Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4152
Peer-review started: March 3, 2022
First decision: April 11, 2022
Revised: April 24, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 14, 2022
Processing time: 159 Days and 19.7 Hours
The emergence of restorative total proctocolectomy has significantly reduced the lifetime colorectal cancer risk associated with familial adenomatous polyposis (FAP). However, adenomas may develop in the ileal pouch over time and may even progress to carcinoma. We evaluated the cumulative incidence, time to development, and risk factors associated with ileal pouch adenoma.
To evaluate the cumulative incidence, time to development, and risk factors associated with pouch adenoma.
In this retrospective, observational study conducted at a tertiary center, 95 patients with FAP who underwent restorative proctocolectomy at our center between 1989 and 2018 were consecutively included. The mean follow-up period was 88 mo.
Pouch adenomas were found in 24 (25.3%) patients, with a median time of 52 mo to their first formation. Tubular adenomas were detected in most patients (95.9%). There were no high-grade dysplasia or malignancies. Of the 24 patients with pouch adenomas, 13 had all detected adenomas removed. Among the 13 patients who underwent complete adenoma removal, four (38.5%) developed recurrence. Among 11 (45.8%) patients with numerous polyps within the pouch, seven (63.6%) exhibited progression of pouch adenoma. The cumulative risks of pouch adenoma development at 5, 10, and 15 years after pouch surgery were 15.2%, 29.6%, and 44.1%, respectively. Severe colorectal polyposis (with more than 1000 polyps) was a significant risk factor for pouch adenoma development (hazard ratio, 2.49; 95% confidence interval: 1.04-5.96; P = 0.041).
Pouch adenomas occur at a fairly high rate in association with FAP after restorative proctocolectomy, and a high colorectal polyp count is associated with pouch adenoma development.
Core Tip: This is a retrospective study that evaluated the cumulative incidence and risk factors for pouch adenoma in association with familial adenomatous polyposis following restorative proctocolectomy. The incidence of pouch adenoma was 25.3%, and the cumulative risk 15 years after pouch surgery was 44.1%. Severe colorectal polyposis was a significant risk factor for pouch adenoma development. In our series, 62% of adenomas did not recur after endoscopic removal, but 63% of patients under observation showed progression. There was no spontaneous adenoma diminution or disappearance. Close endoscopic pouch surveillance is essential, and new pouch adenoma management guidelines are needed.
- Citation: Ryu HS, Yu CS, Kim YI, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Kim JC. Cumulative incidence and risk factors for pouch adenomas associated with familial adenomatous polyposis following restorative proctocolectomy. World J Gastroenterol 2022; 28(30): 4152-4162
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4152
Familial adenomatous polyposis (FAP) is a genetic syndrome caused by an adenomatous polyposis coli (APC) gene mutation that has an incidence of 1 in 10000 births[1]. This genetic variation is characterized by extensive polyposis in the colon and rectum, and patients with FAP have a lifetime colorectal cancer risk of 100% by the age of 35 to 40 years[2]. Hence, prophylactic surgical intervention is required for these individuals, and the currently favorable therapeutic option is a total proctocolectomy and ileal pouch-anal anastomosis (TPC/IPAA)[2]. TPC/IPAA increases life expectancy among patients with FAP by eliminating their metachronous colorectal cancer risk[3]. The postoperative disease course is determined by extracolonic manifestations, including duodenal adenoma and carcinoma, and desmoid tumors[4].
Cancers of the terminal ileum are extremely rare, and this region thus represents a far lower disease risk than the remaining rectal mucosa after ileorectal anastomosis. Notably, however, several studies have found a 35%-57% incidence of ileal pouch adenomas associated with FAP, which can progress to malignancy[5-11]. Given that most of these prior studies involved small sample sizes and were retrospective in nature, definitive results on these cancers are lacking, and the risk factors associated with pouch adenoma development remain to be established. Although these data are limited, the pouch adenoma incidence is reported to be quite high; therefore, long-term periodic surveillance is required. Most clinical guidelines recommend annual endoscopic surveillance of the anal transition zone (ATZ) mucosa after TPC/IPAA as a surgical management strategy for FAP patients. According to the National Comprehensive Cancer Network guidelines, this surveillance interval should be shortened to 6 mo if the adenoma is large or has advanced histology. However, there are currently no specific criteria directing more frequent surveillance. Moreover, with the currently implemented management approaches for pouch adenoma, uncertainty remains regarding the specific indications for endoscopic or surgical intervention[12-14]. More information is, therefore, needed regarding the incidence, natural course, and risk factors associated with pouch adenoma.
We investigated the cumulative incidence and time to development of pouch adenomas. In an FAP cohort after TPC/IPAA, we analyzed the clinical factors associated with pouch adenoma development.
Data on all patients with FAP who underwent pouch surgery at Asan Medical Center, Seoul, South Korea between November 1989 and December 2018 were identified from the hospital database. The FAP patients were identified by the presence of more than 100 colorectal adenomas. The indication for TPC/IPAA was FAP with or without malignancy. We excluded patients with attenuated FAP with fewer than 100 polyps (as per histopathology reports), as well as patients who did not receive TPC/IPAA. Demographic data, surgical details, original histopathology, and details of follow-up pouch endoscopic and pathologic findings were captured retrospectively. The definition of pouch adenoma included lesions that occurred in the ileum above the anastomosis site. Pouch adenoma progression was defined as an increase in the number or size of these lesions, as well as the development of dysplasia or malignancy, evident on histopathological examination. The severity of the duodenal polyposis in each case was assessed using the Spigelman classification[15] (Table 1). The study protocol was approved by the institutional review board of Asan Medical Center (registration No. 2021-0309) in accordance with the Declaration of Helsinki.
| Variable | |
| Age at time of IPAA [yr, median (IQR)] | 32 (24-41) |
| Sex, n (%) | |
| Male | 52 (54.7) |
| Female | 42 (45.3) |
| No. of colorectal polyps [n, median (IQR)] | 350 (150-700) |
| Type of anastomosis, n (%) | |
| Hand-sewn with mucosectomy | 72 (75.8) |
| Double stapling | 23 (24.2) |
| No. of surveillances [n, median (IQR)] | |
| Upper GI endoscopy | 5 (2-8) |
| Sigmoidoscopy | 5 (3-7) |
| Pouch adenomas, n (%) | 24 (25.3) |
| Time to pouch adenoma onset [mo, median (IQR)] | 52 (28.3-114.3) |
| ATZ adenomas, n (%) | 7 (7.4) |
| Gastric polyps, n (%) | 70 (73.7) |
| Duodenal adenomas, n (%) | 46 (48.5) |
| Spigelman stage, n (%) | |
| I | 24 (25.3) |
| II | 12 (12.6) |
| III | 10 (10.5) |
| IV | 0 |
| Missing data | 49 (51.6) |
| Desmoid tumors, n (%) | 23 (24.2) |
| Colorectal cancer at time of IPAA, n (%) | 43 (45.3) |
| Colon | 24 (55.8) |
| Rectum | 19 (44.2) |
An anal mucosectomy leaving a short rectal muscular cuff above the dentate line and transanal hand-sewn ileoanal anastomosis were performed for 72 of our enrolled FAP patients (75.8%). The remaining 23 patients (24.2%) underwent double-stapled anastomosis adjacent to the dentate line at the ATZ. The pouch construction in all patients was J-shaped using two ileal limbs of 15 cm in length.
The regular follow-up protocols for our study patients included clinical examinations, pouch and upper gastrointestinal endoscopy, and abdominoperineal computed tomography. Pouch endoscopy was performed within 1 postoperative year. Subsequently, for patients who underwent a mucosectomy, endoscopic examinations were performed once every 2 years in the absence of any polyps. If polyps were observed during endoscopic follow-up, they were removed regardless of size. In patients with multiple polyps, those larger than 5 mm in diameter were removed, or a biopsy specimen was collected. More intensive follow-up was performed, at intervals of 6-12 mo, if warranted by the size, number, or pathologic characteristics of identified polyps. Patients with colorectal cancer in our cohort were followed at 6-mo intervals for 5 years and annually thereafter. Additionally, testing for carcinoembryonic antigens was conducted every 6 mo, and chest computed tomography was performed annually.
The quantitative variables in this study are expressed as medians with interquartile ranges (IQRs) or ranges, and categorical variables are summarized as frequencies and percentages. The clinicopathological variables for the patients with and without pouch adenomas were compared using the Kruskal-Wallis test and Fisher’s exact test. The time to pouch adenoma formation was defined as the time from the date of surgery to the date of detection of the first histologically confirmed adenoma. Pouch adenoma–free survival was calculated using the Kaplan-Meier method. Multivariate Cox regression analysis based on backward elimination was used to assess the impact of variables on pouch adenoma–free survival with adjustment for variables reported to be associated with pouch adenoma–free survival in previous reports (colorectal polyp burden, time interval after IPAA, presence of desmoid tumors, and presence of gastric polyps and duodenal adenomas) in addition to basic patient characteristics, such as age and sex. Hazard ratios (HRs) with 95% confidence intervals (CIs) were also calculated. Statistical significance was established with a two-sided test at P < 0.05. All statistical analyses were performed using SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, United States).
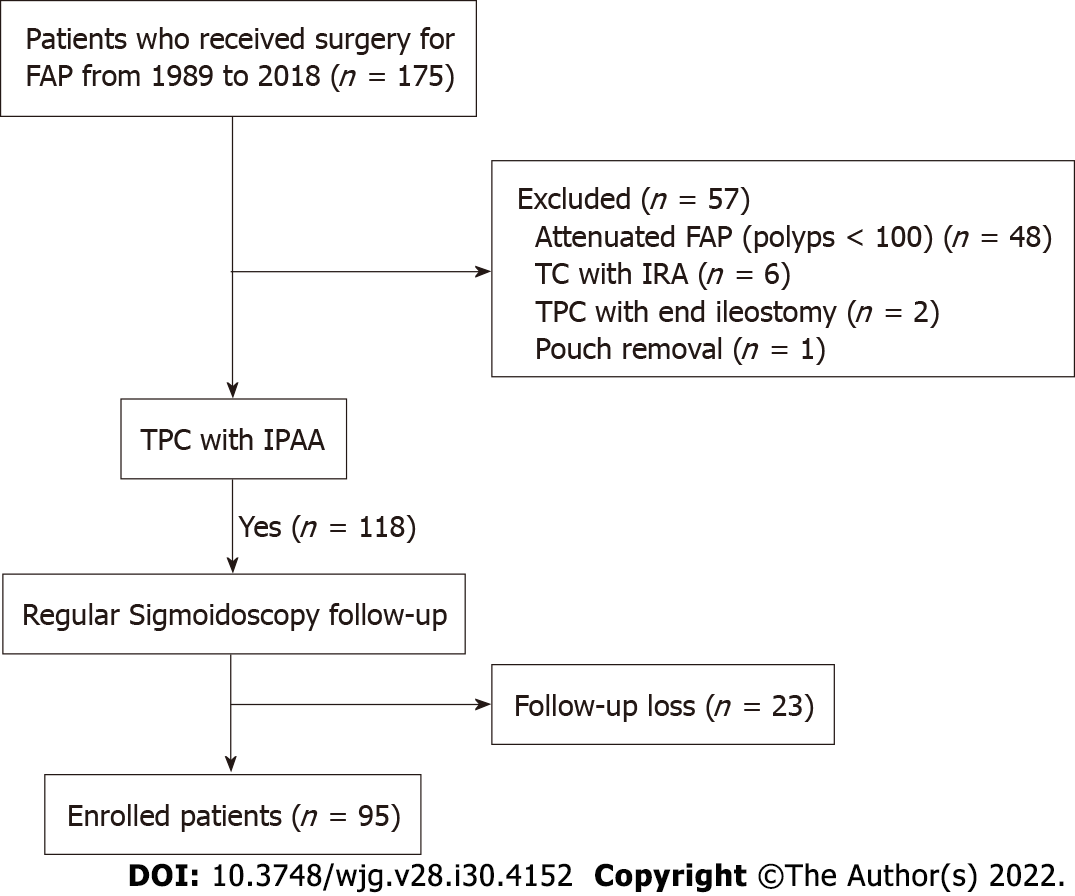
A total of 175 patients with FAP who underwent pouch surgery were identified from the hospital database. Among these patients, we excluded 48 with attenuated FAP with fewer than 100 polyps according to histopathology reports. We also excluded six patients who underwent total colectomy procedures with ileorectal anastomosis, two who underwent end-ileostomy without ileal pouches, and one whose pouch was removed due to postoperative bleeding. We further excluded 23 patients who did not undergo postoperative follow-up sigmoidoscopy. The final study cohort, thus, comprised 95 patients with FAP who underwent TPC/IPAA (Figure 1).
The characteristics of the 95 FAP patients enrolled in this study are summarized in Table 2. The median follow-up period after pouch surgery was 88 mo (IQR, 61-141 mo). The median age at the time of IPAA was 32 years (IQR, 24-41 years). The median number of follow-up endoscopies was 5 (IQR, 3-7). We observed that 24 (25.3%) of our study patients had adenomas that had been histologically confirmed in the pouch mucosa above the anastomosis. The median time to first pouch adenoma detection was 52 mo (IQR, 28.3-114.3 mo). Adenomas arising from the ATZ below the anastomosis were detected in seven (7.4%) patients. Upper gastrointestinal endoscopy was also performed for all patients. Gastric polyps were present in 70 (73.3%) patients; these were mainly fundic gland polyps (75.7%) as per biopsy results. Duodenal adenomas were confirmed in 46 (48.5%) patients. Twenty-four, 12, and 10 patients were classified as having Spigelman stages I, II, and III lesions, respectively, and there were no stage IV cases. Twenty-three (24.2%) patients developed desmoid tumors. At the time of surgery, 43 (45.3%) patients had colorectal cancer.
| Characteristic | Presence of pouch adenomas (n = 24) | Absence of pouch adenomas (n = 71) | P value |
| Age at time of IPAA [yr, median (IQR)] | 29 (20-40) | 32 (26-41) | 0.10 |
| Sex (male), n (%) | 16 (66.7) | 36 (50.7) | 0.17 |
| No. of colorectal polyps [n, median (IQR)] | 700 (325-1000) | 250 (110-500) | 0.001 |
| Colorectal polyps < 1000, n (%) | 16 (66.7) | 64 (90.1) | 0.006 |
| Colorectal polyps ≥ 1000, n (%) | 8 (33.3) | 7 (9.9) | |
| Time interval after IPAA [mo, median (IQR)] | 142 (106-199) | 116 (91-175) | 0.01 |
| Mucosectomy, n (%) | 19 (79.2) | 53 (74.6) | 0.66 |
| Gastric polyps, n (%) | 19 (79.2) | 51 (71.8) | 0.49 |
| Gastric polyp burden, n (%) | 0.31 | ||
| < 20 | 13 (54.2) | 41 (57.7) | |
| 20-49 | 0 | 11 (15.5) | |
| ≥ 50 | 6 (25.0) | 0 | |
| Duodenal adenomas, n (%) | 16 (66.7) | 30 (42.3) | 0.039 |
| Spigelman stage, n (%) | 0.30 | ||
| I | 6 (37.5) | 18 (60.0) | |
| II | 6 (37.5) | 6 (20) | |
| III | 4 (25.0) | 6 (20) | |
| Desmoid tumor, n (%) | 4 (16.7) | 19 (26.8) | 0.32 |
| NSAIDs use, n (%) | 1 (4.2) | 14 (19.7) | 0.07 |
| Colorectal cancer, n (%) | 9 (37.5) | 34 (47.9) | 0.38 |
Information on the underlying germline mutation was available for 40 (42.1%) FAP patients, among whom 28 (70.0%) harbored APC mutations, most commonly within exon 15 of the APC gene (64.3%). No significant difference was found in terms of the distribution of germline mutations between patients with and without pouch adenomas (P = 0.21).
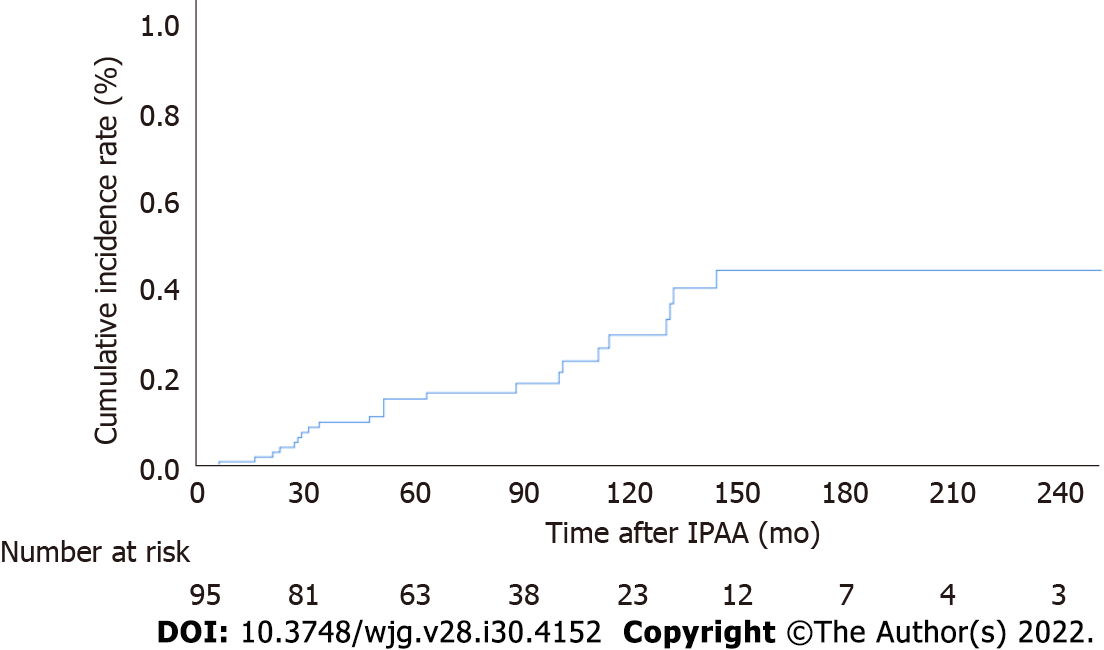
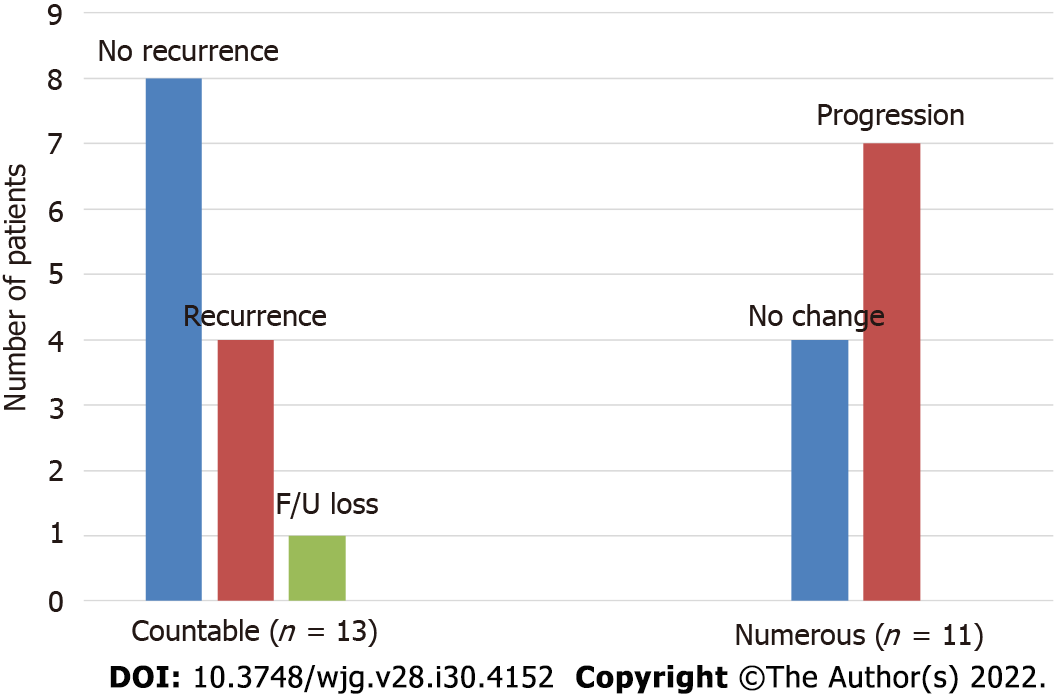
The cumulative incidences of pouch adenomas at 5, 10, and 15 years after IPAA were 15.2%, 29.6%, and 44.1% (95%CI: 7.2%-22.4%, 15.8%-41.1%, and 25.3%-58.2%), respectively (Figure 2). Among the 24 patients with pouch adenomas, 13 (54.2%) had fewer than 12 countable lesions, whereas others had numerous polyps within the pouch. All countable adenomas were removed endoscopically regardless of size. Only one patient had to undergo a transanal excision to remove a pouch adenoma 31 mm in diameter. The median value of the maximum diameter was 3 mm (range, 2.0-31.0 mm). Tubular adenomas were detected in 23 (95.9%) patients, and tubule-villous adenomas were detected in two (8.4%) patients. There were no cases of high-grade dysplasia or malignancy in our study sample. During follow-up for the 13 patients who underwent complete removal of all detected adenomas, eight (61.5%) patients had no recurrence, and four (38.5%) developed recurrent adenomas, which were endoscopically resected. The remaining patient in this group was lost to follow-up, and the disease course after adenoma removal was not documented. For the 11 (45.8%) patients with numerous polyps within the pouch, only those larger than 5 mm were removed, and surveillance biopsy specimens were collected. In four (36.4%) patients, the adenomas remained unchanged, while seven (63.6%) patients exhibited pouch adenoma progression (Figure 3).
Comparisons of the clinical characteristics among the FAP patients according to the presence of pouch adenomas are presented in Table 2. Compared with patients without adenomas, the pouch adenoma group had a significantly higher mean number of colorectal polyps at the time of surgery (700 vs 250, P = 0.001). Severe polyposis involving more than 1000 colorectal polyps was also significantly more common in the pouch adenoma group (P = 0.006). Additionally, patients with pouch adenomas (relative to those without pouch adenomas) were more likely to have duodenal adenomas (66.7% vs 42.3%, P = 0.039). There was no significant intergroup difference in the Spigelman adenoma stage distributions (P = 0.30) and no differences in the gastric polyp status (absent or present) or gastric polyp burden. Nonsteroidal anti-inflammatory drug (NSAID) treatment for desmoid tumors-including with celecoxib and meloxicam—was more common among patients without pouch adenomas, but this difference was not statistically significant (4.2% vs 19.7%, P = 0.07). There were no differences between the clinical characteristics of the study patients when stratified by the presence of colorectal cancer at the time of surgery (P = 0.38).
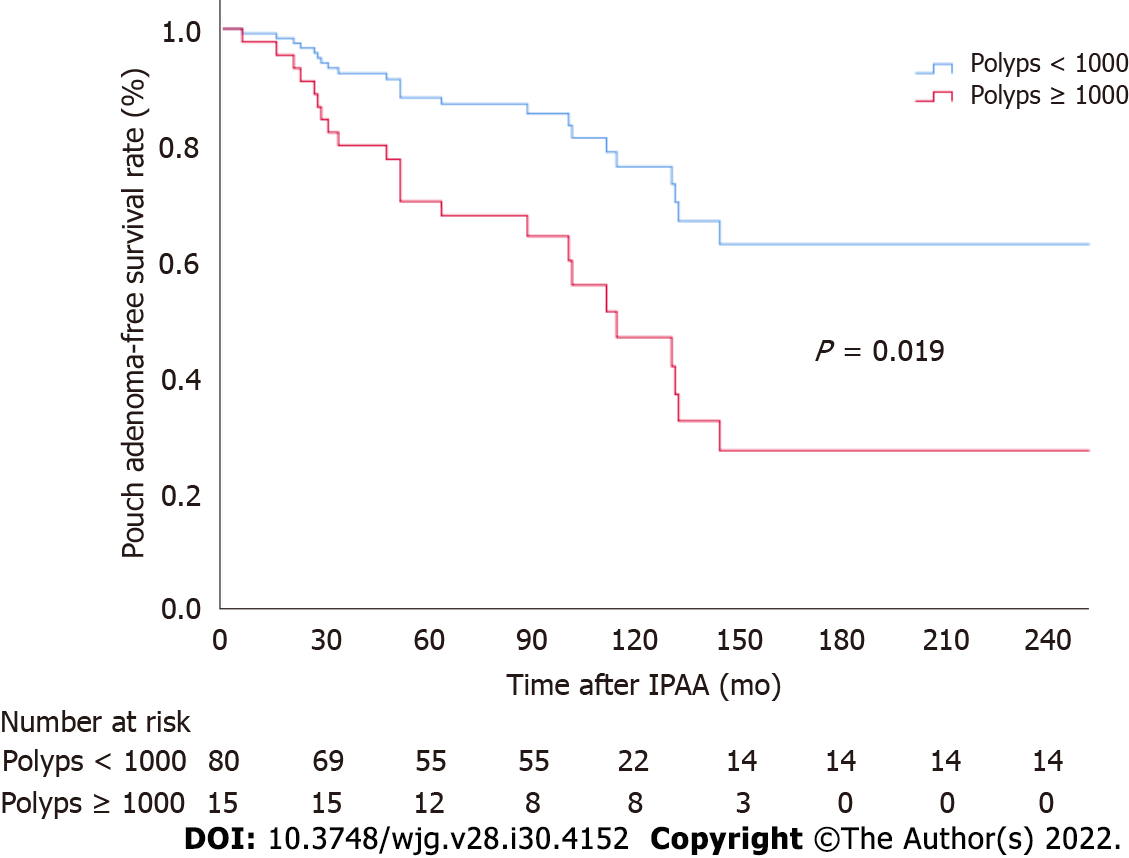
Associations between different patient factors and pouch adenoma–free survival were evaluated (Table 3). Among the clinical factors analyzed, multivariate analysis revealed severe colorectal polyposis (with more than 1000 polyps) to be a significant risk factor for pouch adenoma development (HR, 2.49; 95%CI: 1.04-5.96; P = 0.041) (Figure 4). The presence of gastric polyps or a duodenal adenoma had no significant association with pouch adenoma development (P = 0.28 and 0.54, respectively).
| Variable | Univariate analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.97 (0.93-1.01) | 0.12 | ||
| Sex (male) | 1.46 (0.62-3.44) | 0.39 | ||
| No. of colorectal polyps (≥ 1000) | 2.80 (1.18-6.63) | 0.019 | 2.49 (1.04-5.96) | 0.041 |
| Time interval after IPAA | 0.996 (0.99-1.003) | 0.29 | ||
| Mucosectomy | 1.54 (0.56-4.24) | 0.40 | ||
| Gastric polyps | 1.73 (0.64-4.68) | 0.28 | ||
| Duodenal adenoma | 2.31 (0.99-5.41) | 0.54 | 2.08 (0.88-4.93) | 0.10 |
| Spigelman stage | 0.15 | |||
| I-II | 2.27 (0.92-5.55) | 0.07 | ||
| III-IV | 2.46 (0.73-8.25) | 0.14 | ||
| Desmoid tumor | 0.82 (0.28-2.44) | 0.72 | ||
| NSAID use | 0.29 (0.04-2.13) | 0.22 | ||
| Colorectal cancer | 0.58 (0.25-1.37) | 0.21 | ||
TPC/IPAA is now being implemented as a standard treatment to eliminate the risk of colorectal cancer in patients with FAP. However, the postoperative development of adenomas in the ileal pouch in these individuals has raised new concerns about appropriate postoperative surveillance and management approaches. In this study cohort, the incidence of pouch adenoma was 25.3% at a median follow-up of 88 mo (IQR, 61-141 mo) after IPAA. The cumulative risk of pouch adenoma was 15.2%, 29.6%, and 44.1% at 5, 10, and 15 years after TPC/IPAA, respectively. There were no cases of high-grade dysplasia or carcinoma in our cohort. We found that a higher colorectal polyp burden at the time of surgery was positively associated with the occurrence of pouch adenoma formation (P = 0.001). These results are consistent with the findings reported in prior studies[9,10,16-18].
Several potential risk factors associated with pouch adenoma development have been investigated previously, but they remain controversial. Consistent with the findings of Tonelli et al[10], we found in our present analyses that a high colorectal polyp count at the time of IPAA was positively associated with pouch adenoma development. This result is likely to be assumed to be more aggressive disease. However, other authors have not observed an association between the severity of colonic disease and development of pouch adenoma[5].
Mutations of the APC gene predispose the carrier to benign polyps and malignancies in the upper gastrointestinal tract. Therefore, the risk of developing small bowel adenomas remains in these individuals even after TPC/IPAA. In this context, gastric or duodenal adenomas may be relevant to development of pouch adenoma. Ganschow et el[9] analyzed these variables previously and found that the presence of a gastric adenoma was a significant risk factor for developing a pouch adenoma (P = 0.0019). Several investigator groups have also reported that the presence of a duodenal adenoma is associated with pouch adenoma formation[10,16]. Tonelli et al[10] observed that the severity of duodenal polyposis was also relevant in this regard. As with the findings of other studies, our experience has been that patients who develop pouch adenomas are more likely to have had duodenal adenomas, although this was not observed to be a significant risk factor in our present multivariate analysis.
There has been little research conducted on the association between the site of an APC mutation and the occurrence of pouch adenoma. No such association has been identified in earlier studies[9,10,16], nor was such an association identified in our present analysis. However, in previous reports and in our present series, APC mutation data were not available for all subjects. Moreover, the low number of patients who underwent this mutation analysis in our present series and in prior study cohorts could not yield the statistical power needed to determine whether the APC mutation site is associated with the tendency to develop pouch adenomas. Additionally, a recently published study of genotype-phenotype associations between APC mutations and pouch adenomas found that patients with either indel/deletion mutations or exon 15 mutations had a higher tendency for pouch adenoma formation (P = 0.002 and 0.019, respectively)[19]. Hence, further larger-scale research is required to investigate the association between underlying germline mutations and pouch adenoma development.
Several authors have implicated colonic metaplasia of the ileal mucosa—an adaptive response of the neorectum—as a predisposing condition to the onset of ileal adenoma development[20]. Advanced adenomas with high-grade dysplasia were observed in about 18% of previous study samples[5,18]. However, no case of an advanced adenoma was detected in our present study cohort, possibly because adenomas were aggressively removed from our patients, even if small. If ileal pouch adenomas progress to carcinoma, following the classic adenoma-carcinoma sequence, a higher number and larger size of polyps may be associated with the severity of the dysplasia. Tajika et al[18] reported that the pos
Clinically, the treatment of pouch adenomas depends on the number, size, shape, and histological features—as for the gastric or duodenal polyps. According to the American Society for Gastrointestinal Endoscopy and British Society of Gastroenterology guidelines, all types of gastric or duodenal polyps detected via endoscopy need to be sampled using biopsy forceps for evaluation[21-23]. In the case of adenomatous polyps, these guidelines recommend complete endoscopic removal when it is safe to do so, given the higher probability of malignant transformation[22,23]. Endoscopic follow-up should be repeated at 6 mo for incompletely resected polyps or for those with high-grade dysplasia; it can be conducted again at 12 mo for all other polyps[22]. Although the data are currently limited, 62% of our cohort did not develop adenoma recurrence after adenoma removal, whereas 63% of our patients under observation showed progression. There was no spontaneous diminution or disappearance of any adenoma in our study cohort. Hence, it is important to be aware of the risk of pouch neoplasia when caring for FAP patients after IPAA, and—to enable evaluation and treatment at the same time—we recommend endoscopic resection when the adenoma is detected. If numerous polyps are present, random biopsy sampling is required, and polyps larger than 5 mm should be removed.
Interestingly, our FAP patients who used NSAIDs as a treatment for desmoid tumors rarely developed pouch adenomas. This finding is presumed to be related to the chemopreventive effect of these drugs. Chemoprevention with NSAIDs is a treatment option considered to facilitate the mana
There were some limitations to this study. First, this was a retrospective cohort study with a small sample size, and there may have been unknown confounders. However, as we were investigating a rare disease, there have been few studies to date that have analyzed a substantial number of affected patients. Therefore, in relative terms, our sample size was large, and the patients were followed for a considerable length of time. A second limitation was that whereas all of the patients had a clinical phenotype of 100 or more colorectal polyps and biopsy-confirmed FAP, APC genetic mutation analysis was only performed for about 50% of the patients. This limited the strength of our findings related to these mutations. Additionally, there was no consistent endoscopic treatment standard in our cohort. When a polyp was countable, it was removed regardless of its size. In patients with multiple polyps, however, the individual judgment of the endoscopist determined whether to perform endoscopic resection or observation. The indications used for polyp removal were, therefore, not clear.
Overall, our study findings provide further evidence for the need for standardized endoscopic surveillance of FAP patients following TPC/IPAA. Pouch endoscopy should be performed yearly for these patients, and standardized biopsy and removal protocols are needed if pouch adenomas are observed. Furthermore, surveillance should be tailored depending on the presence and characteristics of the pouch adenoma. In the future, validation of whether an annual endoscopic interval is appropriate is required, as is an evaluation of the feasibility and effectiveness of endoscopic resection of a pouch adenoma.
In conclusion, pouch adenomas occur at a fairly high rate over time in association with FAP after IPAA. A high colorectal polyp count increases the risk of developing a pouch adenoma in this patient population. In our experience, the progression of pouch adenomas to high-grade dysplasia or carcinoma is rare. However, this risk is not negligible, and the long-term risk of this cannot presently be well quantified. Close surveillance of the pouch should thus be mandatory, and new guidelines for the management of pouch adenomas are essential.
Restorative total proctocolectomy is now being implemented as a standard treatment to eliminate the risk of colorectal cancer in patients with familial adenomatous polyposis (FAP). However, the postoperative development of adenomas in the ileal pouch in these individuals has raised new concerns about appropriate postoperative surveillance and management approaches.
More information is needed regarding the incidence, natural course, and risk factors associated with pouch adenoma.
To investigate the cumulative incidence and time to development of pouch adenomas and analyze the clinical factors associated with pouch adenoma development among patients with FAP after restorative proctocolectomy.
A retrospective cohort study was carried out with 95 consecutive patients with FAP who underwent restorative proctocolectomy at Asan Medical Center (Seoul, South Korea) from November 1989 to December 2018.
The cumulative risks of pouch adenoma development at 5, 10, and 15 years after pouch surgery were 15.2%, 29.6%, and 44.1%, respectively. Severe colorectal polyposis (with more than 1000 polyps) was a significant risk factor for pouch adenoma development (hazard ratio, 2.49; 95% confidence interval: 1.04-5.96; P = 0.041).
We recommend endoscopic resection when the adenoma is detected. If numerous polyps are present, random biopsy sampling is required, and polyps larger than 5 mm should be removed. Close surveillance of the pouch should be mandatory, and new guidelines for the management of pouch adenomas are required.
In the future, validation of whether an annual endoscopic interval is appropriate is required, as is an evaluation of the feasibility and effectiveness of endoscopic resection of a pouch adenoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Leo A, Italy; M'Koma AE, United States; Roncucci L, Italy; Sulbaran MN, Brazil S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 295] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Nieuwenhuis MH, Mathus-Vliegen LM, Slors FJ, Griffioen G, Nagengast FM, Schouten WR, Kleibeuker JH, Vasen HF. Genotype-phenotype correlations as a guide in the management of familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2007;5:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Koskenvuo L, Ryynänen H, Lepistö A. Timing of prophylactic colectomy in familial adenomatous polyposis. Colorectal Dis. 2020;22:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Friederich P, de Jong AE, Mathus-Vliegen LM, Dekker E, Krieken HH, Dees J, Nagengast FM, Vasen HF. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Pommaret E, Vienne A, Lefevre JH, Sogni P, Florent C, Desaint B, Parc Y. Prevalence and risk factors for adenomas in the ileal pouch and the afferent loop after restorative proctocolectomy for patients with familial adenomatous polyposis. Surg Endosc. 2013;27:3816-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Zahid A, Kumar S, Koorey D, Young CJ. Pouch adenomas in Familial Adenomatous Polyposis after restorative proctocolectomy. Int J Surg. 2015;13:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Boostrom SY, Mathis KL, Pendlimari R, Cima RR, Larson DW, Dozois EJ. Risk of neoplastic change in ileal pouches in familial adenomatous polyposis. J Gastrointest Surg. 2013;17:1804-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ganschow P, Trauth S, Hinz U, Schaible A, Büchler MW, Kadmon M. Risk Factors Associated With Pouch Adenomas in Patients With Familial Adenomatous Polyposis. Dis Colon Rectum. 2018;61:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Tonelli F, Ficari F, Bargellini T, Valanzano R. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012;55:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Tajika M, Niwa Y, Bhatia V, Tanaka T, Ishihara M, Yamao K. Risk of ileal pouch neoplasms in patients with familial adenomatous polyposis. World J Gastroenterol. 2013;19:6774-6783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Engel C, Frayling I, Friedl W, Hes FJ, Hodgson S, Järvinen H, Mecklin JP, Møller P, Myrhøi T, Nagengast FM, Parc Y, Phillips R, Clark SK, de Leon MP, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen J. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 13. | National Comprehensive Cancer Network. NCCN Genetic/Familial High-Risk Assessment: Colorectal. 2020. Available from: http://www.nccn.org/. |
| 14. | Stjepanovic N, Moreira L, Carneiro F, Balaguer F, Cervantes A, Balmaña J, Martinelli E; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1558-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 474] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Parc YR, Olschwang S, Desaint B, Schmitt G, Parc RG, Tiret E. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001;233:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | van Duijvendijk P, Vasen HF, Bertario L, Bülow S, Kuijpers JH, Schouten WR, Guillem JG, Taat CW, Slors JF. Cumulative risk of developing polyps or malignancy at the ileal pouch-anal anastomosis in patients with familial adenomatous polyposis. J Gastrointest Surg. 1999;3:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tajika M, Tanaka T, Ishihara M, Hirayama Y, Oonishi S, Mizuno N, Kuwahara T, Okuno N, Matsumoto S, Ooshiro T, Kinoshita T, Komori K, Bhatia V, Hara K, Yatabe Y, Niwa Y. Long-term outcomes of metachronous neoplasms in the ileal pouch and rectum after surgical treatment in patients with familial adenomatous polyposis. Endosc Int Open. 2019;7:E691-E698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Kariv R, Rosner G, Fliss-Isakov N, Gluck N, Goldstein A, Tulchinsky H, Zelber-Sagi S. Genotype-Phenotype Associations of APC Mutations With Pouch Adenoma in Patients With Familial Adenomatous Polyposis. J Clin Gastroenterol. 2019;53:e54-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Corfield AP, Warren BF, Bartolo DC, Wagner SA, Clamp JR. Mucin changes in ileoanal pouches monitored by metabolic labelling and histochemistry. Br J Surg. 1992;79:1209-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B; British Society of Gastroenterology. The management of gastric polyps. Gut. 2010;59:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Yang J, Gurudu SR, Koptiuch C, Agrawal D, Buxbaum JL, Abbas Fehmi SM, Fishman DS, Khashab MA, Jamil LH, Jue TL, Law JK, Lee JK, Naveed M, Qumseya BJ, Sawhney MS, Thosani N, Wani SB, Samadder NJ. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020;91:963-982.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 24. | Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |