Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4089
Peer-review started: April 4, 2022
First decision: May 29, 2022
Revised: June 16, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 14, 2022
Processing time: 128 Days and 7.6 Hours
The healthcare burden of inflammatory bowel disease (IBD) is rising globally and there are limited non-invasive biomarkers for accurate and early diagnosis.
To understand the important role that intestinal microbiota play in IBD patho
We performed serological profiling of 100 Crohn’s disease (CD) patients, 100 ulcerative colitis (UC) patients and 100 healthy controls against 1173 bacterial and 397 viral proteins from 50 bacteria and 33 viruses on protein microarrays. The study subjects were randomly divided into discovery (n = 150) and validation (n = 150) sets. Statistical analysis was performed using R packages.
Anti-bacterial antibody responses showed greater differential prevalence among the three subject groups than anti-viral antibody responses. We identified novel antibodies against the antigens of Bacteroidetes vulgatus (BVU_0562) and Streptococcus pneumoniae (SP_1992) showing higher prevalence in CD patients relative to healthy controls. We also identified antibodies against the antigen of Streptococcus pyogenes (SPy_2009) showing higher prevalence in healthy controls relative to UC patients. Using these novel antibodies, we built biomarker panels with area under the curve (AUC) of 0.81, 0.87, and 0.82 distinguishing CD vs control, UC vs control, and CD vs UC, respectively. Subgroup analysis revealed that penetrating CD behavior, colonic CD location, CD patients with a history of surgery, and extensive UC exhibited highest antibody prevalence among all patients. We demonstrated that autoantibodies and anti-microbial antibodies in CD patients had minimal correlation.
We have identified antibody signatures for CD and UC using a comprehensive analysis of anti-microbial antibody response in IBD. These antibodies and the source microorganisms of their target antigens improve our understanding of the role of specific microorganisms in IBD pathogenesis and, after future validation, should aid early and accurate diagnosis of IBD.
Core Tip: We performed the largest serological profiling of anti-microbial antibodies to date in using 100 Crohn’s disease (CD) and 100 ulcerative colitis (UC) patients. We identified novel anti-microbial antibodies with differential prevalence in inflammatory bowel disease (IBD) patients compared with healthy controls. There was minimal correlation between anti-microbial antibodies and our previously reported autoantibodies in CD patients. We combined novel anti-microbial antibodies to build biomarker panels distinguishing CD vs control, UC vs control and CD vs UC with an area under the curve of 0.81, 0.87, and 0.82, respectively. Subgroup analysis revealed that IBD patients with severe disease had the highest antibody prevalence.
- Citation: Shome M, Song L, Williams S, Chung Y, Murugan V, Park JG, Faubion W, Pasha SF, Leighton JA, LaBaer J, Qiu J. Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures. World J Gastroenterol 2022; 28(30): 4089-4101
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4089
Inflammatory bowel disease (IBD) represents a group of intestinal disorders that causes chronic inflammation in the digestive tract. The two main clinical phenotypes are ulcerative colitis (UC) and Crohn’s disease (CD). The public health burden of IBD is rising globally[1]. Early and accurate diagnosis is key to reducing this burden. Gastroenterologists often use a combination of relatively invasive procedures, like ileocolonoscopy with biopsy for diagnosis, and to determine the disease extent and activity. There is a need for serological biomarkers that can reveal the disease state non-invasively. Herein, our objectives were to discover anti-microbial antibody signatures in IBD patients and understand the association of microbial infection with IBD pathogenesis.
IBD is caused by a combination of genetic predisposition, faulty immune responses, and environmental factors[2]. The interaction of microbes with the gut mucosa in a genetically susceptible individual and the corresponding immune response play a pivotal role in the initiation and progression of IBD[3]. After birth, a limited diversity microbial community develops into a complex community due to the influence of diet and environmental factors[4]. During the second or third decade of life, a dysbiosis is observed in IBD patients which leads to an imbalance between commensal and potentially pathogenic microorganisms[5]. The healthy gut microbiota predominately comprises Firmicutes and Bacteroidetes, and to a lesser extent, Actinobacteria and Proteobacteria[6,7]. In IBD, dysbiosis is observed with reduced abundance of Firmicutes and either higher or similar abundance of Proteobacteria. Besides compositional changes, genetic alterations also contribute to gut dysbiosis that leads to disease initiation and progression. For example, NOD2 variants were found in 20%-40% of European and American CD patients[8,9]. NOD2 encodes an intracellular receptor for the bacterial peptidoglycan muramyl dipeptide, which helps maintain the balance of commensal bacterial flora[10].
Immune response to microbes results in the production of antibodies to microbial antigens[11]. Anti-Saccharomyces cerevisiae antibodies (ASCA) are associated with CD patients, with sensitivities and specificities ranging between 55% to 65% and 80% to 95%, respectively[12]. Perinuclear antineutrophil cytoplasmic antibodies (pANCA) are associated with UC patients, with sensitivities and specificities ranging between 50% to 71% and 75% to 98%, respectively[13]. Outer membrane protein of Escherichia coli (OmpC) and flagellin (CBir1) antibodies are prevalent in CD patients, with prevalence ranging between 24%-55% and 50%-56%[13]. The number and response magnitude of anti-microbial antibodies have previously been shown to indicate the presence of IBD, its severity and its clinical course; however, the clinical utility of available antibodies in diagnosis and clinical management of IBD patients has been limited. The techniques used to discover the known anti-microbial antibodies associated with IBD are of low throughput, and have only been applied to test on small number of candidate microorganisms or microbial antigens[14]. We have performed a large-scale comparative profiling of anti-microbial antibodies in CD and UC patients and healthy controls using an innovative protein microarray technology, namely Nucleic Acid Programmable Protein Array. We selected 1570 microbial proteins from our microbial protein collection (DNASU.org) from 50 bacteria and 33 viruses based on preliminary studies in our laboratory and a review of the literature, displayed them on microarrays and probed them against 100 CD, 100 UC and 100 healthy control serum samples.
All the serum samples were acquired from Serum Biobank at Mayo clinic with approval from institutional review board. CD patients were randomly selected, followed by age and gender matched healthy controls and UC patients. The samples (100 CD, 100 UC and 100 controls) were divided evenly into two non-overlapping discovery and validation sets randomly (Table 1). Disease status for study participants was assessed by clinicians at Mayo clinic.
| Discovery set | Validation set | |||||
| CD | UC | HC | CD | UC | HC | |
| N | 50 | 50 | 50 | 50 | 50 | 50 |
| Gender (female, male) | 29, 21 | 29, 21 | 29, 21 | 28, 22 | 28, 22 | 28, 22 |
| Age (median ± SD) | 41 ± 17.66 | 44 ± 17.25 | 42 ± 18.47 | 39.5 ± 17.49 | 44.5 ± 17.23 | 39.5 ± 16.02 |
| Disease behavior (B1/B2/B3) | 9/10/6 | 16/8/2 | ||||
| Disease location (L1/L2/L3/L4) | 12/6/7/0 | 12/7/7/0 | ||||
| Disease extent (E1/E2/E3) | 0/32/18 | 0/34/16 | ||||
| Surgery (Yes, No) | 24, 25 | 8, 42 | 22, 27 | 7, 42 | ||
We analyzed 1570 microbial proteins, of which 1173 proteins were from 50 different species of bacteria, 397 proteins were from 33 different species of viruses and the remaining proteins were autoantigens. These proteins were selected from our large collection of microbial antigens (DNASU.org) with reference to our anti-microbial antibody studies on other diseases (unpublished data). Microbial protein arrays were fabricated as described earlier[15,16]. Briefly, plasmids with genes of interest cloned in the pANT7_cGST expression vector were obtained from the DNASU plasmid repository, prepared, and printed into silicon nanowells using a piezoelectric dispensing system to produce microbial protein arrays. On the day of experiment, proteins were freshly expressed from printed plasmids using an in-vitro transcription and translation protein expression kit (Fisher Scientific) and captured by anti-GST antibody co-printed in each nanowell. After expression, microarrays were incubated with 1:100 diluted serum samples. We randomized the case and control serum samples while profiling on microarray to reduce bias. IgG and IgA anti-microbial protein antibodies were detected by Alexa-647 goat anti-human IgG (H+L) and Cy3 goat anti-human IgA (Jackson ImmunoResearch). After washing and drying, the microarrays were scanned in a Tecan PowerScanner and the raw fluorescence intensity data were extracted using the ArrayPro Analyzer Software. Raw fluorescence intensity of each protein on the microarray was divided by the median intensity of all the proteins on the microarray for normalization. The normalized value was termed as Median Normalized Intensity (MNI) and used for all analysis. Seropositivity of antibody for a particular antigen was defined as MNI ≥ 2 as we have done for our other studies[17,18].
Pairwise comparisons of numbers of IgG or IgA antibodies for each bacterial species among the 3 subject groups were performed using Chi-squared tests to assess statistical significance (Supple
For univariate analysis between two comparison groups, we calculated sensitivity for one group at the 96th percentile of the other group or the MNI of 2, whichever was larger. Antibodies with ≥ 14% sensitivity in the discovery set were selected as candidates for further validation. If an antibody had ≥ 14% sensitivity at 96% specificity in both discovery and validation sets, then it was considered as a “validated marker”. Venn diagram for the overlap of microbial antigen targets were plotted using Venny[19].
We used a three-stage approach to build our multi-antibody panels. In the first stage, we selected all candidate biomarkers that passed the criteria above, i.e., sensitivity was greater or equal than 14% at 96% specificity. Next, we applied the minimum redundancy maximum relevance algorithm to further select biomarkers that were possibly the most important and least correlated[20]. In the third stage, we fit a logistic regression model using the selected biomarkers from the first two stages and generated its receiver operating characteristic curve and AUC value to evaluate the model’s discriminatory performance between CD, UC, and healthy controls.
Pair-wise subgroup comparisons based on the Montreal classification were performed for the odds ratio (OR) of each antibody using the seropositivity threshold defined as the maximum of MNI 2 and the 75th percentile of all samples. Chi-squared tests were used to test global significance between all groups with a slight modification by adding 0.5 to each cell of the table to avoid zero cell counts. P values from the Chi-squared method were adjusted for each pair of comparisons and for all candidate biomarkers. The number of antibodies with significant difference in prevalence among classifications were counted based on OR > 1 and OR < 1 for each pair of classification of CD behavior, CD location, UC extent and the surgery history of CD patients. The difference in total number of antibodies with significant difference between classification groups were computed using two sample proportion test. We did not perform a subgroup analysis for the UC patients based on the surgery history because most (84 out of 100) had no surgeries (Table 1).
Spearman’s rank correlation analysis was performed to assess the correlation between autoantibody and anti-microbial antibody reactivity for CD patients and healthy controls. The R “pheatmap” package was used to generate the heatmap for correlation coefficients.
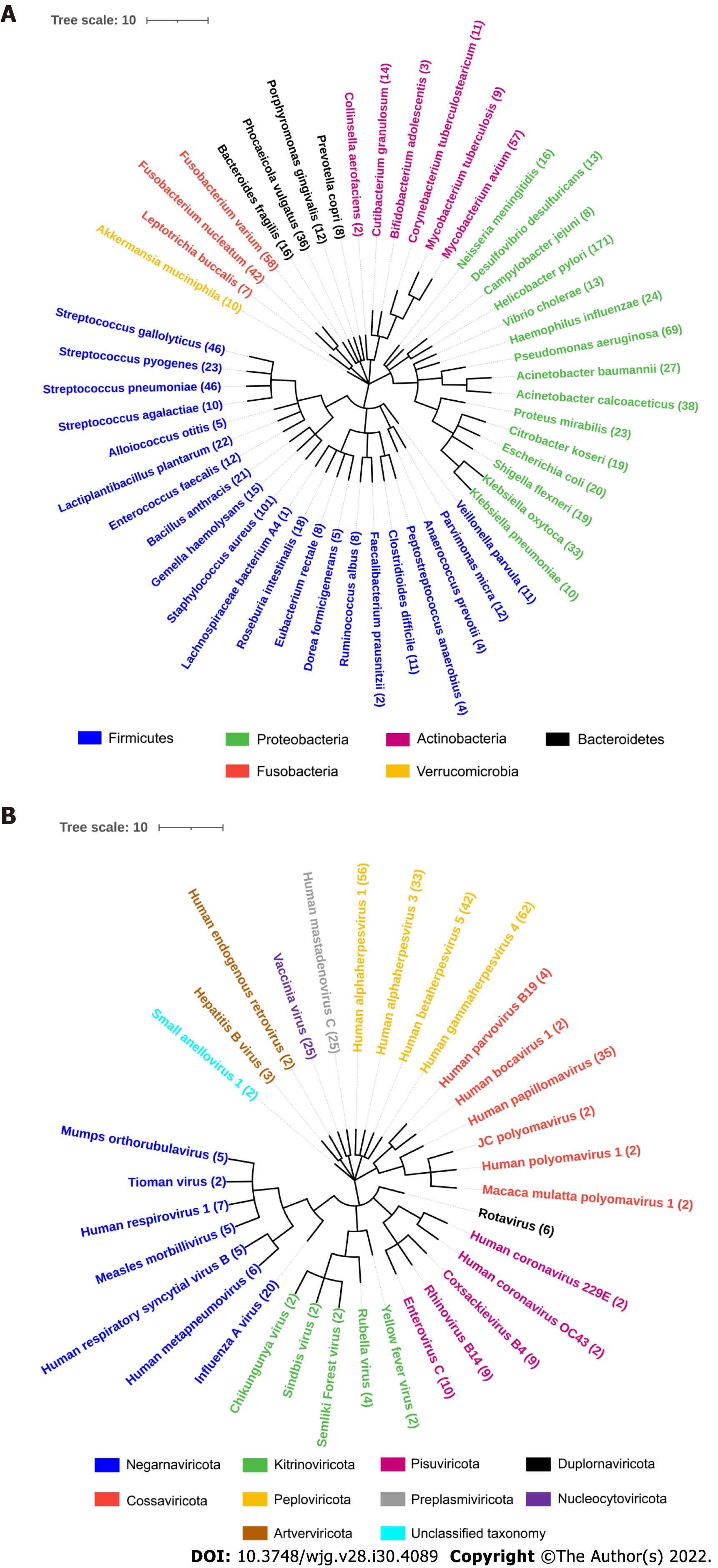
The NCBI Taxonomy browser was used to find the taxonomical details of all the bacteria and viruses used in our study. The taxa were downloaded as phylip tree file and was used as an input in interactive tree of life software. Two phylogenetic trees were created for bacteria and viruses with different colors distinguishing the phylum.
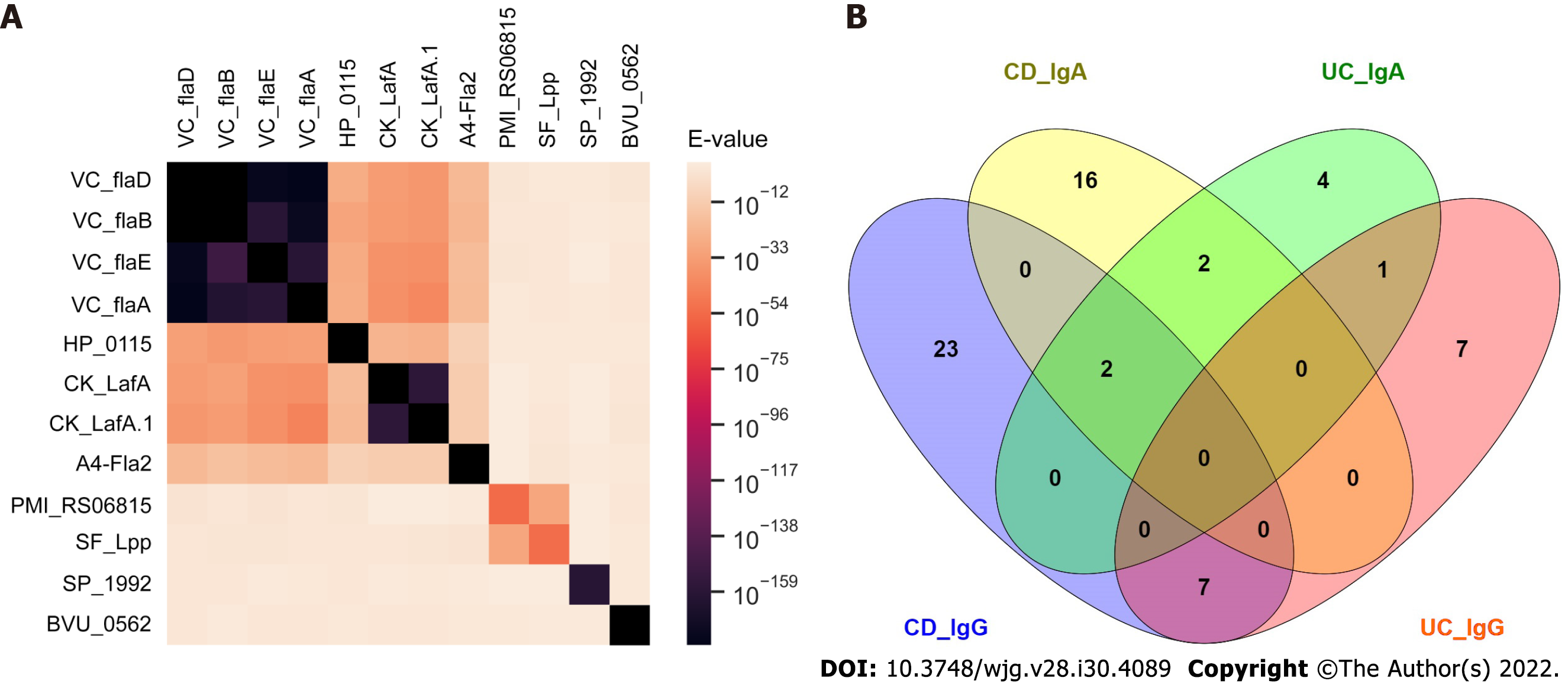
For sequence homology analysis, a pair-wise BLAST analysis was carried out on the antigen protein sequences of validated antibodies for CD vs healthy control analysis. E-values were used to generate a heatmap using Python Seaborn package.
We studied IgG and IgA anti-microbial antibody profiles of 100 CD and 100 UC patients and 100 age-gender matched healthy controls (Table 1) against 1570 microbial antigens including 1173 antigens from 50 different bacteria and 397 antigens from 33 different viruses using our protein microarray platform (Figure 1, Supplementary Table 1). This study provided a representative overview of the anti-microbial antibody response in IBD patients (Supplementary Figure 1). The numbers of IgG antibodies against bacterial proteins from Bacteroidetes vulgatus (B. vulgatus) and Citrobacter koseri (C. koseri) were significantly higher in CD patients compared with those in healthy controls (Chi-square test, P < 0.01) (Supplementary Figure 1). On the contrary, the numbers of IgG antibodies against proteins from several bacteria, such as Streptococcus pneumoniae (S. pneumoniae), Haemophilus influenza (H. influenzae), Staphylococcus aureus (S. aureus), Helicobacter pylori (H. pylori) and Parvimonas micra (P. micra) were significantly lower in CD and UC patients compared with those in healthy controls (Chi-square test, P < 0.05) (Supplementary Figure 1). Overall, fewer IgA anti-microbial antibodies were found than IgG antibodies. The numbers of IgA antibodies against S. pneumoniae, H. influenzae, S. aureus, and H. pylori were significantly lower in UC patients compared with those in healthy controls (Chi-square test, P < 0.01). On the other hand, anti-viral IgG and IgA antibodies showed heterogenous prevalence with no clear trend of differences among CD, UC, healthy controls (data not shown). Therefore, we focused our analysis on anti-bacterial antibodies.
We compared prevalence for individual anti-microbial antibodies between CD patients and healthy controls. We randomly split samples evenly into discovery and the validation sets (Table 1). For antibodies with elevated prevalence in CD patients, 13 IgG antibodies passed the criteria (sensitivity ≥ 14% at 96% specificity) in both discovery and validation sets (Table 2). Anti-A4-Fla2_IgG, a well-studied anti-bacterial flagellin antibody in CD, had the best performance with 47% sensitivity at 96% specificity in the full sample set (Table 2). Beside the flagellins, we found antibodies to four novel target antigens from B. vulgatus (BVU_0562), P. mirabilis (PMI_RS06815), S. flexneri (SF_Lpp) and S. pneumoniae (SP_1992) (Table 2) with no significant sequence homology to flagellins (Figure 2A).
| Antigen | Protein name | Organism | Discovery | Validation | Entire | ||
| Crohn's disease | Bacteria | HP_0115 | Flagellin B | H. pylori | 28 | 48 | 38 |
| BVU_0562 | Uncharacterized protein | B. vulgatus | 26 | 22 | 25 | ||
| CK_LafA | Lateral flagellin | C. koseri | 20 | 22 | 21 | ||
| CK_LafA.1 | Lateral flagellin | C. koseri | 16 | 26 | 24 | ||
| A4-Fla2 | Flagellin | L. bacterium A4 | 40 | 54 | 47 | ||
| PMI_RS06815 | Hypothetical protein | P. mirabilis | 14 | 16 | 15 | ||
| VC_flaD | Flagellin | V. cholerae | 24 | 18 | 19 | ||
| VC_flaB | Flagellin | V. cholerae | 28 | 22 | 24 | ||
| VC_flaE | Flagellin | V. cholerae | 26 | 28 | 23 | ||
| VC_flaA | Flagellin | V. cholerae | 20 | 22 | 21 | ||
| SF_Lpp | Outer membrane lipoprotein | S. flexneri | 14 | 18 | 14 | ||
| SP_1992 | Cell wall surface anchor | S. pneumoniae | 20 | 16 | 18 | ||
| Virus | BILF2 | Glycoprotein BILF2 | Human herpesvirus 4 | 18 | 18 | 18 | |
| Ulcerative colitis | Bacteria | CK_flgG | Flagellar basal-body rod protein | C. koseri | 14 | 16 | 15 |
| A4-Fla2 | Flagellin | L. bacterium A4 | 22 | 16 | 18 | ||
| Virus | BVRF2 | Capsid scaffolding protein | Human herpesvirus 4 | 14 | 16 | 14 | |
| UL139 | Membrane glycoprotein UL139 | Human herpesvirus 5 | 14 | 20 | 17 |
To our surprise, 12 validated IgG antibodies showed elevated prevalence in healthy controls relative to CD patients (Supplementary Table 2). Among these 12 antibodies, anti-bacterial antibodies performed better in differentiating CD patients from healthy controls than anti-viral antibodies (Supplementary Table 2). Antibody against SPy_2009, an anchoring protein located in the cell wall of Streptococcus pyogenes (S. pyogenes), had the highest sensitivity of 24% at 96% specificity in healthy controls relative to CD patients. Seven validated IgA antibodies showed higher prevalence in healthy controls relative to CD patients (Supplementary Table 4).
For anti-microbial antibodies with elevated prevalence in UC patients relative to healthy controls, 4 IgG antibodies passed the criteria in both discovery and validation sets (Table 2). Antibodies to A4-Fla2_IgG and a flagellin from C. koseri had a sensitivity of 18% and 15% respectively. For IgG antibodies with higher prevalence in healthy controls relative to UC patients, 32 antibodies got validated (Supplementary Table 3). Source microorganisms for the target antigens of these 32 antibodies were enriched for S. pneumoniae, S. aureus, and H. influenzae (2-sample proportion test, P < 0.05). 2.7% of the proteins on the microbial protein microarray were from S. pneumoniae while 18.7% of antigens for validated antibodies were from S. pneumoniae, 6.1% of the proteins on the microarrays were from S. aureus while 18.7% of antigens for validated antibodies were from S. aureus, and 1.4% of the proteins on the microarrays were from H. influenzae while 12.5% of antigens for validated antibodies were from H. influenzae. Nine validated IgA antibodies showed higher prevalence in healthy controls relative to UC patients (Supplementary Table 4).
Fewer anti-viral antibodies than anti-bacterial antibodies were validated comparing CD or UC patients with healthy controls (Table 2, Supplementary Tables 2 and 3). Anti-viral antibodies to Rhinovirus B14, Enterovirus C, Influenza A virus, Human metapneumovirus had higher prevalence in healthy controls compared with CD and UC patients (Supplementary Tables 2 and 3).
We found 46 IgG and 22 IgA validated anti-microbial antibodies with higher prevalence in CD patients compared to UC patients while 28 IgG and 9 IgA validated anti-microbial antibodies with higher prevalence in UC patients compared to CD patients. There was minimal overlap of the target antigens of these validated IgG and IgA antibodies (Figure 2B).
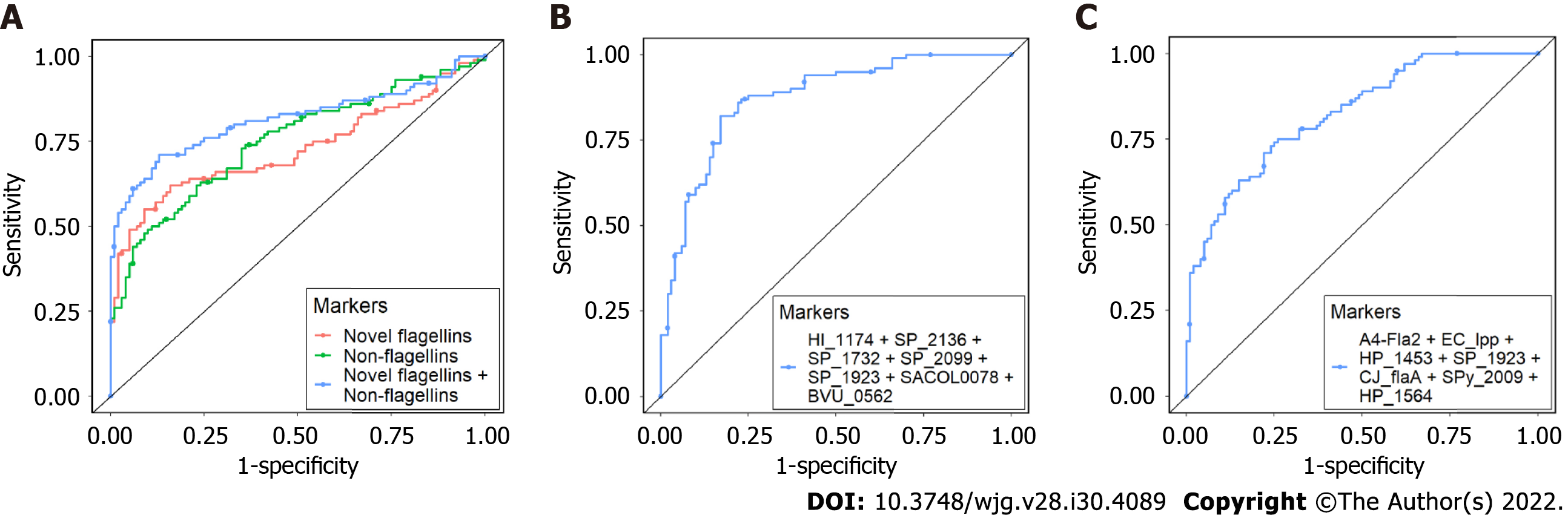
We built multi-antibody panels that could distinguish CD vs control, UC vs control, and CD vs UC with an area under the curve (AUC) of 0.81, 0.87, and 0.82 respectively. For CD vs control, antibodies against novel flagellins (HP_0115, CK_LafA, CK_LafA.1, VC_flaD, VC_flaB, VC_flaE, VC_flaA) had an AUC of 0.73, antibodies against non-flagellins (BVU_0562, SP_1992, PMI_RS06815, and SF_Lpp) had an AUC of 0.75 and the combined AUC of antibodies against novel flagellins and non-flagellins was 0.81 (Figure 3A). For UC vs control, a combination of seven antibodies, four against S. pneumoniae and one each against S. aureus, H. influenzae and B. vulgatus had an AUC of 0.87 (Figure 3B). For CD vs UC, combination of seven antibodies, two against H. pylori and one each against E. coli, S. pneumoniae, S. pyogenes, C. jejuni and L. bacterium A4 had an AUC of 0.82 (Figure 3C).
We investigated the association of CD behavior (B1, B2, B3), CD location (L1, L2, L3), and UC extent (E1, E2, E3) based on the Montreal classification with the anti-microbial antibody prevalence. We calculated the 4th quartile odds ratio for each antibody between the two classification groups and compared the number of antibodies with significant odds ratio (P value < 0.05) in each group. We found B3 (penetrating) had the highest prevalence of antibodies followed by B2 (stricturing) and B1 (non-stricturing, non-penetrating) (Table 3). For CD location, we found L2 had the highest prevalence of antibodies followed by L3 (ileocolonic) and L1 (Table 3). For UC extent, E3 (extensive UC) had higher prevalence of antibodies compared to E2 (left sided UC). In addition to the Montreal classification, we also performed subgroup analysis based on the surgery history of CD patients. We found that patients who had surgery possessed higher prevalence of antibodies compared to those without surgery (Table 3).
| Classification | Comparison | Number of antibodies with | Two sample proportion test | |
| OR > 1 | OR < 1 | |||
| Disease behavior: B1: non-stricturing, non-penetrating; B2: stricturing; B3: penetrating | B1 vs B2 (P < 0.05) | 0 | 32 | P < 0.001 |
| B2 vs B3 (P < 0.05) | 0 | 19 | P < 0.001 | |
| B1 vs B3 (P < 0.05) | 2 | 41 | P < 0.001 | |
| Disease location: L1: ileal; L2: colonic; L3: ileocolonic | L1 vs L2 (P < 0.05) | 0 | 38 | P < 0.001 |
| L2 vs L3 (P < 0.05) | 9 | 5 | P = 0.131 | |
| L1 vs L3 (P < 0.05) | 5 | 16 | P < 0.001 | |
| Disease extent: E2: left sided UC; E3: extensive UC | E2 vs E3 (P < 0.05) | 11 | 39 | P < 0.001 |
| Surgery in CD patients | No vs Yes (P < 0.05) | 6 | 25 | P < 0.001 |
We previously reported novel autoantibodies in CD patients using the same set of CD patients and healthy controls[21]. We profiled both IgG and IgA autoantibodies and anti-microbial antibodies in all 100 CD and 100 healthy controls. It is interesting to note the antibodies showing differences for autoantibodies were mostly IgA, but the anti-microbial antibodies were mostly IgG[21]. Anti-SNRPB_IgA had the highest sensitivity of 20% at 96% specificity among all autoantibodies compared with 47% sensitivity at 96% specificity for the best performing anti-microbial antibody, anti-A4-Fla2_IgG.
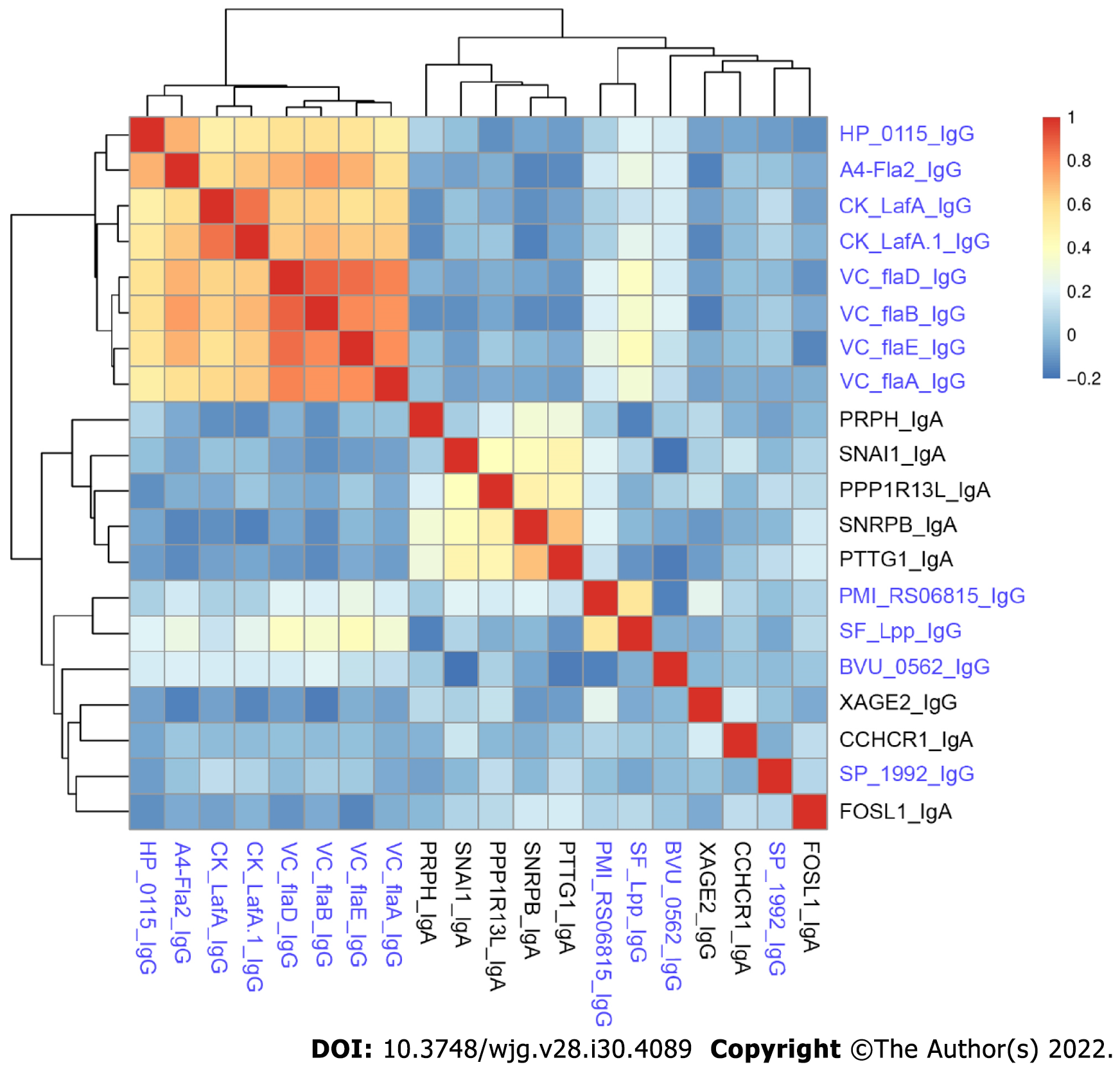
We compared the novel autoantibodies and validated anti-microbial antibody profiles to determine if correlation existed between their reactivity. Overall, we did not observe high correlation between autoantibodies and anti-microbial antibodies in CD patients (Figure 4). Anti-microbial antibodies formed two clusters, one with anti-flagellin antibodies, and the other with SF_Lpp_IgG and PMI_RS06815_IgG. Five autoantibodies, PRPH_IgA, SNAI1_IgA, PPP1R13L_IgA, SNRPB_IgA and PTTG1_IgA, formed a cluster. The remaining antibodies had relatively unique reactivity patterns.
We have performed a microbiomics study to understand the connection between host anti-microbial responses and IBD and to identify antibody signatures that can aid in the accurate diagnosis of IBD. We found antibody responses to novel non-flagellin antigens with elevated prevalence in CD patients compared with healthy controls. On the contrary, we observed many anti-microbial antibodies with lower prevalence in UC patients relative to healthy controls. We built antibody panels that could distinguish CD vs control, UC vs control and CD vs UC with AUCs of 0.81, 0.87, and 0.82, respectively. Lichtenstein et al[22] reported an integrated serological (ASCA-IgA, ASCA-IgG, anti-OmpC, anti-CBir1, anti-I2, pANCA) and genetic (SNP8, SNP12, SNP13) marker panel with an AUC of 0.80 to distinguish CD vs control. A panel of serological markers (ASCA-IgA, ASCA-IgG, ANCA, pANCA, OmpC, and CBir1) built by Plevy et al[23] yields an AUC of 0.78 to distinguish CD vs UC. Our novel antibody marker panels had comparable or better performance in IBD diagnosis or distinguishing CD from UC subtypes. We observed a stronger anti-microbial antibody response with more aggressive disease in both CD and UC patients. Finally, we demonstrated that anti-microbial antibodies and autoantibodies had different reactivity patterns in CD patients.
Our comprehensive study of anti-microbial antibodies in IBD patients provided interesting insight into its pathogenesis. Antibody responses to proteins from B. vulgatus, P. mirabilis, S. flexneri and S. pneumoniae were elevated in CD patients. B. vulgatus has been reported to induce colitis in IBD-susceptible mice[24,25]. P. mirabilis in gut can induce inflammation in cells and a colitis mouse model and has been associated with CD pathogenesis[26]. Our results suggest that B. vulgatus and P. mirabilis may also play a role in human CD development. We observed reduced antibody responses in UC patients to several genera of the Firmicutes phylum including P. micra, S. pyogenes, S. aureus, which were often reduced in abundance in UC patients’ gut microbiota[27]. For several genera belonging to Proteobacteria phylum, such as H. influenzae, H. pylori, K. oxytoca, we observed overall reduced antibody responses; however, their abundance in the gut microbiota of UC patients has been reported to be either increased or remained the same compared with healthy controls[6,28].
Beyond exposure alone, antibody response requires functional immunological interaction between a microorganism and the host; however, anti-microbial antibodies by themselves do not prove causality. As such, source microorganisms whose antibodies show significant changes between IBD patients and healthy controls warrant future confirmation and functional assessment in causing IBD. A4-Fla2 flagellin included in our study showed IBD-specific prevalence with performance similar to that reported in the literature[14,29]. We also identified several antibodies to flagellins with higher prevalence in CD patients relative to healthy controls.
Previous studies mostly focused on antibodies with higher prevalence in IBD patients[30]. Our unbiased data-driven approach revealed the existence of many anti-microbial antibodies with higher prevalence in healthy controls relative to CD and especially UC patients (Supplementary Tables 3 and
We studied the association between anti-microbial antibody prevalence and various disease classifications based on Montreal classification and surgery history. We found more antibodies with significantly higher prevalence in patients with more aggressive disease behaviors relative to those with milder disease behavior. We also found more antibodies with significantly higher prevalence in colonic CD patients relative to those in ileal CD patients. Our results were consistent with previous reports that increasing diversity and magnitude of anti-microbial immune response was correlated with increased frequency of penetrating and/or stricturing disease behavior[13,32]. It is known that the colon has a microbial density of 1011-1012 anaerobic bacteria/gram while the ileum is colonized by 107-108 anaerobic bacteria/gram[7]. Kleessen et al[33] found higher percentage of bacterial invasion of mucosa in colon compared to ileum. CD patients requiring surgery usually had more severe disease compared with those who did not need surgery. Stronger anti-microbial immune response in patients with severe CD or UC suggests a higher abundance of the source microorganisms for the target antigens of the differential antibodies and/or a stronger more conducive immune microenvironment at the disease site in severe disease.
Both autoantibodies and anti-microbial antibodies associated with IBD have been reported[30]. One popular hypothesis for the autoantibody elicitation is molecular mimicry, where anti-microbial antibodies cross react with human proteins. However, we found minimal correlation between the anti-microbial antibodies and the autoantibody profiles in the same set of CD samples. The lack of correlation suggests that IBD-specific autoantibodies and anti-microbial antibodies are elicited independently through different underlying mechanisms, and cross-reactivity may play less of a role in eliciting CD-associated autoantibodies. The breakdown of immune tolerance to human proteins might have occurred due to the damaged gut epithelial cells and the faulty immunological microenvironment partly caused by microbial infections. In addition, the elicitation of autoantibodies may be associated with the infections of multiple microorganisms, and the correlation with individual anti-microbial antibodies may not be great.
Strengths of our study include the broadest analysis to date of IgG and IgA antibodies against individual antigens from many different microorganisms in both CD and UC patients and the use of a two-stage approach with discovery and independent validation of antibody markers. There are some limitations to our study. Except for a few microbes, the number of proteins studied for each species is small, which might limit our interpretation of antibody response in IBD at the species level. Furthermore, many samples used in studies were collected from patients with established disease. Future studies with access to more clinical information, such as information about the use of immunosuppressive or antibiotics treatment, could aid in the interpretation of our results. In addition, samples collected from newly diagnosed patients will strengthen our ability to identify diagnostic markers and microbial connections for IBD development.
In summary, we have demonstrated the power of a microbiomics study of anti-microbial antibodies in IBD for the identification of anti-microbial antibody signatures to improve early accurate diagnosis and help understand IBD etiology. The elucidation of the source microorganisms of the target antigens of the antibody biomarkers could lead to novel strategies for the prevention of IBD.
Early and accurate diagnosis of inflammatory bowel disease (IBD) can benefit the clinical management of IBD patients. The clinical utility of available non-invasive biomarkers is limited.
The gut microbiota plays an important role in the pathogenesis of IBD. Antibody responses to microbial antigens can be exploited to identify better diagnosis markers and improve our understanding of IBD pathology.
This study aimed to compare anti-microbial antibody profiles in Crohn’s disease (CD) and ulcerative colitis (UC) patients and healthy controls.
We employed an innovative protein microarray platform and profiled antibodies against 1570 microbial antigens in 100 CD, 100 UC, and 100 healthy controls.
Antibodies to bacterial proteins were better in distinguishing IBD patients from healthy controls compared with antibodies to viral proteins. We identified a set of novel anti-microbial antibodies against the antigens of Bacteroidetes vulgatus (BVU_0562) and Streptococcus pneumoniae (SP_1992) elevated in CD patients relative to healthy controls. In addition, anti-microbial antibodies against the antigen of Streptococcus pyogenes (SPy_2009) were found to be elevated in healthy controls relative to UC patients. We constructed antibody panels that could distinguish CD vs control, UC vs control, and CD vs UC with an AUC of 0.81, 0.87 and 0.82 respectively. Patients with severe disease had higher prevalence of anti-microbial antibodies. There was minimal correlation among the occurrence of autoantibodies and anti-microbial antibodies in CD patients.
This study discovered novel anti-microbial antibodies with differential prevalence in CD and UC patients relative to healthy controls. In addition, this study revealed the potentially different roles of gut microbiota in CD and UC pathology.
Our study demonstrated the power of a microbiomics approach to identify biomarkers that could aid in the early and accurate detection of IBD non-invasively, and shed light into the role of various microbes in IBD etiology.
The authors would like to thank Jessica Friton for her help with patient sample and data acquisition. The authors would like to thank Shehzad Sheikh for helpful suggestions to improve the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu G, China; Sato Y, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1439] [Article Influence: 287.8] [Reference Citation Analysis (0)] |
| 2. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1112] [Article Influence: 123.6] [Reference Citation Analysis (1)] |
| 3. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 4. | Zuo T, Ng SC. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front Microbiol. 2018;9:2247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 5. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1171] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 6. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3420] [Article Influence: 190.0] [Reference Citation Analysis (1)] |
| 7. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1375] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 8. | Ahmad T, Armuzzi A, Bunce M, Mulcahy-Hawes K, Marshall SE, Orchard TR, Crawshaw J, Large O, de Silva A, Cook JT, Barnardo M, Cullen S, Welsh KI, Jewell DP. The molecular classification of the clinical manifestations of Crohn's disease. Gastroenterology. 2002;122:854-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 412] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3901] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 10. | Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Elkadri AA, Stempak JM, Walters TD, Lal S, Griffiths AM, Steinhart AH, Silverberg MS. Serum antibodies associated with complex inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vermeire S, Joossens S, Peeters M, Monsuur F, Marien G, Bossuyt X, Groenen P, Vlietinck R, Rutgeerts P. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology. 2001;120:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Kuna AT. Serological markers of inflammatory bowel disease. Biochem Med (Zagreb). 2013;23:28-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Takulapalli BR, Qiu J, Magee DM, Kahn P, Brunner A, Barker K, Means S, Miersch S, Bian X, Mendoza A, Festa F, Syal K, Park JG, LaBaer J, Wiktor P. High density diffusion-free nanowell arrays. J Proteome Res. 2012;11:4382-4391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Song L, Wallstrom G, Yu X, Hopper M, Van Duine J, Steel J, Park J, Wiktor P, Kahn P, Brunner A, Wilson D, Jenny-Avital ER, Qiu J, Labaer J, Magee DM, Achkar JM. Identification of Antibody Targets for Tuberculosis Serology using High-Density Nucleic Acid Programmable Protein Arrays. Mol Cell Proteomics. 2017;16:S277-S289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Song L, Song M, Rabkin CS, Williams S, Chung Y, Van Duine J, Liao LM, Karthikeyan K, Gao W, Park JG, Tang Y, Lissowska J, Qiu J, LaBaer J, Camargo MC. Helicobacter pylori Immunoproteomic Profiles in Gastric Cancer. J Proteome Res. 2021;20:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Song L, Song M, Camargo MC, Van Duine J, Williams S, Chung Y, Kim KM, Lissowska J, Sivins A, Gao W, Karthikeyan K, Park J, Leja M, Cohen JI, LaBaer J, Qiu J, Rabkin CS. Identification of anti-Epstein-Barr virus (EBV) antibody signature in EBV-associated gastric carcinoma. Gastric Cancer. 2021;24:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Oliveros JC. VENNY. An interactive tool for comparing lists with Venn Diagrams. Available from: https://bioinfogp.cnb.csic.es/tools/venny/index.html. |
| 20. | Ding C, Peng H. Minimum redundancy feature selection from microarray gene expression data. J Bioinform Comput Biol. 2005;3:185-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 992] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 21. | Wang H, Demirkan G, Bian X, Wallstrom G, Barker K, Karthikeyan K, Tang Y, Pasha SF, Leighton JA, Qiu J, LaBaer J. Identification of Antibody Against SNRPB, Small Nuclear Ribonucleoprotein-Associated Proteins B and B', as an Autoantibody Marker in Crohn's Disease using an Immunoproteomics Approach. J Crohns Colitis. 2017;11:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Lichtenstein GR, Targan SR, Dubinsky MC, Rotter JI, Barken DM, Princen F, Carroll S, Brown M, Stachelski J, Chuang E, Landers CJ, Stempak JM, Singh S, Silverberg MS. Combination of genetic and quantitative serological immune markers are associated with complicated Crohn's disease behavior. Inflamm Bowel Dis. 2011;17:2488-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Plevy S, Silverberg MS, Lockton S, Stockfisch T, Croner L, Stachelski J, Brown M, Triggs C, Chuang E, Princen F, Singh S. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn's disease, and ulcerative colitis patients. Inflamm Bowel Dis. 2013;19:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM Jr, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 25. | Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 551] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 26. | Zhang J, Hoedt EC, Liu Q, Berendsen E, Teh JJ, Hamilton A, O' Brien AW, Ching JYL, Wei H, Yang K, Xu Z, Wong SH, Mak JWY, Sung JJY, Morrison M, Yu J, Kamm MA, Ng SC. Elucidation of Proteus mirabilis as a Key Bacterium in Crohn's Disease Inflammation. Gastroenterology. 2021;160:317-330.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Zakerska-Banaszak O, Tomczak H, Gabryel M, Baturo A, Wolko L, Michalak M, Malinska N, Mankowska-Wierzbicka D, Eder P, Dobrowolska A, Slomski R, Skrzypczak-Zielinska M. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: a pilot study. Sci Rep. 2021;11:2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1675] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 29. | Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 351] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 30. | Mitsuyama K, Niwa M, Takedatsu H, Yamasaki H, Kuwaki K, Yoshioka S, Yamauchi R, Fukunaga S, Torimura T. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol. 2016;22:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Furrie E, Macfarlane S, Cummings JH, Macfarlane GT. Systemic antibodies towards mucosal bacteria in ulcerative colitis and Crohn's disease differentially activate the innate immune response. Gut. 2004;53:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, Wrobel I, Quiros A, Vasiliauskas EA, Grill B, Israel D, Bahar R, Christie D, Wahbeh G, Silber G, Dallazadeh S, Shah P, Thomas D, Kelts D, Hershberg RM, Elson CO, Targan SR, Taylor KD, Rotter JI, Yang H; Western Regional Pediatric IBD Research Alliance. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |