Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5614
Peer-review started: November 16, 2021
First decision: May 10, 2022
Revised: May 21, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: October 14, 2022
Processing time: 329 Days and 22.5 Hours
Estimation of the functional reserve of the remnant liver is important to reduce morbidity and mortality.
To estimate the functional reserve of the remnant liver in patients with hepatocellular carcinoma (HCC).
We reviewed the medical records of 199 patients who underwent resection of HCC. Hepatic clearance of the remnant liver was calculated using fusion images of 99mTc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Posthepatectomy liver failure (PHLF) was classified according to the International Study Group of Liver Surgery. Complications was classified according to Clavien–Dindo classification. We analyzed by the risk factors for PHLF, morbidity and mortality with multivariate analysis.
Twenty-seven (30%) patients had major complications and 23 (12%) developed PHLF. The incidence of major complications increased with increasing albumin–bilirubin (ALBI) grade. The area under the curve values for hepatic clearance of the remnant liver, liver to heart-plus-liver radioactivity at 15 min (LHL15), and ALBI score predicting PHLF were 0.868, 0.629, and 0.655, respectively. The area under the curve for hepatic clearance of the remnant liver, LHL15, and ALBI score predicting major complications were 0.758, 0.594, and 0.647, respectively. The risk factors for PHLF and major complications were hepatic clearance of the remnant liver and intraoperative bleeding.
The measurement of hepatic clearance may predict PHLF and major complications for patients undergoing resection of HCC.
Core tip: Little is known about the association of remnant hepatic clearance with morbidity and mortality. The aim of present study was to evaluate the effectiveness of measuring hepatic clearance of the remnant liver and to determine its association with morbidity and mortality in patients undergoing hepatectomy for hepatocellular carcinoma. Risk factors significantly associated with morbidity and mortality were remnant liver clearance and intraoperative blood loss. Hepatic clearance was associated with posthepatectomy liver failure and the development of major complications. The estimation of hepatic clearance of the remnant liver may provide guidance for determining the extent of resection in a patient-specific manner.
- Citation: Miki A, Sakuma Y, Ohzawa H, Saito A, Meguro Y, Watanabe J, Morishima K, Endo K, Sasanuma H, Shimizu A, Lefor AK, Yasuda Y, Sata N. Clearance of the liver remnant predicts short-term outcome in patients undergoing resection of hepatocellular carcinoma. World J Gastroenterol 2022; 28(38): 5614-5625
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5614
Advances in surgical technique and postoperative care have improved the outcomes of patients undergoing hepatectomy. However, posthepatectomy liver failure (PHLF) can lead to increased rates of morbidity and mortality in patients with hepatocellular carcinoma (HCC) especially in patients with chronic liver damage[1]. Major hepatectomy must be performed to preserve the maximal remnant liver function. However, adequate hepatectomy must be performed to ensure adequate surgical margins around the tumor[2]. Therefore, preoperative assessment of remnant liver function reserve is important to determine the appropriate surgical procedure.
An algorithm including the presence of ascites, serum bilirubin, serum albumin concentration, prothrombin time and encephalopathy is commonly used to determine the indications for resection of HCC. The indocyanine green (ICG) test is the most commonly used test and is considered relatively reliable for assessment of liver functional reserve[3]. However, the Child–Pugh score and ICG test do not accurately predict the development of PHLF[4]. A simple method using conventional data has been reported. The albumin–bilirubin (ALBI) score is an effective predictor of PHLF in patients with HCC compared to that of ICG test[5].
Computed tomography (CT) volumetry can accurately determine the regional liver volume, and has been used to estimate remnant liver function[6]. However, CT volumetry can never reflect the function of the remnant liver. The liver function of each lobe varies with progression of chronic liver disease or steatosis, which indicate that liver function is not distributed homogeneously[7]. Liver function is unevenly modified, resulting from impaired blood circulation[8], biliary stenosis, or induction by the tumor[9]. Changes in portal hemodynamics and a regional reduction in liver function must be considered to determine the optimal surgical procedure[7,10]. A novel method is needed to preoperatively plan for hepatic resection.
Taniguchi et al[11] described that 99mTc-labelled galactosyl-human serum albumin (GSA) hepatic clearance strongly correlates with the degree of liver fibrosis and conventional liver function tests. GSA scintigraphy is widely used to evaluate liver function[10,12-16]. Asialoglycoprotein receptors exist predominantly in the liver on the surface of hepatocytes and are responsible for the metabolism of serum glycoproteins[17]. The receptor density in the liver is closely related to serum asialoglycoprotein level and hepatocyte function[18]. However, little is known about the clinical utility of hepatic clearance for the prediction of PHLF, morbidity and mortality. The aim of present study was to evaluate the effectiveness of measuring hepatic clearance of the remnant liver and to verify risk factors based on the standardized PHLF criteria and complications in patients undergoing hepatectomy.
We included patients who underwent hepatectomy between July 2011 and March 2021 at Jichi Medical University (Shimotsuke, Tochigi, Japan). The protocol for this research project was approved by a suitably constituted Ethics Committee of the institution and it conformed to the provision of the Declaration of Helsinki (Committees of Jichi Medical University, Approval No. A21-029). Blood samples obtained preoperatively were analyzed for conventional liver tests.
The procedures for hepatectomy were categorized according to the Brisbane Nomenclature from the International Hepato-Pancreato-Biliary Association[19]. The anatomical resection was defined as resection of the tumor together with the related portal vein branches and the corresponding hepatic territory. The procedure was classified as a hemihepatectomy, an extended hemihepatectomy (hepatectomy plus removal of additional contiguous segments), a sectionectomy (resection of two Couinaud subsegments), or segmentectomy (resection of one Couinaud subsegment). All other nonanatomical procedures were classified as limited resections.
A three-phase enhanced helical CT scan of the liver was used to confirm tumor location and margins before surgery. A 16-row multi-detector CT scan was performed at 3 mm intervals with 100 mL iohexol (Omnipaque 300; Daiichi Sankyo, Tokyo, Japan) (3 mL/s) injected intravenously.
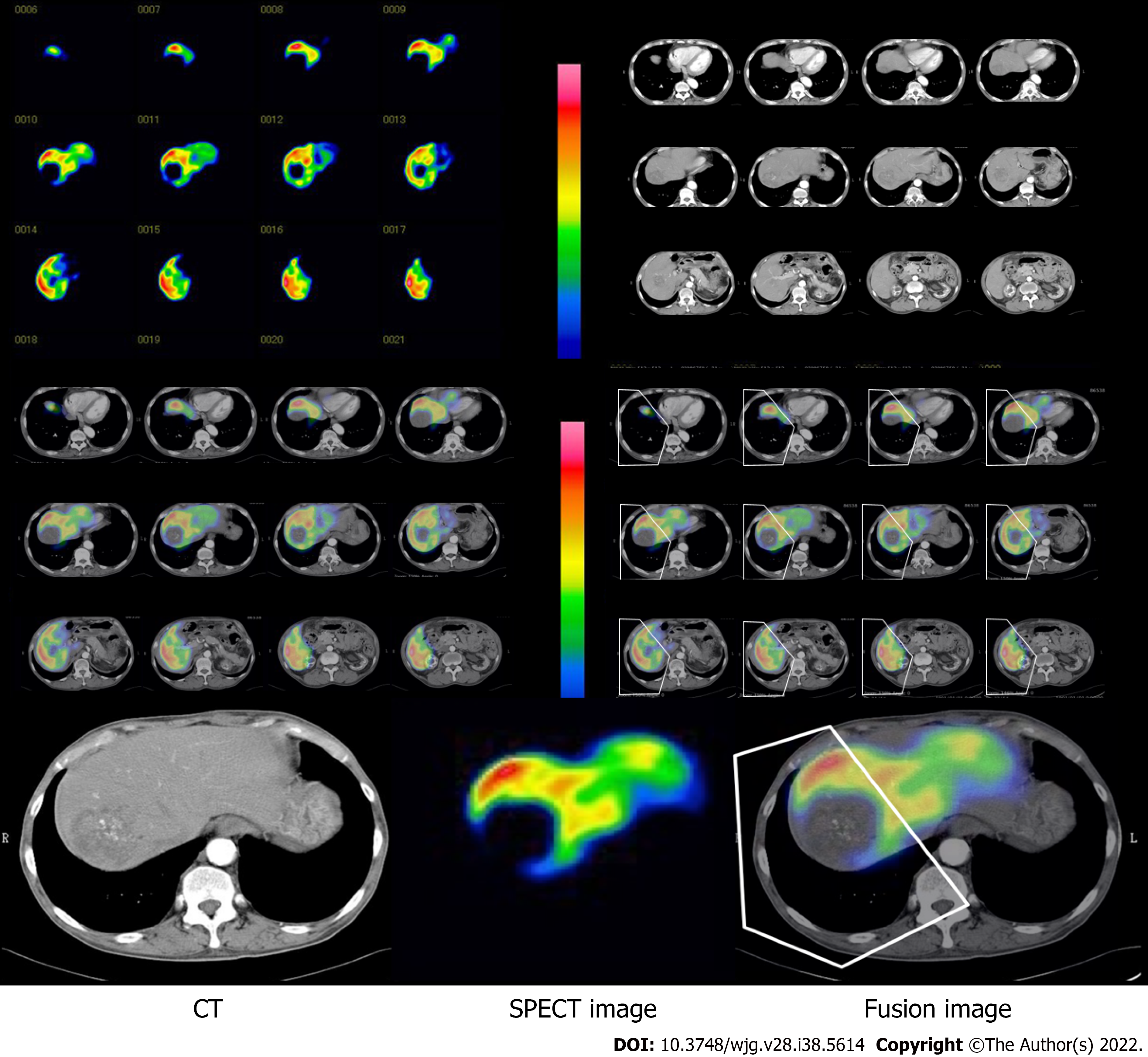
Patients underwent preoperative GSA scintigraphy using a dual-head rotating gamma camera system and a dedicated data processing unit (Prism Axis; Picker Prism International, Cleveland, OH, USA). A single bolus of 3 mg GSA (185 MBq; Nihon Medi-Physics, Tokyo, Japan) was injected intravenously. After confirmation that the detector covered the area in the liver and heart, acquisition of planar images was begun with an acquisition time of 15 s each for 16 min immediately after injection. After acquisition of planar images, dynamic single photon emission CT (SPECT) acquisition was started with an acquisition time of 20 s every 5 min. To generate a set of images equivalent to static SPECT images, projection data from dynamic SPECT were merged. Total liver function was calculated as the total liver GSA clearance, expressed as mL/min by the Patlak plot method.
Region of interest (ROI) was also generated over the entire liver on the tomographic images using iso-count methods (25% cutoff of minimal count) to estimate the liver functional volume (mL). Functional liver volume does not include function parameters.
Hepatic clearance and functional volume of the remnant liver were estimated from the fusion with CT scan images (Figure 1). Images from the CT scan were aligned with the slice of the liver SPECT image with reference to the hepatic vein on every 3-mm liver cross-slice as a landmark on contrast-enhanced helical CT (Figure 1). After the transection line was set on the SPECT images based on the surgical procedure, the remnant liver with the resection line was determined manually. Remnant liver function was calculated from the proportional allocation of voxel count in static SPECT by the Patlak plot method and expressed by GSA clearance (mL/min). Regional functional liver volume (mL) was also calculated from the SPECT data by the outline extraction method[7].
Postoperative complications were defined according to the Clavien–Dindo classification[20]. A major complication was defined as grade IIIa or higher. Postoperative mortality was defined as death within 30 d after surgery. PHLF was defined following the definition of the International Study Group of Liver Surgery[21]. Patients with increased prothrombin time–international normalized ratio (PT-INR) and hyperbilirubinemia (according to the normal cut-off levels defined by the local laboratory) on or after postoperative day 5 were considered to have PHLF. PHLF Grade A resulted in abnormal laboratory parameters and required no change in clinical management. Grade B was a deviation from the regular, postoperative clinical pathway, but patients could be managed without invasive treatment. Grade C resulted in deviation from the regular clinical management and required invasive treatment.
Continuous variables were expressed as mean ± standard deviation. All categorical data were analyzed by Pearson's χ2 test. Normally distributed values were analyzed by Student’s t test. Non-normally distributed values were analyzed using the Mann–Whitney U test. We analyzed the power for the prediction of PHLF, morbidity and mortality with the parameters of GSA scintigraphy with the area under the receiver-operating characteristic (ROC) curves, and the area under the ROC curve was calculated. In multivariate analysis, risk factors for PHLF were determined by logistic regression multivariate analysis with JMP statistical software (version 13; SAS, Inc. Cary, NC, USA). The level of statistical significance was set at P < 0.05.
A total of consecutive 199 patients with HCC were included, including 156 men and 43 women, with a median age of 70 (range, 24–87 years) (Table 1). Among the 199 patients, 94 (47%) had hepatitis C virus infection and 22 (11%) had hepatitis B virus infection. Most patients were Child–Pugh class A (197/199, 99%) and the remaining patients were class B (2/199, 1%). According to ALBI grade, 68% (135/199) of patients were stratified into Grade 1, 32% (64/199) Grade 2, and 1% (2/199) Grade 3. There were 6% of ALBI Grade 1 patients who developed major complications and 18% ALBI Grade 2 patients had major complications (P = 0.04).
| Variables | All patients (n = 199) | No PHLF < Grade B (n = 187) | PHLF ≥ Grade B (n = 12) | P value |
| General | ||||
| Age (yr) | 69.1 ± 9.0 | 69.2 ± 9.12 | 66.9 ± 7.05 | 0.53 |
| Gender (male : female) | 154 : 45 | 144 : 43 | 10 : 2 | 0.61 |
| Child–Pugh class (A : B : C) | 196 : 3 :0 | 184 : 3 : 0 | 12 : 0 : 0 | 0.31 |
| Preoperative laboratory tests | ||||
| Total bilirubin (mg/dL) | 0.85 ± 0.38 | 0.82 ± 0.38 | 1.06 ± 0.32 | 0.01 |
| PT-INR | 1.12 ± 0.20 | 1.12 ± 0.21 | 1.13 ± 0.08 | 0.70 |
| Albumin (mg/dL) | 4.1 ± 0.5 | 4.1 ± 0.5 | 3.9 ± 0.4 | 0.16 |
| AST (IU/L) | 43 ± 41 | 41 ± 40 | 56 ± 47 | 0.11 |
| ALT (IU/L) | 40 ± 34 | 39 ± 33 | 47 ± 40 | 0.31 |
| Choline esterase(U/L) | 262 ± 77 | 267 ± 76 | 226 ± 73 | 0.02 |
| PNI score | 48.6 ± 6.0 | 48.9 ± 6.0 | 46.4 ± 5.6 | 0.08 |
| ALBI score | -2.76 ± 0.4 | -2.78 ± 0.4 | -2.57 ± 0.35 | 0.14 |
| NLR | 2.48 ± 2.03 | 2.50 ± 2.12 | 2.31 ± 1.07 | 0.82 |
| PLR | 0.12 ± 0.08 | 0.13 ± 0.09 | 0.10 ± 0.05 | 0.29 |
| Procedure-related factors | ||||
| Limited resection | 80 (39.8%) | 77 (38.7%) | 3 (1.5%) | 0.43 |
| Segmentectomy | 57 (28.4%) | 51 (25.6%) | 6 (3.0%) | |
| Secteionectomy | 36 (17.9%) | 33 (16.6%) | 1 (0.5%) | |
| Hemihepatectomy | 24 (11.9%) | 22 (11.1%) | 2 (1.0%) | |
| Trisectionectomy | 4 (2.0%) | 4 (2.1%) | 0 (0%) | |
| Operative time (min) | 304 ± 109 | 299 ± 104 | 345 ± 135 | 0.06 |
| Intraoperative blood loss (mL) | 848 ±1006 | 743 ± 889 | 1604 ± 1456 | < 0.001 |
| Liver function tests | ||||
| Total Liver hepatic clearance (mL/min) | 285 ± 98 | 315 ± 106 | 258 ± 91 | 0.02 |
| Total Liver Functional volume (mL) | 1321 ± 280 | 1340 ± 278 | 1202 ± 270 | 0.04 |
| Hepatic clearance of the remnant liver (mL/min) | 248 ± 95 | 261 ± 91 | 149 ± 47 | < 0.001 |
| Functional volume of the remnant liver (mL) | 1057 ± 334 | 1104 ± 317 | 710 ± 239 | < 0.001 |
| LHL15 | 0.92 ± 0.03 | 0.925 ± 0.03 | 0.906 ± 0.05 | 0.02 |
| HH15 | 0.60 ± 0.07 | 0.602 ± 0.07 | 0.651 ± 0.07 | 0.01 |
| Surgical outcome | ||||
| PHLF grade (0 : A : B : C) | 176 : 11 : 8 : 4 | 176 : 11 : 0 : 0 | 0 : 0 : 8 : 4 | - |
| Hospital length of stay (d) | 15 (7-119) | 14 (7-119) | 25 (12-74) | < 0.001 |
Among the 199 patients, 41 (21%) developed postoperative complications (Table 2). The most common complication was PHLF (12%, 23/199), followed by wound infection (5.0%, 10/199). Thirty-three (17%) patients developed minor complications, including Grade I complications in 25 (13%) patients and Grade II complications in eight (4.0%) patients. Major complications occurred in 27 (14%) patients, including Grade IIIa (11%, 21/199), Grade IIIb (1.5%, 3/199), Grade IVa (0.5%, 1/199) and Grade V (1%, 2/199). Eleven patients (5%) had PHLF Grade A, eight (4%) had PHLF Grade B, and four (2%) had PHLF Grade C. Two patients died of PHLF within 30 d after surgery, for a postoperative mortality rate of 1% (Table 2).
| Complication | Clavien–Dindo classification | Total (%) | ||||||
| I | II | IIIa | IIIb | IVa | IVb | V | ||
| Pleural effusion or ascites | 6 | 1 | 7 (3.5) | |||||
| Pneumonia | 1 | 1 | 2 (1.0) | |||||
| Biliary leakage | 1 | 5 | 6 (3.0) | |||||
| Arrhythmia | 1 | 1 (1.0) | ||||||
| Wound infection | 10 | 10 (5.0) | ||||||
| Intra-abdominal abscess | 1 | 8 | 9 (4.5) | |||||
| Intra-abdominal hemorrhage | 1 | 1 | 2 (1.0) | |||||
| PHLF | 8 | 5 | 5 | 2 | 1 | 2 | 23 (11.6) | |
| Total (%) | 25 (12.5) | 8 (4.0) | 21 (10.6) | 3 (1.5) | 1 (0.5) | 0 | 2 (1.0) | 60 (30.0) |
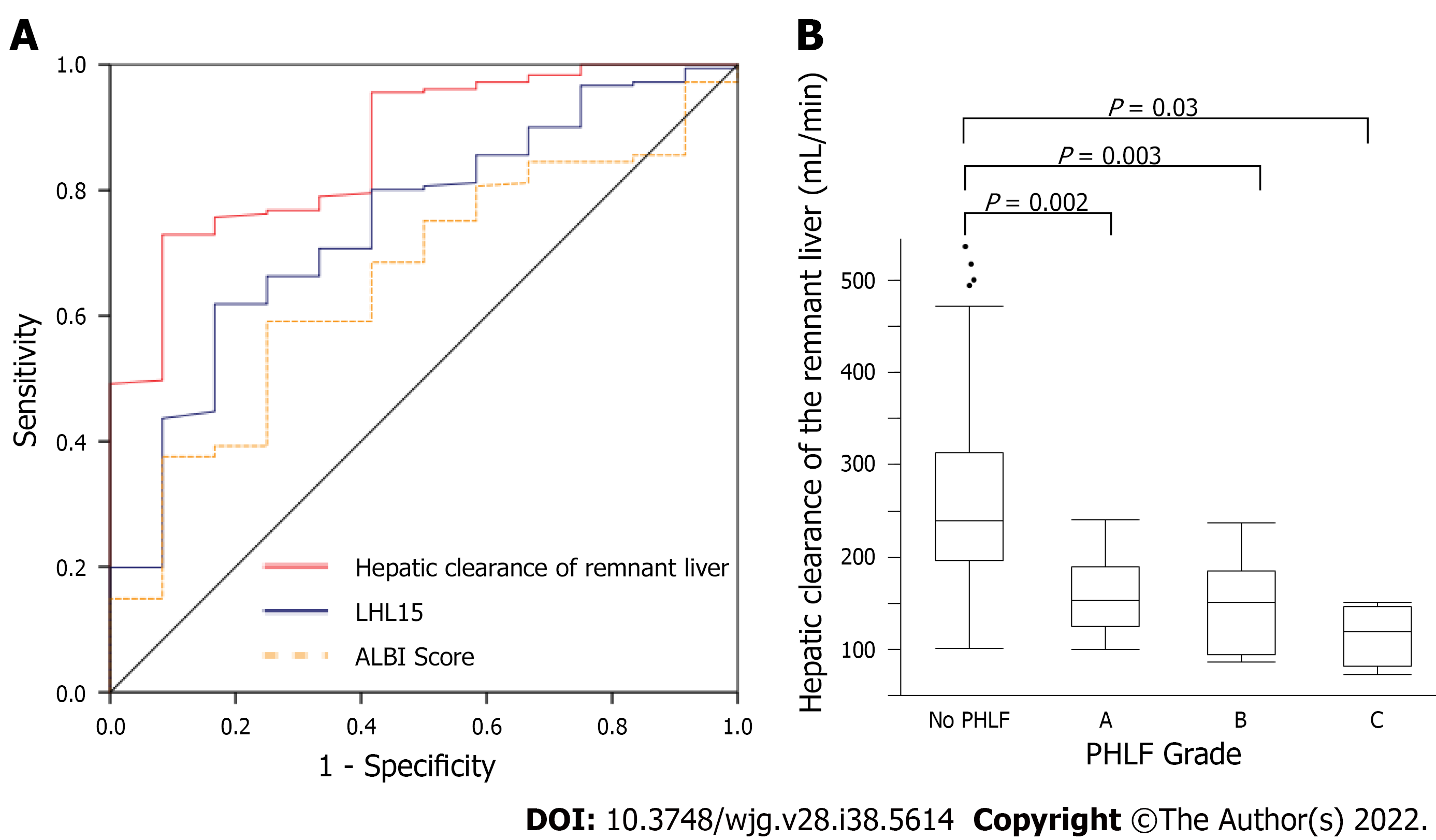
ROC curve analysis of hepatic clearance of the remnant liver, liver to heart-plus-liver radioactivity at 15 min (LHL15), and ALBI score were used to predict the risk of developing PHLF (Figure 2A). The area under the ROC curve for hepatic clearance of the remnant liver, LHL15, and ALBI score for predicting the development of PHLF were 0.868, 0.629, and 0.655, respectively. Hepatic clearance of the remnant liver had the highest area under the curve for predicting the development of PHLF. The cutoff values for predicting PHLF with highest sensitivity and specificity were 192 mL/min (sensitivity, 87.0%; specificity, 76.1%) for hepatic clearance of the remnant liver, 0.91 (sensitivity, 47.8%; specificity, 73.3%) for LHL15, 2.96 (sensitivity, 34.9%; specificity, 95.7%) for ALBI score. The relationship between hepatic clearance of the remnant liver and PHLF grade was evaluated (Figure 2B). The median hepatic clearances of the remnant liver were 239, 153, 150.5 and 119.5 mL/min for no PHLF, Grades A, B and C, respectively. The differences were significant for no PHLF and Grade A (P = 0.002), no PHLF and Grade B (P = 0.003), and no PHLF and Grade C (P = 0.02).
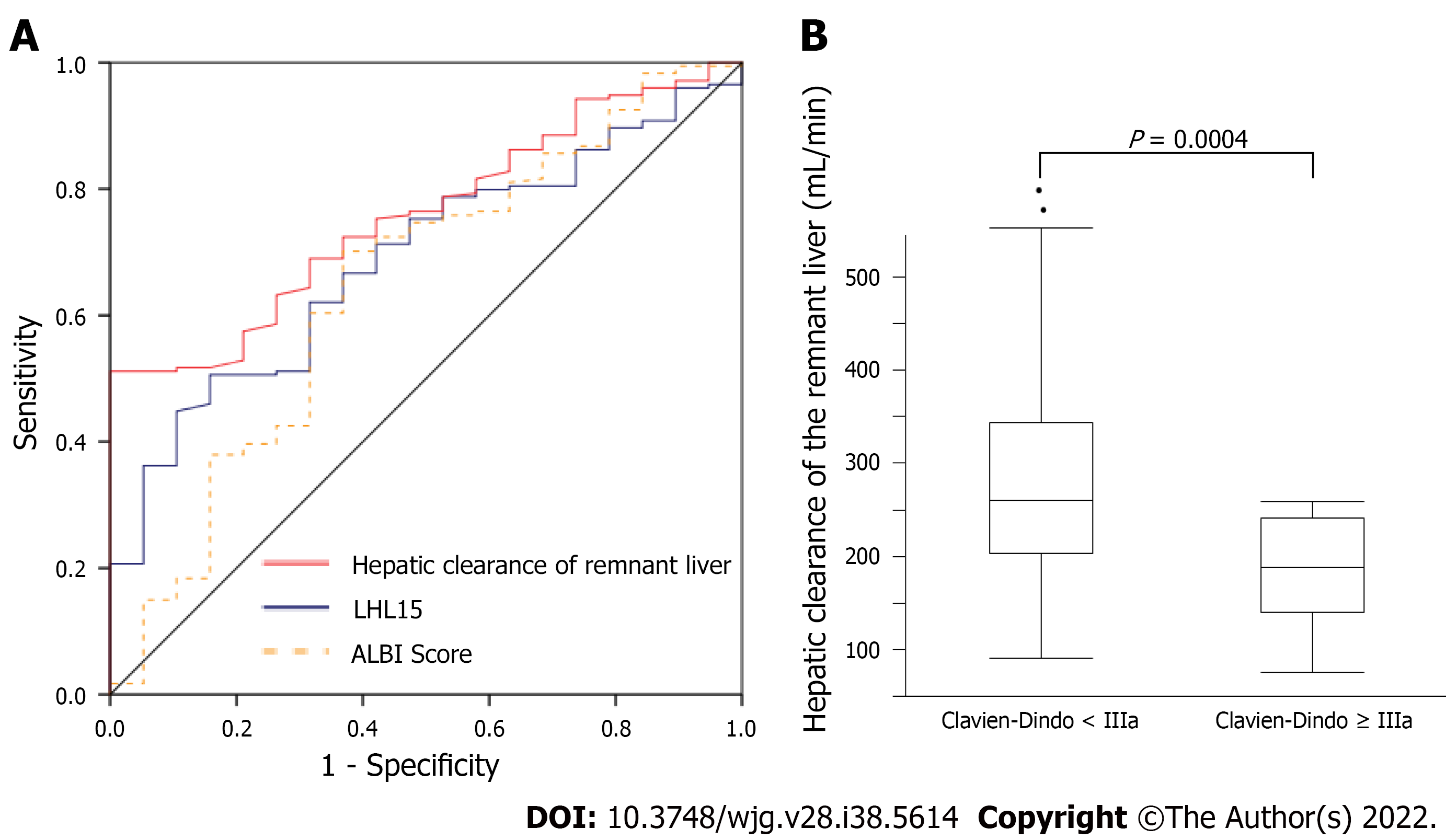
ROC curve analysis of hepatic clearance of the remnant liver, LHL15, and ALBI score were used to predict the risk of developing major complications (Figure 3A). The area under ROC curves for hepatic clearance of the remnant liver, LHL15, and ALBI score for predicting major complications were 0.758, 0.594, and 0.647, respectively. Hepatic clearance of the remnant liver had the highest area under the curve for predicting the development of major complications. The cutoff values for predicting PHLF with highest sensitivity and specificity were 237 mL/min (sensitivity, 100%; specificity, 51.9%) for hepatic clearance of the remnant liver, 0.94 (sensitivity, 84.2%; specificity, 36.2%) for LHL15, 2.63 (sensitivity, 69.3%; specificity, 63.2%) for ALBI score. The relationship between hepatic clearance of the remnant liver and Clavien–Dindo classification was evaluated (Figure 3B). The median hepatic clearances of the remnant liver were 238 and 179 mL/min for Clavien–Dindo < IIIa and Clavien–Dindo ≥ IIIa. The differences were significant for Clavien–Dindo < IIIa and Clavien–Dindo ≥ IIIa (P = 0.0004).
Multivariate regression analysis was performed between variables, with statistically significant differences following the univariate analysis regarding PHLF Grade B or C (Table 3). Hepatic clearance of the remnant liver [P = 0.001, odds ratio (OR): 0.973, 95% confidence interval (CI): 0.952–0.995] and intraoperative blood loss (P = 0.006, OR: 1.001, 95%CI: 1.0002–1.002) were independent risk factors for PHLF Grade B or C.
| Univariate analysis | Multivariate analysis | |||||
| P | OR | 95%CI | P | OR | 95%CI | |
| Age (yr) | 0.53 | 1.02 | 0.96-1.08 | |||
| Gender (male : female) | 0.59 | 0.67 | 0.10-2.66 | |||
| Child–Pugh class | 0.47 | 1.63 | 0.06-1.63 | |||
| PT-INR | 0.86 | 0.79 | 0.05-11.0 | |||
| AST | 0.34 | 0.99 | 0.98-1.00 | |||
| ALT | 0.14 | 0.99 | 0.98-1.00 | |||
| Choline Esterase | 0.24 | 1.00 | 0.99-1.01 | |||
| ALBI score | 0.15 | 0.37 | 0.09-1.41 | 0.375 | 3.31 | 0.37-29.3 |
| Operation time | 0.02 | 1.01 | 0.99-1.00 | 0.110 | 1.010 | 0.99-1.01 |
| Intraoperative blood loss | 0.001 | 1.0007 | 1.0003-1.0012 | 0.033 | 1.001 | 1.0002-1.002 |
| Hepatic clearance of the remnant liver | < 0.001 | 0.972 | 0.958-0.987 | 0.002 | 0.973 | 0.952-0.995 |
| Functional volume of the remnant liver | 0.002 | 0.997 | 0.994-0.999 | 0.351 | 0.998 | 0.995-1.00 |
| LHL15 | 0.02 | 52 | 2.03-1382 | 0.362 | 9.597 | 0.08-1211 |
Multivariate regression analysis was performed between variables, with significant differences following the univariate analysis regarding major complications (Table 4). Hepatic clearance of the remnant liver (P = 0.004, OR: 0.988, 95%CI: 0.979–0.999) and intraoperative blood loss (P = 0.005, OR: 1.0005, 95%CI: 1.0002–1.0014) were independent risk factors for developing major complications.
| Univariate analysis | Multivariate analysis | |||||
| P | OR | 95%CI | P | OR | 95%CI | |
| Age (yr) | 0.68 | 1.01 | 0.96-1.06 | |||
| Gender (male : female) | 0.69 | 0.80 | 0.27-2.36 | |||
| Child–Pugh class | 0.89 | 1.09 | 0.29-2.91 | |||
| PT-INR | 0.97 | 1.03 | 0.09-11.6 | |||
| AST | 0.27 | 0.99 | 0.98-1.00 | |||
| ALT | 0.11 | 0.99 | 0.98-1.00 | |||
| Cholinesterase | 0.58 | 1.00 | 0.99-1.01 | |||
| ALBI score | 0.02 | 3.57 | 1.19-10.7 | 0.790 | 1.22 | 0.286-5.19 |
| Operation time | 0.01 | 1.01 | 1.00-1.01 | 0.166 | 1.004 | 0.991-1.015 |
| Intraoperative blood loss | 0.001 | 1.0006 | 1.0002-1.001 | 0.005 | 1.0005 | 1.0002-1.0014 |
| Hepatic clearance of the remnant liver | < 0.001 | 0.986 | 0.978-0.994 | 0.004 | 0.988 | 0.979-0.999 |
| Functional volume of the remnant liver | 0.003 | 0.998 | 0.996-0.999 | 0.329 | 0.99 | 0.997-1.00 |
| LHL15 | 0.13 | 9.9 | 0.53-183 | 0.914 | 1.27 | 0.02-99.2 |
Hepatic clearance was associated with PHLF and major complications. The independent risk factors for developing PHLF and major complications were the hepatic clearance of the remnant liver, and intraoperative blood loss. The results of this study show that the measurement of hepatic clearance of the remnant liver is reliable for predicting the development of PHLF and major complications.
The results of this study support the use of hepatic clearance of the remnant liver, LHL15, and ALBI score for predicting the development of PHLF and postoperative major complications in patient with HCC. LHL15 and HH15, which are hepatic uptake and blood clearance ratios in GSA scintigraphy, are the most popular and widely used in many institutions. However, they may be insufficient for accurately estimating the degree of liver function because these indices are calculated from planar scintigraphic images, which do not correctly reflect hepatocyte volume[11]. In contrast, hepatic clearance measured by SPECT analysis contains volumetric information and may correctly estimate the hepatocyte volume[11]. LHL15 reflects the function of the whole liver, but the hepatic clearance of remnant liver shows the liver function of remnant liver, therefore, hepatic clearance may reflect functional reserve and short term outcome.
Many studies have investigated the relationship between GSA scintigraphy and PHLF. However, this is the first report comparing residual liver function and major complications using GSA scintigraphy. Patients with lower remnant liver function are at higher risk for PHLF, morbidity and mortality. The quality and volume of the postoperative remnant liver have been shown to be associated with postoperative outcomes[22]. Surgeons should emphasize the remnant liver functional reserve rather than the resected liver volume[12,23]. Elevation of serum bilirubin and PT-INR are associated with morbidity and mortality, regardless of the extent of resection[24]. Liver failure after limited resection can develop. Patients with reduced reserve of the remnant liver are at higher risk for the development of PHLF and major complications[22,25,26]. The extent of surgery should be considered to preserve as much liver function as possible. Moreover PHLF grade C is most severe types of liver failure that may lead to in-hospital death[24]. In ROC curve analysis, the cutoff line of PHLF grade C was 151 mL/min (sensitivity 87.5%, specificity 100%). Patients below the cutoff line should be given special consideration by surgeons before surgery and may not be ideal candidates for hepatic resection. Hepatic clearance of the remnant liver below 100 mL/min is associated with a high mortality rate. Therefore, PTPE should be performed when hepatic clearance of the remnant liver is below 100 mL/min, and surgery should be considered when the clearance is above 100 mL/min. In addition, if hepatic clearance of the remnant liver is greater than 100 mL/min preoperatively, unnecessary PTPE can be avoided.
The risk for developing PHLF and major complications is determined by patient and surgical factors. Intraoperative blood loss is a well-known risk factor for morbidity and mortality after hepatic resection[25,27-30]. Hemorrhage can lead to the development of metabolic acidosis as a consequence of intracellular derangements in oxygen and substrate utilization[29]. Reduced levels of cytokines and humoral factors, such as interleukin-6, hepatocyte growth factors, and growth hormone after extensive blood loss may result in decreased liver regeneration because of loss of growth factors needed for regeneration[28].
The present study had some limitations, including a retrospective design, and being a single center study. Preoperative GSA scintigraphy was routinely performed to estimate total liver function in this hospital. Total liver function, remnant liver function, laboratory data, and liver failure were objectively assessed in advance, which limited the risk of observation bias. Prospective multicenter trials are needed to validate the results of this study.
Lower functional reserve of the remnant liver results in a higher risk of developing PHLF and major complications in patients undergoing resection of HCC. The estimation of hepatic clearance of the remnant liver may provide guidance for determining the extent of resection in a patient-specific manner.
Preoperative assessment of liver function is important to reduce the rate of complications.
Few studies evaluate the relationship of residual liver function to complications and prognosis using hepatic clearance.
To measure hepatic clearance of the remnant liver using 99mTc-galactosyl serum albumin (GSA) scintigraphy, single photon emission computed tomography, to elucidate the association between residual liver function and morbidity and mortality, and to identify risk factors for those factors.
We collected data from 199 patients who underwent resection of hepatocellular carcinoma. Hepatic clearance of the remnant liver was measured using fusion images of 99mTc-labelled GSA liver scintigraphy and computed tomography scans. Risk factors were determined using logistic regression multivariate analysis.
Risk factors for posthepatectomy liver failure, morbidity, and mortality were low hepatic clearance of the remnant liver and intra-operative bleeding.
Lower residual liver function is associated with a poor short-term prognosis.
Preoperative estimation of remnant liver function is useful to determine the surgical approach.
I especially thank Michio Ashizaki for helping patient data acquisition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali SNH, Jordan; Finotti M, Italy S-Editor: Chang KL L-Editor: Kerr C P-Editor: Chang KL
| 1. | Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, Sugimachi K. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Huang J, Hernandez-Alejandro R, Croome KP, Zeng Y, Wu H, Chen Z. Hepatic resection for huge (>15 cm) multinodular HCC with macrovascular invasion. J Surg Res. 2012;178:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Wang YY, Zhao XH, Ma L, Ye JZ, Wu FX, Tang J, You XM, Xiang BD, Li LQ. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Tomimaru Y, Eguchi H, Gotoh K, Kawamoto K, Wada H, Asaoka T, Noda T, Yamada D, Ogawa H, Umeshita K, Nagano H, Doki Y, Mori M. Platelet count is more useful for predicting posthepatectomy liver failure at surgery for hepatocellular carcinoma than indocyanine green clearance test. J Surg Oncol. 2016;113:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Park HJ, Seo KI, Kim SJ, Lee SU, Yun BC, Han BH, Shin DH, Choi YI, Moon HH. Effectiveness of Albumin-bilirubin Score as a Predictor of Post-hepatectomy Liver Failure in Patients with Hepatocellular Carcinoma. Korean J Gastroenterol. 2021;77:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Sugahara K, Togashi H, Takahashi K, Onodera Y, Sanjo M, Misawa K, Suzuki A, Adachi T, Ito J, Okumoto K, Hattori E, Takeda T, Watanabe H, Saito K, Saito T, Sugai Y, Kawata S. Separate analysis of asialoglycoprotein receptors in the right and left hepatic lobes using Tc-GSA SPECT. Hepatology. 2003;38:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Akaki S, Okumura Y, Sasai N, Sato S, Tsunoda M, Kuroda M, Kanazawa S, Hiraki Y. Hepatectomy simulation discrepancy between radionuclide receptor imaging and CT volumetry: influence of decreased unilateral portal venous flow. Ann Nucl Med. 2003;17:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Li XF, Taki J, Kinuya S, Higuchi T, Konishi S, Hwang EH, Shuke N, Nakajima K, Tonami N. Asialoglycoprotein receptor concentration in tumor-bearing livers and its fate early after their sectorial resection. Ann Nucl Med. 2003;17:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Iimuro Y, Kashiwagi T, Yamanaka J, Hirano T, Saito S, Sugimoto T, Watanabe S, Kuroda N, Okada T, Asano Y, Uyama N, Fujimoto J. Preoperative estimation of asialoglycoprotein receptor expression in the remnant liver from CT/99mTc-GSA SPECT fusion images correlates well with postoperative liver function parameters. J Hepatobiliary Pancreat Sci. 2010;17:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Taniguchi M, Okizaki A, Watanabe K, Imai K, Uchida K, Einama T, Shuke N, Miyokawa N, Furukawa H. Hepatic clearance measured with (99m)Tc-GSA single-photon emission computed tomography to estimate liver fibrosis. World J Gastroenterol. 2014;20:16714-16720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Yumoto Y, Yagi T, Sato S, Nouso K, Kobayashi Y, Ohmoto M, Yumoto E, Nagaya I, Nakatsukasa H. Preoperative estimation of remnant hepatic function using fusion images obtained by (99m)Tc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Br J Surg. 2010;97:934-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Ha-Kawa SK, Tanaka Y, Hasebe S, Kuniyasu Y, Koizumi K, Ishii Y, Yamamoto K, Kashiwagi T, Ito A, Kudo M, Ikekubo K, Tsuda T, Murase K. Compartmental analysis of asialoglycoprotein receptor scintigraphy for quantitative measurement of liver function: a multicentre study. Eur J Nucl Med. 1997;24:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Harada K, Mizuguchi T, Katagiri Y, Kawamoto M, Nakamura Y, Meguro M, Ota S, Sasaki S, Miyanishi K, Sonoda T, Mori M, Shinomura Y, Kato J, Hirata K. Area between the hepatic and heart curves of (99m)Tc-galactosyl-human serum albumin scintigraphy represents liver function and disease progression for preoperative evaluation in hepatocellular carcinoma patients. J Hepatobiliary Pancreat Sci. 2012;19:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Shuke N, Okizaki A, Kino S, Sato J, Ishikawa Y, Zhao C, Kinuya S, Watanabe N, Yokoyama K, Aburano T. Functional mapping of regional liver asialoglycoprotein receptor amount from single blood sample and SPECT. J Nucl Med. 2003;44:475-482. [PubMed] |
| 16. | Kwon AH, Ha-Kawa SK, Uetsuji S, Inoue T, Matsui Y, Kamiyama Y. Preoperative determination of the surgical procedure for hepatectomy using technetium-99m-galactosyl human serum albumin (99mTc-GSA) liver scintigraphy. Hepatology. 1997;25:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Kwon AH, Ha-Kawa SK, Uetsuji S, Kamiyama Y, Tanaka Y. Use of technetium 99m diethylenetriamine-pentaacetic acid-galactosyl-human serum albumin liver scintigraphy in the evaluation of preoperative and postoperative hepatic functional reserve for hepatectomy. Surgery. 1995;117:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Sawamura T, Kawasato S, Shiozaki Y, Sameshima Y, Nakada H, Tashiro Y. Decrease of a hepatic binding protein specific for asialoglycoproteins with accumulation of serum asialoglycoproteins in galactosamine-treated rats. Gastroenterology. 1981;81:527-533. [PubMed] |
| 19. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002;4:99; author reply 99-99; author reply100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24856] [Article Influence: 1183.6] [Reference Citation Analysis (0)] |
| 21. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 22. | Safiri S, Ayubi E. Comments on combining albumin-bilirubin score with future liver remnant predicts posthepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018;38:761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, Caridi J. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, Büchler MW, Weitz J. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Kokudo N, Vera DR, Tada K, Koizumi M, Seki M, Matsubara T, Ohta H, Yamaguchi T, Takahashi T, Nakajima T, Muto T. Predictors of successful hepatic resection: prognostic usefulness of hepatic asialoglycoprotein receptor analysis. World J Surg. 2002;26:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Torizuka K, Ha-Kawa SK, Kudo M, Kubota Y, Yamamoto K, Itoh K, Nagao K, Uchiyama G, Koizumi K, Sasaki Y. [Phase III multi-center clinical study on 99mTc-GSA, a new agent for functional imaging of the liver]. Kaku Igaku. 1992;29:159-181. [PubMed] |
| 27. | Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Shirabe K, Motomura T, Takeishi K, Morita K, Kayashima H, Taketomi A, Ikegami T, Soejima Y, Yoshizumi T, Maehara Y. Human early liver regeneration after hepatectomy in patients with hepatocellular carcinoma: special reference to age. Scand J Surg. 2013;102:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Cucchetti A, Siniscalchi A, Ercolani G, Vivarelli M, Cescon M, Grazi GL, Faenza S, Pinna AD. Modification of acid-base balance in cirrhotic patients undergoing liver resection for hepatocellular carcinoma. Ann Surg. 2007;245:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |