Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.4929
Peer-review started: January 27, 2022
First decision: February 24, 2022
Revised: March 5, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: September 14, 2022
Processing time: 222 Days and 19.7 Hours
Despite stringent selection criteria, hepatocellular carcinoma recurrence after liver transplantation (LT) still occurs in up to 20% of cases, mostly within the first 2–3 years. No adjuvant treatments to prevent such an occurrence have been developed so far. However, a balanced use of immunosuppression with minimal dose of calcineurin inhibitors and possible addition of mammalian target of rapamycin inhibitors is strongly advisable. Moreover, several pre- and post-transplant predictors of recurrence have been identified and may help determine the frequency and duration of post-transplant follow-up. When recurrence occurs, the outcomes are poor with a median survival of 12 mo according to most retrospective studies. The factor that most impacts survival after recurrence is timing (within 1–2 years from LT according to different authors). Several therapeutic options may be chosen in case of recurrence, according to timing and disease presentation. Surgical treatment seems to provide a survival benefit, especially in case of late recurrence, while the benefit of locoregional treatments has been suggested only in small retrospective studies. When systemic treatment is indicated, sorafenib has been proved safe and effective, while only few data are available for lenvatinib and regorafenib in second line. The use of immune checkpoint inhibitors is controversial in this setting, given the safety warnings for the risk of acute rejection.
Core tip: Hepatocellular carcinoma (HCC) is becoming the most common indication for liver transplantation (LT). The problem of tumor recurrence after LT, that occurs in up to 20% of cases, is becoming of increasing interest. We reviewed of the available literature on HCC recurrence after LT. The best preventive measures still rely on pretransplant selection criteria, since no dedicated follow-up guidelines exist and no post-LT adjuvant treatments are available. When recurrence occurs, the prognosis is poor. However, aggressive surgical treatment, particularly in the case of late recurrence, may provide a significant survival benefit.
- Citation: Sposito C, Citterio D, Virdis M, Battiston C, Droz Dit Busset M, Flores M, Mazzaferro V. Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma. World J Gastroenterol 2022; 28(34): 4929-4942
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/4929.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.4929
Hepatocellular carcinoma (HCC) is the most common liver cancer and the fourth leading cause of cancer-related mortality[1,2]. From the time of its initial developments in the early 1960s, liver transplantation (LT) appeared as the ideal cure for HCC on liver cirrhosis because of the perspective to cure at the same time both the tumor and the underlying liver disease. However, the first experiences were disappointing with many authors reporting 5-year survival < 40%, mainly because of recurrences of the primary tumor[3-6]. A retrospective review of these discouraging results progressively led to the observation that survival of patients was directly related to the stage of HCC at the time of LT. This was the basis on which a prospective study was conducted in Milan applying a priori restrictive criteria for the selection of HCC candidates for LT (namely a single nodule ≤ 5 cm or two or three nodules ≤ 3 cm, each with no macrovascular invasion at pretransplant imaging). The seminal paper published in 1996 demonstrated that LT under such criteria achieved better long-term results than any other therapy, with outcomes similar to LT for nononcological indications[7]. The so called Milan criteria (MC) were subsequently validated by many other groups reporting 5-year survival rates of ≥ 70%, and became the benchmark for selecting patients with HCC for LT. Pooled recurrence rates have been reported to be around 8% for patients within MC versus 28% for patients beyond these criteria, according to a recent meta-analysis[8]. Thus, HCC recurs in a proportion of recipients who are within MC, while LT may provide cure for some patients who are beyond these criteria. When recurrence occurs, survival is poor and post-LT HCC recurrence is the factor that most affects long-term outcomes in this setting. Considering that HCC represents the most common indication for LT in the USA, and that since the introduction of direct antiviral agents against hepatitis C virus the proportion of patients undergoing LT for HCC is increasing worldwide[9,10], the problem of tumor recurrence will probably affect a growing number of patients. However, to date, treatment of HCC recurrence following LT is largely understudied and dedicated guidelines are lacking. The aim of this paper is to provide a review of the current evidence on therapeutic strategies for patients with HCC recurrence after LT.
Tumor recurrence may be linked to remaining (previously undetected) extrahepatic HCC at the time of LT, or result from the post-LT engraftment of circulating HCC clones[11]. It is extrahepatic in 50%–60% of cases, with lung, bones and adrenal glands being the most frequently affected sites[12]. Timing of HCC recurrence is variable, but in most cases it occurs within 3 years after LT. Early recurrence (< 1 year after LT) is associated with a significantly worse prognosis, while later recurrence might result in better outcomes and even in cure in selected cases[13].
Considering that the risk of post-LT recurrence is strictly related to pretransplant HCC stage and treatment, recurrence is firstly prevented by the application of pre-LT selection criteria able to identify patients at higher risk. Proposals for expansion of MC have been initially developed using tumor morphology, namely size and number of nodules. In fact, these factors have been demonstrated as surrogate markers of microvascular invasion (MVI) and/or poor tumor differentiation, which are the principal determinants of biological aggressiveness and therefore of the risk of post-LT recurrence[14]. Expanded criteria increased the acceptable size and number of HCC nodules with respect to MC, but the considerable heterogeneity coupled with differences in accuracy of liver imaging techniques probably represent the greatest limitation of criteria based only on morphology.
To overcome these limits, criteria incorporating serum markers as surrogates of biological tumor features such as α-fetoprotein (AFP) have been proposed. In particular, by combining the morphological characteristics of the tumor and AFP values it was possible to develop selection criteria for LT definitively exceeding MC, while even decreasing the risk of post-LT recurrence[15,16]. A strategy combining tumor burden with the assessment of response to pre-LT locoregional treatment (LRT) as a marker of favorable tumor biology has gained broader acceptance[17]. For patients beyond MC, a common strategy is to downstage patients by means of LRT or surgical therapy. In fact, patients successfully downstaged within accepted criteria share the same prognosis as patients within the criteria ab initio and so far[18], response to therapies appears as one of the best surrogates of favorable tumor biology and thus an optimal selection tool for candidates for LT[17,19,20]. Patients progressing in the pre-LT period despite LRT have significantly worse post-LT outcomes with respect to patients with stable or responding disease. Finally, tumor differentiation, MVI, presence of circulating cancer cells and genomic markers have also been suggested as selection criteria for LT, but these assessments require biopsy, which might induce tumor seeding. Furthermore, it is well known that tumors are heterogeneous and show areas of varying degrees of differentiation and genomic features.
Considering that post-LT recurrence is mostly asymptomatic, and that early detection of recurrence may have a positive impact on long-term outcomes, post-LT surveillance has an important role in this setting. However, no guidelines from the major Hepato-bilio-pancreatic societies are available and surveillance protocols are mostly center-specific, often with a high heterogeneity between centers as recently reported[21]. Few retrospective studies on post-LT surveillance have been published to date, and several questions regarding frequency, duration, and imaging modality for a cost-effective surveillance remain open.
For imaging modality, cross-sectional imaging of the abdomen [with either multiphase computed tomography (CT) or magnetic resonance imaging] and noncontrast lung CT scan allow detection of the most frequent sites of recurrent HCC[21,22]. Cross-sectional imaging of the brain, bone scintigraphy or positron emission tomography–CT are indicated only in case of clinical suspicion and not on a regular basis, while it seems reasonable to check for AFP levels at each surveillance visit even if no data is available to support this indication.
Given that the majority of recurrences occur within the first 3 years after LT, there is general agreement to indicate surveillance imaging and visits more frequently in this time frame (i.e., every 4–6 mo) and yearly thereafter[22]. Some authors suggest interrupting surveillance after 5 years. However, recurrences (either de novo tumors or true recurrences) have been repeatedly reported up to 10–15 years after LT[23]; considering that late HCC relapse is associated with a better prognosis with respect to earlier events and that it is sometimes curable, it seems reasonable to prolong yearly surveillance for at least ten years.
Ideally, frequency and duration of surveillance would be based on the assessed risk of post-LT HCC recurrence. Several proposals have been made in this sense, and the RETREAT score[24] (that includes AFP at LT, presence of MVI and sum of maximum size + number of vital nodules) is the most recent and promising predictor in terms of discriminative power and validation on a large scale registry. However, no prospective validation is available to date, and the cost-effectiveness of a surveillance program based on the risk of recurrence has yet to be demonstrated.
The impact of surveillance programs on post-recurrence survival has been scarcely studied. A recent multicenter study on 232 patients who experienced HCC recurrence found that increasing number of post-LT surveillance scans (with cut-off at three surveillance scans within the first 2 years) was associated with improved survival and possibility of undergoing potentially curative treatments[25].
Improvements in the management of immunosuppression reduced rejection episodes favoring long-term graft survival; calcineurin inhibitors (CNIs) tacrolimus and cyclosporine played a fundamental role in this improvement. However, several studies demonstrated that CNI exposure is associated with an increased risk of tumor recurrence with a dose-dependent effect[26]. It is likely that the immunosuppression induced by CNIs prevents the immune system from detecting and destroying circulating or dormant HCC cells and therefore, dosage of CNIs should be maintained with the aim of balancing this risk without increasing the risk of rejection episodes.
Mammalian target of rapamycin inhibitors (mTORis) sirolimus and everolimus are another class of immunosuppressants targeting some HCC pathways, which showed antiangiogenic and antiproliferative effects in experimental models[27]. Data from retrospective studies and meta-analyses suggest that, compared to CNIs, the use of mTORis reduces the risk of post-LT HCC recurrence and increases long-term survival. In the most recent meta-analysis including 23 comparative studies [17 observational and 6 randomized controlled trials (RCTs)] with 6495 patients, recurrence-free survival (RFS) was significantly increased with mTORi-based therapy at 1 and 3 years with a nonsignificant increase at 5 years[28]. Overall survival (OS) was also significantly improved, as well as recurrence rate being lower in the mTORi arm without differences based on the type of mTORi. However, only one RCT that compared post-LT immunosuppression containing mTORi (sirolimus) versus not containing mTORi[29]. In this international RCT of 525 patients there appeared to be an advantage in the sirolimus group regarding RFS in the first 3–5 years. However, this benefit was subsequently lost with further follow-up and the trial failed to meet the primary endpoint of demonstrating a significant reduction of recurrences in the mTORi-containing immunosuppression group.
Several attempts had been performed with chemotherapy as an adjuvant treatment to prevent HCC recurrence after LT[30,31]. HCC is a chemoresistant tumor; therefore, cytotoxic systemic therapies have failed to provide any consistent benefit in this setting and have been abandoned in the last decade[32]. Sorafenib, an oral multikinase inhibitor that shows significant improvement in survival of patients with advanced HCC, has been tested in small studies in the setting of adjuvant treatment for HCC after LT. Despite some initial signs of efficacy, with one phase I study showing a significant reduction in the risk of HCC recurrence with a maximum tolerable dose of sorafenib 200 mg twice daily[33], other single-center case series failed to confirm these data and to date no RCTs are available. Lenvatinib, a more recent targeted therapy for advanced HCC, has not been prospectively tested in the adjuvant setting; a small retrospective case series confirmed an acceptable drug safety and patient tolerance but did not show any significant reduction in terms of HCC recurrence[34]. Immune checkpoints inhibitors (ICIs) have emerged as a treatment option for advanced-stage HCC. No studies are available on ICIs as post-LT adjuvant treatment. A recent systematic review and pooled analysis reviewed 14 patients receiving ICIs for recurrent disease after LT for HCC: 11 of them (78.6%) died, and graft rejection was the cause of death in five cases (45.4%). The high rejection rate raises the question of safety of ICIs in transplanted patients, even in the setting of overt recurrence[35]. Thus, to date, no anticancer treatments can be recommended to prevent HCC recurrence after LT, and it is unlikely that they will become available in the near future.
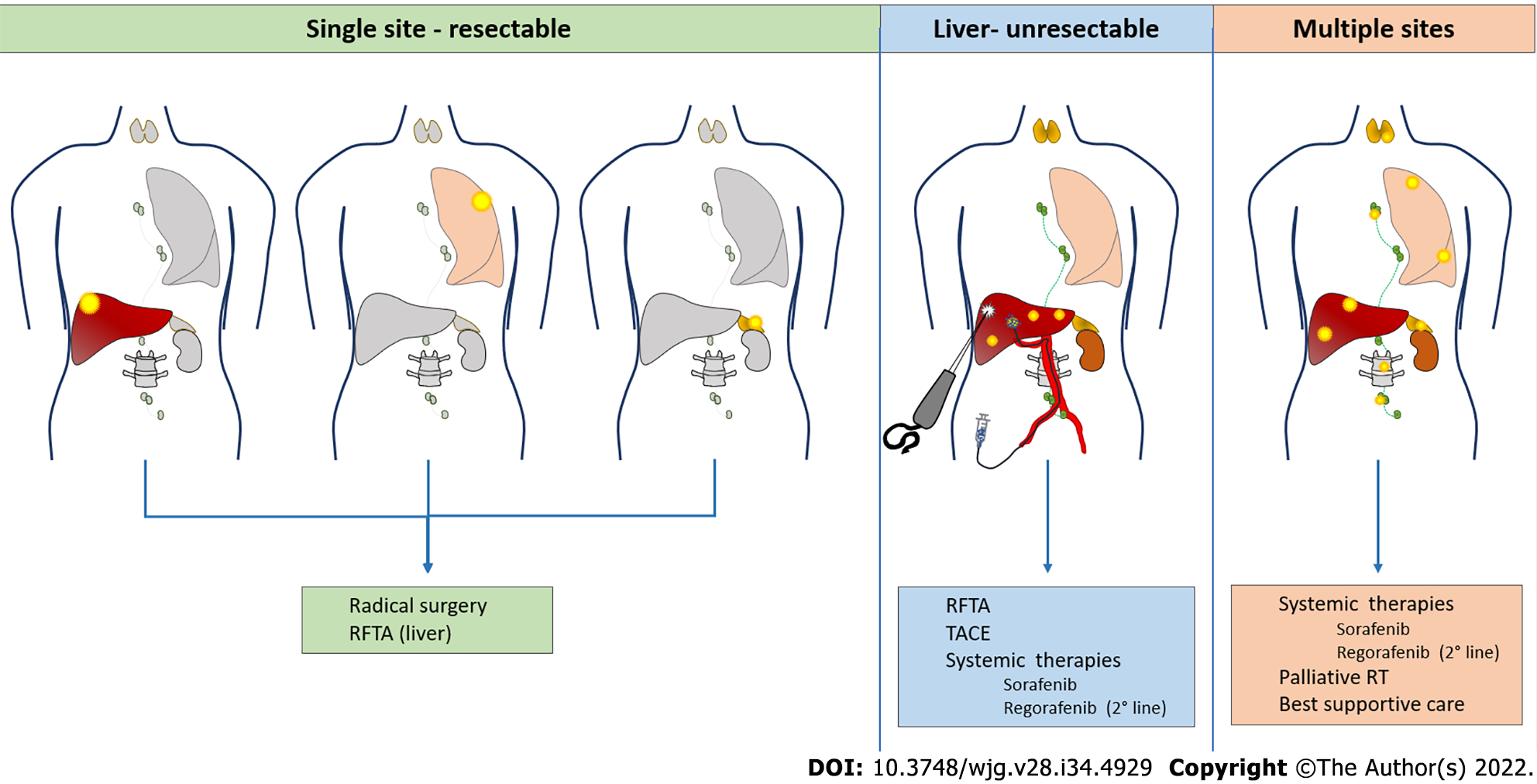
Literature concerning the efficacy of each treatment modality is scarce, with the many limitations related to the small number of patients included, the frequent use of combined treatment and the different patterns of recurrence, all acting as confounding factors. In the majority of cases (50%–60%) recurrence is extrahepatic and affects the following sites: lungs (40%–60%), bones (25%–30%), adrenal glands (10%), lymph nodes (10%) and peritoneum (10%)[12]. Liver-only recurrence occurs in 15%–40% of patients, while combined liver and extrahepatic recurrence accounts for 30%–40% of cases. The therapeutic options clearly depend on location, multifocality and clinical presentation of recurrence (Figure 1).
Liver resection is safe and provides a survival benefit in case of intrahepatic oligorecurrence[36,37], with a median survival of 28–65 mo observed for patients receiving surgery, compared to 5–15 mo in those receiving systemic treatment only[38-41]. Surgical treatment is feasible in 25%–50% of cases with higher morbidity rate (60%–80%) with respect to primary liver resections[42-44], mainly because of the risk of infections in the context of immunosuppression.
Sapisochin et al[39] retrospectively analyzed 121 patients with HCC recurrence after LT, finding that not being amenable to resection or ablation was an independent predictor of poor prognosis [hazard ratio (HR) = 4.7, 95% confidence interval (CI): 2.7–8.3). An Italian multicenter study analyzed 21 patients with recurrence and reported a significantly better 4-year survival rate in patients treated with surgical resection for intra- and extrahepatic recurrence compared to those with unresectable disease (57% vs 14%, P = 0.02)[45]. In another series of 106 patients, treatment for recurrent HCC most commonly included chemotherapy (73.5%), surgical resection (23.3%), external beam radiation (13.6%), and ablation (3.9%), with the majority of patients receiving nonsurgical therapies (59.2%). The highest survival rates at 3 years were observed in patients receiving surgical therapy alone (60%), followed by patients receiving both surgical and nonsurgical therapy (37%), patients receiving only nonsurgical therapy (11%), and patients receiving no treatment (0%)[40]. Time from LT to recurrence is one of the most important prognostic factors, and patients with late recurrence show more favorable 5-year outcomes survival with resection compared to those of patients who recur earlier[46].
Surgery may enhance long-term survival also in patients with pulmonary recurrences amenable to resection, with 5-year survival rates ranging from 34% to 44% in those undergoing metastasectomy[47-52]. A benefit from surgical treatment is also reported for other sites of recurrence in smaller case series, including vertebrae[53], adrenal glands[54,55], lymph nodes[56], peritoneum[57] and pharynx[58]. In patients with multiple recurrences, some benefits have also been gained from repeated resections, probably reflecting less aggressive tumor biology[41].
Radiofrequency ablation (RFA) for liver recurrences may be proposed with a curative intent for small lesions, with the advantages of a percutaneous approach. In a retrospective single-center series, Huang et al[44] compared 15 patients with post-LT HCC recurrence treated surgically with 11 patients treated with RFA. This study demonstrated similar 5-year OS (35% for surgery vs 28% for RFA) but a tendency to a worse 5-year disease-free survival in the RFA group (16% vs 0%). Another study evaluating safety and efficacy of microwave ablation on a series of 11 patients found this technique safe and tolerable, with a 15.8% rate of local tumor progression after treatment and 15.3% survival at 2 years[59].
Multifocal intrahepatic recurrences may be amenable to transarterial chemoembolization (TACE). The largest series collected 28 patients treated by conventional TACE[60]. There were no significant post-treatment complications and the targeted tumor reduced in size by ≥ 25% in 19 patients (67.9%). However, intrahepatic recurrence or extrahepatic metastases occurred in 26 patients (92.9%) within 6 mo. The 3- and 5-year survival rates following TACE were 6% and 0%, respectively, with a mean survival time of 9 mo. A single-center retrospective investigation compared 14 patients treated with TACE with 14 matched controls who did not receive TACE but chemotherapy, radiotherapy or supportive care. Eight of the 14 patients treated with TACE (57%) showed partial tumor response and had a significantly longer survival compared to those who did not[61].
The effectiveness of systemic therapies for HCC recurrence following LT is largely unstudied because these patients have been routinely excluded from clinical trials. Sorafenib has been increasingly administered for treatment of post LT recurrences, and some data have been collected in the literature regarding its safety and efficacy in this setting. In a case–control study from Sposito et al[62], sorafenib provided improved median survival after HCC recurrence untreatable by surgery or LRT with respect to best supportive care (10.6 vs 2.2 mo). A meta-analysis published 2 years later reported a pooled 1-year survival of 36% (range 18%–90%)[63]. The main limitation of sorafenib in transplanted patients is toxicity, often leading to dose reduction, as reported by several studies[62,64]. Close monitoring is warranted for these patients, particularly in case of immunosuppression with mTORis, since this association may lead to severe adverse events[65-70]. Regorafenib may be proposed as a second-line treatment in case of progression under sorafenib[71]. In a recent multicenter study, second line treatment with regorafenib after sorafenib discontinuation provided a median survival of 13.1 mo compared with 5.5 mo with best supportive care in post-LT HCC relapse[72]. Recently approved tyrosine kinase inhibitors (lenvatinib[73] and cabozantinib[74]) and monoclonal antibodies (ramucirumab[75]) will soon be introduced in clinical practice also for the treatment of post-LT recurrence, giving us the opportunity to collect data about their efficacy and toxicity in this setting[76]. Currently, immunotherapy is changing the landscape of systemic therapies for HCC[77-79], but its safety after LT represents a point of concern, since ICIs may cause allograft rejection and other serious adverse events[80-85].
Studies evaluating the outcome of patients with post-LT HCC recurrence mostly consist of small and heterogeneous series burdened by significant biases in terms of transplant criteria, availability of different treatments and patients’ selection to curative and palliative options[86]. Survival of post-LT HCC recurrence is dismal and significantly worse than relapse after resection (median OS around 12 mo vs nearly 2 years in transplanted and resected patients, respectively), and immunosuppression is a potential driver of such a difference[87,88]. A number of factors have an impact on survival, and there is a small subset of patients with more favorable prognosis in whom curative treatments may be undertaken. Table 1 summarizes the results of studies evaluating the prognostic factors and outcome of treatment for HCC relapse after LT.
| Ref. | No. of patients | Type of study | Site of recurrence | Treatment | mTTR | mOS | Negative outcome predictors |
| Sapisochin et al[39], 2015 | 121 (15.5%) | Retrospective multicenter | 18.4% liver, 47.4% extrahepatic, 34.2% liver + extra | 31.4% surgery/ablation, 42.1% palliative, 26.4% BSC | 14 mo | 12.2 mo (1 yr 54%; 3 yr 19%; 5 yr 14%) | No curative treatment. RFS < 1 yr. AFP > 100 ng/mL |
| Ho et al[96], 2020 | 349 (16.4%) | National registry | ≥ 38.1% liver, ≥ 20.3% extrahepatic/(liver + extra) | 4.6% surgery, 6.3% ablation, 32.1% RT, 27.2% TACE, 20.3% Sorafenib, 20.3% BSC | 17.8 mo | 11.2 mo (1 yr 57%; 3 yr 24.7%; 4 yr 19%) | LT era > 2008 (due to listing of downstaged patients). No curative treatment. Sorafenib/RT |
| Hong et al[93], 2019 | 92 (17.3%) (LDLT) | Retrospective multicenter | 37% liver, 34.8% lung, 28.3% bone, 16.3% lymph nodes | 38% surgery, 51.1% TACE, 38% RT, 45.7% Sorafenib | 11.3 mo | 11.7 mo (1 yr 59.5%; 3 yr 23%; 5 yr 11.9%) | TTR < 6 mo. No curative treatment. Multiorgan involvement. Explant tumor size > 5 cm. mTORi (late) |
| Toso et al[92], 2013 | 30 (12.8%) | Retrospective multicenter | 46.6% liver, 43.3% lung, 23.3% bone, 13.3% other | 20% surgery, 10% TACE/RF/PEI, 70% CT/BSC | 14.2 mo | 33 mo | Graft rejection 0-6 mo. TTR |
| Bodzin et al[40], 2017 | 106 (12.4%) | Retrospective multicenter | 37.8% liver, 55.7% lung, 25.5% bone, 3.8% brain | 23.3% surgery, 3.9% RFA, 13.6% RT, 73.5% CT, 17% BSC | 15.8 mo | 10.6 mo | MELD at LT > 23. TTR. > 3 recurrent nodules. Size of recurrence. Bone recurrence. AFP at recurrence. Donor Na. Pre-LT NLR |
| Fernandez-Sevilla et al[41], 2017 | 70 (14.2%) | Retrospective single center | 2.8% liver, 72.9% extrahepatic, 24.3% liver + extra | 31.4% surgery, 8.6% TACE, 28.6% Sorafenib | 17 mo | 19 mo (1 yr 65%; 3 yr 26%; 5 yr 5%) | AFP > 100 ng/mL. Intrahepatic. Multifocal. No surgical treatment |
| Maccali et al[94], 2021 | 105 (16.6%) | Retrospective multicenter | 23.8% liver, 21% liver + extra, 55.2% extrahepatic | 9.5% surgery, 2.9% TACE, 4.8% RT, 44.8% CT/Sorafenib | 13 mo | 6.2 mo | RFS < 1 yr. No surgical, loco-regional or systemic treatment |
| Ekpanyapong et al[95], 2020 | 96 (13.5%) | Retrospective single center | 21.9% liver, 78.1% extrahepatic/(liver + extra) | 27.1% surgery. 5.2% RFA. 1% TACE. 10.4% RT. 39.6% Sorafenib. 16.7% BSC | 17.1 mo | 10.1 mo (1 yr 48%; 3 yr 16%) | AFP > 1000 ng/mL. Poorly differentiated HCC. Bilirubin ≥ 1.2 mg/dL and albumin < 3.5 mg/dL at recurrence. Peritoneal disease |
| Regalia et al[45], 1998 | 21 (15.9%) | Retrospective multicenter | 19% liver, 19% lung, 14% bone, 38% multiple sites | 33.3% surgery, 19% CT, 14.3% RT/CT-RT, 23.8% BSC | 7.8 mo | 1 yr 62%; 3 yr 29%; 4 yr 23% | Related to early recurrence: Explant tumor size > 3 cm; outside Milan Criteria; absence of capsule |
| Kornberg et al[38], 2010 | 16 (26.7%) | Retrospective single center | 25% liver, 25% bone, 31.2% lung,6.2% brain, 6.2% peritoneum, 6.2% adrenal gland | 43.7% surgery, 18.7% RT, 6.2% TACE, 6.2 Sorafenib, 31.2 %BSC | 23 mo | 10.5 mo | No surgical treatment. Early recurrence (< 24 mo) |
| Alshahrani et al[57], 2018 | 232 (15.6%) | Retrospective single center | 31% liver, 57.8% extrahepatic, 13.4% multiple sites | - | - | 1 yr 60.2%; 3 yr 28.3%; 5 yr 20.5%; 10 yr 7% | Early recurrence |
| Taketomi et al[100], 2010 | 17 (16.8%) (LDLT) | Retrospective single center | - | 53% surgery, 47% other | 12.9 mo | 1 yr 76.5%; 3 yr 51.3%; 5 yr 34.2% | No surgical treatment. Early recurrence |
| Roh et al[90], 2014 | 63 (13.8%) | Retrospective single center | 22% liver, 16% lung, 52% multiple sites, 10% other | 6% surgery, 38% local treatment, 16% systemic treatment, 33% combined treatment, 13% BSC | 12.9 mo | 12.2 mo | Bone involvement. Early recurrence (< 6 mo). Multi-organ |
| Valdivieso et al[36], 2010 | 23 (12.6%) | Retrospective single center | 8.7% liver, 21.7% liver + extra, 69.5% extrahepatic | 47.8% surgery, 17.4 systemic treatment, 34.8% BSC | 23.4 mo | R0 33.2 mo, other 11.9 mo | R0 surgical treatment |
| Mehta et al[101], 2020 | 84 (11.6%) | Retrospective multicenter | 26.2% liver, 48.8% extrahepatic, 25% multiple sites | - | 13 mo | - | - |
| Sharma et al[102], 2012 | 17 (18%) | Retrospective single center | 35.3% liver, 64.7% multiple sites | - | 25.2 mo | - | - |
| Shin et al[91], 2010 | 28 (20.3%) (LDLT) | Retrospective single center | 50% liver, 25% extrahepatic, 25% multiple sites | Liver: TACE. Extrahepatic: Systemic therapy/RT | 7.9 mo | 11.7 mo (1 yr 52.8%; 3 yr 15.8%) | Major vascular invasion. Poorly differentiated HCC. No surgical treatment. Bone metastases |
| Schlitt et al[103], 1999 | 39 (56.5%) | Retrospective single center | 23.1% liver, 38.5 liver + extra, 38.5% extrahepatic | 38.4% surgery, 41% BSC, 12.8% systemic treatment, 2.5% TACE, 5.1% RT | 14.5 mo | 8 mo (non-surgical treatment) | - |
| Escartin et al[104], 2007 | 28 (15.2%) | Retrospective single center | 14.3% liver, 46.4% extrahepatic, 39.3% multiple sites | - | - | 7 mo | - |
| Cescon et al[105], 2010 | 34 (12%) | Retrospective single center | 8.8% liver, 20.6% extrahepatic, 70.6% multiple sites | 100% systemic treatment (in combination with: 5.9% surgery, 3% RT, 3% RFA, 3% IA CT) | 12 mo | - | - |
| Roayaie et al[37], 2004 | 57 (18.3%) | Retrospective single center | 15.8 % liver, 52.6% extrahepatic, 31.6% multiple sites | 31.6% surgery, 5.2% TACE, 26.3% systemic treatment, 7% RT, 29.8% BSC | 12.2 mo | 8.7 mo | Bone metastases. No surgical treatment. Early recurrence |
Several studies showed that time from LT to recurrence has a primary role on outcomes, with early relapse being associated with poor prognosis either when defined as occurring within 6 mo[89-93], 1 year[39,94,95] or 2 years[96]. Many factors related to the primary tumor biology and aggressiveness affect time to recurrence and/or post-relapse survival: size (with cutoffs > 30 or > 50 mm)[45,97], staging outside MC[45], bilobar spread[97], absence of peritumoral capsule[45], poorly differentiated tumors[91,94,95], total tumor volume[92], presence of micro- or macrovascular invasion and pre-LT lymphocyte to neutrophil ratio[39,40,91,92]. The use of mTORi in the post-LT setting seems to be related to better post-recurrence outcomes, as shown both in eastern and western series[93,95]. Moreover, a history of graft rejection has also been associated with improved outcomes, possibly due to more active anticancer immunity[92].
It has been suggested that the observed difference in outcomes between early and late recurrences lies in different underlying biological mechanisms. However, this does not turn into a difference in the site of recurrence. In fact, occurrence of extrahepatic, combined intra- and extrahepatic or intrahepatic relapses do not seem to be different in early versus late recurrences[97]. While early relapses may be due to undetected extrahepatic metastases or circulating HCC clones implanting in a target organ during or soon after LT, late recurrences are possibly related to a second hit leading to late engrafting of HCC cells remaining latent during the initial post-LT period. In the latter, immunosuppression may also play a role[23]. As for intrahepatic late relapses, a further mechanism to be considered is de novo occurrence of HCC, usually arising in the context of chronic liver disease or cirrhosis due to recurrence of primary hepatitis, ischemic biliary injury or chronic rejection, several years after LT[98]. In such instances, results are expected to parallel those of nontransplant recipients with localized HCC, in which surgery or LRTs are effective in controlling the disease.
Aside from primary disease features, other studies have focused on the pattern of recurrence as a relevant prognostic factor for post-recurrence survival. As expected, limited disease spread with localized nodules (oligorecurrence), either hepatic or extrahepatic, has been associated to better outcomes than disseminated multifocal recurrence in several series[36-38,41,45,90]. In addition, a different prognostic impact of hepatic versus extrahepatic localization has been repeatedly reported. Hong et al showed that liver involvement as the first recurrence site was associated with worse survival, with fewer patients amenable to resection among intrahepatic rather than extrahepatic localizations[93]. A monocentric French series on 70 HCC recurrences also identified intrahepatic location as an unfavorable prognostic factor, which was confirmed in a Latin American series on 105 post-LT recurrences showing a lower probability of treatment in patients with hepatic relapses[41,94]. It may be speculated that this is related to the biological mechanism underlying tumor relapse, with recurrences due to undetected metastases at the time of LT more likely to occur at extrahepatic sites and associated with decreased burden as compared to circulating HCC clones, biologically more aggressive tumors, and being more likely to implant in the new liver. Of note, peritoneal and bone localizations were also reported as a poor prognostic factors[37,91,95]. Nevertheless, evidence deriving from several studies shows that the best outcomes are observed in patients with unifocal, often extrahepatic disease, easily amenable to surgical resection[36-38,41,45]. In the large series by Sapisochin et al[39] cited above, not being amenable to curative-intent treatment (resection or ablation) was an independent indicator of poor survival, together with AFP ≥ 100 ng/mL at the time of relapse. In another single-center study from the USA by Bodzin et al[40] on 106 recurrences, a prominent prognostic role of recurrence-related factors (AFP at relapse, > 3 nodules, maximum size of recurrence and bone spread) rather than primary disease features was shown. By combining such factors, a risk score model was built, with accurate stratification of recurrent patients into low-risk (median survival of 70.6 mo), medium-risk (12.2 mo) and high-risk (3.4 mo) subgroups.
Due to selection bias of surgical patients towards later recurrences, more favorable localizations, less aggressive disease and better performance status, the independent prognostic role of either recurrence pattern or resectability is questionable. The limited disease spread may make patients more likely to undergo surgical excision on the one hand, or simply reflect a different tumor biology, etiology, and stage of recurrence on the other hand, as compared to more advanced cases of multifocal recurrence. Even when radical resection cannot be undertaken, it is widely accepted that any kind of treatment of recurrence has a positive prognostic impact. In the multicenter Latin American study, propensity score matching was used to evaluate the adjusted treatment effect considering selection bias. Patients treated with both sorafenib and surgery/TACE had better survival compared to the best supportive care regardless of time to recurrence[95]. Although randomized data are unlikely to be available in this context and retrospective comparisons are impaired by intrinsic differences between single site/oligometastatic and disseminated recurrence, surgical treatment remains an independent predictor of improved outcome following post-LT recurrence[37-39,41].
As for primary disease and post-LT outcome, AFP at recurrence as an indicator of disease spread, MVI and biological aggressiveness was frequently reported as a strong predictor of prognosis, with cutoffs varying from 100 to 1000 ng/mL[39-41,95]. The difference in survival for patients with high AFP was evident regardless of curative-intent treatment, confirming its value as a marker of unfavorable biological features, and its ability to guide the clinical management of patients affected by HCC recurrence. Finally, other biochemical markers at recurrence were associated with shorter survival: High bilirubin, possibly as a reflection of graft dysfunction, and low albumin, related to poor nutritional status as a general prognostic factor outlined in several series[96,99].
HCC recurrence after LT is still a dreadful event, occurring in up to 20% of cases. It might be prevented by stringent pretransplant selection criteria incorporating biological markers of aggressiveness (such as response to therapy, serum markers, histological factors) in addition to size and number of tumors. Several advances in this sense have been made in the last decade, allowing patients with HCC broader access to LT with more precise prediction of outcomes. In the post-LT period, surveillance should be driven by post-LT risk stratification, and the RETREAT score seems to be the best cost-effective approach. No adjuvant treatments after LT have been validated to prevent HCC recurrence; however, a balanced use of immunosuppression with minimal dose of CNIs and possibly the addition of mTORi is strongly advisable. Median post-recurrence survival is 12 mo: the interplay between time to recurrence (with a negative impact of earlier events) and the possibility of a radical treatment is the strongest determinant of survival.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang A, Thailand; Kim IH, South Korea S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Wang JJ
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (37)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 3. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R, Madariaga J. Hepatic resection vs transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 633] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 419] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5309] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 8. | Tan DJH, Wong C, Ng CH, Poh CW, Jain SR, Huang DQ, Muthiah MD. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Yang JD, Larson JJ, Watt KD, Allen AM, Wiesner RH, Gores GJ, Roberts LR, Heimbach JA, Leise MD. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol. 2017;15:767-775.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (35)] |
| 10. | Kwong AJ, Kim WR, Flemming JA. De Novo Hepatocellular Carcinoma Among Liver Transplant Registrants in the Direct Acting Antiviral Era. Hepatology. 2018;68:1288-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 13. | Goldaracena N, Mehta N, Scalera I, Sposito C, Atenafu EG, Yao FY, Muiesan P, Mazzaferro V, Sapisochin G. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB (Oxford). 2019;21:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 15. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-94.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 727] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 16. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 17. | Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, Goffette P, Vogel W, Pitton MB, Lerut J; European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK Jr, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 19. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Aggarwal A, Te HS, Verna EC, Desai AP. A National Survey of Hepatocellular Carcinoma Surveillance Practices Following Liver Transplantation. Transplant Direct. 2021;7:e638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Berenguer M, Burra P, Ghobrial M, Hibi T, Metselaar H, Sapisochin G, Bhoori S, Kwan Man N, Mas V, Ohira M, Sangro B, van der Laan LJW. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Verna EC, Patel YA, Aggarwal A, Desai AP, Frenette C, Pillai AA, Salgia R, Seetharam A, Sharma P, Sherman C, Tsoulfas G, Yao FY. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant. 2020;20:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 25. | Lee DD, Sapisochin G, Mehta N, Gorgen A, Musto KR, Hajda H, Yao FY, Hodge DO, Carter RE, Harnois DM. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation. 2020;104:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, Stoupis C, Dufour JF. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Grigg SE, Sarri GL, Gow PJ, Yeomans ND. Systematic review with meta-analysis: sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2019;49:1260-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 30. | Olthoff KM, Rosove MH, Shackleton CR, Imagawa DK, Farmer DG, Northcross P, Pakrasi AL, Martin P, Goldstein LI, Shaked A. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg. 1995;221:734-41; discussion 731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Hsieh CB, Chou SJ, Shih ML, Chu HC, Chu CH, Yu JC, Yao NS. Preliminary experience with gemcitabine and cisplatin adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Fujiki M, Aucejo F, Kim R. Adjuvant treatment of hepatocellular carcinoma after orthotopic liver transplantation: do we really need this? Clin Transplant. 2013;27:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Siegel AB, El-Khoueiry AB, Finn RS, Guthrie KA, Goyal A, Venook AP, Blanke CD, Verna EC, Dove L, Emond J, Kato T, Samstein B, Busuttil R, Remotti H, Coffey A, Brown RS Jr. Phase I trial of sorafenib following liver transplantation in patients with high-risk hepatocellular carcinoma. Liver Cancer. 2015;4:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Han B, Ding H, Zhao S, Zhang Y, Wang J, Gu J. Potential Role of Adjuvant Lenvatinib in Improving Disease-Free Survival for Patients With High-Risk Hepatitis B Virus-Related Hepatocellular Carcinoma Following Liver Transplantation: A Retrospective, Case Control Study. Front Oncol. 2020;10:562103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Ziogas IA, Evangeliou AP, Giannis D, Hayat MH, Mylonas KS, Tohme S, Geller DA, Elias N, Goyal L, Tsoulfas G. The Role of Immunotherapy in Hepatocellular Carcinoma: A Systematic Review and Pooled Analysis of 2,402 Patients. Oncologist. 2021;26:e1036-e1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ, Montejo M, Ortiz de Urbina J. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 38. | Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lázaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 40. | Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 41. | Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Sommacale D, Dondero F, Sauvanet A, Francoz C, Durand F, Farges O, Kianmanesh R, Belghiti J. Liver resection in transplanted patients: a single-center Western experience. Transplant Proc. 2013;45:2726-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Chok KSh. Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol. 2015;7:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, Montalto F, Coppa J, Mazzaferro V. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35:2058-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, Higashiyama M, Ishikawa O, Kodama K, Imaoka S. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Jeong YH, Hwang S, Lee GD, Choi SH, Kim HR, Kim YH, Park SI, Kim DK. Surgical Outcome of Pulmonary Metastasectomy for Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Ann Transplant. 2021;26:e930383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Bates MJ, Farkas E, Taylor D, McFadden PM. Pulmonary resection of metastatic hepatocellular carcinoma after liver transplantation. Ann Thorac Surg. 2008;85:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Han KN, Kim YT, Yoon JH, Suh KS, Song JY, Kang CH, Sung SW, Kim JH. Role of surgical resection for pulmonary metastasis of hepatocellular carcinoma. Lung Cancer. 2010;70:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4687] [Article Influence: 937.4] [Reference Citation Analysis (2)] |
| 52. | Hwang S, Kim YH, Kim DK, Ahn CS, Moon DB, Kim KH, Ha TY, Song GW, Jung DH, Kim HR, Park GC, Namgoong JM, Yoon SY, Jung SW, Park SI, Lee SG. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg. 2012;36:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Hu JG, Lu Y, Lin XJ. En Bloc lumpectomy of T12 vertebra for progressive hepatocellular carcinoma metastases following liver transplantation: A case report. Medicine (Baltimore). 2020;99:e18756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Abdel Wahab M, Shehta A, Ibrahim EM, Eldesoky RT, Sultan AA, Zalata KR, Fathy O, Elshoubary M, Salah T, Yassen AM, Elmorshedi M, Monier A, Farouk A, Shiha U. Adrenalectomy for solitary recurrent hepatocellular carcinoma five years after living donor liver transplantation: A case report. Int J Surg Case Rep. 2019;54:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Jalbani IK, Nazim SM, Tariq MU, Abbas F. Adrenalectomy for solitary metastasis of Hepatocellular carcinoma post liver transplantation: Case report and literature review. Pak J Med Sci. 2016;32:1044-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Ikegami T, Yoshizumi T, Kawasaki J, Nagatsu A, Uchiyama H, Harada N, Harimoto N, Itoh S, Motomura T, Soejima Y, Maehara Y. Surgical Resection for Lymph Node Metastasis After Liver Transplantation for Hepatocellular Carcinoma. Anticancer Res. 2017;37:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Alshahrani AA, Ha SM, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Cho HD, Kwon JH, Kang SH, Lee SG. Clinical Features and Surveillance of Very Late Hepatocellular Carcinoma Recurrence After Liver Transplantation. Ann Transplant. 2018;23:659-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Tohyama T, Sakamoto K, Tamura K, Nakamura T, Watanabe J, Wakisaka H, Takada Y. Pharyngeal metastasis following living-donor liver transplantation for hepatocellular carcinoma: a case report and literature review. World J Surg Oncol. 2020;18:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Zhai H, Liang P, Yu XL, Cheng Z, Han ZY, Liu F, Yu J. Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: An analysis of 11 cases. Int J Hyperthermia. 2015;31:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Ko HK, Ko GY, Yoon HK, Sung KB. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 63. | Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Toso C, Mentha G, Majno P. Integrating sorafenib into an algorithm for the management of post-transplant hepatocellular carcinoma recurrence. J Hepatol. 2013;59:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, Antonelli B, Airoldi A, Manini MA, Sangiovanni A, Rossi G, Donato MF, Saverio Belli L, Lampertico P. Experience With Early Sorafenib Treatment With mTOR Inhibitors in Hepatocellular Carcinoma Recurring After Liver Transplantation. Transplantation. 2020;104:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Piguet AC, Saar B, Hlushchuk R, St-Pierre MV, McSheehy PM, Radojevic V, Afthinos M, Terracciano L, Djonov V, Dufour JF. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Herden U, Fischer L, Schäfer H, Nashan B, von Baehr V, Sterneck M. Sorafenib-induced severe acute hepatitis in a stable liver transplant recipient. Transplantation. 2010;90:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Waidmann O, Hofmann WP, Zeuzem S, Trojan J. mTOR inhibitors and sorafenib for recurrent heptocellular carcinoma after orthotopic liver transplantation. J Hepatol. 2011;54:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | De Simone P, Crocetti L, Pezzati D, Bargellini I, Ghinolfi D, Carrai P, Leonardi G, Della Pina C, Cioni D, Pollina L, Campani D, Bartolozzi C, Lencioni R, Filipponi F. Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2014;46:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, Mazzaferro V. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, Manini MA, López MF, Anders M, Pinter M, Rodríguez MJB, Cristóbal MR, Soteras GA, Piñero F, Villadsen GE, Weinmann A, Crespo G, Mazzaferro V, Regnault H, Giorgio M, González-Diéguez ML, Donato MF, Varela M, Wörns MA, Bruix J, Lampertico P, Reig M. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, Fraile-López M, Czauderna C, Di Costanzo G, Bhoori S, Pinter M, Manini MA, Amaddeo G, Yunquera AF, Piñero F, Blanco Rodríguez MJ, Anders M, Aballay Soteras G, Villadsen GE, Yoon PD, Cesarini L, Díaz-González Á, González-Diéguez ML, Tortora R, Weinmann A, Mazzaferro V, Romero Cristóbal M, Crespo G, Regnault H, De Giorgio M, Varela M, Prince R, Scudeller L, Donato MF, Wörns MA, Bruix J, Sapisochin G, Lampertico P, Reig M. Regorafenib Efficacy After Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Retrospective Study. Liver Transpl. 2021;27:1767-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3822] [Article Influence: 546.0] [Reference Citation Analysis (1)] |
| 74. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1767] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 75. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1247] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 76. | Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 77. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3306] [Article Influence: 413.3] [Reference Citation Analysis (1)] |
| 78. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1339] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 79. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1894] [Article Influence: 270.6] [Reference Citation Analysis (0)] |
| 80. | Kittai AS, Oldham H, Cetnar J, Taylor M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J Immunother. 2017;40:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 81. | Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, Farmer DG, Busuttil RW, Federman N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 82. | Biondani P, De Martin E, Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 83. | DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, Moss AA, Mathur AK, Chascsa DM, Rakela J, Bryce AH, Borad MJ. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 84. | Gassmann D, Weiler S, Mertens JC, Reiner CS, Vrugt B, Nägeli M, Mangana J, Müllhaupt B, Jenni F, Misselwitz B. Liver Allograft Failure After Nivolumab Treatment-A Case Report With Systematic Literature Research. Transplant Direct. 2018;4:e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 85. | Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32:e69-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 86. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 87. | Pelizzaro F, Gambato M, Gringeri E, Vitale A, Cillo U, Farinati F, Burra P, Russo FP. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Shalaby S, Burra P. De novo and recurrent malignancy. Best Pract Res Clin Gastroenterol. 2020;46-47:101680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Jeyarajah DR, Doyle MBM, Espat NJ, Hansen PD, Iannitti DA, Kim J, Thambi-Pillai T, Visser BC. Role of yttrium-90 selective internal radiation therapy in the treatment of liver-dominant metastatic colorectal cancer: an evidence-based expert consensus algorithm. J Gastrointest Oncol. 2020;11:443-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Roh YN, David Kwon CH, Song S, Shin M, Man Kim J, Kim S, Joh JW, Lee SK. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2014;28:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 92. | Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G, Kneteman NM. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Hong SK, Lee KW, Yoon KC, Kim HS, Ahn SW, Kim H, Lee JM, Cho JH, Yi NJ, Suh KS. Different prognostic factors and strategies for early and late recurrence after adult living donor liver transplantation for hepatocellular carcinoma. Clin Transplant. 2019;33:e13703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Maccali C, Chagas AL, Boin I, Quiñonez E, Marciano S, Vilatobá M, Varón A, Anders M, Hoyos Duque S, Lima AS, Menendez J, Padilla-Machaca M, Poniachik J, Zapata R, Maraschio M, Chong Menéndez R, Muñoz L, Arufe D, Figueroa R, Soza A, Fauda M, Perales SR, Vergara Sandoval R, Bermudez C, Beltran O, Arenas Hoyos I, McCormack L, Mattera FJ, Gadano A, Parente García JH, Tani CM, Augusto Carneiro D'Albuquerque L, Carrilho FJ, Silva M, Piñero F. Recurrence of hepatocellular carcinoma after liver transplantation: Prognostic and predictive factors of survival in a Latin American cohort. Liver Int. 2021;41:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Ekpanyapong S, Philips N, Loza BL, Abt P, Furth EE, Tondon R, Khungar V, Olthoff K, Shaked A, Hoteit MA, Reddy KR. Predictors, Presentation, and Treatment Outcomes of Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Large Single Center Experience. J Clin Exp Hepatol. 2020;10:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Ho CM, Lee CH, Lee MC, Zhang JF, Chen CH, Wang JY, Hu RH, Lee PH. Survival After Treatable Hepatocellular Carcinoma Recurrence in Liver Recipients: A Nationwide Cohort Analysis. Front Oncol. 2020;10:616094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | El-Domiaty N, Saliba F, Vibert E, Karam V, Sobesky R, Ibrahim W, Pittau G, Ciacio O, Salloum C, Amer K, Saeed MA, Shawky JA, Sa Cunha A, Rosmorduc O, Cherqui D, Adam R, Samuel D. Early Versus Late Hepatocellular Carcinoma Recurrence After Transplantation: Predictive Factors, Patterns, and Long-term Outcome. Transplantation. 2021;105:1778-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Au KP, Chok KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24:5081-5094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Nagai S, Mangus RS, Kubal CA, Ekser B, Fridell JA, Klingler KR, Maluccio MA, Tector AJ. Prognosis after recurrence of hepatocellular carcinoma in liver transplantation: predictors for successful treatment and survival. Clin Transplant. 2015;29:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 102. | Sharma P, Welch K, Hussain H, Pelletier SJ, Fontana RJ, Marrero J, Merion RM. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig Dis Sci. 2012;57:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G, Raab R, Ringe B, Manns MP, Pichlmayr R. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, Dopazo C, Castro E, Caralt M, Balsells J. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2007;39:2308-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Cescon M, Ravaioli M, Grazi GL, Ercolani G, Cucchetti A, Bertuzzo V, Vetrone G, Del Gaudio M, Vivarelli M, D'Errico-Grigioni A, Dazzi A, Di Gioia P, Lauro A, Pinna AD. Prognostic factors for tumor recurrence after a 12-year, single-center experience of liver transplantations in patients with hepatocellular carcinoma. J Transplant. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |