Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5626
Peer-review started: June 9, 2022
First decision: August 1, 2022
Revised: August 19, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 14, 2022
Processing time: 124 Days and 17.2 Hours
At present, there is insufficient medical evidence to determine whether adjuvant chemotherapy is necessary for T2N0M0 gastric cancer.
To obtain a risk score to assess the need for adjuvant chemotherapy in patients with T2N0M0 gastric cancer.
We identified 325 patients with pathological T2N0M0 stage primary gastric cancer at the National Cancer Center between 2011 and 2018. Univariate and multivariate Cox regression analyses were performed to predict factors affecting prognosis. Vascular invasion, tumor site, and body mass index were assessed, and a scoring system was established. We compared the survival outcomes and benefits of adjuvant chemotherapy between the different subgroups.
Five-year survival rates of the score 0, 1, 2, and 3 groups were 92%, 95%, 80%, and 50%, respectively (P < 0.001). In the score 2-3 group, five-year survival rates for patients in the adjuvant chemotherapy group and postoperative observation group were 95% and 61%, respectively (P = 0.021).
For patients with T2N0M0 stage gastric cancer and two or more risk factors, adjuvant chemotherapy after D2 gastrectomy may have a survival benefit.
Core Tip: It is controversial whether adjuvant chemotherapy is necessary for stage T2N0M0 gastric cancer. In our study, we assessed the risk score of patients with pathologic T2N0M0 gastric cancer after D2 gastrectomy, based on clinicopathological factors, and identified a high-risk subgroup that could benefit from adjuvant chemotherapy. For patients with T2N0M0 stage gastric cancer with two or more risk factors, adjuvant chemotherapy after D2 gastrectomy may have a survival benefit.
- Citation: Xu Q, Kang WZ, Xiong JP, Shao XX, Li WK, Hu HT, Tian YT. A new scoring system to evaluate adjuvant chemotherapy for patients with T2N0M0 gastric cancer after D2 gastrectomy. World J Gastroenterol 2022; 28(38): 5626-5635
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5626
Gastric cancer is one of the most common malignancies worldwide[1-3]. D2 gastrectomy combined with postoperative adjuvant chemotherapy is the main treatment modality for advanced gastric cancer[4-8]. According to the 8th edition of the American Joint Committee on Cancer (AJCC) guidelines for gastric cancer, T2 was defined as tumor invasion of the muscularis propria[9]. It is controversial whether adjuvant chemotherapy is necessary for stage T2N0M0 gastric cancer[10-16]. Previous studies have suggested that patients with stage I gastric cancer cannot benefit from adjuvant chemotherapy[17]. However, there are some risk factors for recurrence of T2N0M0 gastric cancer, such as lymphatic and/or blood vessel invasion, tumor diameter, perineural invasion, proximal tumor location, and poor differentiation[14,18]. Postoperative adjuvant chemotherapy may inhibit the recurrence in these patients. To further clarify the indications for the use of postoperative adjuvant chemotherapy in T2N0M0 gastric cancer, we reviewed 325 patients with T2N0M0 gastric cancer admitted to the National Cancer Center between 2011 and 2018. In this study, we assessed the risk score of patients with pathologic T2N0M0 gastric cancer after D2 gastrectomy based on clinicopathological factors and identified a high-risk subgroup that could benefit from postoperative adjuvant chemotherapy.
We identified 402 patients with pathological T2N0M0 stage primary gastric carcinoma and gastroesophageal junction carcinoma (as defined by the AJCC guidelines, 8th edition) who were admitted to the Department of Pancreatic and Gastric Surgery, National Cancer Center, from 2011 to 2018. Three hundred and twenty-five patients were included in our study, all of whom underwent D2 gastrectomy. A total of 63 patients received post-operative adjuvant chemotherapy. The major chemotherapy regimens included platinum + 5-FU; paclitaxel + platinum + 5-FU; and others. Adjuvant chemotherapy is usually performed for 4-6 cycles. Exclusion criteria included loss to follow-up, lack of adenocarcinoma, neoadjuvant chemotherapy, adjuvant radiotherapy, Siewert I type/Siewert II type gastroesophageal junction carcinoma invading the dentate line, and postoperative survival time < 1 mo. Patients were followed-up by telephone. The follow-up was completed on April 30, 2020. The median follow-up time was 65.4 mo.
Univariate and multivariate Cox regression analyses were performed to screen for prognostic variables. Variables with a P value < 0.05 and < 0.25 in the univariate and multivariable Cox regression analyses were included in the study. Three variables were included in total: Vascular invasion, tumor site, and body mass index (BMI). The tumor site was classified as cardiac or non-cardiac. Cardiac cancer refers to Siewert type II gastroesophageal junction carcinoma that does not invade the dentate line and Siewert type III gastroesophageal junction carcinoma. BMI of < 18.5 or > 23.9, positive result of vascular invasion, and cardiac cancer were defined as risk factors. Each risk factor was assigned one point, and a total of four groups were obtained, which were defined as scores 0, 1, 2, and 3, respectively. We found that patients with a score ≥ 2 had a poor prognosis, and chemotherapy significantly improved prognosis. According to the study results, scores of 2-3 were defined as the high-risk group. The Kaplan-Meier method was used to calculate the 5-year survival rate and compare the overall survival (OS) between the different score groups.
Statistical analysis was performed using the R software 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) and the SPSS 22.0 software (SPSS Inc., Chicago, IL, United States). Each test was bilateral, and statistical significance was set at P < 0.05.
A total of 325 patients were recruited for this study. Table 1 summarizes the clinicopathological characteristics of the patients enrolled in this study. Univariate Cox regression analysis demonstrated that the tumor site (P = 0.022, Table 2), vascular invasion (P < 0.001, Table 2), and BMI (P = 0.036, Table 2) were significant risk factors for OS. Multivariate Cox regression analysis demonstrated that vascular invasion was an independent risk factor for OS (P < 0.001, Table 3).
| Variable | Overall | Adjuvant chemotherapy group | Postoperative observation group | P value |
| 325 | 63 | 262 | ||
| Age (yr) n (%) | 0.609 | |||
| < 40 | 20 (6.2) | 3 (4.8) | 17 (6.5) | |
| ≥ 40 | 305 (93.8) | 60 (95.2) | 245 (93.5) | |
| Sex, n (%) | 0.878 | |||
| Male | 250 (76.9) | 48 (76.2) | 202 (77.1) | |
| Female | 75 (23.1) | 15 (23.8) | 60 (22.9) | |
| Smoking history, n (%) | 0.363 | |||
| Yes | 169 (52.0) | 36 (57.1) | 133 (50.8) | |
| No | 156 (48.0) | 27 (42.9) | 129 (49.2) | |
| Family history of gastric cancer, n (%) | 0.852 | |||
| Yes | 24 (7.4) | 5 (7.9) | 19 (7.3) | |
| No | 301 (92.6) | 58 (92.1) | 243 (92.7) | |
| BMI, n (%) | 0.150 | |||
| < 18.5 or > 23.9 | 176 (54.2) | 29 (46.0) | 147 (56.1) | |
| 18.5-23.9 | 149 (45.8) | 34 (54.0) | 115 (43.9) | |
| Postoperative hospital stay, n (%) | 0.747 | |||
| ≤ 14 d | 285 (87.7) | 56 (88.9) | 229 (87.4) | |
| > 14 d | 40 (12.3) | 7 (11.1) | 33 (12.6) | |
| Tumor site, n (%) | 0.004 | |||
| Cardia cancer | 105 (32.3) | 30 (47.6) | 75 (28.6) | |
| Non-cardia gastric cancer | 220 (67.7) | 33 (52.4) | 187 (71.4) | |
| The degree of differentiation, n (%) | 0.571 | |||
| Poorly differentiated | 121 (37.2) | 24 (38.1) | 97 (37.0) | |
| Moderately differentiated | 178 (54.8) | 36 (57.1) | 142 (54.2) | |
| Highly differentiated | 26 (8.0) | 3 (4.8) | 23 (8.8) | |
| Vascular invasion, n (%) | 0.014 | |||
| Yes | 54 (16.6) | 17 (27.0) | 37 (14.1) | |
| No | 271 (83.4) | 46 (73.0) | 225 (85.9) |
| Clinicopathological features | HR (95%CI) | P value |
| Sex | ||
| Male | Reference | |
| Female | 0.278 (0.066-1.172) | P = 0.081 |
| Smoking history | ||
| Yes | Reference | |
| No | 0.605 (0.276-1.323) | P = 0.208 |
| Family history of gastric cancer | ||
| Yes | Reference | |
| No | 0.550 (0.075-4.057) | P = 0.558 |
| BMI | ||
| 18.5-23.9 | Reference | |
| > 23.9 or < 18.5 | 2.509 (1.060-5.937) | P = 0.036 |
| Postoperative hospital stay | ||
| ≤ 14 d | Reference | |
| > 14 d | 0.990 (0.298-3.292) | P = 0.987 |
| Tumor site | ||
| Cardia cancer | Reference | |
| Non-cardia gastric cancer | 0.411 (0.192-0.878) | P = 0.022 |
| The degree of differentiation | ||
| Poorly differentiated | Reference | |
| Moderately differentiated | 1.330 (0.574-3.082) | P = 0.507 |
| Highly differentiated | 0.857 (0.182-4.043) | P = 0.846 |
| Vascular invasion | ||
| Yes | Reference | |
| No | 0.097 (0.044-0.212) | P < 0.001 |
| Clinicopathological features | HR (95%CI) | P value |
| Sex | ||
| Male | Reference | |
| Female | 0.390 (0.076-1.988) | P = 0.257 |
| Smoking history | ||
| Yes | Reference | |
| No | 0.725 (0.308-1.710) | P = 0.463 |
| Family history of gastric cancer | ||
| Yes | Reference | |
| No | 0.495 (0.058-4.224) | P = 0.521 |
| BMI | ||
| 18.5-23.9 | Reference | |
| > 23.9 or < 18.5 | 1.848 (0.760-4.490) | P = 0.175 |
| Postoperative hospital stay | ||
| ≤ 14 d | Reference | |
| > 14 d | 1.198 (0.350-4.100) | P = 0.960 |
| Tumor site | ||
| Cardia cancer | Reference | |
| Non-cardia gastric cancer | 0.620 (0.277-1.390) | P = 0.246 |
| The degree of differentiation | ||
| Poorly differentiated | Reference | |
| Moderately differentiated | 0.517 (0.206-1.300) | P = 0.161 |
| Highly differentiated | 0.390 (0.077-1.960) | P = 0.305 |
| Vascular invasion | ||
| Yes | Reference | |
| No | 0.106 (0.045-0.246) | P < 0.001 |
Vascular invasion, tumor site, and BMI were assessed in the study, and a scoring system was established.
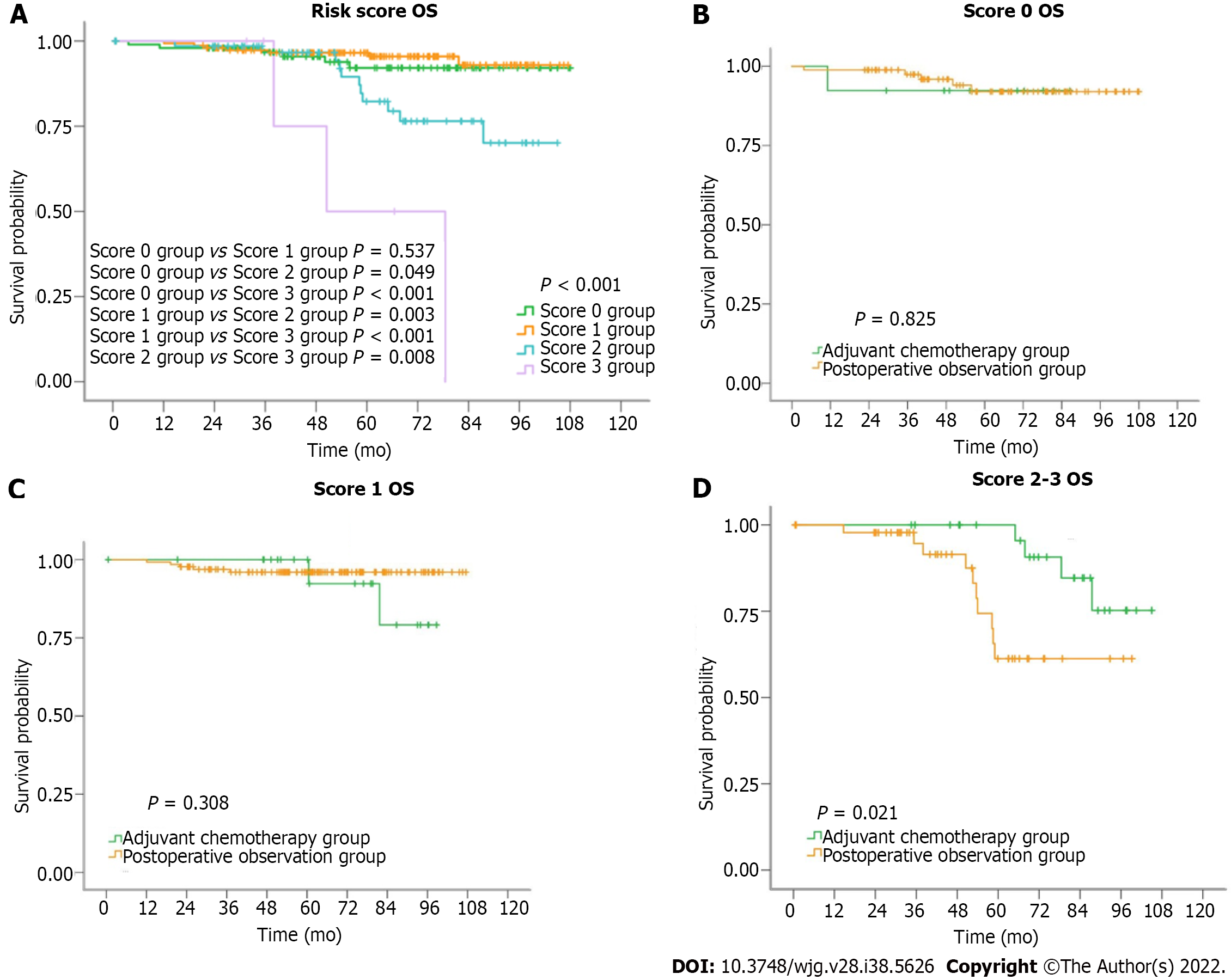
Figure 1A summarizes the survival curves of patients with scores of 0, 1, 2, and 3. There were significant differences among all groups except for the score 0 and 1 groups (score 0 group vs score 1 group, P = 0.537; score 0 group vs score 2 group, P = 0.049; score 0 group vs score 3 group, P < 0.001; score 1 group vs score 2 group, P = 0.003; score 1 group vs score 3 group, P < 0.001; score 2 group vs score 3 group, P = 0.008). For all patients, 5-year survival rates of the adjuvant chemotherapy and postoperative observation groups were 96% and 90%, respectively (P = 0.676, Table 4). Five-year survival rates of the score 0, 1, 2, and 3 groups were 92%, 95%, 80%, and 50%, respectively (P < 0.001, Table 4). In the score 0 and score 1 groups, there were no differences in the 5-year survival rates between the postoperative observation and adjuvant chemotherapy groups. In the score 2-3 group, 5-year survival rates for patients in the adjuvant chemotherapy group and postoperative observation group were 95% and 61%, respectively (P = 0.021, Table 4).
| Group | n | 5-year survival rate | Log rank test | |
| All patients | P = 0.676 | |||
| Adjuvant chemotherapy | 63 | 96% | ||
| Postoperative observation | 262 | 90% | ||
| Risk score | P < 0.001 | |||
| Score 0 group | 98 | 92% | ||
| Score 1 group | 152 | 95% | ||
| Score 2 group | 69 | 80% | ||
| Score 3 group | 6 | 50% | ||
| Score 0 group | P = 0.825 | |||
| Adjuvant chemotherapy | 13 | 92% | ||
| Postoperative observation | 85 | 92% | ||
| Score 1 group | P = 0.308 | |||
| Adjuvant chemotherapy | 22 | 92% | ||
| Postoperative observation | 130 | 96% | ||
| Score 2-3 group | P = 0.021 | |||
| Adjuvant chemotherapy | 28 | 95% | ||
| Postoperative observation | 47 | 61% |
Figure 1B-D summarizes the survival curves of patients with scores of 0, 1, and score 2-3 T2N0M0 gastric cancer in the adjuvant chemotherapy and postoperative observation groups. Table 5 summarizes the distribution of the different risk factors in each risk score group.
| BMI < 18.5 or > 23.9 | Vascular invasion | Cardia cancer | |
| All | 149 | 54 | 105 |
| Score 0 group | 0 | 0 | 0 |
| Score 1 group | 92 | 15 | 45 |
| Score 2 group | 51 | 33 | 54 |
| Score 3 group | 6 | 6 | 6 |
Our study found that adjuvant chemotherapy is necessary for the treatment of T2N0M0 gastric cancer patients with two or more risk factors. The risk factors included vascular invasion, BMI, and tumor site. Based on these results, we obtained a simple risk score to assess the need for adjuvant chemotherapy in patients with T2N0M0 gastric cancer. Patients with a score 2-3 were assigned to the high-risk group.Previous studies have shown that adjuvant chemotherapy can prolong OS in advanced gastric cancer and reduce recurrence[19]. However, evidence of the survival benefits of adjuvant chemotherapy for early gastric cancer is lacking. Although there is no lymph node metastasis in T2N0M0 gastric cancer, some patients still experience recurrence[10-16]. Therefore, it is important to identify patients with stage T2N0M0 gastric cancer who are at high risk of recurrence and may require adjuvant chemotherapy. Univariate Cox regression analysis demonstrated that tumor site (P = 0.022, Table 2), vascular invasion (P < 0.001, Table 2), and BMI (P = 0.036, Table 2) were significant risk factors for OS in patients with T2N0M0 disease. Multivariate Cox regression analysis showed that vascular invasion was an independent prognostic indicator in patients with T2N0M0 disease[14]. Tumor site has been reported to be a prognostic risk factor for stage IB gastric cancer[11]. The 5-year OS rate of patients with stage IB gastric cancer whose tumors are located in the upper third of the stomach is only 81.8%, which is lower than that of patients with stage II disease receiving S-1 adjuvant chemotherapy[5]. Another study that followed 532 patients reported poorer long-term survival in patients with proximal gastric cancer than in those with distal gastric cancer[20]. Proximal gastric cancer has a higher proportion of undifferentiated tumors, and tumors located in this region can metastasize to almost all lymph nodes, except in the five groups[11]. These factors may account for the lower survival rates of patients with proximal gastric cancer. Several studies have shown that BMI affects the prognosis of patients with gastric cancer[21-26]. Low BMI was associated with malnutrition, whereas high BMI was associated with a higher risk of surgery and a higher rate of postoperative complications. A high BMI also increases the risk of stomach cancer[27]. The degree of tumor differentiation was not included in the study, possibly because poorly differentiated tumors do not show significant aggressiveness in the early stages of tumor development.
Based on these findings, we developed a scoring system to assess the need for the use of adjuvant chemotherapy in patients with T2N0M0 gastric cancer. Patients with no or only one risk factor had good prognosis after D2 gastrectomy and did not require adjuvant chemotherapy. Patients in the score 2-3 group had a significantly worse prognosis and could benefit from adjuvant chemotherapy. Our study may help to provide targeted treatment for patients with stage T2N0M0 gastric cancer.This study had some limitations. This was a single-center retrospective study with a lower level of evidence than that of a prospective study. We did not classify the patients into subgroups based on the number of lymph nodes removed. The number of lymph node dissections has a significant effect on OS. For patients with stage T1-2 node-negative gastric cancer, the 5-year survival rate increased by 7.6% for every 10 Lymph nodes examined[28]. No recurrence-free survival or recurrence pattern was observed. We did not discuss the genetic characteristics of patients with gastric cancer included in the study. Genetic characteristics of patients with gastric cancer may influence the efficacy of adjuvant chemotherapy.
For patients with T2N0M0 stage gastric cancer and two or more risk factors, adjuvant chemotherapy after D2 gastrectomy may have a survival benefit. Individualized treatment should be adopted according to examination and pathological results in patients with T2N0M0 gastric cancer.
It is controversial whether adjuvant chemotherapy is necessary for stage T2N0M0 gastric cancer.
To further clarify the indications for the use of postoperative adjuvant chemotherapy in patients with T2N0M0 gastric cancer.
To obtain a risk score to assess the need for adjuvant chemotherapy in patients with T2N0M0 gastric cancer.
Univariate and multivariate Cox regression analyses were performed to predict factors affecting prognosis. Vascular invasion, tumor site, and BMI were assessed, and a scoring system was established. We compared the survival outcomes and benefits of adjuvant chemotherapy between the different subgroups.
Five-year survival rates of the score 0, 1, 2, and 3 groups were 92%, 95%, 80%, and 50%, respectively (P < 0.001). In the score 2-3 group, five-year survival rates for patients in the adjuvant chemotherapy group and postoperative observation group were 95% and 61%, respectively (P = 0.021).
For patients with T2N0M0 stage gastric cancer and two or more risk factors, adjuvant chemotherapy after D2 gastrectomy may have a survival benefit.
Individualized treatment should be adopted according to examination and pathological results in patients with T2N0M0 gastric cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Acikgoz Y, Turkey; Dumitraşcu T, Romania; Hsu JT, Taiwan; Hwang GS, South Korea; Liu YQ, United States; Ntavatzikos A, Greece S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64389] [Article Influence: 16097.3] [Reference Citation Analysis (175)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13204] [Article Influence: 1467.1] [Reference Citation Analysis (3)] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2830] [Article Influence: 566.0] [Reference Citation Analysis (5)] |
| 4. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 5. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 7. | Yoshikawa T, Terashima M, Mizusawa J, Nunobe S, Nishida Y, Yamada T, Kaji M, Fukushima N, Hato S, Choda Y, Yabusaki H, Yoshida K, Ito S, Takeno A, Yasuda T, Kawachi Y, Katayama H, Fukuda H, Boku N, Sano T, Sasako M. Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomised trial. Lancet Gastroenterol Hepatol. 2019;4:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 9. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer. |
| 10. | Yu B, Park JY, Park KB, Kwon OK, Lee SS, Chung HY. Prognostic Factors in Stage IB Gastric Cancer after Surgical Resection. J Gastric Cancer. 2020;20:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Aoyama T, Yoshikawa T, Fujikawa H, Hayashi T, Ogata T, Cho H, Yamada T, Hasegawa S, Tsuchida K, Yukawa N, Oshima T, Oba MS, Morita S, Rino Y, Masuda M. Prognostic factors in stage IB gastric cancer. World J Gastroenterol. 2014;20:6580-6585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Yan J, Hu W, Zhang J, Huo B. Adjuvant chemotherapy provided survival benefit for stage T2N0 gastric cancer with high-risk factors. Neoplasma. 2018;65:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | In H, Kantor O, Sharpe SM, Baker MS, Talamonti MS, Posner MC. Adjuvant Therapy Improves Survival for T2N0 Gastric Cancer Patients with Sub-optimal Lymphadenectomy. Ann Surg Oncol. 2016;23:1956-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Du C, Zhou Y, Huang K, Zhao G, Fu H, Shi Y. Defining a high-risk subgroup of pathological T2N0 gastric cancer by prognostic risk stratification for adjuvant therapy. J Gastrointest Surg. 2011;15:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Saito H, Murakami Y, Miyatani K, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ikeguchi M. Predictive factors for recurrence in T2N0 and T3N0 gastric cancer patients. Langenbecks Arch Surg. 2016;401:823-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Gabriel E, Attwood K, Narayanan S, Brady M, Nurkin S, Hochwald S, Kukar M. Does neoadjuvant/perioperative chemotherapy improve overall survival for T2N0 gastric adenocarcinoma? J Surg Oncol. 2018;117:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Coburn NG, Govindarajan A, Law CH, Guller U, Kiss A, Ringash J, Swallow CJ, Baxter NN. Stage-specific effect of adjuvant therapy following gastric cancer resection: a population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Jin LX, Moses LE, Squires MH 3rd, Poultsides GA, Votanopoulos K, Weber SM, Bloomston M, Pawlik TM, Hawkins WG, Linehan DC, Strasberg SM, Schmidt C, Worhunsky DJ, Acher AW, Cardona K, Cho CS, Kooby DA, Levine E, Winslow ER, Saunders ND, Spolverato G, Maithel SK, Fields RC. Factors Associated With Recurrence and Survival in Lymph Node-negative Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg. 2015;262:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Jiang Z, Sun Y, Zhang W, Cui C, Yang L, Zhou A. Comparison of S-1 plus oxaliplatin (SOX) and capecitabine plus oxaliplatin (XELOX) as adjuvant chemotherapies for stage II and III gastric cancer after D2 resection: A single-center retrospective study. Asia Pac J Clin Oncol. 2020;16:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Piso P, Werner U, Lang H, Mirena P, Klempnauer J. Proximal versus distal gastric carcinoma--what are the differences? Ann Surg Oncol. 2000;7:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Wada T, Kunisaki C, Ono HA, Makino H, Akiyama H, Endo I. Implications of BMI for the Prognosis of Gastric Cancer among the Japanese Population. Dig Surg. 2015;32:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, Hu W, Ba Y, Li SY, Li ZN, Wang KH, Wu J, He Y, Yang JJ, Xie CH, Song XX, Chen GY, Ma WJ, Luo SX, Chen ZH, Cong MH, Ma H, Zhou CL, Wang W, Luo Q, Shi YM, Qi YM, Jiang HP, Guan WX, Chen JQ, Chen JX, Fang Y, Zhou L, Feng YD, Tan RS, Li T, Ou JW, Zhao QC, Wu JX, Deng L, Lin X, Yang LQ, Yang M, Wang C, Song CH, Xu HX, Shi HP; Investigation on the Nutrition Status and Clinical Outcome of Common Cancers (INSCOC) Group. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. 2020;28:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Chen S, Nie RC, OuYang LY, Li YF, Xiang J, Zhou ZW, Chen Y, Peng J. Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lianos GD, Bali CD, Glantzounis GK, Katsios C, Roukos DH. BMI and lymph node ratio may predict clinical outcomes of gastric cancer. Future Oncol. 2014;10:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Feng F, Zheng G, Guo X, Liu Z, Xu G, Wang F, Wang Q, Guo M, Lian X, Zhang H. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer. 2018;18:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Tsekrekos A, Lovece A, Chrysikos D, Ndegwa N, Schizas D, Kumagai K, Rouvelas I. Impact of obesity on the outcomes after gastrectomy for gastric cancer: A meta-analysis. Asian J Surg. 2022;45:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Hirabayashi M, Inoue M, Sawada N, Saito E, Abe SK, Hidaka A, Iwasaki M, Yamaji T, Shimazu T, Shibuya K, Tsugane S; JPHC Study Group. Effect of body-mass index on the risk of gastric cancer: A population-based cohort study in A Japanese population. Cancer Epidemiol. 2019;63:101622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (0)] |