Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4635
Peer-review started: March 24, 2022
First decision: May 30, 2022
Revised: June 8, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 28, 2022
Processing time: 155 Days and 0.1 Hours
Obstructive jaundice (OJ) is caused by bile excretion disorder after partial or complete bile duct obstruction. It may cause liver injury through various mechanisms. Traditional Chinese medicine (TCM) has a lot of advantages in treating OJ. The recovery of liver function can be accelerated by combining Chinese medicine treatment with existing clinical practice. Yinchenhao decoction (YCHD), a TCM formula, has been used to treat jaundice. Although much progress has been made in recent years in understanding the mechanism of YCHD in treating OJ-induced liver injury, it is still not clear.
To investigate chemical components of YCHD that are effective in the treatment of OJ and predict the mechanism of YCHD.
The active components and putative targets of YCHD were predicted using a network pharmacology approach. Gene Ontology biological process and Kyoto Encyclopedia of Genes and Genomes path enrichment analysis were carried out by cluster profile. We predicted the biological processes, possible targets, and associated signaling pathways that YCHD may involve in the treatment of OJ. Thirty male Sprague–Dawley rats were randomly divided into three groups, each consisting of 10 rats: the sham group (Group S), the OJ model group (Group M), and the YCHD-treated group (Group Y). The sham group only received laparotomy. The OJ model was established by ligating the common bile duct twice in Groups M and Y. For 1 wk, rats in Group Y were given a gavage of YCHD (3.6 mL/kg) twice daily, whereas rats in Groups S and M were given the same amount of physiological saline after intragastric administration daily. After 7 d, all rats were killed, and the liver and blood samples were collected for histopathological and biochemical examinations. Total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), and aspartate transaminase (AST) levels in the blood samples were detected. The gene expression levels of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS), and the nucleus positive rate of NF-E2 related factor 2 (Nrf2) protein were measured. Western blot analyses were used to detect the protein and gene expression levels of Nrf2, Kelch-like ECH-associated protein 1, NAD(P)H quinone dehydrogenase 1 (NQO1), and glutathione-S-transferase (GST) in the liver tissues. One-way analysis of variance was used to evaluate the statistical differences using the statistical package for the social sciences 23.0 software. Intergroup comparisons were followed by the least significant difference test and Dunnett’s test.
The effects of YCHD on OJ involve biological processes such as DNA transcription factor binding, RNA polymerase II specific regulation, DNA binding transcriptional activator activity, and nuclear receptor activity. The protective effects of YCHD against OJ were closely related to 20 pathways, including the hepatitis-B, the mitogen-activated protein kinase, the phosphatidylinositol 3-kinase/protein kinase B, and tumor necrosis factor signaling pathways. YCHD alleviated the swelling and necrosis of hepatocytes. Following YCHD treatment, the serum levels of TBIL (176.39 ± 17.03 μmol/L vs 132.23 ± 13.88 μmol/L, P < 0.01), DBIL (141.41 ± 14.66 μmol/L vs 106.43 ± 10.88 μmol/L, P < 0.01), ALT (332.07 ± 34.34 U/L vs 269.97 ± 24.78 U/L, P < 0.05), and AST (411.44 ± 47.64 U/L vs 305.47 ± 29.36 U/L, P < 0.01) decreased. YCHD promoted the translocation of Nrf2 into the nucleus (12.78 ± 0.99 % vs 60.77 ± 1.90 %, P < 0.001). After YCHD treatment, we found a decrease in iNOS (0.30 ± 0.02 vs 0.20 ± 0.02, P < 0.001) and an increase in eNOS (0.18 ± 0.02 vs 0.32 ± 0.02, P < 0.001). Meanwhile, in OJ rats, YCHD increased the expressions of Nrf2 (0.57 ± 0.03 vs 1.18 ± 0.10, P < 0.001), NQO1 (0.13 ± 0.09 vs 1.19 ± 0.07, P < 0.001), and GST (0.12 ± 0.02 vs 0.50 ± 0.05, P < 0.001), implying that the potential mechanism of YCHD against OJ-induced liver injury was the upregulation of the Nrf2 signaling pathway.
OJ-induced liver injury is associated with the Nrf2 signaling pathway. YCHD can reduce liver injury and oxidative damage by upregulating the Nrf2 pathway.
Core tip: Obstructive Jaundice (OJ) may cause liver injury through various mechanisms. Traditional Chinese medicine has lots of advantages in treating OJ. The mechanism of Yinchenhao decoction (YCHD) for treating OJ-induced liver injury has made significant progress in recent years, but it is still unclear. We used the network pharmacology approach to predict the active components and putative targets of YCHD. We created the OJ rat models and through randomized controlled trials, concluded that YCHD could alleviate liver injury and oxidative damage, thereby promoting the translocation of NF-E2 related factor 2 (Nrf2) to the nucleus, and upregulating the Nrf2 signaling pathway.
- Citation: Liu JJ, Xu Y, Chen S, Hao CF, Liang J, Li ZL. The mechanism of Yinchenhao decoction in treating obstructive-jaundice-induced liver injury based on Nrf2 signaling pathway. World J Gastroenterol 2022; 28(32): 4635-4648
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4635.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4635
Obstructive jaundice (OJ), a common disease in clinical practice, is caused by biliary stones or tumors blocking the bile ducts, causing bile not to flow smoothly into the duodenum, causing elevated bilirubin levels in the blood, yellow-stained sclera and skin, urine yellowing, and other relevant symptoms. OJ can cause liver injury, inflammation, intestinal barrier dysfunction, endotoxemia, coagulation dysfunction, decreased immune function, and malnutrition[1,2]. Therefore, understanding the mechanism of OJ is crucial. Although progress has been made in recent years, it remains unclear. The mechanism of liver damage caused by OJ is mainly related to cholestasis, endotoxemia, inflammatory factors, and oxidative stress[3,4]. Numerous endotoxins activate Kupffer cells during OJ, producing reactive oxygen species (ROS) and reactive nitrogen free radicals (RNS)[5], both of which are important in the body’s metabolism.
Oxidative stress is an important pathological mechanism of OJ-induced liver injury. It is present throughout the OJ process[6]. Under normal circumstances, the body’s oxidation and antioxidation systems are in a state of dynamic balance. The production and clearance of active molecules such as ROS and RNS are increased as a result of OJ. The balance between oxidation and antioxidation is dis
NF-E2 related factor 2 (Nrf2) is a key regulator of antioxidant reduction in tissues and cells. Kelch-like ECH-associated protein 1 (Keap1) is a cytoskeletal anchoring protein that acts as a specific inhibitor of Nrf2. Under normal circumstances, Nrf2 is bound to Keap1 in the cytoplasm, which can be rapidly degraded via the ubiquitin-proteasome pathway[11,12]. When the body is stimulated by oxidative stress, Nrf2 is released from sequestration and translocated to the nucleus, where it promotes the transcription of antioxidant and cytoprotective genes. Phase 2 detoxification enzyme genes, such as NAD(P)H quinone dehydrogenase 1 (NQO1), and glutathione-S-transferase (GST) and antioxidant genes, such as heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCS) are among the cytoprotective genes[13]. Yinchenhao decoction (YCHD) is a classic traditional Chinese medicine (TCM) formula from the Treatise on Exogenous Febrile Disease. It consists of Yinchen (Artemisiae Scopariae Herba), Zhizi (Gardenia jasminoides Ellis, Gardeniae Fructus), and Dahuang (Radix Rhei et Rhizoma), which is a common prescription for the treatment of damp-heat jaundice. Studies have shown that YCHD has a positive effect on a variety of stagnant damp-heat diseases of the liver meridian not only on enzymes and proteins in the liver and blood, regulating bile acid, bilirubin, lipid, and glucose metabolism but also on pathological processes such as liver fibrosis, inflammation, and hepatocyte apoptosis directly through liver Kupffer cells, hepatic stellate cells, and other cells. Additionally, it can also regulate intestinal flora and protect the liver.
We used keywords Yinchen, Zhizi, and Dahuang to search the TCM systems pharmacology database and analysis platform (TCMSP) database. According to the standard of oral bioavailability ≥ 30% and drug likeness ≥ 0.18, 13, 16 and 15 active ingredients were obtained. β-Sitosterol is a common component of Yinchen, Zhizi and Dahuang. Yinchen and Zhizi both contain quercetin. After removing the duplicates, YCHD contained 41 active chemical components.
The related targets of OJ were found using the GeneCards database (https://www.genecards.org/). A total of 2183 disease targets were obtained.
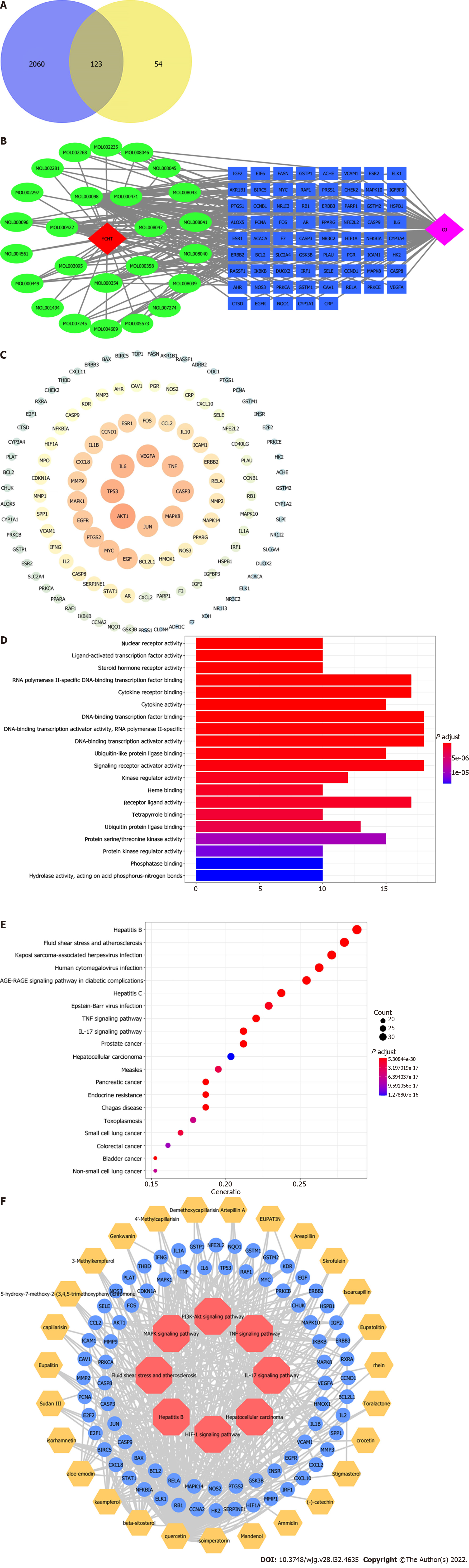
The intersection targets of YCHD and OJ were discovered using R4.0.2 software to analyze the action targets of the main chemical components of YCHD and the targets of OJ. Figure 1A shows the Venn diagram of 123 intersection target genes, or the targets of YCHD acting on OJ. The chemical composition and intersection target of YCHD were entered into the Cytoscape software to construct a Component-Target network of YCHD for treating OJ. The network consists of 153 nodes and 463 edges (Figure 1B).
The protein–protein interaction (PPI) data came from the search tool for the retrieval of interacting genes/proteins database (https://string-db.org), which provides information on predicted and experimental protein interactions. The intersection target’s protein interaction relationship was then obtained. However, one protein was not involved in the interaction. The protein interaction information was then entered into the Cytoscape software to construct and analyze the protein interaction network, yielding the PPI network of the YCHD and OJ intersection target. The network consists of 122 nodes. Protein kinase B (PKB/Akt) 1, tumor protein 53 (TP53), interleukin 6 (IL-6), vascular endothelial growth factor A (VEGFA), caspase-3 (CASP3), and so on can be seen in the picture. They are the core targets of YCHD in the treatment of OJ (Figure 1C).
The R software was used to import data from the Bioconductor database (https://www.biocon
Yinchen, Zhizi, and Dahuang were provided by the particle pharmacy of Tianjin Medical University NanKai Hospital. Serum total bilirubin (TBIL) kits, direct bilirubin (DBIL) kits, alanine transaminase (ALT) kits, and aspartate aminotransferase (AST) kits were purchased from the National Institute of Biochemistry. Thermo Fisher Scientific (Shanghai, China) provided the Nrf2 antibody (PA5-27882), Keap1 antibody (PA5-99434), and protein marker (26617. Anti-NQO1 antibody (ab80588) and Anti-GST3/GST antibody (ab138491) were purchased from Abcam (Shanghai, China). β-Actin (66009-1-Ig) was purchased from Proteintech Group (Wuhan, China). Goat anti-rabbit immunoglobulin G (IgG) (ZB-2301) and anti-mouse IgG (ZB-2305) were purchased from Beijing Zhongshan Gold Bridge Biotechnology (Beijing, China). Poly (vinylidene fluoride) membrane (IPHV00010), electrochemiluminescence (ECL) kit (WBKLS0×500), and radioimmunoprecipitation assay buffer (RIPA) lysis buffer (20-188) were provided by Millipore Corporation (Shanghai, China). 4% paraformaldehyde solution, poly-L-lysine, antigen retrieval solution, goat serum, NOS3 (A-9; sc-376751), NOS2 (A-9; sc-7271), S-A/HRP, DAB kit, and hematoxylin and eosin staining kit were purchased from Santa Cruz Biotechnology (Beijing, China). Bicinchoninic acid (BCA) protein assay kit was provided by Beijing Solarbio Science & Technology (Beijing, China).
According to Shanghan Lun, a dose of YCHD contains Yinchen (82.5 g), Zhizi (14 g), and Dahuang (27.5 g). We boiled it twice after soaking it for 30 min. Then, we concentrated two boiling mixtures to 124 mL each. The final liquid contained 1 g/mL of the crude drug.
Thirty-two healthy SPF-rated Sprague–Dawley rats (200–230 g) were purchased from Beijing HFK Bioscience (license No. SCXK Jin 2020-0008). The experimental protocol was approved by the Animal Research Committee of Tianjin Medical University NanKai Hospital (approval no. NKYY-DWLL-2021-102). All the animals were reared in the Laboratory Animal Center of Tianjin Medical University Nankai Hospital. We strictly followed the rules of the experimental animal center. The rats were fed and acclimatized to laboratory conditions (22–24°C, 12 h/12 h light/dark, 60%–65% humidity, ad libitum access to food and water) for 2 wk prior to experimentation.
Thirty healthy rats were divided into three groups, each with 10 rats: the sham group (Group S), obstructive jaundice model group (Group M), and YCHD-treated group (Group Y). We created OJ models in Groups M and Y by ligating the common bile duct twice and transecting between the sutures. The successful establishment of the bile duct OJ model was demonstrated by the yellowing of the rats’ skin after 3 d. In Group S, the common bile ducts were not clamped and served as a control. From then on, rats in Group Y were given a gavage of YCHD (3.6 mL/kg) twice daily for 1 wk, whereas rats in Groups S and M were given the same amount of physiological saline after intragastric administration daily. Intragastric gavage administration was carried out with conscious animals. All rats were killed on d 10 after the operation, and the liver and blood samples were collected for further research. Every effort was made to alleviate animal suffering. Animal experiments followed were strictly complied with the Guide for the Care and Use of Laboratory Animals.
The blood samples were centrifuged at 3000 r/min for 10 min to obtain the upper serum specimens. The serum levels of TBIL, DBIL, ALT and AST were measured.
The liver tissue was fixed in 4% paraformaldehyde. The samples were sliced into 4–5-μm thick slices and stained with H&E to observe the changes in histology under the microscope after decalcification, dehydration, permeation, and paraffin encapsulation.
Antigen repair, blocking of endogenous peroxidase, incubation of primary and secondary antibodies, DAB staining, restaining of the nucleus, and sealing was performed on dewaxed liver tissue sections. As a result, the nucleus was blue, while the positive DAB expression was brown. The visual field was randomly selected. The levels of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) gene expression, and the nucleus positive rate of Nrf2 protein, were measured.
Liver tissue samples were lysed with RIPA lysis buffer and centrifuged to prepare protein solution. A BCA protein assay kit was used to determine the protein content. Sample feeding, electrophoresis, membrane transfer, and sealing were carried out in order. The specimens were then incubated with the following primary antibodies, anti-Nrf2 (1:500), anti-Keap1 (1:500), anti-NQO1 (1:10000), anti-GST3/GST (1:1000), and β-actin (1:5000). After washing three times with tris buffered saline-Tween 20 (TBST), the membranes were incubated with rabbit horseradish peroxidase (HRP)-conjugated secondary antibody at 4C for 1 h. Electrochemiluminescence was used to detect the proteins, and ImageJ software was used to calculate the average optical density (AOD). The relative content of Nrf2, Keap1, NQO1 and GST in rat liver tissue was calculated by dividing the AOD value of the target protein in the sample by the AOD value of β-actin.
All data were presented as mean ± SD. One-way analysis of variance was used to assess statistical differences using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA). For homogeneity of variance, the least significant difference test was used for homogeneity of variance, and Dunnett’s test was used for nonhomogeneity of variance. The post hoc test above was used to determine the significance of the statistical results. P < 0.05 was considered statistically significant.
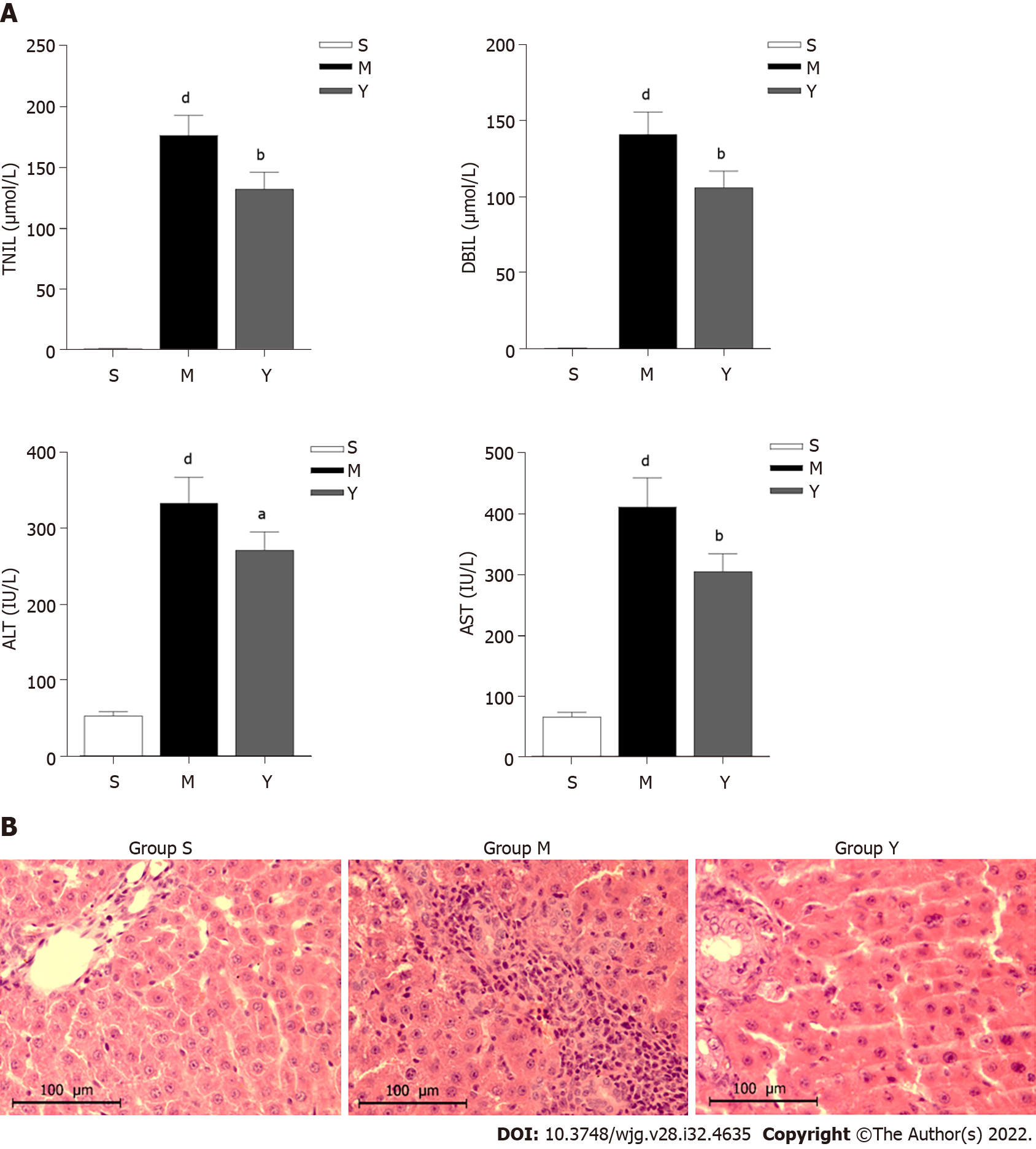
Biochemical markers of liver function include AST, ALT, TBIL and DBIL. The serum levels of TBIL, DBIL, ALT and AST in Groups M and Y were significantly higher than in Group S (Figure 2A). When compared to Group M, serum levels of TBIL, DBIL, ALT and AST decreased after 1 wk of YCHD intervention in Group Y. The liver function of OJ rats was improved after YCDH treatment.
Hepatocytes in Group M were swollen, necrotic, and had neutrophil infiltration and erythrocyte accumulation in the sinusoids, compared with Group S. Group M showed obvious signs of liver injury. The swelling and necrosis of hepatocytes were alleviated in Group Y compared to Group M. After YCHD treatment, the degree of liver injury was significantly reduced (Figure 2B).
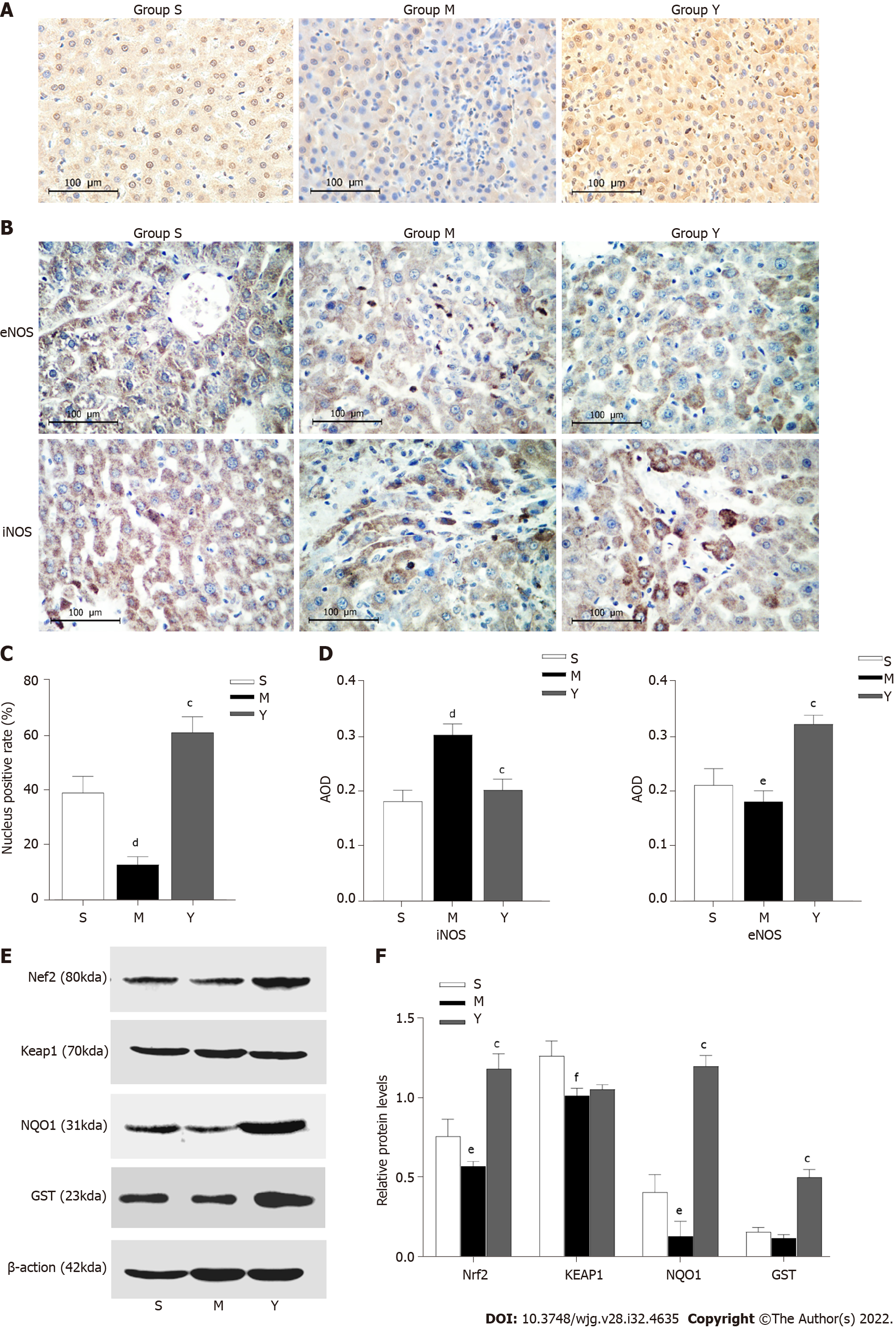
The nucleus positive rate of Nrf2 protein was lower in Group M than in Group S. Compared with Group M, the positive rate in Group Y was significantly higher, suggesting that after the intervention of YCHD, Nrf2 protein was transferred from the cytoplasm to the nucleus, potentially reducing the oxidative damage to OJ liver tissue by regulating the nuclear translocation of Nrf2 (Figure 3).
The AOD of eNOS in Group M decreased when compared to Group S, while that of iNOS increased (Figure 3). Compared with Group M, the AOD of eNOS in Group Y increased, while that of iNOS decreased.
The expression levels of Nrf2, Keap1 and NQO1 in Group M decreased when compared to Group S. Compared with Group M, the expression levels of Nrf2, NQO1 and GST increased in Group Y, but there was no significant difference in the expression of Keap1 between these two groups (Figure 3).
OJ is caused by bile excretion disorder after partial or complete bile duct obstruction. The metabolism of total bile acid (TBA) is disrupted, resulting in an increase in TBA in the blood of up to 60 times the normal amount. Bile buildup causes harmful substances to clump together. Damage in the reticuloendothelial system function causes spillover of bacteria and endotoxin into the systemic circulation[14]. OJ can cause liver damage, inflammation, decreased bowel barrier function, endotoxemia, clotting dysfunction, decreased immune function, and malnutrition. It can cause liver injury through various mechanisms, such as inflammation, endotoxemia and oxidative stress[15]. OJ leads to an increase in the production and clearance of active molecules, such as ROS and RNS. Increased lipid peroxidation and the balance between the hepatic oxidative and antioxidant systems worsen liver injury[16]. OJ causes liver damage and hepatocyte apoptosis by suppressing the protein kinase RNA-like endoplasmic reticulum kinase-induced pathway[17].
YCHD is one of the classic prescriptions for treating liver diseases. The chemical components of YCHD mainly include flavonoids, volatile oils and coumarin, among which the common flavonoids are quercetin, isorhamnetin and cirsimaritin, which belong to a class of compounds with antioxidant, anti-inflammatory, antitumor and other effects. Recent studies have shown that YCHD increases bile acid reabsorption and restore the balance of bile acid excretion, thereby reducing the impact of toxic bile acids on liver injury in rats[18]. YCHD alleviates lithogenic-diet-induced cholelithiasis and improves biliary cholesterol supersaturation to restore biliary cholesterol homeostasis[19]. YCHD could alleviate liver damage by reducing the levels of important cytokines such as TNF-α, myeloid differentiation primary response 88 (MyD88), and nuclear factor B in the Toll-like receptor 4 signaling pathway. YCHD may reduce liver fibrosis by regulating targets in the apoptosis-related TNF, PI3K-Akt and MAPK signaling pathways, promoting hematopoietic stem cell apoptosis while decreasing hematopoietic progenitor cell apoptosis[20]. YCHD showed therapeutic effects on cholangiocarcinoma by regulating related target protein, inhibiting cell proliferation, and increasing the rate of apoptosis[21].
In our study, we demonstrated that YCHD alleviated the swelling and necrosis of hepatocytes, while also regulating serum levels of ALT, AST, TBIL and DBIL. These findings suggest that YCHD can effectively reduce OJ-induced liver injury and improve liver function.
NO plays a dual role in liver damage. NO is still maintained at a physiological level in the early stages of the disease, which can dilate blood vessels, inhibit platelet aggregation, and improve liver blood flow. RNS is an active group whose derivatives revolve around NO. In the physiological state, iNOS is inactive, but in a physiological state, iNOS is activated and expressed, causing it to catalyze L-arginine to produce a large amount of NO[22]. NO can easily interact with O2 to produce free radicals and nitro compounds such as ONOO and its proton form HOONO. Recent studies have shown that with the aggravation of cholestasis, various stimulating factors such as endotoxin and inflammation activate the expression of iNOS in liver tissue, resulting in a large amount of NO, which is cytotoxic and causes liver injury[23]. We found expression of NOS3 (eNOS) and NOS2 (iNOS) in the liver tissue of rats. When OJ occurred, the expression of eNOS decreased, while that of iNOS increased. However, after YCHD intervention, the amount of iNOS decreased, suggesting that YCHD can reduce overexpression of NO by adjusting eNOS and iNOS and keeping it at a physiological level to reduce oxidative damage and improve liver microcirculation.
Nrf2 is an important nuclear transcription factor. Under normal circumstances, Nrf2 is bound to Keap1 in the cytoplasm, where it will be rapidly degraded via the ubiquitin–proteasome pathway. When the body is stimulated by oxidative stress, Nrf2 dissociates from Keap1. A heterodimer is formed when free Nrf2 combines with one of the small Maf (musculoaponeurotic fibrosarcoma oncogene homolog) proteins. Then, it binds to the antioxidant response elements (AREs), which are enhancer sequences in the regulatory regions, to promote the transcription of genes that encode redox balancing factors, detoxifying enzymes, and stress response proteins, such as GSH, GST, NQO1, HO-1 and glutathione cysteine ligase catalytic subunit[13]. Expression of HO-1 and NQO1 reduces oxidative stress by facilitating removal of ROS[24]. p53 induction and apoptosis were reduced in NQO1-deficient mice, and chemically induced tumors susceptibility was increased[25]. The Nrf2/ARE signaling pathway has been shown to effectively remove excessive ROS from the body, inhibiting oxidation and inflammatory reactions and reducing hepatocellular apoptosis. Increased ROS and oxidative stress injury could lead to gastric mucosal damage and malignancies[26,27]. Overexpression of HO-1 increases the production of CO, which protects cardiomyocytes from apoptosis by generating H2O2 in the mitochondria and producing PKB/Akt [28]. The expression of Nrf2 mRNA in silenced neurons for the frataxin gene decreased, and the cells may be more sensitive to oxidative stress[29]. Sulforaphane, an Nrf2 activator, enhances running capacity in rats by upregulating Nrf2 signaling and reduces muscle fatigue by reducing oxidative stress caused by exhaustive exercise[30]. As a result, Nrf2 plays an important role in the response to oxidative stress. Following the intervention of YCHD, the positive rate of Nrf2 in nucleus was significantly increased, suggesting that Nrf2 protein was transferred from the cytoplasm to the nucleus. Nrf2 can only function in the nucleus, forming the Nrf2–Maf heterodimer and binding to ARE to activate antioxidant and metabolic genes. Therefore, YCHD may be able to reduce oxidative damage to OJ liver tissue by regulating the nuclear translocation of Nrf2. As a result, the ability of antioxidant stress is enhanced. We assessed the Nrf2 signaling pathway, which is an important ARE signaling pathway. Expression of Nrf2 was decreased in OJ rats, but significantly increased with YCHD treatment. GSH and NQO1 are the downstream antioxidant factors of the Nrf2 signaling pathway. In the YCHD group, their expression was higher. It was discovered that YCHD protects against OJ-induced oxidative stress by activating the Nrf2 signaling pathway.
We found through network pharmacology research that YCHD has multicomponent, multitarget, and multipathway function characteristics. The mechanism could be related to many biological processes, such as anti-inflammatory activity, inhibition of liver fibrosis, antioxidant activity, and apoptosis. This provides the theoretical basis for further research into the molecular mechanism of action of YCHD in the treatment of OJ. In a future study, the chemical composition of YCHD and its associated targets can be studied accurately using the results predicted by network pharmacology. Measurements of ROS accumulation, antioxidant factors (GSH and NQO1), and iNOS were used to assess the status of oxidative stress caused by OJ. We have demonstrated that YCHD can increase the expression of Nrf2, promote translocation of Nrf2 to the nucleus, reduce overexpression of NO by adjusting eNOS and iNOS, and activate downstream GSH and NQO1expression; all of which would protect liver tissue from oxidative damage. Besides, it can also alleviate liver injury and oxidative damage, promote the translocation of Nrf2 to the nucleus, and upregulate the Nrf2 signaling pathway. In a nutshell, the present study looked into the protective effects of YCHD against OJ-induced liver injury. The Nrf2 signaling was upregulated as a potential mechanism. Although many mechanisms are involved in the occurrence and progression of OJ, we report here for the first time that YCHD can promote the translocation of Nrf2 to the nucleus and upregulate the Nrf2 signaling pathway, which could be a new way to look at antioxidation mechanisms. OJ can be effectively treated using a combination of TCM and modern medicine.
Obstructive jaundice (OJ) may lead to liver injury through various mechanisms. Oxidative stress is an important pathological mechanism of OJ-induced liver injury. It is present throughout the OJ process. Yinchenhao decoction (YCHD), a traditional Chinese medicine (TCM) formula, has been widely used for treating jaundice in clinical practice. Recent studies have confirmed that YCHD can effectively alleviate liver injury. However, the mechanism of YCHD treating OJ-induced liver injury has not yet been fully clarified.
To provide much more scientific evidence for the clinical application of YCHD and promote the combination of TCM and modern medicine to treat diseases more effectively.
We aimed to investigate the effective chemical components of YCHD and predict the mechanism of YCHD in the treatment of OJ-induced liver injury and oxidative damage.
By the network pharmacology approach, we predicted the chemical composition of YCHD and its associated targets. Measurements of inducible nitric oxide synthase (iNOS), endothelial nitric oxide synthase (eNOS), the nucleus positive rate of NF-E2 related factor 2 (Nrf2) protein, and the protein expression levels of Nrf2, Kelch-like ECH-associated protein 1, NAD(P)H quinone dehydrogenase 1 (NQO1) and glutathione-S-transferase (GST) were used to assess the status of oxidative stress caused by OJ.
Network pharmacology research showed that YCHD had multicomponent, multitarget, and multipathway function characteristics. YCHD increased expression of Nrf2, promoted translocation of Nrf2 to the nucleus, reduced overexpression of NO by adjusting eNOS and iNOS, and activated expression of GST and NQO1; all of which protect liver tissue from oxidative damage.
YCHD can alleviate liver injury and reduce oxidative damage. Although various mechanisms are involved in the occurrence and development of OJ, we report that YCHD can promote the translocation of Nrf2 to the nucleus, and upregulate the Nrf2 signaling pathway, which may provide a new perspective for the study of the mechanism of antioxidation. This provides the theoretical basis for further research into the molecular mechanism of action of YCHD in the treatment of OJ.
TCM formulas like YCHD have been widely used for thousands of years. In the near future, more in-depth research on the molecular mechanism of TCM can guide clinical treatment more effectively.
We would like to acknowledge Zhong-Lian Li for his skillful technical assistance and Jing Liang from Chengdu University of traditional Chinese medicine, for her English language proofreading to improve the content of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Cengiz M, Turkey S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Yu HG
| 1. | Atalay E, Ozdemir MT, Tur BK, Erdogdu HI, Sisman P. The effect of alphalipoic acid on oxidative parameters and liver injury in rats with obstructive jaundice. Bratisl Lek Listy. 2019;120:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Unal Y, Tuncal S, Kosmaz K, Kucuk B, Kismet K, Cavusoglu T, Celepli P, Senes M, Yildiz S, Hucumenoglu S. The Effect of Calcium Dobesilate on Liver Damage in Experimental Obstructive Jaundice. J Invest Surg. 2019;32:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Yang R, Zhu S, Pischke SE, Haugaa H, Zou X, Tonnessen TI. Bile and circulating HMGB1 contributes to systemic inflammation in obstructive jaundice. J Surg Res. 2018;228:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 910] [Cited by in RCA: 792] [Article Influence: 72.0] [Reference Citation Analysis (8)] |
| 5. | Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442-448. [PubMed] |
| 6. | Silina EV, Stupin VA, Abramov IS, Bolevich SB, Deshpande G, Achar RR, Sinelnikova TG. Oxidative Stress and Free Radical Processes in Tumor and Non-Tumor Obstructive Jaundice: Influence of Disease Duration, Severity and Surgical Treatment on Outcomes. Pathophysiology. 2022;29:32-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Majima HJ, Indo HP, Suenaga S, Matsui H, Yen HC, Ozawa T. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stress-related diseases. Curr Pharm Des. 2011;17:2190-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Beitz DC. Effect of a mitochondria-targeted vitamin E derivative on mitochondrial alteration and systemic oxidative stress in mice. Br J Nutr. 2011;106:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Zhang Q, Beitz DC. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J Nutr. 2010;140:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1271] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 11. | Turpaev KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc). 2013;78:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Taguchi K, Yamamoto M. The KEAP1-NRF2 System as a Molecular Target of Cancer Treatment. Cancers (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 13. | Yang L, Palliyaguru DL, Kensler TW. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol. 2016;43:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Assimakopoulos SF, Thomopoulos KC, Patsoukis N, Georgiou CD, Scopa CD, Nikolopoulou VN, Vagianos CE. Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest. 2006;36:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Galicia-Moreno M, Rodríguez-Rivera A, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur J Gastroenterol Hepatol. 2009;21:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Wu YL, Li ZL, Zhang XB, Liu H. Yinchenhao decoction attenuates obstructive jaundice-induced liver injury and hepatocyte apoptosis by suppressing protein kinase RNA-like endoplasmic reticulum kinase-induced pathway. World J Gastroenterol. 2019;25:6205-6221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Cai FF, Wu R, Song YN, Xiong AZ, Chen XL, Yang MD, Yang L, Hu Y, Sun MY, Su SB. Yinchenhao Decoction Alleviates Liver Fibrosis by Regulating Bile Acid Metabolism and TGF-β/Smad/ERK Signalling Pathway. Sci Rep. 2018;8:15367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Zhou Q, Hu H, Zhao G, Liu P, Wang Y, Zhang H. Effect and related mechanism of Yinchenhao decoction on mice with lithogenic diet-induced cholelithiasis. Exp Ther Med. 2021;21:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Cai FF, Bian YQ, Wu R, Sun Y, Chen XL, Yang MD, Zhang QR, Hu Y, Sun MY, Su SB. Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomed Pharmacother. 2019;114:108863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Lin T, Liao X, Li Z, Lin R, Qi X, Chen G, Sun L, Lin L. Network pharmacology based research into the effect and mechanism of Yinchenhao Decoction against Cholangiocarcinoma. Chin Med. 2021;16:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Tsuchihashi S, Kaldas F, Chida N, Sudo Y, Tamura K, Zhai Y, Qiao B, Busuttil RW, Kupiec-Weglinski JW. FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am J Transplant. 2006;6:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Picón-Pagès P, Garcia-Buendia J, Muñoz FJ. Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1949-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 24. | Sekine H, Okazaki K, Ota N, Shima H, Katoh Y, Suzuki N, Igarashi K, Ito M, Motohashi H, Yamamoto M. The Mediator Subunit MED16 Transduces NRF2-Activating Signals into Antioxidant Gene Expression. Mol Cell Biol. 2016;36:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Iskander K, Gaikwad A, Paquet M, Long DJ 2nd, Brayton C, Barrios R, Jaiswal AK. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Borrego S, Vazquez A, Dasí F, Cerdá C, Iradi A, Tormos C, Sánchez JM, Bagán L, Boix J, Zaragoza C, Camps J, Sáez G. Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker. Int J Mol Sci. 2013;14:3467-3486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Ma Y, Zhang L, Rong S, Qu H, Zhang Y, Chang D, Pan H, Wang W. Relation between gastric cancer and protein oxidation, DNA damage, and lipid peroxidation. Oxid Med Cell Longev. 2013;2013:543760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 29. | D'Oria V, Petrini S, Travaglini L, Priori C, Piermarini E, Petrillo S, Carletti B, Bertini E, Piemonte F. Frataxin deficiency leads to reduced expression and impaired translocation of NF-E2-related factor (Nrf2) in cultured motor neurons. Int J Mol Sci. 2013;14:7853-7865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Oh S, Komine S, Warabi E, Akiyama K, Ishii A, Ishige K, Mizokami Y, Kuga K, Horie M, Miwa Y, Iwawaki T, Yamamoto M, Shoda J. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci Rep. 2017;7:12902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |