Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.5058
Peer-review started: March 30, 2022
First decision: June 10, 2022
Revised: July 27, 2022
Accepted: August 17, 2022
Article in press: August 17, 2022
Published online: September 14, 2022
Processing time: 160 Days and 18.8 Hours
A gap remains in documenting the impact of anti-tumor necrosis factor therapy on disease burden in ulcerative colitis (UC) patients treated in a real-world setting. The use of patient-reported outcomes (PROs) has been discussed as a primary endpoint in the context of the FDA PRO Guidance, for labelling purposes. Specifically, the efficacy and safety of adalimumab have been demonstrated in pivotal trials; however, data are needed to understand how clinical results translate into improvements in key aspects of the daily lives of UC patients, such as symptoms, health-related quality of life (HRQoL), and disability.
To assess real-world effectiveness of adalimumab on PRO measures in patients with moderate-to-severe UC.
UCanADA was a single arm, prospective, 1-year multicenter Canadian post-marketing observational study in which multiple PRO questionnaires were completed—with psychologic distress/depression symptoms as the primary endpoint—by patients with moderate-to-severe UC. Assessments were performed during patients’ routine care visit schedule, which was at the initiation of adalimumab (baseline), after induction (approximately 8 wk), and 52 wk after baseline. Additional optional assessments between weeks 8 and 52 were collected at least once but no more than two times during this period. Serious safety events and per-protocol adverse events were collected.
From 23 Canadian centres, 100 patients were enrolled and 48 completed the study. Measured with the Patient Health Questionnaire–9 items at week 52, 61.5% (40/65) [95% confidence interval (CI): 49.7%-73.4%] of the patients improved in psychologic distress/depression symptoms, which was slightly higher in completers [65.9% (29/44); 95%CI: 51.9%-79.9%)]. At week 52, clinical response and clinical remission were achieved respectively by 65.7% (44/73) and 47.8% (32/73) of the patients. The odds of improving depressive symptoms for those achieving a clinical remission at week 52 was 7.94 higher compared with those not achieving a clinical remission (CI: 1.42, 44.41; P = 0.018). Significant changes from baseline to weeks 8 and 52 were observed in disability, HRQoL, and fatigue. Meaningful improvement was reported in work impairment.
At week 52, over 60% of the UCanADA patients had depressive symptoms significantly reduced, as well as HRQoL, fatigue symptoms, and work impairment improved. No new safety signals were detected.
Core Tip: In real-world at week 52, over 60% of patients with moderate-to-severe ulcerative colitis treated with adalimumab had their depressive symptoms improved, as well as their quality of life, fatigue symptoms, and work impairment. No new safety signals were detected.
- Citation: Bessissow T, Nguyen GC, Tarabain O, Peyrin-Biroulet L, Foucault N, McHugh K, Ruel J. Impact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study. World J Gastroenterol 2022; 28(34): 5058-5075
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/5058.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.5058
Ulcerative colitis (UC)—an intermittent, idiopathic, and chronic disease of the colon—has a worldwide incidence of 1 to 20 per 100000 individuals and a prevalence of 5 to 500 per 100000 individuals[1,2]. As of 2018 in Canada, it is estimated that 120000 individuals live with this disease.
The burden of UC has been recognized to extend beyond its clinical signs and physical symptoms such as bloody diarrhea and abdominal pain, including the development of anxiety/depression, a decreased health-related quality of life (HRQoL), an impact on work productivity and social interactions, and impairments in sexual function[3-6]. As such, having access to multidisciplinary, collaborative, chronic disease models of care improves patients’ HRQoL[7].
Around 30% of patients with inflammatory bowel disease (IBD) experience psychiatric disorders, and depression/anxiety in these patients have been shown to be three times greater than in the general population[8]. In UC patients, the prevalence of depression symptoms and disorders have been estimated as 16.7% [95% confidence interval (CI): 12.0%-21.4%][9]. Assessing the severity of depression in over 158000 IBD patients, a Patient Health Questionnaire–9 items (PHQ-9) pooled mean score of 7.6 (95%CI: 6.3-8.8, on a 0-27 scale) has been reported, which can be interpreted as a mild depression[9,10].
Among physical symptoms, fatigue, which is not relieved by rest and implies limitations of daily activities[11], has been reported by 42% to 47% of UC patients at diagnosis[12]. Fatigue has been shown to impact IBD patients’ QoL and is experienced by those of all ages, with some studies suggesting a greater burden in women[13-17]. In a recent review on IBD, Nocerino et al[18] documented strong associations between fatigue and sleep disturbance and inadequate sleep, highlighting a proportion of more than 50% of both active and inactive IBD patients reporting sleep deficiency.
Consistent with the increasing inclusion of patient’s voice in all aspects of health care as in UC therapies[19] and aiming to align with the FDA guidance[20], the use of patient-reported outcome (PRO) questionnaires are more and more used as clinical endpoints in IBD studies[21]. The use of PRO instruments helps understand patients’ preferences, which in turn has been shown to be associated with treatment acceptance and adherence[22-25].
In IBD populations, depression/anxiety has been measured with a wide range of tools, including the PHQ-9[9,26-29]. The Inflammatory Bowel Disease Questionnaire (IBDQ) and its short version (SIBDQ) as well as the EuroQol 5-Dimentions, 5 Levels (EQ-5D-5L) questionnaire have been used to assess HRQoL[30-34], fatigue has been assessed by the Functional Assessment Chronic Illness Therapy–Fatigue (FACIT-F) questionnaire[18,35-37], and work productivity with the Work Productivity and Activity Impairment (WPAI) questionnaire[26,30,38-40].
Developed and validated in 2012 in a population-based cohort, the inflammatory bowel disease disability index (IBD-DI) is specific to assess disability in IBD patients[41-43]. On a 0-100 scale, the mean (interquartile range) value of the IBD-DI was 35.3 (Q1 = 19.6; Q3 = 51.8). Higher IBD-DI values were associated with female gender (P < 0.001), clinical disease activity (P < 0.0001), and disease duration (P = 0.02)[42].
To provide real-world data on improvements in daily lives of UC patients, the overall goal of the UCanADA study was to gather evidence on effectiveness, quality of life, disability, and work productivity during an adalimumab treatment. The primary objective was to evaluate psychological distress/depression symptoms using change from baseline in the PHQ-9 after 1 year of a real-world adalimumab treatment in moderate-to-severe UC patients.
In this prospective, single arm, 1-year multicenter Canadian post-marketing observational study, adults (≥ 18 years) with a confirmed diagnosis of UC and a moderate-to-severe disease activity–evidenced by either a Mayo endoscopic subscore (MES) of 2 or 3 from endoscopic investigation in the previous 3-mo closest to the baseline visit, or a Mayo rectal bleeding subscore ≥ 2 and a calprotectin value greater than 250 mg/g–were enrolled if they were prescribed adalimumab as part of their treatment by their treating physician. If a patient was previously treated with vedolizumab or any anti-tumor necrosis factor (TNF) agent (except adalimumab), an appropriate washout period took place per routine practice, which period varied usually from 2 to 3 mo.
Excluded from the study were patients who either previously received adalimumab, used infliximab or any anti-TNF agent and did not clinically respond at any time unless they experienced a treatment limiting reaction; had a history of subtotal colectomy with ileorectostomy or colectomy with ileoanal pouch, Kock pouch, or ileostomy for UC or planned bowel surgery; had a current diagnosis of indeterminate colitis, ulcerative proctitis only, or with a current diagnosis and/or have a history of Crohn’s disease; had other TNF immune-modulated disease; OR had a significant history of renal, neurologic, psychiatric, endocrinologic, metabolic, immunologic, cardiovascular, or hepatic disease that in the opinion of the investigator would adversely affect his/her participating in this study. Also, we excluded from the study pregnant or breast-feeding female patients and patients currently participating in another prospective study including controlled clinical trials.
Eligible patients were approached to participate in the study after a decision to change the patient’s therapy for adalimumab was already made by the treating physician. To participate in the study and to disclose personal health information, all patients were required to sign a patient authorization form (or written informed consent), which was approved by an Independent Ethics Committee/Institutional Review Board (ClinicalTrials.gov Identifier: NCT02506179). The study was conducted between July 2015 and December 2019 in 23 Canadian sites, with approximately half of the sites being community based and the other half academic based.
Patients were followed for 52 wk post initiation of adalimumab treatment (baseline). The assessments were performed during patients’ routine care visits schedule, coinciding approximately to 8 and 52 wk after baseline, in accordance with the Canadian approved label (product monograph) and as per regional requirements. The completers population was defined as patients who received at least one dose of treatment, and at least one follow-up appointment and did not terminate the study early or discontinue.
During these visits, the patients’ medical history and changes in medical conditions, previous and concomitant medications, and disease severity and activity [clinical response defined as simple clinical colitis activity index (SCCAI)[44] decrease from baseline of ≥ 2, clinical remission defined as SCCAI score ≤ 2, endoscopic evaluation (MES), assessment of rectal bleeding (Mayo Rectal bleeding Subscore), and the physician’s global assessment (PGA)] were assessed.
Also, patients were required to fill, on paper at the physician’s office, eight PRO questionnaires for evaluating: The presence and severity of depression (PHQ-9 using a 0-27 scale)[10], the entire spectrum of limitations in functioning in patients (IBD-DI questionnaire evaluating 4 domains of body functions, activity and participation, body structures, and environmental factors)[41], HRQoL (EQ-5D-5L questionnaire comprising mobility, self-care, usual activities, pain/discomfort, and anxiety/depression using a 0-1 scale)[45], SIBDQ questionnaire assessing the social, emotional, bowel, and systemic domains on a 1-7 scale[46], fatigue (FACIT-F questionnaire having a fatigue subscale score with a range from 0 to 52)[47], sleep related outcomes [Medical Outcomes Study Sleep scale (MOS Sleep) 12-item questionnaire including sleep disturbance, sleep awakening short of breath or with headache, sleep adequacy, somnolence, and quantity of sleep/optimal sleep][48], work related outcomes [WPAI: UC V2.0[49] presenting percentages of absenteeism (work time missed), presenteeism (impairment while working), an overall work impairment (overall productivity loss, accounting for both absenteeism and presenteeism), and activity impairment (impairment in activities outside work)], and Valuation of Lost Productivity (VOLP) questionnaire[50], assessing the impact of health conditions on lost productivity in monetary units. The order by which the PRO questionnaires were filled was varied to limit the potential of missing data that would systemically be found for a particular instrument.
Safety assessments included serious adverse events (AEs), any non-serious event of malignancy in patients 30 years of age and younger[51], unusual failure in efficacy, and AEs leading to discontinuation. These were coded using Medical Dictionary for Regulatory Activities version 17.1.
The sample size was calculated assuming a proportion of 15% of patients would improve their PHQ-9 score compared with baseline and change severity category. Using an alpha of 0.05 with a lower CI of 6%, a sample size of 72 patients would be needed. To account for a potential 25% attrition over the course of one year, the sample size was increased to 100 patients. It was anticipated that up to 30% of the 100 moderate-to-severe UC patients newly treated with adalimumab would have prior experience with biologics.
The primary effectiveness endpoint—the proportion of patients with a change in depressive symptoms using the PHQ-9 score from baseline following initiation of adalimumab and after 1 year of treatment—was calculated, and the 95%CIs were estimated. Changes in PHQ-9 scores from baseline were tested by paired sample t-test. Least-square mean (LS mean) of the changes were also estimated by the mixed effect repeated measures models where baseline values were included as a covariate. Changes in severity categories were tested by Bowker’s test (kxk table where k > 2) or McNemar’s test (2 × 2 table).
To understand the independent effect of clinical effectiveness on the probability of improving in PHQ-9 at week 52, a logistic regression analysis was conducted to examine the effect of clinical response and clinical remission adjusting for the baseline PHQ-9 score and other potential prognostic factors. A similar analysis was conducted to assess the association between clinical effectiveness on changes of PHQ-9 scores from baseline. LS mean of the changes associated with clinical response and clinical remission was estimated by the mixed effect repeated measures models using all follow-up visits.
For secondary outcomes, the IBD-DI, EQ-5D-5L, SIBDQ, FACIT-F, and MOS Sleep, scores at baseline, week 8, and week 52 were summarized, and changes in scores from baseline were tested by paired sample t-tests. LS mean of the changes were also estimated by the mixed effect repeated measures models where the baseline value was included as a covariate. Productivity outcomes (WPAI and VOLP) at baseline, week 52, and changes in outcome from baseline were summarized. The 95%CIs for the changes were estimated by bootstrapped percentile CIs based on samples of 10000.
The sensitivity to change for the IBD-DI was evaluated using the effective size (ES) and the standardized response mean (SRM). For both statistics, values of 0.020, 0.50, and 0.80 or greater were used to represent small, moderate, and large, respectively. The association between the change in PROs (EQ-5D-5L, SIBDQ, FACIT-F, and MOS Sleep) and clinical response/remission (effectiveness) were assessed using a mixed model for repeated measures using observations from all follow-up visits with the baseline value included in the model as a covariate. All models with repeated measures included a random intercept with the effectiveness variable (fixed, forced-in), visit (fixed, forced-in), baseline value of the PRO measure (fixed, forced-in) and other covariates. Cross-sectional regression models included an intercept with the effectiveness variable (forced-in), baseline value of the PRO measure (fixed) and other covariates. Least squares means, P value and 2-sided 95%CI of the difference between the two groups defined by the clinical effectiveness were determined. Additional details on the statistical analysis used to determine the correlation between effectiveness (clinical response and remission) rates and PRO measures are provided in the Supplementary material section.
Missing data were imputed only for the sensitivity analysis of the primary outcome. For missing responses on PRO questionnaire items, missing data were handled per the imputation solutions provided in the coding of the PRO instruments. To assess the impact of missing data on the primary endpoint estimate, the sensitivity analysis was performed using two imputation methods: Non-responder imputation (NRI), defined as patients who did not provide week 52 effectiveness data or dropped out of the study prior to week 52 were considered as no improvement; and last observation carried forward (LOCF) defined as the last effectiveness assessment prior to week 52 was used for those missing week 52 assessment.
All calculations and analyses were performed using SAS version 9.4 (Cary, NC: SAS Institute Inc.) under the Windows 10 Enterprise operating system at the Centre for Health Evaluation and Outcome Sciences, in Vancouver, Canada.
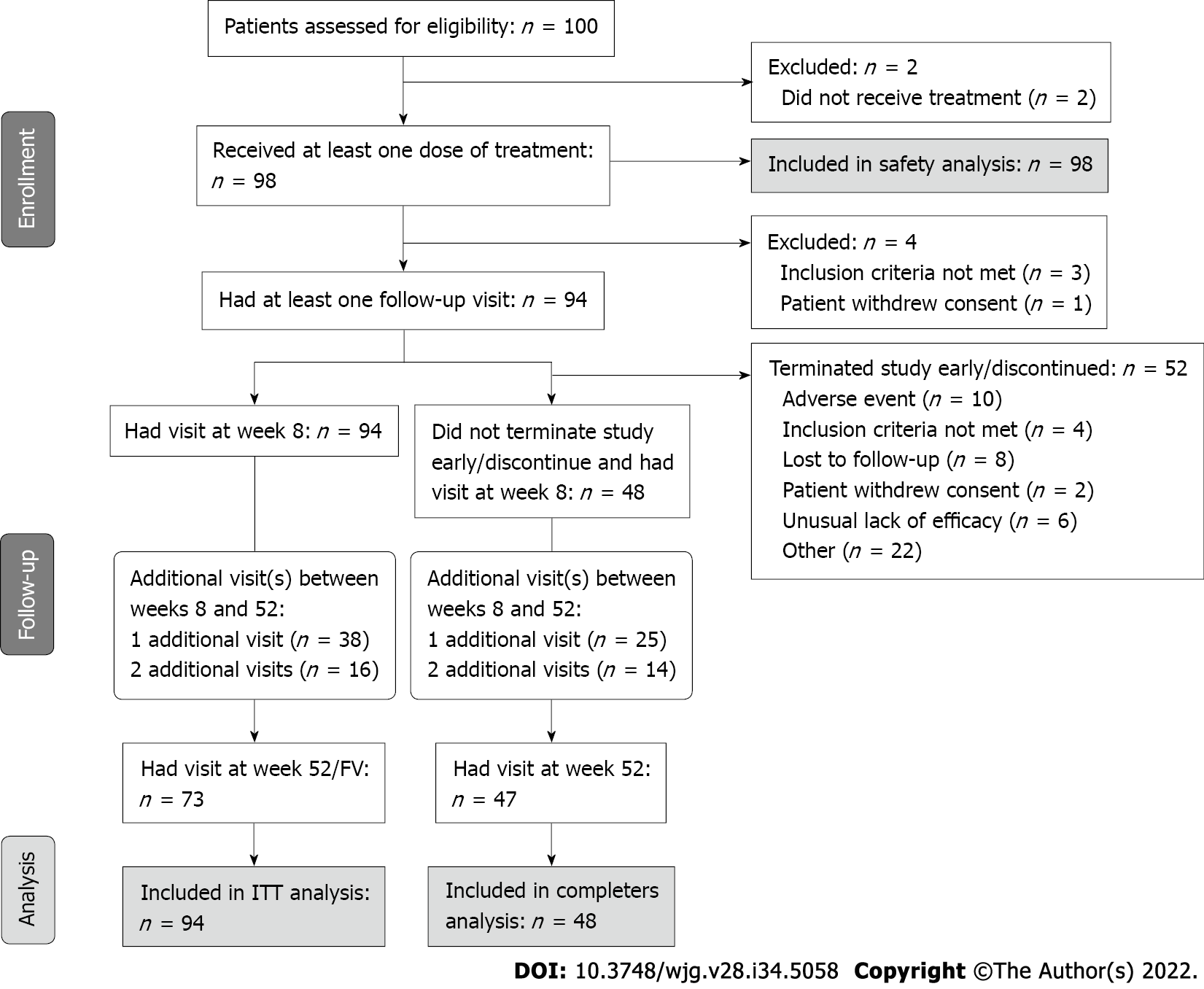
One hundred patients from 23 Canadian sites were included in the study (Figure 1). Respectively, 94, 48, and 98 patients were included in the effectiveness population [intent-to-treat (ITT) population], the completers population, and the safety population. Patients in the ITT population had a mean age (SD) of 42.5 (15.3) years and a mean body mass index of 25.4 (4.5) kg/m2 (Table 1). The majority was White (93.6%) and male (59.6%). The mean age at UC diagnosis was 34.5 (15.3) years, and the mean duration of disease was 7.9 (8.0) years. Forty-eight (48%) patients completed the study.
| Characteristics | N | n (%) or mean ± SD | |
| Age | 94 | 42.5 (15.3) | |
| Gender | Male | 94 | 56 (59.6) |
| BMI (kg/m2) | 91 | 25.4 (4.5) | |
| Race | American Indian/Alaska Native | 94 | 1 (1.1) |
| Asian | 5 (5.3) | ||
| White | 88 (93.6) | ||
| Employment status | Disability | 2 (2.1) | |
| Employed (fulltime, part time < 35 h/week) | 63 (67.0) | ||
| Homemaker | 3 (3.2) | ||
| Retired | 12 (12.8) | ||
| Student | 6 (6.4) | ||
| Temporary leave of absence | 1 (1.1) | ||
| Unemployed | 6 (6.4) | ||
| Unknown | 1 (1.1) | ||
| Tobacco use | Current smoker | 94 | 2 (2.1) |
| Former smoker | 34 (36.2) | ||
| Never smoked | 55 (58.5) | ||
| Unknown | 3 (3.2) | ||
| Alcohol use | Non-drinker | 94 | 20 (21.3) |
| Ex-drinker | 5 (5.3) | ||
| Light (less than 2 drinks per day) | 61 (64.9) | ||
| Moderate (2-4 drinks per day) | 5 (5.3) | ||
| Unknown | 3 (3.2) | ||
| Age at UC diagnosis (yr) | 98 | 34.5 (15.3) | |
| Disease duration (yr) | 98 | 7.9 (8.0) | |
| Family history of UC | No | 98 | 59 (60.2) |
| Yes | 20 (20.4) | ||
| Unknown | 19 (19.4) | ||
| Montreal classification of extent of UC | E2 | 97 | 55 (56.7) |
| (prior 3 mo) | E3 | 42 (43.3) | |
| Mayo Endoscopic Subscore | 1 | 90 | 1 (1.1) |
| (prior 3 mo) | 2 | 66 (73.3) | |
| 3 | 23 (25.6) | ||
| Endoscopy (prior 6 mo) | Yes | 98 | 91 (92.9) |
| UC-related ED visit (prior 6 mo) | Yes | 98 | 13 (13.3) |
| UC-related hospitalization (prior 6 mo) | Yes | 98 | 12 (12.2) |
| Previous biologic use | Yes | 44 | 5 (11.4) |
| Medication use: | Corticosteroids | 98 | 63 (64.3) |
| Since UC diagnosis to prior 6 mo | Imuran (azathioprine) | 37 (37.8) | |
| 6-MP | 8 (8.2) | ||
| 5-ASA | 83 (84.7) | ||
| Methotrexate | 5 (5.1) | ||
| Cyclosporine | 1 (1.0) | ||
| Medication use: | Corticosteroids | 98 | 61 (62.2) |
| Since prior 6 mo to current | Imuran (azathioprine) | 39 (39.8) | |
| 6-MP | 5 (5.1) | ||
| 5-ASA | 67 (68.4) | ||
| Methotrexate | 8 (8.2) | ||
| Cyclosporine | 0 (0.0) |
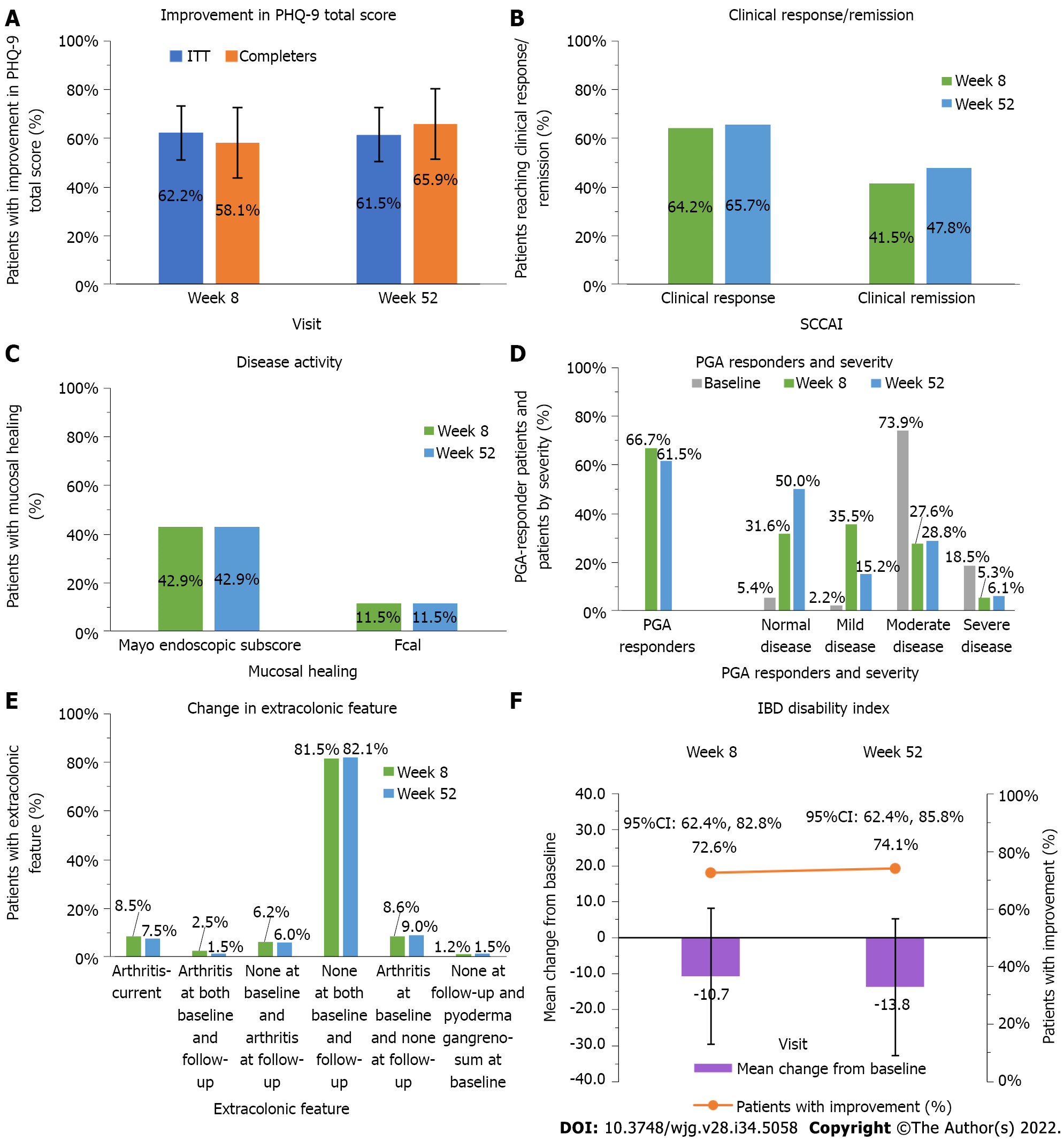
Following routine care treatment with adalimumab, the proportion of patients who improved in psychological distress/depressive symptoms using the PHQ-9 total score at week 52—defined as a change in PHQ-9 total score from baseline, the study primary endpoint—was 61.5% (40/65) (95%CI: 49.7%-73.4%) for the ITT population and 65.9% (29/44) (95%CI: 51.9%-79.9%) for the completers population (Figure 2A). To assess the impact of missing data, the sensitivity analyses conducted on the primary endpoint showed that the proportions of patients who improved in psychological distress/depressive symptoms, using the NRI and LOCF imputation methods, were similar to the proportion obtained using the original non-imputed data analysis, with the ITT population (Supplementary Table 1).
Overall, changes from baseline in the PHQ-9 total score were significant at weeks 8 and 52 (P = 0.010), with changes slightly higher for the completers population [-2.5 (6.1) and -3.4 (6.8) at weeks 8 and 52] than in the ITT population [-2.2 (6.1) -2.4 (7.1)] on a 0-27 scale (Table 2). The proportion of patients with a PHQ-9 total score 10 (yellow flag category, i.e., moderate or more severe depression) was 25.6% (21/82) at week 8, which slightly increased to 29.2% (19/65) at week 52 for the ITT population. These proportions were lower and stable over time (18%-19%) for the completers population. For patients with severe depressive symptoms (red flag category), the proportions improved from 19.1% (18/94) and 16.7% (8/48) at baseline to 12.3% (8/65) and 2.3% (1/44) at week 52 for the ITT population and completers population, respectively.
| Outcome | ITT | Completers | ||||
| Baseline (n = 94) | Week 8 (n = 94) | Week 52/final visit (n = 73) | Baseline (n = 48) | Week 8 (n = 48) | Week 52/final visit (n = 47) | |
| PHQ-9 total score | ||||||
| n | 94 | 82 | 65 | 48 | 43 | 44 |
| mean ± SD | 8.8 (6.3) | 6.8 (5.3) | 6.5 (5.6) | 8.2 (6.7) | 5.8 (5.6) | 4.6 (4.3) |
| Median | 8.0 | 6.0 | 5.0 | 5.0 | 5.0 | 3.0 |
| Min, Max | 0.0, 26.0 | 0.0, 26.0 | 0.0, 22.0 | 1.0, 26.0 | 0.0, 26.0 | 0.0, 15.0 |
| Change from baseline | ||||||
| n | 82 | 65 | 43 | 44 | ||
| mean ± SD | -2.2 (6.1) | -2.4 (7.1) | -2.5 (6.1) | -3.4 (6.8) | ||
| P value | 0.002 | 0.008 | 0.010 | 0.002 | ||
| PHQ-9 category, n (%) | ||||||
| Minimal | 35 (37.2) | 31 (37.8) | 28 (43.1) | 23 (47.9) | 21 (48.8) | 25 (56.8) |
| Mild | 23 (24.5) | 30 (36.6) | 18 (27.7) | 8 (16.7) | 14 (32.6) | 11 (25.0) |
| Moderate | 18 (19.1) | 14 (17.1) | 11 (16.9) | 9 (18.8) | 4 (9.3) | 7 (15.9) |
| Moderately severe | 11 (11.7) | 4 (4.9) | 7 (10.8) | 4 (8.3) | 2 (4.7) | 1 (2.3) |
| Severe | 7 (7.4) | 3 (3.7) | 1 (1.5) | 4 (8.3) | 2 (4.7) | 0 (0.0) |
| P value | 0.280 | 0.681 | 0.610 | 0.542 | ||
| PHQ-9: Yellow flag category, n (%) | ||||||
| 36 (38.3) | 21 (25.6) | 19 (29.2) | 17 (35.4) | 8 (18.6) | 8 (18.2) | |
| P value | 0.028 | 0.083 | 0.021 | 0.059 | ||
| PHQ-9: Red flag category, n (%) | ||||||
| Yes | 18 (19.1) | 7 (8.5) | 8 (12.3) | 8 (16.7) | 4 (9.3) | 1 (2.3) |
| P value | 0.013 | 0.134 | 0.180 | 0.034 | ||
The PHQ-9 questionnaire items that showed the highest improvement from baseline were ‘Poor appetite or overeating’ (response ‘Not at all’ increased by 23.3% from baseline to week 52, and response ‘Nearly every day’ decreased by 20.3% from baseline to week 52), ‘Little interest or pleasure in doing things’ (response ‘Not at all’ increased by 15.3% from baseline to week 52, and response ‘Nearly every day’ decreased by 12.1% from baseline to week 52), and ‘Feeling tired or having little energy’ (response ‘Not at all’ increased by 8.2% from baseline to week 52, and response ‘Nearly every day’ decreased by 20.3% from baseline to week 52) (Supplementary Table 2).
The proportions of patients who achieved a clinical response (decrease from baseline ≥ 2 in SCCAI score) remained similar throughout the study [64.2% (52/81) at week 8 and 65.7% (44/67) at week 52], and the proportion who achieved clinical remission (SCCAI score ≤ 2) slightly increased [41.5% (34/82) at week 8 and 47.8% (32/67) at week 52] in the ITT population (Figure 2B). For the completers population, these proportions increased during the study for both the clinical response and clinical remission, reaching 85.4% (35/41) and 73.2% (30/41), respectively, at week 52 (Supplementary Table 3).
Similarly, the proportions of patients who achieved endoscopic healing remained constant between weeks 8 and 52 [42.9% (6/14) had a Mayo endoscopic score of 0 or 1 and 11.5% (3/26), had a fecal calprotectin concentration < 50 μg/g] in the ITT population (Figure 2C), whereas in the completers population, the proportions increased over time [71.4% (5/7) and 80.0% (8/10), measured with the Mayo endoscopic score and 7.7% (1/13) and 11.8% (2/17) measured with the fecal calprotectin concentration, at weeks 8 and 52, respectively] (Supplementary Table 3).
No major changes were observed over time in the extracolonic feature, with the majority of patients not having arthritis at both baseline and follow-up visit [81.5% (66/81) at week 8 and 82.1% (55/67) at week 52] (Figure 2D). The proportion of patients who had arthritis at baseline and none at follow-up was 8.6% (7/81) at week 8 and 9.0% (6/67) at week 52. Similar proportions were observed in the completers population (Supplementary Table 3).
The proportion of patients who were PGA responders, defined as a decrease from baseline of ≥ 1 point, varied from 66.7% (50/75) to 61.5% (40/65) for the ITT population (Figure 2E) and from 73.2% (30/41) to 83.7% (36/43) for the completers population, from week 8 to week 52, respectively (Supplementary Table 3). While at baseline the majority of patients had moderate disease [73.9% (68/92)], at week 8 the highest proportion of patients had mild disease [35.5% (27/76)], and at week 52, 50.0% (33/66) of patients were assessed as normal (Figure 2E). The proportion of patients assessed with severe disease remained comparable between week 8 [5.3% (4/76)] and week 52 [6.1% (4/66)]. Similar results were reported for the completers population (Supplementary Table 3).
A proportion of 5.5% (4/73) of patients reported complications including hospitalization and surgery at week 52 in the ITT population (Supplementary Table 4), and none were reported among the completers population (Supplementary Table 3). The proportion of patients with current steroid use decreased between week 8 and week 52 from 29.4% (25/85) to 19.2% (14/73) in the ITT population and from 22.2% (10/45) to 12.8% (6/47) in the completers population.
The mean change from baseline in IBD-DI increased from -10.7 (17.21) at week 8 to -13.8 (22.24) at week 52 (P < 0.001), with proportions of patients who improved disability increasing from 72.6% (53/73) to 74.1% (40/54) over time in the ITT population (Figure 2F). Results from the completers population were slightly higher [mean change from baseline = -14.69 (17.99) and -20.09 (17.74) and improved disability = 81.4% (35/43) and 88.9% (32/36) at weeks 8 and 52, respectively] (Supplementary Table 5).
The IBD-DI was moderately sensitive to change for the ITT population, varying from -0.61 to -0.77 for ES and had a SRM of -0.62 (Supplementary Table 6). For the completers population, the IBD-DI was greatly sensitive to change. The ES varied from -0.75 to 1.08 and the SRM varied from -0.82 to -1.13
A correlation analysis showed that at week 52 an improvement in PHQ-9 total score was associated with the baseline PHQ-9 score, with a higher baseline score predicting a greater improvement on the PHQ-9 (Table 3). No associations were detected between an improvement in PHQ-9 and clinical response; however, an association was measured with clinical remission in the ITT analysis (OR: 7.94; 95%CI: 1.42-44.41; P = 0.018), though this was not statistically significant in the completer analysis.
| Analysis population | Parameter | Model coefficient (SE) | Est. odds ratio (95%CI) | P value |
| Clinical response | ||||
| ITT | Baseline PHQ-9 total score | 0.17 (0.06) | 1.19 (1.05-1.34) | 0.005 |
| Clinical response at week 52: Yes versus No | 0.30 (0.65) | 1.35 (0.38-4.84) | 0.648 | |
| UC duration (years) | 0.11 (0.05) | 1.11 (1.00-1.24) | 0.051 | |
| Completers | Baseline PHQ-9 total score | 0.20 (0.10) | 1.22 (1.00-1.48) | 0.049 |
| Clinical response at week 52: Yes versus No | -1.29 (1.29) | 0.28 (0.02-3.43) | 0.317 | |
| UC duration (years) | 0.14 (0.08) | 1.15 (0.98-1.35) | 0.079 | |
| Clinical remission | ||||
| ITT | Baseline PHQ-9 total score | 0.28 (0.09) | 1.33 (1.11-1.59) | 0.002 |
| Clinical remission at week 52: Yes versus No | 2.07 (0.88) | 7.94 (1.42-44.41) | 0.018 | |
| UC duration (years) | 0.11 (0.06) | 1.11 (1.00-1.24) | 0.054 | |
| Completers | Baseline PHQ-9 total score | 0.22 (0.11) | 1.24 (1.00-1.53) | 0.045 |
| Clinical remission at week 52: Yes versus No | 1.39 (1.07) | 4.00 (0.50-32.37) | 0.193 | |
| UC duration (years) | 0.14 (0.08) | 1.15 (0.98-1.36) | 0.095 | |
A regression analysis between the PHQ-9 total score and clinical response/remission at week 52 showed an association at week 52 using the ITT population (P < 0.001), but not in the completer population (P ≥ 0.098) (Supplementary Table 7).
For the other PRO tools used to assess the impact of the adalimumab treatment, changes from baseline at weeks 8 and 52 were significant for the EQ-5D-5L utility score, SIBDQ total score, FACIT-F total score, MOS Sleep Problems Index I and Sleep Problems Index II (P = 0.049) measured in the ITT population (Table 4) and the completers population (Supplementary Tables 8 and 9). A regression analysis showed that changes in the PRO measures between baseline and week 52 were all significantly associated with clinical outcomes (P < 0.001), except for the MOS Sleep measures (P ≥ 0.064) (Supplementary Table 10).
| PRO measure | Baseline | Change from baseline | |||||
| n (%) or mean ± SD | N | Week 8, n (%) or mean ± SD | P value | N | Week 52, n (%) or mean ± SD | P value | |
| EQ-5D-5L | 0.78 (0.17) | 83 | 0.05 (0.17) | 0.021 | 65 | 0.06 (0.24) | 0.049 |
| SIBDQ | |||||||
| Total score | 4.26 (1.08) | 83 | 0.60 (1.08) | < 0.001 | 65 | 0.71 (1.24) | < 0.001 |
| Social function | 4.40 (1.91) | 82 | 0.93 (1.78) | < 0.001 | 64 | 1.09 (2.11) | < 0.001 |
| Emotional function | 4.32 (0.78) | 83 | 0.23 (0.82) | 0.013 | 65 | 0.20 (0.93) | 0.093 |
| Bowel symptoms | 4.25 (1.33) | 83 | 0.69 (1.50) | < 0.001 | 65 | 0.93 (1.66) | < 0.001 |
| Systemic symptoms | 4.11 (1.63) | 83 | 0.63 (1.33) | < 0.001 | 65 | 0.68 (1.56) | < 0.001 |
| FACIT-F | |||||||
| Fatigue subscale | 30.10 (13.76) | 83 | 3.78 (12.29) | 0.006 | 65 | 5.41 (13.87) | 0.003 |
| Physical fatigue | 17.67 (6.59) | 83 | 2.44 (6.27) | < 0.001 | 65 | 3.86 (7.11) | < 0.001 |
| Social impact of fatigue | 20.69 (5.02) | 82 | 0.52 (3.97) | 0.234 | 65 | 1.32 (4.60) | 0.024 |
| Emotional fatigue | 15.37 (4.71) | 83 | 1.10 (4.24) | 0.021 | 65 | 2.06 (5.26) | 0.002 |
| Functional fatigue | 15.65 (5.54) | 83 | 2.07 (5.43) | < 0.001 | 65 | 2.58 (6.36) | 0.002 |
| Trial outcome index | 63.42 (24.02) | 83 | 8.29 (21.79) | < 0.001 | 65 | 11.85 (24.85) | < 0.001 |
| FACT-G total score | 69.38 (16.99) | 82 | 6.16 (15.58) | < 0.001 | 65 | 9.82 (18.58) | < 0.001 |
| FACIT-F total score | 99.48 (29.04) | 82 | 9.99 (26.34) | < 0.001 | 65 | 15.23 (30.64) | < 0.001 |
| MOS Sleep | |||||||
| Sleep problems index I | 40.46 (19.16) | 83 | -4.14 (15.89) | 0.020 | 65 | -6.56 (16.21) | 0.002 |
| Sleep problems index II | 42.30 (19.69) | 83 | -3.47 (15.66) | 0.047 | 65 | -4.92 (16.75) | 0.021 |
| Sleep disturbance scale | 41.52 (25.58) | 83 | -3.67 (20.82 | 0.112 | 65 | -4.22 (22.30) | 0.132 |
| Snoring scale | 30.22 (33.08) | 81 | 0.00 (24.49) | 1.000 | 63 | 2.54 (25.78) | 0.437 |
| Short of breath scale | 11.06 (19.37) | 83 | 2.41 (21.50) | 0.310 | 65 | 3.08 (19.12) | 0.199 |
| Sleep adequacy | 42.13 (26.96) | 83 | 8.07 (26.01) | 0.006 | 65 | 11.69 (26.31) | < 0.001 |
| Somnolence scale | 43.12 (27.35) | 83 | -1.37 (22.42) | 0.581 | 65 | -4.00 (25.24) | 0.206 |
| Sleep quantity | 6.73 (1.41) | 82 | 0.13 (1.29) | 0.371 | 63 | 0.34 (1.31) | 0.042 |
| WPAI1 | |||||||
| Work time missed (%) | 18.9 (31.1) | 48 | -6.7 (30.9) | 37 | -9.4 (35.0) | ||
| Work impairment while working (%) | 39.5 (28.5) | 46 | -14.8 (33.1) | 38 | -14.5 (35.8) | ||
| Overall work impairment (%) | 44.2 (30.1) | 42 | -16.2 (30.2) | 35 | -14.5 (34.4) | ||
| Activity impairment (%) | 46.0 (31.9) | 83 | -16.9 (29.8) | 64 | -16.7 (33.6) | ||
| VOLP | |||||||
| Any paid work productivity loss in the past x months (%) | 45 (76.3) | 58 | -5 (-4.6) | 47 | -17 (-16.7) | ||
| Paid work productivity loss in the past x months (hours) | 98.5 (122.7) | 51 | 13.5 (127.8) | 42 | -42.2 (115.7) | ||
| Any unpaid work productivity loss in the past 7 d (%) | 20 (29.0) | 61 | -8 (-9.3) | 48 | -7 (-1.9) | ||
| Unpaid work productivity loss in the past 7 d (hours) | 3.9 (11.6) | 61 | -3.4 (12.4) | 48 | -1.9 (14.8) | ||
| Any costs of lost productivity in the past x month (%) | 47 (79.7) | 58 | -5 (-7.3) | 47 | -15 (-11.6) | ||
| Total costs of lost productivity in the past x months ($) | 6075.8 (8890.9) | 51 | -1328 (5594.9) | 42 | -1998 (7299.7) | ||
The SIBDQ items of social function and bowel symptoms improved the most from baseline [mean change at week 52, 1.09 (2.11) and 0.93 (1.66), respectively on a 1-7 scale] (P < 0.001) (Table 4). Observing the FACIT-F measures, patients reported gaining more over time from the physical fatigue [3.86 (7.11)] than from the functional fatigue [2.58 (6.36)], emotional fatigue [2.06 (5.26)], and social impact of fatigue [1.32 (4.60)], at week 52 (P = 0.024). For the MOS Sleep subscales, only the sleep adequacy subscale significantly improved over time, with a mean change from baseline of 11.69 (5.26) at week 52 (P < 0.001).
All the WPAI scores improved from baseline, and the ones that improved the most were activity impairment [-16.9% (29.8) at week 8 and -16.7% (33.6) at week 52], i.e., activities performed outside of work, and overall work impairment [-16.2% (30.2) at week 8 and -14.5% (34.4) at week 52], which combines absenteeism and presenteeism at work (Table 4). Similar results were reported for the completers (Supplementary Table 11).
For the VOLP, the most important changes were reported at week 52 and were related to paid work [paid work productivity loss in the past x months = -42.2 (115.7) hours and any paid work productivity loss in the past x months = -17% (-16.7%)] and lost productivity [any costs of lost productivity in the past x month = -15% (11.6%) hours and total costs of lost productivity in the past x months ($) = -1998 (-7299.7) $]. Study completers reported slightly higher results (Supplementary Table 11).
The safety profile was consistent with the known safety profile of adalimumab. During the study, 18 (18.4%) patients experienced at least one AE (Table 5). The AEs reported by more than 1% of patients were: Colitis ulcerative [6 (6.1%) patients], drug ineffective [6 (6.1%) patients], haematochezia [2 (2.0%) patients], and arthralgia [2 (2.0%) patients]. Each of the severe AEs was experienced by only one (1.0%) patient, which included one event each of anal fissure, colitis, dysphagia, and mouth ulceration. One patient experienced two events of severe oesophagitis.
| Events (n = 55) | Patients (n = 98) | |
| All AEs | 55 | 18 (18.4%) |
| Severe AEs | 10 (18.2%) | 5 (5.1%) |
| AEs related to study drug | 17 (30.9%) | 12 (12.2%) |
| Mild | 5 (29.4%) | 4 (4.1%) |
| Moderate | 6 (35.3%) | 6 (6.1%) |
| Severe | 4 (23.5%) | 2 (2.0%) |
| Not provided | 2 (11.8%) | 2 (2.0%) |
| Serious AEs | 27 (49.1%) | 7 (7.1%) |
| Number of patients with AEs | ||
| Resulting in hospitalization | 6 (6.1%) | |
| Resulting in study drug discontinuation | 15 (15.3%) | |
| Malignancy in patients ≤ 30 yr | 0 | 0 |
| Death | 0 | 0 |
Two (2.0%) patients experienced serious treatment-related AEs that were assessed by the investigator to be reasonably possibly related to adalimumab: 1 (1.0%) patient experienced two events of severe oesophagitis that led to hospitalization and prolongation of hospitalization, and one event of severe aggravated colitis that led to hospitalization; 1 (1.0%) patient experienced severe injection site pain. There was one report of cutaneous basal cell cancer in a 63-year-old male. Monitored as per protocol safety variable, there were no reports of malignancy in patients 30 years of age and younger. No death was reported during the study, and no new signal or unexpected trend was identified for the patient population.
To fill an information gap on Canadian real-world data on the effectiveness of adalimumab on PRO measures in moderate-to-severe UC patients, and consistent with the FDA guidelines on the use of PRO measures to support labelling claims[20], the UCanADA study enrolled 100 patients from 23 centres using as a primary endpoint the change from baseline in depressive symptoms at week 52, measured by the PHQ-9 questionnaire.
The PHQ-9 measures in study UCanADA showed that over 60% of the study population improved in psychological distress/depression symptoms during the real-world adalimumab treatment, with most gains observed at week 52 in the completers population (65.9%). Significant changes from baseline were observed at week 8, which were maintained at week 52 and were slightly higher for the completers population (P = 0.010). Despite this improvement, these scores may be interpreted as a remaining mild depression in patients[10] and not necessarily a clinically meaningful change[52-54], which indicates a potential relevance to offer psychological support to this population[7,55]. In the present observational study, while patients who had a preliminary failure to biologics were excluded, patients with secondary failures were included, which may have led to the inclusion of patients with greater psychological burden than biologic-naïve patients.
Our results show a significant change from baseline in PHQ-9 score earlier during treatment than those of a recently reported cohort of 1804 UC outpatients, who were included regardless of treatment assignment or disease activity[26]. However, a similar decrease in PHQ-9 score was measured in UC patients at least 30 d after being initiated on an anti-TNF therapy (including infliximab, adalimumab, or certolizumab) and/or immunomodulator therapy (methotrexate or azathioprine)[29].
At week 52, clinical response and clinical remission were achieved respectively by 65.7% and 47.8% of the ITT population, and 85.4% and 73.2% of the completers population. These results are comparable to those from the InspirADA study at week 8, in which 463 moderate-to-severe UC patients from 92 international sites were treated with adalimumab following usual clinical practice[40].
To our knowledge, UCanADA is the first study reporting associations between PHQ-9 scores and clinical response/remission in UC patients in a real-world setting. A regression analysis showed that in the ITT population, the odds of improving depressive symptoms for those achieving a clinical remission at week 52 was 7.94 higher compared to those not achieving a clinical remission (OR: 7.94; 95%CI: 1.42-44.41; P = 0.018).
These results–as well as the significant associations measured between the PHQ-9 total score and clinical response/remission at week 52 (P < 0.001) and between clinical response/remission and IBD-DI, EQ-5D-5L, SIBDQ total score, and FACIT-F fatigue subscale (P = 0.002)–are consistent with the other findings showing a relationship between disease activity and HRQoL[34,35,56]. In a 6-mo study including 199 UC patients, a consistent and almost linear relationship was demonstrated between SCCAI values and the EQ-5D-5L index values (correlation: ρ: -0.53; P < 0.001)[34].
Other studies conducted in UC patients reported correlations between disease activity indexes and HRQoL measures. Aniwan et al[35] reported a good correlation between the SIBDQ and the combination of self-rated rectal bleeding and stool frequency using the 6-point partial Mayo score (ClinPRO2) and MES, from a study on 90 UC patients (r = -0.70; P < 0.01). Assessed on 110 UC patients, a significant correlation has been reported between SIBDQ and SCCAI and MES alone (r = −0.79 and r = −0.58, respectively)[56]. Consistent with our findings, these support an association between clinical remission and improved HRQoL.
To the PHQ-9 item ‘Feeling tired or having little energy’ between baseline and week 52, the proportion of patients feeling it ‘Nearly every day’ decreased by 20%, and those feeling it ‘Not at all’ increased by 8%. These results are in line with the significant decrease in fatigue shown in the FACIT-F total score and fatigue subscale (P = 0.006).
As fatigue has been reported to be strongly associated with sleep disturbances in IBD patients[18], not surprisingly our scores from the MOS sleep problems indexes I and II also significantly improved during the study, as well as sleep adequacy and sleep quantity subscores (P = 0.042 at week 52). Using the NIH PROMIS questionnaire in 160 patients with IBD using either and anti-TNF or vedolizumab, Stevens et al[57] reported significant and meaningful improvement in sleep quality by week 6 (P = 0.009), which was paralleled by a significant reduction in depression (P < 0.05), as measured in the UCanADA study population. These results reinforce the need to assess sleep disorders a part of an algorithmic approach for the systemic workup of fatigue[18].
Also related to fatigue, in a study including 1185 IBD patients (462 with UC), Williet et al[58] reported a strong correlation between FACIT-F score and IBD-DI measure (r = -0.78) as well as overall work impairment (r = -0.70). Similar trends were observed in UCanADA, i.e., a significant improvement in fatigue (FACIT-F total score; P < 0.001) mirrored by a significant improvement in IBD-DI measures (P < 0.001) as well as a meaningful improvement in overall work impairment during the study. Our IBD-DI measures are similar to those reported from other UC populations[43]. In a meta-analysis including 3167 IBD patients, Lo et al[43] reported a significant lower disease disability rates in patients on biological treatment than those on corticosteroids (P < 0.01).
At week 8 and week 52, there was a gain between 15% and 17% in work impairment, overall work impairment, and activity impairment in the UCanADA study population. These represent less gains than those reported from the InspirADA population at week 26; however, they were twice as high as the minimal clinically important difference of 7% in WPAI outcome[59].
Limitations of the research methods used in this study are related to, but may not have been limited to, the observational nature of the study with regards to missing data, which has been alleviated with the use of sensitivity analyses for the primary endpoint. This study consisted of a small cohort of patients, and only 48 (48%) patients completed the study. However, the results between the ITT population and completers population were fairly consistent. The PRO questionnaires being self-administered provide subjective data as opposed to objective data. The collection of secondary PROs data may be subject to a recall bias.
At week 52 in a real-world setting, adalimumab was effective in reducing depressive symptoms in patients with UC, with more than 60% of the patients achieving an improvement the PHQ-9 with a mean improvement of 2.4 points. A broad range of PROs including HRQoL and work productivity also significantly improved during the study. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
The efficacy and safety of adalimumab have been demonstrated in pivotal trials, but there remained a need to assess more holistically how the clinical results translate into concrete improvements in key aspects of the daily lives of ulcerative colitis (UC) patients, such as symptoms, health-related quality of life (HRQoL), and disability.
Although some patient-reported outcomes (PROs) from existing studies may have items capturing some of these aspects, limited data was available for adalimumab in UC, specifically on psychological distress/depression, disability, fatigue, and pain or sleep quality in real-life setting.
The overarching goal for the UCanADA study was to assess the real-life effectiveness of adalimumab on PRO measures, while taking the opportunity to use the inflammatory bowel disease disability index to assess the impact of adalimumab on key components of patients’ functioning when affected with moderate-to-severe UC.
UCanADA was a single arm, prospective, 1-year multicenter Canadian post-marketing observational study in which multiple PRO questionnaires were completed—with psychologic distress/depression symptoms as the primary endpoint—by patients with moderate-to-severe UC. Assessments were performed during patients’ routine care visit schedule, which was at the initiation of adalimumab (baseline), after induction (approximately 8 wk), and 52 wk after baseline. Additional optional assessments between weeks 8 and 52 were collected at least once but no more than two times during this period. Serious safety events and per-protocol adverse events were collected.
One hundred patients were included in this final analysis, with 94 (94%) patients included in the efficacy population (identified as the intent-to-treat (ITT) population), 48 (48%) patients included in the completers’ population, and 98 (98%) patients included in the safety population. The primary endpoint–the proportion of patients who achieved a change from baseline, defined as an improvement in total severity score relative to baseline, in the Patient Health Questionnaire–9 items (PHQ-9) measure at week 52–was 61.5% [40/65 patients; 95% confidence interval (CI): 49.7%-73.4%] for the ITT population and 65.9% (29/44 patients; 95%CI: 51.9%-79.9%) for completers. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
At week 52, adalimumab, used in a real-life study, was effective in reducing depressive symptoms in patients with UC, with more than 60% of the patients achieving an improvement the PHQ-9 with a mean improvement of 2.4 points. Thus, the treatment with adalimumab contributed to reducing the depressive symptoms frequently experienced in patients with UC as well as improving a broad range of PROs such as HRQoL and work productivity, as assessed with PRO instruments. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
Improvements in PHQ-9 were associated with clinical remission. Beyond the PHQ-9, significant improvements in several PROs were observed suggesting an improvement in HRQoL and work productivity as well. The population in the study, as well as the inclusion and exclusion criteria, was representative of the target population. In addition, coinciding the study visits with the patient’s routine care visit schedule helped increase generalizability of the PRO instruments by decreasing the impact on real life.
AbbVie and the authors thank all study investigators (Dr. Kenneth Atkinson, Columbia Gastroenterology Management Ltd.; Dr. Guy Aumais, CHUM-Hôpital Maisonneuve-Rosemont; Dr. Rahman Bacchus, Dr. Rahman Bacchus Medicine Corporation; Dr. Andrew Bellini, Bellini Medicine Professional Corporation; Dr. Edmond-Jean Bernard, Le Centre des Maladies Inflammatoires et Intestinales de
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Innocenti T, Italy; Kaewput W, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. BMJ. 2013;346:f432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 2. | Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 915] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 3. | McCormick JB, Hammer RR, Farrell RM, Geller G, James KM, Loftus EV Jr, Mercer MB, Tilburt JC, Sharp RR. Experiences of patients with chronic gastrointestinal conditions: in their own words. Health Qual Life Outcomes. 2012;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies Revisited: A Systematic Review of the Comorbidity of Depression and Anxiety with Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 5. | Regueiro M, Greer JB, Szigethy E. Etiology and Treatment of Pain and Psychosocial Issues in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:430-439.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Yarlas A, Rubin DT, Panés J, Lindsay JO, Vermeire S, Bayliss M, Cappelleri JC, Maher S, Bushmakin AG, Chen LA, DiBonaventura M. Burden of Ulcerative Colitis on Functioning and Well-being: A Systematic Literature Review of the SF-36® Health Survey. J Crohns Colitis. 2018;12:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Jones JL, Nguyen GC, Benchimol EI, Bernstein CN, Bitton A, Kaplan GG, Murthy SK, Lee K, Cooke-Lauder J, Otley AR. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J Can Assoc Gastroenterol. 2019;2:S42-S48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Zhang CK, Hewett J, Hemming J, Grant T, Zhao H, Abraham C, Oikonomou I, Kanakia M, Cho JH, Proctor DD. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1732-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. 2016;87:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 10. | Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21545] [Cited by in RCA: 28894] [Article Influence: 1203.9] [Reference Citation Analysis (0)] |
| 11. | Barsevick AM, Cleeland CS, Manning DC, O'Mara AM, Reeve BB, Scott JA, Sloan JA; ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes). ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39:1086-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Grimstad T, Norheim KB, Isaksen K, Leitao K, Hetta AK, Carlsen A, Karlsen LN, Skoie IM, Gøransson L, Harboe E, Aabakken L, Omdal R. Fatigue in Newly Diagnosed Inflammatory Bowel Disease. J Crohns Colitis. 2015;9:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Romberg-Camps MJ, Bol Y, Dagnelie PC, Hesselink-van de Kruijs MA, Kester AD, Engels LG, van Deursen C, Hameeteman WH, Pierik M, Wolters F, Russel MG, Stockbrügger RW. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis. 2010;16:2137-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Cohen BL, Zoëga H, Shah SA, Leleiko N, Lidofsky S, Bright R, Flowers N, Law M, Moniz H, Merrick M, Sands BE. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39:811-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, Miller N, Rogala L, Rawsthorne P, Lix L, Bernstein CN. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Jonefjäll B, Simrén M, Lasson A, Öhman L, Strid H. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United European Gastroenterol J. 2018;6:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Nocerino A, Nguyen A, Agrawal M, Mone A, Lakhani K, Swaminath A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv Ther. 2020;37:97-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Daperno M, Armuzzi A, Danese S, Fries W, Liguori G, Orlando A, Papi C, Principi M, Rizzello F, Viscido A, Gionchetti P. Unmet Medical Needs in the Management of Ulcerative Colitis: Results of an Italian Delphi Consensus. Gastroenterol Res Pract. 2019;2019:3108025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Food and Drug Administration. Guidance for industry patient-reported outcome measures: Use in medical product development to support labeling claims. In: Services USDoHaH, editor, 2009: 39. |
| 21. | Bojic D, Bodger K, Travis S. Patient Reported Outcome Measures (PROMs) in Inflammatory Bowel Disease: New Data. J Crohns Colitis. 2017;11:S576-S585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Bastemeijer CM, Voogt L, van Ewijk JP, Hazelzet JA. What do patient values and preferences mean? Patient Educ Couns. 2017;100:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Coello PA, Brożek J, Wiercioch W, Etxeandia-Ikobaltzeta I, Akl EA, Meerpohl JJ, Alhazzani W, Carrasco-Labra A, Morgan RL, Mustafa RA, Riva JJ, Moore A, Yepes-Nuñez JJ, Cuello-Garcia C, AlRayees Z, Manja V, Falavigna M, Neumann I, Brignardello-Petersen R, Santesso N, Rochwerg B, Darzi A, Rojas MX, Adi Y, Bollig C, Waziry R, Schünemann HJ. Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach. Health Qual Life Outcomes. 2017;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Postmus D, Mavris M, Hillege HL, Salmonson T, Ryll B, Plate A, Moulon I, Eichler HG, Bere N, Pignatti F. Incorporating patient preferences into drug development and regulatory decision making: Results from a quantitative pilot study with cancer patients, carers, and regulators. Clin Pharmacol Ther. 2016;99:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Ho M, Saha A, McCleary KK, Levitan B, Christopher S, Zandlo K, Braithwaite RS, Hauber AB; Medical Device Innovation Consortium’s Patient Centered Benefit-Risk Steering Committee. A Framework for Incorporating Patient Preferences Regarding Benefits and Risks into Regulatory Assessment of Medical Technologies. Value Health. 2016;19:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Ghosh S, Sensky T, Casellas F, Rioux LC, Ahmad T, Márquez JR, Vanasek T, Gubonina I, Sezgin O, Ardizzone S, Kligys K, Petersson J, Suzuki Y, Peyrin-Biroulet L. A Global, Prospective, Observational Study Measuring Disease Burden and Suffering in Patients with Ulcerative Colitis Using the Pictorial Representation of Illness and Self-Measure Tool. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Moon JR, Lee CK, Hong SN, Im JP, Ye BD, Cha JM, Jung SA, Lee KM, Park DI, Jeen YT, Park YS, Cheon JH, Kim H, Seo B, Kim Y, Kim HJ; MOSAIK study group of the Korean Association for Study of Intestinal Diseases. Unmet Psychosocial Needs of Patients with Newly Diagnosed Ulcerative Colitis: Results from the Nationwide Prospective Cohort Study in Korea. Gut Liver. 2020;14:459-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Geiss T, Schaefert RM, Berens S, Hoffmann P, Gauss A. Risk of depression in patients with inflammatory bowel disease. J Dig Dis. 2018;19:456-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Horst S, Chao A, Rosen M, Nohl A, Duley C, Wagnon JH, Beaulieu DB, Taylor W, Gaines L, Schwartz DA. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci. 2015;60:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Armuzzi A, Tarallo M, Lucas J, Bluff D, Hoskin B, Bargo D, Cappelleri JC, Salese L, daCosta DiBonaventura M. The association between disease activity and patient-reported outcomes in patients with moderate-to-severe ulcerative colitis in the United States and Europe. BMC Gastroenterol. 2020;20:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Walter E, Hausberger SC, Groß E, Siebert U. Health-related quality of life, work productivity and costs related to patients with inflammatory bowel disease in Austria. J Med Econ. 2020;23:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Teich N, Grümmer H, Jörgensen E, Liceni T, Holtkamp-Endemann F, Fischer T, Hohenberger S. Golimumab in real-world practice in patients with ulcerative colitis: Twelve-month results. World J Gastroenterol. 2020;26:2852-2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Paschos P, Katsoula A, Salanti G, Giouleme O, Athanasiadou E, Tsapas A. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther. 2018;48:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Panés J, Domènech E, Aguas Peris M, Nos P, Riestra S, Juliá de Páramo B, Cea-Calvo L, Romero C, Marín-Jiménez I. Association between disease activity and quality of life in ulcerative colitis: Results from the CRONICA-UC study. J Gastroenterol Hepatol. 2017;32:1818-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Aniwan S, Bruining DH, Park SH, Al-Bawardy B, Kane SV, Coelho Prabhu N, Kisiel JB, Raffals LE, Papadakis KA, Pardi DS, Tremaine WJ, Loftus EV Jr. The Combination of Patient-Reported Clinical Symptoms and an Endoscopic Score Correlates Well with Health-Related Quality of Life in Patients with Ulcerative Colitis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Saraiva S, Cortez-Pinto J, Barosa R, Castela J, Moleiro J, Rosa I, da Siva JP, Dias Pereira A. Evaluation of fatigue in inflammatory bowel disease - a useful tool in daily practice. Scand J Gastroenterol. 2019;54:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Villoria A, García V, Dosal A, Moreno L, Montserrat A, Figuerola A, Horta D, Calvet X, Ramírez-Lázaro MJ. Fatigue in out-patients with inflammatory bowel disease: Prevalence and predictive factors. PLoS One. 2017;12:e0181435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Christiansen LK, Lo B, Bendtsen F, Vind I, Vester-Andersen MK, Burisch J. Health-related quality of life in inflammatory bowel disease in a Danish population-based inception cohort. United European Gastroenterol J. 2019;7:942-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Yarlas A, Maher SM, Bayliss MS, Lovley A, Cappelleri JC, DiBonaventura MD. Psychometric validation of the work productivity and activity impairment questionnaire in ulcerative colitis: results from a systematic literature review. J Patient Rep Outcomes. 2018;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Travis S, Feagan BG, Peyrin-Biroulet L, Panaccione R, Danese S, Lazar A, Robinson AM, Petersson J, Pappalardo BL, Bereswill M, Chen N, Wang S, Skup M, Thakkar RB, Chao J. Effect of Adalimumab on Clinical Outcomes and Health-related Quality of Life Among Patients With Ulcerative Colitis in a Clinical Practice Setting: Results From InspirADA. J Crohns Colitis. 2017;11:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, Kostanjsek N, Stucki G, Colombel JF; International Programme to Develop New Indexes for Crohn's Disease (IPNIC) group. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 42. | Gower-Rousseau C, Sarter H, Savoye G, Tavernier N, Fumery M, Sandborn WJ, Feagan BG, Duhamel A, Guillon-Dellac N, Colombel JF, Peyrin-Biroulet L; International Programme to Develop New Indexes for Crohn's Disease (IPNIC) group; International Programme to Develop New Indexes for Crohn's Disease (IPNIC) group. Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. Gut. 2017;66:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 43. | Lo B, Prosberg MV, Gluud LL, Chan W, Leong RW, van der List E, van der Have M, Sarter H, Gower-Rousseau C, Peyrin-Biroulet L, Vind I, Burisch J. Systematic review and meta-analysis: assessment of factors affecting disability in inflammatory bowel disease and the reliability of the inflammatory bowel disease disability index. Aliment Pharmacol Ther. 2018;47:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1041] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 45. | Kind P. The EuroQol instrument - an index of health-related quality of life. In: Spilker B, editor Quality of Life and Pharmacoeconomics in Clinical Trials 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers, 1996. |
| 46. | Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571-1578. [PubMed] |
| 47. | Tinsley A, Macklin EA, Korzenik JR, Sands BE. Validation of the functional assessment of chronic illness therapy-fatigue (FACIT-F) in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Hays R, Stewart A. Sleep Measures. In: Stewart A, Ware J, editors. Measuring functioning and well-being; the medical outcomes study approach Duke edition: Duke University Press; 1992: 235–259. |
| 49. | Zhang W, Bansback N, Boonen A, Young A, Singh A, Anis AH. Validity of the work productivity and activity impairment questionnaire--general health version in patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Zhang W, Bansback N, Kopec J, Anis AH. Measuring time input loss among patients with rheumatoid arthritis: validity and reliability of the Valuation of Lost Productivity questionnaire. J Occup Environ Med. 2011;53:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | U.S. Food and Drug Administration. FDA Drug Safety Communication: UPDATE on Tumor Necrosis Factor (TNF) blockers and risk for pediatric malignancy. 2018. [cited 10 January 2022]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-update-tumor-necrosis-factor-tnf-blockers-and-risk-pediatric. |
| 52. | Round JM, Lee C, Hanlon JG, Hyshka E, Dyck JRB, Eurich DT. Changes in patient health questionnaire (PHQ-9) scores in adults with medical authorization for cannabis. BMC Public Health. 2020;20:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Hudgens S, Floden L, Blackowicz M, Jamieson C, Popova V, Fedgchin M, Drevets WC, Cooper K, Lane R, Singh J. Meaningful Change in Depression Symptoms Assessed with the Patient Health Questionnaire (PHQ-9) and Montgomery-Åsberg Depression Rating Scale (MADRS) Among Patients with Treatment Resistant Depression in Two, Randomized, Double-blind, Active-controlled Trials of Esketamine Nasal Spray Combined With a New Oral Antidepressant. J Affect Disord. 2021;281:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Turkoz I, Alphs L, Singh J, Jamieson C, Daly E, Shawi M, Sheehan JJ, Trivedi MH, Rush AJ. Clinically meaningful changes on depressive symptom measures and patient-reported outcomes in patients with treatment-resistant depression. Acta Psychiatr Scand. 2021;143:253-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, Kvern B, Lazarescu A, Liu L, Paterson WG, Sidani S, Vanner S. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J Can Assoc Gastroenterol. 2019;2:6-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 56. | Theede K, Kiszka-Kanowitz M, Nordgaard-Lassen I, Mertz Nielsen A. The Impact of Endoscopic Inflammation and Mucosal Healing on Health-related Quality of Life in Ulcerative Colitis Patients. J Crohns Colitis. 2015;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Stevens BW, Borren NZ, Velonias G, Conway G, Cleland T, Andrews E, Khalili H, Garber JG, Xavier RJ, Yajnik V, Ananthakrishnan AN. Vedolizumab Therapy Is Associated with an Improvement in Sleep Quality and Mood in Inflammatory Bowel Diseases. Dig Dis Sci. 2017;62:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Williet N, Sarter H, Gower-Rousseau C, Adrianjafy C, Olympie A, Buisson A, Beaugerie L, Peyrin-Biroulet L. Patient-reported Outcomes in a French Nationwide Survey of Inflammatory Bowel Disease Patients. J Crohns Colitis. 2017;11:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 59. | Binion DG, Louis E, Oldenburg B, Mulani P, Bensimon AG, Yang M, Chao J. Effect of adalimumab on work productivity and indirect costs in moderate to severe Crohn's disease: a meta-analysis. Can J Gastroenterol. 2011;25:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |