ulcerative colitis patients downregulate Tim-3-mediated inhibitory effects on monocytes in vitro. Microb Pathog. 2019;128:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 890] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 49. | Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 50. | Ackermann JA, Nys J, Schweighoffer E, McCleary S, Smithers N, Tybulewicz VL. Syk tyrosine kinase is critical for B cell antibody responses and memory B cell survival. J Immunol. 2015;194:4650-4656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Iwata S, Tanaka Y. B-cell subsets, signaling and their roles in secretion of autoantibodies. Lupus. 2016;25:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Li N, Wu J, Wu Y, Xu Y, Yao R, Li G, Zhang J, Zhou Y, Yin L, Yin Y, Yu T, Wang J. Further delineation of primary B cell immunodeficiency caused by novel variants of the BLNK gene in two Chinese patients. Clin Immunol. 2020;214:108387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 53. | Kometani K, Yamada T, Sasaki Y, Yokosuka T, Saito T, Rajewsky K, Ishiai M, Hikida M, Kurosaki T. CIN85 drives B cell responses by linking BCR signals to the canonical NF-kappaB pathway. J Exp Med. 2011;208:1447-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |