Published online Aug 14, 2022. doi: 10.3748/wjg.v28.i30.4061
Peer-review started: January 24, 2022
First decision: April 10, 2022
Revised: April 21, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 14, 2022
Processing time: 197 Days and 21.6 Hours
Chronic liver disease is characterized by several hematological derangements resulting in a complex and barely rebalanced haemostatic environment. Thrombocytopenia is the most common abnormality observed in these patients and recent advances have led to researchers focus the attention on the multifactorial origin of thrombocytopenia and on the key role of thrombopoietin (TPO) in its physio
Core Tip: Recent advances have shed light on the pathophysiology of thrombocytopenia in chronic liver disease and on the key role of thrombopoietin (TPO). Severe thrombocytopenia complicates the management of patients with liver disease by increasing the potential risk of bleeding for invasive procedures, possibly delaying lifesaving interventions. In the very last years, novel agents such as the TPO-receptor agonists avatrombopag and lusutrombopag have been developed in order to increase platelet production as an alternative to platelet transfusions, with positive efficacy and safety outcomes.
- Citation: Gallo P, Terracciani F, Di Pasquale G, Esposito M, Picardi A, Vespasiani-Gentilucci U. Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures. World J Gastroenterol 2022; 28(30): 4061-4074
- URL: https://www.wjgnet.com/1007-9327/full/v28/i30/4061.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i30.4061
Thrombocytopenia, usually defined as any decrease in platelet count below the lower normal limit of 150000/μL, is the most common haematological abnormality in patients with chronic liver disease[1]. Current data report a prevalence ranging from 6% to 78%, which progressively increases from patients with compensated to those with decompensated cirrhosis[2]. The clinical significance of mild (100000/μL-150000/μL) and moderate (50000/μL-100000/μL) thrombocytopenia is minimal and does not interfere with the regular clinical practice. Otherwise, severe thrombocytopenia (< 50000/μL) can be associated with many sequelae and could have a negative impact on the management of patients with advanced chronic liver disease.
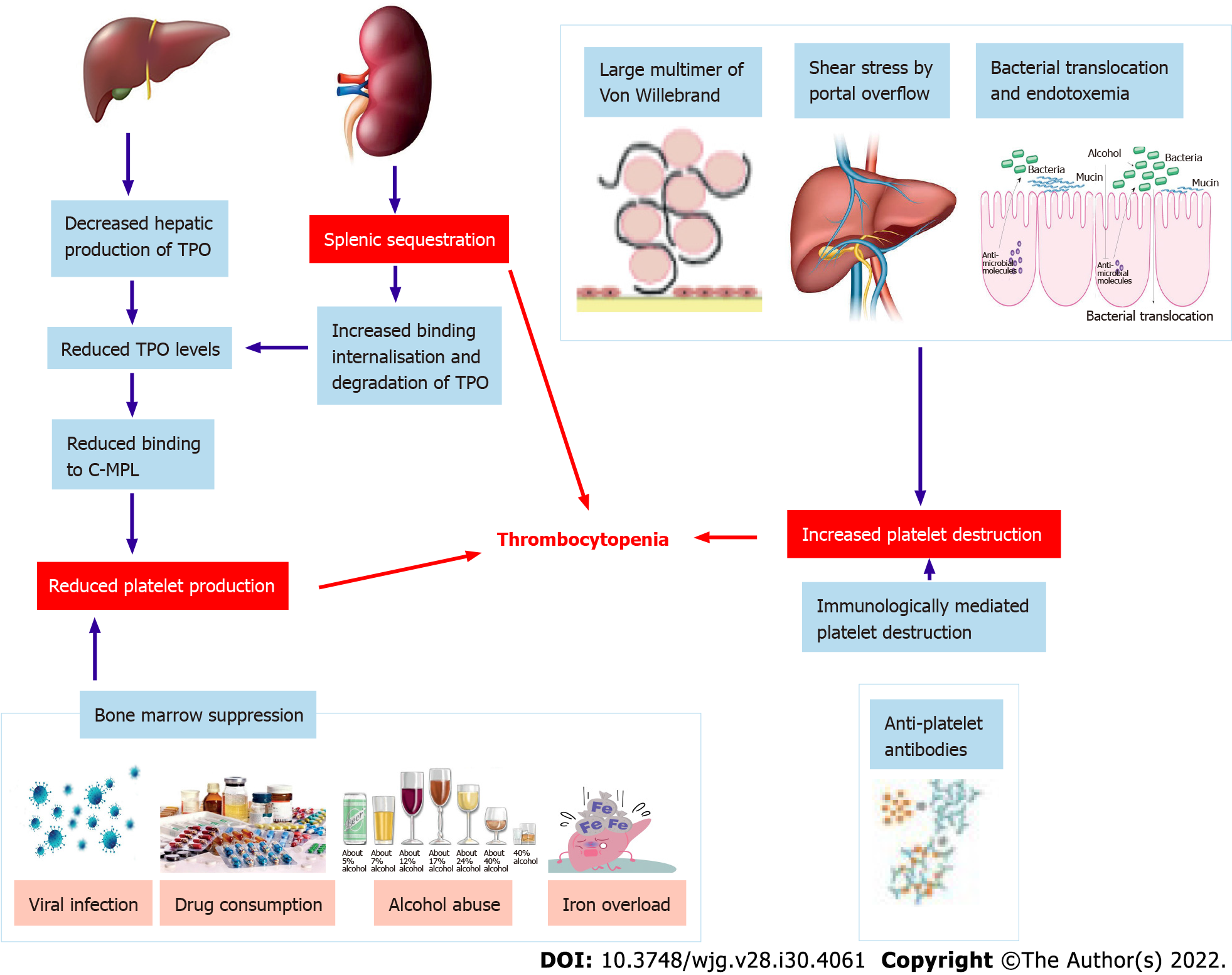
In chronic liver disease, thrombocytopenia has been classically attributed to hypersplenism[1,3]. Over the last decades, however, advances in the understanding of thrombopoiesis have led to a wider and better understanding of its physiopathology. As a result, thrombocytopenia is considered a more complex and multifactorial process involving multiple different mechanisms. These are generically divided into those leading to decreased production or increased destruction of thrombocytes and splenic sequestration[1] (Figure 1).
Decreased platelet production is a consequence of decreased production of thrombopoietin (TPO) and direct bone marrow suppression. Currently, the production of TPO is believed to play a pivotal role in thrombopoiesis. TPO is primarily produced in the liver and, after being secreted into the circulation, it binds to the surface of platelets and megakaryocytes through the c-MPL receptor[4]. TPO-receptor ligation activates a number of intracellular signalling pathways via Janus kinase type 2 and tyrosine kinase 2[5], which ultimately lead to the differentiation of bone marrow stem cells into mature megakaryocytes and to the production of platelets which are released into the peripheral circulation[6]. Of note, after binding to its receptor, TPO is internalized and destroyed in order to reduce further platelet and megakaryocyte exposure[4]. Platelet production is therefore mainly regulated by platelet levels in the blood through a negative feedback circuit[1]. The role of a decreased hepatic production of TPO in the development of thrombocytopenia in chronic liver disease is supported by the immediate increase in TPO levels and platelet production after liver transplantation[7]. Animal models and human clinical studies have confirmed decreased expression of TPO mRNA in liver tissue with the progression of cirrhosis[8], which is probably associated with specific regulatory mechanisms for the expression of TPO gene and is not regulated by bone marrow[9]. Moreover, a correlation between reduced c-MPL expression and the progression of liver cirrhosis has been demonstrated and may play an additional role in the development of thrombocytopenia[10]. Some chronic liver diseases may also cause decreased platelet production through direct bone marrow suppression or toxicity, as observed during viral infection (particularly hepatitis C virus (HCV) infection[11], alcohol abuse[12], iron overload[12], and drug consumption[1,13]).
Increased platelet destruction is a multifactorial process that may involve decreased levels of A disintegrin-like and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS13), immunologically mediated platelet destruction, and bacterial activity. ADAMTS13 is a metalloproteinase produced by hepatic stellate cells, whose physiological role is to cleave large von Willebrand factor (vWF) multimers[14]. In cirrhosis, decreased levels and activity of ADAMTS13 drive the accumulation of vWF multimers, which mediates an enhancement of shear-stress induced platelet aggregation[14]. Additionally, anti-platelet antibodies are a frequent finding in patients with liver cirrhosis, being detectable in up to 64% of cases[15]. The inverse relationship between platelet-associated immunoglobulin G (IgG) levels and platelet count evidences that immunologic destruction contributes to the genesis of thrombocytopenia at least in some chronic liver diseases[16]. Immune-mediated thrombocytopenia is most likely to occur in the course of autoimmune liver diseases (particularly primary biliary cholangitis) and HCV infection[1,17].
HCV can cause immune-mediated thrombocytopenia through multiple mechanisms[1]. First, it can be associated with idiopathic thrombocytopenic purpura (ITP), as supported by a prevalence of anti-HCV antibodies of approximately 10% in patients with ITP[18]. The virus can also directly bind to platelets interacting with multiple surface receptors, leading to the attachment of anti-HCV antibodies to platelets. This will ultimately determine either platelet phagocytosis by the reticuloendothelial system or alterations in the platelet membrane epitopes that induce the production of anti-platelet antibodies[19]. Finally, HCV infection can be associated with the production of cryoglobulins, which can accelerate platelet clearance by the reticuloendothelial system[20]. Thrombocytopenia can be found in about 48% of patients with bacterial infection and sepsis[21], confirming that the inflammatory cascade plays a role in the development of thrombocytopenia. This is confirmed in the hospitalized cirrhotic population[22]. In sepsis, thrombocytopenia is mainly dependent on the increased activation of the coagulative system, resulting in clot formation and platelet consumption[23].
Hypersplenism has been classically considered the main determinant of thrombocytopenia during chronic liver disease[1,3], even after that many other physiopathologic mechanisms have been progressively identified. During chronic liver disease, the inception of portal hypertension causes a redistribution of splanchnic venous blood flow, ultimately responsible for congestion of the spleen and consequent enlargement of the organ, leading to a significant increase of the splenic pool of platelets[24]. Actually, hypersplenism is the clinical syndrome in which splenomegaly is associated with splenic hyperactivity, i.e., a reduction in one or more peripheral blood cell types, in patients with an appropriate proliferative bone marrow response. This syndrome can be reverted with splenectomy[1,24].
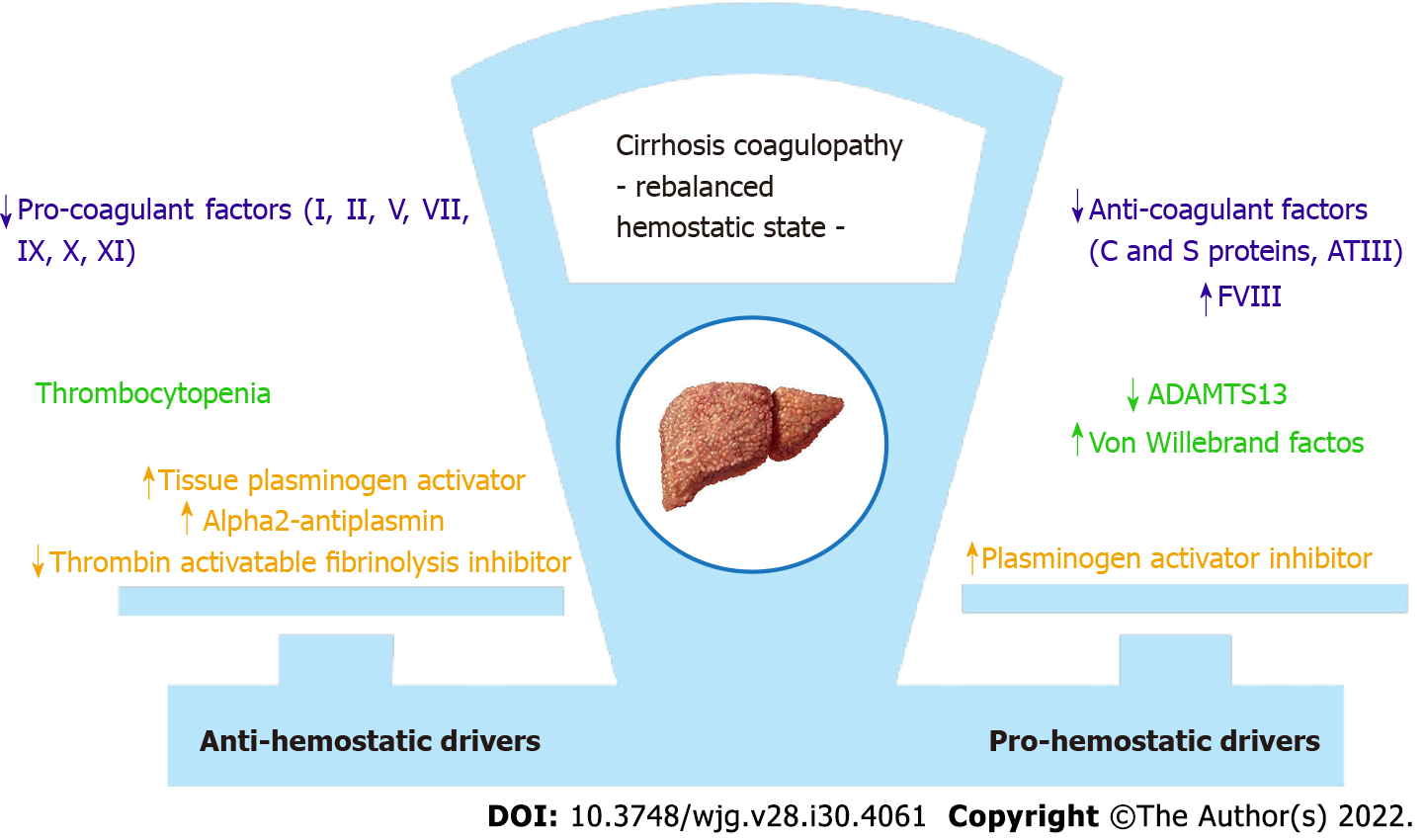
Chronic liver disease is characterized by alterations of the entire hemostatic system[25]. Thrombocytopenia is just one face of a wider coagulation disorder whose relevance is mirrored by the inclusion of coagulation indices in all functional and prognostic scores of liver disease[26]. Traditionally, coagulopathy in cirrhosis was considered as a bleeding diathesis disorder[27], alongside the well-known thrombocytopenia, and the impaired coagulation tests were perceived as indicators of hemorrhagic risk[28]. In the last decades, several studies led to significant changes in knowledge, with a renewed vision concerning the coagulopathy of liver cirrhosis. A new paradigm of a balanced, albeit precarious, hemostatic state has emerged and the net effect is a rebalanced equilibrium[26,27], which can be easily disturbed by many different clinical events, alternatively leading to hemorrhagic as well as to thrombotic manifestations, with the latter being even more frequent indeed[27] (Figure 2).
It has been shown that the reduction in liver-derived pro-coagulant factors is counteracted by the concomitant decrease of the liver-derived anti-coagulant ones, especially protein C[28,29]. Thrombocytopenia and platelet abnormal function are offset by increased vWF and decreased ADAMTS13 levels. In cirrhosis, even if diminished in number, platelets are able to support normal thrombin generation at least until they are in the range 50-60000/mL, therefore assuring a normal primary hemostasis. This is possible thanks to the compensatory action of vWF and to the upregulation of intracellular activating signalling pathways[27], leading to an enhanced thrombocyte response. Furthermore, decreased clearance of tissue plasminogen activator and plasminogen activator inhibitor, and decreased synthesis of alpha 2-antiplasmin and thrombin activable fibrinolysis inhibitor are all factors contributing to hyperfibrinolysis. The latter is observed in up to 30% of patients with advanced liver disease[1], confirming a re-arrangement of the whole hemostatic system.
In cirrhotic patients, even though reduced in number, platelets are still able to ensure an adequate haemostatic function. Consequently, the sole platelet count is not able to predict bleeding risk in liver cirrhosis. Actually, for patients with cirrhosis undergoing invasive diagnostic or therapeutic procedures, the risk of procedure-related bleeding remains a clinical issue[30], and risk stratification is a great challenge. This is mainly due to the inaccuracy, in the context of cirrhosis, of the laboratory tests that are routinely used for the assessment of the hemocoagulative system[25,27,30]. Indeed, it is now well established that the standard clotting tests do not reflect the actual bleeding risk[30-32], and current evidence does not support prothrombin time (PT)/international normalized ratio (INR) as clinical targets[25,33]. Conversely, assessing platelet count and fibrinogen levels before high-risk procedures is recommended, as well as it is the pre-procedural correction of these parameters, having these laboratory parameters been proposed as more reliable indicators of the bleeding risk in patients with cirrhosis[34].
Moreover, many studies have shown that cirrhotic portal hypertension and kidney injury are more essential in determining the risk of bleeding[25,35]. As a matter of fact, renal failure can lead to platelet impairment resulting from reduced adhesive and aggregative capacities via alterations of serotonin concentration, of calcium flow and of thromboxane metabolism[25]. Patient with cirrhosis can also develop accelerated intravascular coagulation and fibrinolysis, described as a bleeding entity similar to disseminated intravascular coagulation, but different for the imbalance between pro- and anti-fibrinolytic factors, resulting in hyper-fibrinolysis with an increased bleeding risk[35]. Despite all such evidence, most current guidelines still recommend correcting elevated PT/INR values through plasma transfusions, while tests capable of better capturing the hemostatic function of cirrhotic patients (thrombin generation tests, thromboelastography, etc.) are not readily available in everyday clinical practice[25].
The management of thrombocytopenia in chronic liver disease has been a primary and challenging endpoint for decades. In the 1960s, surgical splenorenal shunts were performed with this purpose, but they were soon abandoned due to high mortality rates and the risk of liver decompensation[36]. Total and partial splenectomy were therefore developed. They gained popularity in the 1990s thanks to limited complication rates, mainly after the introduction of the laparoscopic technique. Later, less invasive techniques, namely, splenic artery embolization or spleen radiofrequency ablation, opened new scenarios[36].
In the past, open splenectomy has been considered among the strategies for treating thrombocytopenia. Actually, this procedure was associated with a high risk of bleeding and consequent hepatic decompensation, particularly following the open technique. For these reasons, subsequent studies supported the laparoscopic technique over open surgical splenectomy or the shunt techniques over splenectomy[37,38]. Overall, also with less invasive procedures, the rate of complications range from 2.5% to 17%, and the risk of portal and splenic vein thrombosis is elevated (about 10%)[39]. Altogether, due to their invasive nature and to the high risk of complications, these strategies are restricted to rare and specific cases, while they have been virtually abandoned in the ordinary clinical practice.
Splenic artery embolization has been introduced since the 1970s as an alternative to splenectomy in surgically unfit patients. For the increased risk of developing splenic abscesses after total embolization, partial splenic embolization has become the preferred option for candidate patients[40], with a lower risk of complications, sepsis and mortality compared to total splenectomy[36,41]. Unfortunately, the extent of the beneficial effect on platelet count depends on the amount of the splenic mass embolized, which is proportional to the complications observed.
Radiofrequency ablation of the spleen is a minimally invasive procedure with promising results in patients with cirrhosis and severe thrombocytopenia[36]. The main benefits of this minimally invasive procedure are cost-effectiveness and lower complication rates over other invasive procedures. The major side effects are hemorrhagic shock and intra-abdominal bleeding, while complications such as splenic abscess or rupture are not a concern with this procedure[42]. Besides, to date, more clinical trials with longer follow-up would be needed to estimate the effectiveness of this strategy.
Shunt procedures [portocaval shunt, splenorenal shunt, and transjugular intrahepatic portosystemic shunt (TIPS)] are other possible techniques which have been experimented in chronic liver disease. They are aimed at decreasing splenic congestion and, therefore, platelet sequestration[36]. To date, however, these approaches are not supported by available studies; indeed, there is an absence of clear benefits on the platelet number and well-known complications. Actually, the use of these procedures, in particular TIPS, is currently restricted to selected cases where the aim is not to increase platelet levels, but to control bleeding from oesophageal varices or to manage refractory ascites[43].
Platelet transfusion has become the mainstay of treatment in patients with chronic liver disease and severe thrombocytopenia who require an invasive procedure[44]. The choice to transfuse platelets is variable and controversial, depending upon patient comorbidities and the risk of bleeding. Most data suggest that invasive procedures may be performed without a significantly increased risk of bleeding in patients with more than 50000/μL platelets, while there is less consensus about the risk in patients with a lower count, as reflected also in the latest guidelines and a recent retrospective study[45,46]. In a sub-analysis of 2740 liver biopsies from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis trial, there were only 16 bleeding events (0.6%), and the highest bleeding risk (5.3%) was recognized for platelet counts less than 60000/μL[47]. Conversely, in another study evaluating the safety of liver biopsies (177 patients), the frequency of bleeding in patients with a platelet count lower than 50000/μL was not significantly different from that in those with a normal platelet count, and the only independent risk factor for bleeding was an underlying malignancy[32].
Guidelines vary as to the threshold below which periprocedural bleeding risk justifies intervening to treat thrombocytopenia; however, transfusions are more frequently indicated with a platelet threshold < 50000/μL[48]. Although platelet transfusion has been the standard of care for correcting thrombocytopenia for a long time, relevant side effects limit its use. The most common issues include febrile or allergic reactions, risk of infection (even if very low), hemolysis, transfusion-related graft-versus-host disease, and alloimmunization[41,49]. Even the procedure of transfusion itself may be critical. Potential risks include errors in patient identification, in blood typing, and in cross-matching[50]. Concerning efficacy, platelet transfusions determine an overall modest increase in platelet count, without a significant impact on the risk of bleeding[51], and treatment effect has a limited duration. Moreover, up to 70% of patients receiving repeated platelet transfusions become refractory to subsequent ones. Finally, platelet transfusions are limited by donor supply, and the reduced availability may have a great impact on the management and clinical outcomes of these patients.
Recent advances in understanding the physiopathology of thrombocytopenia in chronic liver disease, with the discovery of the central role of TPO in thrombocytopoiesis, has led to the development of many drugs with TPO activity.
Two recombinant TPOs that stimulate platelet production in humans and showed potential promise were recombinant human TPO and pegylated recombinant megakaryocyte growth and development factor[52,53]. They have shown clinical benefits in clinical trials of haematological patients without safety concerns, but have been withdrawn from clinical development for the induction of neutralizing antibodies[53]. Similarly, the use of recombinant human cytokines was limited because of side effects and the occurrence of toxicities as observed after the subcutaneous injection of the recombinant human interleukin-1, approved by the Food and Drug Administration (FDA) for the treatment of the thrombocytopenia induced by chemotherapy for solid tumors, which can cause cardiovascular side effects and flu-like symptoms[54].
Activation of thrombopoiesis through TPO receptor agonists is an alternative method to stimulate platelet production with the use of drugs, which are not homologous to endogenous TPO but activate the same receptor working on a different site. These drugs have been primarily investigated in patients with chronic ITP and subsequently in chronic liver disease for treating thrombocytopenia before invasive procedures. The effect of TPO agonists is mediated by the interaction with TPO receptors on megakaryocytes, specifically c-MPL ligand-mediated activation of Janus kinases and signal transducer and activator of transcription proteins and mitogen-activated protein kinase pathways[55].
Romiplostin: Romiplostin is a polypeptide linked to an IgG heavy-chain Fc molecule and has no amino acid sequence homology to endogenous TPO. It acts by competing with the same site of the TPO-receptor and activating intracellular transcriptional pathways aimed to increase platelet production[55]. Due to a different molecular structure, its use has not been associated with antibodies reacting against endogenous TPO. This drug is administered subcutaneously once a week, but it is currently approved only for treatment of ITP when refractory to other drugs[54,56].
Indeed, most of the experience with romiplostin was derived from clinical studies in patients with refractory ITP[57], while only anecdotal case reports and small series involved patients with chronic liver disease[58-61]. A single-centre study in Egypt involved 35 patients with HCV-related cirrhosis and severe thrombocytopenia who required elective surgery[60]. Patients received romiplostin once weekly for a maximum of 4 wk. The primary endpoint - achieving a threshold of platelet count of 70000/μL - was reached in 94% of patients, and 20% of them had maintained a count > 50000/μL 3 mo after the last injection. Headache was reported as the only adverse event. In another single centre, prospective, randomized, double-blind study, 65 subjects with chronic liver disease and thrombocytopenia (less than 60000/μL) undergoing percutaneous liver biopsy received a TPO agonist or platelet transfusion[61]. Romiplostin determined significantly higher pre-biopsy and post-biopsy platelet counts compared to eltrombopag and platelet transfusion, and it was cost-effective and safe.
Eltrombopag: Eltrombopag is an orally available, small non-peptide TPO mimetic molecule more largely studied for use in liver disease. Its binding to a specific human transmembrane domain of TPO-receptor induces proliferation and differentiation of megakaryocytes and precursor cells[62]. It is taken orally once daily and is approved for thrombocytopenia: (1) In chronic ITP refractory to other treatments; (2) In HCV chronic hepatitis candidates for treatment with interferon-based regimens; and (3) In patients with severe aplastic anaemia[63].
Eltrombopag safely increased platelet number in patients with cirrhosis and HCV infection[64]. In a phase II multicentre randomized trial, eltrombopag was effective in increasing platelet count to more than 100000/μL at week 4 in 75%-95% of patients, compared to 0% of patients in the placebo group. Consequently, these patients were significantly more likely to initiate and complete 12 wk of antiviral treatment with respect to those on placebo (36%-65% vs 6%). In a phase II trial in Japan on 38 patients with chronic liver disease, eltrombopag increased platelet count in a dose-dependent manner[65].
The most recently published data in chronic liver disease are derived from the Eltrombopag Evaluated for Its Ability to Overcome Thrombocytopenia and Enable Procedures study, a phase 3 double-blind, placebo controlled trial that assessed the utility of this drug to increase platelet count and reduce the need for transfusion in patients undergoing elective procedures[66]. In this trial, 86% of patients recruited had liver cirrhosis with a platelet count less than 50000/μL. Patients were randomized to receive 75 mg eltrombopag daily or placebo in the 2 wk preceding the invasive procedure performed within 5 d from the last dose. Primary endpoint was the number of subjects who did not require a platelet transfusion before, during, and up to 7 d after the procedure. This was achieved in 72% (104/145) of subjects who received eltrombopag, compared to 19% (28/147) in the placebo group (P < 0.001). However, this study was prematurely terminated since six patients in the treatment group developed thrombotic events (2 patients in placebo, odds ratio for eltrombopag 3.04, 95% confidence interval: 0.62-14.82). Post-hoc analysis suggested an association of platelet count > 200000/μL with the occurrence of portal vein thrombosis[66].
The other two international phase III trials included patients with chronic hepatitis C and platelet counts less than 75000/μL[67]. Eltrombopag to Initiate and Maintain Interferon Antiviral Treatment to Benefit Subjects with Hepatitic C-Related Liver Disease (ENABLE)-1 and ENABLE-2 assessed the ability of eltrombopag to increase platelet count and, so, allow subjects to initiate and maintain antiviral treatment with pegylated interferon and ribavirin. In both trials, significantly more patients on eltrombopag achieved a sustained virological response at 24 wk of antiviral therapy, with similar adverse events. However, the absolute benefit over placebo was less than 10% and the use of this drug was associated with an increased risk of hepatic decompensation (10% vs 5% placebo) and thromboembolic events (3% vs 1% placebo). The most frequent adverse events reported were anaemia, pyrexia, and neutropenia.
Avatrombopag: Avatrombopag is an orally available drug that has a similar mode of action to eltrombopag, and it does not compete with endogenous TPO for its receptor site-binding. It is taken once daily with food for 5 d a week, with the dose adjusted according to baseline platelet count. Differently from eltrombopag, it exhibits significant drug-drug interactions based on the cytochrome P450 2C9 (CYP2C9) and CYP3A cytochrome systems[68]. In a phase II study, 130 patients with chronic liver disease and platelet count less than 60000/μL received two different formulations of the drug 1 wk prior to an elective invasive procedure, and both groups of avatrombopag-treated patients achieved the primary endpoint of an platelet increase of > 20000/μL from baseline, and to > 50000/μL, at least once during the treatment days 4-8.
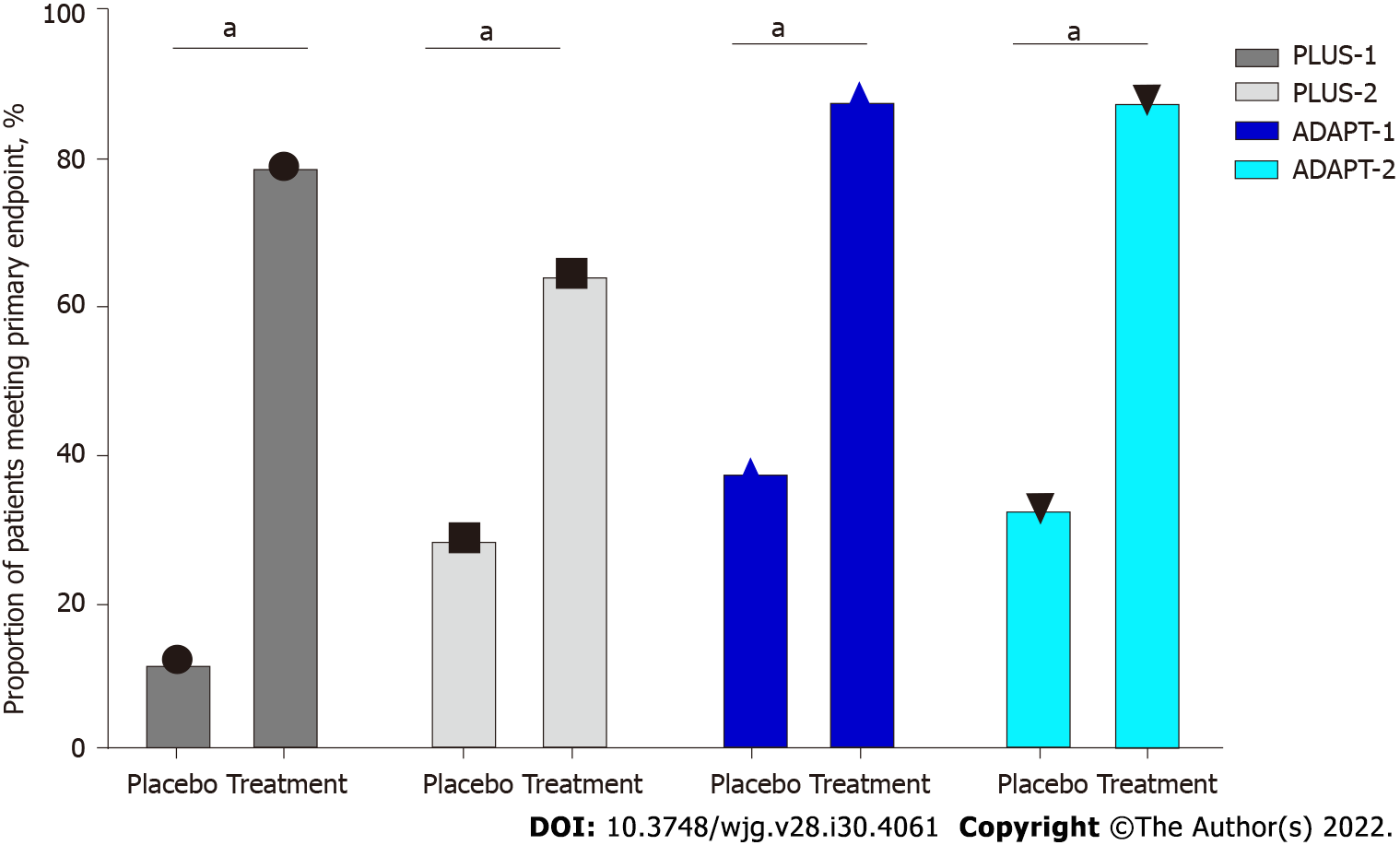
After that, avatrombopag was approved by the FDA in 2018 and the European Medicines Agency (EMA) in 2019 for the treatment of severe thrombocytopenia in patients with chronic liver disease undergoing invasive procedures, with the recommendation to take it 10-13 d before the procedure scheduled within 5-8 d from the last dose administration[69]. The safety and efficacy were evaluated in two pivotal randomized phase 3 studies[69]. ADAPT-1 and ADAPT-2 (Table 1) randomized 430 patients with cirrhosis and severe thrombocytopenia undergoing scheduled procedures to receive avatrombopag at different doses (according to platelet baseline count) or placebo for 5 d. The primary endpoint was the need for platelet transfusion or rescue procedures for bleeding in the 7 d after the procedures[70]. Significantly more patients met this endpoint in avatrombopag groups: In ADAPT-1, 65.6% and 88.1% compared to 22.9% and 38.2% of patients receiving placebo; in ADAPT-2, 68.6% and 87.9% compared to 34.9% and 33.3% (P < 0.001 for both) (Figure 3). Overall, the safety profile was similar to placebo and the most frequent adverse events were nausea, fatigue, abdominal pain, pyrexia, and headache. Serious adverse events occurred in 16%-19% of patients with avatrombopag and 6%-14% of those with placebo, including one patient who developed portal vein thrombosis during post-treatment follow-up. Finally, the safety and efficacy of avatrombopag were recently confirmed in a real-world setting, where cirrhotic patients mainly undergoing esophageal varices band ligation received the drug without requiring platelet transfusion, and with a good profile of adverse events[71,72].
| Key characteristic | ADAPT 1-2 |
| Key inclusion criteria | Chronic liver disease (MELD score ≤ 24) and thrombocytopenia (platelet counts < 50000/μL). Age ≥ 18 yr |
| Key exclusion criteria | ADAPT-1/2: History of thrombosis or hematologic disorders. Significant cardiovascular disease. Portal or splenic mesenteric system thrombosis at screening; portal vein blood flow < 10 cm/s at screening. Platelet transfusion within 7 d of screening. Use of heparin, warfarin, FANS, aspirin, verapamil, or antiplatelet therapy with ticlopidine or glycoprotein IIb/IIIa antagonists, or erythropoietin-stimulating agents within 7 d of screening. Advanced hepatocellular carcinoma (BCLC C or D) |
| Dosing | Low baseline platelet count cohort (≤ 40000/μL): 60 mg avatrombopag or placebo once daily with a meal on days 1-5. High baseline platelet count cohort (40000-50000/μL): 40 mg avatrombopag or placebo once daily with a meal on days 1-5 |
| Type of study | ADAPT-1/2: Global, multicenter, randomized, double-blind, placebo- controlled, phase 3 studies |
| Patients number | ADAPT-1: 231; ADAPT-2: 204 |
| Endpoints | Efficacy assessment: (1) Primary: Proportion of patients not requiring a platelet transfusion or rescue procedure for bleeding bleeding in the 7 d after the procedures; and (2) Secondary: Proportion of patients achieving the target platelet count of 50000/μL on procedure day; the change in platelet count from baseline to procedure day. Safety assessment: The incidence of adverse events adverse drug reactions, treatment-emergent adverse events |
Lusutrombopag: Lusutrombopag is an orally administered synthetic small molecule that acts as an agonist of human TPO, activating the signal transduction pathways to upregulate platelet production. Earlier studies have demonstrated that lusutrombopag raises platelet count and is a manageable drug that does not require food restrictions, and has no clinically significant drug-drug interactions[73,74]. Furthermore, it has demonstrated a dose-proportional pharmacokinetic with no clinically significant differences in the pharmacokinetic grounded on age, liver function (Child-Pugh classes A and B), and renal function (creatinine clearance greater than 30 mL/min)[75]. It is primarily metabolized by CPY4 enzymes, including CYP4A11, and it is mainly excreted by the faecal route (83% of dose). Moreover, it has a low potential to inhibit or induce transporter systems and CYP enzymes[73]. The recommended dose of the drug is 3 mg once daily for 7 d, beginning 8-14 d prior to the scheduled procedure.
Lusutrombopag was approved in Japan in 2015 for use in patients with thrombocytopenia and chronic liver disease who needed invasive procedures and received a positive opinion from the EMA Committee for Medicinal Products for Human Use in 2018[76]. The approval of this drug was based on the results of Lusutrombopag for the Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures trial (L-PLUS-1), a phase 3 double-blind study, carried out in Japan with 96 patients with chronic liver disease and severe thrombocytopenia (platelet count < 50000/μL) undergoing invasive procedures[77] (Table 2). In this study, the proportion of patients that did not require pre-operative platelet transfusions was significantly greater in the lusutrombopag group vs placebo (79.2 % vs 12.5%, respectively, P < 0.0001) (Figure 3). The median platelet count reached more than 50000/μL after 5 d in the drug group, with the greatest value observed after a mean of 13.4 d. Moreover, no significant concerns were raised in this study, and no significant adverse drug reactions were observed.
| Key characteristic | L-PLUS 1-2 |
| Key inclusion criteria | Chronic liver disease (Child Pugh A or B) and thrombocytopenia (platelet counts < 50000/μL). Age ≥ 18 yr (≥ 20 yr in L-PLUS 2) |
| Key exclusion criteria | L-PLUS 1: (1) Hematopoietic tumors; aplastic anemia, myelodysplastic syndrome, myelofibrosis, congenital, immune, or drug-induced thrombocytopenia; (2) Splenectomy, any other causes of thrombocytopenia; (3) History of portal vein thrombosis; (4) Active malignant tumor other than primary hepatic cancer; (5) Therapies that could influence platelet count (L-PLUS 1); (6) Chronic liver disease with Child-Pugh C; (7) Portal vein tumor embolism; and (8) Past or present thrombosis or prothrombotic condition. L-PLUS 2: (1) Hematopoietic tumors; aplastic anemia, myelodysplastic syndrome, myelofibrosis, congenital, immune, or drug-induced thrombocytopenia; (2) Portal vein thrombosis within 28 d prior to randomization or a history of portal vein thrombosis; absence of hepatopetal blood flow in the main trunk of the portal vein as demonstrated by doppler ultrasonography within 28 d prior to randomization; (3) Chronic liver disease with Child-Pugh C; (4) Portal vein tumor embolism; and (5) Past or present thrombosis or prothrombotic condition, history of splenectomy |
| Dosing | 3 mg lusutrombopag or placebo once daily for up to 7 d |
| Type of study | L-PLUS 1: Double-blind, parallel-group, phase 3 study; L-PLUS 2: Global, phase 3, randomized, double-blind, placebo-controlled study |
| Patients number | L-PLUS 1: 96; L-PLUS 2: 215 |
| Endpoints | Efficacy assessment: (1) Primary: Proportion of patients who required no platelet transfusions before the primary invasive procedure and no rescue therapy for bleeding; and (2) Secondary: Rate of response (defined as proportion of patients who achieved platelet count of more than 50000/μL with an increase of ≥ 20000/μL from baseline at any time during the study); the duration of sustained platelet count increase; time courses of changes in platelet count. Safety assessment: The incidence of adverse effects, adverse drug reactions, treatment-emergent adverse effects, bleeding-related adverse effects, and thrombotic events |
The safety and efficacy of this drug were confirmed in a larger study, the L-PLUS-2[78]: 215 patients with chronic liver disease and a platelet count < 50000/μL were randomly assigned to once-daily lusutrombopag at a dosage of 3 mg or placebo for ≤ 7 d before an invasive procedure. The procedure was performed within 7 d after the last dose. In the intention to treat analysis, significantly more patients in the lusutrombopag group (64.8%) met this endpoint compared with placebo (29%, P < 0001). This percentage was greater in the per-protocol analysis, with the endpoint reached in 72.5% of patients in the active drug group vs 20% in the placebo group (P < 0.0001). The median duration of the achievement of a target of platelet count > 50000/μL was 19.2 d in the lusutrombopag group vs 0 d for the patients who received placebo. Moreover, the median maximum change in platelets from baseline was over 4 times higher for patients treated with the active drug, who did not receive platelet transfusions, compared with patients who did receive transfusions (45000 vs 11000/μL)[78]. Finally, 47.7% of patients in the lusutrombopag group and 48.6% in the placebo group had at least one adverse event. These side effects were mainly mild or moderate in severity and the most common were headache, abdominal pain, fatigue, peripheral edema, and nausea. There were only three mild bleeding events in three patients in the lusutrombopag group (2.8%) vs seven bleeding events (4 moderate and 1 severe; 5.6%) with placebo. Only three thromboembolic events were recorded (1 in the lusutrombopag group), which were not related to platelet count.
The first real-life study in Japan enrolled 25 patients with cirrhosis, who were treated with lusutrombopag prior to invasive treatments (radiofrequency ablation, transarterial chemoembolization, and endoscopic variceal ligation)[79]. In this group, platelet count significantly increased compared with baseline (82000 ± 26000 vs 41000 ± 11000 /μL). The proportion of patients who needed platelet transfusions before procedures was very low (only 4, 16%) compared to those not treated with lusutrombopag (69 patients, 54%). Moreover, platelet counts after treatment and before invasive procedures were lower in patients with a count less than 30000/μL, and this cut-off, together with a spleen index > 40 cm2, was predictive of a lower response rate to the drug[80]. This was probably due to a larger number of platelets sequestered in the spleen in this subgroup of patients. No haemorrhagic complications were observed, and only a single case of recurrent portal vein thrombosis was observed and successfully treated.
Another real-life setting retrospective study was carried out in patients with chronic liver disease and severe thrombocytopenia. In this study[81], 74.2% of patients who received treatment did not require platelet transfusion before invasive procedures. This percentage increased to 82.1% of treatments if patients who repeated lusutrombopag use more times were included, thus demonstrating the efficacy of repeated use of the drug. Furthermore, only one serious adverse event was observed during/after treatment, i.e., one case of portal thrombosis disappearing after anticoagulation. Notably, this study confirmed that a lower platelet count at baseline was a predictive factor for failure to reach the target of > 50000/μL platelets. Indeed, median basal platelet count was higher in responders vs non-responders (38000/μL vs 12000/μL).
Moreover, the safety and efficacy of repeated use of lusutrombopag have been confirmed also in 66 patients who underwent radiofrequency ablation for recurrence of hepatocellular carcinoma[82]. Later, others reports have confirmed the efficacy and safety of lusutrombopag in real life in patients with thrombocytopenia due to chronic liver disease. In a case report, Kaneko et al[83] showed lusutrombopag to be a successful substitute for platelet transfusion in a patient with chronic liver disease undergoing endoscopic spinal surgery. Kawata et al[84] reported three patients treated with lusutrombopag before tooth extraction: Platelet count increased, preventing the need for transfusion in two of three cases. There were no adverse events. In addition, the efficacy and safety of the drug have been confirmed in a retrospective Japanese study based on hospital administrative databases. Here the incidence of bleeding events was lower in the lusutrombopag group than in the platelet transfusion group (3.7% vs 8.2%, P < 0.001), with a consequently lower average medical cost[85]. Finally, real-world data for adverse events (spontaneously reported by healthcare professionals and consumers in a database including about 4000 patients exposed to lusutrombopag from December 2015 to April 2018) confirm the efficacy and the safety of the drug (93% of patients did not require pre-procedural platelet transfusion; 1.2% of serious adverse events, with 0.4% cases of portal vein thrombosis)[86].
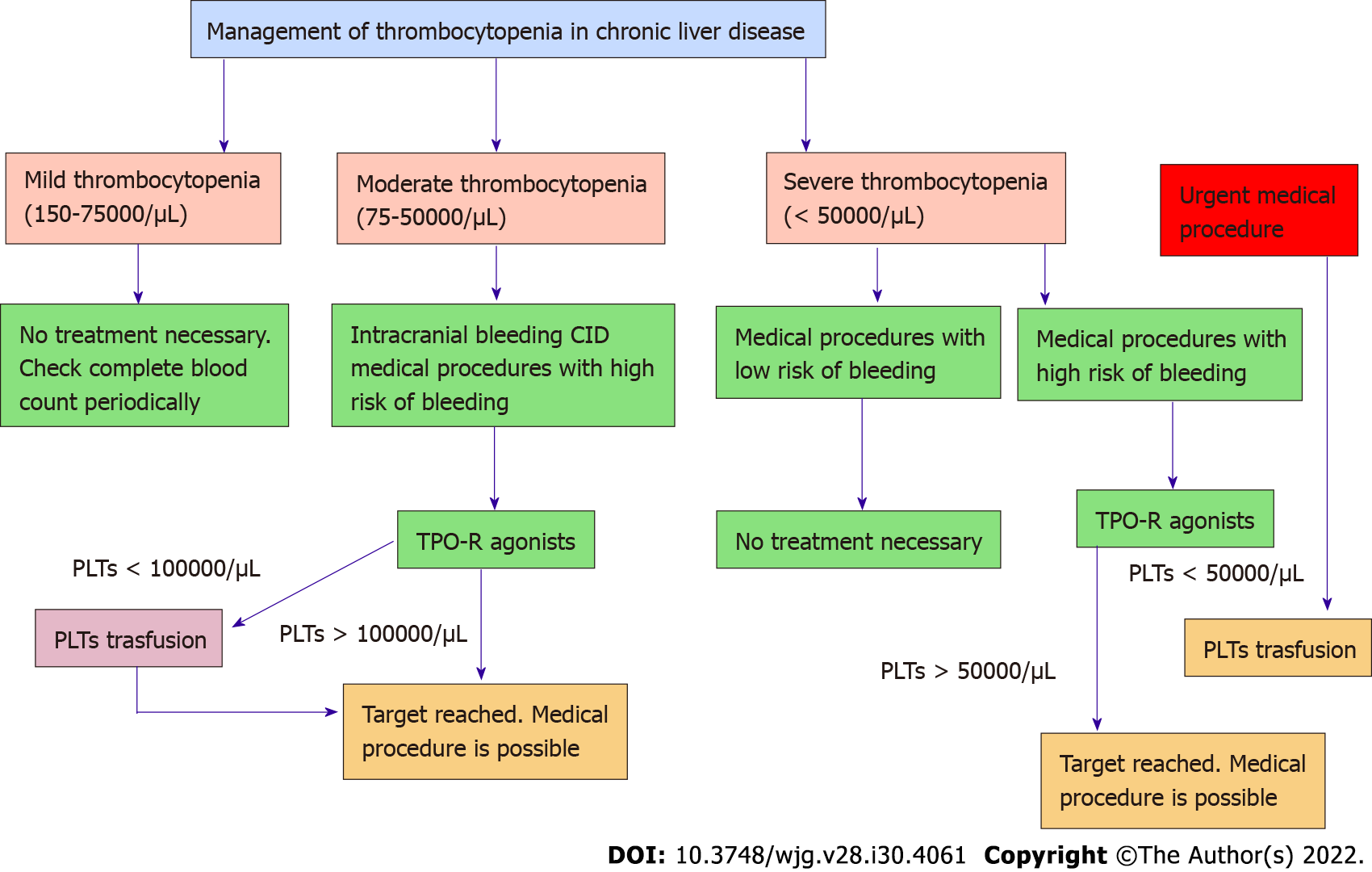
Thrombocytopenia represents one of the main coagulation disorders in patients with chronic liver disease. Recent awareness in its physiopathology shed light on the central role of TPO. The development of TPO receptor agonists has opened a new scenario in the management of patients with liver disease who need invasive procedures (Figure 4). Avatrombopag and lusutrombopag have demonstrated their efficacy and safety in increasing platelet count without an increased risk of thrombosis. Moreover, they reduce the overall clinical risk associated with platelet transfusion without any delay in the management of these patients. With careful logistical planning and coordination between drug availability and medical procedures, patients with chronic liver disease and severe thrombocytopenia should now be able to undergo more easily invasive procedures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishikawa T, Japan; Jiang W, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95:2936-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 554] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Saur SJ, Sangkhae V, Geddis AE, Kaushansky K, Hitchcock IS. Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood. 2010;115:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Sattler M, Durstin MA, Frank DA, Okuda K, Kaushansky K, Salgia R, Griffin JD. The thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinases. Exp Hematol. 1995;23:1040-1048. [PubMed] |
| 6. | Rouyez MC, Boucheron C, Gisselbrecht S, Dusanter-Fourt I, Porteu F. Control of thrombopoietin-induced megakaryocytic differentiation by the mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:4991-5000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Martin TG 3rd, Somberg KA, Meng YG, Cohen RL, Heid CA, de Sauvage FJ, Shuman MA. Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med. 1997;127:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Ishikawa T, Ichida T, Matsuda Y, Sugitani S, Sugiyama M, Kato T, Miyazaki H, Asakura H. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol. 1998;13:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Ishikawa T, Ichida T, Matsuda Y, Sugitani S, Sugiyama M, Kato T, Miyazaki H, Asakura H. Expression of hepatic thrombopoietin mRNA in primary cultured hepatocytes and in rats with acute liver injury or bone marrow suppression with or without cirrhosis. J Gastroenterol Hepatol. 2000;15:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ishikawa T, Ichida T, Sugahara S, Yamagiwa S, Matsuda Y, Uehara K, Kato T, Miyazaki H, Asakura H. Thrombopoietin receptor (c-Mpl) is constitutively expressed on platelets of patients with liver cirrhosis, and correlates with its disease progression. Hepatol Res. 2002;23:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986;78:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O. Haemochromatosis. Nat Rev Dis Primers. 2018;4:18016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (2)] |
| 13. | Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 353] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Uemura M, Fujimura Y, Matsumoto M, Ishizashi H, Kato S, Matsuyama T, Isonishi A, Ishikawa M, Yagita M, Morioka C, Yoshiji H, Tsujimoto T, Kurumatani N, Fukui H. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol. 1995;50:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Sanjo A, Satoi J, Ohnishi A, Maruno J, Fukata M, Suzuki N. Role of elevated platelet-associated immunoglobulin G and hypersplenism in thrombocytopenia of chronic liver diseases. J Gastroenterol Hepatol. 2003;18:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 17. | Bassendine MF, Collins JD, Stephenson J, Saunders P, James OF. Platelet associated immunoglobulins in primary biliary cirrhosis: a cause of thrombocytopenia? Gut. 1985;26:1074-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Pawlotsky JM, Bouvier M, Fromont P, Deforges L, Duval J, Dhumeaux D, Bierling P. Hepatitis C virus infection and autoimmune thrombocytopenic purpura. J Hepatol. 1995;23:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Panzer S, Seel E. Is there an increased frequency of autoimmune thrombocytopenia in hepatitis C infection? Wien Med Wochenschr. 2003;153:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 329] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013;1:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2003;497-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Jandl JH, Aster RH. Increased splenic pooling and the pathogenesis of hypersplenism. Am J Med Sci. 1967;253:383-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019;157:34-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 26. | Under the auspices of the Italian Association for the Study of Liver Diseases (AISF) and the Italian Society of Internal Medicine (SIMI). Hemostatic balance in patients with liver cirrhosis: Report of a consensus conference. Dig Liver Dis. 2016;48:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing Concepts of Cirrhotic Coagulopathy. Am J Gastroenterol. 2017;112:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 28. | Tripodi A. Hemostasis abnormalities in cirrhosis. Curr Opin Hematol. 2015;22:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 951] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 30. | Thakrar SV, Mallett SV. Thrombocytopenia in cirrhosis: Impact of fibrinogen on bleeding risk. World J Hepatol. 2017;9:318-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Violi F, Ferro D. Clotting activation and hyperfibrinolysis in cirrhosis: implication for bleeding and thrombosis. Semin Thromb Hemost. 2013;39:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8:899-902; quiz e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ. Review article: the prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell SH, Violi F; faculty of the 7th International Coagulation in Liver Disease. Concepts and Controversies in Haemostasis and Thrombosis Associated with Liver Disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost. 2018;118:1491-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 35. | Joist JH. AICF and DIC in liver cirrhosis: expressions of a hypercoagulable state. Am J Gastroenterol. 1999;94:2801-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Gangireddy VG, Kanneganti PC, Sridhar S, Talla S, Coleman T. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol. 2014;28:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Yamamoto S, Hidemura R. Surgical treatment of portal hypertension--with special reference to the feature of intrahepatic circulatory disturbances. Jpn Circ J. 1964;28:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Shigekawa Y, Uchiyama K, Takifuji K, Ueno M, Hama T, Hayami S, Tamai H, Ichinose M, Yamaue H. A laparoscopic splenectomy allows the induction of antiviral therapy for patients with cirrhosis associated with hepatitis C virus. Am Surg. 2011;77:174-179. [PubMed] |
| 39. | Hassn AM, Al-Fallouji MA, Ouf TI, Saad R. Portal vein thrombosis following splenectomy. Br J Surg. 2000;87:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 40. | Witte CL, Ovitt TW, Van Wyck DB, Witte MH, O'Mara RE, Woolfenden JM. Ischemic therapy in thrombocytopenia from hypersplenism. Arch Surg. 1976;111:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 42. | Liu Q, Ma K, He Z, Dong J, Hua X, Huang X, Qiao L. Radiofrequency ablation for hypersplenism in patients with liver cirrhosis: a pilot study. J Gastrointest Surg. 2005;9:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 44. | Saab S, Brown RS Jr. Management of Thrombocytopenia in Patients with Chronic Liver Disease. Dig Dis Sci. 2019;64:2757-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 375] [Article Influence: 93.8] [Reference Citation Analysis (1)] |
| 46. | Ronca V, Barabino M, Santambrogio R, Opocher E, Hodson J, Bertolini E, Birocchi S, Piccolo G, Battezzati P, Cattaneo M, Podda GM. Impact of Platelet Count on Perioperative Bleeding in Patients With Cirrhosis Undergoing Surgical Treatments of Liver Cancer. Hepatol Commun. 2022;6:423-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, Dienstag JL; HALT–C Trial Group. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 48. | ASGE Standards of Practice Committee; Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (2)] |
| 49. | Murphy MF, Waters AH. Clinical aspects of platelet transfusions. Blood Coagul Fibrinolysis. 1991;2:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Kaufman RM, Assmann SF, Triulzi DJ, Strauss RG, Ness P, Granger S, Slichter SJ. Transfusion-related adverse events in the Platelet Dose study. Transfusion. 2015;55:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Nuttall GA, Stubbs JR, Oliver WC Jr. Transfusion errors: causes, incidence, and strategies for prevention. Curr Opin Anaesthesiol. 2014;27:657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457-3469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 53. | Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241-3248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 478] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 54. | Demetri GD. Targeted approaches for the treatment of thrombocytopenia. Oncologist. 2001;6 Suppl 5:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Miyazaki H. Update on thrombopoietin in preclinical and clinical trials. Curr Opin Hematol. 1998;5:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | U.S. Food and Drug Administration. NPLATE® (romiplostim). [cited 24 December 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125268s163lbl.pdf. |
| 57. | Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, Liebman HA, Slovick FT, de Wolf JT, Bourgeois E, Guthrie TH Jr, Newland A, Wasser JS, Hamburg SI, Grande C, Lefrère F, Lichtin AE, Tarantino MD, Terebelo HR, Viallard JF, Cuevas FJ, Go RS, Henry DH, Redner RL, Rice L, Schipperus MR, Guo DM, Nichol JL. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 622] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 58. | Voican CS, Naveau S, Perlemuter G. Successful antiviral therapy for hepatitis C virus-induced cirrhosis after an increase in the platelet count with romiplostim: two case reports. Eur J Gastroenterol Hepatol. 2012;24:1455-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Castellote J, Girbau A, Arajol C, Xiol X. Romiplostim in chronic liver disease with severe thrombocytopenia undergoing an elective invasive procedure. Rev Esp Enferm Dig. 2011;103:556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Moussa MM, Mowafy N. Preoperative use of romiplostim in thrombocytopenic patients with chronic hepatitis C and liver cirrhosis. J Gastroenterol Hepatol. 2013;28:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Basu P, Nair T, Jafri M, Shah James N, Farhat S, Foustin S. Single use of romiplostim thrombopoietin analogue in severe thrombocytopenia for outpatient percutaneous liver biopsy in patients with chronic liver disease—a randomized double blinded prospective trial. Gut. 2012;56:38. [DOI] [Full Text] |
| 62. | Dieterich DT, Bernstein D, Flamm S, Pockros PJ, Reau N. Review article: a treatment algorithm for patients with chronic liver disease and severe thrombocytopenia undergoing elective medical procedures in the United States. Aliment Pharmacol Ther. 2020;52:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | U.S. Food and Drug Administration. PROMACTA® (eltrombopag). [cited 24 December 2021]. Available from: https://www.novartis.us/sites/www.novartis.us/files/promacta.pdf. |
| 64. | McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC, Campbell FM, Theodore D, Blackman N, Jenkins J, Afdhal NH; TPL102357 Study Group. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 65. | Kawaguchi T, Komori A, Seike M, Fujiyama S, Watanabe H, Tanaka M, Sakisaka S, Nakamuta M, Sasaki Y, Oketani M, Hattori T, Katsura K, Sata M. Efficacy and safety of eltrombopag in Japanese patients with chronic liver disease and thrombocytopenia: a randomized, open-label, phase II study. J Gastroenterol. 2012;47:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, Campbell F, Hyde D, Brainsky A, Theodore D; ELEVATE Study Group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 67. | Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A, Rodriguez-Torres M, Rugina S, Bakulin I, Lawitz E, Shiffman ML, Tayyab GU, Poordad F, Kamel YM, Brainsky A, Geib J, Vasey SY, Patwardhan R, Campbell FM, Theodore D. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442-52.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Moore AH. Thrombocytopenia in Cirrhosis: A Review of Pathophysiology and Management Options. Clin Liver Dis (Hoboken). 2019;14:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | U.S. Food and Drug Administration. DOPTELET® (avatrombopag). [cited 24 December 2021]. Available from: https://doptelet.com/themes/pdf/prescribing-information.pdf. |
| 70. | Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, Allen LF, Hassanein T. Avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients With Chronic Liver Disease and Thrombocytopenia. Gastroenterology. 2018;155:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 71. | Verma D, Yum JJ, LeRoy K, McDaniel TK, Saab S. Real-life experience with avatrombopag. Dig Med Res. 2021;4. [DOI] [Full Text] |
| 72. | Saab S, McDaniel TK, Bau SN, Patel RP. Efficacy of repeat doses of avatrombopag: a case series. Dig Med Res. 2019;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | U.S. Food and Drug Administration. MULPLETA® (lusutrombopag). [cited 24 December 2021]. Available from: https://doptelet.com/themes/pdf/prescribing-information.pdf. |
| 74. | Tateishi R, Seike M, Kudo M, Tamai H, Kawazoe S, Katsube T, Ochiai T, Fukuhara T, Kano T, Tanaka K, Kurokawa M, Yamamoto K, Osaki Y, Izumi N, Imawari M. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2019;54:171-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 75. | Katsube T, Ishibashi T, Kano T, Wajima T. Population Pharmacokinetic and Pharmacodynamic Modeling of Lusutrombopag, a Newly Developed Oral Thrombopoietin Receptor Agonist, in Healthy Subjects. Clin Pharmacokinet. 2016;55:1423-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Kim ES. Lusutrombopag: First Global Approval. Drugs. 2016;76:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Hidaka H, Kurosaki M, Tanaka H, Kudo M, Abiru S, Igura T, Ishikawa T, Seike M, Katsube T, Ochiai T, Kimura K, Fukuhara T, Kano T, Nagata T, Tanaka K, Kurokawa M, Yamamoto K, Osaki Y, Izumi N, Imawari M. Lusutrombopag Reduces Need for Platelet Transfusion in Patients With Thrombocytopenia Undergoing Invasive Procedures. Clin Gastroenterol Hepatol. 2019;17:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 78. | Peck-Radosavljevic M, Simon K, Iacobellis A, Hassanein T, Kayali Z, Tran A, Makara M, Ben Ari Z, Braun M, Mitrut P, Yang SS, Akdogan M, Pirisi M, Duggal A, Ochiai T, Motomiya T, Kano T, Nagata T, Afdhal N. Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2). Hepatology. 2019;70:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 79. | Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, Yasui Y, Tamaki N, Takaura K, Komiyama Y, Higuchi M, Kubota Y, Wang W, Okada M, Shimizu T, Watakabe K, Enomoto N, Izumi N. Real-life experience of lusutrombopag for cirrhotic patients with low platelet counts being prepared for invasive procedures. PLoS One. 2019;14:e0211122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Uojima H, Arase Y, Itokawa N, Atsukawa M, Satoh T, Miyazaki K, Hidaka H, Sung JH, Kako M, Tsuruya K, Kagawa T, Iwakiri K, Horie R, Koizumi W. Relationship between response to lusutrombopag and splenic volume. World J Gastroenterol. 2018;24:5271-5279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Nomoto H, Morimoto N, Miura K, Watanabe S, Takaoka Y, Maeda H, Sasaki T, Koyashiki Y, Kurata H, Numao N, Isoda N, Yamamoto H. Lusutrombopag is effective and safe in patients with chronic liver disease and severe thrombocytopenia: a multicenter retrospective study. BMC Gastroenterol. 2020;20:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Ishikawa T, Okoshi M, Tomiyoshi K, Kojima Y, Horigome R, Imai M, Nozawa Y, Iwanaga A, Sano T, Honma T, Yoshida T. Efficacy and safety of repeated use of lusutrombopag prior to radiofrequency ablation in patients with recurrent hepatocellular carcinoma and thrombocytopenia. Hepatol Res. 2019;49:590-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Kaneko T, Takano Y, Ishibashi K. Lusutrombopag as a substitute for platelet transfusion for thrombocytopenia associated with chronic liver disease in a patient undergoing endoscopic spinal surgery: A case report. Medicine (Baltimore). 2021;100:e24094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Kawata Y, Endou M, Isozaki Y, Kitamura T, Fukushima Y, Sato T. Three cases of patients with chronic liver disease complicated by thrombocytopenia who were treated with lusutrombopag before tooth extraction. J Oral Max Surg Med. 2021;33:463-466. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Yoshida M, Tateishi R, Hiroi S, Hongo Y, Fujiwara M, Kitanishi Y, Iwasaki K, Takeshima T, Igarashi A. Effects of Lusutrombopag on Post-invasive Procedural Bleeding in Thrombocytopenic Patients with Chronic Liver Disease. Adv Ther. 2022;39:379-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Brown RS, Izumi N, Kano T, Ochiai T, Kurosaki M, Violi F, Shrestha P. Lusutrombopag is a safe treatment option for thrombocytopenia in patients with chronic liver disease undergoing a scheduled invasive procedure: pooled safety analysis from 3 studies. Proceedings of the 24th Congress of the European Hematology Association (EHA); 2019 Jun 13-16; Amsterdam, Netherlands. Netherlands: EHA Library. |