Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4698
Peer-review started: March 2, 2022
First decision: April 12, 2022
Revised: May 5, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: August 28, 2022
Processing time: 176 Days and 23.6 Hours
Pancreatic cancer, as the one of most fatal malignancies, remains a critical issue in the global burden of disease.
To estimate trends in pancreatic cancer incidence and mortality worldwide in the last three decades.
A descriptive epidemiological study was done. Pancreatic cancer incidence and mortality data were obtained from the database of the World Health Organi
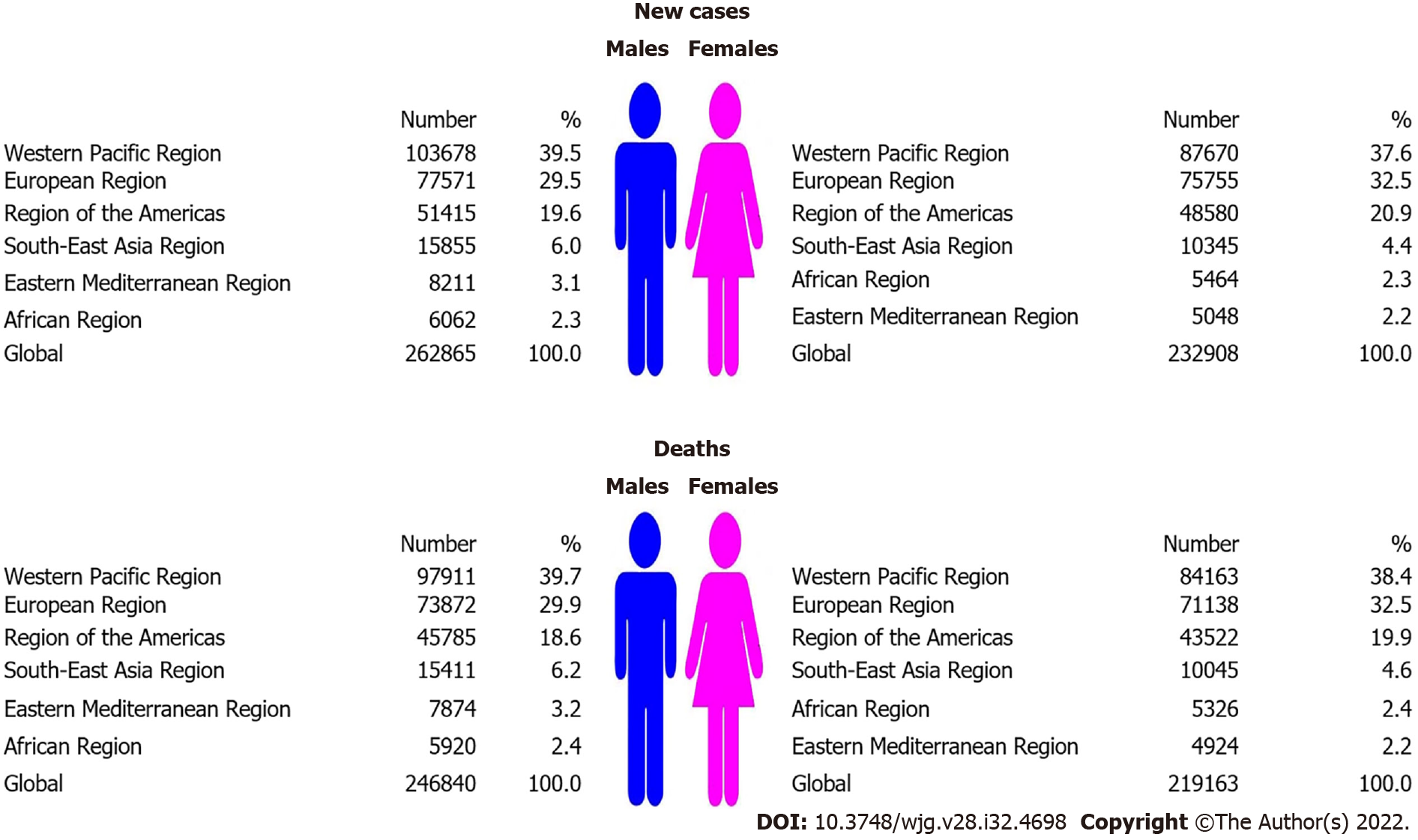
A total of 495773 (262865 male and 232908 female) new cases and 466003 (246840 male and 219163 female) deaths from pancreatic cancer were reported worldwide in 2020. In both sexes, most of the new cases (191348; 38.6% of the total) and deaths (182074; 39.1% of the total) occurred in the Western Pacific Region. In both sexes, the highest ASRs were found in the European Region, while the lowest rates were reported in the South-East Asia Region. The general pattern of rising pancreatic cancer incidence and mortality was seen across countries worldwide in observed period. Out of all countries with an increase in pancreatic cancer incidence, females in France and India showed the most marked rise in incidence rates (AAPC = +3.9% and AAPC = +3.7%, respectively). Decreasing incidence trends for pancreatic cancer were observed in some countries, but without significance. Out of all countries with an increase in pancreatic cancer mortality rates, Turkmenistan showed the most marked rise both in males (AAPC = +10.0%, 95%CI: 7.4–12.5) and females (AAPC = +6.4%, 95%CI: 3.5–9.5). The mortality trends of pancreatic cancer were decreasing in both sexes only in Canada and Mexico.
Further research is needed to explain the cause of large international differences in incidence and mortality trends of pancreatic cancer in last three decades.
Core tip: Pancreatic cancer, as one of the most fatal malignancies, remains a critical issue in global burden of disease. About 500000 new cases and 470000 deaths from pancreatic cancer were recorded in 2020 across the world. Globally, the rate of pancreatic cancer incidence in 2020 was 4.9 per 100000 for both sexes together, while mortality rate was 4.5 per 100000. Increasing trends in both incidence and mortality of pancreatic cancer were observed in most countries across the world. Further efforts to explore the reasons for differences in international pattern in pancreatic cancer incidence and mortality could be critical for global cancer control.
- Citation: Ilic I, Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J Gastroenterol 2022; 28(32): 4698-4715
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4698.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4698
Pancreatic cancer is one of the most lethal cancers, with insidious onset, aggressive nature and poor prognosis[1-3]. The 5-year overall survival rate of pancreatic cancer is low (~10%), since more than half of the cases are confirmed at an advanced stage[3,4]. Based on GLOBOCAN 2020 estimates, pancreatic cancer remains a large part of the global burden of disease, and ranks as the 12th most common malignancy (2.6% of all cancers) and the 7th leading cause of cancer mortality (4.7% of all cancers)[1,2]. Age-standardized incidence and mortality rates are about fivefold higher in countries with high/very high Human Development Index than in low/medium countries[2].
World Health Organization (WHO) estimated pancreatic cancer as the third leading cancer-related cause of death in people of all ages for both sexes in the USA, Germany, Italy, Austria, Czechia, Finland, Hungary, Malta, Spain and Switzerland in 2020[2]. In four countries (Finland, Qatar, Benin and Guadeloupe), pancreatic cancer was the second leading cause of death of all cancers in 2020 in females aged ≥ 70 years[2]. The Global Burden of Disease (GBD) Study ranked pancreatic cancer as the seventh top cause of absolute years of life lost (YLLs) among all cancers in both sexes in 2017, while it ranked ninth in 2007 among the top causes for YLLs worldwide[5]. Also, the GBD study showed that the disability adjusted life years caused by pancreatic cancer have more than doubled from 1990 to 2017 globally[6].
The age patterns were similar for both incidence and mortality of pancreatic cancer, with the rates increasing with increasing age; the burden moderately grew after 30 years of age and came to peak after 80 years of age, with similar trend in males and females[3,5-7]. In 27 countries of the EU and in the UK, between 1990 and 2019, incidence and mortality from pancreatic cancer significantly increased by 0.65% and 0.55% per year, respectively[8].
WHO and the United Nations Sustainable Development Goals, published in 2015, target to reduce premature mortality rate attributed to cancer by one third by 2030[9]. However, according to Ferlay et al[10], by 2025 there will be more deaths annually from pancreatic cancer in the EU countries than deaths from breast cancer, and it will become the third most important cause of cancer death in the EU after lung and colorectal cancer. In addition, a particularly important issue is how the COVID-19 pandemic will affect the management of patients with pancreatic cancer that can be disrupted or delayed, particularly in the context of treatment selection and postoperative care to reduce the risk of SARS-CoV-2-associated morbidity and mortality[11,12].
Recent estimates suggest that pancreatic cancer is becoming a critical public health challenge and a growing health service issue both in developed and developing countries[1,10,13]. The aim of this manuscript was to assess international patterns in incidence and mortality of pancreatic cancer.
This descriptive epidemiological study comprised the annual underlying cause of disease and death data to describe trends in incidence and mortality from pancreatic cancer in the world in last three decades.
Data of pancreatic cancer incidence and mortality were extracted from the WHO databases[2], i.e. the GLOBOCAN estimates of cancer incidence and mortality generated by the International Agency for Research on Cancer. Pancreatic cancer estimates included site code C25, based on the 10th revision of the International Classification of Diseases and Related Health Problems to classify cause of disease and death (ICD-10)[14].
The WHO databases provide a comprehensive and comparable assessment of incidence and mortality of pancreatic cancer[2]. These databases provide high-quality statistics based on national vital registries worldwide. The methods used to compile the GLOBOCAN 2020 estimates involved predictions, modeling of incidence-to-mortality ratios, and approximation from neighboring countries. For pancreatic cancer, we extracted all incidence and mortality data available from WHO databases in all years available since 1991. The WHO estimates comprise only national mortality data series that meet the minimal inclusion criteria according to the WHO-defined medium data quality level, based on degree of population coverage, completeness and accuracy[15]. Level of data quality was classified using criteria described by WHO[14]: data of high quality (ICD-9 or ICD-10 coding is used and completeness is > 90% and ill-defined codes appear on < 10% of registrations), medium quality (completeness is 70%–90% OR ill-defined codes appear on 10%–20% of registrations OR non-ICD codes used although completeness is > 90% and ill-defined codes appear on < 10% of registrations) and low quality (completeness < 70% OR ill-defined codes appear on > 20% of registrations).
This study presented the incidence and mortality figures for 185 WHO Member States, as well as for six WHO regions (Africa, Americas, South-East Asia, Europe, Eastern Mediterranean, and Western Pacific). First analysis of incidence and mortality for pancreatic cancer was for 2020. The trends analyses included only countries with data for pancreatic cancer incidence/mortality available in the observed period (from 1991 or later) continuously, provided that there were data for at least 15 years in a row continuously. Countries with missing values in any year of trend analysis were excluded from the analysis. Specifics about the availability of incidence and mortality data of pancreatic cancer since 1991, as well as the quality classification of data for different countries by years in observed period are defined in Supplementary Table 1. In this study, the trend analysis included only countries with high/medium data quality.
For pancreatic cancer incidence and mortality, all figures were adjusted by age and presented as age-standardized rates (ASRs, per 100000 persons) calculated by the direct method of standardization by age and sex, using the World standard population according to the Segi–Doll World reference population[2].
Trends in pancreatic cancer incidence and mortality rates were calculated using Joinpoint regression analysis software version 4.9.0.0 (National Cancer Institute, Bethesda, MD, USA – March 2021, available through the Surveillance Research Program of the US National Cancer Institute), proposed by Kim et al[16]. The joinpoint regression analysis was used to assess the magnitude and direction of temporal trends in incidence and mortality of pancreatic cancer. The joinpoint regression analysis detected points, the so-called joinpoints, at which there was a significant change in trend in rates of pancreatic cancer, using the calendar year as a regression variable. The ASR was used as the dependent variable, while the by-variables were sex and age group. A Monte Carlo permutation test for multiple comparisons was used and 4499 randomly selected data sets, which finds the best fit line for each segment[16]. The analysis started with a minimum of zero joinpoints (i.e., a straight line) and tested whether a change in the trend was significant by testing more joinpoints up to the maximum of four (five segments)[16]. The resulting regression equation was: y = a + bx, where y = ln(rate) and x = calendar year, with slope a and y intercept b, whereby average annual percent change (AAPC) was estimated as 100 × (eb 1). The Grid Search method was selected[17]. The trend of pancreatic cancer incidence and mortality in each country was presented with a straight line in the whole period, even if there were changes in trends in the observed period. The AAPC over the entire study period with the corresponding 95% confidence interval (95%CI) was determined[18]. In describing the direction of temporal trends, the terms significant increase or significant decrease were used, in order to signify that the slope of the trend was statistically significant (P < 0.05, on the basis of the statistical significance of the AAPC compared to zero). Additionally, analysis was performed by sex and age (30–49, 50–69, ≥ 70 years) in selected countries. Trends were not evaluated separately among persons aged < 30 years due to the small number of pancreatic cancer cases in this age group. A line diagram of joinpoint analysis for individual countries provided information about the number of the joinpoints for the trends in incidence and mortality of pancreatic cancer by sex, by describing of annual percent change and the corresponding 95%CI.
This study was approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (No. 01-14321).
Globally, the total number of new pancreatic cancer cases was 495 773 in 2020: it was diagnosed in 262 865 (53%) males and 232 908 (47%) females (Figure 1). The total number of pancreatic cancer deaths was 466 003 (246 840 males and 219 163 females). Male to female ratio was: 1.1:1 both for incidence and mortality. In both sexes, most new cases (191 348; 38.6% of the total) and deaths (182 074; 39.1% of the total) occurred in the Western Pacific Region. In males, the least number of pancreatic cancer new cases (6062; 2.3% of total) and deaths (5920; 2.4% of total) was recorded in the African region. In females, the least number of pancreatic cancer new cases (5048; 2.2% of total) and deaths (4924; 2.2% of total) was recorded in the Eastern Mediterranean Region.
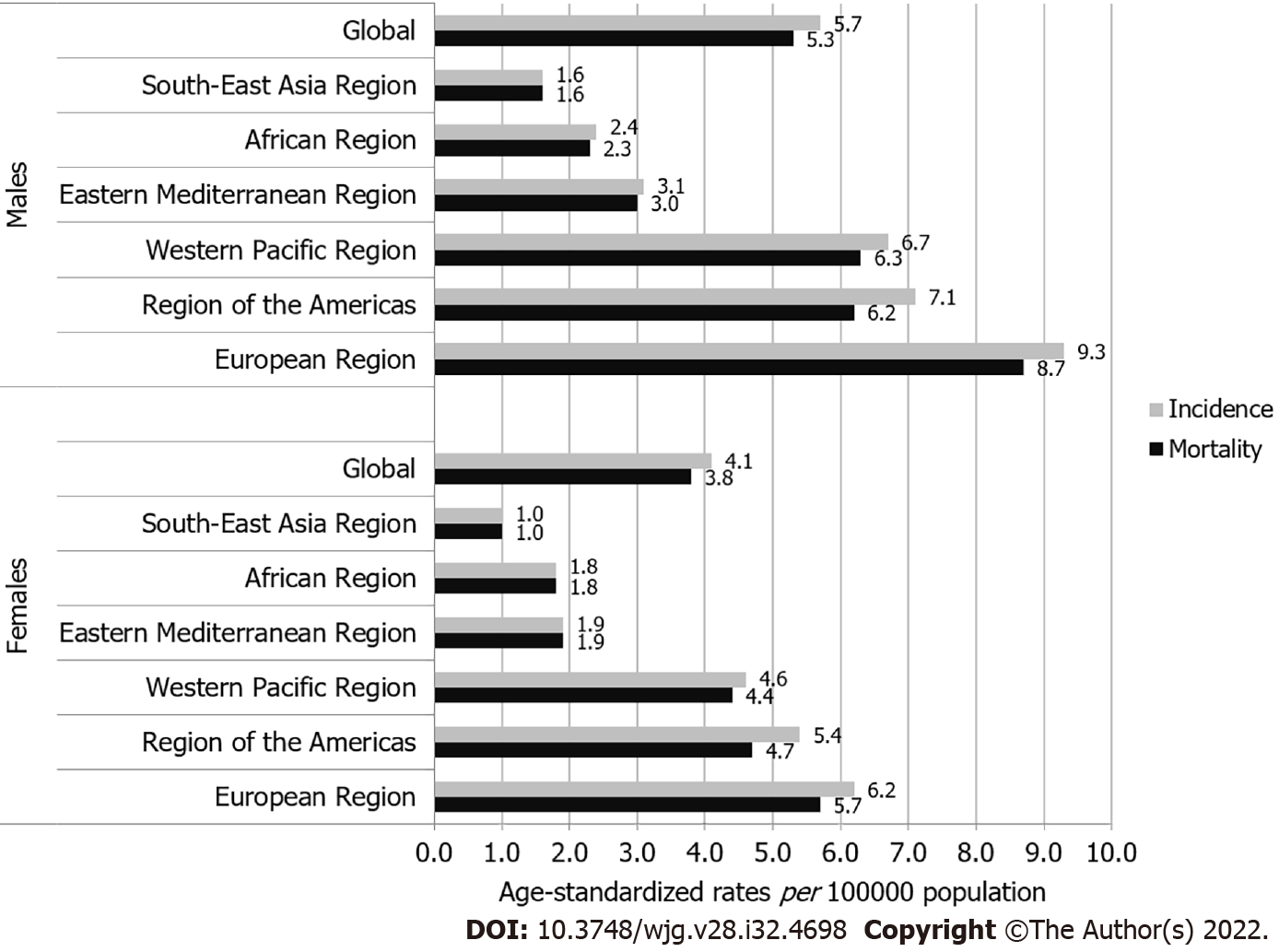
The global ASR of incidence of pancreatic cancer was 4.9 per 100000 population in both sexes (5.7 in males vs 4.1 in females), and ASR of mortality was 4.5 (5.3 in males vs 3.8 in females) (Figure 2). In both sexes, the highest incidence ASRs was found in the European Region (9.3 in males vs 6.2 in females), while the lowest rates were in the South-East Asia Region (1.6 in males vs 1.0 in females). Also in both sexes, the highest mortality ASRs were found in the European Region (8.7 in males vs 5.7 in females), while the lowest rates were in the South-East Asia Region (1.6 in males vs 1.0 in females).
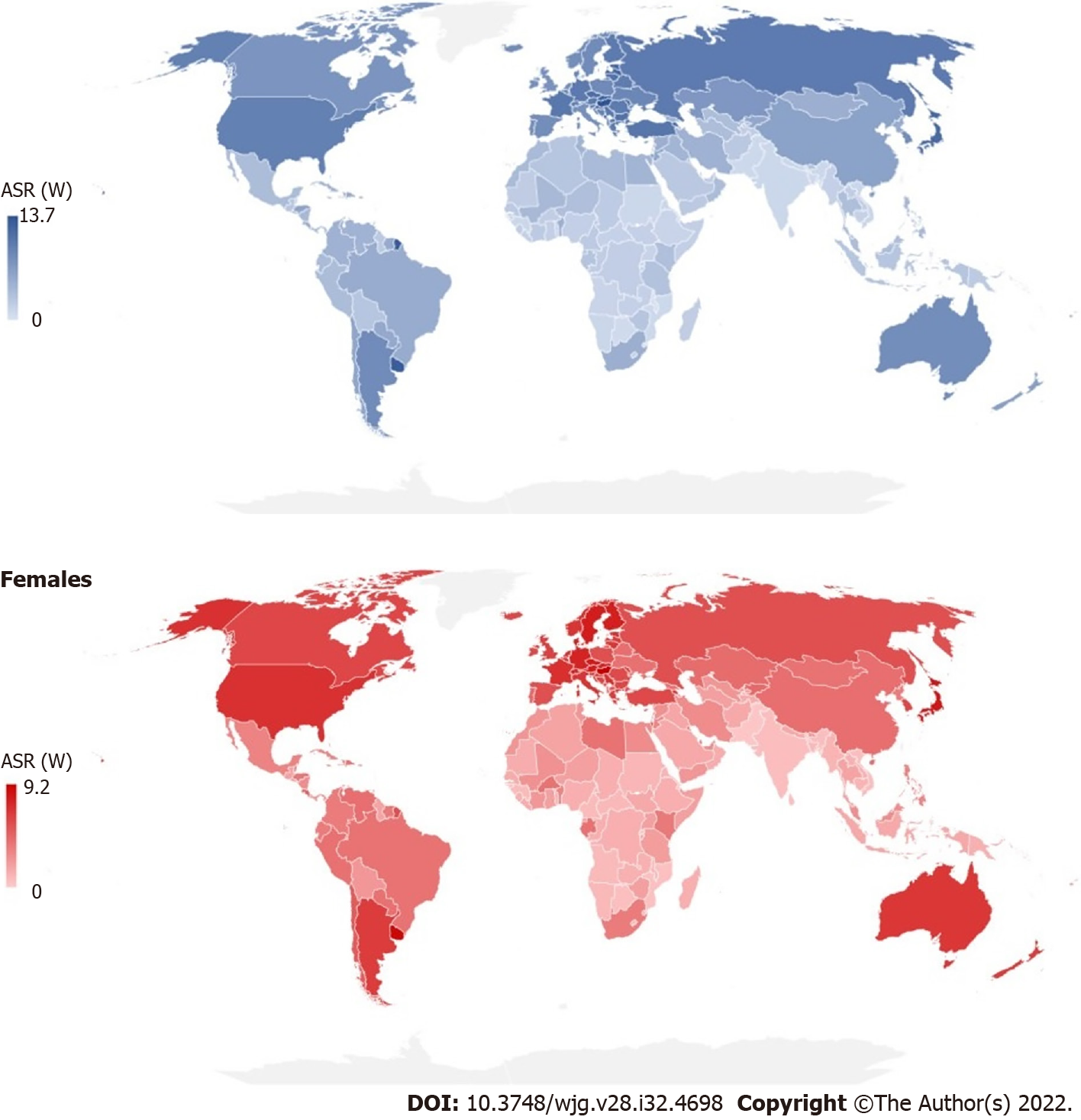
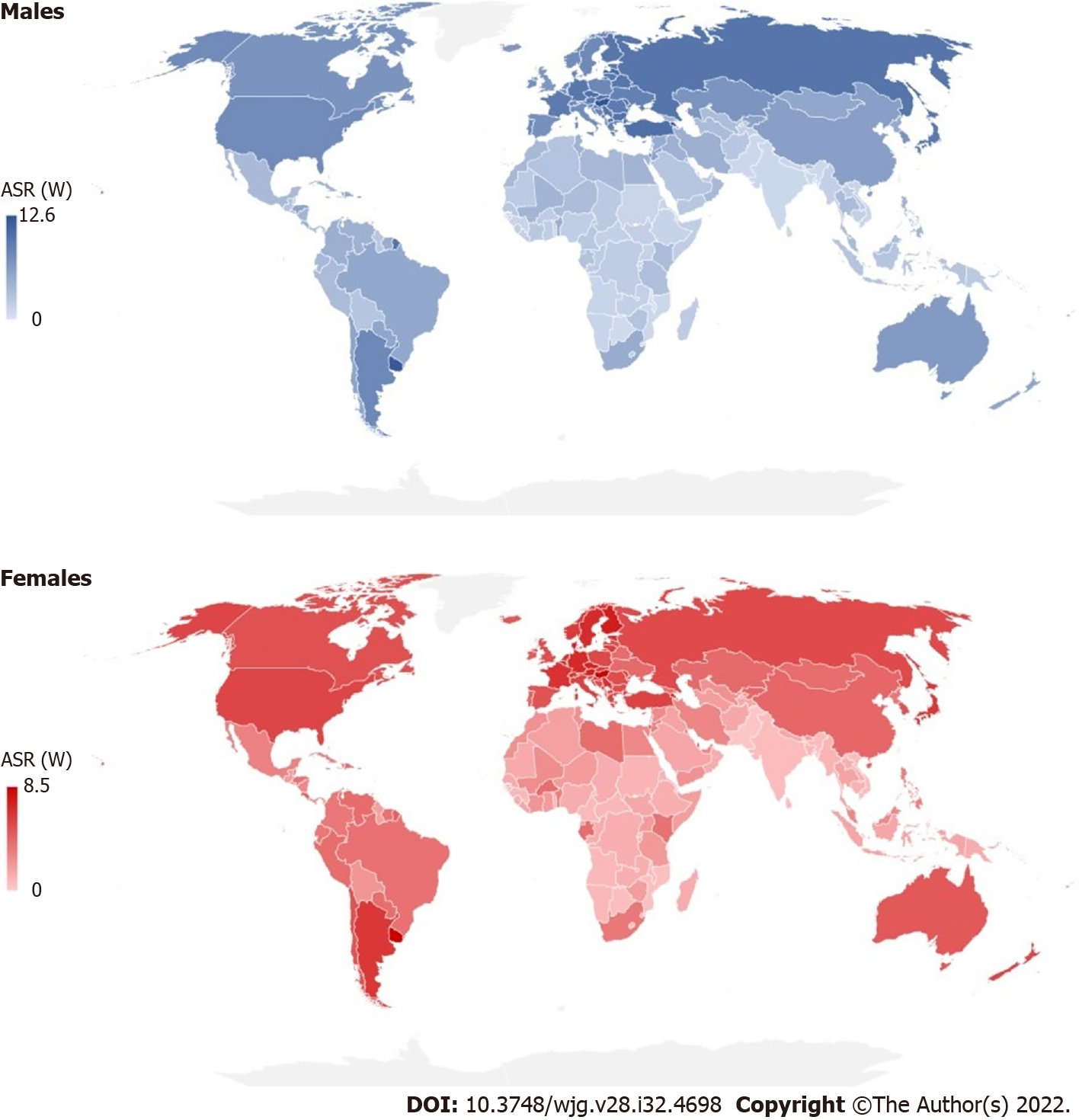
There were significant international variations in incidence and mortality of pancreatic cancer in 2020 (Figures 3 and 4). The highest ASRs of incidence and mortality of pancreatic cancer in both sexes in 2020 were in Hungary (11.2 and 10.2, respectively). The highest incidence ASRs in males and females were in Hungary (13.7 and 9.2 per 100000), followed by Uruguay (12.8 and 8.9), while the lowest rates (equally about 0.5) were reported in Botswana, Eswatini and Malawi in males, and in India, Sri Lanka and Pakistan in females. The highest mortality ASR in males was in Hungary (12.6) and in females in Uruguay (8.5), while the lowest rate was in Malawi in males (0.46) and in Pakistan in females (0.30). In comparison to males, the incidence and mortality ASRs were lower in females in countries across the world in 2020: The only exceptions were for females in Bahrain, Belize, Brunei, Burkina Faso, Cabo Verde, Eswatini, Gabon, Ghana, Kenya, Libya, Malawi, Nepal, New Zealand, Peru, Qatar, Gambia, Uganda and United Arab Emirates where rates higher than in males were recorded.
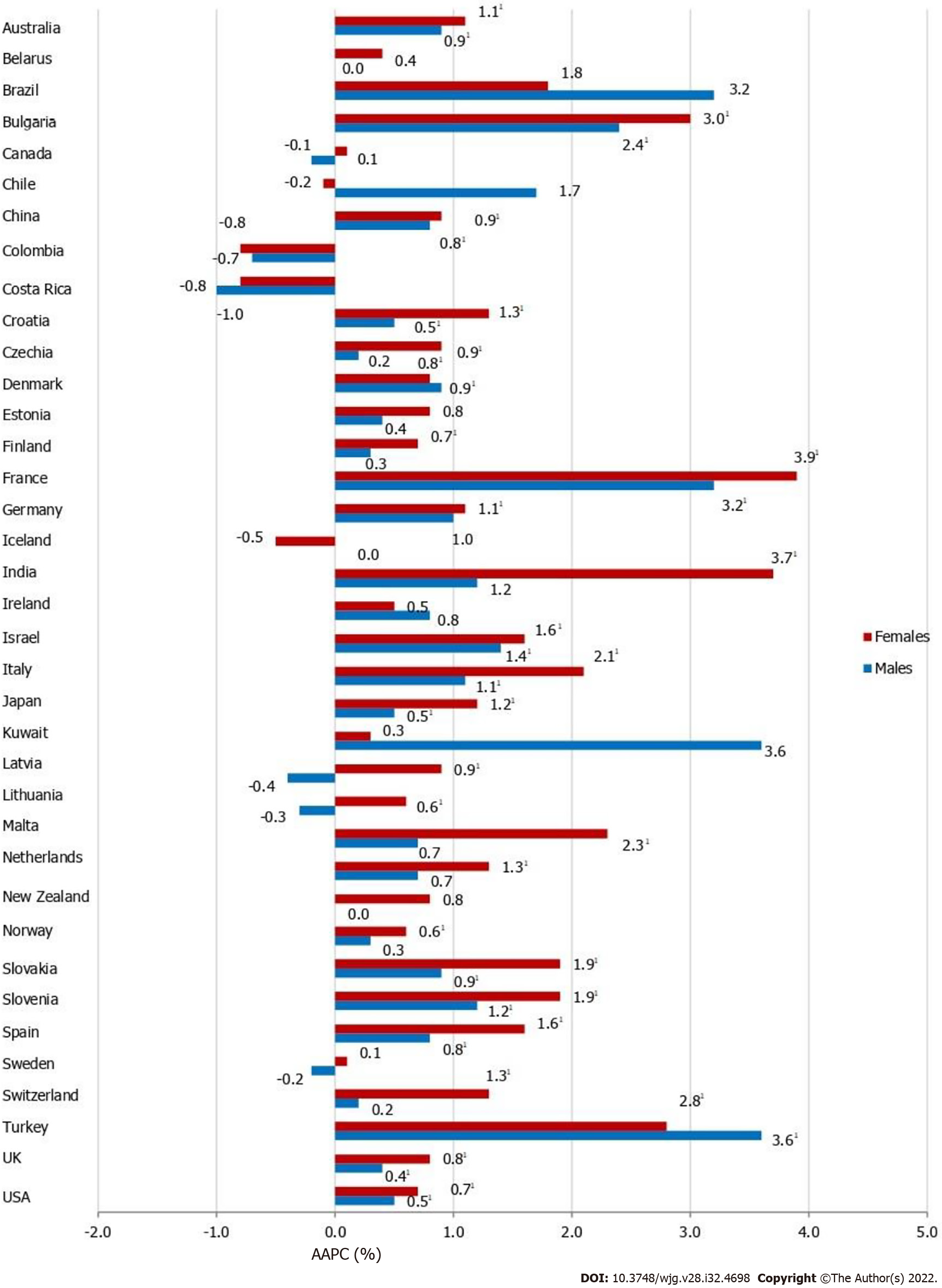
Most of the countries showed a meaningful increase in pancreatic cancer incidence in both males and females, but the magnitude of increasing incidence trends for pancreatic cancer was higher in females than in males in almost all countries (Figure 5). Out of all countries with an increase in pancreatic cancer incidence, females in France and India showed the most marked rise in incidence rates (AAPC = +3.9% and AAPC = +3.7%, respectively). In males in Kuwait and Turkey the most marked rise in incidence rates was recorded (both equally by AAPC = +3.6%). In some countries, pancreatic cancer incidence trends were significantly increasing in females (Czechia, Germany, India, Latvia, Lithuania, Malta, Netherlands, Norway and Switzerland), in contrast to males. Decreasing trends of pancreatic cancer incidence were observed in some countries but without significance. In some of the most developed countries (such as the UK and Sweden), in both sexes significantly increasing trends in pancreatic cancer incidence rates were observed in the last decade, which followed a previous period characterized by a declining incidence trend (Supplementary Figure 1). In contrast to that, two decades of continuous growth, a downward trend of pancreatic cancer incidence rates in both sexes happened in the more recent decade in Denmark.
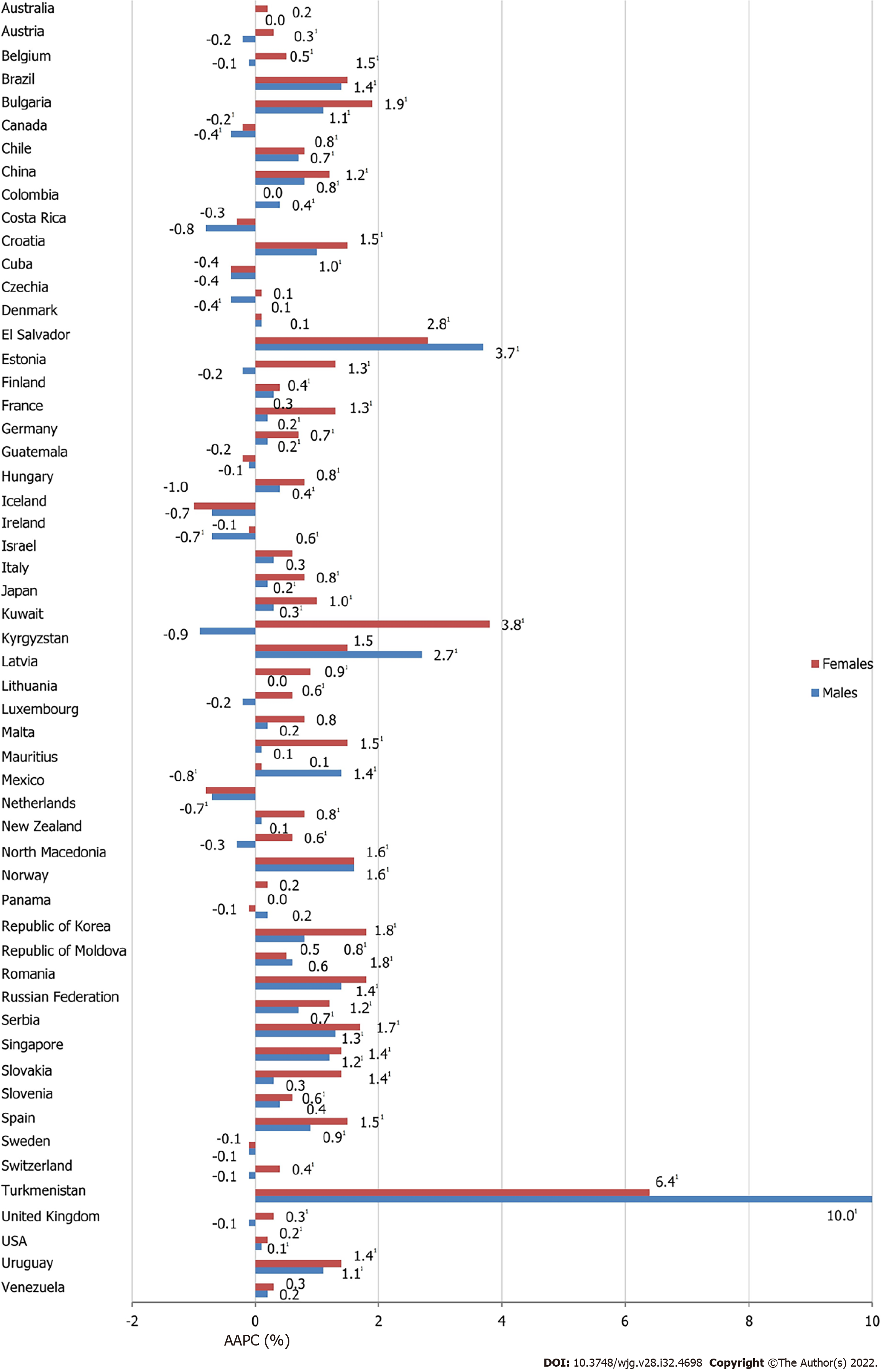
The majority of analyzed countries showed a significant increase in pancreatic cancer mortality in both males and females, but the magnitude of mortality increasing trends for pancreatic cancer was higher in females than in males in almost all countries since 1991 (Figure 6). Out of all countries with an increase in pancreatic cancer mortality rates, Turkmenistan showed the most marked rise both in males (AAPC = +10.0%, 95%CI: 7.4–12.5) and females (AAPC = +6.4%, 95%CI: 3.5–9.5). In some countries, pancreatic cancer mortality trends were significantly increasing in males (Colombia, Kyrgyzstan and Mauritius), in contrast to females. Our results suggested that there were only two countries where significant decreasing pancreatic cancer mortality trends were observed in both sexes: Canada (by 0.4% per year in males and by 0.2% per year in females) and Mexico (by 0.7% in males and 0.8% in females). However, there were only two countries where decreasing pancreatic cancer mortality trends were observed in males only: Czechia (by 0.4% per year) and Ireland (by 0.7%). In some of the most developed countries (such as the USA, UK and Germany) significantly increasing trends in pancreatic cancer mortality rates were observed since the 1990s (Supplementary Figure 2).
The greatest increase in pancreatic cancer incidence was noted in people aged ≥ 70 years in France in both males (by +4.2% per year) and females (by +4.9%) (Table 1). An increase was observed in most of the countries in both sexes for persons aged ≥ 50 years. A decreasing trend in incidence of pancreatic cancer was recorded only in females aged ≥ 70 years in Canada (by 0.3% per year). Also, a decreasing trend in pancreatic cancer incidence in males aged ≥ 70 years was noted only in Canada (by 0.4% per year). In males aged 30–49 years, incidence rates decreased mainly in transitional countries (Czechia by 1.9%, Estonia 2.1%, Latvia 2.2%, Lithuania 2.2% and Slovakia 1.5%). In almost all countries a significantly increasing trend in mortality of pancreatic cancer was recorded both in males and females aged ≥ 70 years. A similar pattern was observed in people aged 50–69 years. The greatest increase in pancreatic cancer mortality was in people aged ≥ 70 years in Turkmenistan in both males (by +12.8%) and females (by +11.4%). Large disparities were observed in changes of rates among the young age group, where mortality rates decreased over the study period for most countries. The favorable change was higher in transitional countries (Czechia by 3.3% in males, 2.7% in females; Latvia by 2.7% in males, 4.7% in females).
| Countries2 | Incidence | Mortality | ||||||||||
| Males | Females | Males | Females | |||||||||
| 30-49 | 50-69 | 70+ | 30-49 | 50-69 | 70+ | 30-49 | 50-69 | 70+ | 30-49 | 50-69 | 70+ | |
| Australia | +1.81 | +0.81 | +0.71 | +1.71 | +1.31 | +0.81 | 0.2 | -0.1 | +0.41 | -0.2 | -0.0 | +0.81 |
| Austria2 | -0.3 | -0.2 | -0.0 | 0.4 | 0.3 | +0.31 | ||||||
| Belarus2 | 0.7 | -0.1 | -0.2 | 0.7 | 0.2 | 0.5 | ||||||
| Belgium2 | -1.21 | -0.2 | 0.2 | 0.6 | +0.61 | +0.61 | ||||||
| Brazil | 2.5 | +4.21 | 1.3 | - | 2.5 | -0.0 | +0.51 | +1.41 | +1.61 | +2.01 | +1.61 | +1.61 |
| Bulgaria | 0.2 | +2.91 | +2.51 | +2.91 | +2.81 | +3.11 | -1.21 | +1.51 | +2.11 | 1.1 | +1.81 | +2.61 |
| Canada | 0.2 | -0.2 | -0.41 | 0.7 | 0.3 | -0.31 | -0.3 | -0.81 | -0.1 | 0.0 | -0.61 | +0.31 |
| Chile | - | 4.0 | -1.3 | - | 0.3 | -0.8 | +2.21 | 0.4 | +1.11 | 0.6 | +0.81 | +1.21 |
| China3 | 0.6 | +0.81 | +1.01 | -0.3 | 0.4 | +1.61 | +2.01 | 0.5 | +1.11 | - | 0.4 | +1.81 |
| Colombia | - | 0.2 | -1.7 | -0.1 | -0.9 | -0.8 | 0.9 | 0.3 | 0.3 | 0.6 | -0.5 | +0.71 |
| Costa Rica | -0.7 | -1.81 | -0.3 | -0.3 | -0.9 | -0.5 | 0.2 | -1.4 | -0.9 | -1.8 | 0.4 | -1.71 |
| Croatia | -1.0 | +0.71 | 0.7 | 0.6 | +1.81 | +0.81 | -1.2 | +0.81 | +1.71 | 0.1 | +1.31 | +2.11 |
| Cuba2 | 1.3 | -0.1 | -0.81 | 0.4 | 0.3 | -0.3 | ||||||
| Czechia | -1.91 | +0.31 | +0.61 | 0.4 | +1.11 | +0.71 | -3.31 | -0.2 | +0.41 | -2.71 | +0.51 | +0.41 |
| Denmark | -0.7 | +1.11 | +1.01 | 0.8 | +0.71 | +0.91 | -1.91 | +0.51 | 0.5 | -1.6 | 0.2 | +0.71 |
| El Salvador2 | +5.81 | +2.91 | +4.71 | -1.1 | +4.21 | +3.01 | ||||||
| Estonia | -2.11 | 0.1 | +1.71 | -1.5 | 0.7 | 1.3 | -3.21 | -1.01 | -1.61 | - | 0.4 | +3.31 |
| Finland | -1.41 | +0.91 | 0.3 | -0.4 | +1.31 | +0.51 | -2.51 | +0.71 | +0.51 | -2.51 | +0.91 | +0.61 |
| France | +1.71 | +2.91 | +4.21 | +2.81 | +3.51 | +4.91 | -0.51 | +0.31 | +0.41 | +1.61 | +1.31 | +1.11 |
| Germany | -1.2 | 1.1 | +1.21 | 0.9 | 1.4 | 0.7 | -0.2 | 0.2 | +0.61 | +0.71 | +0.81 | +0.81 |
| Guatemala2 | -4.11 | 0.4 | 0.7 | -1.6 | 0.3 | -0.5 | ||||||
| Hungary2 | -2.01 | +0.61 | +0.51 | -0.5 | +1.01 | +0.91 | ||||||
| Iceland | - | -0.1 | 0.4 | - | -0.1 | -0.3 | - | -1.4 | 0.8 | -1.2 | -0.1 | |
| India2,3 | 0.1 | 0.9 | 2.4 | 3.7 | +4.01 | +4.51 | ||||||
| Ireland | 0.9 | +1.31 | -0.0 | 1.5 | 0.3 | +0.71 | -1.2 | -1.11 | -0.0 | -2.1 | -0.91 | +0.91 |
| Israel | 1.3 | +1.21 | +1.61 | +4.21 | +1.81 | +1.21 | -1.0 | 0.2 | +1.01 | 0.7 | 0.6 | +1.01 |
| Italy | 1.4 | +0.91 | +1.41 | +4.51 | +1.81 | +2.11 | -0.4 | 0.0 | +0.71 | 0.5 | +0.61 | +1.31 |
| Japan | -1.2 | +0.71 | +0.51 | 0.8 | +1.61 | +1.01 | -1.41 | +1.01 | +0.71 | -1.01 | +1.71 | +1.51 |
| Kuwait | - | 3.1 | 6.6 | -0.0 | -1.6 | - | - | 1.3 | -1.9 | - | 2.7 | 2.1 |
| Kyrgyzstan2 | -0.1 | 0.2 | +5.71 | 3.1 | 0.6 | +3.21 | ||||||
| Latvia | -2.21 | 0.0 | -0.4 | -2.3 | +1.41 | 0.7 | -2.71 | -0.2 | -0.2 | -4.71 | -0.1 | +2.01 |
| Lithuania | -2.21 | -0.1 | -0.1 | 1.5 | 0.1 | +1.31 | -0.7 | -0.51 | 0.2 | 1.0 | 0.0 | +1.3 |
| Luxembourg2 | - | -0.2 | 1.0 | - | 0.5 | 1.2 | ||||||
| Malta | - | 1.2 | 0.2 | - | 3.0 | +2.31 | - | 1.4 | -0.7 | - | +2.01 | +1.81 |
| Mauritius2 | -1.4 | +1.41 | +2.51 | - | 0.4 | 0.4 | ||||||
| Mexico2 | 0.5 | -0.81 | -0.71 | -0.6 | -1.51 | -0.0 | ||||||
| Netherlands | 0.5 | +0.61 | 0.5 | 0.6 | +1.61 | +1.01 | -0.5 | -0.51 | 0.3 | 0.6 | +1.01 | +0.71 |
| New Zealand | 2.0 | -0.3 | 0.4 | 0.8 | 0.1 | 0.9 | 1.0 | -0.9 | 0.3 | 1.2 | -0.5 | +1.21 |
| North Macedonia2 | 0.2 | +1.21 | +2.11 | 1.3 | +1.61 | +1.91 | ||||||
| Norway | -0.8 | 0.4 | 0.2 | 0.2 | +1.31 | 0.3 | -0.4 | -0.1 | 0.3 | -1.8 | 0.2 | +0.51 |
| Panama2 | - | -0.1 | 0.4 | 0.3 | 0.5 | -0.5 | ||||||
| Republic of Korea | - | -0.51 | -0.3 | +2.71 | 0.4 | 0.1 | +4.51 | |||||
| Republic of Moldova2 | -0.1 | 0.3 | 0.6 | -2.2 | -0.3 | +1.71 | ||||||
| Romania2 | -0.8 | +1.41 | +2.21 | -0.1 | +1.51 | +3.01 | ||||||
| Russian Federation2 | -1.61 | 0.0 | +1.31 | -0.2 | -0.0 | +2.11 | ||||||
| Serbia2 | -0.6 | +1.01 | +2.11 | 0.5 | +1.11 | +2.81 | ||||||
| Singapore2 | 0.6 | 0.8 | +2.11 | - | 0.5 | +2.11 | ||||||
| Slovakia | -1.51 | +1.01 | +1.61 | 0.7 | +1.51 | +2.31 | -2.21 | 0.1 | +0.91 | -0.2 | +0.91 | +1.91 |
| Slovenia | 0.6 | +1.21 | +1.41 | 2.8 | +1.91 | +1.81 | -1.5 | +0.71 | +1.11 | 0.8 | 0.4 | +1.11 |
| Spain | 0.2 | +1.31 | 0.3 | 2.0 | +2.41 | +0.91 | -0.3 | +0.71 | +1.21 | +1.51 | +1.41 | +1.51 |
| Sweden | -0.4 | 0.0 | -0.4 | 0.2 | 0.1 | 0.0 | -2.21 | 0.0 | +0.41 | -1.81 | -0.2 | +0.61 |
| Switzerland | 1.2 | 0.9 | -1.0 | 1.1 | 0.6 | +1.91 | -0.7 | 0.2 | -0.2 | 0.7 | 0.1 | +0.81 |
| Turkey2 | 2.3 | +3.41 | +4.51 | 1.6 | +3.61 | +2.31 | ||||||
| Turkmenistan2 | - | +8.11 | +12.81 | 3.2 | +11.41 | |||||||
| United Kingdom | 0.3 | +0.41 | +0.41 | +1.11 | +0.71 | +0.91 | -0.61 | -0.2 | 0.2 | -0.3 | -0.0 | +0.71 |
| United States | 0.3 | +0.61 | +0.51 | +1.61 | +0.71 | +0.41 | -0.0 | -0.0 | +0.41 | +0.71 | -0.1 | +0.51 |
| Uruguay2 | -2.3 | +1.61 | +1.31 | 2.4 | +1.01 | +1.81 | ||||||
| Venezuela2 | -1.81 | 0.8 | 0.3 | -0.1 | 0.5 | 0.4 | ||||||
In 2020, the highest incidence and mortality rates of pancreatic cancer were reported in developed countries. The pattern of rising pancreatic cancer incidence and mortality was seen across countries worldwide in the last three decades, in both developed and transitioned populations. Increasing trend was most pronounced among persons aged ≥ 50 years, similarly among males and females. However, it is promising that pancreatic cancer mortality rates were declining in some countries such as Canada and Mexico.
In both sexes together, the highest incidence and mortality rates of pancreatic cancer in 2020 were reported in the European region (7.6 cases and 7.1 deaths per 100000 persons, respectively) and the Americas region (6.2 cases and 5.4 deaths per 100000 persons, respectively), while the lowest were recorded in South-East Asia (equally 1.3 cases and deaths per 100000 persons). Since due to delayed disease detection and limited effectiveness of therapies the survival for pancreatic cancer remains poor[3,4,19], its mortality is still largely determined by its incidence. Besides, the overall survival rates vary little between developed and developing countries[20]. Since the risk of pancreatic cancer occurrence increases linearly with age and the average age at the time of diagnosis of pancreatic cancer is 70 years[2-6], significant geographic differences in pancreatic cancer incidence and mortality rates could be explained by differences in life expectancy at birth by region. Although improving continuously in all regions in the past decades, life expectancy at birth in 2019 was the highest in countries in Northern America and Europe, and the lowest in South Asia and Africa[21]. Also, the GBD study showed the association of age-standardized incidence and mortality rates for pancreatic cancer with development status at the national level (measured by the Socio-demographic Index, that composes the total fertility rate among women under the age of 25 years, mean education for individuals aged ≥ 15 years, and income per capita) in 2017[6]. Based on the current evidence, international variations in incidence rates of pancreatic cancer are mainly attributed to exposure to environmental factors, particularly tobacco smoking, obesity, diabetes mellitus, alcohol consumption[6,22,23]. Additionally, the migrant effect among Japanese in Hawaii[24] and the discrepancies in pancreatic cancer incidence in Chinese populations[25], which share a similar genetics, but have lived in different regions, indicate that cancer development was mainly determined by environmental factors. Based on the WHO 2015 estimates, the prevalence of tobacco smoking was the highest in countries in the European Region (35% in Greece, equally 33% in the Russian Federation and Serbia), intermediate (~20%) in Western Pacific and Eastern Mediterranean Regions, and lowest (~10%) in Africa and the Americas[26]. Based on the results of the GBD Study, pancreatic cancer deaths worldwide were primarily attributable to smoking (25.9% in males, 16.1% in females), high fasting plasma glucose (9.3% in males, 8.6% in females), and high body mass index (5.0% in males, 7.4% in females) in 2017[6]. In both sexes together in 2017, fraction of pancreatic cancer age-standardized deaths attributable to smoking was the highest in central Europe (28.2% of all pancreatic cancer deaths), while the highest fraction attributable to high fasting plasma glucose was observed in Oceania (16.6%), and the highest fraction attributable to high body mass index was observed in high-income North America (10.2%)[6]. Similarly, the correlation analysis of risk factors for pancreatic cancer for 48 countries showed that higher incidence and mortality rates among men were significantly associated with higher prevalence of smoking, alcohol drinking, physical inactivity, obesity and high cholesterol levels[27]. At the same time, pancreatic cancer rates among women were linked with higher prevalence of smoking, alcohol consumption and high cholesterol. In contrast, the correlation analysis indicated absence of association between the prevalence of diabetes mellitus and the incidence and mortality for pancreatic cancer. A recent meta-analysis involving 23 cohort studies indicated that diabetes mellitus was associated with a 52% excess risk for pancreatic cancer[28]. However, the issue of the validity of a cancer certificate always exists and the impact of variations in data quality on incidence rate cannot be ruled out, especially for pancreatic and other cancers with poorer prognosis and in older age groups[29,30]. Pancreatic cancer incidence rates in the world in 2020 indicate the possibility that there is an underestimation of the incidence rates in many countries, mainly in developing countries[2]. Many countries reported an incidence rate that was equal to the mortality rate of pancreatic cancer in 2020, while in a few countries, the mortality rate was higher than the incidence rate[2]. Besides, in many countries, cancer registries were not populational, but sometimes reported data only from urban/metropolitan area.
Our study showed unfavorable trends in pancreatic cancer incidence rates in both males and females in many countries in the world in the last three decades. The incidence was noticeably increasing throughout European countries, USA, Australia and Japan, while stable trends in incidence were noted in Canada, Sweden and Ireland. One previous study showed that pancreatic cancer incidence rates gradually increased in males and females among all ages in the USA, Canada, the Netherlands, Australia and New Zealand from the mid-1990s until 2014[31]. In the same study, a decreasing trend of pancreatic cancer incidence in males was noticed only in Iceland and Croatia, while in women there was a significant increasing trend in the incidence of pancreatic cancer in most of the countries from the 1990s onwards[31]. The pancreatic cancer incidence in both sexes in China increased continuously from 1990 to 2019[32]. The observed increasing trends in pancreatic cancer incidence could be attributed to population growth and aging, as well as changes in the prevalence of risk factors for cancer[6,33,34]. The GBD study demonstrated that there was a link between increasing trends in incidence for pancreatic cancer and increases in development status at the national level in period 1990–2017[6]. Also, one previous study indicated that increasing trends in pancreatic cancer incidence in both sexes were correlated with socioeconomic development (measured by Human Development Index and Gross Domestic Product per capita)[35]. In the 1980s, a rapid social and economic transition from the lifestyle that characterized socialistic countries (such as countries of the former Eastern Bloc, etc.) to an industrialized westernized lifestyle took place[36-38]. It is hypothesized that, with a lag phase, undergoing different states of social and economic transition has contributed to the sharply increasing trends of pancreatic cancer incidence that have been observed in eastern and southern European countries, China and Brazil since the 2000s. Based the results of the Czech MONICA and post-MONICA studies, cigarette smoking prevalence declined significantly only in males (from 45.0% in 1985 to 23.9% in 2016/17), while there was no change in the prevalence of smoking in females (from 20.9% to 25.9%) over the analyzed period[38]. Throughout the entire 30-year survey period, for both sexes significantly decreased prevalence of dyslipidemia and the consumption of meat and fat was observed, with increase in vegetable oil intake and fresh fruit and vegetables, whereas a significant increase in body mass index was observed in males only[38]. Both the incidence and mortality trends of pancreatic cancer significantly increased in China during the last decades, which could be due to the rise of the prevalence of overweight to 28.1%, diabetes to 11.2%, and smoking to 28.1% of adults: namely, China consumes about 40% of the world’s total cigarettes, and that predominantly among men, with a large increase in consumption over the past few decades[32,39]. A large-scale nationwide cohort study in the Republic of Korea, which included 9 514 171 adults during a median follow-up period of 7.3 years, indicated that diabetes and alcohol consumption were associated with an increased risk of pancreatic cancer[40]. The influence of the improvement of medical imaging on the diagnosis of pancreatic cancer, that is, on the increasing trends of the incidence of pancreatic cancer during the last few decades cannot be ruled out[41].
Pancreatic cancer mortality rates across the countries showed an increasing trend in both sexes. A decreasing trend, however, was found in both sexes only in Canada and Mexico, and a level-off was observed in Denmark, Norway, Sweden, Panama, Moldova and Venezuela. With the exception of some developing countries (Latvia, Kyrgyzstan, Mauritius, Turkmenistan, Nicaragua and Colombia), in all other populations the magnitude of the increase in mortality from pancreatic cancer was higher in women. While the increased trends of pancreatic cancer mortality have long been observed in developed countries (such as the USA, Japan, Germany, France, Italy and Spain)[33], in recent years, a precipitous increase has also been reported in developing countries (such as Kyrgyzstan, Turkmenistan, Bulgaria and Serbia)[42,43]. Some of these changes have been associated with western lifestyle and environmental factors that are linked to an increased risk of pancreatic cancer occurrence (including smoking, diabetes, alcohol consumption, obesity, physical inactivity, and high-energy food intake)[8,22,23,27,35,38,44]. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades mostly reflect different phases of the smoking epidemic across countries, and among males and females[26]. In support of that, our study indicated that mortality rates from pancreatic cancer showed a slower rise among women and a drop among men in China in the last decade (from 1998 to 2012), which could be affected by reduced prevalence of tobacco consumption[44,45]. Also, significantly decreasing trends in pancreatic cancer mortality in both sexes in Mexico (especially since 2008) can partially be attributed to changes in smoking patterns which are resulting from new tobacco tax/laws which were implemented in Mexico in 2007/2008[46]. Unfortunately, due to the silent nature of disease, late diagnosis, and limited treatment options, and the metastatic potential of pancreatic cancer cells, and its poor prognosis, most pancreatic cancers are diagnosed at an advanced stage, which is not amenable to treatment[42,43]. Differences in the international patterns in incidence and mortality trends of pancreatic cancer in the last three decades could be pertinent to impel continuous efforts to identify novel risk factors (particularly the modifiable risk factors) and implement policies for more effective pancreatic cancer control[34]. Global inequalities in standard medical practices (prevention/diagnosis/therapy) and their impact on pancreatic cancer mortality have not yet been systematically explored[47].
In our study, significantly lower favorable pancreatic cancer incidence trends in the young age group (30–49 years) were observed in women compared to men in all countries. Both older age groups (50–69 and ≥ 70 years), for both men and women, generally showed similar trends in the incidence of pancreatic cancer. Other studies have reported similar results[29,30]. Similar to incidence, significantly less favorable trends in mortality of pancreatic cancer in the young age group (30–49 years) were observed in women compared to men in all countries. In both older age groups (50–69 and ≥ 70 years), both men and women generally showed similarly increasing trends in the mortality of pancreatic cancer. It is particularly promising that in males aged 30–49 years, pancreatic cancer mortality trends were falling in most of the countries. Some studies also reported an increasing trend in mortality for pancreatic cancer in the past decade, especially among women and those aged ≥ 50 years in some countries[24,28]. Although in countries where there is a significant trend in increasing incidence of pancreatic cancer, this increase was recorded at the age of ≥ 50 years. Of particular concern is the significant trend in increasing incidence of pancreatic cancer in the youngest group (30–49 years), which is registered in our study mainly in developed countries (UK, USA, Australia, France, Israel, Italy and Bulgaria). Also, although the trend in increasing mortality from pancreatic cancer was recorded at the age of ≥ 50 years, the significant trend in increasing mortality from pancreatic cancer among the youngest group (30–49 years) in women is particularly worrying (mainly in developed countries such as the USA, France, Germany, Spain and Brazil) and in males in Chile, China, Brazil and El Salvador. Although there is no unified definition, some authors defined the onset of pancreatic cancer at an age of ≤ 50 years as early onset[48,49]. Similar to our results, one study found a significantly increasing trend in incidence of pancreatic cancer among persons younger than 40 years only in women in four high-income countries (Canada, UK, France and the Netherlands)[27]. Despite the fact that some studies suggest an association with cigarette smoking, genetic factors, obesity and metabolic disorders, the reasons for the increasing trends in incidence and mortality of early-onset pancreatic cancer are still not well understood[50-52]. Population data on tobacco use from 12 European countries revealed that in both men and women, smoking prevalence was the highest among young adults aged 25–44 years, and decreased with increasing age[50]. Results of two prospective US cohort studies indicated that established risk factors were more strongly associated with earlier-onset pancreatic cancer (i.e., among those aged ≤ 60 years), while association attenuated among older people[51]. The question of whether the frequency of performing diagnostic and therapeutic procedures, as well as autopsies at a younger age has an impact on the trend in incidence and mortality from earlier-onset pancreatic cancer should also be clarified in future research[53].
However, certain comparisons of trends may not be feasible due to the unavailability or incom
Along with either increasing or stable current incidence and mortality trends of pancreatic cancer in many countries, with the aging population and the increase in prevalence of certain risk factors (cigarette smoking, overweight/obesity, diabetes and alcohol consumption), and taking into consideration that screening for pancreatic cancer is currently not recommended, it would be difficult to expect that the UN Sustainable Development Goal of reducing mortality from cancers (hence pancreatic cancer) by one-third by 2030 can be achieved[8]. Additionally, pancreatic cancer survival is still consistently low[20]. Since the COVID-19 pandemic began there has been concern regarding the possible delay in the diagnosis/treatment of pancreatic cancer, as indicated by a recent study in Portugal[54] about the pancreatic cancer having been diagnosed at a later stage during the pandemic, which is a matter that should be elucidated in future research. In summary, the marked international differences in rates and trends in incidence and mortality of pancreatic cancer indicate the need for introducing a more effective public health approach to prevention and improvements in diagnostic and treatment practices worldwide.
This study analyzed both incidence and mortality figures of pancreatic cancer for 185 WHO member states for 2020. Our study analyzed international patterns in trends of incidence and mortality of pancreatic cancer in the last three decades by sex and age groups. One of the major strengths of our study was the use of the GLOBOCAN database and the large population-based datasets, whereby the trends analysis was conducted only for countries with quality of data that was classified as high or medium. Finally, the described international patterns and trends in pancreatic cancer incidence and mortality over time may be useful in generating hypotheses about risk factors for pancreatic cancer.
However, several possible limitations in this study should be considered. First, there is always a question of reliability and validity of cancer certificates. Also, the quality of data varied significantly from country to country. Additionally, comparison between some countries could be difficult because statistical data quality might change and differ over time. An issue of under-reporting of pancreatic cancer, particularly in developing countries, could introduce bias in the assessment of incidence and mortality trends. Availability of data for the incidence/mortality of pancreatic cancer for each country is different, and in some countries limited. Not all of the data are available for all of the years in the analyzed period (since 1991). Therefore, the data of incidence and mortality are taken for different intervals for some countries, which makes it difficult to compare trends across countries. Besides, our trend analysis did not cover countries with statistical data classified as low quality, which therefore did not allow comparisons for a number of countries. In addition, for some countries, data on pancreatic cancer were available for incidence or mortality only, so greater caution in the inference about trends is recommended. Finally, an epidemiological fallacy was an inherent limitation of this study. Possible modes of mitigation for the above-mentioned limitations include greater diagnostic accuracy of pancreatic cancer, better quality of death certification, implementation of mandatory population-based cancer registry, further reduction of the proportion of uncertain causes of death, introduction of registration of autopsies (e.g., part of pancreatic cancer cases diagnosed at autopsy was missing on the death certificates), provision of survival data for pancreatic cancer patients, as well as the improvements and availability of pancreatic cancer treatment across countries[55-57]. Based on accurate assessment of epidemiological characteristics of pancreatic cancer, policy-makers could provide a better planning for funding, access to more effective treatment and care strategies, scientific and clinical advances in development of effective screening tests, improving early diagnosis and improving survival[55]. Despite these limitations, this study can help assess the international patterns in incidence and mortality trends of pancreatic cancer, which still need to be elucidated in analytical epidemiological studies in the future.
Increasing trends in pancreatic cancer incidence and mortality were observed in the last three decades in many countries, in both sexes and most age groups. Further efforts to explore the reasons of differences in international pattern of pancreatic cancer incidence and mortality could be critical for global disease control.
Pancreatic cancer, as the one of most fatal malignancies, remains a critical issue in the global burden of disease.
The main motivation of this study was to describe the international patterns in incidence and mortality trends of pancreatic cancer in the last three decades. Accurate assessment of pancreatic cancer burden is crucial for more effective disease control.
The aim of this study was to estimate trends in incidence and mortality due to pancreatic cancer in the world in the last three decades.
A descriptive epidemiological study was done. Pancreatic cancer incidence and mortality data were obtained from the World Health Organization database. The age-standardized rates (expressed per 100000) were presented. To estimate trends of incidence and mortality of pancreatic cancer, joinpoint regression analysis was used: the average annual percent change (AAPC) with the corresponding 95% confidence interval (95%CI) was calculated.
The general pattern of rising pancreatic cancer incidence and mortality was seen across countries worldwide. Out of all countries with an increase in pancreatic cancer incidence, females in France and India showed the most marked rise in incidence rates (AAPC = +3.9% and AAPC = +3.7%, respectively). Decreasing pancreatic cancer incidence trends have been observed in some countries but without significance. Out of all countries with an increase in pancreatic cancer mortality rates, Turkmenistan showed the most marked rise both in males (AAPC = +10.0%, 95%CI: 7.4%–12.5%) and females (AAPC = +6.4%, 95%CI: 3.5%–9.5%). The mortality trends of pancreatic cancer were decreasing in both sexes only in Canada and Mexico.
The increasing trends in incidence and mortality from pancreatic cancer were observed across the world, particularly in developed countries. The favorable pattern in trends in incidence and mortality of pancreatic cancer was reported in only a few countries.
Further research is needed to explain the cause of large international differences in incidence and mortality trends of pancreatic cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dhamnetiya D, India; Zimmitti G, Italy S-Editor: Gao CC L-Editor: Kerr C P-Editor: Li X
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Ferlay J, Colombet M and Bray F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. [cited 7 Feb 2022]. In: International Agency for Research on Cancer [Internet]. Available from: http://ci5.iarc.fr. |
| 3. | Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2018. [cited 22 Feb 2022]. In: National Cancer Institute [Internet]. Available from: https://seer.cancer.gov/csr/1975_2018/. |
| 4. | Wang H, Liu J, Xia G, Lei S, Huang X. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: a population-based retrospective study. Sci Rep. 2020;10:7048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1749] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 6. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 7. | Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci. 2020;16:820-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | Yu J, Yang X, He W, Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int J Cancer. 2021;149:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | UN General Assembly. Transforming our world: the 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. [cited 7 Feb 2022]. In: UN General Assembly [Internet]. Available from http://www.refworld.org/docid/57b6e3e44.html. |
| 10. | Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 11. | Pergolini I, Demir IE, Stöss C, Emmanuel K, Rosenberg R, Friess H, Novotny A. Effects of COVID-19 Pandemic on the Treatment of Pancreatic Cancer: A Perspective from Central Europe. Dig Surg. 2021;38:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | McKay SC, Pathak S, Wilkin RJW, Kamarajah SK, Wigmore SJ, Rees J, Dunne DFJ, Garcea G, Ahmad J, de Liguori Carino N, Sultana A, Silva M, Lykoudis P, Nasralla D, Milburn J, Shah N, Kocher HM, Bhogal R, Baron RD, Navarro A, Halle-Smith J, Al-Sarireh B, Sen G, Jamieson NB, Briggs C, Stell D, Aroori S, Bowles M, Kanwar A, Harper S, Menon K, Prachalias A, Srinivasan P, Frampton AE, Jones C, Arshad A, Tait I, Spalding D, Young AL, Durkin D, Ghods-Ghorbani M, Sutcliffe RP, Roberts KJ. Impact of SARS-CoV-2 pandemic on pancreatic cancer services and treatment pathways: United Kingdom experience. HPB (Oxford). 2021;23:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Klein AP. Pancreatic cancer: a growing burden. Lancet Gastroenterol Hepatol. 2019;4:895-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. International classification of disease and related health problems: 10th revision. [cited 7 Feb 2022]. In: World Health Organization [Internet]. Available from: https://www.who.int/standards/classifications/classification-of-diseases. |
| 15. | Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171-177. [PubMed] |
| 16. | Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 17. | Lerman PM. Fitting segmented regression models by grid search. Appl Stat. 1980;29:77-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670-3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 766] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 19. | Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 20. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3415] [Article Influence: 487.9] [Reference Citation Analysis (1)] |
| 21. | GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1083] [Article Influence: 216.6] [Reference Citation Analysis (1)] |
| 22. | Lugo A, Peveri G, Bosetti C, Bagnardi V, Crippa A, Orsini N, Rota M, Gallus S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur J Cancer. 2018;104:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 24. | Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14:431-439. [PubMed] |
| 25. | Liu Z, Shi O, Cai N, Jiang Y, Zhang K, Zhu Z, Yuan H, Fang Q, Suo C, Franceschi S, Zhang T, Chen X. Disparities in Cancer Incidence among Chinese Population vs Migrants to Developed Regions: A Population-Based Comparative Study. Cancer Epidemiol Biomarkers Prev. 2019;28:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | World Health Organization. WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. [cited 7 Feb 2022]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/handle/10665/178574. |
| 27. | Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, Ng K, Chong C, Zheng ZJ, Wong MCS. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology. 2021;160:744-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 302] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 28. | Pang Y, Kartsonaki C, Guo Y, Bragg F, Yang L, Bian Z, Chen Y, Iona A, Millwood IY, Lv J, Yu C, Chen J, Li L, Holmes MV, Chen Z. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Møller B, Jerm MB, Larønningen S, Johannesen TB, Seglem AH, Larsen IK, Myklebust TÅ. The validity of cancer information on death certificates in Norway and the impact of death certificate initiated cases on cancer incidence and survival. Cancer Epidemiol. 2021;75:102023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Rahu K, McKee M, Mägi M, Rahu M. The fall and rise of cancer registration in Estonia: The dangers of overzealous application of data protection. Cancer Epidemiol. 2020;66:101708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Luo G, Zhang Y, Guo P, Ji H, Xiao Y, Li K. Global Patterns and Trends in Pancreatic Cancer Incidence: Age, Period, and Birth Cohort Analysis. Pancreas. 2019;48:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Zhu B, Wu X, Guo T, Guan N, Liu Y. Epidemiological Characteristics of Pancreatic Cancer in China From 1990 to 2019. Cancer Control. 2021;28:10732748211051536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Lucas AL, Malvezzi M, Carioli G, Negri E, La Vecchia C, Boffetta P, Bosetti C. Global Trends in Pancreatic Cancer Mortality From 1980 Through 2013 and Predictions for 2017. Clin Gastroenterol Hepatol. 2016;14:1452-1462.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Tsai HJ, Chang JS. Environmental Risk Factors of Pancreatic Cancer. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung JJY. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7:3165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (4)] |
| 36. | Shkolnikov VM, Churilova E, Jdanov DA, Shalnova SA, Nilssen O, Kudryavtsev A, Cook S, Malyutina S, McKee M, Leon DA. Time trends in smoking in Russia in the light of recent tobacco control measures: synthesis of evidence from multiple sources. BMC Public Health. 2020;20:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Psota M, Bandosz P, Gonçalvesová E, Avdičová M, Bucek Pšenková M, Studenčan M, Pekarčíková J, Capewell S, O'Flaherty M. Explaining the decline in coronary heart disease mortality rates in the Slovak Republic between 1993-2008. PLoS One. 2018;13:e0190090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Cífková R, Bruthans J, Wohlfahrt P, Krajčoviechová A, Šulc P, Jozífová M, Eremiášová L, Pudil J, Linhart A, Widimský J Jr, Filipovský J, Mayer O Jr, Škodová Z, Poledne R, Stávek P, Lánská V. 30-year trends in major cardiovascular risk factors in the Czech population, Czech MONICA and Czech post-MONICA, 1985 - 2016/17. PLoS One. 2020;15:e0232845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Liu S, Zhang M, Yang L, Li Y, Wang L, Huang Z, Chen Z, Zhou M. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. 2017;71:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 40. | Park JH, Han K, Hong JY, Park YS, Park JO. Association between alcohol consumption and pancreatic cancer risk differs by glycaemic status: A nationwide cohort study. Eur J Cancer. 2022;163:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Defossez G, Uhry Z, Delafosse P, Dantony E, d'Almeida T, Plouvier S, Bossard N, Bouvier AM, Molinié F, Woronoff AS, Colonna M, Grosclaude P, Remontet L, Monnereau A; French Network of Cancer Registries (FRANCIM). Cancer incidence and mortality trends in France over 1990-2018 for solid tumors: the sex gap is narrowing. BMC Cancer. 2021;21:726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 43. | Seoane-Mato D, Nuñez O, Fernández-de-Larrea N, Pérez-Gómez B, Pollán M, López-Abente G, Aragonés N. Long-term trends in pancreatic cancer mortality in Spain (1952-2012). BMC Cancer. 2018;18:625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5702] [Cited by in RCA: 5050] [Article Influence: 1010.0] [Reference Citation Analysis (1)] |
| 45. | Zhang K, Tartarone A, Pérez-Ríos M, Novello S, Mariniello A, Roviello G, Zhang J. Smoking burden, MPOWER, future tobacco control and real-world challenges in China: reflections on the WHO report on the global tobacco epidemic 2021. Transl Lung Cancer Res. 2022;11:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Hernández-Garduño E. The impact of tobacco tax/law implementation on pancreatic cancer mortality in Mexico, 1999-2015. Ecancermedicalscience. 2018;12:ed85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Noel M, Fiscella K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity. 2019;3:532-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 48. | Raimondi S, Maisonneuve P, Löhr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1894-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Piciucchi M, Capurso G, Valente R, Larghi A, Archibugi L, Signoretti M, Stigliano S, Zerboni G, Barucca V, La Torre M, Cavallini M, Costamagna G, Marchetti P, Ziparo V, Delle Fave G. Early onset pancreatic cancer: risk factors, presentation and outcome. Pancreatology. 2015;15:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 50. | Gallus S, Lugo A, Liu X, Behrakis P, Boffi R, Bosetti C, Carreras G, Chatenoud L, Clancy L, Continente X, Dobson R, Effertz T, Filippidis FT, Fu M, Geshanova G, Gorini G, Keogan S, Ivanov H, Lopez MJ, Lopez-Nicolas A, Precioso J, Przewozniak K, Radu-Loghin C, Ruprecht A, Semple S, Soriano JB, Starchenko P, Trapero-Bertran M, Tigova O, Tzortzi AS, Vardavas C, Vyzikidou VK, Colombo P, Fernandez E; TackSHS Project Investigators. Who Smokes in Europe? J Epidemiol. 2021;31:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Yuan C, Kim J, Wang QL, Lee AA, Babic A; PanScan/PanC4 I-III Consortium, Amundadottir LT, Klein AP, Li D, McCullough ML, Petersen GM, Risch HA, Stolzenberg-Solomon RZ, Perez K, Ng K, Giovannucci EL, Stampfer MJ, Kraft P, Wolpin BM. The age-dependent association of risk factors with pancreatic cancer. Ann Oncol. 2022;33:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 52. | McWilliams RR, Maisonneuve P, Bamlet WR, Petersen GM, Li D, Risch HA, Yu H, Fontham ET, Luckett B, Bosetti C, Negri E, La Vecchia C, Talamini R, Bueno de Mesquita HB, Bracci P, Gallinger S, Neale RE, Lowenfels AB. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas. 2016;45:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 53. | LaPelusa M, Shen C, Arhin ND, Cardin D, Tan M, Idrees K, Geevarghese S, Chakravarthy B, Berlin J, Eng C. Trends in the Incidence and Treatment of Early-Onset Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 54. | Brito M, Laranjo A, Sabino J, Oliveira C, Mocanu I, Fonseca J. Digestive Oncology in the COVID-19 Pandemic Era. GE Port J Gastroenterol. 2021;579:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, Sørensen HT. Nordic Health Registry-Based Research: A Review of Health Care Systems and Key Registries. Clin Epidemiol. 2021;13:533-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 56. | Gupta N, Yelamanchi R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol. 2021;27:3158-3181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 57. | Fest J, Ruiter R, van Rooij FJ, van der Geest LG, Lemmens VE, Ikram MA, Coebergh JW, Stricker BH, van Eijck CH. Underestimation of pancreatic cancer in the national cancer registry - Reconsidering the incidence and survival rates. Eur J Cancer. 2017;72:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |