Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4668
Peer-review started: March 13, 2022
First decision: June 11, 2022
Revised: June 23, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 28, 2022
Processing time: 165 Days and 19.4 Hours
Dendrobium officinale is an herb of Traditional Chinese Medicine (TCM) commonly used for treating stomach diseases. One formula of Granule Dendrobii (GD) consists of Dendrobium officinale and American Ginseng (Radix Panacis quinquefolii), and is a potent TCM product in China. Whether treatment with GD can promote gastric acid secretion and alleviate gastric gland atrophy in chronic atrophic gastritis (CAG) requires verification.
To determine the effect of GD treatment on CAG and its potential cellular mechanism.
A CAG model was induced by feeding rats N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) for 12 wk. After oral administration of low, moderate, and high doses of GD in CAG rats for 8 wk, its effects on body weight, gastric mucosa histology, mucosal atrophy, intestinal metaplasia, immunohistochemical staining of proliferating cell nuclear antigen (PCNA) and B-cell lymphoma-2, and hemoglobin and red blood cells were examined.
The body weights of MNNG-induced CAG model rats before treatment (143.5 ± 14.26 g) were significantly lower than that of healthy rats (220.2 ± 31.20 g, P < 0.01). At the 8th week of treatment, the body weights of rats in the low-, moderate-, and high-dose groups of GD (220.1 ± 36.62 g) were significantly higher than those in the untreated group (173.3 ± 28.09 g, all P < 0.01). The level of inflammation in gastric tissue of the high-dose group (1.68 ± 0.54) was significantly reduced (P < 0.01) compared with that of the untreated group (3.00 ± 0.00, P < 0.05). The number and thickness of gastric glands in the high-dose group (31.50 ± 6.07/mm, 306.4 ± 49.32 µm) were significantly higher than those in the untreated group (26.86 ± 6.41/mm, 244.3 ± 51.82 µm, respectively, P < 0.01 and P < 0.05), indicating improved atrophy of gastric mucosa. The areas of intestinal metaplasia were significantly lower in the high-dose group (1.74% ± 1.13%), medium-dose group (1.81% ± 0.66%) and low-dose group (2.36% ± 1.08%) than in the untreated group (3.91% ± 0.96%, all P < 0.01). The expression of PCNA in high-dose group was significantly reduced compared with that in untreated group (P < 0.01). Hemoglobin level in the high-dose group (145.3 ± 5.90 g/L), medium-dose group (139.3 ± 5.71 g/L) and low-dose group (137.5 ± 7.56 g/L) was markedly increased compared with the untreated group (132.1 ± 7.76 g/L; P < 0.01 or P < 0.05).
Treatment with GD for 8 wk demonstrate that GD is effective in the treatment of CAG in the MNNG model by improving the histopathology of gastric mucosa, reversing gastric atrophy and intestinal metaplasia, and alleviating gastric inflammation.
Core Tip: Symptomatic treatment is mainly adopted for chronic atrophic gastritis (CAG) in modern medicine, and no drugs are available to promote gastric acid secretion and alleviate gastric gland atrophy. Our study shows that treatment with Granule Dendrobii (GD) for 8 wk was effective in reducing weight loss, gastric mucosal inflammation, atrophy and intestinal metaplasia, and loss of hemoglobin and erythrocytes in a rat model of CAG induced by N-methyl-N'-nitro-N-nitrosoguanidine. GD also alleviated the overexpression of PCNA and B-cell lymphoma-2 in CAG rats. These results provide new evidence on the use of GD not only for improving clinical symptoms but also for normalizing the abnormal histological changes of CAG.
- Citation: Wu Y, Li Y, Jin XM, Dai GH, Chen X, Tong YL, Ren ZM, Chen Y, Xue XM, Wu RZ. Effects of Granule Dendrobii on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats. World J Gastroenterol 2022; 28(32): 4668-4680
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4668
Chronic atrophic gastritis (CAG), characterized by thinning mucosal layer and loss or atrophy of gastric mucosal glands, is considered a premalignant change of gastric cancer (GC)[1]. It has been reported that the prevalence of CAG and intestinal metaplasia (IM) is 16% and 13%, respectively, and increases to 27% in countries with a high GC incidence[2]. The incidence of GC in patients with CAG or IM is 0.004%-0.3% per person each year, indicating that these patients have a higher risk of GC[3]. At present, the treatment of CAG in modern medicine mainly focuses on symptomatic treatment, and no drugs are available to promote gastric acid secretion and alleviate gastric gland atrophy. Therefore, it is essential to look for alternative therapies to reduce symptoms in CAG patients and improve their quality of life[4]. Traditional Chinese medicine (TCM) is often used for the treatment of CAG and has shown efficacy. However, there is a lack of scientific research on the effect of TCM in improving gastric mucosa atrophy and IM.
Dendrobium officinale is an herb of TCM commonly used for treating stomach diseases. The TCM literature states that it has the following functions: ”thickening the stomach and intestines” (improving stomach function) and ”smoothing stomach qi” (enhancing gastric motility and accelerating gastric emptying). One formula of Granule Dendrobii (GD) consisting of Dendrobium officinale and American Ginseng (Radix Panacis quinquefolii) is a potent TCM product in China. An earlier clinical study found that GD treatment of CAG reversed gastric mucosa atrophy and improved gastric mucosa and IM[5]. To provide scientific evidence for the clinical application of this product, we studied the effect of GD on gastric mucosa atrophy and IM reversal, as well as changes in proliferating cell nuclear antigen (PCNA) and B-cell lymphoma-2 (Bcl-2) in a CAG model induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) in rats.
Herbal product under investigation: Granule Dendrobii (GD, Li Zuan Brand, Zhejiang Tian Huang Pharmaceutical Co., Ltd, China) 3 g per bag, contained 0.5g of crude Dendrobium Officinale and 0.4 g of American Ginseng (Radix Panacis Quinquefolii). Based on the theory of TCM, this formula has the functions of enhancing energy metabolism of the stomach and restoring its nutrient supply to improve the production of body fluid, which makes it suitable for treating the type of CAG that has lower levels of energy production and nutrient supply. The dosage for clinical treatment was 18 g/d (equivalent to 5.4 g herbs/d).
Herbal product as control: Yangweishu Granule (YWS, prodeuced by Shenlu Shuanghe Pharmaceutical Co., Ltd, Hefei, China) 10 g per bag contained dangshen (Radix Codonopsis), chenpi (Pericarpium Citri Reticulatae), huangjing (Polygonatum sibiricum), shanyao (Rhizoma Dioscoreae), xuanshen (Radix Scrophulariae), wumei (Fructus Mume), shanzha (Fructus Crataegi), beishashen (Glehnia littoralis), ganjiang (Rhizoma Zingiberis), tusizi (Cuscuta Chinensis), baizhu (Rhizoma Atractylodis Macrocephalae), dextrin and saccharose. The dosage for clinical treatment was 20 g/d.
All experiments were performed on Wistar rats (50% male) obtained from the Shanghai Slack Laboratory Animal Co., Ltd. All the animals were kept in a temperature (25 ± 2 ℃)- and humidity-controlled animal facility on a 12-h light/dark cycle, with food and water supplied ad libitum. The protocol was designed to minimize pain or discomfort to the animals. All procedures were approved by the Animal Care and Use Committee of the Institutional Guide for the Care and Use of Laboratory Animals at Zhejiang Institute of Traditional Chinese Medicine.
MNNG (Tokyo Chemical Industry, Japan) was added to the drinking water for six-week-old Wistar rats (50% male). The MNNG stock solution was made every week by mixing MNNG with distilled water to a concentration of 1 g/L and kept in a refrigerator in the dark. For daily use, the stock solution was diluted to 167 µg/mL with distilled water and stored in 200 mL opaque water bottles for the rats to drink freely. The bottles were changed daily. As earlier studies established the development of CAG in 2 to 3 mo, with pathological changes from initial erosive and inflammation of the gastric mucosa in several weeks to gradual atrophy and dysplasia, the rats were provided with MNNG for a period of 12 wk for model generation.
To confirm the development of CAG in this model, 4 MNNG model animals and 4 normal animals were sacrificed for gastric histopathological examination at the end of model generation. The rats were then randomly divided into the following five groups. Three treatment groups were fed with 0.6 g, 1.2 g, and 2.4 g/kg body weight/day of GD as the low (12 rats), medium (12 rats) and high (14 rats) dosage groups, respectively. The fourth group was fed with physiological saline at the same volume as the untreated model group (14 rats), and the last group was fed with YWS (12 rats) granule 4.0 g/kg/d as a positive control group. In addition, 14 normal rats were given physiological saline at the same volume as the normal control group. Two rats were reserved for the high-dose group, the normal control group and the untreated model group in order to sacrifice the animals for pathological pre-examination to determine the termination point of the course when the treatment was almost finished. Each group was given its corresponding treatment daily for 8 wk.
The day after the last administration, all the rats were anesthetized and blood was taken from the abdominal aorta. The stomach was quickly removed and fixed in formalin. The longitudinal length and full layer of the anterior stomach to the duodenum was collected. A strip of tissue from the lateral wall was taken as the standard pathological sample. After paraffin embedding, the tissue was sliced into 4 µm sections and stained with Hematoxylin-Eosin (HE)[6], Alcian Blue (AB)[7], and immunohistochemistry against PCNA (antibody against gastric epithelial cell proliferating cell nuclear antigen, NeoMarkers, United States) and Bcl-2 (anti-Bcl-2 antibody, Millipore, United States).
PCNA and Bcl-2 staining: After dewaxing, the sections were incubated with 3% H2O2 deionized water for 10 min, followed by incubation with primary antibodies at room temperature or 37 ℃ for 1 to 2 h. After rinsing in PBS, secondary antibodies were added and incubated at room temperature or 37 ℃ for 20 min. The sections were rinsed in PBS, and processed for color rendering dehydration, transparency and sealing.
Gastric mucosal inflammation: The effect of treatment on gastric mucosal inflammation induced by MNNG in CAG rats was evaluated using a semi-quantitative method. Specifically, after the entire gastric mucosa was observed under the microscope low power view, 10 fields of view in the gastric antrum were selected to determine inflammation. According to the diagnostic criteria for gastritis proposed by Houston in 1994[8,9], the degree of inflammatory cell infiltration is divided into seven grades from 0 to 3. Grade 0: no inflammation; Grade 0.5: inflammation between 0 to 1 observed under a microscope; Grade 1: multiple chronic inflammatory cell infiltrations seen in the pit of the stomach or the bottom of the inherent gland; Grade 1.5: inflammation between Grade 1 to 2 observed under a microscope; Grade 2: more inflammatory cells in the gastric mucosa from the fovea to the myometrium; Grade 2.5: inflammation between Grade 2 to 3 observed under a microscope; Grade 3: numerous inflammatory cells seen in the gastric mucosa.
Changes in gland thickness and gland number on gastric mucosa: Five fields of gastric antrum mucosa were obtained from each section, and the gland thickness in each field was measured with a micrometer. The average value of the five fields in each section was determined (μm). At 100X power, the total number of proper glands in the gastric antrum between 0.2-1.2 mm of the pyloric ring in rats were counted and expressed as "units/mm".
The number of gastric primary cells and parietal cells: At 400× power, the mean numbers of gastric primary cells and parietal cells from 5 fields in the gastric body mucosa starting from 1.0 mm below the border between the anterior stomach and the gastric body were counted from each section.
Intestinal metaplasia: Intestinal metaplasia was evaluated by analyzing images from AB stained sections. Five photos of gastric mucosa in the gastric antrum were randomly obtained from each section using a Nikon ECLIPSE Ti microfilming system at a magnification of 400×. Each group was imaged under the same conditions. A Leica QWin color<RG> image analysis system was used for analysis. Under the same conditions, the proportion (%) of the total positively stained area over the total analyzed area of each photo was calculated. An average proportion was calculated from 5 photos in each rat and used for statistical comparisons among all the experimental groups.
As pathological changes in CAG are correlated with proliferation and apoptosis of the gastric mucosa, we examined the expression of PCNA and Bcl-2 proteins in gastric mucosa. PCNA is an intraconuclear protein that is expressed in a small amount during the quiescence phase of DNA synthesis. It is an important indicator of cell proliferation because its changes are consistent with intracellular DNA replication. Bcl-2 is a gene that inhibits apoptosis. Its overexpression can inhibit cell apoptosis and is indicative of a favorable precondition for the transformation of malignant cells. Positive staining of PCNA was located in the nucleus, while positive staining of Bcl-2 was located in the cytoplasm and was occasionally accompanied by nuclear staining. Based on previous studies[10-14], we used a semi-quantitative grading method to quantify positive cells. Proportions of positive cells were calculated by counting the numbers of positive cells and total cells in five fields randomly observed under a high-power view (400 ×). A score was assigned according to the percentage of positive cells: 0 for 5%, 1 point for 6%-25%, 2 points for 26%-50%, and 3 points for > 50%. Then the intensity of staining of positive cells was scored: 0 for non-staining, 1 point for light yellow, 2 points for brownish yellow, and 3 points for brown. Finally, these points were added together. 0 was negative “-”, 1-2 points were weakly positive “+”, 3-4 points were moderately positive “++”, and 5-6 points were strongly positive “+++”.
As reduced iron absorption and utilization due to gastric mucosal atrophy in CAG often lead to decreases in hemoglobin and erythrocyte count in rats, we examined whether treatment with GD would also improve these parameters in CAG rats. After the rats were anesthetized with pentobarbital, blood was collected from the abdominal aorta. Whole blood was placed in a disposable vacuum blood collection anticoagulant tube and routine blood tests were carried out using an XT-1800iv five-classification hemocyte analyzer (Hisen Meikang Co., Japan). Reports of the analysis were automatically generated.
Statistical analyses were performed using SPSS software 17.0 (SPSS Inc., Chicago, United States). Quantitative data were expressed as the mean ± SD. Differences among multiple groups were compared by one-way ANOVA, and pairwise comparisons between groups were followed by the least significant difference if the variances were homogeneous, and the Dunnett T3 method was used otherwise. Numerical data were analyzed with the χ2 test.
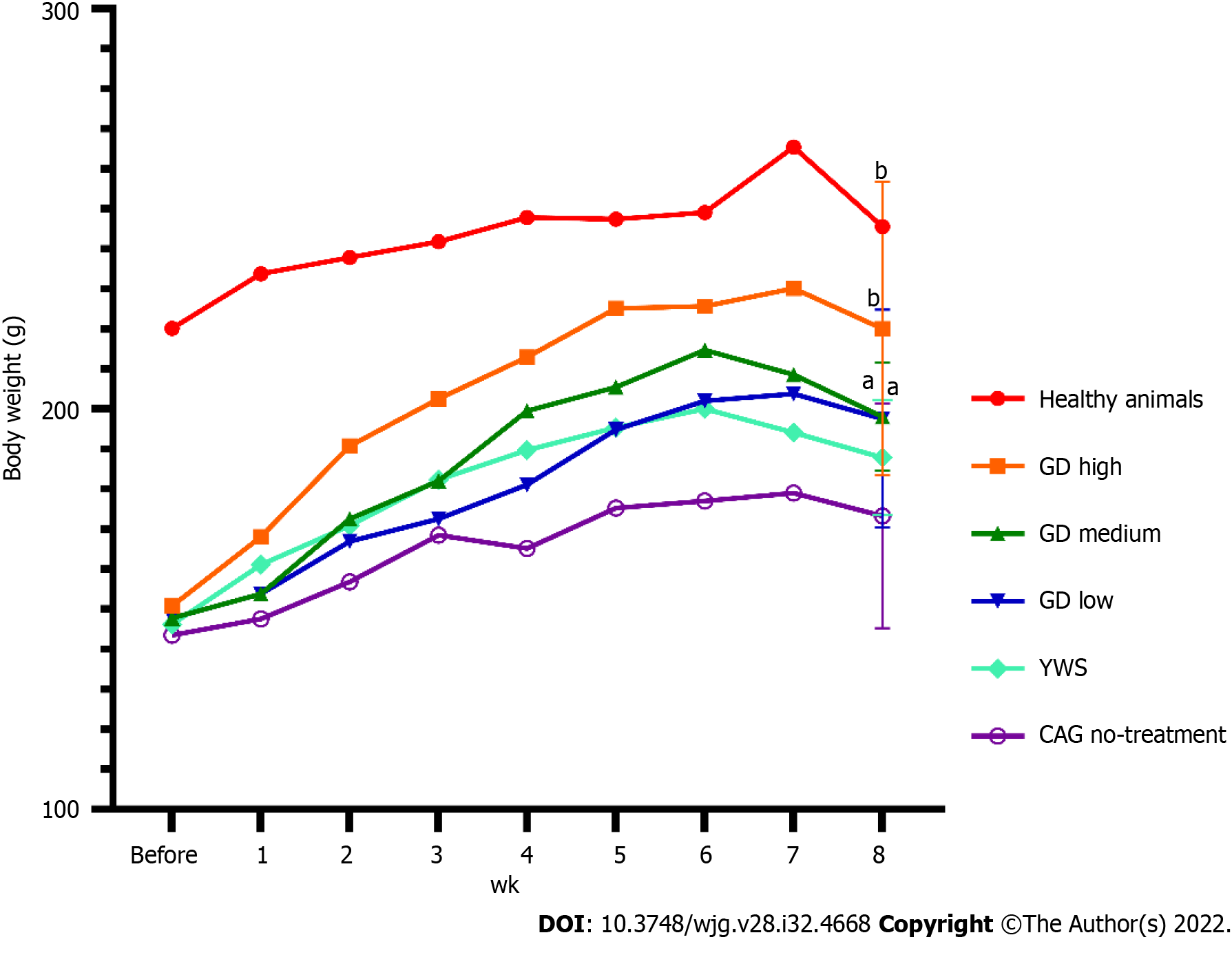
The mean body weight of rats with MNNG-induced CAG before treatment was 143.5 ± 14.3 g, which was significantly lower than that of the normal rats (Figure 1, 220.2 ± 31.2 g, P < 0.01). On completion of model generation, there were no significant differences in body weight among all the treatment groups (Figure 1, P > 0.05).
At the end of treatment, the body weights of all the treatment groups (except YWS) had significantly recover when compared with those of the untreated group (Figure 1, P < 0.05). At the 8th wk of treatment, the mean body weights of groups treated with high (220.1 ± 36.6 g, P < 0.01), medium (198.1 ± 13.5 g, P < 0.05), and low doses (197.6 ± 27.3 g, P < 0.05) of GD were significantly higher than that of the untreated group (173.3 ± 28.1 g). The results at 8 wk after treatment indicated that GD treatment had the effect of reducing weight loss in the MNNG model of CAG.
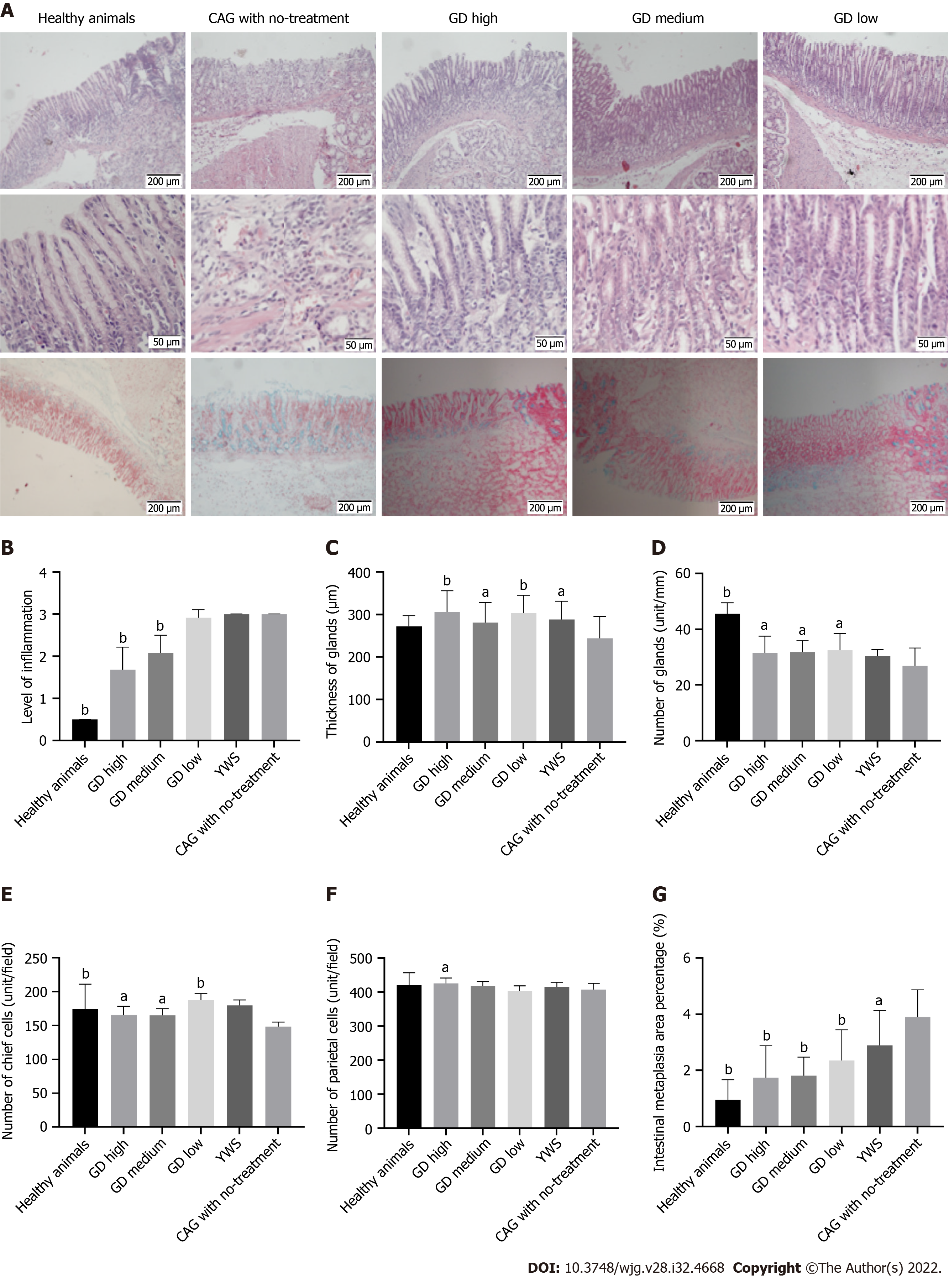
In normal rats, the gastric mucosal glands were relatively thick and numerous, and the epithelial cells were neatly arranged without defect or exfoliation. The glands were regular in shape and basically the same in size. AB staining showed a small amount of blue staining in the deep mucosa without IM. In the CAG group, the gastric mucosa was atrophic and thinned with reduced glands. There was edema and surface exfoliation. The infiltration of many acute and chronic inflammatory cells was visible in the interstitium. Hyperemia and thickening of the mucosal myometrium were observed. Atypical hyperplasia was seen, with cells of irregular size and shape and a disordered arrangement. AB staining revealed that the entire mucosa was stained blue in the presence of IM. In the CAG rats treated with high-dose GD, the pathological changes were reduced and atrophy was improved. The epithelial cells were well arranged without defect or exfoliation. The shapes of the glands were regular with similar size and shape. AB staining showed a very light blue color in the deep part of the gastric antrum mucosa, suggesting almost no IM (Figure 2A). In the CAG rats treated with medium-dose GD, the pathological changes were similar to those in the high-dose group with significant improvement. Such changes could be classified as mild to moderate superficial gastritis. AB staining showed a normal staining pattern with no IM, and was similar to that in the high-dose group (Figure 2A). In the CAG rats treated with low-dose GD, the pathological changes as shown by HE staining were slightly alleviated with improvements in atrophy compared with the untreated group, but inflammatory cell infiltration was observed in the mucosal layer. These changes were classified as mild to moderate superficial gastritis. On AB staining, there was a small amount of blue staining in the deep mucosa with IM (Figure 2A).
After 8 wk of GD treatment in CAG rats, the levels of gastric mucosal inflammation in the high-dose (1.68 ± 0.54) and medium-dose (2.08 ± 0.42) groups were significantly lower than those in the low-dose, no-treatment, and YWS groups (Figure 2B, P < 0.01 for all comparisons).
At 8 wk after CAG generation, significant decreases in the thickness and number of glands in the gastric mucosa were observed. The number (31.50 ± 6.07 unit/mm) and thickness (306.4 ± 49.32 μm) of glands in gastric tissue of the high-dose group were significantly higher than those of the no-treatment group (26.86 ± 6.41 unit/mm, 244.3 ± 51.82 μm, respectively), indicating a significant reduction in the atrophy of gastric mucosa in the treatment group (Figure 2C and D, P < 0.01 or P < 0.05). The glandular thickness of gastric tissue in the medium- and low-dose groups was also significantly improved compared with that in the no-treatment group (Figure 2C, P < 0.01 or P < 0.05).
At 8 wk after model preparation, there was a significant decrease in the number of chief cells in the CAG group when compared with the healthy animal group (P < 0.05). After 8 wk of treatment, the numbers of chief cells (165.5 ± 12.65 unit/field) and parietal cells (425.4 ± 16.0 unit/field) in gastric mucosa in the high-dose group were significantly higher than those in no-treatment group (chief cells: 148.7 ± 6.38 unit/field; parietal cells: 407.2 ± 18.6 unit/field), indicating a protective effect of GD treatment on chief cells and parietal cells in gastric mucosa. The number of chief cells in gastric mucosa of the medium- and low-dose groups was also significantly higher than the no-treatment group (Figure 2E, P < 0.01 and P < 0.05, respectively); but the number of parietal cells in gastric mucosa of the medium- and low-dose groups was not significantly higher than that in the no-treatment group (Figure 2F, P > 0.05).
At 8 wk after model preparation, quantitative analysis of gastric mucosa in the CAG rats showed that the percentage of IM increased significantly (0.95 ± 0.72% and 3.91 ± 0.96% in the healthy animal and CAG groups, respectively, P < 0.05). After treatment with GD for 8 wk, the areas of IM in the high-dose (1.74 ± 1.13%), medium-dose (1.81 ± 0.66%), and low-dose (2.36 ± 1.08%) groups were significantly lower than that in the no-treatment group (P < 0.01 for all), suggesting a significant improvement in IM after treatment with GD (Figure 2G).
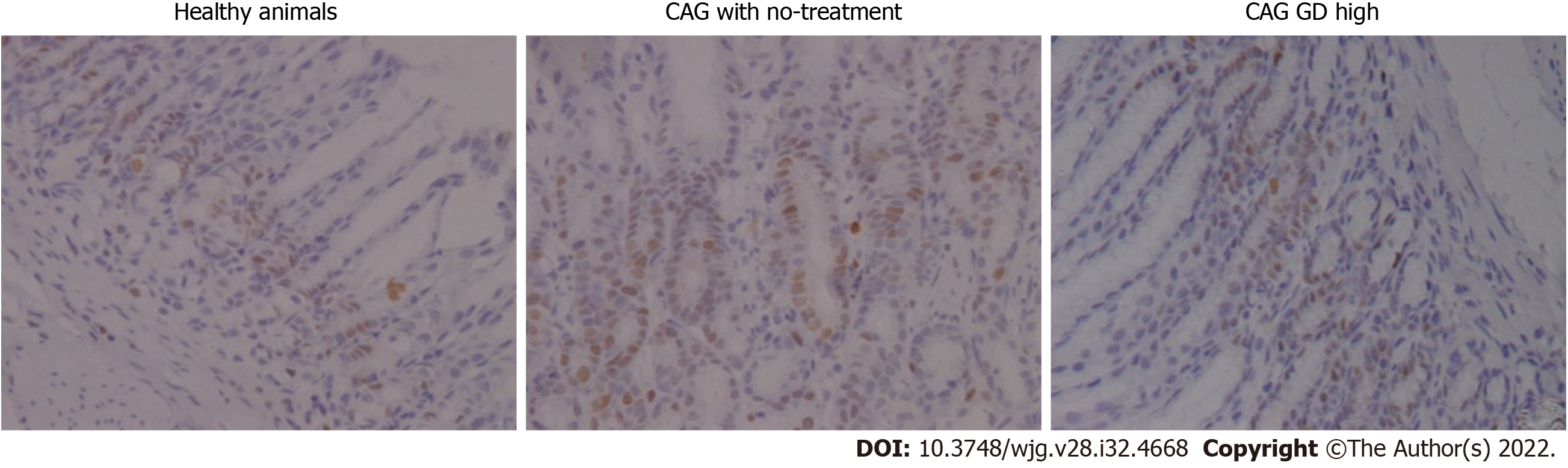
In healthy rats, the number of PCNA expressing cells in gastric mucosa was low and mainly located in the proliferation zone, with regular distribution and weak staining. In the CAG rats without treatment, the number of PCNA expressing cells in gastric mucosa was increased and distributed in nearly all layers with deeper staining. In the high dose group, the expression and distribution of PCNA were similar to that of the healthy animal group, with less PCNA expressing cells mainly located in the proliferation zone than the CAG with no-treatment group (Figure 3).
Table 1 shows a summary of the expression of PCNA and Bcl-2 in gastric mucosa. PCNA expression in the CAG group was significantly higher than that in the healthy animal group (P < 0.01), while its expressions in the high-dose and low-dose groups was significantly lower than that in the CAG group (P < 0.01). Similarly, the expression of Bcl-2 was significantly higher in the CAG group than in the healthy animals group (P < 0.01). The expression of Bcl-2 in the high-dose, medium-dose, and low-dose groups was lower than that of the no-treatment group, but without statistical significance (P > 0.05).
| Grouping | PCNA | Bcl-2 | ||||||||||
| +++ | ++ | + | - | n | P value | +++ | ++ | + | - | n | P value | |
| GD high | 1 | 3 | 10 | 0 | 14 | < 0.01 | 3 | 4 | 7 | 0 | 14 | > 0.05 |
| GD medium | 5 | 4 | 3 | 0 | 12 | > 0.05 | 2 | 8 | 2 | 0 | 12 | > 0.05 |
| GD low | 3 | 2 | 7 | 0 | 12 | < 0.01 | 1 | 6 | 5 | 0 | 12 | > 0.05 |
| YWS | 4 | 3 | 5 | 0 | 12 | < 0.05 | 2 | 7 | 3 | 0 | 12 | > 0.05 |
| Healthy animals | 0 | 0 | 14 | 0 | 14 | < 0.01 | 0 | 3 | 11 | 0 | 14 | < 0.01 |
| CAG no-treatment | 9 | 5 | 0 | 0 | 14 | - | 5 | 7 | 2 | 0 | 14 | - |
| 6 groups of χ2-test | χ2 value | 38.96 | 20.97 | |||||||||
| P value | P < 0.01 | P < 0.05 | ||||||||||
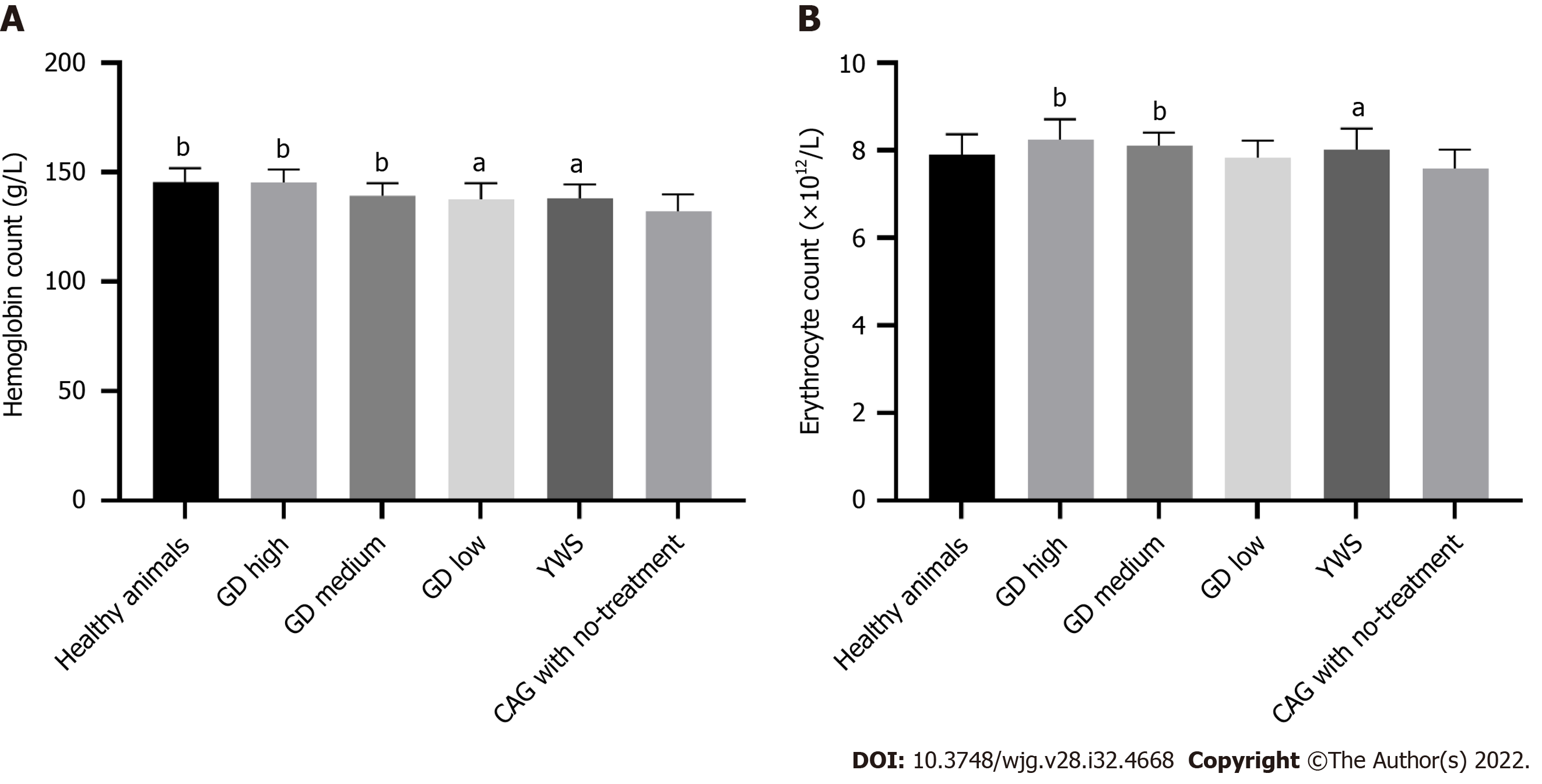
As shown in Figure 4A, hemoglobin in the CAG group (132.1 ± 7.76) was significantly reduced compared with that in the healthy animal group (145.6 ± 6.26, P < 0.01). In the high-dose (145.3 ± 5.90), medium-dose (139.3 ± 5.71) and low-dose groups (137.5 ± 7.56) of GD after 8 wk of treatment, hemoglobin was significantly higher than that in the no-treatment group (P < 0.01 or P < 0.05). In addition, there was a dose-dependent relationship between the increase in hemoglobin and the dose of GD used. The increase in hemoglobin level in the high-dose group was significantly greater than that in the medium and low-dose group (Figure 4A, P < 0.05). Erythrocyte count in the untreated group was lower than that in the healthy animal group, but the difference was not statistically significant (Figure 4B, P > 0.05). After 8 wk of treatment, the high-dose group and the medium-dose group had significant increases in erythrocyte counts compared with the no-treatment group (P < 0.01). In addition, there was a dose-dependent relationship between the increase in erythrocyte count and dose of GD used. The increase in erythrocyte count in the high dose group was significantly greater than that in the low dose group (Figure 4B, P < 0.05). These results suggest that treatment with GD for 8 wk increased hemoglobin and erythrocytes in CAG rats.
Treatment with GD for 8 wk resulted in significant improvements in body weight, gastric mucosal inflammation, atrophy, and intestinal metaplasia in rats with MNNG-induced CAG. GD also increased the reduced levels of hemoglobin and erythrocytes in CAG rats. Furthermore, GD improved gastric mucosal lesions and improved the secondary pathological changes of CAG including reduced hemoglobin and red blood cell count, and overall body condition. It was observed that GD alleviated the overexpression of PCNA and Bcl-2 in model rats, which maybe one of the therapeutic mechanisms related to the reduction of gastric mucosal inflammation and mucosal damage.
Rodent models of CAG can be induced by chemical stimulation (ethanol, ammonia water, sodium deoxycholate, etc.), autoimmunity against gastric mucosa homogenization, Helicobacter pylori and MNNG. Among these, the carcinogen (MNNG) induction method is widely used to generating a CAG model[15-17], with lesion severity being adjustable according to the length of induction time. Specifically, mild atrophy of gastric mucosa generally develops within 25 wk in rats, the CAG model with IM established within 35 wk[18], while early GC develops in 7-9 mo. As the CAG rat model induced by MNNG is stable, relatively severe, and resistant to natural recovery, it is credible for testing the efficacy of therapeutic treatment.
Dendrobium officinale extraction has been shown to effectively reduce the incidence of GC in rats and may be valuable in the prevention of GC[19]. Dendrobium officinale polysaccharide, the main active ingredient of Dendrobium officinale, can effectively inhibit the progression of premalignant gastric lesions induced by MNNG[20]. The formula of GD is mild. The combination of Dendrobium candidum and American ginseng has the functions of tonifying Qi, and Yin, and nourishing the stomach to promote the production of body fluid, making it suitable for the treatment and recovery of digestive diseases. Such functions are also in line with records in relevant traditional literature. Treating CAG with TCM is relatively safe and feasible during long-term use, while the effect is holistic and comprehensive, and research in this direction has significant prospects[21].
In recent years, treatment with traditional Chinese herbal medicine has been reported to improve CAG gastric mucosal atrophy and IM. In Helicobactor pylori positive CAG, a TCM herbal formula was shown to improve clinical symptoms and efficacy rate, and reverse atrophy in gastric mucosa in CAG patients[22]. Fuzi Lizhong decoction can reverse IM due to CAG to a certain extent through a mechanism related to the regulation of shh gene expression of the diseased gastric mucosa[23]. Recent research reported that notoginsenoside R1, an ingredient of Panax notoginseng, can improve CAG by increasing Bcl-2 expression and decreasing Bax expression in gastric tissue of rats with CAG induced by MNNG combined with an irregular diet[24]. Morroniside, an extract from Cornus officinalis, was shown to relieve gastric mucosa injury due to CAG by preventing inflammation[25]. Modified Sijunzi Decoction (MSD) relieved the symptoms of CAG, improved the pathologic changes in CAG including fatigue and tiredness symptoms in CAG patients[26]. Berberine, an isoquinoline alkaloid from Rhizoma coptidis, has an inhibitory effect on gastritis[27] and GC cells[28], and significantly improves the pathological characteristics of gastric tissue, and alleviated serum biochemical indices[29]. These studies show that in addition to the formula we used in this study (Dendrobium officinale and American ginseng), fuzi Lizhong Decoction, notoginsenoside R1, morroniside, MSD, berberine, or TCM treatment aimed at invigorating spleen, soothing the liver, promoting blood circulation and detoxification, also have varying degrees of anti-inflammatory effects and reverse gastric mucosal atrophy and IM.
Some researchers believe that it is difficult to achieve the effect of reversing gastric mucosal atrophy and IM in CAG or they doubt the reported efficacy. For example, clinical studies have shown that eradication of Helicobacter pylori can improve chronic gastritis. Although some studies have shown certain improvement in gastric atrophy, IM seems reversible[30]. Some people also think that advanced atrophic gastritis may be irreversible[31]. As the conclusions by different authors vary widely, further long-term prospective studies in different ethnic and geographical environments are needed to provide more reliable evidence on the reversibility of gastric atrophy and IM[32].
As discussed above, it is believed that it is difficult to reverse gastric mucosal atrophy and gastric mucosal IM in CAG. However, as this conclusion is mainly based on observations of gastric mucosal atrophy and gastric mucosal IM following eradication of Helicobactor pylori, the question of whether a different treatment (i.e., TCM) will be effective in reversing gastric atrophy and IM remains to be answered.
Our histological data support that treatment with GD for 8 wk was effective in reversing gastric mucosa atrophy and IM. Whether this effect can be duplicated in human CAG patients requires confirmation in clinical studies with large sample sizes, and strict control and rigorous experimental design. The results will provide new evidence on the use of GD not only for improving clinical symptoms but also for normalizing abnormal histological changes in CAG.
This study shows that treatment with GD for 8 wk was effective in reducing weight loss, gastric mucosal inflammation and atrophy, and intestinal metaplasia in a rat model of CAG induced by MNNG. GD also reduced the decreases in hemoglobin level and erythrocytopenia in this model. The treatment resulted in alleviation of the overexpression of PCNA and Bcl-2 in the model rats which may be an underlying mechanism that contributes to the reduction in gastric mucosal inflammation and mucosal damage.
Dendrobium officinale is often used to treat stomach diseases. One formula of Granule Dendrobii (GD) consisting of Dendrobium officinale and American ginseng (Radix Panacis quinquefolii) is a potent Traditional Chinese Medicine product in China for chronic atrophic gastritis (CAG) as it reverses gastric mucosa atrophy and improves gastric mucosa intestinal metaplasia (IM).
This study determined the effect of GD treatment on CAG and its potential cellular mechanism to provide a reference for future treatment.
To study the effect and cellular mechanism of GD in the treatment of CAG, and to provide scientific evidence for the clinical application of GD.
A rat model of CAG induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) was established, and treatment with GD resulted in weight loss, gastric mucosa atrophy and intestinal metaplasia reversal, as well as changes in PCNA and B-cell lymphoma-2 (Bcl-2) after 8 wk of treatment.
Treatment with GD for 8 wk resulted in significant improvements in body weight, gastric mucosal inflammation, atrophy, and IM in rats with CAG induced by MNNG. GD also increased the reduced levels of hemoglobin and erythrocytes in CAG rats.
GD improved gastric mucosal lesions and the secondary pathological changes of CAG including hemoglobin and red blood cell reductions, and overall body condition. GD also alleviated the overexpression of PCNA and Bcl-2 in model rats, which maybe one of the therapeutic mechanisms related to the reduction in gastric mucosal inflammation and mucosal damage.
Our research group will continue to confirm whether the effect of GD can be duplicated in CAG patients by clinical studies with large sample sizes, strict control and rigorous experimental design, and further study the molecular and cellular mechanism of GD.
The authors would like to acknowledge Dr. Chun-Li Zhang and Dr. Min Yang from Tongde Hospital of Zhejiang Province for their professional advice on pathological work, as well as Peng Ren from Jiangsu Yanchang Science and Technology Co., Ltd for statistical analysis assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Herold Z, Hungary; Nakajima N, Japan; Sahin Y, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Marques-Silva L, Areia M, Elvas L, Dinis-Ribeiro M. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Liu Y, You P, Feng G. Occurrence of gastric cancer in patients with atrophic gastritis during long-term follow-up. Scand J Gastroenterol. 2018;53:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Li Y, Zhang Y, Meng H, Liao M, Su Z, Zhai M, Jiang L, Li P, Ding X. Efficacy and safety of acupuncture therapy for chronic atrophic gastritis: A meta-analysis and trial sequential analysis protocol. Medicine (Baltimore). 2019;98:e17003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Wu RZ, Chen JX, Xia L, Xu H, Yao LY, Fu HZ, Chen XL, Xie K. Clinical study on Tiepi Fengdou Granules (Capsules) in treating chronic atrophic gastritis with deficiency of qi and yin. Shanghai Zhongyiyao Zazhi. 2004;38:28-29. [DOI] [Full Text] |
| 6. | Jiang JY, Liu DJ, Liu MX. The protective effect of NF-κB signaling pathway inhibitor PDTC on mice with chronic atrophic gastritis. Scand J Gastroenterol. 2021;56:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Qin R, Wang NN, Chu J, Wang X. Expression and significance of homeodomain protein Cdx2 in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2012;18:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 8. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3545] [Article Influence: 122.2] [Reference Citation Analysis (3)] |
| 9. | Khakoo SI, Lobo AJ, Shepherd NA, Wilkinson SP. Histological assessment of the Sydney classification of endoscopic gastritis. Gut. 1994;35:1172-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Qi SN, Xu S, Chen J, Lv NH. Lesion mutants of p53, MDM2 and PCNA protein expression in gastric mucosal and the relationship with helicobacter pylori infection. Shandong Yiyao. 2009;49:1-4. |
| 11. | Meng J, Yang JX, Lu XF, Dai X, Fang J. Xiao Pi granules on the expression of Bcl-2 and bax in gastric mucosa of rats with Chronic atrophic gastritis and atypical hyperplasia. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2007;15:214-217. |
| 12. | Meng J, Yang JX, Lu XF, Dai X, Fang J. Xiao pi Granules on the expression of PCNA in rats with chronic atrophic gastritis with atypical hyperplasia. Liaoning Zhongyi Zazhi. 2007;33:369-371. |
| 13. | He XL, Peng CF, Huang ZM, Guo LJ. The effect of Cynanchum auriculatum Royle ex Wight on the expression of p53 and PCNA protein in rats induced by chronic atrophic gastritis with atypical hyperplasia. Shizhen Guoyi Guoyao. 2010;21:2090-2391. |
| 14. | Jiang XY, Qian LP, Zheng XJ, Xia YY, Jiang YB, Sun DY. Effect of Ginkgo biloba extract on proto-oncogene expression in rats with gastric mucosal precancerous lesions. Weichangbingxue He Ganbingxue Zazhi. 2010;19:982-984. |
| 15. | Zhu X, Liu S, Zhou J, Wang H, Fu R, Wu X, Wang J, Lu F. Effect of Astragalus polysaccharides on chronic atrophic gastritis induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats. Drug Res (Stuttg). 2013;63:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Huang K, Zhong G, Huang Y, Li S, Qu S, Zhang J. Acupuncture Decreases NF-κB p65, miR-155, and miR-21 and Increases miR-146a Expression in Chronic Atrophic Gastritis Rats. Evid Based Complement Alternat Med. 2016;2016:9404629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Kubo Y, Matsui H, Ninomiya T, Mizukami Y, Onji M. Non-invasive approach for diagnosing atrophic gastritis using the 13C-bicarbonate breath test. Int J Mol Med. 2001;7:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Yin J, Yi J, Yang C, Xu B, Lin J, Hu H, Wu X, Shi H, Fei X. Chronic atrophic gastritis and intestinal metaplasia induced by high-salt and N-methyl-N'-nitro-N-nitrosoguanidine intake in rats. Exp Ther Med. 2021;21:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zhao Y, Liu Y, Lan XM, Xu GL, Sun YZ, Li F, Liu HN. Effect of Dendrobium officinale Extraction on Gastric Carcinogenesis in Rats. Evid Based Complement Alternat Med. 2016;2016:1213090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Zhao Y, Li B, Wang G, Ge S, Lan X, Xu G, Liu H. Dendrobium officinale Polysaccharides Inhibit 1-Methyl-2-Nitro-1-Nitrosoguanidine Induced Precancerous Lesions of Gastric Cancer in Rats through Regulating Wnt/β-Catenin Pathway and Altering Serum Endogenous Metabolites. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Ding PJ, Liu HY. Research progress of TCM treatment for Chronic Atrophic Gastritis. Shiyong Zhongyi Neike Zazhi. 2010;24:6-8. |
| 22. | Zeng J, Ge Y, Guo H, Xia SJ. Treatment of pylori pyrolytic gastritis with the method of invigorating the spleen, soothing blood circulation and activating blood. Jilin Zhongyiyao. 2017;37:907-911. [DOI] [Full Text] |
| 23. | Liu XY, Liu XM, Yang ZB. Clinical efficacy and mechanism of Fuzilizhong Decoction in the treatment of chronic atrophic gastritis intestinal metaplasia. Yunnan Zhongyi Xueyuan Xuebao. 2016;39:54-57. |
| 24. | Luo C, Sun Z, Li Z, Zheng L, Zhu X. Notoginsenoside R1 (NGR1) Attenuates Chronic Atrophic Gastritis in Rats. Med Sci Monit. 2019;25:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Zhang J, Wang H. Morroniside protects against chronic atrophic gastritis in rat via inhibiting inflammation and apoptosis. Am J Transl Res. 2019;11:6016-6023. [PubMed] |
| 26. | Tian G, Wu C, Li J, Liang B, Zhang F, Fan X, Li Z, Wang Y, Liu D, Lai-Han Leung E, Chen J. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res. 2019;144:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Wu X, Li X, Dang Z, Jia Y. Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor. J Cell Biochem. 2018;119:5373-5381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Hu Q, Li L, Zou X, Xu L, Yi P. Berberine Attenuated Proliferation, Invasion and Migration by Targeting the AMPK/HNF4α/WNT5A Pathway in Gastric Carcinoma. Front Pharmacol. 2018;9:1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Tong Y, Zhao X, Wang R, Li R, Zou W, Zhao Y. Therapeutic effect of berberine on chronic atrophic gastritis based on plasma and urine metabolisms. Eur J Pharmacol. 2021;908:174335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Zivny J, Wang TC, Yantiss R, Kim KH, Houghton J. Role of therapy or monitoring in preventing progression to gastric cancer. J Clin Gastroenterol. 2003;36:S50-60; discussion S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Genta RM, Graham DY. Intestinal metaplasia, not atrophy or achlorhydria, creates a hostile environment for Helicobacter pylori. Scand J Gastroenterol. 1993;28:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Genta RM. Atrophy, metaplasia and dysplasia: are they reversible? Ital J Gastroenterol Hepatol. 1998;30 Suppl 3:S324-S325. [PubMed] |