Published online Oct 14, 2022. doi: 10.3748/wjg.v28.i38.5573
Peer-review started: May 30, 2022
First decision: August 1, 2022
Revised: August 16, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 14, 2022
Processing time: 134 Days and 15.4 Hours
Ischemia-reperfusion injury (IRI) is a major risk associated with liver surgery and transplantation, and its pathological mechanism is complex. Interleukin-1 receptor antagonist (IL-1ra) can protect the liver from IRI. However, the regulatory mechanism of IL-1ra expression is still unclear.
To identify the mechanism that could protect the liver in the early stage of IRI.
To screen the key genes in hepatic IRI, we performed RNA sequencing and gene enrichment analysis on liver tissue from mice with hepatic IRI. Subsequently, we verified the expression and effect of IL-1ra in hepatic IRI. We also used promoter mutagenesis and chromatin immunoprecipitation assay to search for the trans
We identified IL-1ra as a key regulator in hepatic IRI. The expression of IL-1ra was significantly upregulated after hepatic IRI both in vivo and in vitro. Furthermore, we found that HIF-1α regulated Il-1ra transcription in response to hypoxia. Increased HIF-1α accumulation promoted IL-1ra expression, whereas inhibition of HIF-1α exhibited the opposite effect. We also confirmed a predominant role for hypoxia response element in the regulation of Il1ra transcription by HIF-1α activation. Of note, we demonstrated that IP protects against hepatic IRI by inducing IL-1ra expression, which is mediated through HIF-1α.
We demonstrated that ischemia or hypoxia leads to increased expression of IL-1ra through HIF-1α. Importantly, IP protects the liver from IRI via the HIF-1α–IL-1ra pathway.
Core Tip: Ischemia-reperfusion injury (IRI) is a major risk associated with liver surgery and trans
- Citation: Wang ZY, Liu Y, Li SP, Li JJ, Zhang Z, Xiao XC, Ou Y, Wang H, Cai JZ, Yang S. Hypoxia inducible factor 1α promotes interleukin-1 receptor antagonist expression during hepatic ischemia-reperfusion injury. World J Gastroenterol 2022; 28(38): 5573-5588
- URL: https://www.wjgnet.com/1007-9327/full/v28/i38/5573.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i38.5573
Hepatic ischemia (IS)-reperfusion injury (IRI) is a common complication in liver surgery and transplantation[1]. IRI usually results from restoration of the blood supply after brief IS[2]. The sterile inflammatory response induced by hypoxic stress leads to hepatic IRI[3]. The pathogenesis of hepatic IRI is complex and affected by various factors[4,5], which involve the damage-associated molecular patterns, innate immune response, and inflammation. Among them, the inflammation caused by interleukin (IL)-1β plays an important role[6,7]. During hepatic IRI, injured cells activate the inflammasome pathway, allowing the IL-1β precursor to be cleaved into its mature form by caspase-1 and subsequently released. Mature IL-1β is a broad-acting proinflammatory cytokine that increases the recruitment of endothelial adhesion molecules to innate immune cells and promotes the development of inflammatory phenotypes[8]. However, blocking the action of IL-1 can reduce hepatic damage[9,10]. As a natural anti-inflammatory factor, IL-1 receptor antagonist (IL-1ra) is highly expressed in a variety of inflammatory diseases and is closely related to the occurrence and progression of inflammation[11-14]. Several studies have shown a potential protective effect of IL-1ra in inflammation. For example, IL-1ra competes with IL-1β to bind to IL-1 receptor I, thus playing an anti-inflammatory role[15]. However, the transcriptional regulatory mechanism of IL-1ra during IRI remains unclear.
As a transcription factor, hypoxia-inducible factor (HIF)-1α is an important molecule for cell regulation in the hypoxic environment[16,17]. There have been a few reports confirming that HIF-1α plays an irreplaceable role in hepatic IRI[18]. HIF-1α can regulate the expression of multiple genes in cells after hypoxia[19], and consequently reduce organ IRI by regulating metabolism[20]. Furthermore, the mechanism of HIF-1α protection against hepatic IRI deserves further study.
Ischemic preconditioning (IP) was first reported by Murry et al[21] in 1986, who demonstrated that multiple IP reduces cell death after coronary artery occlusion. Although the protective effect of IP on hepatic IRI has been subsequently reported[22], its mechanism of action remains unclear. Therefore, it is necessary to confirm the mechanism underlying this protective effect, which would be of great clinical significance. In the present study, we found that IL-1ra was expressed in hepatic tissue during IRI and was regulated by IS-induced HIF-1α. We also confirmed that the protective effect of IP was exerted precisely via the HIF-1α–IL-1ra pathway, leading to inhibition of IL-1β signaling and subsequent reduction in hepatic IRI.
2-MeOE2 (S1233) and DMOG (S7483) were purchased from Selleck (China). Percoll (17089101) was purchased from GE Healthcare (United States). Collagenase IV (C8160) was purchased from Solarbio (China). Mouse monoclonal antibody for β-actin (sc-47778) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). A rabbit monoclonal antibody for IL-1ra (ab124962) was purchased from Abcam (Cambridge, MA, United States). Rabbit antibodies for HIF-1α (36169), BAX (14796), Bcl-2 (3498), and cleaved caspase-3 (9664) were purchased from Cell Signaling Technology (Danvers, MA, United States). ELISA kits for detecting mouse alanine transaminase (ALT) (JL12668), aspartate transaminase (AST) (JL13793), and IL-1ra (JL20255) were purchased from Jonln (China). Recombinant IL-1ra (200-01RA) was purchased from PeproTech (United States).
Wild-type male C57BL/6 mice, aged 6-8 wk, were purchased from Beijing HFK Bioscience (Beijing, China). The mice were raised in standard conditions[23] and received humanitarian care. The mice were acclimated to the rearing environment for 3-4 d before experimentation.
Mice were randomly grouped and marked. The mice were weighed and anesthetized with 1% pentobarbital (50 mg/kg). For the hepatic IR experiments, mice were divided into six groups (n = 5 each): Sham group: The portal vein was exposed, and no blood vessels were clamped; ischemia reperfusion (IS) 1.5 h group: We only clamped the left and middle lobe vessels for 1.5 h; IR 3-24 h group: Reperfusion was performed for the corresponding time after the vessels had been clamped for 1.5 h; IP group: We briefly clamped the vessel for 10 min and released it for 10 min before IS; this was named an IP cycle; shIl1ra group: Adenovirus carrying Il1ra shRNA was injected via the tail vein 48 h before IR. At the end of the experiment, the ischemic hepatic lobes were isolated for subsequent processing or stored at -80 °C. The animal study protocol was approved by the Animal Ethical and Welfare Committee (protocol code: IRM-DWLL-2020173 and date of approval: September 15, 2020).
Nonparenchymal cells (NPCs) and hepatocytes were isolated from mice by collagenase digestion and differential centrifugation using Percoll, as previously described[24].
The left lobe samples of mouse livers were fixed in formaldehyde solution, dehydrated, paraffin-embedded, and cut into 4-μm-thick sections. The IL-1ra levels were determined by immunohistochemistry. The histological analysis was performed using the histochemistry score. A section was selected from the left lobe in every liver, and five views (200 ×) were captured for calculation in each section.
Mouse liver parenchymal cells, AML12 (SCSP-550; American Type Culture Collection, Manassas, VA, United States), were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (11965092; Gibco) supplemented with 10% fetal bovine serum (FBS) (10099133; Gibco) and 1% penicillin/streptomycin at 37 °C in an atmosphere containing 5% CO2. HEK293T cells were cultured in DMEM (11320033; Gibco) supplemented with 10% FBS, 1% sodium pyruvate, 1% non-essential amino acids, and 2% glutamine.
AML12 cells were subjected to hypoxia and reoxygenation to simulate the ischemic and reperfusion environment in vivo. During hypoxia, cells were cultured in a 37 °C incubator with 95% N2 and 5% CO2. After a specific time period, cells were removed and placed back into the 37 °C incubator with 5% CO2. To interfere with HIF-1α, cells were treated with DMOG or 2-MeOE2 at a concentration of 100 μM for 6 h before subsequent experiments were performed.
The mouse Il1ra promoter (-2113/+143) sequences were obtained by polymerase chain reaction (PCR) from mouse genomic DNA and cloned into the pGL3-basic vector (Promega, Madison, WI, United States). Mutagenesis of HRE-I and HRE-II in the mouse Il1ra promoter was performed using a Quick-Change® Lightning Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, United States). The primer sequences are listed in Table 1. The mouse Il1ra shRNA plasmid was purchased from Hanbio Biotechnology (Shanghai, China), and packaged into adenoviral particles.
| Gene | Forward sequence | Reverse sequence |
| Il1ra | TCTTGGGCATCCACGGG | GAGGCTCACAGGACGGTCAG |
| β-actin | GTGACGTTGACATCCGTAAAGA | GCCGGACTCATCGTACTCC |
| Il1ra pro-2.1K | CGGAGCTCGCCAGCAAGATTTTAAGTGATTCT | CCAGATCTGGGTGAGCTAAACAGGACACAAGGT |
| Il1ra pro-1.5K | CGGAGCTCCCCAACAACTTCCAGACTTCCCTC | CCAGATCTGGGTGAGCTAAACAGGACACAAGGT |
| Il1ra pro-1.1K | CGGAGCTCATCAGTCTAAGGCTGGGCAGGGAG | CCAGATCTGGGTGAGCTAAACAGGACACAAGGT |
| Il1ra pro-0.6K | CGGAGCTCCCCAGCTCAAATGCCACCATTCTC | CCAGATCTGGGTGAGCTAAACAGGACACAAGGT |
| Il1ra Mut1 | TTTATGCACATTCCCTCTTTCAGC | AGAGGGAATGTGCATAAACTTGT |
| Il1ra Mut2 | GATATGGACTTGCCATTTTGACTC | AAATGGCAAGTCCATATCTTTCTT |
Total RNA from liver tissue and AML12 cells was extracted using Trizol (Life Technologies, Carlsbad, CA, United States). cDNA was synthesized using reverse transcriptase (Takara, Japan). The specific product of Il1ra was amplified by qPCR using the TransStart Green Q-PCR SuperMix Kit (TransGen, China). β-Actin was used as a normalization control. The primer sequences are listed in Table 1.
Tissues and cell lysates were prepared in RIPA buffer with protease and phosphatase inhibitors. The immunoblotting procedures can be found in the literature[25].
Cells were transfected with wild-type or mutant mouse Il1ra promoters, followed by treatment with DMOG or 2-MeOE2 under hypoxia in 24-well plates. Lysates were prepared 48 h after transfection, and the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega). The luciferase activity was normalized to the values for Renilla luciferase.
Chromatin immunoprecipitation (ChIP) assays were performed using an EZ-ChIP kit (Millipore, Billerica, MA, United States). The primers and antibodies used in these experiments are shown in Table 1.
Statistical analyses were performed using GraphPad Prism 7.0 (San Diego, CA, United States). All the data are presented as the mean ± SEM and represent three or five independent experiments. One-way analysis of variance was used to compare means among treatment groups. Student’s t-test for unpaired observations was applied. P < 0.05 was considered significant.
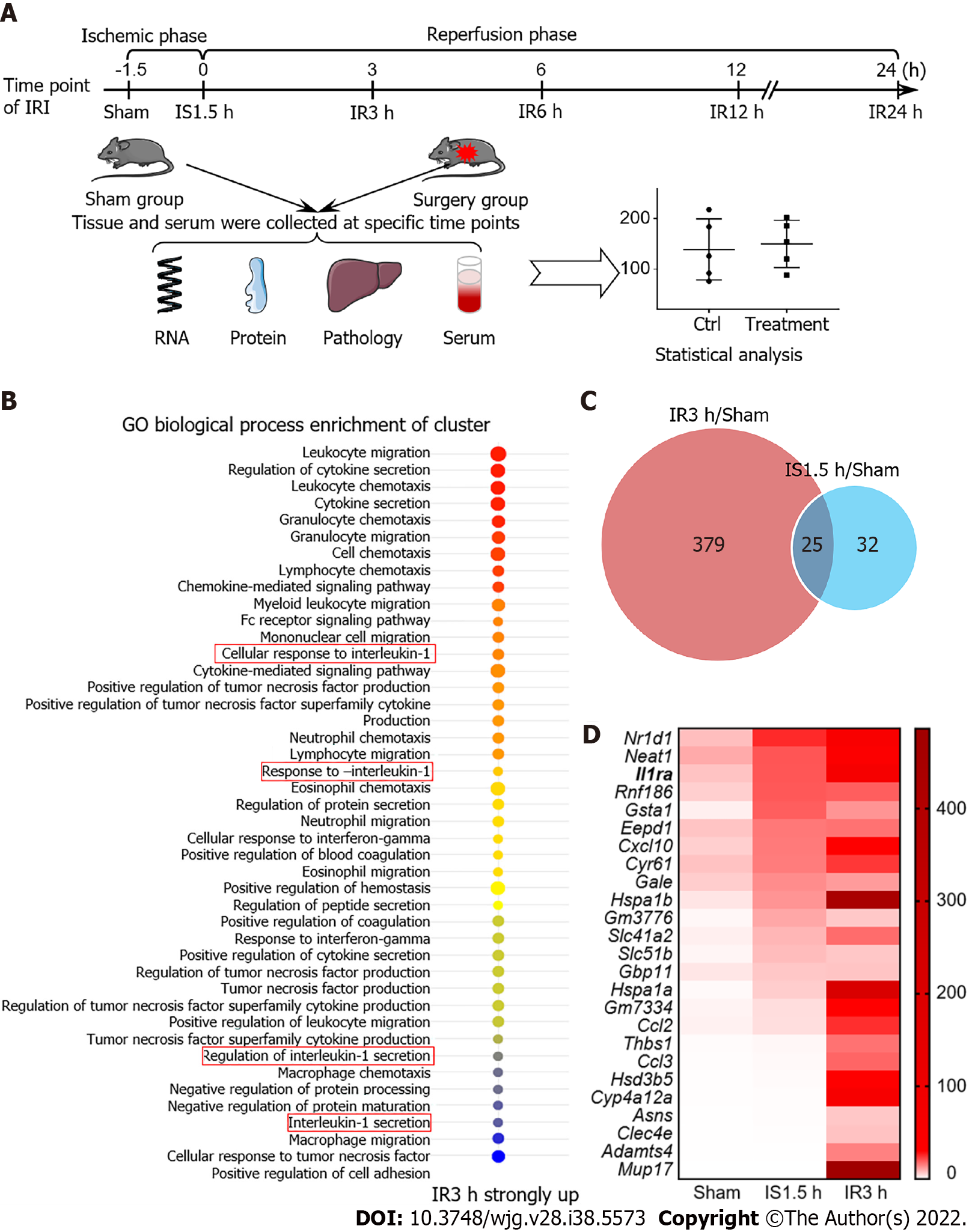
To screen key genes that potentially play protective roles in the liver in IRI, we constructed a mouse model of hepatic IRI and performed transcriptome sequencing using mRNAs of the liver from the Sham, IS 1.5 h, and IR 3 h groups (Figure 1A). Gene enrichment analysis showed that, after IR 3h, a group of genes enriched in the IL-1 pathway were upregulated (Figure 1B). Expression of 25 genes was significantly increased (> 2-fold) in both IS and IR periods (Figure 1C). These genes were then ranked in descending order of ischemic expression abundance, and Il1ra, as a key antagonist of the IL-1 signaling, was shown to be one of the highly differentially expressed genes (Figure 1D). Thus, we chose Il1ra for further investigation on its regulatory mechanism in the hepatic IRI.
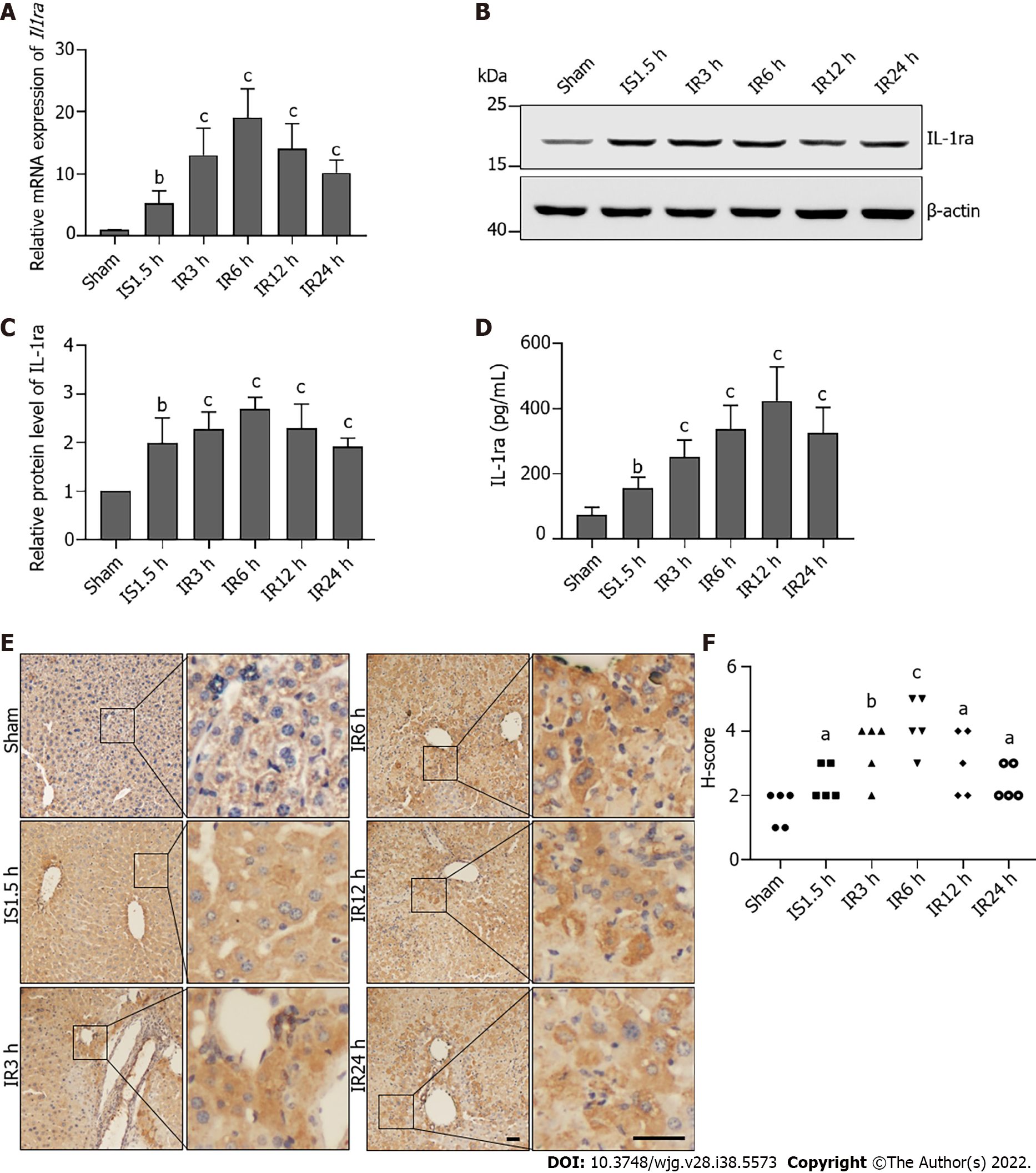
We detected IL-1ra expression in mice with IRI. This confirmed that mRNA expression of Il1ra was increased in mice that experienced hepatic IRI as compared with the Sham group (Figure 2A). Upregulation of IL-1ra in liver tissue was confirmed at the protein level by immunoblotting (Figures 2B and C), ELISA (Figure 2D), and immunohistochemistry (Figures 2E and F), demonstrating that expression of IL-1ra is significantly elevated in response to hepatic IRI in mice.
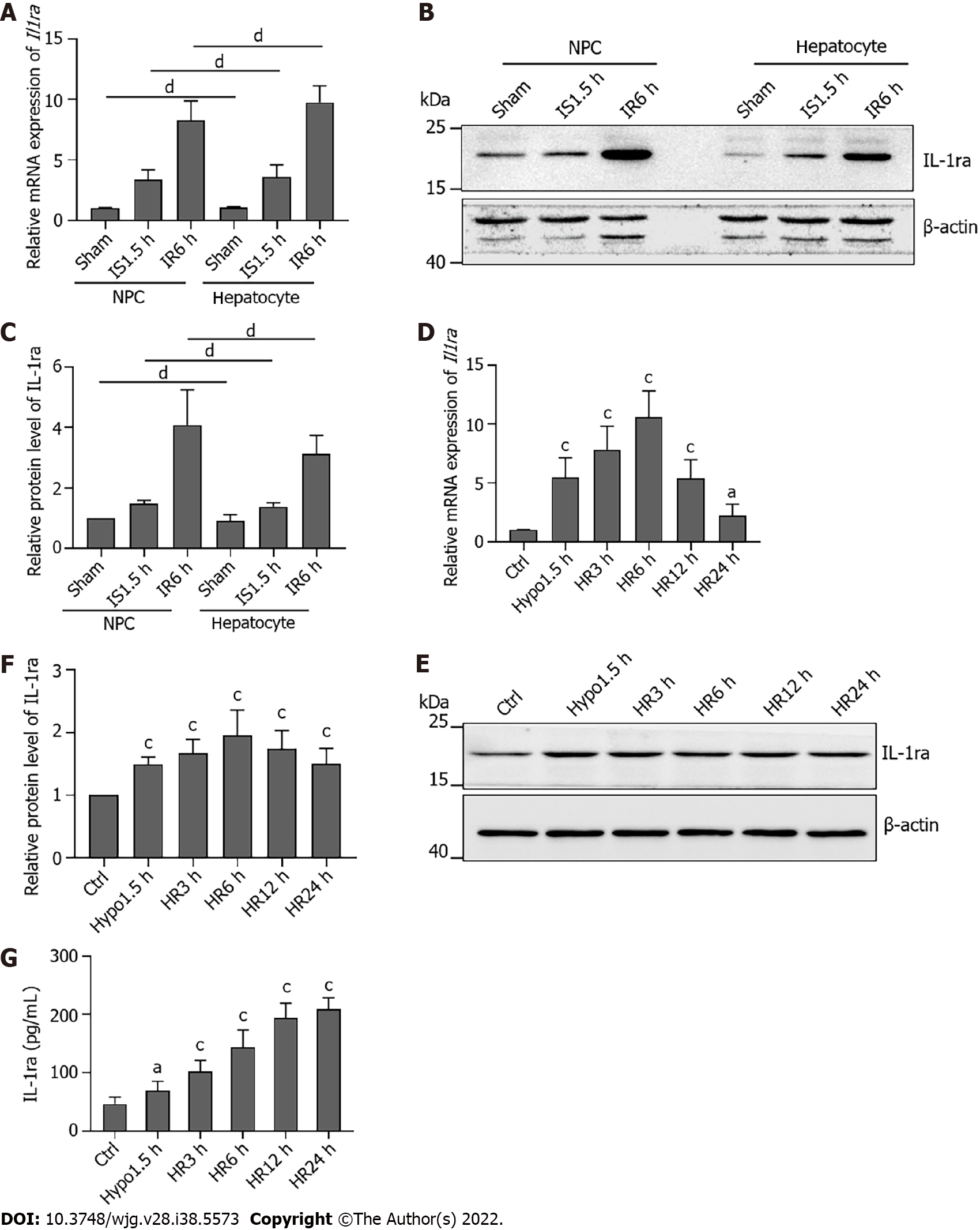
The liver is composed of several different embryonic-derived cell types, including hepatocytes, bile duct epithelial cells, stellate cells, Kupffer cells, and sinusoidal endothelial cells[26]. The liver consists of 80% hepatocytes, and 20% of the other cells are collectively called NPCs. Hepatocytes are the major epithelial cell population of the liver and perform various physiological functions. Therefore, we aimed to identify whether there is a difference in the expression of IL-1ra in hepatocytes and NPCs after IRI. To do so, gradient centrifugation was used to separate hepatocytes and NPCs from mouse liver tissue, and expression of IL-1ra was detected. There was no significant difference in the expression of IL-1ra at both the mRNA (Figure 3A) and protein (Figures 3B and C) levels between hepatocytes and NPCs after IR treatment.
To confirm these results in vitro, we performed the hypoxia–reoxygenation experiments in the mouse hepatic cell line AML12 to simulate IRI. We found that the mRNA (Figure 3D) and protein (Figures 3E and F) expression of IL-1ra was increased in AML12 cells upon hypoxia and reoxygenation. Similarly, the content of IL-1ra in the culture medium of the hypoxia-reoxygenation group was significantly higher than that in the control group (Figure 3G). Together, these data suggested that IL-1ra is highly expressed in both hepatocytes and NPCs after IRI.
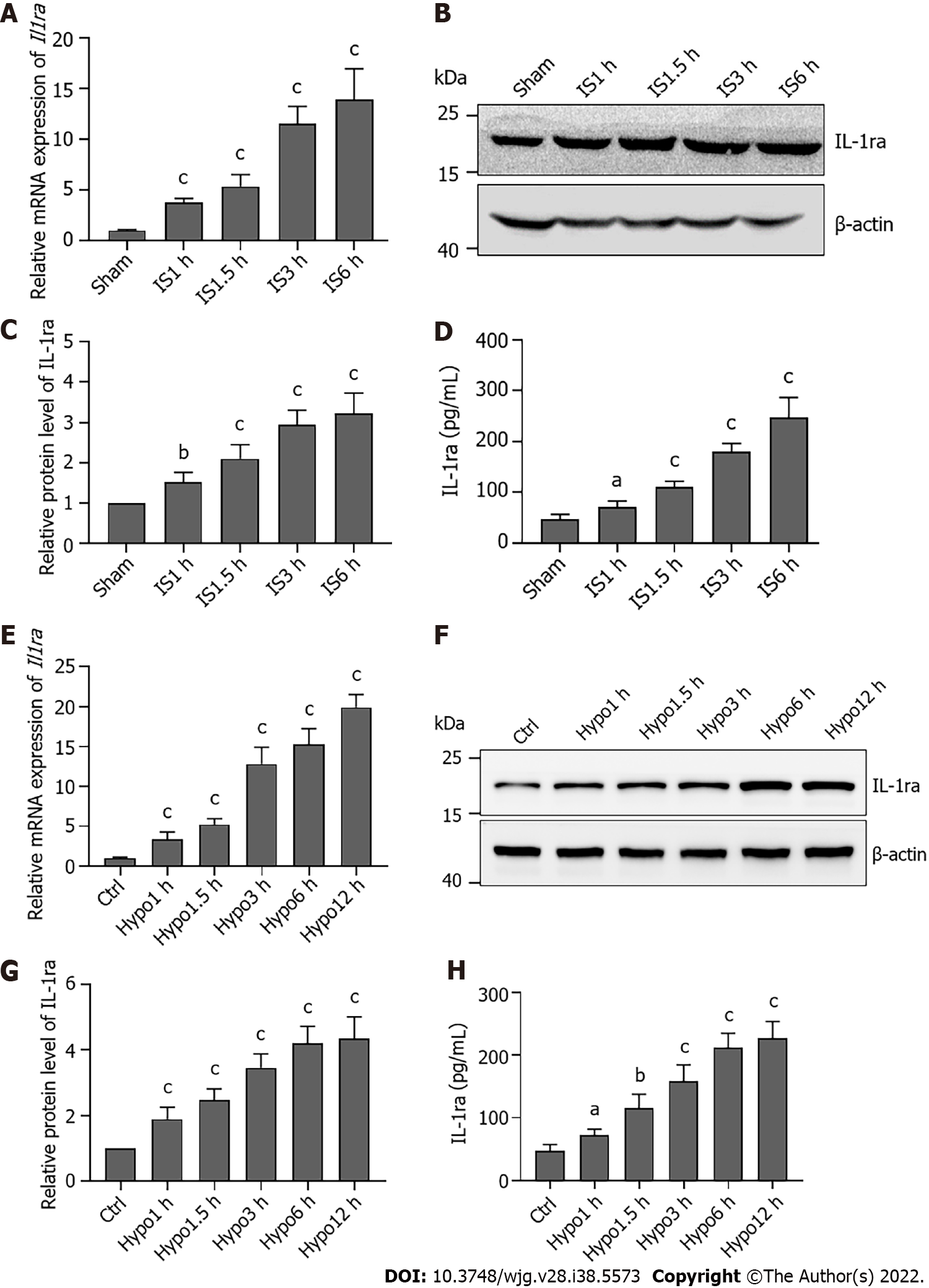
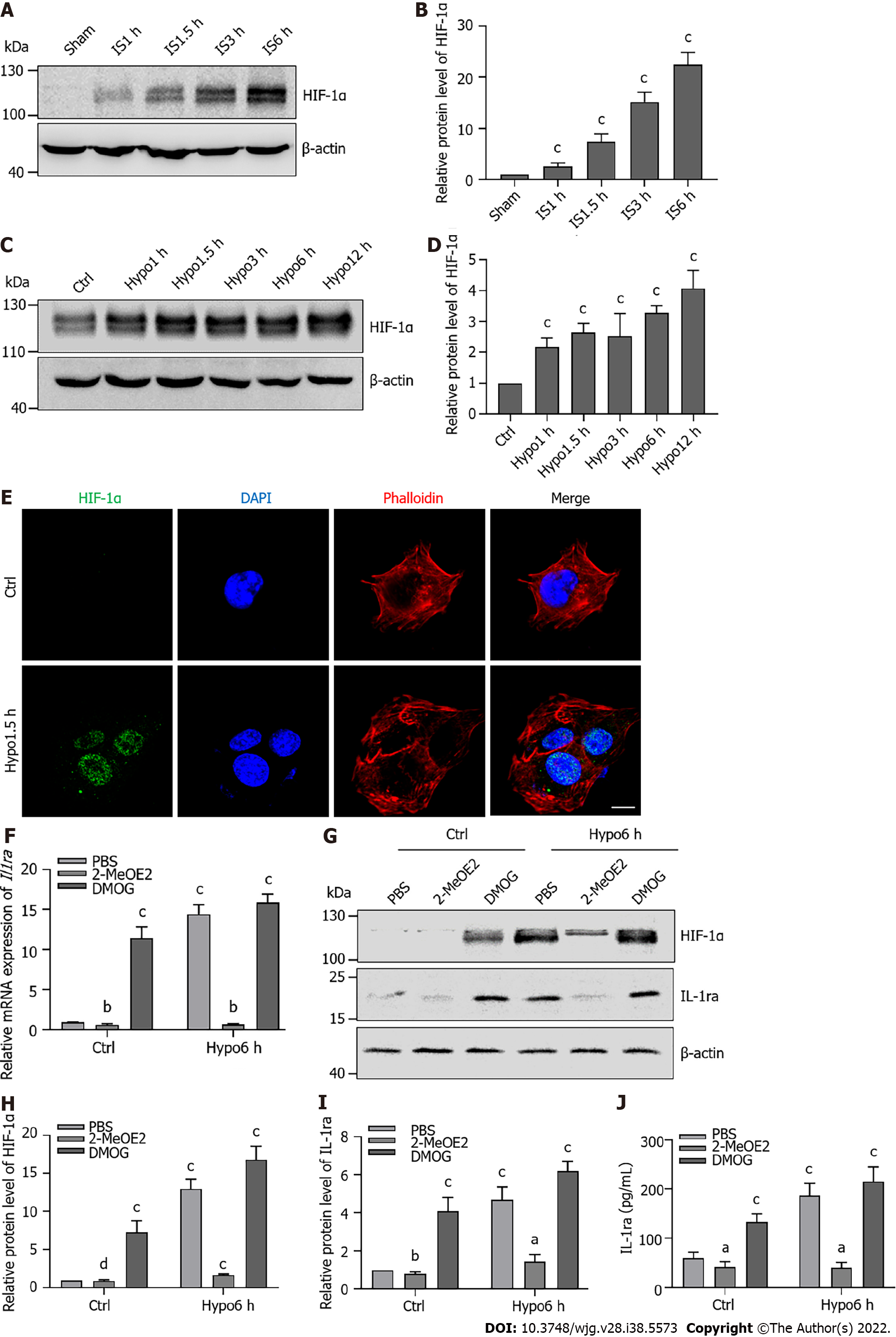
To verify the regulation of IL-1ra expression in the early stage of hepatic IRI, we constructed hypoxic models at different time points in vivo and in vitro. Compared with the Sham group, ischemic treatment in mice increased IL-1ra expression in a time-dependent manner at both the mRNA (Figure 4A) and protein (Figures 4B-D) levels. Similar results were also revealed in AML12 cells (Figures 4E-H), demonstrating that IS and hypoxia could promote the expression of IL-1ra.
HIF-1α, as a transcriptional regulator, plays an important role under hypoxic conditions. Therefore, we speculated that HIF-1α would regulate the expression of IL-1ra. Thus, we moved to evaluate the relationship between HIF-1α and IL-1ra expression in hepatic IRI. Increased expression of HIF-1α was observed upon ischemic treatment in mice in a time-dependent manner (Figures 5A and B). Similar results were confirmed in AML12 cells (Figures 5C and D). Immunofluorescence analysis indicated that HIF-1α was localized in the nuclei of AML12 cells after 1.5 h of hypoxia (Figure 5E).
We treated AML12 cells with 2-MeOE2 (a specific HIF-1α inhibitor) or DMOG (a specific HIF-1α agonist). 2-MeOE2 reduced the expression of Il-1ra mRNA during hypoxia, while DMOG increased Il-1ra expression under normoxia (Figure 5F). Immunoblotting (Figures 5G-I) and ELISA (Figure 5J) confirmed that 2-MeOE2 reduced the protein levels of HIF-1α and IL-1ra in AML12 cells; however, DMOG had the opposite effect. These results indicated that HIF-1α independently promotes the expression of IL-1ra.
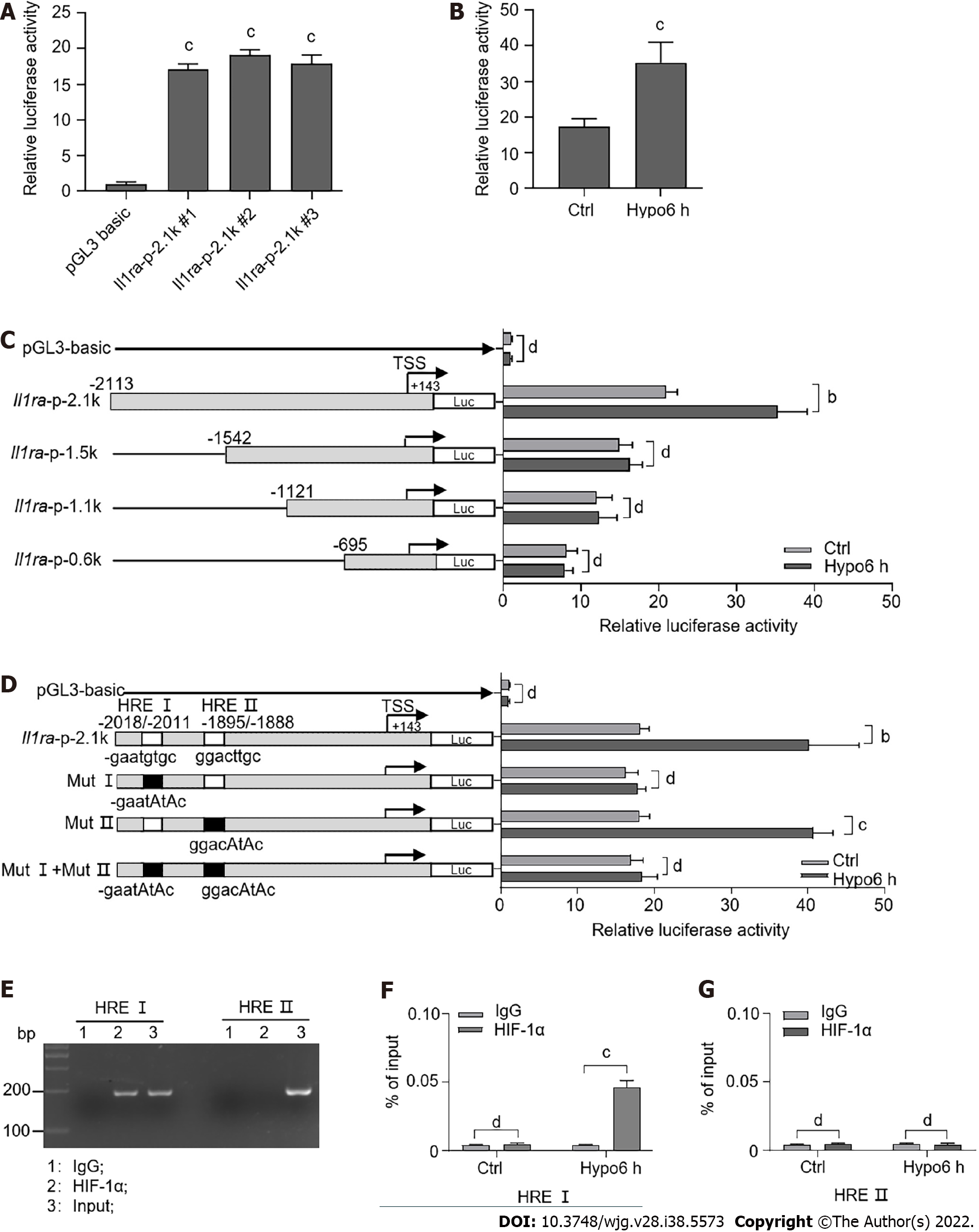
To investigate the regulatory mechanism of IL-1ra by HIF-1α, we transfected a mouse Il1ra promoter-reporter construct into AML12 cells (Figure 6A). Upon treatment with hypoxia, we found that Il1ra promoter activity was significantly increased (Figure 6B). To explore the transcriptional regulatory elements of the Il1ra promoter in response to hypoxia, we generated a series of truncated Il1ra promoter–reporter constructs. Il1ra-p-2.1k promoter activity was significantly increased upon hypoxic treatment, while the truncated promoter activity of Il1ra-p-1.5k, Il1ra-p-1.1k, and Il1ra-p-0.6k was not altered by hypoxia (Figure 6C).
We used the JASPAR database to predict the binding site of HIF-1α on the Il1ra-p-2.1k promoter and found two possible binding elements, HRE I (-2018/-2011) and HRE II (-1895/-1888) (Figure 6D). Site-directed mutagenesis showed that mutation of HRE II did not affect hypoxia-induced activation of the Il1ra promoter, while mutation of either HRE I or HRE I + II eliminated this effect (Figure 6D). Importantly, we used the ChIP assay to verify that HIF-1α was able to be recruited to the HRE I element in the Il1ra promoter (Figures 6E and F).
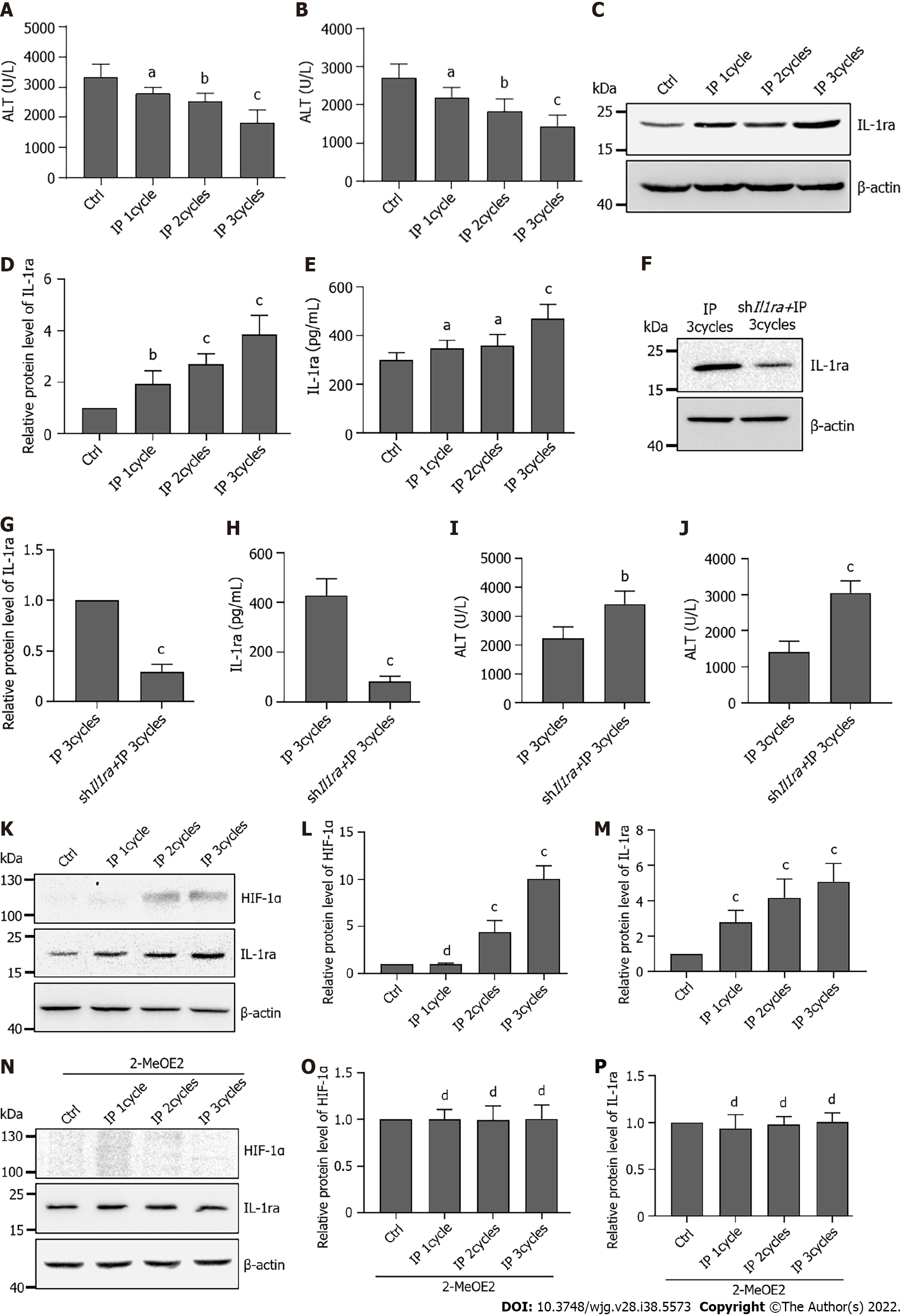
It has been reported that IP can alleviate hepatic IRI[27]. However, the protective mechanism of IP is not yet clear. Here, we speculated that IL-1ra might play a potential role in the regulation of IP. Thus, the mice were subjected to IP with the indicated cycles. Compared with the control group, the IP group had significantly lower ALT and AST levels in their serum, which were further reduced by increased IP cycles (Figures 7A and B). Similar results of IL-1ra upregulation were observed by immunoblotting (Figures 7C and D) and ELISA (Figure 7E).
To verify whether IL-1ra plays a protective role in IP, we injected adenovirus carrying Il1ra shRNA via the tail vein to knock down the expression of IL-1ra in mice. Expression of IL-1ra in the liver and serum of mice injected with adenovirus was significantly decreased (Figures 7F-H). The ALT and AST levels were increased by knockdown of IL-1ra in the liver (Figures 7I and J).
It has been reported that HIF-1α plays an important role in IP[28], and we thus speculated that the expression of IL-1ra in IP is regulated by HIF-1α. We found that IP induced accumulation of HIF-1α in the liver (Figures 7K-M); however, 2-MeOE2 significantly attenuated this effect (Figures 7M-P). These results collectively suggested that IP might exert a protective effect on the liver from IRI through regulating the HIF-1α–IL-1ra signaling.
Hepatic IRI is a common complication of liver surgery. Previous studies have demonstrated that the IL-1 signaling pathway plays a pivotal role in the regulation of hepatic IRI. Here, we extended the study, showing that the expression of IL-1ra, an inhibitor of the IL-1 signaling pathway, is increased in response to hepatic IRI, especially in the ischemic stage. Mechanistically, hypoxia induces the transcriptional expression of IL-1ra through HIF-1α accumulation. Of note, we proved that IP could protect the liver from IRI by promoting IL-1ra expression in a HIF-1α-dependent manner. Thus, our study has provided additional evidence for the regulatory mechanism of IL-1ra during IS, indicating a practical strategy for alleviating hepatic IRI.
Previous studies have shown that hepatic IRI not only causes damage to the liver itself, but also leads to the abnormal functioning of various organs, and induces systemic inflammatory response syndrome[29]. Therefore, the question of how to reduce or even eliminate hepatic IRI has received constant attention among clinicians and researchers. Hepatic IRI is a complex pathophysiological process with numerous and relational influencing factors. Among them, the early inflammatory response plays a crucial role in the occurrence and development of hepatic IRI[30], because it is located in the upstream region of the injury response chain. Upon activation, downstream factors often promote each other and thus aggravate the injury[31]. If the activation of early inflammation can be reduced or even blocked, it may be possible to prevent the occurrence and development of hepatic IRI at source.
Current studies have shown that many proinflammatory cytokines are released in the early stage of hepatic IRI[4]. For example, blocking the activation of proinflammatory IL-1 signaling significantly prevents IRI[32]. IL-1 is a major inflammatory response mediator[33]. Experimental results have shown that large amounts of IL-1β and tumor necrosis factor-α are produced in the tissue after hepatic IS and reperfusion[34]. At the same time, this can also stimulate the secretion of inflammatory cells, leading to the inflammatory destruction of local tissues. IL-1β and the nucleotide-binding and leucine-rich repeat protein 3 (NLRP3) inflammasome play a role through high mobility group box 1 protein[35], nuclear factor-κB and Toll-like receptor 4 in lesions caused by warm IRI[7]. Of note, NLRP3 may be involved in IRI independently of the inflammasome pathway by recruiting neutrophils. Macrophages secrete pro-IL-1β, which is subsequently cleaved and activated by neutrophil-derived proteases in a mouse model. Mature IL-1β is required to induce inflammation during hepatic IR, and the interaction between macrophages and neutrophils is essential in this process[6]. Therefore, the antagonism of proinflammatory IL-1 cytokines has important therapeutic potential for reducing IRI.
IL-1ra is a naturally occurring polypeptide. Recent research has focused on the role of IL-1ra in various pathophysiological states or the effects of recombinant IL-1ra administration in animals and humans[36]. It has been reported that the delivery of the Il-1ra gene to the rat liver via the adenovirus vector or lipofection can significantly reduce IR-induced proinflammatory cytokine production and hepatocyte injury[15]. This is consistent with our results showing that the knockdown of IL-1ra significantly increased the damage during hepatic IRI, suggesting a potential inhibitory effect of IL-1ra on inflammation in response to hepatic IR.
In the process of IRI, IS is a prerequisite. It is precisely regulated because the depletion of oxygen and energy in the ischemic period cause a series of injuries in the subsequent reperfusion period. In the state of hepatic IS, the metabolic mode switches from aerobic to anaerobic, the redox process in hepatocytes is blocked, the ATP-dependent cellular metabolic activity gradually stops, and the intracellular ATP is rapidly depleted. At the same time, the absence of oxygen causes HIF-1α to gradually accumulate. The increased transport of HIF-1α into the nucleus triggers the expression of genes involved in oxygen transport, oxygen utilization, glycolytic metabolism, cell death, cell survival, and other processes that affect cell survival during IS. Recent studies have demonstrated that HIF-1α can alleviate IRI by regulating the expression of inducible NO synthase[37]. HIF-1α may also enhance tissue anti-inflammatory effects by increasing heme oxygenase-1 expression, thereby reducing damage[38]. In this study, we further showed that HIF-1α, as an important protective factor in IRI, could protect the liver from injury by promoting the expression of IL-1ra and subsequently inhibiting activation of the IL-1 signaling pathway. In terms of the mechanism, we found that HIF-1α recognizes and binds to the basic helix-loop-helix protein sequence in the Il1ra promoter region, resulting in the increased transcription of Il1ra.
Recent studies have shown that IP has broad protective effects against IRI[39]. However, due to the complexity of IRI, the underlying cellular and molecular mechanisms of IP remain largely unknown. In the present study, we provided evidence that IP promotes the expression and release of IL-1ra into the hepatic microenvironment, thus blocking the downstream activation of IL-1β and protecting the liver from the cascading effects of inflammatory factors. We demonstrated that IP can induce the accumulation of HIF-1α, which increases with the number of IP cycles. These results would explain the protective effect of IP at a mechanistic level and lay a theoretical foundation for the better application of IP.
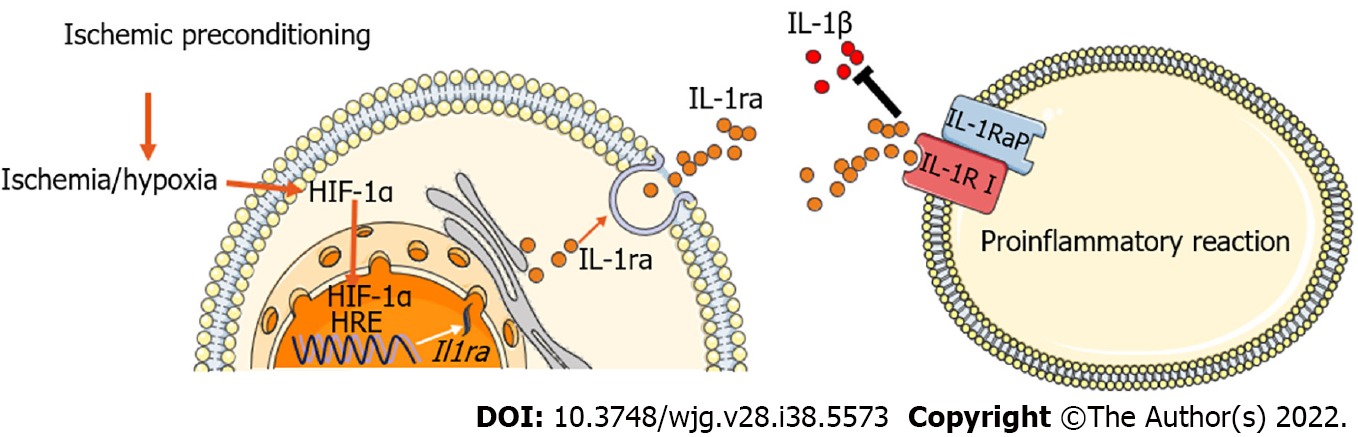
Our study demonstrated a protective effect of IL-1ra on hepatic IRI through the transcriptional regulation of IL-1ra by HIF-1α. Importantly, we found that IP can regulate the expression of IL-1ra through HIF-1α at early stage of hepatic IRI, thereby blocking the inflammatory IL-1 signaling pathway to protect the liver from IRI (Figure 8).
Ischemia-reperfusion injury (IRI) is associated with transplant failures, graft dysfunction, and a relatively poor prognosis. Interleukin-1 receptor antagonists (IL-1ra) play an important role in protecting the liver from IRI. However, the mechanism of its regulatory expression remains unclear.
Inhibition of hepatic inflammation by promoting the expression of IL-1ra is one of the key targets for the treatment of hepatic IRI.
To investigate the regulatory mechanism of hepatocyte-derived IL-1ra expression in the process of hepatic IRI to provide a therapeutic target for hepatic IRI.
A 70% hepatic IRI model was established in mice, and AML12 cells were subjected to hypoxia/ reoxygenation for the simulation of IRI in vitro. The Il-1ra promoter-reporter system was constructed to detect the regulatory effect of hypoxia. The ischemic preconditioning (IP) model was established to investigate its regulatory effect on IL-1ra.
IL-1ra is a key regulator of hepatic IRI. Il-1ra expression was significantly up-regulated after hepatic IRI in vivo and in vitro. Hypoxia inducible factor (HIF)-1α regulates Il-1ra transcription during hypoxia. IP prevents hepatic IRI by inducing the expression of IL-1ra, which is mediated by HIF-1α.
Ischemia or hypoxia leads to increased IL-1ra expression through HIF-1α. In addition, IP protects the hepatic tissue from IRI through the HIF-1α-IL-1ra pathway.
HIF-1α-IL-1ra pathway is a potential mechanism of hepatic protection during hepatic IRI, and also a key target for the treatment of hepatic IRI.
The authors would like to thank Yi Shi and Wei Sun (Medical College of Nankai University) for their skillful technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Katada K, Japan; Tzeng IS, Taiwan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2266] [Article Influence: 377.7] [Reference Citation Analysis (0)] |
| 2. | Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68-78. [PubMed] |
| 3. | Ji H, Liu Y, Zhang Y, Shen XD, Gao F, Busuttil RW, Kuchroo VK, Kupiec-Weglinski JW. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology. 2014;60:2052-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 467] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 5. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 657] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 6. | Sadatomo A, Inoue Y, Ito H, Karasawa T, Kimura H, Watanabe S, Mizushina Y, Nakamura J, Kamata R, Kasahara T, Horie H, Sata N, Takahashi M. Interaction of Neutrophils with Macrophages Promotes IL-1β Maturation and Contributes to Hepatic Ischemia-Reperfusion Injury. J Immunol. 2017;199:3306-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Kamo N, Ke B, Ghaffari AA, Shen XD, Busuttil RW, Cheng G, Kupiec-Weglinski JW. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1053] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 9. | Gehrke N, Hövelmeyer N, Waisman A, Straub BK, Weinmann-Menke J, Wörns MA, Galle PR, Schattenberg JM. Hepatocyte-specific deletion of IL1-RI attenuates liver injury by blocking IL-1 driven autoinflammation. J Hepatol. 2018;68:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 10. | Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1359] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 11. | Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 529] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 12. | Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 758] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 13. | Thompson RC, Dripps DJ, Eisenberg SP. Interleukin-1 receptor antagonist (IL-1ra) as a probe and as a treatment for IL-1 mediated disease. Int J Immunopharmacol. 1992;14:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Dayer JM. Evidence for the biological modulation of IL-1 activity: the role of IL-1Ra. Clin Exp Rheumatol. 2002;20:S14-S20. [PubMed] |
| 15. | Harada H, Wakabayashi G, Takayanagi A, Shimazu M, Matsumoto K, Obara H, Shimizu N, Kitajima M. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation. 2002;74:1434-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | McGettrick AF, O'Neill LAJ. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020;32:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 427] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 17. | Schumacker PT. Hypoxia-inducible factor-1 (HIF-1). Crit Care Med. 2005;33:S423-S425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Guo Y, Feng L, Zhou Y, Sheng J, Long D, Li S, Li Y. Systematic review with meta-analysis: HIF-1α attenuates liver ischemia-reperfusion injury. Transplant Rev (Orlando). 2015;29:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892-10903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1292] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 20. | Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 787] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 21. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5538] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 22. | Liu A, Fang H. Ischemic Preconditioning on Liver Ischemia Reperfusion Injury: How Far is the Bedside from the Bench? J Invest Surg. 2020;33:884-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Ferreira-Silva M, Faria-Silva C, Carvalheiro MC, Simões S, Marinho HS, Marcelino P, Campos MC, Metselaar JM, Fernandes E, Baptista PV, Fernandes AR, Corvo ML. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Alchera E, Chandrashekar BR, Clemente N, Borroni E, Boldorini R, Carini R. Ischemia/Reperfusion Injury of Fatty Liver Is Protected by A2AR and Exacerbated by A1R Stimulation through Opposite Effects on ASK1 Activation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Jiang H, Zhou C, Zhang Z, Wang Q, Wei H, Shi W, Li J, Wang Z, Ou Y, Wang W, Wang H, Zhang Q, Sun W, Sun P, Yang S. Jagged1-Notch1-deployed tumor perivascular niche promotes breast cancer stem cell phenotype through Zeb1. Nat Commun. 2020;11:5129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Lloris-Carsí JM, Cejalvo D, Toledo-Pereyra LH, Calvo MA, Suzuki S. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc. 1993;25:3303-3304. [PubMed] |
| 28. | Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Quesnelle KM, Bystrom PV, Toledo-Pereyra LH. Molecular responses to ischemia and reperfusion in the liver. Arch Toxicol. 2015;89:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 373] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 31. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 693] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 32. | Zhu P, Duan L, Chen J, Xiong A, Xu Q, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum Gene Ther. 2011;22:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Wang J, Yu S, Li J, Li H, Jiang H, Xiao P, Pan Y, Zheng J, Yu L, Jiang J. Protective role of N-acetyl-l-tryptophan against hepatic ischemia-reperfusion injury via the RIP2/caspase-1/IL-1β signaling pathway. Pharm Biol. 2019;57:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Saleh H, El-Shorbagy HM. Chitosan protects liver against ischemia-reperfusion injury via regulating Bcl-2/Bax, TNF-α and TGF-β expression. Int J Biol Macromol. 2020;164:1565-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Zhong W, Rao Z, Rao J, Han G, Wang P, Jiang T, Pan X, Zhou S, Zhou H, Wang X. Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell. 2020;19:e13186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Carter DB, Deibel MR Jr, Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 446] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 37. | Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol. 2004;287:H2369-H2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA 3rd, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542-H548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Suzuki S, Inaba K, Konno H. Ischemic preconditioning in hepatic ischemia and reperfusion. Curr Opin Organ Transplant. 2008;13:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |