Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5589
Peer-review started: June 8, 2022
First decision: September 2, 2022
Revised: September 10, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: October 14, 2022
Processing time: 125 Days and 23.9 Hours
The prognosis of gastric cancer in an advanced stage remains poor. The exact efficacy of the use of intraoperative sustained-release chemotherapy with 5-fluorouracil (5-FU) in advanced-stage gastric cancer is still unelucidated.
To explore the long-term survival benefit of using sustained-release 5-FU implants in stage II and stage III gastric cancer patients.
Patients with gastric cancer in a locally advanced stage and who underwent an R0 radical resection between Jan 2014, to Dec 2016, in this single institution were included. Patients with pathological diagnoses other than adenocarcinoma were excluded. All included patients were grouped according to whether intraoperative sustained-release (SR) chemotherapy with 5-FU was used or not (NSR). The primary end-point was 5-year overall survival. Kaplan–Meier method with log-rank test was used to analyze the overall survival of patients and Cox analysis was used to analyze prognosis factors of these patients.
In total, there were 563 patients with gastric cancer with locally advanced stage, who underwent an R0 radical resection. 309 patients were included in the final analysis. 219 (70.9%) were men, with an average age of 58.25 years. Furthermore, 56 (18.1%) received neoadjuvant chemotherapy, and 191 (61.8%) were in TNM stage III. In addition, 158 patients received intraoperative sustained-release chemotherapy with 5-FU and were included in the SR group, while the other 161 patients were included in the NSR group. The overall complication rate was 12.94% in the whole group and 10.81%, 16.46% in SR and NSR groups, respectively. There were no significant differences between the two groups in overall survival and complication rate (P > 0.05). The multivariate cox analysis indicated that only N Stage and neoadjuvant therapy were independent influencing factors of survival.
Intraoperative sustained-release chemotherapy usage with 5-FU, did not improve the survival of patients who underwent an R0 radical resection in locally advanced stage of gastric cancer.
Core Tip: Intraoperative drug administration shows promise in preventing the recurrence of gastric cancer. Sustained-release 5-fluorouracil (5-FU) implant is a new convenient and continuous drug release method that ensures locally high drug concentration for approximately 1 mo. 5-FU implants are widely used to treat various gastric tumors. However, presently, only retrospective research and a small-scale clinical study conducted in China have reported on its use in patients with gastric cancer. In our real-world study, we collected complete datasets, making this the largest study in China. Unfortunately, 5-FU implants did not improve the long-term survival of gastric cancer patients.
- Citation: Wu YZ, Wu M, Zheng XH, Wang BZ, Xue LY, Ding SK, Yang L, Ren JS, Tian YT, Xie YB. No long-term survival benefit with sustained-release 5-fluorouracil implants in patients with stages II and III gastric cancer. World J Gastroenterol 2022; 28(38): 5589-5601
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5589
Gastric cancer is the fifth most common newly diagnosed cancer and the third most common cause of cancer mortality worldwide[1-3]. In China, there are approximately 400000 new patients with gastric cancer, and nearly 300000 people die of gastric cancer every year[4]. At present, locally advanced gastric cancer with the American Joint Committee on Cancer TNM stages II and III accounts for the majority of these cases[5]. For locally-advanced gastric cancer patients, the current standard treatment is D2 gastrectomy, followed by adjuvant chemotherapy[6]. However, even in those who received standard treatment, 30–60% of them may relapse either locally or distantly[7].
Patients with stages II and III gastric cancer have been found to have a tendency to relapse after treatment[8]. According to the findings of a study by Yago et al[9], the 5-year recurrence-free survival rate was 88.3% for stage IIA, 73.8% for stage IIB, 67.4% for stage IIIA, 55.7% for stage IIIB, and 29.9% for stage IIIC. Of the three major recurrence patterns namely hematogenous, peritoneal, and lymph nodal recurrences after curative gastrectomy, the latter two account for the majority of the cases[10-13]. In order to decrease local recurrence after resection, methods, such as extended peritoneal lavage[14], hyperthermic intraperitoneal perfusion chemotherapy[15], peritoneal lavage with 5-FU, and continuous intraperitoneal chemotherapy using a retained tube or pump, have been developed[16]. Furthermore, it has been reported that intraoperative intraperitoneal chemotherapy can reduce the mortality risk[17]. In addition, increasing the temperature of chemotherapeutic drugs has a synergistic effect with the increase of intraperitoneal chemotherapy. The CYTO-CHIP study group reported that compared with the routine method of cytoreductive surgery combined with hyperthermic intraperitoneal chemo
Moreover, optimistic results concerning the use of sustained-release 5-FU implants in the treatment of gastric cancer have been shown. Recently, a multi-center, randomized, open-label, controlled clinical study showed that for cTNM stage III gastric cancer patients, the use of intraoperative 5-FU implants, combined with postoperative adjuvant chemotherapy, may reduce the risk of peritoneal recurrence and prolong progression-free survival significantly[22]. Another retrospective study with 5-FU implants in advanced gastric cancer patients showed that the use of 5-FU implants may improve 5-years overall survival (OS) and progression-free survival rates after surgery in gastric cancer patients[21]. However, these studies had limitations. The sample size of these studies was limited, the follow-up times were insufficient, and the rate of loss of follow-up was extremely high. In order to explore the long-term survival benefit of the use of sustained-release 5-FU implants, we conducted a real-world study using our own clinical data from a single institution.
In total, we treated 563 stages II and III gastric cancer patients between January 2014 and December 2016. The inclusion criteria were the presence of clinical TNM stage II and stage III, pathologically diagnosed adenocarcinoma of the stomach, a complete record of history, and age between 18–80 years. The exclusion criteria were the presence of gastric remnant cancer, distant metastases, positive peritoneal cytology, palliative resection, and any unsuitable condition for 5-FU chemotherapy. The primary clinical outcome was 5-year OS. Information on the patients’ age, sex, body mass index, tumor (TNM Stage, Borrmann classification, Lauren classification, pathological classification, differentiation, number of resected lymph nodes, number of positive lymph nodes, as well as the extent of nerve invasion, and presence of a vascular tumor thrombus), treatment (resections, neoadjuvant therapy, and use of 5-FU implants), postoperative complications (pulmonary infections, anastomotic fistulas, bleeding, abdominal infections, intestinal obstructions, and admissions to the intensive care unit), and their follow-up results were extracted from our hospital’s electronic records.
All our patients underwent a standard D2 gastric resection with or without neoadjuvant therapy. In addition, all the patients received 6–8 cycles of adjuvant chemotherapy with an S-1 + oxaliplatin regimen. In the sustained-release (SR) group, sustained-release 5-FU implants in a fixed dosage of 1000 mg were placed intraoperatively near the tumor bed after resection. Other patients were divided into non-sustained-release (NSR) group. The placement of 5-FU implants near vessels and anastomotic stoma should be avoided.
The continuous variables were presented as central tendencies (means or medians) and dispersions (standard deviations or interquartile ranges). For the group comparisons of the numeric variables, the Student’s t-test was used when the data were normally distributed, and the Mann–Whitney test for the variables in which distribution was not normal. When the categorical predictors were compared between the groups, we used Pearson’s χ2 test or Fisher’s exact test. The survival analysis included the use of the Kaplan–Meier estimator for OS. A Cox regression analysis was performed to obtain the crude and adjusted hazard ratios for OS. Significance level for all tests was reached when the two-tailed P value was < 0.05. Statistical analyzes were performed using the IBM SPSS Statistics (version 26.0, IBM Inc., Armonk, NY, United States), and Prism software (version 9, GraphPad Software Inc., San Diego, CA, United States).
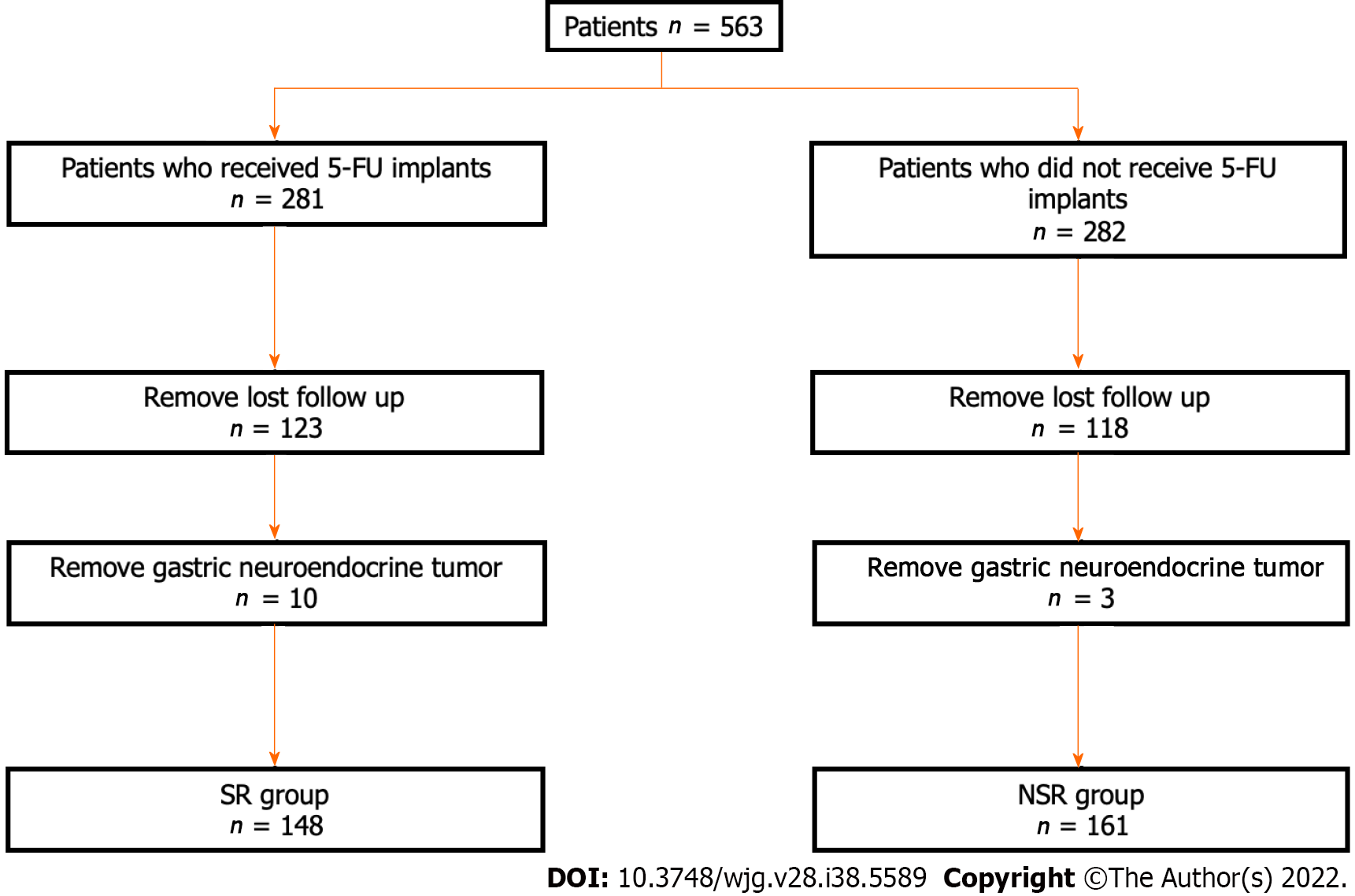
In total, 241 patients with incomplete data and 13 patients with a pathological diagnosis of gastric neuroendocrine tumor were excluded. Finally, 309 patients were included in this study, and they were divided into two groups according to whether sustained-release 5-FU was used or not. Therefore, there were 158 patients in the SR group and 164 patients in the NSR group (Figure 1).
The average age of the whole group was 58.25 ± 11.2 years, with male patients being the majority (70.9%). All the patients were diagnosed pathologically as having a stomach adenocarcinoma of TNM stages II and III. Furthermore, 32.4% of patients had signet ring cell carcinoma. All of the patients underwent an R0 resection surgery. As shown in Table 1, we compared the differences in the clinicopathological characteristics between the two patient cohorts. There were no significant differences between the two groups with respect to age, sex, body mass index, and TNM stage. Similarly, there were no differences between the two groups with respect to their pathological characteristics such as the Borrmann classification, Lauren classification, and differentiation. Although the number of resected lymph nodes in the SR group was higher than that in the NSR (P = 0.0145), the number of positive lymph nodes number was similar (P = 0.2319). Lymph node dissection was satisfactory in both groups, even though there was a gap between the two groups (Table 1).
| SR group (n = 148) | NSR group (n =161) | P value | |
| Age (yr) | 57.15 ± 10.71 | 59.27 ± 11.52 | 0.2917 |
| Gender, n (%) | 0.781 | ||
| Male | 106 (71.6) | 113 (70.2) | |
| Female | 42 (28.4) | 48 (29.8) | |
| BMI (kg/m2) | 23.97 ± 3.591 | 23.68 ± 3.488 | 0.9716 |
| Neoadjuvant therapy, n (%) | 0.52 | ||
| Yes | 29 (19.6) | 27 (16.8) | |
| No | 119 (80.4) | 134 (83.2) | |
| Cardiovascular disease, n (%) | 0.503 | ||
| Yes | 32 (21.6) | 40 (24.8) | |
| No | 116 (78.4) | 121 (75.2) | |
| Diabetes, n (%) | 0.093 | ||
| Yes | 10 (6.8) | 20 (12.4) | |
| No | 138 (93.2) | 141 (87.6) | |
| T stage, n (%) | 0.699 | ||
| T1 and T2 | 23 (15.5) | 21 (13.0) | |
| T3 and T4 | 96 (64.9) | 113 (70.2) | |
| yp T1 and T2 | 5 (3.4) | 3 (1.9) | |
| yp T3 and T4 | 24 (16.2) | 24 (14.9) | |
| N stage, n (%) | 0.533 | ||
| N0 and N1 | 44 (29.7) | 51 (31.7) | |
| N2 and N3 | 75 (50.7) | 83 (51.6) | |
| yp N0 and yp N1 | 11 (7.4) | 15 (9.3) | |
| yp N2 and yp N3 | 18 (12.2) | 12 (7.5) | |
| M stage, n (%) | > 0.999 | ||
| M0 | 148 (100.00) | 161 (100) | |
| TNM stage, n (%) | 0.91 | ||
| Stage II | 57 (38.5) | 61 (37.9) | |
| Stage III | 91 (61.5) | 100 (62.1) | |
| Borrmann classification, n (%) | 0.207 | ||
| Superficial type | 3 (2.0) | 1 (0.6) | |
| Type I | 10 (6.8) | 9 (5.6) | |
| Type II | 41 (27.7) | 36 (22.4) | |
| Type III | 73 (49.3) | 78 (48.4) | |
| Type IV | 2 (1.4) | 9 (5.6) | |
| NA | 19 (12.8) | 28 (17.4) | |
| Lauren classification, n (%) | 0.782 | ||
| Intestinal type | 42 (28.4) | 48 (29.8) | |
| Diffuse type | 64 (43.2) | 69 (42.9) | |
| Mixed type | 34 (23.0) | 39 (24.2) | |
| NA | 8 (5.4) | 5 (3.1) | |
| Differentiation, n (%) | 0.666 | ||
| Low | 88 (59.5) | 91 (56.5) | |
| Low-medium | 36 (24.3) | 39 (24.2) | |
| Medium | 19 (12.8) | 26 (16.1) | |
| Medium-high and high | 2 (1.4) | 4 (2.5) | |
| NA | 3 (2.0) | 1 (0.6) | |
| Nerve invasion, n (%) | 0.26 | ||
| Yes | 73 (49.3) | 92 (57.1) | |
| No | 24 (16.2) | 27 (16.8) | |
| NA | 51 (34.5) | 42 (26.1) | |
| Vascular tumor thrombus, n (%) | 0.23 | ||
| Yes | 57 (38.5) | 57 (38.5) | |
| No | 40 (27.0) | 54 (33.5) | |
| NA | 51 (34.5) | 42 (26.1) | |
| Pathological classification, n (%) | 0.481 | ||
| Signet-ring cell carcinoma | 45 (30.4) | 55 (34.2) | |
| Another adenocarcinoma | 103 (69.6) | 106 (65.8) | |
| Resection type, n (%) | 0.057 | ||
| Total gastrectomy | 53 (35.8) | 54 (33.5) | |
| Proximal gastrectomy | 7 (4.7) | 20 (12.4) | |
| Distal gastrectomy | 88 (59.5) | 87 (54.0) | |
| R0 resection | 148 (100.00) | 161 (100) | > 0.999 |
| Resected lymph nodes number | 39.21 ± 14.13 | 35.73 ± 13.53 | 0.0145 |
| Positive lymph nodes number | 7.393 ± 8.864 | 6.856 ± 9.743 | 0.2319 |
Table 2 shows the major postoperative complications, such as pulmonary inflections, anastomotic fistulas, postoperative bleeding, abdominal infections, and intestinal obstructions. There were no significant differences between the two groups with respect to the incidence of postoperative complications (P > 0.05).
| SR group (n = 148), n (%) | NSR group (n = 161), n (%) | P value | |
| Pulmonary infection | 3 (2.0) | 6 (3.8) | 0.583 |
| Anastomotic fistula | 6 (4.0) | 6 (3.8) | 0.882 |
| Postoperative bleeding | 1 (0.7) | 3 (1.9) | 0.675 |
| Abdominal infection | 3 (2.0) | 9 (5.6) | 0.105 |
| Intestinal obstruction | 3 (2.0) | 1 (0.6) | 0.556 |
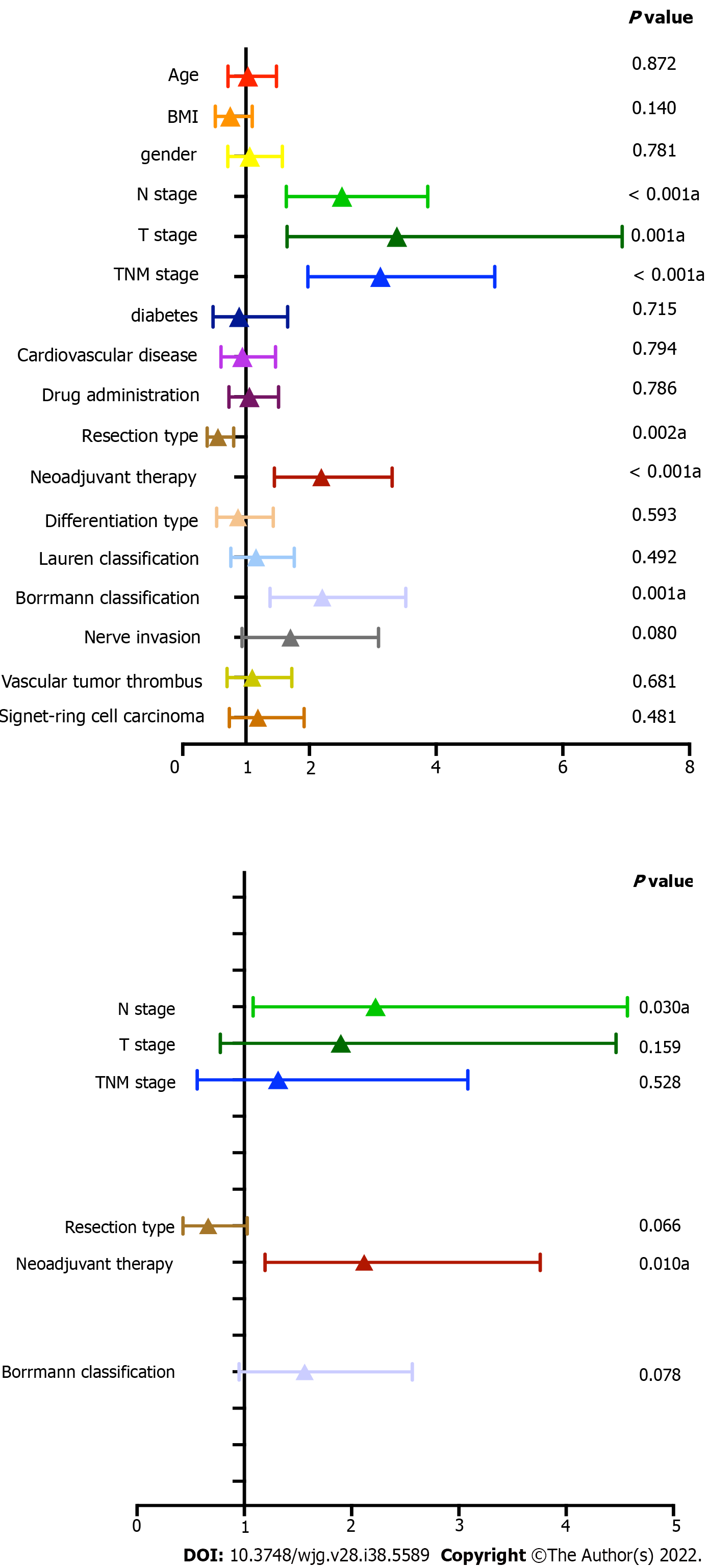
Figure 2 shows the results of the univariate and multivariate Cox analyses. From the univariate analysis, N stage (P < 0.001), T stage (P = 0.001), TNM stage (P < 0.001) neoadjuvant therapy (P < 0.001), Borrmann classification (P = 0.001), and resection type (P = 0.002) were found to be influencing prognostic factors in this group of patients. As per the multivariate Cox analysis, only N stage (P = 0.030) and neoadjuvant therapy were found to be independent influencing prognostic factors (Figure 2 and Table 3). The use of sustained-release 5-FU implants was not an independent influencing prognostic factor in this group (P = 0.786).
| Univariate analysis | Multivariate analysis | |||||
| Characteristics | HR | 95%CI | P value | HR | 95%CI | P value |
| Gender | ||||||
| Male | Reference | |||||
| Female | 1.058 | 0.712-1.572 | 0.781 | |||
| Age, yr | ||||||
| < 60 | Reference | |||||
| ≥ 60 | 1.03 | 0.717-1.481 | 0.872 | |||
| Neoadjuvant therapy | ||||||
| No | Reference | |||||
| Yes | 2.188 | 1.448-3.306 | < 0.001 | 2.118 | 1.194-3.759 | 0.01 |
| BMI | ||||||
| Normal < 24 | Reference | |||||
| Abnormal ≥ 24 | 0.752 | 0.516-1.098 | 0.14 | |||
| Cardiovascular disease | ||||||
| No | Reference | |||||
| Yes | 0.943 | 0.606-1.467 | 0.794 | |||
| Diabetes | ||||||
| No | Reference | |||||
| Yes | 0.891 | 0.479-1.658 | 0.715 | |||
| Drug administration | ||||||
| No | Reference | |||||
| Yes | 1.052 | 0.731-1.513 | 0.786 | |||
| Resection type | ||||||
| Total gastrectomy | Reference | |||||
| Partial gastrectomy | 0.558 | 0.387-0.804 | 0.002 | 0.663 | 0.428-1.027 | 0.066 |
| Borrmann classification | ||||||
| Superficial type, Type I and II | Reference | |||||
| Type III and IV | 2.203 | 1.378-3.522 | 0.001 | 1.562 | 0.951-2.566 | 0.078 |
| Lauren classification | ||||||
| Intestinal type | Reference | |||||
| Others | 1.158 | 0.762-1.762 | 0.492 | |||
| Differentiation type | ||||||
| Low | Reference | |||||
| Others | 0.874 | 0.535-1.430 | 0.593 | |||
| TNM stage | ||||||
| Stage II | Reference | |||||
| Stage III | 3.12 | 1.977-4.926 | < 0.001 | 1.315 | 0.561-3.084 | 0.528 |
| T stage | ||||||
| T1 and T2 | Reference | |||||
| T3 and T4 | 3.382 | 1.649-6.937 | 0.001 | 1.9 | 0.777-4.645 | 0.159 |
| N stage | ||||||
| N0 and N1 | Reference | |||||
| N2 and N3 | 2.516 | 1.636-3.869 | < 0.001 | 2.224 | 1.082-4.572 | 0.03 |
| Signet-ring cell carcinoma | ||||||
| Yes | Reference | |||||
| No | 1.188 | 0.736-1.916 | 0.481 | |||
| Nerve invasion | ||||||
| No | Reference | |||||
| Yes | 1.703 | 0.938-3.093 | 0.08 | |||
| Vascular tumor thrombus | ||||||
| No | Reference | |||||
| Yes | 1.099 | 0.701-1.722 | 0.681 | |||
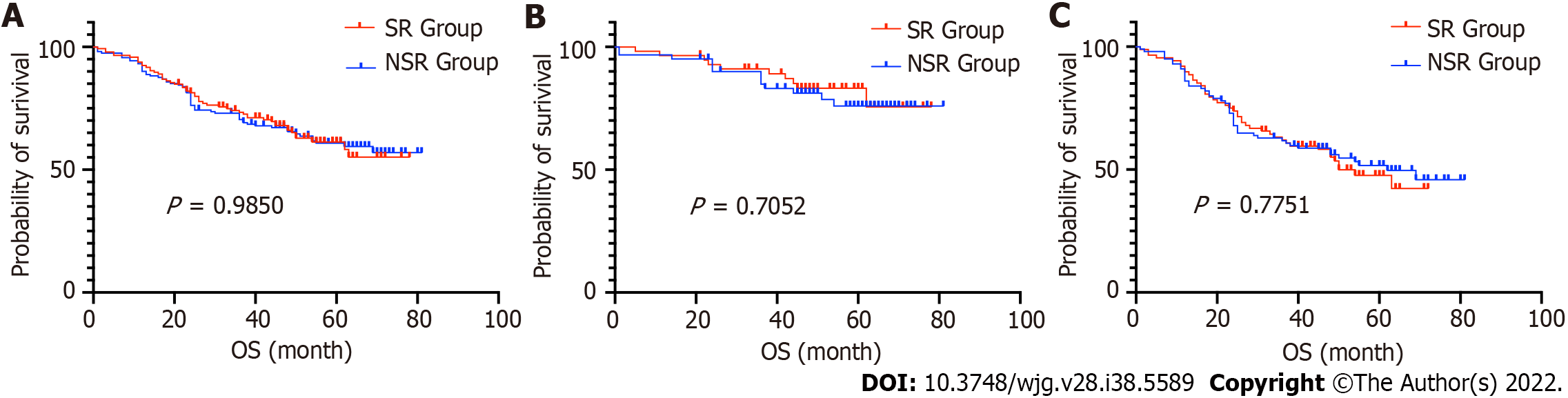
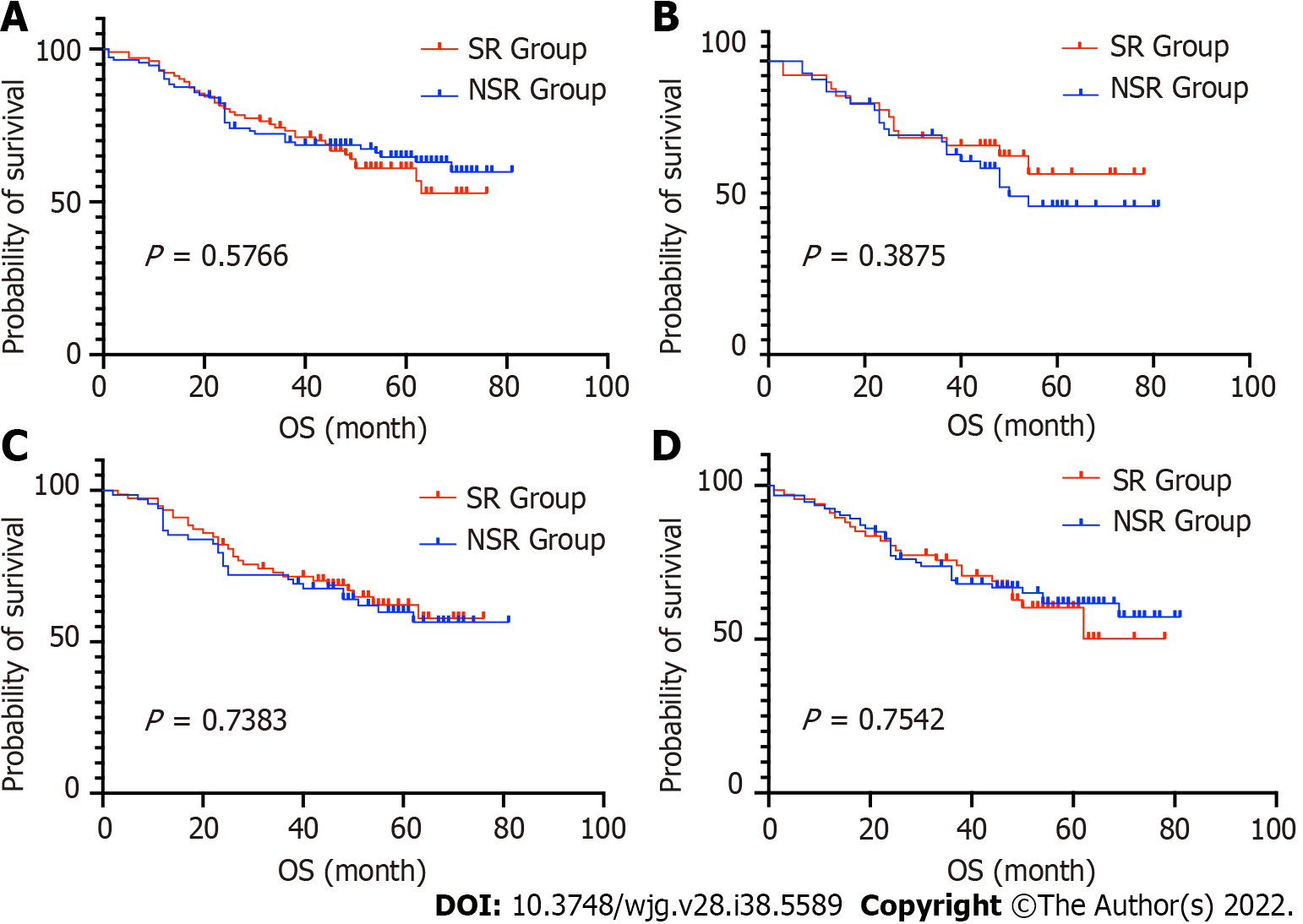
The 5-years OS rate of the SR and NSR groups were 68.2% and 67.9%, respectively (P = 0.9850). The median survival time for both groups was not obtained, as patient mortality numbers were too low to calculate. Furthermore, we performed subgroup comparisons according to the patient's age, sex, and TNM stage. There were no significant differences in any of the subgroups (P > 0.05) (Figures 3 and 4, Table 3).
Gastric cancer remains one of the most lethal cancers in China[23,24]. Almost 70% of the relapses occur within 2 years and more than 90% within 5 years[7,25]. In clinical practice, the recurrence patterns are classified as locoregional, peritoneal, and hematogenous. As reported by Wu, approximately all of the recurrences (99.5%) occurred within 7 years after the surgery, with 53.5% having had peritoneal dissemination, 43.3% hematogenous metastases, and 28.6% distant lymphatic spread[26]. The locoregional and peritoneal recurrences, which usually occur in gastric cancer patients, were found to be a critical problem. Peritoneal recurrence has been reported as the most common recurrence pattern in locally advanced gastric cancer patients, which accounts for 50% of the cases and occurs within 2 years after surgery[27]. In another study, almost 60% of the gastric cancer patients experienced a recurrence, which included 32.4% locoregional recurrences, 13.7% peritoneal metastases, and 44.3% distant metastases[28].
To reduce locoregional recurrences, intraperitoneal chemotherapy has been used widely worldwide. Different from other methods, the use of intraoperative sustained-release 5-FU is promising because of the convenient usage and a long effective time of approximately 1 mo. The use of sustained-release 5-FU implants has shown survival benefits in different types of digestive system tumors such as colon cancer and primary hepatic cellular cancer[29,30]. Furthermore, a few studies on the treatment of gastric cancer, which showed similar results, were also reported by Chinese researchers[21,22]. However, P values of survival benefits obtained in previous studies on gastric cancer were lower, but close to 0.05. Therefore, we conducted this real-world study to confirm the findings.
In the present study, the use of sustained-release 5-FU implants did not improve the long-term survival of gastric cancer patients with either TNM stage II or III. The estimated 5-year survival of patients with gastric cancer with TNM stage III in SR and NSR group was 50% and 49%, respectively (P = 0.775), slightly higher than that of previous studies[31,32]. This may have been due to the R0 radical resection decreasing the risk of local-regional recurrence and compromising the effects of the sustained-release drug. Therefore, we may hypothesize that in cases of incomplete resections, such as an R1 resection, or if the lymph node dissection did not reach the D2 resection standard, the use of a sustained-release drug may improve survival. Previous results of drug use in the treatment of unresectable tumors, such as pancreatic cancer and colorectal cancer, provided evidence for this hypothesis[33].
Similar to the findings of previous studies, the use of the sustained-release 5-FU implants did not increase postoperative complications, and systemic toxicity was rare in the present study. The use of 5-FU implants is usually recommended in gastric cancer patients, particularly at TNM stage T4 or N1–3[34]. In our study, the selection criteria were based on preoperative image examinations. Patients treated with 5-FU implants were in stage II and III, which is in accordance with the recommendations from the Chinese expert consensus[34].
Our study had some limitations. First, the sample size was insufficient. However, to date it remains the largest group studied. Second, selection biases existed. Many patients had incomplete history records, which may have contributed to a contrary result. Finally, the exact relapse time and the first site of recurrence were recorded incompletely. Owing to a lack of analysis of the relapses, the results were compromised. In order to understand the survival benefit of the sustained-release 5-FU plants on gastric cancer, a randomized controlled large-scale study is needed.
While the use of intraoperative sustained-release chemotherapy with 5-FU did not improve the survival of patients in an advanced stage of gastric cancer who underwent an R0 radical resection, it was a safe method, and it did not increase the complication rate.
The prognosis of gastric cancer in an advanced stage remains poor. The exact efficacy of the use of intraoperative sustained-release chemotherapy with 5-fluorouracil (5-FU) in advanced-stage gastric cancer is still unelucidated.
To explore the long-term survival benefit of using sustained-release 5-FU implants in stage II and stage III gastric cancer patients.
To explore the long-term survival benefit of using sustained-release 5-FU implants in stage II and stage III gastric cancer patients.
All included patients were grouped according to whether intraoperative sustained-release (SR) chemotherapy with 5-FU was used or not (NSR). The primary end-point was 5-year overall survival. Kaplan–Meier method with log-rank test was used to analyze the overall survival of patients and Cox analysis was used to analyze prognosis factors of these patients.
309 patients were included in the final analysis. In addition, 158 patients received intraoperative sustained-release chemotherapy with 5-FU and were included in the SR group, while the other 161 patients were included in the NSR group. The overall complication rate was 12.94% in the whole group and 10.81%, 16.46% in SR and NSR groups, respectively. There were no significant differences between the two groups in overall survival and complication rate (P > 0.05).
Intraoperative sustained-release chemotherapy usage with 5-FU, did not improve the survival of patients who underwent an R0 radical resection in locally advanced stage of gastric cancer.
5-FU implants did not improve survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Liu YQ, United States; Mishra TS, India; Sempokuya T, United States; Socea B, Romania S-Editor: Wu YXJ L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, Teoh A, Liang P. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open. 2021;4:e2118457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 3. | Lin Y, Zheng Y, Wang HL, Wu J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988-2012) and Predictions to 2030. Gastroenterology. 2021;161:116-127.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. Guojia Aizheng Zhongxin Zazhi. 2022;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 951] [Article Influence: 317.0] [Reference Citation Analysis (1)] |
| 5. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 6. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 7. | Li JH, Zhang SW, Liu J, Shao MZ, Chen L. Review of clinical investigation on recurrence of gastric cancer following curative resection. Chin Med J (Engl). 2012;125:1479-1495. [PubMed] |
| 8. | Nakauchi M, Vos E, Tang LH, Gonen M, Janjigian YY, Ku GY, Ilson DH, Maron SB, Yoon SS, Brennan MF, Coit DG, Strong VE. Outcomes of Neoadjuvant Chemotherapy for Clinical Stages 2 and 3 Gastric Cancer Patients: Analysis of Timing and Site of Recurrence. Ann Surg Oncol. 2021;28:4829-4838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Yago A, Haruta S, Ueno M, Hamada Y, Ogawa Y, Ohkura Y, Urabe M, Udagawa H. Adequate period of surveillance in each stage for curatively resected gastric cancer: analyzing the time and rates of recurrence. Gastric Cancer. 2021;24:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Roviello F, Marrelli D, De Manzoni G, Morgagni P, Di Leo A, Saragoni L, De Stefano A. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113-1119. |
| 11. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 12. | Nakanishi Y, Ohara M, Domen H, Shichinohe T, Hirano S, Ishizaka M. Differences in risk factors between patterns of recurrence in patients after curative resection for advanced gastric carcinoma. World J Surg Oncol. 2013;11:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 488] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Li S, Li L, Tan B, Wang J, Xue S. The benefits of surgery plus extensive intraoperative peritoneal lavage (EIPL) for patients with gastric cancer compared with surgery alone: a systematic review and meta-analysis. Updates Surg. 2022;74:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Zhang JF, Lv L, Zhao S, Zhou Q, Jiang CG. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages. Ann Surg Oncol. 2022;29:3170-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1086] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 17. | Huang JY, Xu YY, Sun Z, Zhu Z, Song YX, Guo PT, You Y, Xu HM. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:4379-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O; FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Kwon OK, Chung HY, Yu W. Early postoperative intraperitoneal chemotherapy for macroscopically serosa-invading gastric cancer patients. Cancer Res Treat. 2014;46:270-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Li L, Li C, Zhou J. Effective sustained release of 5-FU-loaded PLGA implant for improving therapeutic index of 5-FU in colon tumor. Int J Pharm. 2018;550:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Ge J, Liu T, Lei T, Li X, Song K, Azizi S, Liu H, Tang M. Retrospective Cohort Study of Intraoperative Administration of Sustained-Release 5-Fluorouracil Implants in Advanced Gastric Cancer Patients. Front Pharmacol. 2021;12:659258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Xu Y, Zhang R, Li C, Sun Z, Deng J, Wang X, Ding X, Wang B, Xue Q, Ke B, Zhan H, Liu N, Liu Y, Liang H, Xue Y, Xu H. Intraperitoneal Chemotherapy Using Fluorouracil Implants Combined With Radical Resection and Postoperative Adjuvant Chemotherapy for Stage III Gastric Cancer: A Multi-Center, Randomized, Open-Label, Controlled Clinical Study. Front Oncol. 2021;11:670651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Gao K, Wu J. National trend of gastric cancer mortality in China (2003-2015): a population-based study. Cancer Commun (Lond). 2019;39:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Zeng H, Ran X, An L, Zheng R, Zhang S, Ji JS, Zhang Y, Chen W, Wei W, He J; HBCR Working Group. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877-e887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 25. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2856] [Article Influence: 571.2] [Reference Citation Analysis (5)] |
| 26. | Wu CW, Lo SS, Shen KH, Hsieh MC, Chen JH, Chiang JH, Lin HJ, Li AF, Lui WY. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Liu D, Lu M, Li J, Yang Z, Feng Q, Zhou M, Zhang Z, Shen L. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Yuan H, Zheng B, Tu S. Clinical research of intraperitoneal implantation of sustained-release 5-fluorouracil in advanced colorectal cancer. World J Surg Oncol. 2015;13:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Chen J, Zhang J, Wang C, Yao K, Hua L, Zhang L, Ren X. Safety of implanting sustained-release 5-fluorouracil into hepatic cross-section and omentum majus after primary liver cancer resection. Int J Immunopathol Pharmacol. 2016;29:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Liang Y, Zhao L, Chen H, Lin T, Chen T, Zhao M, Hu Y, Yu J, Liu H, Li G. Survival analysis of elderly patients over 65 years old with stage II/III gastric cancer treated with adjuvant chemotherapy after laparoscopic D2 gastrectomy: a retrospective cohort study. BMC Cancer. 2021;21:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Huang H, Wang W, Chen Z, Jin JJ, Long ZW, Cai H, Liu XW, Zhou Y, Wang YN. Prognostic factors and survival in patients with gastric stump cancer. World J Gastroenterol. 2015;21:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Shim IK, Yi HJ, Yi HG, Lee CM, Lee YN, Choi YJ, Jeong SY, Jun E, Hoffman RM, Cho DW, Kim SC. Locally-applied 5-fluorouracil-loaded slow-release patch prevents pancreatic cancer growth in an orthotopic mouse model. Oncotarget. 2017;8:40140-40151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Chen HQ, Gong JP, He YL, Huang CM, Liang H, Meng XL, Xu HM, Xue YW, Yan M, Zhou ZW. Expert consensus on intraoperative regional sustained-release chemotherapy for advanced gastric cancer. Zhonghua Wichang Waike Zazhi. 2012;15:981-983. |