Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4516
Peer-review started: January 14, 2022
First decision: April 12, 2022
Revised: May 14, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 28, 2022
Processing time: 223 Days and 11.5 Hours
Barrett’s esophagus (BE) is a condition that results from replacement of the damaged normal squamous esophageal mucosa to intestinal columnar mucosa and is the most significant predisposing factor for development of esophageal adenocarcinoma. Current guidelines recommend endoscopic evaluation for screening and surveillance based on various risk factors which has limitations such as invasiveness, availability of a trained specialist, patient logistics and cost. Trans-nasal endoscopy is a less invasive modality but still has similar limitations such as limited availability of trained specialist and costs. Non-endoscopic modalities, in comparison, require minimal intervention, can be done in an office visit and has the potential to be a more ideal choice for mass public screening and surveillance, particularly in patents at low risk for BE. These include newer generations of esophageal capsule endoscopy which provides direct visualization of BE, and tethered capsule endomicroscopy which can obtain high-resolution images of the esophagus. Various cell collection devices coupled with biomarkers have been used for BE screening. Cytosponge, in combination with TFF3, as well as EsophaCap and EsoCheck have shown promising results in various studies when used with various biomarkers. Other modalities including circulatory microRNAs and volatile organic compounds that have demonstrated favorable outcomes. Use of these cell collection methods for BE surveillance is a potential area of future research.
Core Tip: This review summarizes the non-endoscopic modalities available for the screening and surveillance of Barrett’s esophagus which include esophageal imaging devices (trans-nasal endoscopy, esophageal capsule, tethered capsule endomicroscopy), cell collection devices (Cytosponge, Esophacap, Esocheck), circulatory micro-RNAs and volatile organic compounds. There is promise using some of the noninvasive modalities for mass screening in BE and a role in surveillance is yet to be determined.
- Citation: Shahsavari D, Kudaravalli P, Yap JEL, Vega KJ. Expanding beyond endoscopy: A review of non-invasive modalities in Barrett’s esophagus screening and surveillance. World J Gastroenterol 2022; 28(32): 4516-4526
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4516.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4516
The esophagus is normally lined by stratified squamous epithelium. The mucosa of the esophagus is regularly exposed to gastric acid and bile through reflux from the stomach which can result in mucosal damage. The injury is usually repaired by regeneration of the squamous mucosa. However, in some patients, the mucosal damage is repaired resulting in a metaplastic columnar epithelium with gastric and intestinal features. The condition is termed Barrett’s esophagus (BE) and is recognized as the major risk factor for the development of esophageal adenocarcinoma (EAC)[1]. It is estimated that as many as 5.6% of United States adults have BE based on modeling using EAC rates[2]. Of note, endoscopic prevalence is widely variable based on geographic location of the population studied[3-6]. Current estimates are likely an underrepresentation given that upper endoscopy is required for diagnosis. Mean age at BE identification is approximately 55 years and is two to threefold more common in men than in women[7,8]. Among patients undergoing upper endoscopy, compared to Caucasians, African Americans have a significantly lower BE prevalence[9,10]. Accepted BE risk factors besides Caucasian race include age > 50, male, chronic (> 5 years) or frequent (> once weekly) gastroesophageal reflux disease (GERD), smoking, obesity (Body mass index > 35), and family history of BE or EAC (first degree relative)[11].
Endoscopy is currently the mainstay for BE diagnosis and management. An endoscopic approach is inappropriate for mass screening as it is not cost effective resulting in missed opportunities to discover patients with undiagnosed BE. Current screening guidelines target individuals with GERD and multiple risk factors for endoscopy to detect BE then enroll those with it into a surveillance program. However, this approach does not take into consideration that most EAC cases do not have a diagnosis of BE prior[12]. This provides an excellent opportunity for non-endoscopic techniques to be used as a public health tool for screening (identification of disease) and surveillance. Individuals belonging to the low risk BE strata are best suited for non-endoscopic modalities as they require minimal intervention whereas the high risk BE group would require more precise but also more invasive endoscopic based technologies[13]. Non-endoscopic techniques provide cost-effective, less invasive screening tools and more importantly increase the ease of accessibility to screening opportunities.
Screening for BE is recommended in patients with multiple known risk factors including chronic or frequent GERD, age greater than 50 years, male sex, Caucasian race, smoking, obesity along with family history of a 1st degree relative with BE or EAC. Currently, screening the general population with only GERD symptoms is not recommended per society guidelines[14,15].
Currently, high-definition white light endoscopy is the mainstay of BE screening and surveillance. Despite being safe and well-tolerated, it is invasive and associated with higher costs and side effects[16]. Trans-nasal endoscopy (TNE, Figure 1) has been proposed as a less invasive alternative to screen for BE. TNE is an ultra-thin endoscope (diameter < 6 mm) used in the outpatient setting to directly visualize the distal esophagus. This has occurred in primary care offices[17,18] and mobile vans for community BE screening[19]. Drawbacks of TNE are limited availability, cost, decontamination facility for reuse, and need for trained operators.
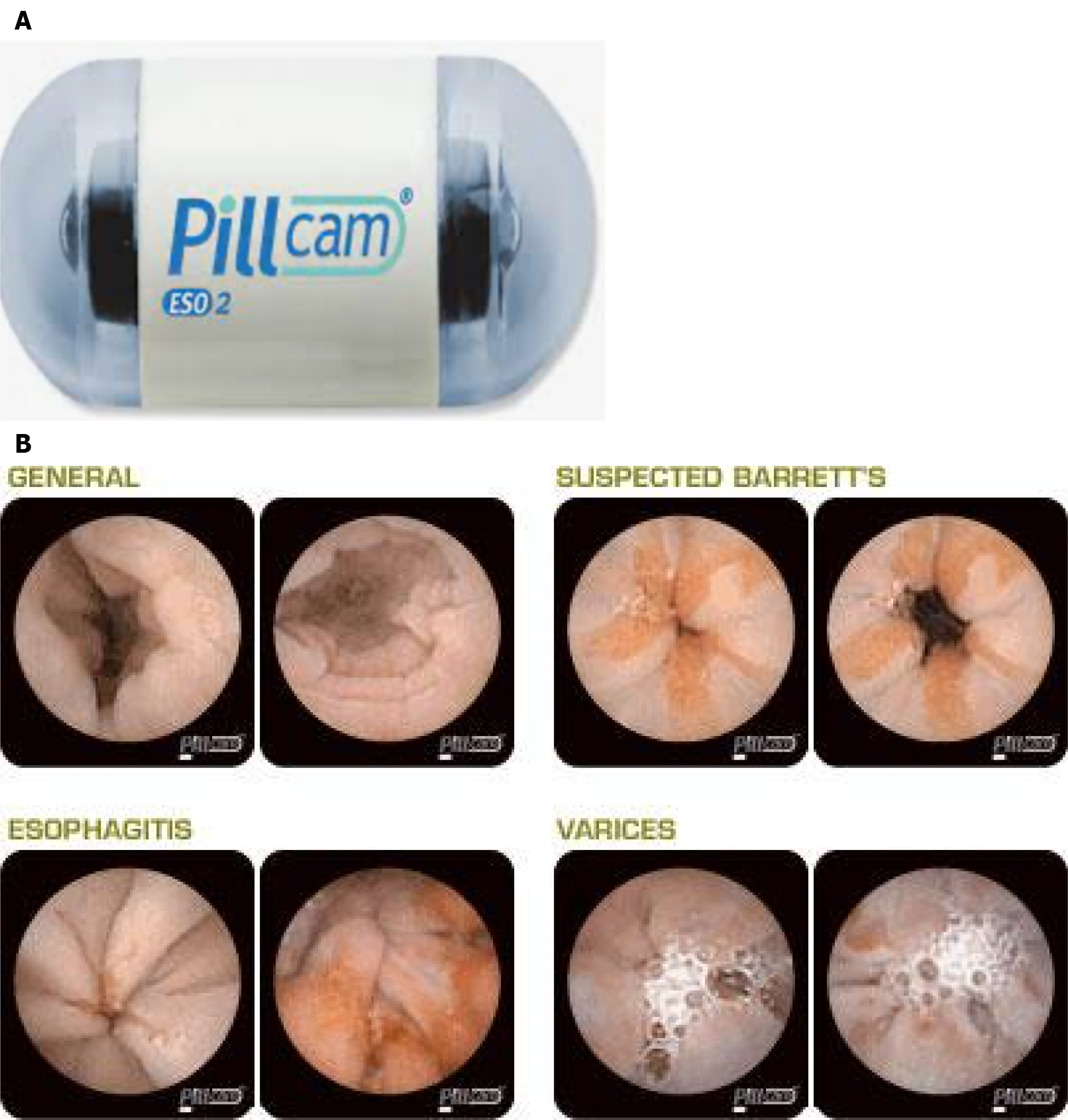
Esophageal capsule endoscopy (ECE), similar to small bowel capsule endoscopy, consists of a wireless capsule containing a camera, battery, and radio transmitter. Images are transmitted to a digital receiver and transferred to a computer for analysis[20]. A meta-analysis of 9 studies comprising of 618 patients showed a pooled sensitivity of 78% and specificity of 86% (EGD as the reference had sensitivity and specificity of 78% and 90% respectively)[21]. The suboptimal diagnostic accuracy is contributed to rapid esophageal transit time. Newer versions of ECE have been developed to overcome this issue and allow prolonged imaging. PillCam ESO (Medtronic Inc, Minneapolis, MN) was initially approved by Food and Drug Administration in 2004 and a second-generation device (PillCam ESO2, Figure 2A) with cameras at both ends of the capsule. The second-generation device captures images (PillCam ESO2, Figure 2B) at a rate of 18 frames per seconds (fps)[22]. A third-generation capsule (PillCam UGI) with a wider angle of view (174°) and higher recording rate (35 fps) is under investigation with pilot data suggesting inferiority to standard endoscopy regarding BE detection[23]. Another solution for the rapid esophageal transit issue is the detachable string magnetically controlled capsule endoscopy (also known as wireless magnetically controlled capsule endoscopy or WMCCE) which has been shown to be feasible and well tolerated in various studies[24-26]. A recent prospective multicenter study showed sensitivity of 92% and specificity of 80% for high-risk esophageal varices[27]. With regards to cost-effectiveness of ECE compared to traditional endoscopy, the results have been equivocal[28-30]. More studies are needed to determine of this modality is viable and cost-effective for BE screening.
Another variant of ECE is optical coherence tomography tethered capsule endomicroscopy (OTC-TCE, Figure 3) which uses optical-frequency domain imaging technology that rapidly acquires high-resolution, 3-dimensional, cross-sectional images of the entire esophagus[31,32]. In a proof-of-concept study of 13 subjects (7 normal volunteers and 6 with known BE), no complications were reported, and 12/13 patients reported preference of this method over conventional endoscopy[33]. The feasibility and safety of this method was further demonstrated in a recent multi-center study of 147 patients with known BE, and a blinded comparison of maximum extent of BE measured by OTC-TCE and EGD showed a strong correlation (r = 0.77-0.79, P < 0.05)[34]. In this study, high-quality microscopic images of the entire esophageal wall were obtained in the majority of the cases (93.7%). Larger prospective studies are needed to assess diagnostic yield and cost-effectiveness in the general population setting.
Various devices have been designed for esophageal cell collection. These samples can be analyzed cytologically and coupled with various biomarkers.
The Cytosponge (Medtronic, Minneapolis, MN, Figure 4) is a 30 mm polyurethane sponge, compressed withing a gelatin capsule and attached to a string[13]. Once patient swallows the capsule and it reaches the stomach, the capsule opens up and reveals the sponge. As the string is pulled back, the Cytosponge collects cells from the lining of the entire esophagus and oropharynx. Although several biomarkers have been used with Cytosponge including a multi-gene next-generation sequencing panel, differentially methylated genes, and microRNAs, the most well-established biomarker used with Cytosponge has been trefoil factor family protein 3 (TFF3) immunohistochemical staining[35-37].
In a multicenter case-control study of 11 United Kingdom hospitals (total subjects = 1,110; 463 dyspepsia controls and 647 BE patients), Cytosponge-TFF3 was performed prior to endoscopy[38]. BE was diagnosed with specificity of 92.4% and sensitivity of 79.9% (87.2% in patients with larger circumferential BE than 3 cm). Fitzgerald et al[39] in the BEST3 trial, a multicenter RCT study in 109 United Kingdom general practice clinics, demonstrated in patients with GERD symptoms, Cytosponge-TTF3 results in improved detection of BE, treatable dysplasia, and early cancer. In a systematic review of 13 studies, this method was shown to be cost-effective and well-tolerated through multiple patient populations[40]. Another patient-level review of 5 prospective trials assessing Cytosponge performance in patients with reflux disease, BE and eosinophilic esophagitis, also showed tolerability and safety of this device [41].
The advantage of Cytosponge is that it is not operator-dependent, it is quick, and does not require specialized equipment or extensive training, so it could easily be applied to a primary care setting[40].
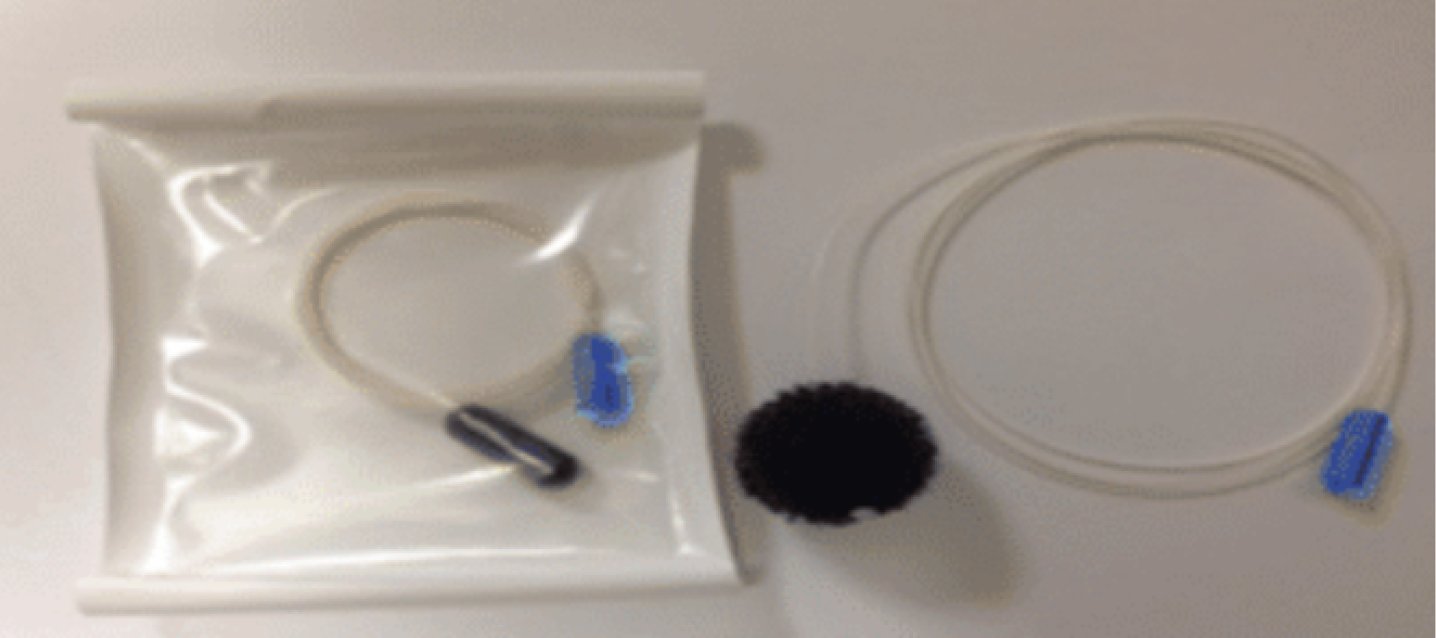
EsophaCap (CapNostics, Concord, NC, Figure 5) is a sponge on string device similar to Cytosponge, albeit smaller and softer[42]. EsophaCap has been used in a pilot trial using a panel of 2 methylated DNA markers (MDM), 3-VAV2 and zinc finger protein 682-ZNF682, on whole esophageal brushings (49 BE case and 36 controls), and in 40 subjects (20 BE cases and 20 controls) randomly assigned to swallow EsophaCap[42]. Overall, 80% of MDM candidates showed high accuracy for BE (AUCs 0.84-0.94) with sensitivity and specificity of 100%. The EsophCap was swallowed and withdrawn in 98% of subjects with no reported major complications, and 32% had minimal abrasions. More recently, in the same group conducted a multi-center case-cohort study of 268 subjects swallowed the capsule, but 201 met the inclusion criteria (112 cases and 89 controls) using the two previously mentioned MDMs and included 3 additional markers (NDRG4, FER1L4, and ZNF568)[43]. Cross-validated sensitivity and specificity were 92% and 94% respectively. EsophaCap was well tolerated in most patients (performed mostly by non-physicians) and 95% preferred the device over endoscopy. Currently a case-control trial is ongoing to identify potential biomarkers for the early detection of BE, esophageal carcinoma (both adenocarcinoma and squamous cell carcinoma) using EsophaCap (ClinicalTrial.gov ID: NCT04214119).
EsoCheck (Lucid Diagnostics, New York, NY, Figure 6) is a balloon-based sampling device which consists of a collapsible balloon attached to thin silicone catheter connected to a syringe[13]. Once EsoCheck is swallowed and is in the stomach, the balloon is inflated by injecting air into the catheter and withdrawn through the distal 3-6 cm of the esophagus, collecting epithelial cells. After sampling the area described above, the balloon is deflated which leads to its retraction into to the capsule, thereby protecting the sample from bio-contamination from the mid or proximal esophagus as well as oropharynx.
Several biomarkers including MDMs have been used with EsoCheck. In a pilot study, Moinova et al[44] performed a genome-wide screening and identified high-frequency methylation within the CCNA1 DNA locus. They tested CCNA1 and VIM DNA methylation (already an established BE biomarker) using EsoCheck in 173 individuals with or without BE and showed an AUC = 0.95 for discriminating metaplasia and neoplasia vs normal individuals for both, and with both biomarkers combined, the panel had a sensitivity of 95% and specificity of 91%. The results were replicated in an independent validation cohort of 149 subjects. The device was generally well-tolerated but 28 (18%) of subjects could not swallow the pill and 9% had poor DNA yield.
A new multi-center, single-arm trial is underway to study of the screening efficacy of a new generation EsoCheck device in combination with EsoGuard (2-marker MDM panel) in at risk population (ClinicalTrial.gov ID: NCT042293458).
MicroRNAs (miRNAs) are short (approximately 18-25 nucleotides in length) non-coding RNAs which regulate gene expression by binding to mRNAs to inhibit their translation or facilitate their degradation[45]. miRNAs play role in cell growth, differentiation and migration and can be dysregulated in malignancy[46]. Several miRNAs have been shown to be differently expressed in patients with BE. In a quantitative real-time PCR analysis of 60 disease/normal-paired tissues from 30 patients with esophagitis or BE, miR-143, miR-145, miR-194, and miR-215 were significantly higher, while miR-203 and miR-205 were lower in BE tissues[47]. Analysis on circulating miRNA levels confirmed that miR-194 and miR-215 were significantly upregulated in BE patients. Additionally, serum miR-130a was also shown to be elevated in BE and EAC patients[48]. Pavlov et al[49] in a study of 69 patients showed serum miR-320e and miR-199a-3p to be significantly lower in BE compared to patients with normal epithelium. Investigators have recently reported miR-4485-5p as a novel biomarker of esophageal dysplasia worthy of continued investigation[50]. These markers provide a non-invasive method, requiring only a patient’s peripheral blood sample and likely increasing acceptability and tolerability, but validation studies are needed in larger cohorts to demonstrate adequate sensitivity as well as specificity for widespread use.
Detection of cancer through exhaled breath using volatile organic compounds (VOC) has shown promising results for various cancers[51,52]. Two techniques have been used in patients with BE or esophageal cancer, which are gas chromatography-mass spectrometry and electronic nose (E-nose) apparatus[53-56]. The gas chromatography method was used to analyze exhaled breath samples from 81 patients (including 48 esophageal cancer patients) and 129 controls (including 16 patients with BE), and although this method was able to discriminate esophageal cancer from controls (AUC = 0.97), it was not able to identify patients with BE[56]. The drawback of this method is that it is costly and labor-intensive. The electronic nose apparatus, which consists of an array of 3 metal oxide sensors, uses a chemical to electrical interface to measure VOC profiles associated with various diseases and can be combined with machine learning[57]. In cross-sectional study of 122 patients with dysplastic BE, breath samples while in a fasting state were analyzed in real-time using the e-nose device[54]. Subjects were at various stages of treatment or surveillance. The data was introduced into an artificial neuronal network to discriminate differences in subjects stratified by the presence or absence of BE on biopsies. The test showed the ability to detect BE with a sensitivity of 82%, specificity of 80%, and accuracy of 81% (AUC = 0.79). In another proof-of-concept study of 402 patients (129 patients with BE and 141 patients with GERD symptoms), 5-minute breath samples were collected, and the test was able to identify BE patients with a sensitivity of 91% and specificity of 74%[53]. Other advantages of E-nose compared to the gas chromatography-mass spectrometry is cost and portability. More validation studies at the general population level are needed for use as a potential BE screening method.
Circulatory tumor DNA (CtDNA), miniscule amounts of fragmented DNA originating from tumor cells, has been proposed as a part of multi-cancer early detection (MCED) project. The Circulating Cell-free Genome Atlas trial in a prospective, case-controlled observational study showed that a blood-based MCED test using CtDNA in combination with machine learning could detect cancer signals for multiple cancer types and predict cancer signal origin with promising accuracy[58]. Most recently, a large multi-center MCED trial called PATHFINDER study began recruiting participants across 31 United States sites to examine DNA methylation patterns in blood samples to detect various cancers including esophageal cancers[59]. Since BE is a pre-malignant condition, more specific studies need to be conducted in the future to assess feasibility.
Patients with BE are currently recommended to enter a surveillance endoscopic program based on histological findings and degree of dysplasia[14,60]. This has been associated with a significant mental burden on these patients, presenting as various forms of anxiety and stress related to thoughts of disease progression as well as potential implications of the test, with many finding the program physically burdensome and intrusive[61]. This opens up opportunity for non-invasive surveillance options, which could even be performed in the setting of regular outpatient clinic visits. These modalities can potentially facilitate access to care for a larger population of patients and increase compliance similar to non-invasive tests for colorectal cancer screening programs[62]. As such, non-endoscopic cell collection methods have the potential for use in BE surveillance but so far, no studies have compared their use with conventional endoscopy for the purpose of BE surveillance, leaving this important area ripe for future investigation.
Despite advances in screening and surveillance programs for BE, their lack of efficiency is demonstrated by the fact that only one in ten cases of EAC is diagnosed within a surveillance program. Upper endoscopy remains the gold standard but barriers exist to function as an effective screening tool which include high cost, invasiveness, possible complications, need for a trained specialist, and patient desirability. Screening guidelines have been published by several societies that are based on risk factors and do not include the general population. Less invasive modalities have been proposed for BE screening (Table 1). TNE has been used in outpatient settings including offices and mobile medical vans with some success. ECE has shown promising results, and newer generations have attempted to circumvent the issue of rapid esophageal transit with higher frame rates, wider angle view, and magnetic control. OTC-TCE is capable in acquiring high-resolution, 3-dimensional cross-sectional esophageal images with safety and feasibility been demonstrated in multi-center studies. Various cell collection devices coupled with biomarkers have been used for BE screening. Cytosponge, in combination with TFF3, is a cost-effective and well-tolerated method. Similar devices including EsophaCap and EsoCheck have shown promising results when used with various biomarkers and multi-center large-scale trials are currently underway. Circulatory MicroRNAs have been proposed for BE screening as they are expressed differently in this population. VOC using gas chromatography-mass spectrometry and electronic nose have been used to identify BE or EAC with varying success. Finally, CtDNA is now being used as a part of “multi-cancer early detection” campaign which may play a role in detection of pre-cancer states such as BE. Using these non-invasive methods could also play a role as a surveillance tool once patients are identified with BE. Large future studies are desired to demonstrate the efficacy and feasibility for the BE surveillance population.
| Device name | Application | Detection method | Company | FDA approved | Commercially available |
| Esophageal capsule endoscopy (ECE) | Swallowed | Direct visualization | Given imaging | Yes (First generation: 2004) | Three generations (SB1, SB2, SB3) |
| Tethered capsule endomicroscopy (OTC-TCE) | Swallowed | Direct visualization | N/A | No | No |
| Cytosponge | Swallowed | Cell collection | Medtronic | Yes (2018) | Yes |
| Esophacap | Swallowed | Cell collection | PAVmed (previously CapNostics) | Yes (2021) | No |
| EsoCheck | Swallowed | Cell collection | PAVmed (Lucid Diagnostics) | Yes (2019) | Yes |
| Circulatory microRNAs | Blood sample | miRNA collection | N/A | No | No |
| Volatile organic compounds (VOC) | Breath sample | VOC collection | Aeonose (Netherlands) | No | No |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American College of Gastroenterology; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Deng K, China; Okasha H, Egypt; Spadaccini M, Italy S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and Cells of Origin of Barrett's Esophagus. Gastroenterology. 2019;157:349-364.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett's esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Hirota WK, Loughney TM, Lazas DJ, Maydonovitch CL, Rholl V, Wong RK. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 336] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 320] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Ormsby AH, Kilgore SP, Goldblum JR, Richter JE, Rice TW, Gramlich TL. The location and frequency of intestinal metaplasia at the esophagogastric junction in 223 consecutive autopsies: implications for patient treatment and preventive strategies in Barrett's esophagus. Mod Pathol. 2000;13:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Spechler SJ. Barrett's esophagus. Semin Gastrointest Dis. 1996;7:51-60. [PubMed] |
| 8. | Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett's esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol. 2008;6:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Khoury JE, Chisholm S, Jamal MM, Palacio C, Pudhota S, Vega KJ. African Americans with Barrett's esophagus are less likely to have dysplasia at biopsy. Dig Dis Sci. 2012;57:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Qumseya BJ, Bukannan A, Gendy S, Ahemd Y, Sultan S, Bain P, Gross SA, Iyer P, Wani S. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Wenker TN, Tan MC, Liu Y, El-Serag HB, Thrift AP. Prior Diagnosis of Barrett's Esophagus Is Infrequent, but Associated with Improved Esophageal Adenocarcinoma Survival. Dig Dis Sci. 2018;63:3112-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Yusuf A, Fitzgerald RC. Screening for Barrett's Oesophagus: Are We Ready for it? Curr Treat Options Gastroenterol. 2021;1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1053] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 15. | American Gastroenterological Association; Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 16. | Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Wilkins T, Gillies RA. Office-based ultrathin esophagogastroduodenoscopy in a primary care setting. J Am Board Fam Pract. 2004;17:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Cho S, Arya N, Swan K, Cirocco M, Kandel G, Kortan P, Marcon N. Unsedated transnasal endoscopy: a Canadian experience in daily practice. Can J Gastroenterol. 2008;22:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Sami SS, Dunagan KT, Johnson ML, Schleck CD, Shah ND, Zinsmeister AR, Wongkeesong LM, Wang KK, Katzka DA, Ragunath K, Iyer PG. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett's esophagus screening in the community. Am J Gastroenterol. 2015;110:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Fernandez-Urien I, Carretero C, Armendariz R, Muñoz-Navas M. Esophageal capsule endoscopy. World J Gastroenterol. 2008;14:5254-5260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett's esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Park J, Cho YK, Kim JH. Current and Future Use of Esophageal Capsule Endoscopy. Clin Endosc. 2018;51:317-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Duvvuri A, Desai M, Vennelaganti S, Higbee A, Gorrepati VS, Dasari C, Chandrasekar VT, Vennalaganti P, Kohli D, Sathyamurthy A, Rai T, Sharma P. Diagnostic accuracy of a novel third generation esophageal capsule as a non-invasive detection method for Barrett's esophagus: A pilot study. J Gastroenterol Hepatol. 2021;36:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Xiu H, Lu Y, Liu X, Liu F, Zhang L, Zhao C, Sun X. Detachable string magnetically controlled capsule endoscopy for complete observation of the upper gastrointestinal tract. Eur J Gastroenterol Hepatol. 2021;33:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Song J, Bai T, Zhang L, Xiang XL, Xie XP, Hou XH. Better view by detachable string magnetically controlled capsule endoscopy for esophageal observation: a retrospective comparative study. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Chen YZ, Pan J, Luo YY, Jiang X, Zou WB, Qian YY, Zhou W, Liu X, Li ZS, Liao Z. Detachable string magnetically controlled capsule endoscopy for complete viewing of the esophagus and stomach. Endoscopy. 2019;51:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Wang S, Huang Y, Hu W, Mao H, McAlindon ME, Liu Y, Yang L, Zhang C, Xu M, He C, Dang T, Wu B, Ji D, Zhang L, Mao X, Liu C, Xu D, Li Y, Li G, Han J, Lv F, Liang X, Jin S, Zhang S, Tai FWD, Xu Q, Yang C, Wang G, Wang L, Li B, Yang H, Xie P, Deng L, Ren L, Chang Z, Wang X, Wang S, Gao X, Li J, Zhu L, Wang F, Zhang G, Jiang X, Pan J, Meng W, Li X, Hou J, Dray X, Liao Z, Qi X. Detachable string magnetically controlled capsule endoscopy for detecting high-risk varices in compensated advanced chronic liver disease (CHESS1801): A prospective multicenter study. Lancet Reg Health West Pac. 2021;6:100072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Gerson L, Lin OS. Cost-benefit analysis of capsule endoscopy compared with standard upper endoscopy for the detection of Barrett's esophagus. Clin Gastroenterol Hepatol. 2007;5:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Rubenstein JH, Inadomi JM, Brill JV, Eisen GM. Cost utility of screening for Barrett's esophagus with esophageal capsule endoscopy vs conventional upper endoscopy. Clin Gastroenterol Hepatol. 2007;5:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Lin OS, Schembre DB, Mergener K, Spaulding W, Lomah N, Ayub K, Brandabur JJ, Bredfeldt J, Drennan F, Gluck M, Jiranek GC, McCormick SE, Patterson D, Kozarek RA. Blinded comparison of esophageal capsule endoscopy vs conventional endoscopy for a diagnosis of Barrett's esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc. 2007;65:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 31. | Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, Suter MJ, Chan RC, Evans JA, Jang IK, Nishioka NS, de Boer JF, Bouma BE. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Gora MJ, Quénéhervé L, Carruth RW, Lu W, Rosenberg M, Sauk JS, Fasano A, Lauwers GY, Nishioka NS, Tearney GJ. Tethered capsule endomicroscopy for microscopic imaging of the esophagus, stomach, and duodenum without sedation in humans (with video). Gastrointest Endosc. 2018;88:830-840.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS, Kava LE, Rosenberg M, Bouma BE, Tearney GJ. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19:238-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 34. | Dong J, Grant C, Vuong B, Nishioka N, Gao AH, Beatty M, Baldwin G, Baillargeon A, Bablouzian A, Grahmann P, Bhat N, Ryan E, Barrios A, Giddings S, Ford T, Beaulieu-Ouellet E, Hosseiny SH, Lerman I, Trasischker W, Reddy R, Singh K, Gora M, Hyun D, Quénéhervé L, Wallace M, Wolfsen H, Sharma P, Wang KK, Leggett CL, Poneros J, Abrams JA, Lightdale C, Leeds S, Rosenberg M, Tearney GJ. Feasibility and Safety of Tethered Capsule Endomicroscopy in Patients With Barrett's Esophagus in a Multi-Center Study. Clin Gastroenterol Hepatol. 2022;20:756-765.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Katz-Summercorn A, Anand S, Ingledew S, Huang Y, Roberts T, Galeano-Dalmau N, O'Donovan M, Liu H, Fitzgerald RC. Application of a multi-gene next-generation sequencing panel to a non-invasive oesophageal cell-sampling device to diagnose dysplastic Barrett's oesophagus. J Pathol Clin Res. 2017;3:258-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Chettouh H, Mowforth O, Galeano-Dalmau N, Bezawada N, Ross-Innes C, MacRae S, Debiram-Beecham I, O'Donovan M, Fitzgerald RC. Methylation panel is a diagnostic biomarker for Barrett's oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut. 2018;67:1942-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Li X, Kleeman S, Coburn SB, Fumagalli C, Perner J, Jammula S, Pfeiffer RM, Orzolek L, Hao H, Taylor PR, Miremadi A, Galeano-Dalmau N, Lao-Sirieix P, Tennyson M, MacRae S, Cook MB, Fitzgerald RC. Selection and Application of Tissue microRNAs for Nonendoscopic Diagnosis of Barrett's Esophagus. Gastroenterology. 2018;155:771-783.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Ross-Innes CS, Debiram-Beecham I, O'Donovan M, Walker E, Varghese S, Lao-Sirieix P, Lovat L, Griffin M, Ragunath K, Haidry R, Sami SS, Kaye P, Novelli M, Disep B, Ostler R, Aigret B, North BV, Bhandari P, Haycock A, Morris D, Attwood S, Dhar A, Rees C, Rutter MD, Sasieni PD, Fitzgerald RC; BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med. 2015;12:e1001780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 39. | Fitzgerald RC, di Pietro M, O'Donovan M, Maroni R, Muldrew B, Debiram-Beecham I, Gehrung M, Offman J, Tripathi M, Smith SG, Aigret B, Walter FM, Rubin G; BEST3 Trial team, Sasieni P. Cytosponge-trefoil factor 3 vs usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396:333-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 40. | Iqbal U, Siddique O, Ovalle A, Anwar H, Moss SF. Safety and efficacy of a minimally invasive cell sampling device ('Cytosponge') in the diagnosis of esophageal pathology: a systematic review. Eur J Gastroenterol Hepatol. 2018;30:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Januszewicz W, Tan WK, Lehovsky K, Debiram-Beecham I, Nuckcheddy T, Moist S, Kadri S, di Pietro M, Boussioutas A, Shaheen NJ, Katzka DA, Dellon ES, Fitzgerald RC; BEST1 and BEST2 study investigators. Safety and Acceptability of Esophageal Cytosponge Cell Collection Device in a Pooled Analysis of Data From Individual Patients. Clin Gastroenterol Hepatol. 2019;17:647-656.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Iyer PG, Taylor WR, Johnson ML, Lansing RL, Maixner KA, Yab TC, Simonson JA, Devens ME, Slettedahl SW, Mahoney DW, Berger CK, Foote PH, Smyrk TC, Wang KK, Wolfsen HC, Ahlquist DA. Highly Discriminant Methylated DNA Markers for the Non-endoscopic Detection of Barrett's Esophagus. Am J Gastroenterol. 2018;113:1156-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Iyer PG, Taylor WR, Johnson ML, Lansing RL, Maixner KA, Hemminger LL, Cayer FK, Yab TC, Devens ME, Slettedahl SW, Broderick BT, Mahoney DW, McGlinch MC, Berger CK, Foote PH, Giakomopoulos M, Allawi H, Smyrk TC, Wang KK, Katzka DA, Wolfsen HC, Burke JA, Ahlquist DA, Kisiel JB. Accurate Nonendoscopic Detection of Barrett's Esophagus by Methylated DNA Markers: A Multisite Case Control Study. Am J Gastroenterol. 2020;115:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Moinova HR, LaFramboise T, Lutterbaugh JD, Chandar AK, Dumot J, Faulx A, Brock W, De la Cruz Cabrera O, Guda K, Barnholtz-Sloan JS, Iyer PG, Canto MI, Wang JS, Shaheen NJ, Thota PN, Willis JE, Chak A, Markowitz SD. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 45. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27765] [Article Influence: 1322.1] [Reference Citation Analysis (0)] |
| 46. | Clark RJ, Craig MP, Agrawal S, Kadakia M. microRNA involvement in the onset and progression of Barrett's esophagus: a systematic review. Oncotarget. 2018;9:8179-8196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Cabibi D, Caruso S, Bazan V, Castiglia M, Bronte G, Ingrao S, Fanale D, Cangemi A, Calò V, Listì A, Incorvaia L, Galvano A, Pantuso G, Fiorentino E, Castorina S, Russo A. Analysis of tissue and circulating microRNA expression during metaplastic transformation of the esophagus. Oncotarget. 2016;7:47821-47830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Wang L, Ji F, Liu G, Wang W, Li Z, Yue Y, Wang Z. Upregulation of circulating miR130a is correlated with development of Barrett's esophagus and esophageal adenocarcinoma. Onco Targets Ther. 2019;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Pavlov K, Kluiver J, Meijer C, Boersma-van Ek W, Kruyt FAE, Karrenbeld A, Kleibeuker JH, Peters FTM, van den Berg A. Circulating miRNAs in patients with Barrett's esophagus, high-grade dysplasia and esophageal adenocarcinoma. J Gastrointest Oncol. 2018;9:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Craig MP, Rajakaruna S, Paliy O, Sajjad M, Madhavan S, Reddy N, Zhang J, Bottomley M, Agrawal S, Kadakia MP. Differential MicroRNA Signatures in the Pathogenesis of Barrett's Esophagus. Clin Transl Gastroenterol. 2020;11:e00125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (2)] |
| 51. | Hanna GB, Boshier PR, Markar SR, Romano A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:e182815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 52. | Krilaviciute A, Heiss JA, Leja M, Kupcinskas J, Haick H, Brenner H. Detection of cancer through exhaled breath: a systematic review. Oncotarget. 2015;6:38643-38657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 53. | Peters Y, Schrauwen RWM, Tan AC, Bogers SK, de Jong B, Siersema PD. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut. 2020;69:1169-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Chan DK, Zakko L, Visrodia KH, Leggett CL, Lutzke LS, Clemens MA, Allen JD, Anderson MA, Wang KK. Breath Testing for Barrett's Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology. 2017;152:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Markar SR, Wiggins T, Antonowicz S, Chin ST, Romano A, Nikolic K, Evans B, Cunningham D, Mughal M, Lagergren J, Hanna GB. Assessment of a Noninvasive Exhaled Breath Test for the Diagnosis of Oesophagogastric Cancer. JAMA Oncol. 2018;4:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 56. | Kumar S, Huang J, Abbassi-Ghadi N, Mackenzie HA, Veselkov KA, Hoare JM, Lovat LB, Španěl P, Smith D, Hanna GB. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann Surg. 2015;262:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 57. | Chan DK, Leggett CL, Wang KK. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J Gastroenterol. 2016;22:1639-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, Chung G, Clement J, Gao J, Hunkapiller N, Jamshidi A, Kurtzman KN, Seiden MV, Swanton C, Liu MC. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 517] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 59. | Nadauld LD, McDonnell CH 3rd, Beer TM, Liu MC, Klein EA, Hudnut A, Whittington RA, Taylor B, Oxnard GR, Lipson J, Lopatin M, Shaknovich R, Chung KC, Fung ET, Schrag D, Marinac CR. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 60. | ASGE Standards of Practice Committee; Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, Agrawal D, Buxbaum JL, Fishman DS, Gurudu SR, Jue TL, Kripalani S, Lee JK, Khashab MA, Naveed M, Thosani NC, Yang J, DeWitt J, Wani S; ASGE Standards of Practice Committee Chair. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335-359.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 61. | Britton J, Hamdy S, McLaughlin J, Horne M, Ang Y. Barrett's oesophagus: A qualitative study of patient burden, care delivery experience and follow-up needs. Health Expect. 2019;22:21-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Adler A, Geiger S, Keil A, Bias H, Schatz P, deVos T, Dhein J, Zimmermann M, Tauber R, Wiedenmann B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |