Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.5007
Peer-review started: May 20, 2022
First decision: July 13, 2022
Revised: July 19, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 14, 2022
Processing time: 110 Days and 4.9 Hours
Slow transit constipation (STC) is a common intestinal disease with increasing incidence. STC results from various factors, such as the enteric nervous system and metabolic changes. As a classical formula of traditional Chinese medicine, Ji-Chuan decoction (JCD) has been extensively and effectively used in STC tre
To explore the integrated regulatory pattern of JCD against STC through hyphenated techniques from metabolism, network pharmacology and molecular methods.
STC model mice were generated by intragastric administration of compound diphenoxylate (10 mg/kg/d) for 14 d. The STC mice in the low dose of JCD (3.04 g/kg), middle dose of JCD (6.08 g/kg) and high dose of JCD (12.16 g/kg) groups were orally administered JCD solution once a day for 2 wk. The acetylcholine (ACH) level was examined by enzyme-linked immunosorbent assay. The pathological features of colon tissue were observed by hematoxylin and eosin staining. The differentially expressed metabolites and metabolic pathways were tested by nontargeted metabolomics. The main targets and core ingredients of JCD were identified by network pharmacology, and the expression of AKT was confirmed by immunohistochemistry. Finally, the pathways involved in JCD treatment were predicted using a combination of differentially expressed metabolites and targets, and intestinal glial cell apoptosis was demonstrated by immunofluorescence.
JCD significantly promoted intestinal motility, increased the levels of the excitatory neurotransmitter ACH and reduced intestinal inflammation in STC mice. Untargeted metabolomics results showed that JCD significantly restored metabolic dysfunction and significantly affected taurine and hypotaurine metabolism. Network pharmacology and molecular experiments showed that JCD regulates AKT protein expression, and the core component is quercetin. Combined analysis demonstrated that apoptosis may be an important mechanism by which JCD relieves constipation. Further experiments showed that JCD reduced enteric glial cell (EGC) apoptosis.
This work demonstrated that reducing EGC apoptosis may be the critical mechanism by which JCD treats STC. These findings call for further molecular research to facilitate the clinical application of JCD.
Core Tip: Slow transit constipation (STC) model mice, which were established with compound diphenoxylate, were effectively treated with Ji-Chuan decoction (JCD). The results show that JCD can promote intestinal motility, increase acetylcholine content, reduce enteric inflammation, improve metabolic dysfunction, and reduce enteric glial cell apoptosis. This work demonstrated that reducing enteric glial cell apoptosis may be the critical mechanism by which JCD treats STC. These findings call for further molecular research to facilitate the clinical application of JCD.
- Citation: Wang XM, Lv LX, Qin YS, Zhang YZ, Yang N, Wu S, Xia XW, Yang H, Xu H, Liu Y, Ding WJ. Ji-Chuan decoction ameliorates slow transit constipation via regulation of intestinal glial cell apoptosis. World J Gastroenterol 2022; 28(34): 5007-5022
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/5007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.5007
Chronic constipation is a common complaint and can generally be divided into defecatory disorder, mixed constipation, normal transit constipation and slow transit constipation (STC). STC is the major type of chronic constipation characterized by a substantial increase in bowel transit time. STC has become an epidemic that particularly impacts the quality of life of elderly patients[1]. Despite high morbidity worldwide, the etiology of STC is poorly understood. An accumulation of publications indicates that multiple factors have been documented in the pathogenesis of STC[2-4]. Consequently, an interdisciplinary approach is necessary for exploring its pathological characteristics and developing therapies based on multiple components and multiple targets, such as traditional Chinese medicine (TCM)[5,6]. The active components of Ma-Zi-Ren-Wan could safely and effectively relieve the severity of functional constipation. Based on network pharmacology[7], the anti-STC mechanism of Gui-ren-Run-chang granules, another TCM formula, is associated with repairing the SCF/c-kit pathway and reducing aquaporin-4 expression in the colon[8].
Ji-Chuan decoction (JCD) is a representative TCM formula that originated from Jing-Yue-Zhang in the Ming Dynasty. It has been extensively used for STC and other gastroenteric disorders for hundreds of years[9]. JCD, as a typical TCM prescription, is composed of six herbs and includes multiple bioactive ingredients and complex targets. Studies have shown that JCD can effectively shorten colonic transit time, improve anorectal dynamics, regulate gastrointestinal neurotransmitters, alleviate constipation symptoms in STC patients, and improve the quality of life of STC patients[9,10]. After 1 mo of continuous use, only a few patients experienced adverse reactions such as dizziness (1.7%) and dry mouth (1.7%)[10]. Our clinical practice confirmed that JCD was a useful formula for STC therapy. Cistanche deserticola (C. deserticola) is the monarch drug for JCD, and the combination of geniposide and Lactobacillus plantarum KSFY06 has been shown to have anti-montmorillonite-induced constipation effects in Kunming mice[11,12]. However, direct experimental study of the effects of JCD against STC remains to be performed. In this work, we attempt to assess the laxative effect of JCD against STC through a multiomics approach, including metabolomics and TCM network pharmacology, to explore the integrated therapeutic mechanism of JCD.
Rabbit anti-AKT monoclonal antibody (No: bs-0115R) was provided by Bioss. The biotin secondary antibody anti-rabbit working solution (Goat, SP-9001) was provided by Beijing Zhongshan Jinqiao Biological Technology Co., Ltd. Glial fibrillary acidic protein (GFAP) (mouse, code: ab4648) and Caspase3 (rabbit, code: ab4051) were purchased from Abcam. CY3-labeled goat anti-mouse immunoglobulin G (IgG) (code: 202110) and fluorescein isothiocyanate-labeled goat anti-rabbit IgG (code: GB22303) were purchased from Servicebio.
The JCD solution was prepared according to the protocol described by Cui et al[13]. A clinical packet of JCD is composed of six Chinese herbs, i.e., Angelica sinensis (15 g), C. deserticola (9 g), Achyranthes bidentata (A. bidentata) (6 g), Fructus aurantii (F. aurantii) (4.5 g), Alisma orientalis (3 g) and Cimicifuga heracleifuga (3 g). Six packets of JCD were purchased from Chengdu Hospital of Integrated TCM and Western Medicine and identified by Dr. Wan Lin from Chengdu University of TCM. These herbs were then soaked in 2430 mL ddH2O (w/v 1:10) for 30 min and decocted for 20 min. Combined solutions following three decoctions were filtered by a 0.22-μm filter, concentrated to 0.81 g of crude herb per milliliter by a vapor evaporator, and stored at -20 °C for further use. In the past, scholars have studied the hyphenated to liquid chromatography characteristic fingerprints of JCD substances[14].
This experiment was approved by the Animal Ethics Committee, Chengdu University of TCM (license 2016-16). Thirty C57BL/6J male mice, aged 7 wk and weight 20.21 ± 2.10 g, were purchased from Chongqing Evansville Laboratory Animal Co., Ltd. (Chongqing, China). The animals were adaptively fed for 7 d in an environment with relative humidity of 45%-55%, a 12-h light/dark cycle and temperature of 22 ± 2 °C. The mice were then randomly divided into six groups, with five animals in each group: Healthy control (HC), STC model (STC), positive drug treatment (mosapride, MSP), low dose of JCD (JCDL), middle dose of JCD (JCDM) and high dose of JCD (JCDH). Mice in the HC group were orally administered normal saline (0.1 mL/10 g/d) as a negative control. The other mice were induced as the STC models by oral administration of compound diphenoxylate (10 mg/kg/d) for 14 d. After model identification, mice in the MSP group were orally administered with MSP (2.5 mg/kg); mice in the JCDL (3.04 g/kg), JCDM (6.08 g/kg) and JCDH (12.16 g/kg) groups were orally administered with JCD; and normal saline (0.1 mL/10 g/d) was gavaged in the HC and STC groups. Each mouse was administered treatments once a day for 14 d. Body weight, food intake and water intake were monitored every week. At the end of experiment, the feces of each mouse were collected under sterile procedures, frozen in liquid nitrogen and stored at -80 °C for further use.
The number of defecation particles within 6 h was counted, and the wet weight of the stool samples was evaluated. Then, the dry weight of the stool was weighed after drying at 60 °C for 12 h in a desiccator, and the moisture content of the stool was calculated. The colonic samples were harvested after euthanasia[15]. The acetylcholine (ACH) concentrations were detected by enzyme-linked immunosorbent assay.
The intestinal propulsive rate was measured after the last administration as follows[16]: All mice were fasted for 12 h and allowed free access to water. Then, mice were fed charcoal powder in 10% acacia gum. After 30 min, the abdomen was opened and the intestines were removed. The length from the pylorus to the ileocecal junction, as well as the charcoal transport distance were measured. The intestinal propulsive rate was calculated by the following formula[17]: Charcoal transit ratio (%) = distance of charcoal transport (cm)/length from pylorus to ileocecal junction (cm) × 100%.
The collected tissue samples were fixed with 10% formalin, dehydrated with alcohol, and embedded in paraffin wax. The embedded tissues were then sliced into 5-μm slices using a microtome (Leica, Buffalo Grove, United States) and stained with hematoxylin and eosin (HE). The pathological features were imaged by a digital microscope (Xiamen, China), and the optical densities were quantified using Image-Pro Plus 6.0.
Metabolomics was performed with a Vanquish UHPLC (Thermo, Germany) coupled with a Q Exactive™ HF (Thermo, Germany) platform (Novogene, Beijing, China) as previously described. Fecal samples (100 mg) were ground in liquid nitrogen, incubated on ice for 5 min, and centrifuged at 15000 × g for 20 min at 4 °C. The supernatant was diluted with liquid chromatography-mass spectrometry grade water to a final concentration of 53% methanol. After another centrifugation step, the supernatant was injected into the liquid chromatography-tandem mass spectrometry system for analysis. Samples were injected into a Hypesil Gold column (C18) using a 17-min linear gradient at a flow rate of 0.2 mL/min. The eluents in positive polarity mode were Eluent A (0.1% FA in water) and Eluent B (methanol). The eluents for negative polarity mode were Eluent A (5 mmol/L ammonium acetate, pH 9.0) and Eluent B (methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2%-100% B, 12.0 min; 100% B, 14.0 min; 100%-2% B, 14.1 min; and 2% B, 17 min. The Q ExactiveTM HF mass spectrometer was operated in positive/negative mode with a spray voltage of 3.2 kV, a capillary temperature of 320 °C, a sheath gas flow of 40 arb, and an auxiliary gas flow of 10 arb. Then, the data were matched to the mzCloud, mzVault, and MassList databases for accurate qualitative and relative quantitative results. The threshold for differential metabolites was set as variable importance in the projection (VIP) > 1.0, fold change (FC) > 1.5 or < 0.667 and P value < 0.05. The metabolic functions and relevant metabolic pathways were enriched by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/).
TCMSP (https://tcmsp-e.com/) was applied to find the chemical components and corresponding targets of JCD, with OB ≥ 30% and DL ≥ 0.18. The UniProt database (https://www.UniProt.org/) was used to correct potential targets. The chemical composition and corresponding targets of JCD were checked by BAT-MAN (http://bionet.ncpsb.org.cn/batman-tcm/index.php), with a score cutoff over 500. Then, the targets obtained from TCMSP and BAT-MAN were combined and deduplicated. Taking “slow transfer constipation” as the keyword, the target points with a score greater than 5.0 were collected from the GeneCard database (https://www.genecards.org/), and all the targets collected from OMIM (https://omim.org/) were included in the follow-up study. The common genes of drug targets and disease genes were put into the STRING11.0 (https://string-db.org/) database to construct a protein-protein interaction (PPI) network. The relevant data were imported into Cytoscape (3.7.1) software in tsv format, and the cytoHubba plug-in was used to display the top 30 nodes in the betweenness algorithm as Hub nodes and to display them in Excel. The distribution pattern and expression levels of AKT, which is one of the most important hub nodes in colonic tissues, were analyzed by immunohistochemistry. Rabbit anti-AKT antibody (Bioss; 1:100) was applied overnight at 4 °C, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG incubation at room temperature for 30 min, and diaminobenzidine was used for staining. The graphic processing software Image-Pro plus 6.0 was used to quantify the expression of AKT. Five fields of each slide were randomly observed, and the optical density values were determined. The components acting on AKT are considered core components of JCD.
The HGNC database (https://www.genenames.org/) was applied to transform the obtained JCD target genes into mouse genes. The Joint-Pathway analysis of MetaboAnalyst 5.0 was used to enrich differentially expressed metabolites (DEMs) and target genes associated with JCD. Except for those nominated by other diseases, the top 25 pathways were selected for integrated analysis. The degree of apoptosis of enteric glial cells (EGCs) in colon tissue was analyzed by immunofluorescence. Rabbit anti-caspase3 antibody (Abcam; 1:100) was applied overnight at 4 °C, and mouse anti-GFAP antibody (Abcam; 1:100) was stained at 37 °C for 30 min followed by 4’,6-diamidino-2-phenylindole for 10 min at room temperature. ImageJ graphics processing software was used to calculate the apoptosis of EGCs. Five fields of each slide were randomly observed, and densitometric values were estimated.
Data processing and statistical analyses were performed by Graph Pad Prism 7 (GraphPad, La Jolla, CA, United States). Data are expressed as the mean ± SD. One-way analysis of variance was used to compare the data of six groups. Student’s t test was used for the pairwise comparison of data. The results were considered significant when P < 0.05.
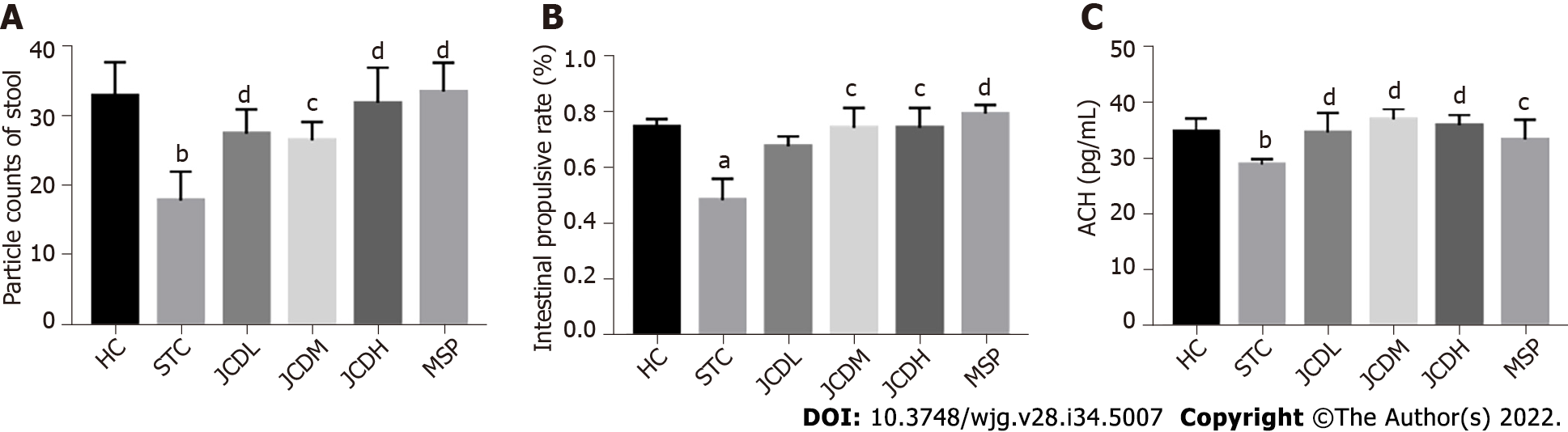
General restoration of STC symptoms was observed after JCD treatment. The food intake, water intake and body weight in the JCDH and JCDM groups were similar to those in the HC group (Supple
| Grouping/feces index | Wet weight (g/mou) | Dry weight (g/mou) | Moisture content (%/mou) |
| HC | 0.26 ± 0.02 | 0.18 ± 0.01 | 0.30 ± 0.04 |
| STC | 0.12 ± 0.01a | 0.10 ± 0.01c | 0.18 ± 0.01c |
| JCDL | 0.25 ± 0.01 | 0.18 ± 0.01 | 0.29 ± 0.02 |
| JCDM | 0.26 ± 0.02a | 0.18 ± 0.02c | 0.30 ± 0.02c |
| JCDH | 0.26 ± 0.02a | 0.18 ± 0.01c | 0.31 ± 0.02c |
| MSP | 0.27 ± 0.03b | 0.19 ± 0.02c | 0.30 ± 0.01c |
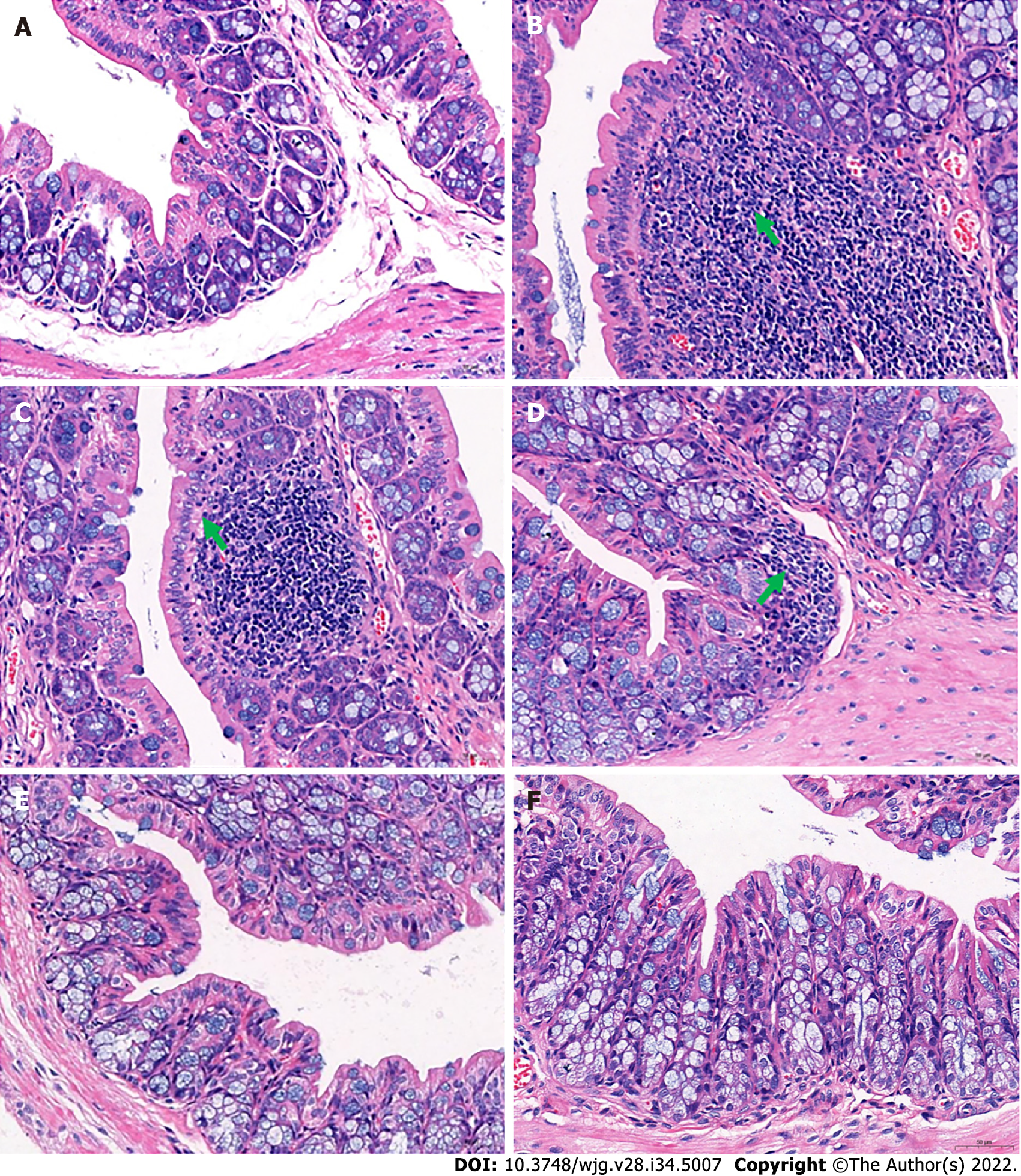
In addition, pathological observations demonstrated that JCD significantly reduced constipation-associated intestinal inflammation. Pathological observation demonstrated typical pathology of intestinal inflammation in STC mice, with inflammatory infiltration and necrosis in the distal colon. JCD repaired the colonic injuries in a dose-dependent manner (Figure 2). Briefly, the results showed that JCD exerts its effects by enhancing intestinal motility, promoting excitatory neurotransmitters and inhibiting intestinal inflammation in STC mice. Therefore, it is considered that the modeling was successful.
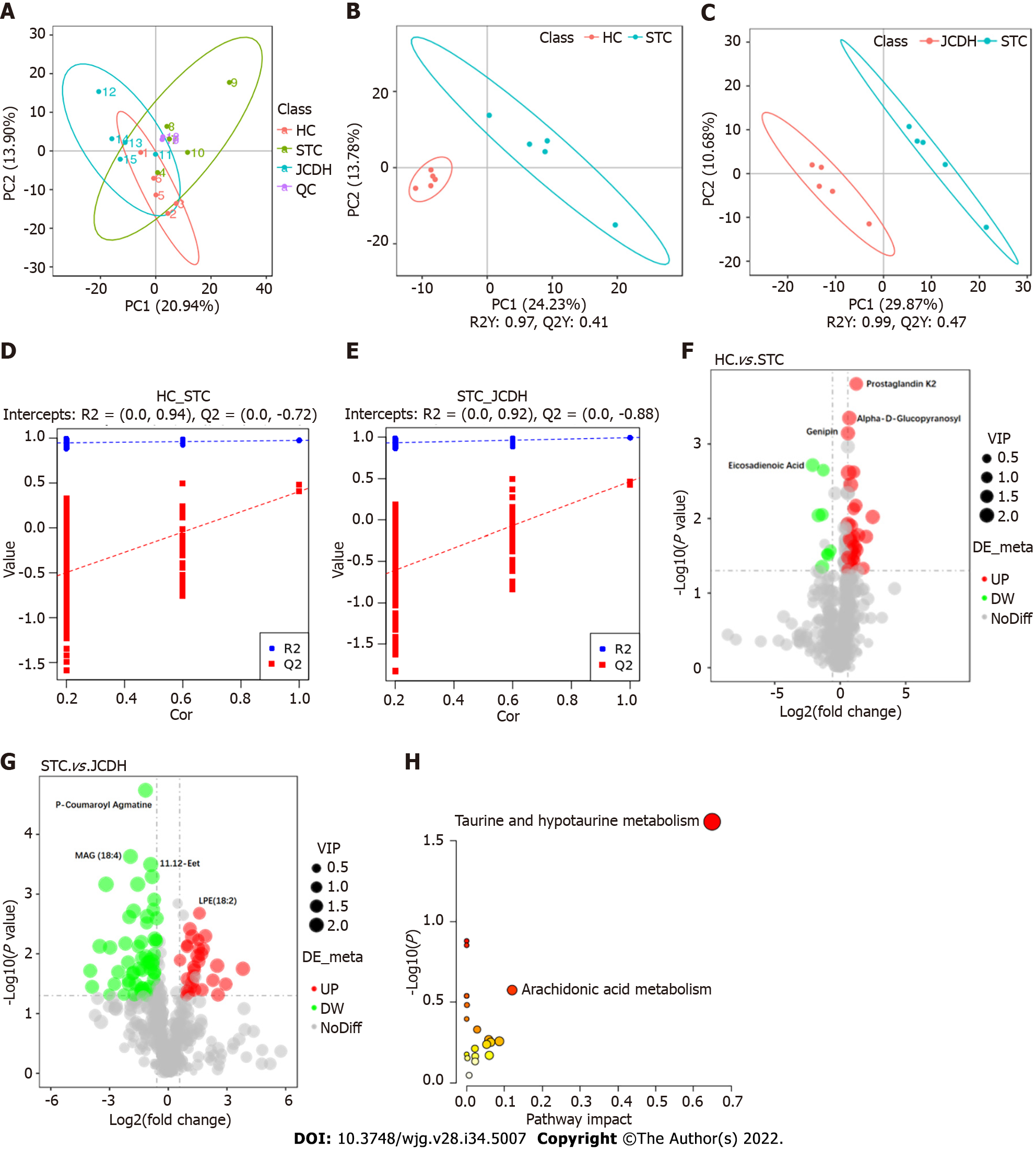
Forty-two DEMs (Supplementary Table 2) (negative ion mode) between the HC and STC groups and 86 DEMs (Supplementary Table 3) between the STC and JCDH groups were identified (the sample chromatogram in negative ion mode is shown in Supplementary Figure 1). Among the DEMs, eighteen were altered by the modeling process but recovered after JCDH treatment (Supplementary Table 4). As shown in Figure 3A, the closely focused cluster of quality control (QC) samples indicated the reproducibility of the experiments. Figures 3B and C display the fecal DEMs observed after modeling and JCDH treatment. Compared with the STC group, the R2 (evaluation of the modeling ability) and Q2 (description of the predictive ability) of the HC and JCDH groups indicated that the orthogonal partial least squares-discrimination analysis model had high predictability and reliability. Compared with the STC group, the R2 intercepts of the HC and JCDH groups were 0.94 and 0.92, respectively, and the Q2 intercepts were 0.72 and 0.88, respectively (Figures 3D and E). Figures 3F and G show the DEMs by volcano maps. Furthermore, taurine and hypotaurine metabolism were the main pathways impacted by JCDH treatment (Figure 3H).
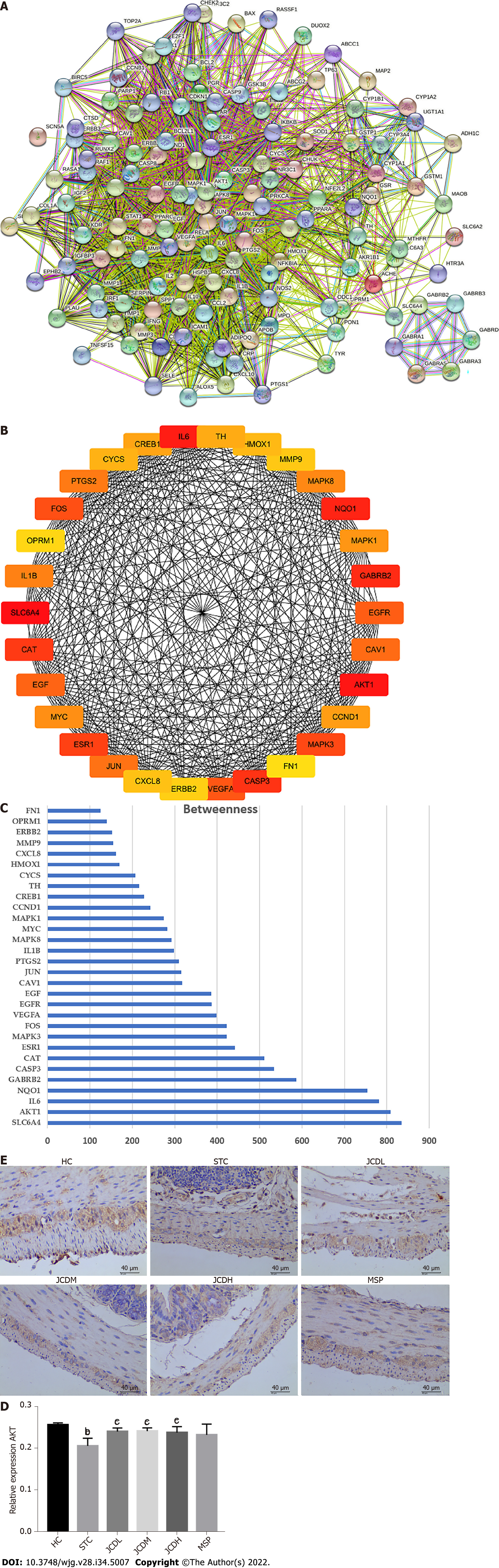
To further determine the therapeutic mechanism of JCD in STC, we performed a network pharmacology analysis. After data screening, 45 active pharmaceutical ingredients (Supplementary Table 2), 280 related targets, and 1372 disease targets were obtained. Comparing the goals related to JCD and STC, 89 common goals were identified and registered in the STRING database. Cytoscape 3.7.2 software was used to construct a PPI network, including 132 nodes and 2492 interactive edges (Figure 4A). According to the betweenness centrality value, the top 30 hub nodes were identified (Figures 4B and C), and AKT was considered one of the important targets. Immunohistochemistry confirmed that colonic AKT expression was significantly suppressed in STC mice compared with the HC group, whereas JCD significantly reversed this effect (Figures 4D and E). The chemical that acts on AKT is considered to be the core component of JCD, i.e., the quercetin from A. bidentata and C. deserticola, baicalein, kaempferol and wogonin from A. bidentata, and naringenin from F. aurantii (Table 2).
| Molecular name | Oral bioavailability (%) | Drug like |
| Baicalein | 33.52 | 0.21 |
| Kaempferol | 41.88 | 0.24 |
| Naringenin | 59.29 | 0.21 |
| Quercetin | 46.43 | 0.28 |
| Wogonin | 30.68 | 0.23 |
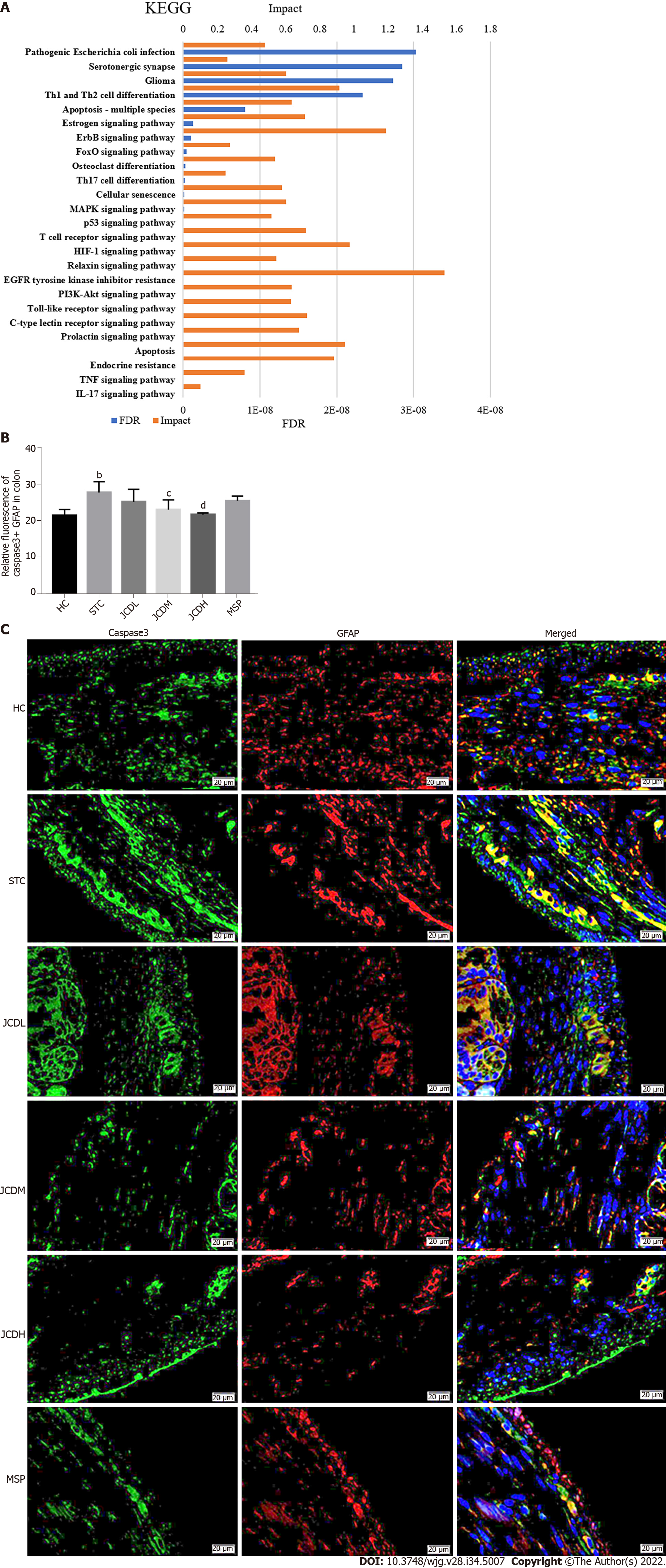
To comprehensively probe the pharmacological mechanism of JCD, a joint-pathway analysis was performed based on DEMs and common genes from network pharmacology. Twenty-five remarkable pathways are presented in Figure 5A. Apoptosis may be an important signaling pathway in the treatment of STC by JCD. GFAP is an EGC marker. Immunofluorescence double-labeling of GFAP and caspase3 showed that compared with the HC group, the apoptotic rate of EGCs in the STC group was significantly increased, while those in the JCDH and JCDM groups were significantly decreased (Figures 5B and C), confirming the predicted results.
JCD is an established TCM formula that is a particularly effective therapy for STC differentiated by TCM as Spleen and Kidney Yang deficiency syndrome[9], yet its pharmaceutical mechanism remains unclear. We explored the therapeutic mechanism of JCD in a dose-dependent manner and observed that the best effect was achieved by JCDH. The compound diphenoxylate is widely used to induce STC in animal models and can inhibit the peristaltic reflex of intestinal mucosa. It is easier to induce constipation than the commonly used loperamide and is more available than morphine[17]. It is easier to induce constipation than other loperamides and easier to obtain than morphine[17]. The holistic view is shared between systems biology and TCM[18]; therefore, we used key disciplines of systems biology, such as metabolomics and network pharmacology, to effectively reveal the integrated mechanism of JCD for STC.
Constipation refers to laborious defecation, dry and hard stools and low stool volume. Effective drugs will increase key indices of constipation, such as the quantity and water content of the feces and the intestinal propulsive rate[19,20]. In this study, we observed that JCD substantially improved almost all indices tested in this work (Figures 1A and B, Table 1). The intestinal propulsive rates, extensively used to evaluate the pathological degree and curative effect of constipation, were significantly decreased after diphenoxylate treatment. Similar anti-constipation effects were obtained among the JCDM, JCDH and the positive reagent MSP groups (Figure 1B). In addition, the three JCD doses could statistically increase the concentration of excitatory neurotransmitter (ACH) (Figure 1C), suggesting that the increase in excitatory neurotransmitter is a critical approach for anti-constipation by JCD[3]. Our colonic path
The metabolome results showed that the pathways involving taurine and hypotaurine metabolism were important for the anti-constipation effect of JCD. Taurine is a sulfur-containing α-amino acid with anti-inflammatory properties that can be sequentially converted into homocysteine, cystathionine, cysteine and/or hypotaurine[22,23]. The concentration of taurine was significantly reduced in loperamide-induced constipation rats, and red liriope ameliorated constipation and increased the level of taurine[24,25]. Our work also showed that the contents of taurine and its metabolite hypotaurine were negatively associated with the manifestation of STC and positively associated with intestinal transit rates[26]. However, the molecular mechanism of taurine on gastrointestinal motility and the regulatory mechanisms of JCD remain to be further explored.
PPI network prediction showed that AKT is the core target of JCD for STC therapy. In our experiments, STC mice showed downregulated expression of AKT, while all three doses of JCD treatment reversed the abnormally reduced expression levels. AKT plays an important role in cell survival and apoptosis. Previous studies have shown that high glucose levels can induce EGC apoptosis through the AKT pathway, which is closely related to intestinal motility[27]. Another study showed that EGCs could protect the nervous system from hyperglycemia-induced damage by activating the Akt/GSK-3β pathway[28]. Therefore, we speculate that the treatment of STC with JCD may be related to EGC apoptosis.
Experimental evidence in humans and animals suggests that EGCs play a key role in regulating gastrointestinal motility and transit[29-32]. A cohort study of twenty-six STC patients showed reduced EGCs compared with ten healthy volunteers[33]. EGC can increase the expression of Akt and ZO-1 by releasing glial cell-derived neurotrophic factor, indirectly regulating the integrity of the intestinal epithelial barrier, reducing intestinal inflammation and improving delayed colonic transit[34,35]. Another study showed that enteric glial LPAR1 signaling regulates gastrointestinal motility through EGCs and may contribute to chronic intestinal pseudo-obstruction in humans[36]. Activation of opioid receptors in EGCs may be associated with morphine-induced constipation[37]. Previous studies have shown that the key components of JCD are associated with neural apoptosis, and many of them also involve changes in AKT protein. For example, wogonin, a key component of JCD identified in this paper, can prevent hippocampal injury after brain trauma through antioxidation and anti-apoptosis, which has been shown to occur through the PI3K/Akt/nuclear factor E2-related factor 2 (Nrf2)/HO-1 pathway[38]. Baicalein reduces sevoflurane-induced neurodegeneration, improves learning and memory retention in rats, and modulates the PI3/Akt/GSK-3β and JNK/ERK signaling pathways[39]. Kaempferol prevents cerebral ischemia-reperfusion injury by interfering with oxidative and inflammatory stress-induced apoptosis[40]. Quercetin is involved in neuroprotection by regulating Nrf2, paraoxonase 2, JNK, tumour necrosis factor alpha, PGC-1α, MAPKs, CREB and PI3K/Akt[41]. Naringenin can effectively inhibit Aβ25-35-induced neuronal injury in PC12 cells by regulating the ER and PI3K/Akt pathways[42]. To our knowledge, the current work is the first to demonstrate that JCD can improve constipation by reducing EGC apoptosis. Our work applied a multiomics strategy to explore the therapeutic mechanism of JCD in the treatment of STC and found some interesting evidence that remains to be elucidated in more detail in the future. The JCDH group of mice exhibited a better effect, suggesting that a suitable dose needs to be further evaluated.
This work demonstrated that reduced enteric EGC apoptosis may be the critical mechanism of JCD in STC therapy. These findings call for further molecular research to facilitate the clinical application of JCD.
Slow transit constipation (STC) is a common intestinal disorder without an effective therapeutic regimen. Ji-Chuan Decoction (JCD) is an established formula for STC. However, its pharmacological mechanism is still unclear.
To determine the ingredients and mechanism of JCD for STC treatment.
To explore the integrated regulatory pattern of JCD against STC through hyphenated techniques from metabolism, network pharmacology and molecular methods.
STC model mice were generated by gavage of diphenoxylate for 14 d. STC mice in the low- (3.04 g/kg), medium- (6.08 g/kg) and high-dosage (12.16 g/kg) JCD groups were orally administered. The acetylcholine (ACH) level was detected by enzyme-linked immunosorbent assay. AKT expression and enteric glial cell (EGC) apoptosis were demonstrated by immunofluorescence. The differentially expressed metabolites were tested by nontargeted metabolomics. The targets and core ingredients were identified by network pharmacology.
JCD significantly promotes intestinal motility, increases colonic ACH content and reduces inflammation in STC mice. It markedly restores the misaligned metabolites, including taurine/hypotaurine, and rescues AKT expression with quercetin. Inhibition of EGC apoptosis is a potential mechanism by which JCD relieves constipation.
Regulating gut metabolites and reducing EGC apoptosis in STC mice may be the key mechanism of JCD for STC treatment.
Further investigation into the molecular interactions among the JCD ingredients and metabolites, intestinal microbiota and host response in STC mice is necessary.
The authors would like to acknowledge Yao Zheng-Hong (Novogene, Beijing, Master of Agriculture), for skillful technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khayyat YM, Saudi Arabia; Kumar A, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Nelson AD, Camilleri M, Chirapongsathorn S, Vijayvargiya P, Valentin N, Shin A, Erwin PJ, Wang Z, Murad MH. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut. 2017;66:1611-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Yeh KM, Johansson O, Le H, Rao K, Markus I, Perera DS, Lubowski DZ, King DW, Zhang L, Chen H, Liu L. Cystic fibrosis transmembrane conductance regulator modulates enteric cholinergic activities and is abnormally expressed in the enteric ganglia of patients with slow transit constipation. J Gastroenterol. 2019;54:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Russell JP, Mohammadi E, Ligon C, Latorre R, Johnson AC, Hoang B, Krull D, Ho MW, Eidam HS, DeMartino MP, Cheung M, Oliff AI, Kumar S, Greenwood-Van Meerveld B. Enteric RET inhibition attenuates gastrointestinal secretion and motility via cholinergic signaling in rat colonic mucosal preparations. Neurogastroenterol Motil. 2019;31:e13479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kim JE, Park JW, Kang MJ, Choi HJ, Bae SJ, Choi Y, Lee YJ, Seo S, Hong JT, Hwang DY. Laxative Effect of Spicatoside A by Cholinergic Regulation of Enteric Nerve in Loperamide-Induced Constipation: ICR Mice Model. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Zhong LLD, Zheng G, Da Ge L, Lin CY, Huang T, Zhao L, Lu C, Lu AP, Bian ZX. Chinese herbal medicine for constipation: zheng-based associations among herbs, formulae, proprietary medicines, and herb-drug interactions. Chin Med. 2016;11:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Whiting RL, Ford AC. Efficacy of traditional chinese medicine in functional constipation. Am J Gastroenterol. 2011;106:1003; author reply 1003-1003; author reply 1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Huang T, Ning Z, Hu D, Zhang M, Zhao L, Lin C, Zhong LLD, Yang Z, Xu H, Bian Z. Uncovering the Mechanisms of Chinese Herbal Medicine (MaZiRenWan) for Functional Constipation by Focused Network Pharmacology Approach. Front Pharmacol. 2018;9:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Ali MZ, Mehmood MH, Haneef M, Saleem M, Ishrat G, Siddiqi HS, Gilani AU, Ahmed M. A flavonoid driven phyto-pharmacological effects of Capparis decidua Edgew. in rodents. Pak J Pharm Sci. 2020;33:333-342. [PubMed] |
| 9. | Dai L, Zhong LL, Ji G. Irritable bowel syndrome and functional constipation management with integrative medicine: A systematic review. World J Clin Cases. 2019;7:3486-3504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zhang SX, Zhang XA, An YK. Effect of Jichuanjian on Gastrointestinal Function,Serum Intestinal Neurotransmitters andIntestinal Flora in Elderly with Chronic Functional Constipation. Chin J ETMF. 2018;24:169-174. [DOI] [Full Text] |
| 11. | Gan Y, Liang J, Diao W, Zhou X, Mu J, Pang L, Tan F, Zhao X. Lactobacillus plantarum KSFY06 and geniposide counteract montmorillonite-induced constipation in Kunming mice. Food Sci Nutr. 2020;8:5128-5137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Wang Y, Yang S, Xiao Y, Guan H, Yue X, Wang X, Li X. The Difference of Chemical Components and Biological Activities of the Raw Products slices and the Wine Steam-Processed Product from Cistanche deserticola. Evid Based Complement Alternat Med. 2019;2019:2167947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Cui H, Li Y, Wang Y, Jin L, Yang L, Wang L, Liao J, Wang H, Peng Y, Zhang Z, Liu X. Da-Chai-Hu Decoction Ameliorates High Fat Diet-Induced Nonalcoholic Fatty Liver Disease Through Remodeling the Gut Microbiota and Modulating the Serum Metabolism. Front Pharmacol. 2020;11:584090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Shi C, Li X, Feng J, Huang Y, Wang Y, Chen J, Li S. Study on HPLC characteristic fingerprint of substance benchmark of classical famou sprescription of Jichuan Decoction. Zhong Cao Yao. 2020;51:3930-3936. |
| 15. | Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012;50:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Nagakura Y, Naitoh Y, Kamato T, Yamano M, Miyata K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur J Pharmacol. 1996;311:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Yang A, Yan Y, He H, Gao Y, He K, Shi H, Liu M. Comparative study on four models for preparing functional constipation in mice. China Med Eng. 2019;27:1-4. [DOI] [Full Text] |
| 18. | Meng X, Ma J, Kang AN, Kang SY, Jung HW, Park YK. A Novel Approach Based on Metabolomics Coupled With Intestinal Flora Analysis and Network Pharmacology to Explain the Mechanisms of Action of Bekhogainsam Decoction in the Improvement of Symptoms of Streptozotocin-Induced Diabetic Nephropathy in Mice. Front Pharmacol. 2020;11:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 19. | Qian Y, Zhao X, Kan J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp Ther Med. 2013;6:228-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1375] [Article Influence: 105.8] [Reference Citation Analysis (1)] |
| 21. | Ren X, Liu L, Gamallat Y, Zhang B, Xin Y. Enteromorpha and polysaccharides from enteromorpha ameliorate loperamide-induced constipation in mice. Biomed Pharmacother. 2017;96:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Obeid OA, Johnston K, Emery PW. Plasma taurine and cysteine levels following an oral methionine load: relationship with coronary heart disease. Eur J Clin Nutr. 2004;58:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Kim JE, Lee YJ, Kwak MH, Jun G, Koh EK, Song SH, Seong JE, Kim JW, Kim KB, Kim S, Hwang DY. Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats. Lab Anim Res. 2014;30:35-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kim JE, Lee YJ, Ryu SH, Park JW, Kang MJ, Choi HJ, Bae SJ, Choi Y, Kang HG, Kim KB, Kim S, Lim Y, Hwang DY. Metabolomics approach to serum biomarker for laxative effects of red Liriope platyphylla in loperamide-induced constipation of SD rats. Lab Anim Res. 2019;35:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Lee DS, Jo HG, Kim MJ, Lee H, Cheong SH. Laxative Effects of Taurine on Loperamide-Induced Constipation in Rats. Adv Exp Med Biol. 2019;1155:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Liu G, He F, Zhang L, Yang K, Yu H, Zhou J, Gan H. MicroRNA 375 modulates hyperglycemia-induced enteric glial cell apoptosis and Diabetes-induced gastrointestinal dysfunction by targeting Pdk1 and repressing PI3K/Akt pathway. Sci Rep. 2018;8:12681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Luo P, He WX, Li C, Chang MJ. Enteric glial cells exert neuroprotection from hyperglycemia-induced damage via Akt/GSK3β pathway. Neuroreport. 2021;32:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, De Giorgio R. Enteric glial cells: recent developments and future directions. Gastroenterology. 2014;147:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:433-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G. Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice. Gastroenterology. 2017;153:1068-1081.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 32. | Grubišić V, Gulbransen BD. Enteric glia: the most alimentary of all glia. J Physiol. 2017;595:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, Xie Q, Huang XL, Gan HT. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Liu GX, Yang YX, Yan J, Zhang T, Zou YP, Huang XL, Gan HT. Glial-derived neurotrophic factor reduces inflammation and improves delayed colonic transit in rat models of dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2014;19:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Ahmadzai MM, McClain JL, Dharshika C, Seguella L, Giancola F, De Giorgio R, Gulbransen BD. LPAR1 regulates enteric nervous system function through glial signaling and contributes to chronic intestinal pseudo-obstruction. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Gao H, Zhang Y, Li Y, Chang H, Cheng B, Li N, Yuan W, Li S, Wang Q. μ-Opioid Receptor-Mediated Enteric Glial Activation Is Involved in Morphine-Induced Constipation. Mol Neurobiol. 2021;58:3061-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Feng Y, Ju Y, Yan Z, Ji M, Yang M, Wu Q, Wang L, Sun G. Protective role of wogonin following traumatic brain injury by reducing oxidative stress and apoptosis via the PI3K/Nrf2/HO1 pathway. Int J Mol Med. 2022;49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Wang S, Zhou Y. Baicalein Inhibits Neuroapoptosis Via Pathways in Sevoflurane Induced Rats. Transl Neurosci. 2018;9:88-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Wang J, Mao J, Wang R, Li S, Wu B, Yuan Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front Pharmacol. 2020;11:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 41. | Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, Najda A, Walasek-Janusz M, Kamel M, Albadrani GM, Akhtar MF, Saleem A, Abdel-Daim MM. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother. 2021;140:111729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 42. | Zhang N, Hu Z, Zhang Z, Liu G, Wang Y, Ren Y, Wu X, Geng F. Protective Role Of Naringenin Against Aβ25-35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell Mol Neurobiol. 2018;38:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |