Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4471
Peer-review started: April 4, 2022
First decision: May 29, 2022
Revised: June 14, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 21, 2022
Processing time: 134 Days and 8.1 Hours
Irritable bowel syndrome (IBS) is an important health care concern. Alterations in the microbiota of the gut-brain axis may be linked to the pathophysiology of IBS. Some dietary intake could contribute to produce various metabolites including D-amino acids by the fermentation by the gut microbiota. D-amino acids are the enantiomeric counterparts of L-amino acids, in general, which could play key roles in cellular physiological processes against various oxidative stresses. Therefore, the presence of D-amino acids has been shown to be linked to the protection of several organs in the body. In particular, the gut microbiota could play significant roles in the stability of emotion via the action of D-amino acids. Here, we would like to shed light on the roles of D-amino acids, which could be used for the treatment of IBS.
Core Tip: The potential efficacy of D-amino acids for the treatment of irritable bowel syndrome is shown here.
- Citation: Ikeda Y, Taniguchi K, Sawamura H, Tsuji A, Matsuda S. Promising role of D-amino acids in irritable bowel syndrome. World J Gastroenterol 2022; 28(31): 4471-4474
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4471
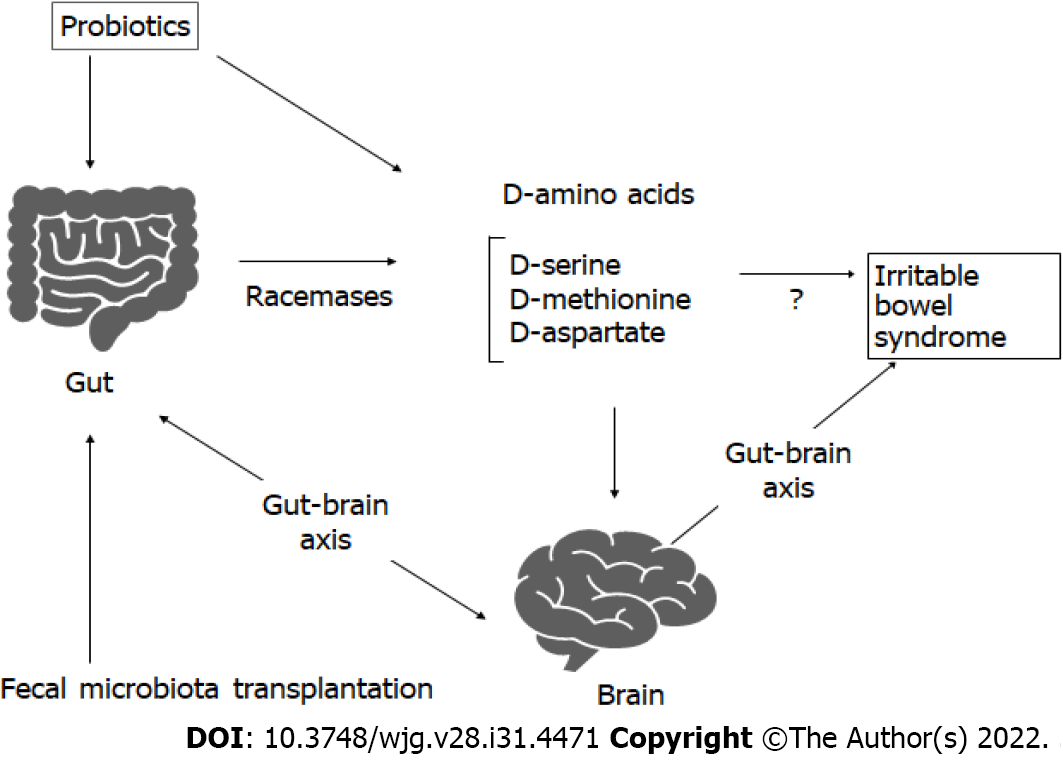
With great interest, we have read the article by Mamieva et al[1]. As irritable bowel syndrome (IBS) could exacerbate the patients’ quality of life, it is a considerable health care concern. Although the underlying pathophysiological mechanisms are not clear, the role of low-grade inflammation and mucosal immune activation appears to be obvious in the signs of IBS. IBS is a functional gastrointestinal disorder, and some probiotic supplementation may reduce the symptoms[2]. In addition, fecal microbiota transplantation expects recommendations for the treatment of IBS, suggesting that alterations in the gut microbiota-brain axis are linked to the pathophysiology of IBS (Figure 1). It has been revealed that some cytokines and neurotransmitters as well as several microbial metabolites including short chain fatty acids (SCFAs) such as acetate, lactate, butyrate, and propionate produced by the bacteria in the gut could modulate the integrity of brain function[1]. The bidirectional communication between the gut microbiota and the brain is well-known as the gut-brain axis, which could play an important role in the stability of emotion[3]. As shown in the article by Mamieva et al[1], the microbiota could influence the pathogenetic factors of IBS through the production of several microbial metabolites. Here, we would like to add the efficacy of D-amino acids for the alteration of IBS condition.
Mice treated with D-serine prior to the induction of colitis exhibited a reduction in the colonic inflammation that was not seen in mice fed L-serine[4]. In addition, D-serine efficiently suppressed the progression of chronic colitis. Therefore, D-serine might have effective properties as a preventive strategy and/or a treatment for colitis[4]. In addition, several studies have shown the significance of D-amino acids in clinical usage[5]. For example, D-methionine protects against the intestinal damage through anti-oxidative and anti-inflammatory effects, which could improve the gut microbiome imbalance by enhancing the growth of beneficial bacteria[6]. Protective effects of low-dose D-serine have been also shown to suppress the renal damage, which may promote the proliferation of kidney epithelial cells[7]. In addition, D-cysteine administration could defend the kidney from ischemia-reperfusion injury, which may be beneficial for the treatment of several renal diseases[8]. Gastro-protective effect with D-cysteine but not with L-cysteine has been shown via the effects of decreasing cellular damage, edema, and epithelium loss[9]. Treatment with D-aspartate may bring positive effects in the nervous system[10]. Furthermore, D-cycloserine is a glutamatergic N-methyl-D-aspartate receptor agonist which has been revealed to support the stability of emotion[11]. Furthermore, the activity of ovarian development with D-tryptophan is more effective than that with L-tryptophan[12]. These data suggest that D-amino acids could have beneficial and/or protective effects on various tissues, which might be favorable to the treatment of IBS (Figure 1). In particular, the emotional stability via the action of D-amino acids seems to be important[13], because it has been shown that different types of physiological and/or psychological stressors are known to contribute to the development, maintenance, and exacerbation of IBS[14].
The gut microbiota has a large genetic capacity to produce D-amino acids which are utilized as nutrients to support bacterial growth[15]. D-amino acids are essential elements of peptidoglycans in the cell wall of bacteria. Hence, higher levels of D-amino acids have been basically related to the mass of the gut microbiota[16]. Many bacterial species encode specific racemases that can convert L-amino acids to D-amino acids, which are frequently present in the peptidoglycan-containing bacteria in the gut microbiota[17]. Accordingly, the lumen of the gastro-intestinal tract in mammals may be rich in free D-amino acids that might be derived from such bacteria or fermented foods. Probably, the source of D-amino acids in mammals may mostly be from their gut microbiota. For example, D-alanine production is linked to the relative abundance of bacterial species such as Enterococcus and Lactobacillus in the gut microbiota[18]. Therefore, the metabolism of D-amino acids in the body might be modified by the alteration of gut bacterial communities affecting the host health and/or homeostasis[19]. Reduction of the amount of several D-amino acids may promote senescence through the increase of reactive oxygen species production[20,21].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gobin I; Ji G, China; Mamieva Z, Russia S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Mamieva Z, Poluektova E, Svistushkin V, Sobolev V, Shifrin O, Guarner F, Ivashkin V. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J Gastroenterol. 2022;28:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (6)] |
| 2. | Lee J, Park SB, Kim HW, Lee HS, Jee SR, Lee JH, Kim TO. Clinical Efficacy of Probiotic Therapy on Bowel-Related Symptoms in Patients with Ulcerative Colitis during Endoscopic Remission: An Observational Study. Gastroenterol Res Pract. 2022;2022:9872230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 4. | Asakawa T, Onizawa M, Saito C, Hikichi R, Yamada D, Minamidate A, Mochimaru T, Asahara SI, Kido Y, Oshima S, Nagaishi T, Tsuchiya K, Ohira H, Okamoto R, Watanabe M. Oral administration of D-serine prevents the onset and progression of colitis in mice. J Gastroenterol. 2021;56:732-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Müller C, Fonseca JR, Rock TM, Krauss-Etschmann S, Schmitt-Kopplin P. Enantioseparation and selective detection of D-amino acids by ultra-high-performance liquid chromatography/mass spectrometry in analysis of complex biological samples. J Chromatogr A. 2014;1324:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Wu CH, Ko JL, Liao JM, Huang SS, Lin MY, Lee LH, Chang LY, Ou CC. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther Adv Med Oncol. 2019;11:1758835918821021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, Miyake T, Sakai N, Kitajima S, Toyama T, Shinozaki Y, Sagara A, Miyagawa T, Hara A, Shimizu M, Kamikawa Y, Sato K, Oshima M, Yoneda-Nakagawa S, Yamamura Y, Kaneko S, Miyamoto T, Katane M, Homma H, Morita H, Suda W, Hattori M, Wada T. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Souza LK, Araújo TS, Sousa NA, Sousa FB, Nogueira KM, Nicolau LA, Medeiros JV. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric Oxide. 2017;64:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | de Rosa V, Secondo A, Pannaccione A, Ciccone R, Formisano L, Guida N, Crispino R, Fico A, Polishchuk R, D'Aniello A, Annunziato L, Boscia F. D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Levinson CA, Rodebaugh TL, Fewell L, Kass AE, Riley EN, Stark L, McCallum K, Lenze EJ. D-Cycloserine facilitation of exposure therapy improves weight regain in patients with anorexia nervosa: a pilot randomized controlled trial. J Clin Psychiatry. 2015;76:e787-e793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Kobayashi K, Maezawa T, Tanaka H, Onuki H, Horiguchi Y, Hirota H, Ishida T, Horiike K, Agata Y, Aoki M, Hoshi M, Matsumoto M. The identification of ᴅ-tryptophan as a bioactive substance for postembryonic ovarian development in the planarian Dugesia ryukyuensis. Sci Rep. 2017;7:45175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Kitagishi Y, Matsuda S. D-Amino Acids as a Biomarker in Schizophrenia. Diseases. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 16. | Sasabe J, Miyoshi Y, Rakoff-Nahoum S, Zhang T, Mita M, Davis BM, Hamase K, Waldor MK. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat Microbiol. 2016;1:16125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Cava F, Lam H, de Pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011;68:817-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 18. | Gilmore MS, Skaugen M, Nes I. Enterococcus faecalis cytolysin and lactocin S of Lactobacillus sake. Antonie Van Leeuwenhoek. 1996;69:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kawase T, Nagasawa M, Ikeda H, Yasuo S, Koga Y, Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr. 2017;117:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Nagano T, Yamao S, Terachi A, Yarimizu H, Itoh H, Katasho R, Kawai K, Nakashima A, Iwasaki T, Kikkawa U, Kamada S. d-amino acid oxidase promotes cellular senescence via the production of reactive oxygen species. Life Sci Alliance. 2019;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Canteros MG. D-Arginine as a neuroprotective amino acid: promising outcomes for neurological diseases. Drug Discov Today. 2014;19:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |