Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.5023
Peer-review started: May 24, 2022
First decision: June 27, 2022
Revised: July 9, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: September 14, 2022
Processing time: 105 Days and 12.8 Hours
Data that assess maternal and infant outcomes in hepatitis C virus (HCV)-infected mothers are limited.
To investigate the frequency of complications and the associated risk factors.
We performed a cohort study to compare pregnancy and fetal outcomes of HCV-viremic mothers with those of healthy mothers. Risk factors were analyzed with logistic regression.
Among 112 consecutive HCV antibody-positive mothers screened, we enrolled 79 viremic mothers. We randomly selected 115 healthy mothers from the birth registry as the control. Compared to healthy mothers, HCV mothers had a significantly higher frequency of anemia [2.6% (3/115) vs 19.0% (15/79), P < 0.001] during pregnancy, medical conditions that required caesarian section [27.8% (32/115) vs 48.1% (38/79), P = 0.004], and nuchal cords [9.6% (11/115) vs 34.2% (27/79), P < 0.001]. In addition, the mean neonatal weight in the HCV group was significantly lower (3278.3 ± 462.0 vs 3105.1 ± 459.4 gms; P = 0.006), and the mean head circumference was smaller (33.3 ± 0.6 vs 33.1 ± 0.7 cm; P = 0.03). In a multivariate model, HCV-infected mothers were more likely to suffer anemia [adjusted odds ratio (OR): 18.1, 95% confidence interval (CI): 4.3-76.6], require caesarian sections (adjusted OR: 2.6, 95%CI: 1.4-4.9), and have nuchal cords (adjusted OR: 5.6, 95%CI: 2.4-13.0). Their neonates were also more likely to have smaller head circumferences (adjusted OR: 2.1, 95%CI: 1.1-4.3) and lower birth weights than the average (≤ 3250 gms) with an adjusted OR of 2.2 (95%CI: 1.2-4.0). The vertical transmission rate was 1% in HCV-infected mothers.
Maternal HCV infections may associate with pregnancy and obstetric complications. We demonstrated a previously unreported association between maternal HCV viremia and a smaller neonatal head circumference, suggesting fetal growth restriction.
Core Tip: Although hepatitis C virus (HCV) affects a significant number of pregnant women, there is limited data regarding the impact of HCV active infection on pregnancy and infant outcomes. The current cohort study compared maternal complications and fetal development of HCV mothers with detectable levels of HCV RNA with those of healthy mothers. The study demonstrates a previously unreported association between maternal HCV viremia and a smaller neonate head circumference. In addition, HCV viremia was an independent predictor for negative maternal outcomes including anemia during pregnancy, medical conditions that required caesarian section, and nuchal cords. These findings increase the need for close antenatal surveillance in HCV mothers with viremia for maternal complications and delayed fetal development.
- Citation: Pan CQ, Zhu BS, Xu JP, Li JX, Sun LJ, Tian HX, Zhang XH, Li SW, Dai EH. Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China. World J Gastroenterol 2022; 28(34): 5023-5035
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/5023.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.5023
Hepatitis C virus (HCV) infection is a common infectious disease that affects the liver and remains a significant global health burden[1]. Although spontaneous viral clearance may occur in approximately 15% of patients who have acute HCV infection, the majority develop a chronic HCV infection. Among patients who have chronic hepatitis C, approximately 10%-15% will progress to cirrhosis within the first 20 years of infection, which eventually becomes decompensated without appropriate therapy and places them at high risk of developing liver cancer[2]. The prevalence of antibodies to HCV (HCV-Ab) in pregnant women is 0.1% to 2.4%, although it is much higher in some endemic areas[3]. The proportion of pregnant women with HCV-Ab positivity and active infection with viremia is approximately 60% to 70%[3].
Globally, up to 8% of pregnant women are infected with HCV in highly endemic areas[4]. In the United States, surveillance published in 2017 revealed a nationwide increase in HCV infection among pregnant women, which is an increasing but potentially modifiable threat to maternal and child health[5]. The proportion of infants born to HCV-infected women is also increasing in the United States[6]. It has been reported that vertical transmission is the most common mechanism of HCV infection in children, occurring in approximately 6% of infants born to women with HCV infection[7]. The risk of HCV vertical transmission increases if the maternal serum HCV viral load is above 105 copies/mL[8,9]. In addition, published studies have suggested that vertical transmission encompasses several potential transmission routes from an infected woman to her newborn, including intrauterine, intrapartum, and postnatal routes[10-13]. According to the American Association for the Study of Liver Diseases guidelines, all pregnant women should be tested for HCV infections, ideally at the time of initiation of prenatal care[14].
Although HCV affects a significant number of pregnant women, there are limited data regarding the impact of HCV active infection on pregnancy and infant outcomes. Prior studies of HCV and pregnancy have focused on the vertical transmission rates of HCV infection using limited assessments of the effects of chronic HCV infections on maternal health, complications during delivery, and fetal complications[15]. Therefore, there are data gaps in supporting strategies for the clinical management of mothers with HCV infections during pregnancy. Additionally, the identification of adverse consequences could improve current perinatal care and monitoring recommendations. With that in mind, we conducted a retrospective cohort study to compare the frequency and severity of adverse maternal outcomes during pregnancy, as well as fetal and infant outcomes, between mothers with HCV viremia and healthy mothers.
This is a single-center retrospective observational cohort study conducted at a tertiary referral university hospital located in Shijiazhuang city of Hebei province in China, which receives referrals from different levels of community medical clinics and health facilities in the city. The study site mainly included mothers with infectious diseases, including hepatitis B, hepatitis C, and human immunodeficiency virus (HIV). The Institutional Review Board approved the study, and the need for informed consent was waived. Local standards of care for prenatal care include regular clinic visits approximately every 4 to 6 wk during pregnancy for mothers who are infected with chronic viral hepatitis. Mothers received a symptom-directed physical exam, blood tests, and ultrasonography exams from the early second trimester to delivery. Viral hepatitis and HIV screening were performed at the first prenatal visit (often during the first or early second trimester), and hospital delivery was mandated in the entire province except in an emergency event.
In the current study, patients who attended the services in the prenatal care clinic from November 1, 2011 to May 31, 2020 were screened for eligibility. Adult patients (age > 18 years old) who had a diagnosis of HCV-Ab positivity for at least six months and detectable levels of HCV RNA (> 15 IU/mL) during prenatal screening were eligible for enrollment. Major exclusion criteria were the following: Coinfection with hepatitis B virus, hepatitis D virus, or HIV; current or history of intravenous drug use or sexually transmitted diseases; liver cancer; autoimmune liver disease; primary biliary cirrhosis; and alcohol-related liver diseases (consumption of more than 20 g/day of alcohol for > 5 years). Patients with other liver diseases, including inherited liver diseases and drug-induced liver injury, were also excluded. For each patient included in the HCV group, a healthy mother was identified and selected from the Delivery Suite Registry at random. The selection was based on their infants' date of birth (± 30 d) matched to those of the HCV mothers with similar baseline values (matched for gestational days and parity). Based on the ratio of approximately 1:1 to match the number of subjects enrolled in the HCV group, a similar number of subjects was included in the healthy mother group. All patients included in the study were not smoking, drinking alcohol, or using any recreational drugs since these variables may affect the infants' outcomes.
Laboratory measurements for subjects in the study were all performed by the central laboratory in the medical center. HCV-Ab was tested by a chemiluminescent microparticle immunoassay (Autobio, Zhengzhou, China). Serum HCV RNA levels were measured with the real-time quantitative polymerase chain reaction method by using the Cobas TaqMan polymerase chain reaction assay according to the laboratory manuals (Roche Diagnostics, United States). An undetectable level was defined as below the lowest level of quantitation = 15 IU/mL. The comprehensive chemistry panel was tested using a HITACHI 7600 fully automatic biochemical analyzer, with the ULN of alanine aminotransferase (ALT) set at 40 U/L (Wako Pure Chemical Industries, Ltd. Japan).
Using an electronic medical record system and paper charts, we collected the following maternal data: Patients’ demographic information and pertinent clinical data, including a history of liver disease or hepatocellular carcinoma, pregnancy or obstetric complications; medication lists; positive physical findings, including pelvimetry, labor outcomes, and modes of delivery; laboratory results of completed blood count, coagulation tests, chemistry panels with ALT, and virological tests; and imaging results if available. Pertinent data were assessed at all visits starting from gestational week 12 with a four-week interval before delivery, at delivery, and at postpartum weeks 12, 24, and 36. Perinatal information for fetal development, including birth weight, height, Apgar scores, gestational age, and perinatal complications such as birth trauma and neonatal jaundice, was extracted from the neonatal records. Infant outcomes, such as intrauterine growth restriction, birth defects, macrosomia, low birth weight, and meconium staining stool, were collected.
The primary assessment was to analyze the frequency of maternal complications (both pregnancy and obstetrics complications) and negative fetal outcomes in HCV-infected mothers with viremia vs those in healthy mothers. In addition, vertical transmission rates were analyzed among mothers with HCV infection. Secondary assessments were the association between demographic or clinical features and negative maternal or fetal outcomes in a multivariable logistic regression analysis. The current study used the following criteria to define the vertical transmission of HCV and considered the transmission confirmed if any of the following occurred: (1) Detection of HCV RNA in an infant who is 3 to 6 months old; (2) Detection of HCV RNA in the infant on at least 2 occasions; (3) Finding elevated serum aminotransferase levels in an HCV-Ab positive child (ULN = 40 U/mL); or (4) Confirming an identical viral genotype between mother and child[3].
Data analyses were performed using the Statistical Package for Social Science for Windows, Version 25.0 (SPSS Inc., IBM, New York, United States). Frequencies and percentages were used to summarize the categorical variables. Fisher’s exact tests or chi-squared tests were used when comparing data between and within groups. Depending on the underlying distribution of the data, descriptive values are expressed as the means ± SD or medians and interquartile ranges. The student's t-test was used to assess continuous variables between groups. The maternal outcomes or infant outcomes were calculated per pregnant mother and/or per infant when appropriate. The baseline demographic or characteristic variables were analyzed as independent variables, whereas the negative maternal or infant outcomes were considered dependent variables. Risk factors identified from the univariate analysis (P value < 0.05) were further analyzed in the multivariate logistic regression model. The aforementioned risk factors associated with negative outcomes are presented with crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs). All tests were two-tailed with a 95%CI, and a P value < 0.05 was considered significant.
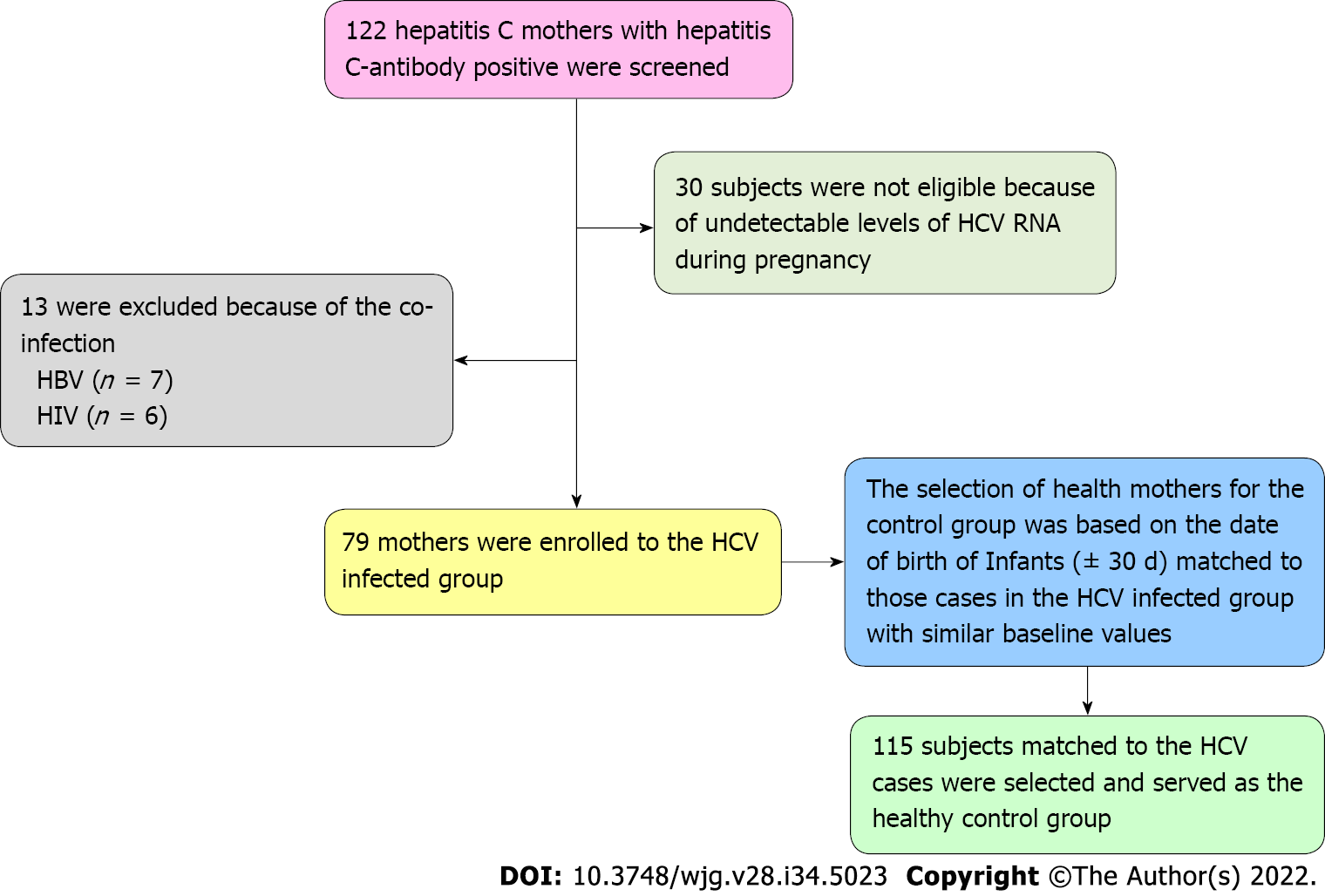
Among the 122 consecutive HCV antibody-positive pregnant women screened, 30 were not eligible due to undetectable levels of HCV RNA throughout the pregnancy. In addition, 13 patients were excluded because they were coinfected with HIV (n = 6) or hepatitis B virus (n = 7). As a result, seventy-nine patients who had HCV viremia during pregnancy were eligible for the HCV group. In addition, 115 healthy mothers were identified and selected from the Delivery Suite Registry at random (delivery date ± 30 d matched to those cases in the HCV-infected group with similar baseline variables). As a result, our cohort consisted of 194 pregnant women with 79 and 115 mothers in the HCV group and a healthy mother (noninfected) group, respectively. The patient selection process is shown in Figure 1. All patients in the HCV group had no clinical indicator for liver decompensation. The clinical characteristics of the study patients are presented in Table 1. The demographic characteristics were well matched between the two groups in the majority of variables, including pre-pregnancy mean BMI, the number of parities or pluralities, mean gestational days, and mean ALT at delivery. However, mothers in the healthy group had a significantly older mean age (29.4 ± 4.9 vs 25.8 ± 4.7 years, P < 0.001), a low frequency of intertuberous diameter < 8.5 cm (29.6% vs 48.1%, P = 0.009) and were taller (160.9 ± 4.0 vs 159.6 ± 3.8 cm, P < 0.001) than those in the HCV group.
| Variables presented with n (%), mean ± SD, or specified | HCV mothers with viremia (n = 79)1 | Healthy mothers (n = 115)1 | P value |
| Maternal characteristics | |||
| Age (yr) | 25.8 ± 4.7 | 29.4 ± 4.9 | < 0.001 |
| < 20 | 2/79 (2.5) | 0/115 (0.0) | 0.003 |
| 20-34 | 73/79 (92.4) | 94/115 (81.7) | |
| ≥ 35 | 4/79 (5.1) | 21/115 (18.3) | |
| Height, cm | 159.6 ± 3.8 | 160.9 ± 4.0 | 0.02 |
| Pre-pregnancy BMI | 27.4 ± 3.4 | 27.5 ± 3.1 | 0.80 |
| < 23 | 4/79 (5.1) | 3/115 (2.6) | 0.44 |
| 23-29 | 53/79 (67.1) | 86/115 (74.8) | |
| ≥ 29 | 22/79 (27.8) | 26/115 (22.6) | |
| History of miscarriage | 1/79 (1.3) | 1/115 (0.9) | > 0.99 |
| History of stillbirth | 2/79 (2.5) | 2/115 (1.7) | > 0.99 |
| Previous uterine surgery | 28/79 (35.4) | 35/115 (30.4) | 0.46 |
| Parity | |||
| 0-1 | 47/79 (59.5) | 62/115 (53.9) | 0.44 |
| ≥ 2 | 32/79 (40.5) | 53/115 (46.1) | |
| Plurality | |||
| 0-1 | 47/79 (59.5) | 62/115 (53.9) | 0.44 |
| > 2 | 32/79 (40.5) | 53/115 (46.1) | |
| Gestational (days) | 273.8 ± 9.5 | 276.0 ± 9.1 | 0.11 |
| Interspinous distance, median (IRQ), cm | 25.0 (24.0, 25.0) | 25.0 (24.0, 25.0) | 0.23 |
| Intercristal distance, median (IRQ), cm | 27.0 (27.0, 28.0) | 27.0 (27.0, 28.0) | 0.40 |
| External conjugate, median (IRQ), cm | 20.0 (20.0, 21.0) | 20.0 (20.0, 21.0) | 0.33 |
| Intertuberous diameter, median (IRQ), cm | 8.5 (8.0, 8.5) | 8.5 (8.0, 8.5) | 0.006 |
| < 8.5 | 38/79 (48.1) | 34/115 (29.6) | 0.009 |
| ≥ 8.5 | 41/79 (51.9) | 81/115 (70.4) | |
| Hemoglobin, median (IRQ), g/L | 111.0 (100.0, 119.0) | 110.0 (100.0, 119.0) | 0.88 |
| < 110 | 37/79 (46.8) | 51/115 (44.3) | 0.73 |
| ≥ 110 | 42/79 (53.2) | 64/115 (55.7) | |
| Platelet, median (IRQ), × 109/L | 207.0 (166.0, 242.0) | 201.0 (167.0, 242.0) | 0.95 |
| Prothrombin time, median (IRQ), seconds | 10.6 (10.1, 11.1) | 10.5 (10.0, 11.1) | 0.40 |
| ALT at delivery, U/L | 26.3 ± 20.1 | 16.6 ± 56.7 | 0.15 |
| HCV RNA at delivery, IU/mL | 19, 217, 509 ± 35, 745, 723 | - | - |
Data from gestational week 12 to delivery about pregnancy or obstetric complications and maternal laboratory abnormalities were analyzed. The following pregnancy and obstetric complications were identified in both groups (Table 2): Preterm labor, preeclampsia, eclampsia, gestational hypertension, anemia, abnormal renal or thyroid function, oligohydramnios, gestational diabetes, nuchal cord, umbilical cord prolapses, postpartum hemorrhage, premature rupture of membranes, and cesarean section due to medical needs. When comparing the aforementioned outcomes or laboratory abnormalities between the HCV-infected and healthy individuals, a significantly higher frequency of anemia during pregnancy was observed in the HCV group [19.0% (15/79) vs 2.6% (3/115), P < 0.001]. In addition, a significantly higher frequency of nuchal cords [34.2% (27/79) vs 9.6% (11/115); P < 0.001] and cesarean sections due to medical needs [48.1% (38/79) vs 27.8% (32/115); P = 0.004] was reported in the HCV group. The frequencies of other pregnancy or obstetric complications did not differ between the two groups (Table 2).
| Variables, n (%) | HCV-infected group (n = 79), % | Healthy mother group (n = 115), % | P value |
| Pregnancy complications | |||
| Preterm (< 37 wk) | 1/79 (1.3) | 0/115 (0.0) | > 0.99 |
| Preeclampsia | 3/79 (3.8) | 0/115 (0.0) | 0.07 |
| Eclampsia | 0/79 (0.0) | 2/115 (2.5) | 0.17 |
| Gestational hypertension | 0/79 (0.0) | 4/115 (3.5) | 0.15 |
| Abnormal thyroid function | 4/79 (5.1) | 1/115 (0.9) | 0.16 |
| Gestational diabetes | 4/79 (5.1) | 4/115 (3.5) | 0.72 |
| Oligohydramnios | 6/79 (7.6) | 3/115 (2.6) | 0.16 |
| Abnormal renal function | 49/79 (62.0) | 64/115 (55.7) | 0.38 |
| Anemia during pregnancy | 15/79 (19.0) | 3/115 (2.6) | < 0.001 |
| Obstetric complications | |||
| Rates of cesarean section1 | 38/79 (48.1) | 32/115 (27.8) | 0.004 |
| Nuchal cord | 27/79 (34.2) | 11/115 (9.6) | < 0.001 |
| Umbilical cord prolapses | 1/79 (1.3) | 0/115 (0.0) | 0.41 |
| Postpartum hemorrhage | 1/79 (1.3) | 0/115 (0.0) | 0.41 |
| Premature rupture of membranes | 3/79 (3.8) | 4/115 (3.5) | > 0.99 |
When comparing the fetal and infant outcomes between the HCV-infected and healthy mother groups (Table 3), we observed a significantly lower mean ± SD body weight in neonates who were born to HCV-infected mothers (3105.1 ± 459.4 vs 3278.3 ± 462.0 gms; P = 0.006). However, the frequency of low birth weight (< 2500 g) did not differ between the two groups [8.9% (7/79) vs 3.5% (4/115); P = 0.20]. The other variables did not differ between the two groups. In addition, neonates in the HCV group had a significantly smaller mean head circumference (33.1 ± 0.7 vs 33.3 ± 0.6 cm; P = 0.03). The other measurements did not differ between the two groups, which included gestational weeks, the percentage of neonates that reached full-term or small for gestational age at delivery, and the mean height at birth. There were no miscarriages, stillbirths, birth defects, or Apgar scores < 7 at 5 min after birth in the entire cohort.
| Infant characteristics at birth1 variables presented with n (%) or mean ± SD | Infants from HCV-infected mothers (n = 79) | Infants from healthy mothers | P value |
| Gestational weeks | 39.1 ± 1.4 | 39.4 ± 1.3 | 0.11 |
| Full-term birth | 74/79 (93.7) | 107/115 (93.0) | 0.86 |
| Meconium staining positive | 8/79 (10.1) | 11/115 (9.6) | > 0.99 |
| Height at birth, cm | 49.0 ± 1.6 | 49.4 ± 1.0 | 0.05 |
| Head circumference, cm | 33.1 ± 0.7 | 33.3 ± 0.6 | 0.03 |
| Weight at birth, gms | 3105.1 ± 459.4 | 3278.3 ± 462.0 | 0.006 |
| ≤ 3250 gms | 49/79 (62.0) | 52/115 (45.2) | 0.02 |
| < 2500 gms | 7/79 (8.9) | 4/115 (3.5) | 0.20 |
| Small for gestational age2 | 1/79 (1.3) | 1/115 (0.9) | > 0.99 |
| Birth defects | 0/79 (0.0) | 0/115 (0.0) | > 0.99 |
| Apgar score < 7 at 5 min | 0/79 (0.0) | 0/115 (0.0) | > 0.99 |
| HCV-Ab (+) at birth, n (%) | 79/79 (100) | - | - |
| Detectable HCV RNA, n (%) | 1/79 (1.27) | - | - |
Among infants who were born to HCV-infected mothers, all were tested HCV-Ab positive at birth, and one had a detectable level of HCV RNA (2165 IU/mL). All infants in the study cohort were breastfed. Their HCV-Ab became negative beyond six months, except for the one who had HCV viremia at birth. This infant continued to have HCV antibodies and detectable levels of HCV RNA measured at the ages of three months and nine months, meeting the criteria of chronic hepatitis C infection. The HCV transmission rate in our study was 1.3% (n = 1/79). In the review of maternal characteristics, the mother was 25 years old with a maternal HCV RNA level of 2.58 × 5 Log10 IU/mL at delivery. She had a history of blood transfusion and was diagnosed with chronic HCV infection during prenatal screening. Her pregnancy was uneventful, with normal levels of ALT throughout the entire pregnancy. She delivered a girl with normal physical development at gestational week 39 plus 5 d.
When comparing the pregnancy and obstetric complications between the two groups, we found that a significantly higher frequency of anemia, nuchal cord, and cesarean section due to medical needs occurred among HCV-infected mothers. The crude and adjusted ORs with 95%CIs of each risk factor are presented in Table 4. The analyses indicated that HCV infection was the only factor associated with anemia (adjusted OR: 18.1, 95%CI: 4.3-76.6), increased numbers of C sections due to medical needs (adjusted OR: 2.6, 95%CI: 1.4-4.9), and nuchal cords during pregnancies (adjusted OR: 5.6, 95%CI: 2.4-13.0). Since a significantly smaller head circumference and lower mean birth weight were the only two negative fetal outcomes identified in infants from HCV-infected mothers, we analyzed the maternal risk factors (Table 5) and found that maternal HCV infection was associated with these negative outcomes. The adjusted ORs of maternal HCV infection associated with a smaller head circumference and birth weight ≤ 3250 gms were 2.1 (95%CI: 1.1-4.3) and 2.2 (95%CI: 1.2-4.0), respectively.
| Clinical variables | Case/exposed1 | Crude OR (95%CI); P value | Adjusted OR (95%CI); P value |
| C-section2 | |||
| Age, n (%) | |||
| < 35 | 59/194 (30.4) | 1 | 1 |
| ≥ 35 | 11/194 (5.7) | 1.5 (0.6-3.4); P = 0.38 | 1.5 (0.6-3.9); P = 0.42 |
| Nulliparity, n (%) | |||
| No | 37/194 (19.1) | 1 | 1 |
| Yes | 33/194 (17.0) | 0.6 (0.3-1.0); P = 0.06 | 0.6 (0.3-1.1); P = 0.08 |
| BMI, n (%) | |||
| < 30 | 57/194 (29.4) | 1 | 1 |
| ≥ 30 | 13/194 (6.7) | 1.3 (0.6-2.7); P = 0.56 | 1.2 (0.5-2.7); P = 0.65 |
| HCV infection, n (%) | |||
| No | 32/194 (16.5) | 1 | 1 |
| Yes | 38/194 (19.6) | 2.4 (1.3-4.4); P = 0.004 | 2.6 (1.4-4.9); P = 0.003 |
| Intertuberous diameter, n (%) | |||
| ≥ 8.5 | 41/194 (21.1) | 1 | 1 |
| < 8.5 | 29/194 (14.9) | 1.3 (0.7-2.2); P = 0.41 | 2.6 (1.4-4.9); P = 0.65 |
| Nuchal cord | |||
| Age, n (%) | |||
| < 35 | 33/194 (17.0) | 1 | 1 |
| ≥ 35 | 5/194 (2.6) | 1.0 (0.4-2.9); P = 0.96 | 2.8 (0.8-10.3); P = 0.11 |
| Nulliparity, n (%) | |||
| No | 13/194 (6.7) | 1 | 1 |
| Yes | 25/194 (12.9) | 1.6 (0.8-3.5); P = 0.19 | 2.0 (0.9-4.8); P = 0.11 |
| BMI, n (%) | |||
| < 30 | 32/194 (16.5) | 1 | 1 |
| ≥ 30 | 6/194 (3.1) | 0.9 (0.4-2.5); P = 0.90 | 0.7 (0.2-1.9); P = 0.44 |
| HCV infection, n (%) | |||
| No | 11/194 (5.7) | 1 | 1 |
| Yes | 27/194 (13.9) | 4.9 (2.3-10.7); P < 0.001 | 5.6 (2.4-13.0); P < 0.001 |
| Intertuberous diameter, n (%) | |||
| ≥ 8.5 | 20/194 (10.3) | 1 | 1 |
| < 8.5 | 18/194 (9.3) | 1.3 (0.7-2.4); P = 0.35 | 1.3 (0.6-2.8); P = 0.51 |
| Maternal anemia during pregnancy | |||
| Age, n (%) | |||
| < 35 | 19/194 (9.8) | 1 | 1 |
| ≥ 35 | 3/194 (1.5) | 1.1 (0.3-3.9); P = 0.91 | 2.5 (0.5-13.3); P = 0.28 |
| Nulliparity, n (%) | |||
| No | 13/194 (6.7) | 1 | 1 |
| Yes | 9/194 (4.6) | 0.5 (0.2-1.2); P = 0.13 | 0.5 (0.2-1.4); P = 0.18 |
| BMI, n (%) | |||
| < 30 | 20/194 (10.3) | 1 | 1 |
| ≥ 30 | 2/194 (1.0) | 0.5 (0.1-2.1); P = 0.33 | 0.4 (0.1-1.8); P = 0.21 |
| HCV infection, n (%) | |||
| No | 3/194 (1.5) | 1 | 1 |
| Yes | 19/194 (9.8) | 11.8 (3.4-41.6); P < 0.001 | 18.1 (4.3-76.6); P < 0.001 |
| Intertuberous diameter, n (%) | |||
| ≥ 8.5 | 13/194 (6.7) | 1 | 1 |
| < 8.5 | 9/194 (4.6) | 1.2 (0.5-3.0); P = 0.35 | 0.8 (0.3-2.3); P = 0.72 |
| Clinical variables | Case/exposed1 | Crude OR (95%CI) | Adjusted OR (95%CI) |
| Head circumference ≤ 33 cm at birth | |||
| Maternal age, n (%) | |||
| < 35 | 118 /194 (60.8) | 1 | 1 |
| ≥ 35 | 18 /194 (9.3) | 1.1 (0.4-2.8); P = 0.82 | 2.0 (0.7-5.7); P = 0.19 |
| Nulliparity, n (%) | |||
| No | 53/194 (27.3) | 1 | 1 |
| Yes | 83 /194 (42.8) | 1.9 (1.0-3.6); P = 0.04 | 2.4 (1.2-4.9); P = 0.01 |
| BMI, n (%) | |||
| < 30 | 120/194 (61.9) | 1 | 1 |
| ≥ 30 | 16/194 (8.2) | 0.4 (0.2-0.8); P = 0.008 | 0.3 (0.1-0.7); P = 0.003 |
| HCV infection, n (%) | |||
| No | 75/194 (38.7) | 1 | 1 |
| Yes | 61/194 (31.4) | 1.8 (0.9-3.5); P = 0.08 | 2.1 (1.1-4.3); P = 0.03 |
| Intertuberous diameter, n (%) | |||
| ≥ 8.5 | 85/194 (43.8) | 1 | 1 |
| < 8.5 | 51/194 (26.3) | 1.1 (0.6-2.0); P = 0.86 | 1.0 (0.5-2.0); P = 0.75 |
| Weight at birth ≤ 3250 gms | |||
| Age, n (%) | |||
| < 35 | 88/194 (45.4) | 1 | 1 |
| ≥ 35 | 13/194 (6.7) | 1.0 (0.4-2.3); P > 0.99 | 1.7 (0.6-4.3); P = 0.29 |
| Nulliparity, n (%) | |||
| No | 39/194 (20.1) | 1 | 1 |
| Yes | 62/194 (32.0) | 1.6 (0.9-2.8); P = 0.13 | 1.8 (0.9-3.4); P = 0.07 |
| BMI, n (%) | |||
| < 30 | 88/194 (45.4) | 1 | 1 |
| ≥ 30 | 13 /194 (6.7) | 0.6 (0.3-1.2); P = 0.16 | 0.5 (0.2-1.1); P = 0.09 |
| HCV infection HCV, n (%) | |||
| No | 52/194 (26.8) | 1 | 1 |
| Yes | 49/194 (25.3) | 2.0 (1.1-3.5); P = 0.02 | 2.2 (1.2-4.0); P = 0.01 |
| Intertuberous diameter, n (%) | |||
| ≥ 8.5 | 61/194 (31.4) | 1 | 1 |
| < 8.5 | 40/194 (20.6) | 1.2 (0.7-2.2); P = 0.46 | 1.1 (0.6-2.1); P = 0.66 |
Although HCV vertical transmission can occur in up to 5.8% of mother-infant pairs[16], many children can clear HCV infection spontaneously[17]. The disease can also be cured with oral antiviral therapy starting at the age of 3[17]. Therefore, the clinical landscape of managing HCV-infected mothers has recently shifted from addressing HCV vertical transmission to the assessment and management of negative pregnancy or neonatal outcomes. Published studies have linked several negative pregnancy outcomes to maternal HCV infection, including intrahepatic cholestasis[18-20], gestational diabetes[21-23], the premature rupture of the membranes[24,25], the requirement for cesarean delivery[24,25], preterm delivery[23], small for gestational age[26], and low birth weight[26]. However, its effect on restriction or disturbance of intrauterine fetal growth remains inconclusive[21,23,26-28].
Our study assessed both maternal and fetal outcomes in viremic mothers with HCV infections. To our knowledge, this is the first study from China to assess pregnancy outcomes in HCV viremic mothers. We found that HCV might be associated with a higher frequency of nuchal cords and a smaller neonatal head circumference, which has not been reported in the literature before. We also found that HCV viremia was linked to pregnancy anemia, cesarean sections due to medical needs, and low gestational weight in neonates. In addition, we observed that lower birth weight was associated with maternal infection, which was consistent with published data from the United States and Europe[23,26]. In the context of all infants in our study being breastfed, the HCV vertical transmission rate was 1.27%, which was within the range (0.2%-6%) of the already published studies[16,29].
Early studies by Jaffery et al[30] and Bohman et al[31] found that fetal outcomes did not differ between HCV-positive mothers and healthy controls[30,31]. However, in a study by Salemi et al[29], the risk of an adverse neurological outcome was higher in infants born to HCV mothers, including feeding difficulties (OR: 1.32, 95%CI: 1.06-1.64) and neonatal seizures (OR: 1.74, 95%CI: 0.98-3.10)[29]. The aforementioned studies have limitations of lacking a well-defined study population because the diagnosis of HCV was based on the HCV antibody, and HCV RNA was not always tested. Paternoster et al[24] observed that intrahepatic cholestasis was more common in HCV-RNA-positive mothers than in HCV-RNA-negative mothers, suggesting that HCV viremia may lead to different outcomes[19]. In addition, cofounders such as intravenous drug use or sexually transmitted diseases may not be adjusted in studies based on pregnancy registries[26,32]. These factors could contribute to the discrepancies among the study findings. In the context of the paucity of data and infrequency of fetal negative events, Huang et al[33] performed a meta-analysis and found that low birth weight was linked to maternal HCV infection (OR: 1.97, 95%CI: 1.43-2.71)[33].
In our study, birth weight ≤ 3250 gms was associated with HCV exposure. There was also a trend of HCV-exposed neonates with a birth weight of < 2500 gms. More importantly, our cohort demonstrates a previously unreported association between maternal HCV viremia and a smaller neonatal head circumference. Our findings provide new evidence supporting the intrauterine restriction of fetal growth in a well-defined HCV population, which enrolled only HCV-infected mothers with detectable levels of HCV RNA who had no history of intravenous drug use or sexually transmitted diseases.
Although the mechanism of fetal growth restriction is not fully understood, several studies have suggested that HCV-induced inflammation in the placenta may cause fetal development restriction. In an in vitro study, HCV infected a human cytotrophoblast and changed its ultrastructure dramatically upon infection[33]. In addition, Hurtado et al[34] observed that the cytotoxicity of natural killer cells and natural killer T cells was enhanced in the placenta, and placental natural killer T-cell cytotoxicity was further increased by HCV infections[34]. Several population-based retrospective cohort studies reported higher rates of gestational diabetes in HCV-infected mothers than in noninfected mothers[35,36], but the association was limited to women with excessive weight gain during pregnancy. Our study did not show such complications, which is likely because our patients are Asians with a much lower body mass index than those in other studies[21,24,26].
In this cohort study, some limitations should be addressed. Being a single-center retrospective design, this study has a limited capacity when adjusting or balancing all covariates between the HCV-exposed and HCV nonexposed groups. Additionally, we did not have HCV genotype data. However, published studies in China indicated that the majority of Chinese patients with HCV had genotype 1[36]. Second, cohort data about HCV antibody-positive but nonviremic mothers are limited: These mothers were not enrolled in our study due to the small number of patients in our center (n = 30, Figure 1). Further studies in this subgroup will add to the understanding of pregnancy outcomes. Third, the liver fibrosis stages for patients with HCV infection were not assessed in this study, although all patients had no clinical indications of liver decompensation. Therefore, future studies might be needed to investigate whether HCV-infected patients with advanced fibrosis have negative maternal and fetal outcomes. Last, the frequency of negative events in HCV-infected mothers could be underestimated due to the maternal mean age being younger than that of healthy mothers.
In conclusion, our study demonstrates a previously unreported association between maternal HCV viremia and a smaller neonatal head circumference. Given our new findings on the intrauterine restriction of fetal growth from HCV exposure, screening all mothers during pregnancy for HCV should be a mandatory practice. More importantly, our findings indicate a need for close antenatal surveillance for maternal complications and delayed fetal development in HCV mothers with viremia. Last, our data support that preconception health management should include HCV screening, so HCV infection can be treated before pregnancy to improve the health of both the mothers and infants.
Hepatitis C virus (HCV) infection remains a significant global health burden, and there is a high proportion of women with antibodies to HCV positive whose active infection with viremia. In addition, HCV infection among pregnant women is an increasing but potentially modifiable threat to maternal and child health.
Although HCV affects a significant number of pregnant women, there is limited data regarding the impact of HCV active infection on pregnancy and infant outcomes. Therefore, there are data gaps in supporting strategies for clinical management of mothers with HCV infections during pregnancy.
We conducted a retrospective cohort study to compare the frequency and severity of adverse maternal outcomes during pregnancy, as well as fetal and infant outcomes between mothers with HCV viremia and healthy mothers.
A retrospective observational cohort study was conducted to compare pregnancy and fetal outcomes of HCV-viremic mothers with those of healthy mothers. After HCV mothers with viremia and healthy mothers were enrolled, we collected their demographic information and pertinent clinical data using an electronic medical record system and paper charts. Perinatal information for fetal development and infant outcomes were extracted from the neonatal records. Data analyses were performed using the Statistical Package for Social Science for Windows, Version 25.0 (SPSS Inc., IBM, New York, United States).
Our study enrolled 79 viremic mothers and 115 healthy mothers. Compared to healthy mothers, HCV mothers had a significantly higher frequency of anemia, caesarian section, and nuchal cords during pregnancy. In addition, the mean neonatal weight and head circumference in the HCV group was significantly lower. In a multivariate model, similar results were found.
Our study demonstrates the association between maternal HCV viremia and a smaller neonate head circumference. We also confirmed the high frequency of pregnancy and obstetric complications in HCV viremic mothers.
Multi-center and large sample studies are needed to verify these results in the future and to investigate if HCV-infected patients with advanced fibrosis have negative maternal and fetal outcomes.
We thank the medical staff in the Department of Obstetrics and Gynecology, The Fifth Hospital of Shijiazhuang, for their support and assistance during our study. We also like to thank Mr. Andrew Park (Northern Valley Regional High School at Old Tappan, United States) for proofreading the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han GR, China; Lewis-Ximenez L, Brazil; Tamori A, Japan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C 2002 (June 10-12, 2002). Gastroenterology. 2002;123:2082-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36:S106-S113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Spera AM, Eldin TK, Tosone G, Orlando R. Antiviral therapy for hepatitis C: Has anything changed for pregnant/Lactating women? World J Hepatol. 2016;8:557-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C Virus Infection Among Women Giving Birth - Tennessee and United States, 2009-2014. MMWR Morb Mortal Wkly Rep. 2017;66:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, Huang X, Leake JA, Ward JW, Vellozzi C. Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep. 2016;65:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M, Bettocchi S, Selvaggi L. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Hayashida A, Inaba N, Oshima K, Nishikawa M, Shoda A, Hayashida S, Negishi M, Inaba F, Inaba M, Fukasawa I, Watanabe H, Takamizawa H. Re-evaluation of the true rate of hepatitis C virus mother-to-child transmission and its novel risk factors based on our two prospective studies. J Obstet Gynaecol Res. 2007;33:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, Vierucci A. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 180] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Mok J, Pembrey L, Tovo PA, Newell ML; European Paediatric Hepatitis C Virus Network. When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed. 2005;90:F156-F160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Arshad M, El-Kamary SS, Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period--are they opportunities for treatment? J Viral Hepat. 2011;18:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Pembrey L, Newell ML, Tovo PA; EPHN Collaborators. The management of HCV infected pregnant women and their children European paediatric HCV network. J Hepatol. 2005;43:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | AASLD. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. [cited 10 February 2022]. Available from: https://wwwhcvguidelinesorg/evaluate/testing-and-linkage. |
| 15. | Society for Maternal-Fetal Medicine (SMFM); Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Duan Z, Jia JD, Hou J, Lou L, Tobias H, Xu XY, Wei L, Zhuang H, Pan CQ. Current challenges and the management of chronic hepatitis C in mainland China. J Clin Gastroenterol. 2014;48:679-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Reddick KL, Jhaveri R, Gandhi M, James AH, Swamy GK. Pregnancy outcomes associated with viral hepatitis. J Viral Hepat. 2011;18:e394-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003-2005 Washington state birth cohort. Am J Obstet Gynecol. 2008;199:38.e1-38.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Money D, Boucoiran I, Wagner E, Dobson S, Kennedy A, Lohn Z, Krajden M, Yoshida EM. Obstetrical and neonatal outcomes among women infected with hepatitis C and their infants. J Obstet Gynaecol Can. 2014;36:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Dibba P, Cholankeril R, Li AA, Patel M, Fayek M, Dibble C, Okpara N, Hines A, Ahmed A. Hepatitis C in Pregnancy. Diseases. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Epstein RL, Espinosa C. Hepatitis C Virus in Neonates and Infants. Clin Perinatol. 2021;48:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 367] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Paternoster DM, Fabris F, Palù G, Santarossa C, Bracciante R, Snijders D, Floreani A. Intra-hepatic cholestasis of pregnancy in hepatitis C virus infection. Acta Obstet Gynecol Scand. 2002;81:99-103. [PubMed] |
| 25. | Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Rac MW, Sheffield JS. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin North Am. 2014;41:573-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. Am J Perinatol. 2013;30:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Floreani A. Hepatitis C and pregnancy. World J Gastroenterol. 2013;19:6714-6720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Salemi JL, Whiteman VE, August EM, Chandler K, Mbah AK, Salihu HM. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J Viral Hepat. 2014;21:e144-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Jaffery T, Tariq N, Ayub R, Yawar A. Frequency of hepatitis C in pregnancy and pregnancy outcome. J Coll Physicians Surg Pak. 2005;15:716-719. [PubMed] |
| 31. | Bohman VR, Stettler RW, Little BB, Wendel GD, Sutor LJ, Cunningham FG. Seroprevalence and risk factors for hepatitis C virus antibody in pregnant women. Obstet Gynecol. 1992;80:609-613. [PubMed] |
| 32. | Berkley EM, Leslie KK, Arora S, Qualls C, Dunkelberg JC. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Huang QT, Hang LL, Zhong M, Gao YF, Luo ML, Yu YH. Maternal HCV infection is associated with intrauterine fetal growth disturbance: A meta-analysis of observational studies. Medicine (Baltimore). 2016;95:e4777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Nie QH, Gao LH, Cheng YQ, Huang XF, Zhang YF, Luo XD, Wang JQ, Wang YY. Hepatitis C virus infection of human cytotrophoblasts cultured in vitro. J Med Virol. 2012;84:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Hurtado CW, Golden-Mason L, Brocato M, Krull M, Narkewicz MR, Rosen HR. Innate immune function in placenta and cord blood of hepatitis C--seropositive mother-infant dyads. PLoS One. 2010;5:e12232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Trevisanuto D, Peruzzetto C, Cavallin F, Vedovato S, Cosmi E, Visentin S, Chiarelli S, Zanardo V. Fetal placental inflammation is associated with poor neonatal growth of preterm infants: a case-control study. J Matern Fetal Neonatal Med. 2013;26:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |