Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4201
Peer-review started: January 16, 2022
First decision: April 12, 2022
Revised: April 26, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 14, 2022
Processing time: 205 Days and 18.2 Hours
Previous meta-analyses, with many limitations, have described the beneficial nature of minimal invasive procedures.
To compare all modalities of esophagectomies to each other from the results of randomized controlled trials (RCTs) in a network meta-analysis (NMA).
We conducted a systematic search of the MEDLINE, EMBASE, Reference Citation Analysis (https://www.referencecitationanalysis.com/) and CENTRAL databases to identify RCTs according to the following population, intervention, control, outcome (commonly known as PICO): P: Patients with resectable esophageal cancer; I/C: Transthoracic, transhiatal, minimally invasive (thoracolaparoscopic), hybrid, and robot-assisted esophagectomy; O: Survival, total adverse events, adverse events in subgroups, length of hospital stay, and blood loss. We used the Bayesian approach and the random effects model. We presented the geometry of the network, results with probabilistic statements, estimated intervention effects and their 95% confidence interval (CI), and the surface under the cumulative ranking curve to rank the interventions.
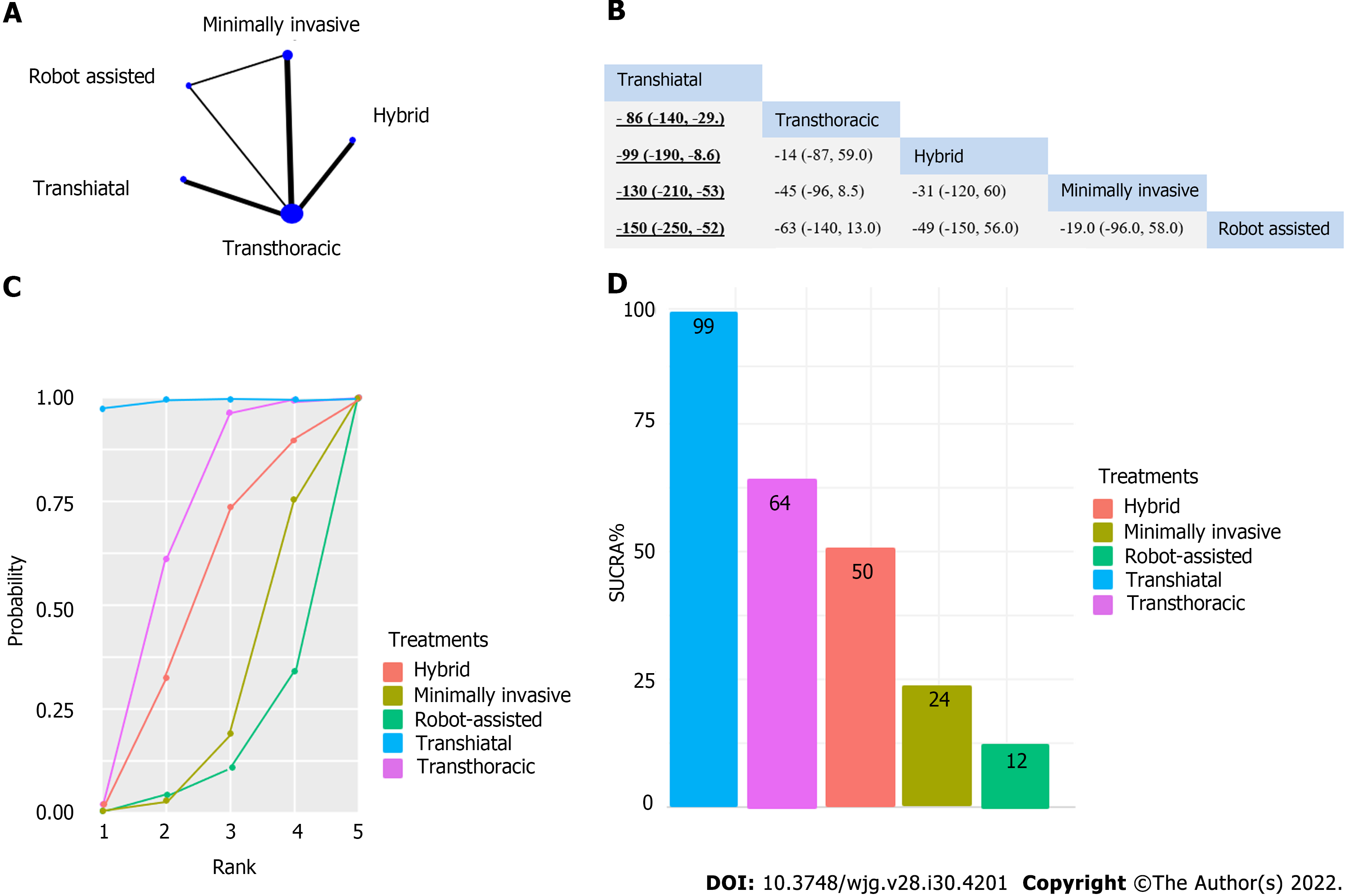
We included 11 studies in our analysis. We found a significant difference in postoperative pulmonary infection, which favored the minimally invasive intervention compared to transthoracic surgery (risk ratio 0.49; 95%CI: 0.23 to 0.99). The operation time was significantly shorter for the transhiatal approach compared to transthoracic surgery (mean difference -85 min; 95%CI: -150 to -29), hybrid intervention (mean difference -98 min; 95%CI: -190 to -9.4), minimally invasive technique (mean difference -130 min; 95%CI: -210 to -50), and robot-assisted esopha
Based on our results, the implication of minimally invasive esophagectomy should be favored.
Core Tip: Minimally invasive laparoscopic techniques should be the preferred approach for the treatment of esophageal cancer, due to the lower incidence of postoperative pulmonary complications.
- Citation: Szakó L, Németh D, Farkas N, Kiss S, Dömötör RZ, Engh MA, Hegyi P, Eross B, Papp A. Network meta-analysis of randomized controlled trials on esophagectomies in esophageal cancer: The superiority of minimally invasive surgery. World J Gastroenterol 2022; 28(30): 4201-4210
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4201
Esophageal cancer is the eighth most common type of cancer worldwide[1], with an incidence of 5.2 per 100000 for squamous cell cancer (SCC) and 0.7 per 100000 for adenocarcinoma (AC)[2]. While the prognosis varies between the two histological diagnoses, both AC and SCC are associated with poor clinical outcomes, with a 5-year survival rate of 20%[3].
Surgical therapy plays an essential role in the treatment of esophageal cancer. However, it cannot be routinely used due to the late diagnosis, as symptoms usually occur when the cancer is already unresectable[4]. Traditionally, open surgical interventions are performed, including transhiatal and transthoracic techniques. A meta-analysis comparing these two open surgical modalities did not find a significant difference in 5-year survival[5]. While both techniques are successful in terms of removing the neoplasm, open esophagectomies are associated with significant limitations, most importantly, postoperative morbidity[6,7].
A transition to non-open surgical techniques has been the trend in almost every field of surgery in recent years[8]. A wide variety of non-open techniques are available, including minimally invasive surgery (thoracolaparoscopic) surgery or even robot-assisted esophagectomy[9,10]. In the form of hybrid surgical intervention, a combination of open and non-open technique is available[11].
Previous meta-analyses have compared the different types of surgical techniques, with variable success and significant limitations[12-19]. To date, convincing evidence is missing regarding the optimal surgical approach of resectable esophageal cancer, as it is presented in a recent guideline[20].
Network meta-analysis (NMA) is a relatively novel methodology, which allows the direct and indirect comparison of multiple interventions, thus providing more information than traditional meta-analyses. Indirect comparisons can be made in the case of missing trials comparing two interventions if those are compared with a third intervention[21]. Several meta-analyses were carried out focusing on esophageal cancer surgery, but none of those addressed the problem of the wide variety of surgical techniques.
The purpose of our study was to provide objective evidence considering the surgical treatment of resectable esophageal cancer by comparing each treatment modality in the form of an NMA and possibly rank the different approaches.
The NMA was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-NMA guideline[22].
The NMA protocol was registered in advance in PROSPERO under the number CRD42020160978. Analyses of the mortality and quality of life could not be carried out due to the low number of reporting articles. The risk of bias was assessed using an updated risk assessment tool.
We conducted a systematic search of the MEDLINE (via PubMed), EMBASE, Reference Citation Analysis (https://www.referencecitationanalysis.com/) and Cochrane Central Register of Controlled Trials (CENTRAL) from initiation until 2019 November to identify studies, comparing at least two types of esophagectomies from transthoracic, transhiatal, hybrid, laparoscopic or robot-assisted approach treating esophageal cancer without the restriction of histological subtype and an NMA was performed. The following search key was used: (((esophagus OR oesophagus OR esophageal OR oesophageal) AND (tumor OR tumour OR malign* OR cancer OR adenocarcinoma OR carcinoma)) AND (esophagectomy OR oesophagectomy OR Ivor-Lewis OR „Ivor Lewis” OR hybrid OR laparoscop* OR „minimal invasive”)) AND random*. We also reviewed the reference lists of eligible articles for further studies. Only randomized controlled trials (RCTs) were included.
After the removal of duplications, two independent reviewers (Szakó L, Engh MA) executed the selection first by title, second by abstract, last by full text following pre-discussed aspects. Data extraction was done by the same two independent reviewers (Szakó L, Engh MA) onto a pre-established Excel worksheet (Office 365, Microsoft, Redmond, WA, United States). Extracted data consisted of the year of publication, name of the first author, study design, country, applied surgical modalities, mortality, overall survival rate (referred as survival), adverse events (AEs), blood loss, length of hospitalization, length of surgical procedure, and demographic data including age, male-female ratio, and SCC/AC ratio. Disagreements regarding both selection and data extraction were resolved by consensus. If consensus could not be reached, a third reviewer (Dömötör RZ) resolved the disagree
The Bayesian method was used to perform pairwise meta-analyses and NMAs. All analyses were carried out using a random effects model. To ensure the interpretability of the NMA results (pooled of direct and indirect data), we presented the geometry of the network, the results with probabilistic statements, and estimates of intervention effects along with their corresponding 95% confidence intervals (CIs), as well as forest plots for ranking the interventions, we chose to use the surface under the cumulative ranking (SUCRA) curve, which provides a numerical summary of the rank distribution of each treatment.
The risk of bias assessment was performed at the individual study level, according to the Revised Cochrane risk-of-bias tool for RCTs[23].
The Grading of Recommendations Assessment, Development, and Evaluation system was used to assess the certainty of evidence into four levels: high, moderate, low, and very low. The certainty of the evidence was classified into four levels: high, moderate, low, and very low. Two independent reviewers (Szakó L, Engh MA) decided the overall quality of the evidence[24]. Disagreements were resolved by consensus. If consensus could not be reached, a third reviewer (Dömötör RZ) resolved the disagreement.
The database search yielded 3335 records, of which 2002 articles were left after removing duplicates. Twenty-one full-text articles were screened for eligibility. Finally, we included 11 RCTs (25-35), including 1525 patients, in the quantitative synthesis (Figure 1). Baseline characteristics of the enrolled studies are presented in Table 1[25-35].
| Ref. | Year | Country | Design | Compared interventions | Number of patients | Male/female ratio | Age in yr, mean | Squamous cell cancer/adenocarcinoma ratio | Inclusion criteria |
| Straatman et al[25] | 2012 | Netherlands, Spain, Italy | Multicenter | MI-TT | 59-56 | 43/16-46/17 | 62.3–61.8 | 24/35-19/36 | cT1-3, N0-1, M0 |
| van der Sluis et al[26] | 2019 | Netherlands | Single center | RA-TT | 54-55 | 46/8-42/13 | 64-65 | 13/41-12/43 | T1-4a, N0-3, M0 |
| Mariette et al[27] | 2019 | France | Multi center | H-TT | 103-104 | 88/15-175/32 | 59-61 (median) | 46/57-84/123 | T1-3, N0-1, M0 |
| Guo et al[28] | 2013 | China | Single center | MI-TT | 111-110 | 68/43-72/38 | 57.3-60.8 | No information | T1-3, N0-1, M0 |
| Ma et al[29] | 2018 | China | Single center | MI-TT | 47-97 | 36/11-83/14 | 61-59.3 | 43/0-91/2 | Resectable cancer |
| Jacobi et al[30] | 1997 | Germany | Single center | TH-TT | 16-16 | No information | 54-55 | 13/3-13/3 | Resectable cancer |
| Goldminc et al[31] | 1993 | Australia | Single center | TH-TT | 32-35 | 31/1-33/2 | 57.4-57.4 | 32/0-35/0 | Resectable squamous cell cancer |
| Chu et al[32] | 1997 | China | Single center | TH-TT | 20-19 | 18/2-17/2 | 60.7-63.9 | No information | Lower third resectable cancer |
| Hulscher et al[33] | 2002 | Netherlands | Multicenter | TH-TT | 106-114 | 92/14-97/17 | 69-64 | 0/106-0/114 | Resectable adenocarcinoma |
| Yang et al[35] | 2016 | China | Single center | MI-TT | 120-120 | 82/38-87/33 | 62.5 -67.8 | 75/45-72/48 | T1-3, N0-1, M0 |
| Paireder et al[34] | 2018 | Austria | Single center | H-TT | 14-12 | 10/4-10/2 | 64.5-62.5 (median) | 4/10-1/11 | Siewert I-II, resectable squamous cell cancer |
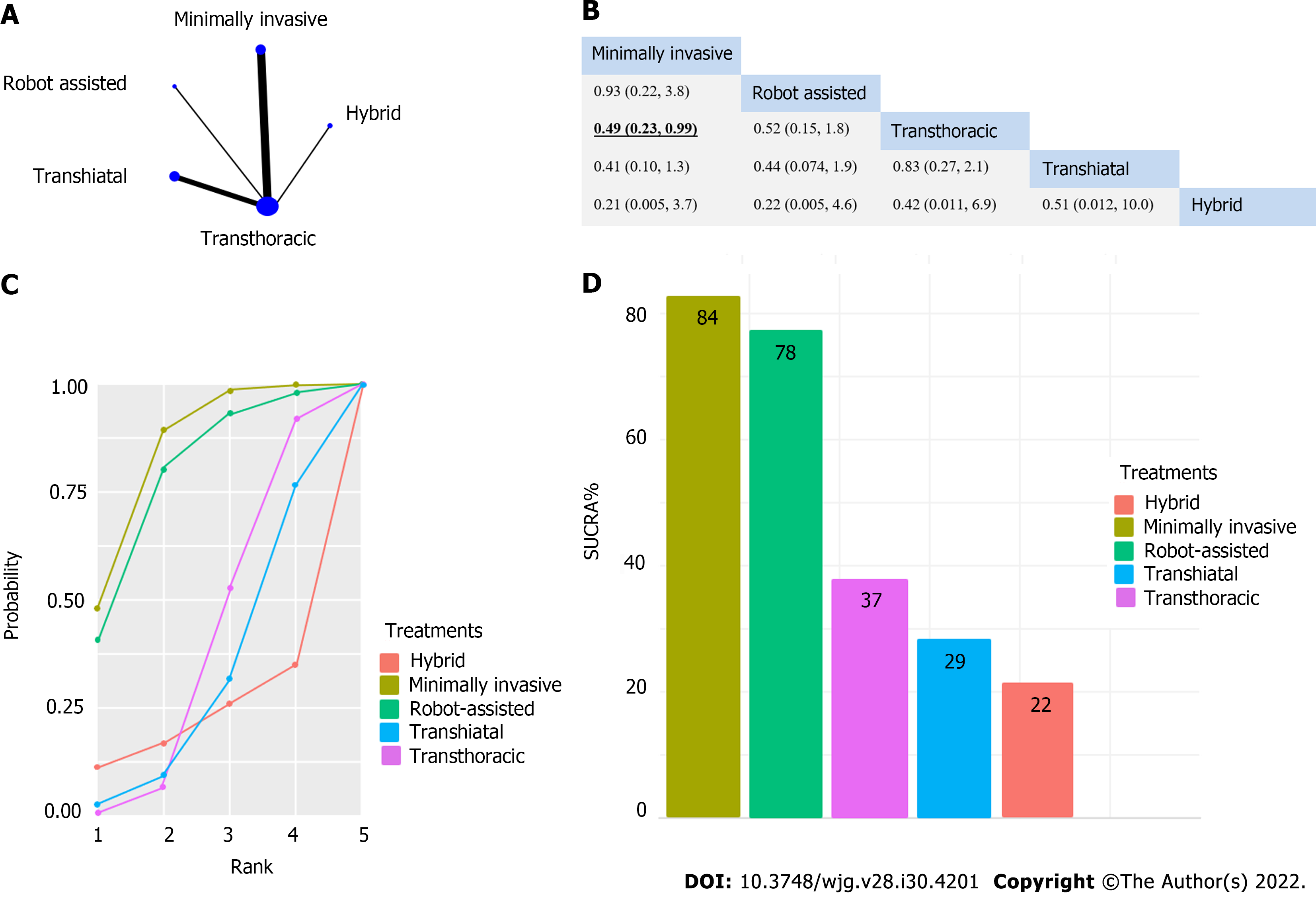
A significant difference was found for pulmonary infection, which favored the minimally invasive intervention compared to transthoracic surgery (relative risk [RR]: 0.49, 95%CI: 0.23-0.99) (Figure 2). Operation time was significantly shorter for the transhiatal approach compared to transthoracic surgery (mean difference: -86 min, 95%CI: -150 to -29 min), hybrid intervention (mean difference -99 min, 95%CI: -190 to -9.4 min), minimally invasive technique (mean difference -130 min, 95%CI: -210 to -53 min), and robot-assisted esophagectomy (mean difference -150 min, 95%CI: -250 to -52 min) (Figure 3). We did not find significant differences regarding survival (Supplementary Figures 1-5), total AEs (Supplemen
Results of the risk of bias assessment for the outcome of survival were assessed following the Cochrane Risk of Bias Assessment Tool 2. Details are shown in Table 2.
| Ref. | Randomization process | Deviation from intended intervention | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall |
| Straatman et al[25] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| van der Sluis et al[26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Mariette et al[27] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Guo et al[28] | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Ma et al[29] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | High risk |
| Jacobi et al[30] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | High risk |
| Goldminc et al[31] | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Chu et al[32] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | High risk |
| Hulscher et al[33] | Low risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Yang et al[35] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | High risk |
| Paireder et al[34] | Low risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
The results of the certainty of evidence are presented in Supplementary Table 1.
Our NMA confirmed the superiority of the minimally invasive esophagectomy over transthoracic open surgery regarding one of the main complications during these procedures, namely pulmonary infection. On the other hand, non-open surgical techniques require significantly more time to perform compared to open techniques. While statistically significant results were only achieved in the case of pulmonary infection, a clear tendency was demonstrated by the SUCRA curves, showing a preference for non-open techniques, which is also supported by the individual studies.
The results of previous meta-analyses and systematic reviews are not congruent regarding the comparison of minimally invasive and open surgical techniques. Kauppila et al[14] described the superiority of minimally invasive esophagectomy (MIE) regarding quality of life (QoL), which our work failed to analyze, as there were not enough RCTs reporting on QoL. Guo et al[13] also described the advantages of minimally invasive techniques regarding total complication rate, intraoperative blood loss, wound infection, and pulmonary infection, supporting our findings. MIE was also favorable in the analysis of Wang et al[19] considering blood loss. Besides blood loss and hospital stay, fewer respiratory complications were also shown by MIE in a meta-analysis conducted by Nagpal et al[15]. The work of Yibulayin et al[18] also supports the superiority of MIE in terms of in-hospital mortality and postoperative morbidity. By contrast, Dantoc et al[12] focused on oncological outcomes in their meta-analysis, where significant differences could not be proven. Sgourakis et al[17] showed that open surgery was more beneficial in terms of anastomotic stricture, while morbidity favored MIE. Oor et al[16] also described the benefit of open surgery in the case of hiatal hernia. The above comprehensive studies show that the inclusion of non-randomized studies carries a notable limitation.
Although the results of our analysis are only supportive in terms of pulmonary complication, the future perspectives are promising regarding minimally invasive esophagectomy, as the limelight shifts towards robot-assisted surgical techniques. The technique is time consuming, but with the development of new robotic platforms, the benefit of less AEs and more precise procedure will overcome this limitation[36]. The steep learning curve will be possibly managed by allowing the intervention to be carried out only in larger centers, as it has been seen in northern countries[37]. Despite the missing cumulative evidence, minimal invasive techniques have become the gold standard interventions for esophageal cancer since the TIME study. The results of this RCT provide evidence for using minimally invasive surgery for patients with resectable esophageal cancer aimed toward improving postoperative outcomes (especially pulmonary complication) and QoL with comparable oncologic results[25].
Considering the strengths of our analysis, by the inclusion of only RCTs, we managed to achieve a higher quality of evidence than previous works. Furthermore, a thorough methodology was applied. With the application of NMA, we were also able to make indirect comparisons. To date, this work is the most comprehensive review of the available RCTs.
One of the limiting factors of our study was the low number of cases and limited number of direct comparisons. Other limitations were the different enrollment criteria of the individual studies considering the histological subtype and stage of esophageal cancer. Furthermore, our analysis included many indirect comparisons, with weak direct comparisons. Additionally, we only included studies published until 2019.
We emphasize the application of MIE over open surgical techniques. Further analyses should focus on the outcomes of robot-assisted esophagectomies, and direct comparisons should be carried out between robot-assisted esophagectomy and thoracolaparoscopic intervention. Following recent trends, the centralization of upper gastrointestinal surgery is suggested, thus achieving the possibility of the implementation of such techniques without the limitation originating from the low number of cases and the learning curve of minimally invasive techniques.
While practice is already shifting towards the application of minimally invasive techniques, it should be noted that clear evidence is still needed to form guidelines. As we aimed to fill this void, we were only able to prove the beneficial nature of these techniques regarding pulmonary infection. To further assess any other potential differences between the techniques, RCTs and systematic analysis of these trials are needed.
The differences considering esophagectomies as the most applied curative methodology in the case of esophageal cancer are not clearly described. Minimally invasive techniques have become more popular in the belief of their superiority, although objective evidence is missing.
Recent guidelines are not yet clear considering the usage of minimally invasive esophagectomies. The authors wanted to provide the most objective evidence available, considering the differences between every subtype of minimally invasive and open esophagectomies.
The authors aimed to find every randomized controlled trial (RCT) providing comparative information about at least two types of esophagectomies, and pool the results using NMA.
After establishing our clinical question using the population, intervention, control, outcome (commonly known as (PICO) framework a systemic search was carried out using three different databases. The results of the search were pooled, duplications were removed, suitable studies were selected, from which the data extraction was carried out onto a data sheet. With the help of biostatisticians, a network meta-analysis was performed. The quality of the included studies was assessed, as well as the grade of evidence.
Eleven articles were included in our analysis, according to which the minimally invasive surgical technique was superior compared to the transthoracic open approach in terms of pulmonary infection, while transthoracic surgery took less time to perform than any other surgical technique.
The authors conclude that minimally invasive surgical techniques should be performed, whenever possible, for resectable esophageal cancer.
The conduction of additional RCTs evaluating the same problem would be welcomed, while we hope that our work will help clinicians in the decision-making of the selection of the right surgical technique.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Z, China; Scurtu RR, Romania; Tsujinaka S, Japan S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Cai YX
| 1. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 2. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1027] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 3. | Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin K A. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Available from: https://seer.cancer.gov/csr/1975_2016/. |
| 4. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 5. | Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta-analysis. Ann Surg. 2011;254:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10:71-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Morita M, Nakanoko T, Fujinaka Y, Kubo N, Yamashita N, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Shirabe K, Maehara Y. In-hospital mortality after a surgical resection for esophageal cancer: analyses of the associated factors and historical changes. Ann Surg Oncol. 2011;18:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Himal HS. Minimally invasive (laparoscopic) surgery. Surg Endosc. 2002;16:1647-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Levy RM, Wizorek J, Shende M, Luketich JD. Laparoscopic and thoracoscopic esophagectomy. Adv Surg. 2010;44:101-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | van Hillegersberg R, Seesing MF, Brenkman HJ, Ruurda JP. Robot-assisted minimally invasive esophagectomy. Chirurg. 2017;88:7-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Allaix ME, Long JM, Patti MG. Hybrid Ivor Lewis Esophagectomy for Esophageal Cancer. J Laparoendosc Adv Surg Tech A. 2016;26:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg. 2012;147:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 13. | Guo W, Ma X, Yang S, Zhu X, Qin W, Xiang J, Lerut T, Li H. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc. 2016;30:3873-3881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 14. | Kauppila JH, Xie S, Johar A, Markar SR, Lagergren P. Meta-analysis of health-related quality of life after minimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg. 2017;104:1131-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T. Is minimally invasive surgery beneficial in the management of esophageal cancer? Surg Endosc. 2010;24:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Oor JE, Wiezer MJ, Hazebroek EJ. Hiatal Hernia After Open versus Minimally Invasive Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23:2690-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Sgourakis G, Gockel I, Radtke A, Musholt TJ, Timm S, Rink A, Tsiamis A, Karaliotas C, Lang H. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55:3031-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol. 2016;14:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 19. | Wang B, Zuo Z, Chen H, Qiu B, Du M, Gao Y. The comparison of thoracoscopic-laparoscopic esophagectomy and open esophagectomy: A meta-analysis. Indian J Cancer. 2017;54:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50-v57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 21. | Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 577] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 22. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5279] [Article Influence: 527.9] [Reference Citation Analysis (1)] |
| 23. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15191] [Article Influence: 2531.8] [Reference Citation Analysis (0)] |
| 24. | Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A, Bousquet J, Guyatt GH, Schünemann HJ; GRADE Working Group. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 583] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 25. | Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, Rosman C, van Berge Henegouwen MI, Gisbertz SS, van der Peet DL. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg. 2017;266:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 26. | van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N, Mook S, Vleggaar FP, Borel Rinkes IHM, Ruurda JP, van Hillegersberg R. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg. 2019;269:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 27. | Mariette C, Markar S, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrere N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G; FRENCH, FREGAT. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg. 2020;271:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Guo M, Xie B, Sun X, Hu M, Yang Q, Lei Y. A comparative study of the therapeutic effect in two protocols: video-assisted thoracic surgery combined with laparoscopy versus right open transthoracic esophagectomy for esophageal cancer management. Zhongde Linchuang Zhongliuxue Zazhi. 2013;12:68-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Ma G, Cao H, Wei R, Qu X, Wang L, Zhu L, Du J, Wang Y. Comparison of the short-term clinical outcome between open and minimally invasive esophagectomy by comprehensive complication index. J Cancer Res Ther. 2018;14:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Jacobi CA, Zieren HU, Müller JM, Pichlmaier H. Surgical therapy of esophageal carcinoma: the influence of surgical approach and esophageal resection on cardiopulmonary function. Eur J Cardiothorac Surg. 1997;11:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Goldminc M, Maddern G, Le Prise E, Meunier B, Campion JP, Launois B. Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg. 1993;80:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 200] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Chu KM, Law SY, Fok M, Wong J. A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg. 1997;174:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 34. | Paireder M, Asari R, Kristo I, Rieder E, Zacherl J, Kabon B, Fleischmann E, Schoppmann SF. Morbidity in open versus minimally invasive hybrid esophagectomy (MIOMIE): Long-term results of a randomized controlled clinical study. Eur Surg. 2018;50:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Yang ZQ, Lu HX, Zhang JH, Wang J. Comparative study on long-term survival results between minimally invasive surgery and traditional resection for esophageal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2016;20:3368-3372. [PubMed] |
| 36. | van Boxel GI, Kingma BF, Voskens FJ, Ruurda JP, van Hillegersberg R. Robotic-assisted minimally invasive esophagectomy: past, present and future. J Thorac Dis. 2020;12:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Jeremiasen M, Linder G, Hedberg J, Lundell L, Björ O, Lindblad M, Johansson J. Improvements in esophageal and gastric cancer care in Sweden-population-based results 2007-2016 from a national quality register. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |