Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4075
Peer-review started: November 9, 2021
First decision: April 16, 2022
Revised: May 4, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 14, 2022
Processing time: 274 Days and 4.5 Hours
Clostridioides difficile (C. difficile) is the most common pathogen causing health care-associated infections. C. difficile TcdA and TcdB have been shown to activate enteric neurons; however, what population of these cells is more profoundly influenced and the mechanism underlying these effects remain unknown.
To characterize a specific population of TcdA-affected myenteric neurons and investigate the role of the P2X7 receptor in TcdA-induced ileal inflammation, cell death, and the changes in the enteric nervous system in mice.
Swiss mice were used to model TcdA-induced ileitis in ileal loops exposed to TcdA (50 μg/Loop) for 4 h. To investigate the role of the P2X7 receptor, Brilliant Blue G (50 mg/kg, i.p.), which is a nonspecific P2X7 receptor antagonist, or A438079 (0.7 μg/mouse, i.p.), which is a competitive P2X7 receptor antagonist, were injected one hour prior to TcdA challenge. Ileal samples were collected to analyze the expression of the P2X7 receptor (by quantitative real-time polymerase chain reaction and immunohistochemistry), the population of myenteric enteric neurons (immunofluorescence), histological damage, intestinal inflammation, cell death (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling), neuronal loss, and S100B synthesis (immunohistochemistry).
TcdA upregulated (P < 0.05) the expression of the P2X7 receptor gene in the ileal tissues, increasing the level of this receptor in myenteric neurons compared to that in control mice. Comparison with the control mice indicated that TcdA promoted (P < 0.05) the loss of myenteric calretinin+ (Calr) and choline acetyltransferase+ neurons and increased the number of nitrergic+ and Calr+ neurons expressing the P2X7 receptor. Blockade of the P2X7 receptor decreased TcdA-induced intestinal damage, cytokine release [interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α], cell death, enteric neuron loss, and S100B synthesis in the mouse ileum.
Our findings demonstrated that TcdA induced the upregulation of the P2X7 receptor, which promoted enteric neuron loss, S100B synthesis, tissue damage, inflammation, and cell death in the mouse ileum. These findings contribute to the future directions in understanding the mechanism involved in intestinal dysfunction reported in patients after C. difficile infection.
Core Tip: There is a knowledge gap regarding the population of enteric neurons affected by TcdA and the role of the P2X7 receptor, which is a low-sensitivity adenosine triphosphate-gated cation channel, in TcdA-induced alterations in enteric neurons and enteric glial cell (EGC)-derived mediators, particularly S100B. The findings of the present study demonstrated the mechanism of P2X7 receptor-driven enteric neuronal loss induced by TcdA in the mouse ileum. TcdA promoted the upregulation of the P2X7 receptor, which promoted cell death in enteric neurons and induced the release of proinflammatory mediators, which in turn promoted S100B synthesis in EGCs. However, the blockade of the P2X7 receptor abrogated ileal damage induced by TcdA.
- Citation: Santos AAQA, Costa DVS, Foschetti DA, Duarte ASG, Martins CS, Soares PMG, Castelucci P, Brito GAC. P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice. World J Gastroenterol 2022; 28(30): 4075-4088
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4075.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4075
Clostridioides difficile (C. difficile) continues to be the leading cause of nosocomial diarrhea worldwide[1]. TcdA, TcdB, and C. difficile binary toxin are the main virulence factors of C. difficile infection-related intestinal damage. These toxins have been shown to play an important role in secretory diarrhea and inflammation during the infection[2,3]. The clinical disease ranges from mild diarrhea to toxic megacolon, colonic perforation, and death.
Intestinal dysfunction has been identified in patients after the acute phase of C. difficile infection[4-7]. Growing evidence suggests that the enteric nervous system (ENS) plays an important role in the regulation of intestinal inflammation. Alterations in the ENS components, including enteric neurons and glia, contribute to the amplification of inflammatory immune response and intestinal dysfunction under inflammatory conditions.
The P2X7 receptor is a low-sensitivity adenosine triphosphate (ATP)-gated cation channel expressed by several cell types, such as macrophages[8], EGCs[9], and enteric neurons[10]. Once activated, the P2X7 receptor increases the intracellular Ca2+ concentrations, which in turn promote the release of proinflammatory cytokines and neuromodulators[11,12]. Additionally, high levels of the P2X7 receptor have been reported in enteric neurons during colitis induced by dinitrobenzene sulfonic acid[13] and intestinal ischemia[10].
TcdA and TcdB have been shown to excite enteric neurons, stimulating the release of substance P and vasoactive intestinal peptide via the inhibition of noradrenergic transmission and the interleukin (IL)-1β pathway, respectively, resulting in neutrophil recruitment and secretory diarrhea[14-16]. However, there is a knowledge gap regarding the population of enteric neurons affected by TcdA and the role of the P2X7 receptor in TcdA-induced alterations in enteric neurons and enteric glial cell (EGC)-derived mediators, particularly S100B.
In the present study, we characterized the population of myenteric neurons affected by TcdA during ileitis in mice. In addition, we investigated the role of the P2X7 receptor in ileal damage, inflammation, and enteric glial and neuronal changes in TcdA-induced ileitis in mice. Our hypothesis was that TcdA affects specific types of neurons and induces reactive gliosis and that activation of P2RX7 is involved not only in ileal damage and inflammation but also in the activation of enteric glia and neuronal loss induced by this toxin.
Swiss mice (8-week-old) were provided by the central vivarium of the Federal University of Ceara. All mice were maintained under standard conditions at 24 °C at a 12-h light-dark cycle, and all groups were provided water and food ad libitum. All mouse procedures were conducted according to current regulations regarding animal experiments approved by the local Animals Care and Use Committee (protocol no. 7028200418).
A mouse model of TcdA-induced ileitis was established as described previously with[17] some modifications. Swiss mice (n = 5 per group) were fasted for 4 h with free access to water and deeply anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). After a midline laparotomy, a single 4-cm ileal loop was ligated and injected with 50 μg of TcdA in 100 μL of phosphate-buffered saline (PBS). The control loops were injected with 100 μL of PBS alone. After 4 h, the mice were euthanized, and the ileal loops were removed for subsequent analysis. Alternatively, some mice were injected with Brilliant Blue G (BBG, Sigma–Aldrich, 50 mg/kg, i.p.)[10], a nonspecific P2X7 receptor antagonist, or with A438079 (Abcam, 10 μM/200 μL, i.p.), a competitive P2X7 receptor antagonist[18], one hour prior to PBS or TcdA (50 μg) injection in the ileal loops. The experimental groups were as follows: Control (loops were injected with 100 μL of PBS alone), TcdA (loops were injected with 50 μg of TcdA in 100 μL of PBS), BBG (injected with BBG one hour prior to the injection of 100 μL of PBS in the loop), A438079 (injected with A438079 one hour prior to the injection of 100 μL of PBS in the loop), TcdA + BBG (injected with BBG one hour prior to the injection of 50 μg of TcdA in 100 μL of PBS), and A438079 (injected with A438079 one hour prior to the injection of 50 μg of TcdA in 100 μL of PBS).
TcdA was provided by Prof. Carlos Quesada from the University of Costa Rica. BBG was kindly provided by Dr. Patricia Castelucci from the University of São Paulo. A43807 was kindly provided by Dr. Henning Ulrich from the University of São Paulo.
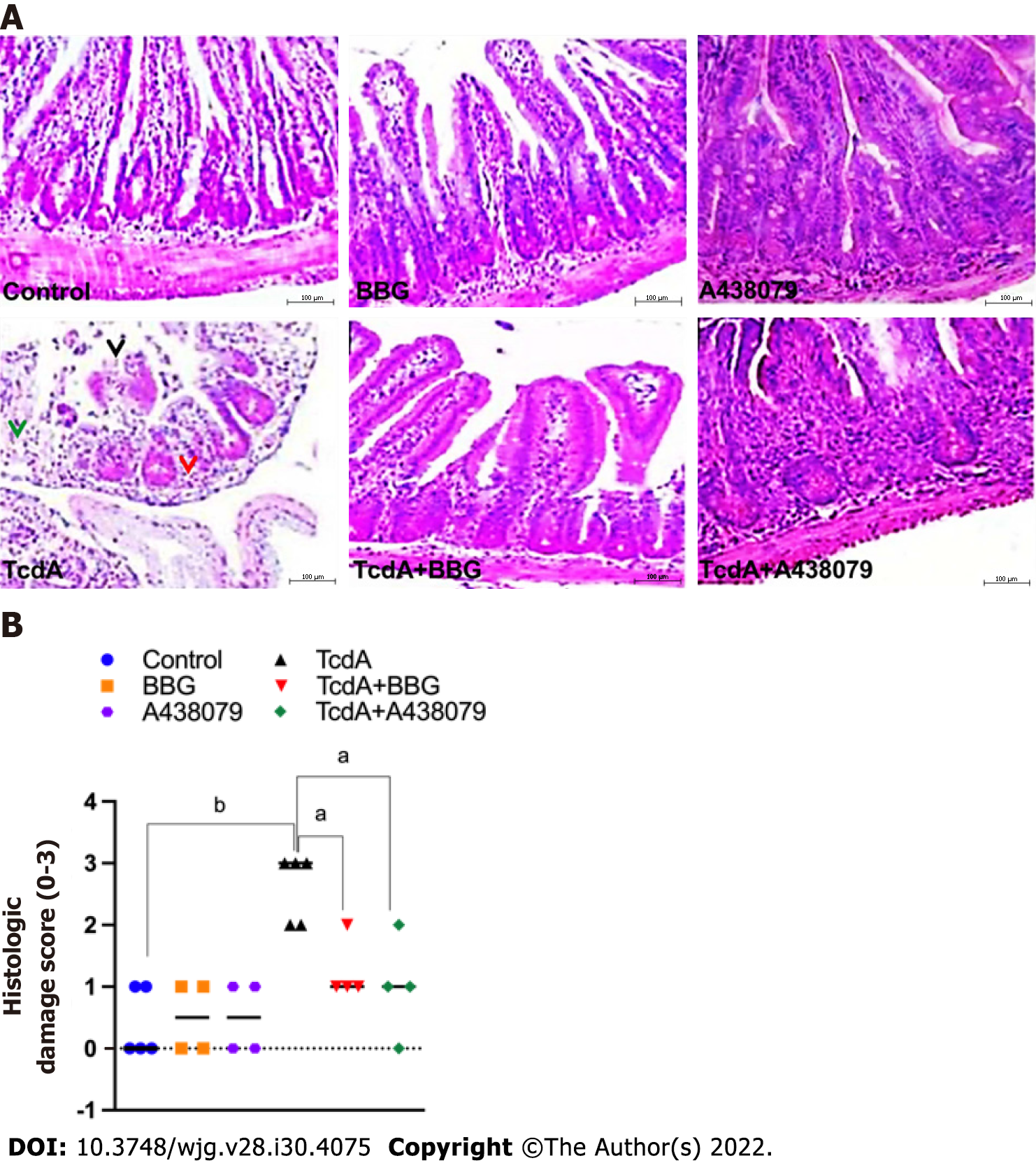
The ileal samples were fixed in 10% formalin solution for 20 h and processed by the NEMPI-UFC Research Histology Core. The severity of ileal damage was measured by a blinded expert in histopathology based on a scoring system ranging from 0 to 3 as described previously with some modifications as follows: (0) Absence of alterations; (1) Mild loss of the integrity of the villi, mild edema, and neutrophil infiltration; (2) Partial loss of the villi, moderate edema, and neutrophil infiltration; and (3) Complete loss of the villi, extensive edema, and intense neutrophil infiltration[19].
Fresh ileal samples were flushed with PBS, dissected, and opened along the mesenteric border. Then, the samples were fixed in 4% paraformaldehyde (in 0.2 M sodium phosphate buffer, pH 7.4) overnight at 4 °C. Then, the samples were washed three times with 100% dimethyl sulfoxide for 10 min, followed by three washes with PBS for 10 min each. All samples were stored at 4 °C in PBS containing 0.1% sodium azide. The fixed tissues were dissected to remove the mucosa, submucosa, and circular layers, yielding longitudinal muscle-myenteric plexus whole mounts as described previously[10].
Whole-mount preparations of the ileal myenteric samples were preincubated in 10% horse serum in PBS containing 1.5% Triton X-100 for 45 min at room temperature to reduce nonspecific binding and permeabilize the tissue. The antibodies used in the present study are described in Table 1. Double labeling was achieved using the combinations of primary antibodies (Table 1) overnight at 4 °C. Then, the samples were washed (with PBS three times for 10 min each) and incubated with secondary antibodies (Table 1). After washing with PBS, the samples were mounted in glycerol buffer (in 0.5 M sodium carbonate, pH 8.6). The immunostaining images were acquired using confocal microscopy by a Zeiss confocal scanning laser system installed on a Zeiss Axioplan 2 microscope. The images were acquired at a resolution of 512 × 512 pixels, and the thickness of each optical section was 0.5 μm. The Z-stacks of immunoreactive cells were captured as a series of optical sections with a center spacing of 0.2 μm. Confocal images were collected using Zeiss LSM 5 image processing software and further processed using Corel Photo Paint and Corel Draw software[10].
| Antigen | Host | Dilution | Manufacturer |
| nNOS | Sheep | 1:2000 | Millipore (AB1529/Lot 2488802) |
| ChAT | Goat | 1:50 | Millipore (AB144P/Lot 1978747) |
| P2X7 receptor | Rabbit | 1:100 | Millipore (AB5246/Lot 2361386) |
| Calretinin | Goat | 1:100 | Molecular |
| Donkey anti-rabbit IgG Alexa 594 | Donkey | 1:200 | Probes (A21206/Lot 1182675) |
| Donkey anti-sheep IgG Alexa 488 | Donkey | 1:400 | Molecular |
| Probes (A11016/Lot93D1-1) |
The antigen colocalization was determined in fluorescently labeled preparations. Initially, the neurons were identified by immunofluorescence. Then, the filter was switched, and the labeling of the second antigen was evaluated. The proportion of the neurons labeled with the antigen pairs was thus determined. The cohort size was 100 neurons, and the data were collected from the preparations obtained from five mice per experimental group. The percentage of double immunoreactive neurons was calculated and is expressed as the mean ± standard error of the mean (SEM). The density of the neurons immunoreactive (neurons/cm2) to the P2X7 receptor, neuronal nitric oxide synthase (nNOS), calretinin (Calr), and choline acetyltransferase (ChaT) and neuronal morphological profiles were assessed in the whole-mount preparations at 100 × magnification. The number of the cell bodies of immunoreactive neurons in the myenteric ganglia in each visual microscopic field (0.04909 mm2) was estimated. To quantify two whole-mount preparations (1.0 cm2 each), the counts were estimated in 40 microscopic fields selected at random for each antigen in each animal. The perikaryon profile areas (μm2) of 50 randomly selected neurons from each animal were obtained using a semiautomatic morphometry device and measured using the Image-Pro Plus software package.
Immunostaining of S100B (an enteric glia-derived mediator), HuC/D (a neuronal marker), and the P2X7 receptor was performed in paraffin-embedded ileal formalin-fixed sections (4-μm thick) using the streptavidin-biotin-peroxidase method; the sections were mounted on poly(L)-lysine-coated microscope slides as described previously[20]. Briefly, the samples were deparaffinized and rehydrated by incubation with xylene and graded alcohol solutions, respectively. Then, the samples were immersed in antigen retrieval solution (EnVisionTM FLEX target retrieval solution, pH = 6.0; Dako, Denmark A/S) for 20 min on a PT Link system (Dako), incubated in 3% hydrogen peroxide (EnVisionTM FLEX peroxidase-blocking reagent; Dako) to block endogenous peroxidase for 15 min at room temperature, and washed with PBS. Then, the samples were incubated with primary antibodies (rabbit anti-P2X7 receptor (Invitrogen), mouse anti-HuC/D (Invitrogen), or goat anti-S100B (Santa Cruz Biotechnology, 1:100) in antibody diluent solution (EnVisionTM FLEX antibody diluent; Dako) overnight at 4 °C. Then, the samples were incubated with EnVisionTM FLEX/HRP (Dako) as recommended by the manufacturer. P2X7 receptor, HuC/D, and S100B were detected using the chromogen 3,3′-diaminobenzidine (DAB, EnVisionTM FLEX DAB+ chromogen; Dako). The negative control sections were processed simultaneously as described above; however, the primary antibody was replaced with antibody diluent solution (EnVisionTM FLEX antibody diluent; Dako). The slides were counterstained with Mayer’s hematoxylin. The images were acquired by a Leica DM100 microscope and analyzed using Adobe Photoshop 8.0 software. The percentages of P2X7 receptor-, S100B- and HuC/D-stained tissue sections were measured by using Adobe Photoshop as described previously[20].
Total RNA was isolated from the ileum using an Aurum™ total RNA fatty and fibrous tissue kit (Bio-Rad, CA, United States), and 1 μg of the RNA was reverse transcribed using iScript™ (Bio-Rad) according to the manufacturer's instructions. Real-time polymerase chain reaction (qPCR) was performed on a 7900HT fast real-time PCR system (Applied Biosystems) using the following specific primers (IDT, Coralville, IA): P2X7 receptor (forward: GCACGAATTATGGCACCGTC and reverse: CCCCACCCTCTGTGACATTCT) and GAPDH (forward: TGCACCACCAACTGCTTAG and reverse: GGATGCAGGGATGATGTTC)[21]. The reaction mixture was prepared in a final volume of 20 μL as follows: 10 μL of master mix iQTM SYBR® Green (Applied Biosystems), 2 μL of each primer (200 nM), 1 μL of cDNA, and 5 μL of nuclease-free water. The gene amplification included the following steps: 10 min at 95 °C (initial denaturation), 15 s at 95 °C and 60 s at 60 °C for 40 cycles; thus, a melting curve was obtained. Relative gene expression was determined using the 2-ΔΔCt method with GAPDH as a housekeeping gene.
Ileal samples were processed for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) using an ApopTagR S 7100 kit (Merck Millipore, Germany) to quantify apoptotic and necrotic cells. Briefly, paraffin-embedded sections were hydrated and incubated with proteinase K (Sigma, United States, 20 mg/mL) for 15 min at room temperature. Endogenous peroxidase was blocked with 3% hydrogen peroxide in PBS for 5 min at room temperature. After a washing step, the sections were incubated with TdT buffer containing TdT enzyme and reaction buffer in a humidified chamber at 37 °C for 1 h. The specimens were incubated for 10 min at room temperature with stop/wash buffer and then incubated with an anti-digoxigenin–peroxidase conjugate at room temperature in a humidified chamber for 30 min. After washing with PBS, the slides were covered with peroxidase substrate (DAB) to develop the color and were counterstained with methyl green.
To measure inflammatory markers, the ileal samples were processed to evaluate the levels of IL-1β, IL-6, keratinocyte chemoattractant (KC, a human IL-8 analog), and tumor necrosis factor (TNF)-α by enzyme-linked immunosorbent assay (ELISA) using a mouse cytokine kit (R&D Systems) according to the manufacturer’s instructions. The absorbance of the samples was detected at 450 nm using an ELISA plate reader (Biotech Epoch, United States). The data are expressed as pg per mg of tissue.
The results are expressed as the mean ± SEM calculated by GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA). The differences between more than two experimental groups were evaluated using one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test. Student's t test was performed to analyze the differences between two groups. The histopathological score data were compared by using Kruskal–Wallis nonparametric test followed by Dunn’s test. Statistical significance was defined as P < 0.05.
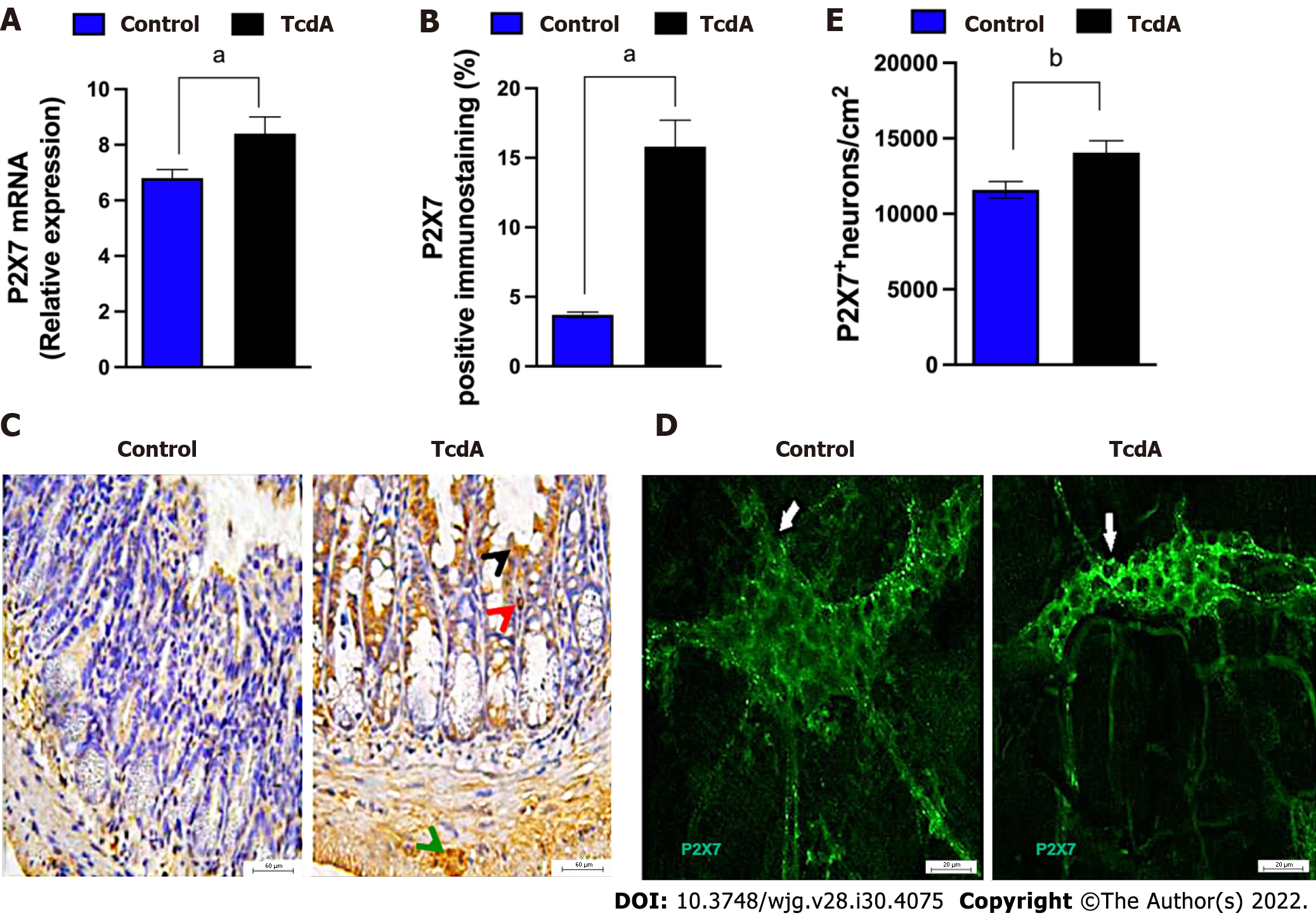
Initially, we investigated whether TcdA alters the expression of the P2RX7 gene in the ileum of mice by using qPCR. We demonstrated that TcdA upregulated the P2X7 receptor in the ileum of mice compared with that in control mice (P < 0.05, Figure 1A). The data of the assay of the P2X7 receptor protein by immunofluorescence showed an increase in the percentage of positive P2X7 receptor immunostaining in the ileal samples of TcdA-challenged mice compared with that in the control samples (P < 0.05, Figure 1B). An increase in the expression of the P2X7 receptor was observed in epithelial cells, the lamina propria, and the myenteric plexus (Figure 1C).
Enteric neurons are an important component of the myenteric plexus, which is a part of the ENS; hence, we investigated whether the level of the P2X7 receptor is increased in these cells in the myenteric plexus by using immunofluorescence analysis. Comparison with the control group indicated an increase in the density of enteric neurons expressing the P2X7 receptor in the ileum myenteric plexus in mice challenged with TcdA (P = 0.01, Figure 1D and E).
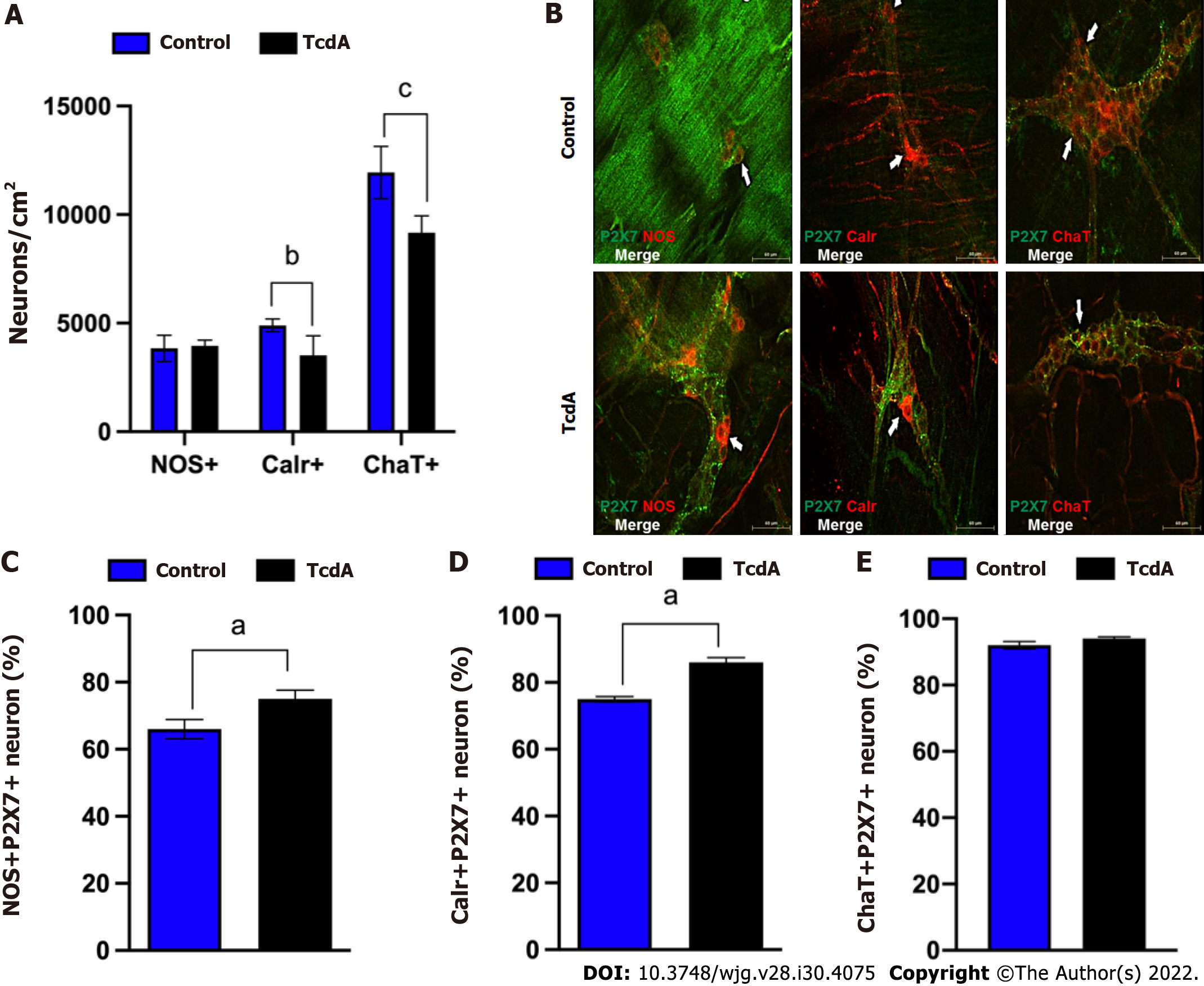
Subsequently, to better understand how TcdA affects the myenteric enteric neuron population, we immunostained the ileum myenteric plexus to detect nNOS, Calr, and ChaT, which were stained in the main population of enteric neurons. As shown in Figure 2A, the density of Calr+ (P < 0.03) and ChaT+ neurons (P < 0.002) in the ileum myenteric plexus of mice challenged with TcdA was decreased compared to that in control mice. In addition, all these subtypes of the neurons expressed the P2X7 receptor (Figure 2B).
Comparison with the control group of mice indicated that the enteric neuron population expressing the P2X7 receptor in the ileum myenteric plexus had a higher number of nNOS+P2RX7+ and Calr+P2RX7+ neurons, but not of ChaT+P2RX7+ neurons (P < 0.05, Figure 2C-E).
Overall, these findings indicated that TcdA decreased the enteric neuron population, specifically Calr+ and ChaT+ cells and upregulated the P2X7 receptor in a specific population (nNOS and Calr) of the neurons in the ileum myenteric plexus in mice.
Then, we blocked the P2X7 receptor by pretreating mice using a pharmacological approach, including administration of BBG and A438079 one hour prior to the challenge with TcdA to determine whether P2X7 receptor activity is required for ileal damage induced by TcdA. Hematoxylin and eosin-stained slides of ileum samples were analyzed for evidence of epithelial damage, edema, and neutrophil infiltration, with a maximal severity score of 3 (Figure 3). TcdA induced complete epithelial disruption, extensive edema, and intense neutrophil infiltration in the ileum of mice, resulting in a high damage score (score = 3) compared with those in the undamaged ileum in control mice (P < 0.007, Figure 3). However, both P2X7 receptor antagonists (BBG and 438079) induced a substantial decrease in the ileal damage promoted by TcdA, resulting in a reduction in the damage score (score = 1) compared to that in untreated TcdA-challenged mice (P < 0.04, Figure 3).
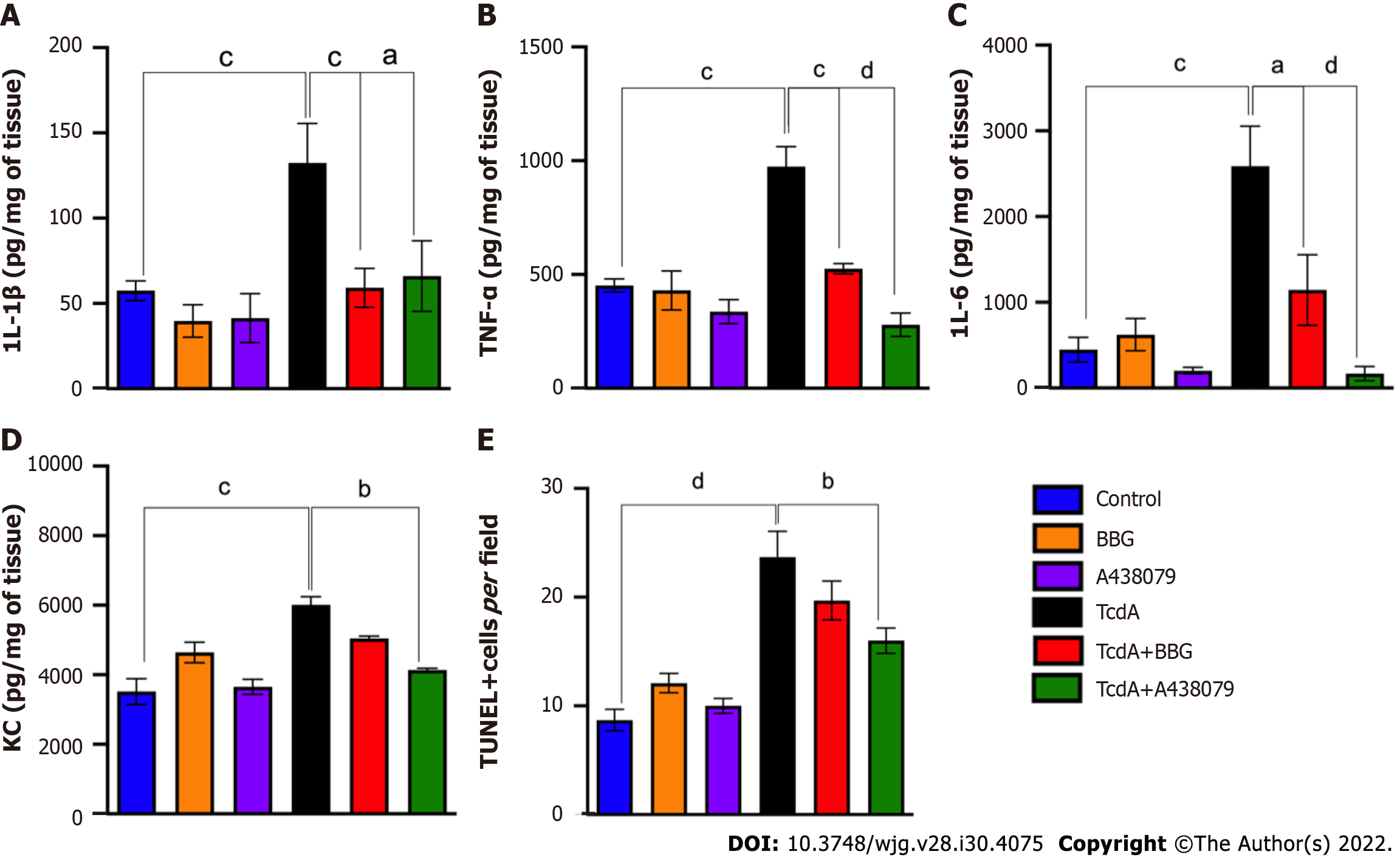
Subsequently, we evaluated whether P2X7 receptor activity is involved in ileal inflammation and cell death induced by TcdA in mice. We demonstrated that both P2X7 receptor blockers (BBG and A438079) reversed a TcdA-induced increase in IL-1β (P = 0.008 and P = 0.03, Figure 4A), TNF-α (P = 0.0002 and P = 0.0001, Figure 4B), and IL-6 (P = 0.03 and P < 0.0001, Figure 4C) in the ileal samples of mice. However, comparison with TcdA-challenged mice, which were not pretreated with the blockers, indicated that blockade of the P2X7 receptor by A438079, but not by BBG, decreased the levels of KC (P = 0.01, Figure 4D) and the number of TUNEL+ cells (P = 0.01, Figure 4E) in the ileum of mice challenged with TcdA.
Overall, these data indicated that the P2X7 receptor was involved in intestinal damage, inflammation, and cell death induced by TcdA in mice.
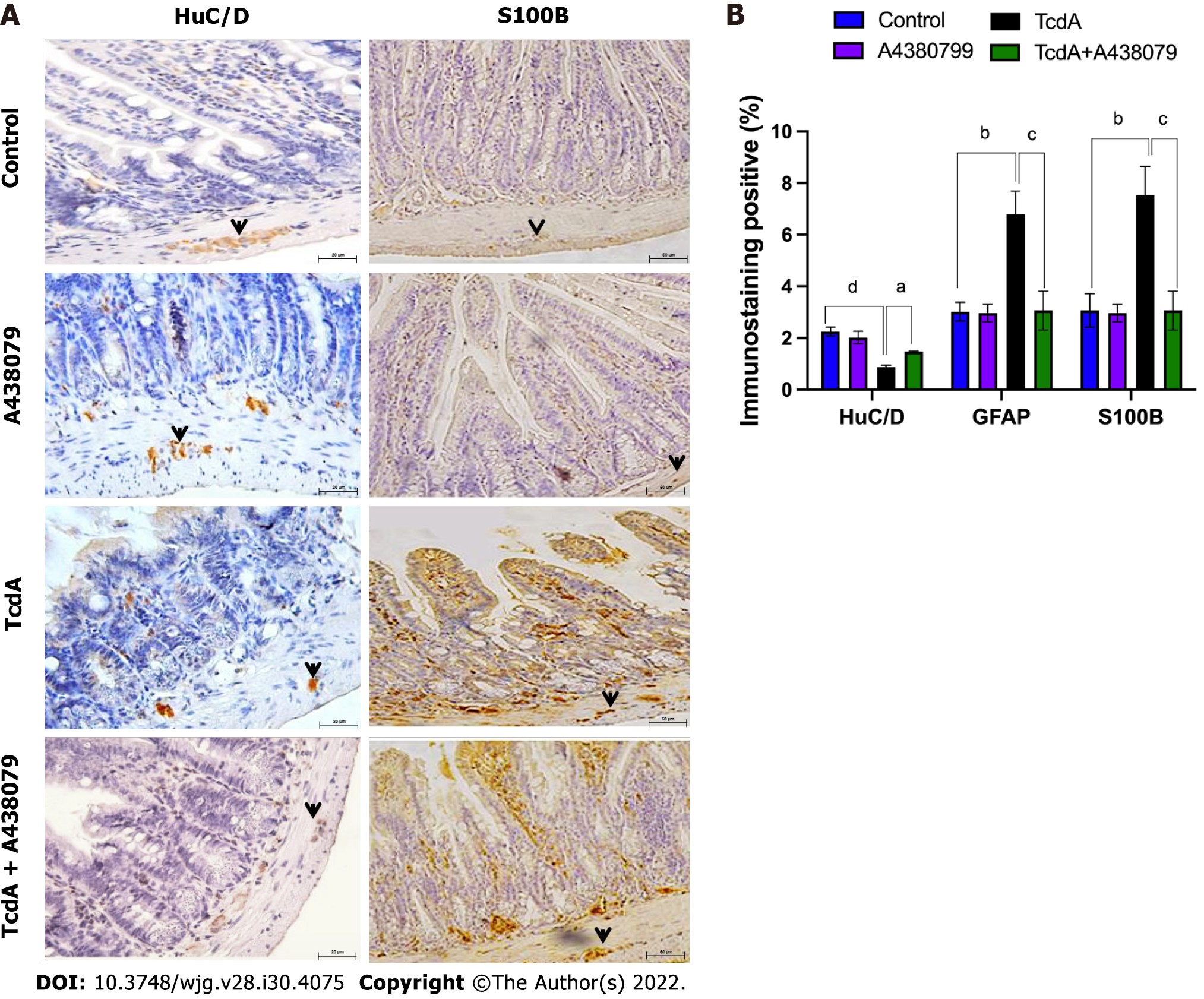
Since we demonstrated that TcdA promoted a decrease in ileum enteric neurons in mice, we assessed whether the P2X7 receptor accounts for this alteration. Comparison with TcdA-challenged mice, which were not pretreated with the blockers, indicated that a P2X7 receptor blocker (A438079) increased the percentage of positive immunostaining of HuC/D, which is a pan-marker of enteric neurons, in the ileum of mice challenged with TcdA (Figure 5).
Furthermore, we evaluated whether the activation of the P2X7 receptor is required to induce S100B expression in the ileum of TcdA-challenged mice; high levels of S100B are released by EGCs under inflammatory conditions. As shown in Figure 5, the P2X7 receptor antagonist A438079 induced a considerable decrease in the percentage of S100B-positive immunostaining in the ileum of mice challenged with TcdA compared with that in the ileum TcdA-challenged mice, which were not pretreated with the blockers (P = 0.009).
Overall, these data indicated that the P2X7 receptor was involved in enteric neuronal loss and S100B synthesis induced by TcdA in mice.
The data of the present study indicated that TcdA upregulated the P2X7 receptor in the ileum of mice. An increase in the expression of P2RX7 has been reported in colonic biopsies from Crohn’s disease patients[21] and in preclinical models of intestinal inflammation, such as colitis induced by trinitrobenzene sulfonic (TNBS) acid[22] and sepsis[23]. Thus, the upregulation of the P2X7 receptor is a common phenomenon under inflammatory conditions[24].
In the present study, we also demonstrated that the level of the P2X7 receptor was increased in the epithelial layer, lamina propria, and myenteric plexus. In the myenteric plexus, we detected an increase in the density of neurons expressing the P2X7 receptor, including the nNOS+ and Calr+ subtypes. In addition to enteric neurons, other cell types can express the P2X7 receptors, such as mast cells, T cells, and dendritic cells[21]. However, we focused on enteric neurons in the myenteric plexus because this component of the ENS is a major functional unit of the system that moves the luminal contents along the intestine by coordinating muscle contraction and relaxation[25]. In addition, C. difficile infection is characterized by intense diarrhea in the acute phase of the disease, and the mechanism of these events is poorly understood.
We demonstrated that TcdA promoted neuron loss specifically by reducing the density of the Calr+ and ChaT+ neuronal populations. Acetylcholine, which is synthesized in a reaction of choline with acetyl-CoA catalyzed by ChaT, is the primary transmitter in excitatory motor neurons, intrinsic afferent neurons, and interneurons, and Calr is the primary transmitter in excitatory cholinergic neurons[26]. Excitatory motor neurons are involved in coordinated muscle contraction[25]; thus, a reduction in the density of Calr+ and ChaT+ neuronal populations induced by TcdA may be involved in the functional disorders manifested after C. difficile infection. Accordingly, a study performed in the United States military personnel reported functional gastrointestinal disorders (including gastroesophageal reflux disease, dyspepsia, irritable bowel syndrome, or constipation) after C. difficile infection recovery[6]. In the present study, alterations in the myenteric enteric neuron population induced by TcdA could have been related to these post-C. difficile infection-related intestinal dysfunctions. However, more studies are needed to better understand how these alterations specifically contribute to intestinal dysfunction induced by C. difficile infection.
In the present study, we also showed that the activation of the P2X7 receptor was involved in the TcdA-induced loss of enteric neurons because inhibition of the receptor by known P2RX7 antagonists (BBG and A438079) resulted in a substantial decrease in the loss of these ENS cells during ileitis induced by TcdA. In agreement with these data of the present study, another study demonstrated that the activation of P2RX7 promotes cell death in mucosal regulatory T cells in colitis induced by TNBS[27]. The P2X7 receptor regulates cell death pathways, such as apoptosis, pyroptosis, necrosis, and auto
ATP released from dead cells can increase the activation of the P2X7 receptor and promote the secretion of proinflammatory cytokines, such as IL-1β, which in turn can induce the secretion of other cytokines[29,30]. In the present study, the blockade of the P2X7 receptor markedly decreased IL-1β, IL-6, KC, and TNF-α synthesis in the TcdA-challenged mouse ileum, suggesting that this receptor plays an important role in inflammation induced by C. difficile toxin. Similarly, in a model of TNBS-induced colitis, P2X7 receptor blockade reduces the severity of inflammation by decreasing the infiltration of macrophages in the lamina propria[31]. In contrast, deletion of P2RX7 increases the susceptibility to toxoplasmic ileitis[32], suggesting that the activation of this receptor plays a role in the response against intracellular pathogens. In contrast, C. difficile releases toxins, which in turn enter the cells to inhibit Rho GTPases, and the P2RX7 antagonists have positive effects in this case.
In addition, we demonstrated that blockade of the P2X7 receptor decreased S100B synthesis in the ileum of mice challenged with TcdA. S100B functions as a proinflammatory mediator when released at higher levels by activating the nuclear activation factor-κB[20] and is an important mediator during C. difficile infection[33]. In the myenteric plexus, EGCs express S100B[34] and are involved in the control of motility and epithelial barrier[35]. In a rat glioblastoma cell line, IL-6 promotes S100B synthesis[36]. In the present study, a reduction in proinflammatory cytokines related to P2X7 receptor blockade could have contributed to a decrease in S100B synthesis induced by TcdA, which in turn reduced intestinal inflammation and neuronal death.
Additional studies are needed, for example, using a C. difficile infection model, to explore how P2RX7 blockage can influence the C. difficile infection outcome and to better understand physiological benefits of this blockade for relief of intestinal permeability and dysmotility during the infection. However, it is important to emphasize that investigations of the role of this receptor in the damaging effects induced by one of the main virulence factors released by C. difficile will help to understand the pathogenesis of these effects and to develop alternative cotreatments to control the deleterious and exacerbated host response to the C. difficile toxins.
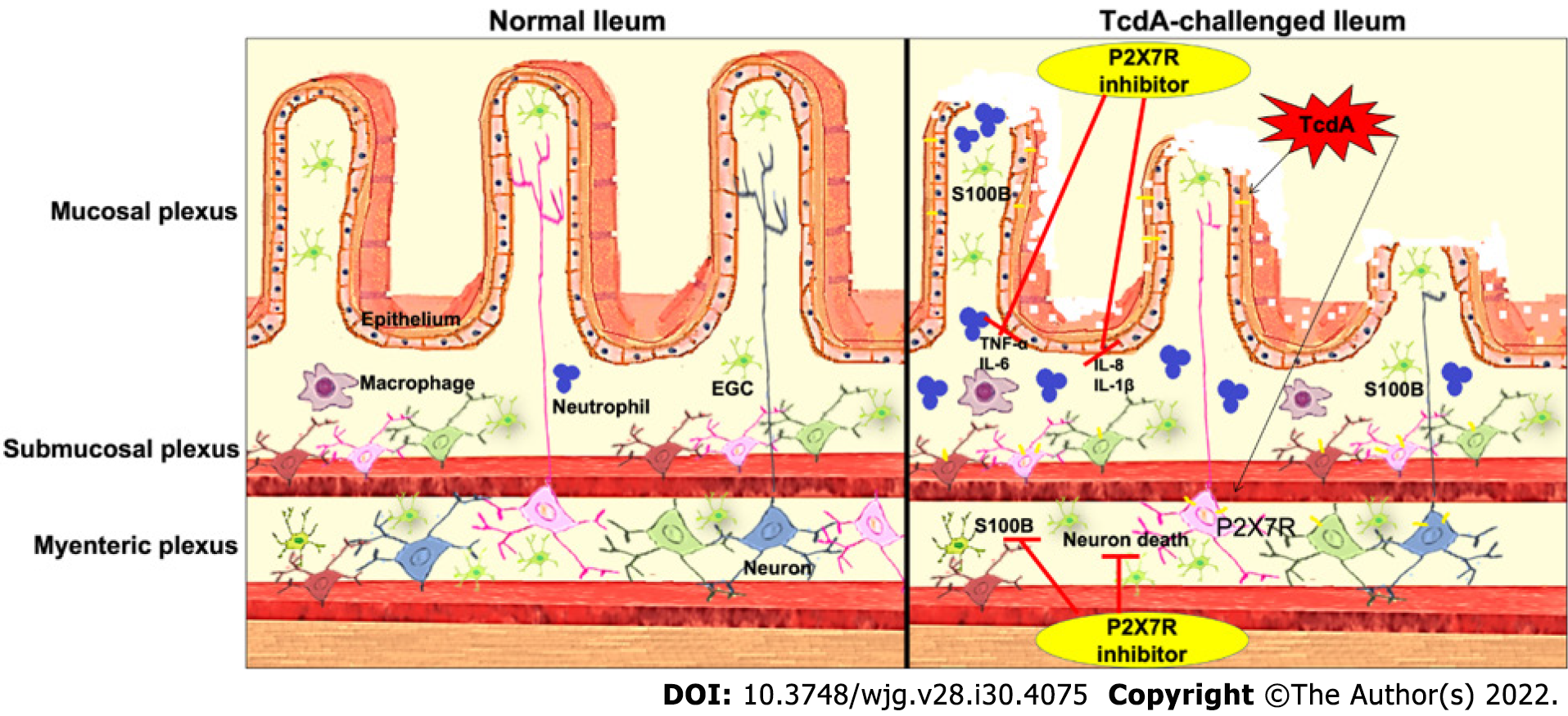
In conclusion, the results of the present study revealed the mechanism of P2X7 receptor-driven loss of enteric neurons induced by TcdA in the mouse ileum. TcdA promoted the upregulation of the P2X7 receptor, which promoted cell death in enteric neurons and induced the release of proinflammatory mediators (IL-1β, IL-6, KC, and TNF-α) in epithelial/immune cells, which in turn promoted S100B synthesis in EGCs. However, blockade of the P2X7 receptor abrogated ileal damage induced by TcdA (Figure 6). Overall, the findings of the present study open new avenues to better understand how C. difficile toxins promote the changes in the ENS components that can be related to intestinal dysfunction after C. difficile infection.
The P2X7 receptor, a low-sensitivity adenosine triphosphate-gated cation channel, is expressed in several cell types, including enteric neurons. Once activated, the P2X7 receptor promotes the release of proinflammatory cytokines and neuromodulators. High levels of P2X7 receptor have been reported in enteric neurons during experimental colitis.
There is a knowledge gap regarding the population of enteric neurons affected by TcdA and the role of the P2X7 receptor in TcdA-induced alterations in enteric neurons and enteric glial cell-derived mediators, particularly S100B.
We characterized the population of myenteric neurons affected by TcdA during ileitis in mice. In addition, we investigated the role of the P2X7 receptor in ileal damage, inflammation, and the changes in enteric glia and neurons in TcdA-induced ileitis in mice.
Swiss mice were used to model TcdA-induced ileitis in ileal loops exposed to TcdA (50 μg/Loop) for 4 h. To investigate the role of the P2X7 receptor Brilliant Blue G (50 mg/kg, i.p.), which is a nonspecific P2X7 receptor antagonist or A438079 (0.7 μg/mouse, i.p.), which is a competitive P2X7 receptor antagonist, were injected one hour prior to TcdA challenge. Ileum samples were collected to analyze the expression of the P2X7 receptor (quantitative real-time polymerase chain reaction and immunohistochemistry), the population of myenteric enteric neurons (immunofluorescence), histological damage, intestinal inflammation, cell death (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling), neuronal loss, and S100B synthesis (immunohistochemistry).
TcdA upregulated (P < 0.05) the expression of the P2RX7 gene in the ileal tissues, increasing the level of this receptor in myenteric enteric neurons compared with that in control mice. Comparison with control mice indicated that TcdA promoted (P < 0.05) the loss of myenteric calretinin+ (Calr) and choline acetyltransferase+ neurons and increased the number of nitrergic+ (nitric oxide synthase+) and Calr+ neurons expressing the P2X7 receptor. Blockade of the P2X7 receptor decreased TcdA-induced intestinal damage, cytokine release (interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α), cell death, enteric neuron loss, and S100B synthesis in the mouse ileum.
The findings of the present study demonstrated that TcdA induced the upregulation of the P2X7 receptor, which promoted enteric neuron loss, S100B synthesis, tissue damage, inflammation, and cell death in the ileum of mice.
These findings contribute to future directions in understanding the mechanism involved in intestinal dysfunction reported in patients after Clostridioides difficile infection.
The authors would like to thank Darlyane V S Costa for kindly drawing Figure 6 and Socorro França and Flávia A Silva for their technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lin L, China; Shen ZY, China; Wang HD, China S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Kumar R, Goomber S, Kaur J. Engineering lipases for temperature adaptation: Structure function correlation. Biochim Biophys Acta Proteins Proteom. 2019;1867:140261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Jank T, Giesemann T, Aktories K. Clostridium difficile glucosyltransferase toxin B-essential amino acids for substrate binding. J Biol Chem. 2007;282:35222-35231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Bilverstone TW, Garland M, Cave RJ, Kelly ML, Tholen M, Bouley DM, Kaye P, Minton NP, Bogyo M, Kuehne SA, Melnyk RA. The glucosyltransferase activity of C. difficile Toxin B is required for disease pathogenesis. PLoS Pathog. 2020;16:e1008852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Solomon K. The host immune response to Clostridium difficile infection. Ther Adv Infect Dis. 2013;1:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Walker AS, Eyre DW, Wyllie DH, Dingle KE, Griffiths D, Shine B, Oakley S, O'Connor L, Finney J, Vaughan A, Crook DW, Wilcox MH, Peto TE; Infections in Oxfordshire Research Database. Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis. 2013;56:1589-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Gutiérrez RL, Riddle MS, Porter CK. Increased risk of functional gastrointestinal sequelae after Clostridium difficile infection among active duty United States military personnel (1998-2010). Gastroenterology. 2015;149:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Grubišić V, Verkhratsky A, Zorec R, Parpura V. Enteric glia regulate gut motility in health and disease. Brain Res Bull. 2018;136:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Liu YH, Chang YC, Chen LK, Su PA, Ko WC, Tsai YS, Chen YH, Lai HC, Wu CY, Hung YP, Tsai PJ. The ATP-P2X7 Signaling Axis Is an Essential Sentinel for Intracellular Clostridium difficile Pathogen-Induced Inflammasome Activation. Front Cell Infect Microbiol. 2018;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Mendes CE, Palombit K, Tavares-de-Lima W, Castelucci P. Enteric glial cells immunoreactive for P2X7 receptor are affected in the ileum following ischemia and reperfusion. Acta Histochem. 2019;121:665-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Palombit K, Mendes CE, Tavares-de-Lima W, Barreto-Chaves ML, Castelucci P. Blockage of the P2X7 Receptor Attenuates Harmful Changes Produced by Ischemia and Reperfusion in the Myenteric Plexus. Dig Dis Sci. 2019;64:1815-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 889] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 12. | Miras-Portugal MT, Sebastián-Serrano Á, de Diego García L, Díaz-Hernández M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J Neurosci. 2017;37:7063-7072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 14. | Neunlist M, Barouk J, Michel K, Just I, Oreshkova T, Schemann M, Galmiche JP. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1beta-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1049-G1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Xia Y, Hu HZ, Liu S, Pothoulakis C, Wood JD. Clostridium difficile toxin A excites enteric neurones and suppresses sympathetic neurotransmission in the guinea pig. Gut. 2000;46:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A. 1994;91:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 200] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile- induced enteritis. J Clin Invest. 1998;101:1547-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Glaser T, de Oliveira SL, Cheffer A, Beco R, Martins P, Fornazari M, Lameu C, Junior HM, Coutinho-Silva R, Ulrich H. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One. 2014;9:e96281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Koon HW, Ho S, Hing TC, Cheng M, Chen X, Ichikawa Y, Kelly CP, Pothoulakis C. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother. 2014;58:4642-4650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Costa DVS, Bon-Frauches AC, Silva AMHP, Lima-Júnior RCP, Martins CS, Leitão RFC, Freitas GB, Castelucci P, Bolick DT, Guerrant RL, Warren CA, Moura-Neto V, Brito GAC. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci Rep. 2019;9:665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun. 2012;3:1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 22. | Marques CC, Castelo-Branco MT, Pacheco RG, Buongusto F, do Rosário A Jr, Schanaider A, Coutinho-Silva R, de Souza HS. Prophylactic systemic P2X7 receptor blockade prevents experimental colitis. Biochim Biophys Acta. 2014;1842:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Wu X, Ren J, Chen G, Wu L, Song X, Li G, Deng Y, Wang G, Gu G, Li J. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci Rep. 2017;7:4364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Liu X. Research progress of P2X7 receptor in inflammatory bowel disease. Scand J Gastroenterol. 2019;54:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell. 2020;182:1606-1622.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 26. | Fung C, Vanden Berghe P. Functional circuits and signal processing in the enteric nervous system. Cell Mol Life Sci. 2020;77:4505-4522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Figliuolo VR, Savio LEB, Safya H, Nanini H, Bernardazzi C, Abalo A, de Souza HSP, Kanellopoulos J, Bobé P, Coutinho CMLM, Coutinho-Silva R. P2X7 receptor promotes intestinal inflammation in chemically induced colitis and triggers death of mucosal regulatory T cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Bidula S, Dhuna K, Helliwell R, Stokes L. Positive allosteric modulation of P2X7 promotes apoptotic cell death over lytic cell death responses in macrophages. Cell Death Dis. 2019;10:882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Oliveira-Giacomelli Á, Petiz LL, Andrejew R, Turrini N, Silva JB, Sack U, Ulrich H. Role of P2X7 Receptors in Immune Responses During Neurodegeneration. Front Cell Neurosci. 2021;15:662935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Soare AY, Freeman TL, Min AK, Malik HS, Osota EO, Swartz TH. P2RX7 at the Host-Pathogen Interface of Infectious Diseases. Microbiol Mol Biol Rev. 2021;85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Neves AR, Castelo-Branco MT, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CM, Carneiro AJ, Coutinho-Silva R, de Souza HS. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn's disease. Inflamm Bowel Dis. 2014;20:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Miller CM, Zakrzewski AM, Robinson DP, Fuller SJ, Walker RA, Ikin RJ, Bao SJ, Grigg ME, Wiley JS, Smith NC. Lack of a Functioning P2X7 Receptor Leads to Increased Susceptibility to Toxoplasmic Ileitis. PLoS One. 2015;10:e0129048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Costa DVS, Moura-Neto V, Bolick DT, Guerrant RL, Fawad JA, Shin JH, Medeiros PHQS, Ledwaba SE, Kolling GL, Martins CS, Venkataraman V, Warren CA, Brito GAC. S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides difficile Infection by Modulating Inflammatory Response. Front Cell Infect Microbiol. 2021;11:739874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Boesmans S, Decoster L, Schallier D. Pemetrexed-induced radiation recall dermatitis of the breast. Anticancer Res. 2014;34:1179-1182. [PubMed] |
| 35. | Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | de Souza DF, Wartchow K, Hansen F, Lunardi P, Guerra MC, Nardin P, Gonçalves CA. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |