Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3793
Peer-review started: January 18, 2022
First decision: March 8, 2022
Revised: April 10, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 7, 2022
Processing time: 197 Days and 3.8 Hours
Chronic Hepatitis B is a highly prevalent disease worldwide and is estimated to cause more than 800000 annual deaths from complications such as cirrhosis and hepatocellular carcinoma (HCC). Although universal hepatitis B vaccination programs may have reduced the incidence and prevalence of chronic hepatitis B and related HCC, the disease still imposes a significant healthcare burden in many endemic regions such as Africa and the Asia-Pacific region. This is especially concerning given the global underdiagnosis of hepatitis B and the limited availability of vaccination, screening, and treatment in low-resource regions. Demographics including male gender, older age, ethnicity, and geo
Core Tip: While many studies in the past have analyzed the impact of various epidemiological and socioeconomic factors on viral hepatitis and hepatocellular carcinoma (HCC), this minireview is the first to adopt a global perspective in highlighting the impact of both epidemiologic and socioeconomic factors on current trends in chronic hepatitis B and related HCC. We highlight trends in incidence, prevalence and mortality of chronic hepatitis B seen throughout the world in the past few decades and the disparity in healthcare distribution and outcomes between different populations.
- Citation: Gnyawali B, Pusateri A, Nickerson A, Jalil S, Mumtaz K. Epidemiologic and socioeconomic factors impacting hepatitis B virus and related hepatocellular carcinoma. World J Gastroenterol 2022; 28(29): 3793-3802
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3793
In 2019, the World Health Organization (WHO) estimated the worldwide prevalence of chronic hepatitis B virus (HBV) to be around 296 million with the incidence of new HBV infection to be 1.5 million each year. HBV is more prevalent with high burden of disease in the regions of Africa, Western Pacific and South-East Asia compared to North America and Europe. Furthermore, fewer people in the African and South-East Asian regions knew about their HBV status and had access to treatment compared to the latter regions[1].
Hepatocellular carcinoma (HCC) in unsuspected HBV patients is a major cause of increased morbidity and mortality in low-income countries with limited resources[2,3]. While it may be too early to see the true impact of the global HBV vaccination initiative led by the WHO or treatment efforts for HBV-related HCC, evidence thus far demonstrates decreased burden of HBV and HCC in children and suggests treatment with antivirals can reduce HCC risk in some patients[4-8]. There continues to be great disparity in access to vaccines, treatment, and screening programs worldwide, however. Even where there is access, the risk of HCC in treated chronic HBV is not fully mitigated[9,10]. While concomitant liver diseases are certainly at play including co-infection with hepatitis C, aflatoxin exposure, metabolic syndrome, and alcohol use disorder, chronic HBV-related HCC has a significant global disease burden that disproportionately impacts people of different regions and demographics[9,11]. The aim of this review is to examine the impact of epidemiologic and socioeconomic factors on chronic HBV-related HCC from a global perspective. Strategies to address the resulting disparities in disease outcomes will also be discussed.
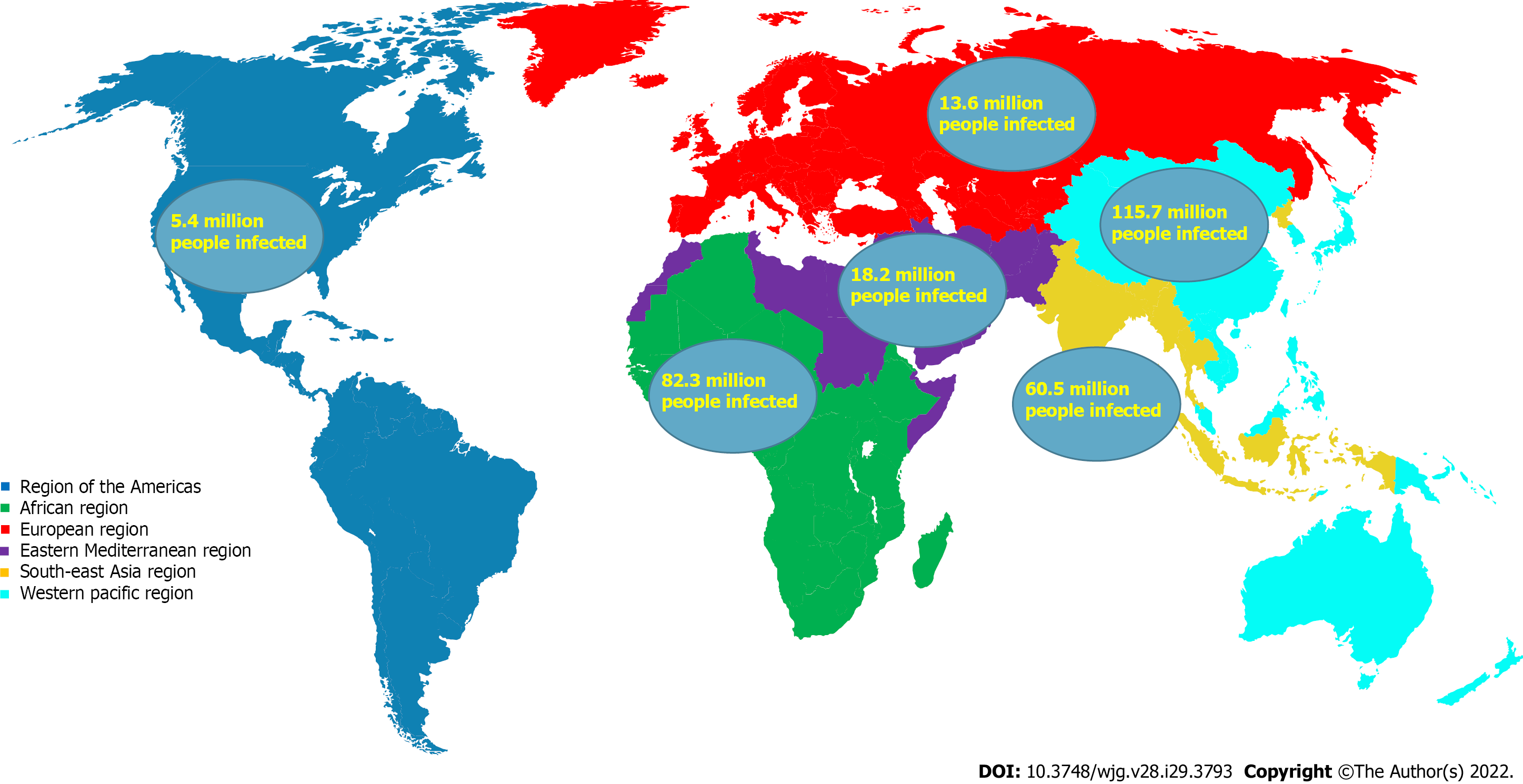
There is significant global variation in the prevalence of chronic HBV between regions. As a result, the incidence and prevalence of HBV related HCC are also quite variable between regions and correlate with rate of HBV infection[12]. About 50%-80% of HCC can be linked to HBV worldwide, and the high-risk HCC regions represent 80% of the global burden of HCC[13]. Regions such as Africa, Southeast Asia, and the Western Pacific, are considered high-risk HCC regions owed partially due to high seroprevalence of chronic HBV which is estimated at 5%-10%. On the contrary, North America and Western Europe are low-risk HCC regions with an HBV prevalence of < 1%. The Middle East and the Indian subcontinent are intermediate-risk regions with HBV prevalence of 2%-5%[14]. Data illustrating the geographic distribution of chronic HBV by prevalence is seen in Table 1 and Figure 1.
| Geographic region[22,40] | People living with hepatitis B infection |
| African region | 82.3 million (62.1-114.7 million) |
| Region of the Americas | 5.4 million (3.1-12.0 million) (2.1 million in Latin America and the Caribbean) |
| Eastern Mediterranean region | 18.2 million (14.4-23.8 million) |
| European region | 13.6 million (10.2-22.1 million) |
| South-East Asia region | 60.5 million (45.3-120.9 million) |
| Western Pacific region | 115.7 million (95.2-141.9 million) |
Prevalence of HBV in Asia and Africa within high-risk regions is not uniform. In Africa, particularly West Africa and Sub-Saharan Africa, complications of chronic HBV including cirrhosis and HCC are frequent and fatal due to a relative lack of vaccination, surveillance and treatment[15]. Sub Saharan Africa had an estimated vaccination rate of less than 10% and fewer than 1% of HBV infections were diagnosed in 2019[9].
In East Asia, China has a high prevalence of HBV-related HCC, estimated to be 50% of the world’s burden of HCC. Mongolia has the highest incidence rate of HCC in the world at 93.7 per 100000 with HBV being the predominant risk factor[9]. However, countries of low HCC incidence also exist in the Asia-Pacific region such as Japan, India, Singapore, and Pakistan. Interestingly, Japan has a low incidence of HBV where hepatitis C virus (HCV) is the major contributor of HCC, estimated to account for 80%-90% of cases[11,14,16].
Data from Asian nations such as India, China, Thailand and Nepal show that there are evident disparities in HBV prevalence within population sub-groups[17,18]. Surveys of the Tibetan population in Nepal’s Kathmandu valley showed prevalence of chronic HBV of 10%-20% compared to the overall prevalence of 0.9% in Nepal[18]. The general population of China has an estimated HBV prevalence of 6.89% while Western provinces have a higher prevalence at 8.92%[19]. The hill tribe of Chiang Rai province in Thailand see a significantly higher prevalence of chronic HBV at 26.6% while the overall prevalence in Thailand is 5.1%[17,20]. Although Southeast Asia as a region has a higher prevalence of HBV infection at 3.0%, there are variations in prevalence between ethnicities within the region. Given the relatively high prevalence rate of chronic HBV infection in Southeast Asia compared to Europe and the Americas, the incidence of liver cancer being highest in these regions is not surprising[11,21]. Some explanations for the disparities seen in the various regions and ethnic groups include unequal access to vaccines, limited health care programs, lack of health literacy, and elements of culture and religion that also serve a role[17,19,21].
Immigration from endemic countries is the main contributor to chronic HBV cases in the United States, which has a prevalence rate of 0.5% compared to 3.8% globally[21]. An estimated 70% of HBV infections in the United States are among foreign-born individuals with an estimated 730000 to 2.2 million living with chronic HBV[22]. Screening studies of foreign-born immigrants from Africa, the Middle East, and Asia have shown a higher chronic HBV prevalence rate of 10%-15% compared to 0.27%-0.50% for the general population[11,12,23,24]. Chronic HBV infection is disproportionately higher amongst Asian Americans in the United States[25]. Asian Americans comprise roughly 5%-6% of the population in the United States but 50% of the cases of chronic HBV[22].
Studies have also shown ethnic variation in HBV-related HCC within the United States, owing to differing sizes of immigrant populations from endemic countries. While rates of HCC in Asian and Pacific Islanders, once elevated are now declining, the incidence of HCC in Hispanics, Whites and African Americans continues to increase, driven mainly by HCV and non-alcoholic steatohepatitis (NASH) related cirrhosis[7,23,26-28]. Southern and Western states have the highest incidence rates of HCC in the United States[28]. While Asian Americans have the lowest mortality rates from HCC of all ethnicities, the highest mortality rates are seen in African Americans[29,30]. This apparent disparity may be partially explained by the etiology of HCC which include alcohol use disorder and HCV related cirrhosis, as well as health care disparities and access to quality care among African Americans. Recent studies show a disparity even after liver transplantation in patients with HCC, with African Americans consistently having worse survival than Asian Americans and White American patients[31].
Western Europe, like the United States, has a growing diversity in its population. Most chronic HBV infections in Western European countries are due to migrant populations, with an HBV prevalence estimated to be around 4% for migrant populations and < 1% for the general population[23,27]. There is also a greater prevalence of cirrhosis and HCC in the foreign-born population[32]. A population-based study of HCC in England found a higher proportion of HCC in non-white ethnicities, particularly due to viral hepatitis-related HCC[33]. Similar findings are reported in studies of other low HCC risk countries such as Austria, Finland, Netherlands, Germany, the United Kingdom and Denmark in which migrant populations are over-represented in cases of chronic HBV and HCC[32,34].
There is a reported heterogeneous distribution in chronic HBV prevalence amongst Latin American countries. Owed to variation in the endemicity of HBV and underreporting, some studies report an estimated 7-12 million people infected in Central and South America and the Caribbean[35]. In contrast, a report by the Pan American Health Organization and the WHO in 2016 suggested chronic HBV seroprevalence in Latin America being 0.33% with an estimated 2.1 million infected in the general population. However, the variation in HBV serum antigen (HBsAg) seroprevalence ranged from 0.20% to 13.55% among the numerous countries[36].
In the majority of Latin America, HCV and alcoholic liver disease are the leading causes of HCC. HBV is more endemic to certain countries such as Brazil, Argentina, and Peru.
Systematic reviews and retrospective studies have shown HBV-related HCC accounting for 12%-14% of HCC in South and Central America with the countries Peru and Brazil having 20%-60% of their HCC cases related to HBV infection[37-39].
Although Latin American countries are more varied in HBV prevalence and range from low to high prevalence, overall HCC risk and burden is similarly as low as the rest of the Americas in a global context[11,36].
Chronic HBV has a greater prevalence in males than females across all geographic regions[13]. Males also have a greater incidence, prevalence, and mortality from HCC than females across geographic location and age; studies have reported a 2 to 3 times increased risk of developing HCC in males compared to females[26,27,40,41].
Regions of higher chronic HBV prevalence such as sub-Saharan Africa and Southeast Asia, tend to also have a higher male to female ratio of HCC incidence. In the United States, both sexes have shown a trend of rising incidence rates of HCC since 1975, with HBV estimated to account for 10%-15% of HCC cases[42]. The gender disparity is not completely understood but is believed to be partially due to many overlapping risk factors that are more common in males, including alcoholism, diabetes, viral hepatitis, and tobacco use[43]. In the United States, heavy alcohol usage and tobacco use is much more common in males with both being independent risk factors for incidence of HCC[43,44]. In contrast, metabolic syndrome is more common in women, with one retrospective study reporting it accountable for 32% of HCC burden in the United States[27,44].
Despite controlling for other risk factors, male sex continues to remain an independent risk factor for HCC. Studies have linked higher testosterone levels to greater incidence of HCC in chronic HBV patients and estrogen replacement therapy to reduced risk of developing HCC[42,45,46]. Serum testosterone level has been associated with upregulated inflammatory activity while estrogen has shown to have an anti-inflammatory effect by inhibition of the NF-kB pathway[46-48]. Estrogen may be protective against development of HBV-related HCC through decreasing HBV RNA transcription which could explain higher viral loads seen in male carriers of HBV[12,47]. However, studies have failed to demonstrate a benefit in survival from hormonal therapy such as flutamide, an anti-androgen, and leuprorelin, a gonadotropin-releasing hormone agonist which has anti-androgen effects[48].
The average age of chronic HBV patients has continued to increase over time. A study comparing chronic HBV patients derived from an insurance claims database of Medicaid and Medicare patients found that the median age had risen from 44.1 to 50.2 years for Medicaid patients and 48.1 to 51.8 for Medicare patients[49]. This trend has also been seen in studies from other countries. A large territory-wide cohort study conducted in Hong Kong found the mean age for Chronic HBV had increased from 41 in 2000-2004 to 55 in 2014-2017[50]. Chronic HBV is now presenting at an older median age due to longer life expectancy, under diagnosis of HBV, under screening, and delayed treatment[21,51]. There is also improved vaccine-induced immunity in the 20-49 years age group which is predicted to cause a continual upward shift in the median age of diagnosis[49].
In populations at low risk for HBV infection, such as in Western Europe and the United States where HBV in not endemic, HCC is rarely seen before the fourth decade of life with a mean age of diagnosis around 65 years[12]. In contrast, endemic regions such as Southeast Asia and sub-Saharan Africa, where > 80% of HCC cases occur, have mean ages of diagnosis about one decade earlier[42]. China has a mean age of diagnosis of HCC around 55-59 years[12]. In countries in Sub-Saharan Africa, mean age of diagnosis of HCC is 35-50 years, and found to be almost 20 years later in those Black Africans who migrate from a rural to city setting[12,52].
With the advent of the HBV vaccine in 1981, and the universal vaccination programs that began in the 1990s, there was a new focus on vaccination of newborns. As of 2020, 190 WHO member countries vaccinated newborns as part of their routine vaccination schedules, and global coverage with all 3 doses of HBV vaccine was estimated to be 83%[53]. This campaign also included recommendations to vaccinate high-risk adult populations, adolescents who had missed immunization, and advocated for societal awareness of the risks and consequences of HBV[22]. Asian males who are HBV carriers continue to present with HCC at a relatively young age[54]. This may be related to viral factors such as genotype. Genotype B is more commonly seen in the Asian demographic and has been associated with onset of HCC in patients under 50 years of age, with one study from Taiwan finding that more than 90% under 35 years of age had genotype B HBV[47,55]. Genotype C is associated with the highest risk of developing HCC in patients aged > 50 years[56]. Genotype F has been seen in Alaskan Native populations to have the greatest risk of developing HCC at a lower median age, with the annual incidence rate amongst men at 387/100000 and 63/100000 for women[57]. In contrast, genotypes A, D are less frequently associated with development of HCC and more common in North American and European populations[58].
Patients belonging to low socio-economic status are at significant disadvantage due to low health literacy, limited healthcare resources and access-including lack of insurance or ability to pay for care, especially for care of preventable diseases such as HBV. Hepatitis B is very infectious as it can be transmitted by contact with blood or bodily fluids, sexual intercourse and vertically from mother to baby. Vertical transmission is the most common mode of transmission in the developing world and can be dramatically reduced by HBV vaccination and use of anti-viral medications during third trimester[7]. Horizontal transmission seems to be more common in low prevalence regions[13,22]. While Hepatitis B viral load is considered one of the strongest predictors of HCC risk and can be managed with anti-viral medications, unfortunately, even in low-risk regions such as United States, roughly 3% of people currently living with chronic HBV are on treatment[14,21]. Several studies have shown the impact of socioeconomic status (SES) on health outcomes in cancer with populations of lower SES and less wealthy nations having significantly lower survival rates[32,59-61]. One retrospective analysis of European nations found nearly 20% variation in all-cancer relative survival between the least wealthy and most wealthy nations[61]. Similarly, low SES groups are associated with a variety of risk factors for poor outcomes in chronic HBV-related HCC. Despite 5-year survival rate for HCC in the United States increasing to 18% in 2019, the greatest benefits in survival and mortality are seen in groups with higher SES, while higher HCC incidence, later stage of diagnosis, and lower survival rates are seen in low-SES status groups[28,40]. Disadvantaged groups are typically minority ethnic groups such as African Americans and Hispanics as they tend to live in areas with the highest rates of family poverty, unemployment and high-school dropouts and thus may be associated with greater risk for HCC due to less access to screening and treatment[62].
Among patients with Medicaid insurance, the presence of comorbidities such as obesity, diabetes, alcohol, and tobacco use disorders combined with lower educational attainment contribute to liver disease such as alcoholic and NASH (ASH/NASH) and significantly overlap with liver disease from chronic HBV[63,64].
Globally, chronic HBV and HCC cases are underdiagnosed. The 2016 Polaris Observatory study estimated that only 10% of infected people were diagnosed with HBV[51]. Chronic HBV infection is often asymptomatic and requires greater emphasis on screening, especially considering that HBV can lead to HCC in patients without cirrhosis[13,42,65]. There is a geographic disparity in screening: About 2% of people with HBV knew their status in 2019 in Africa vs 22% in the Americas and 19% in the European region[21]. Additionally, there is likely underestimation of the true number of chronic HBV in the United States and other regions because high-risk populations have historically been under-represented in national surveys and surveillance studies, especially considering the large influx of yearly immigration to the US[24].
Early detection of tumors from screening in chronic HBV improves overall survival. A Randomized Controlled Trial study in Shanghai found that biannual screening in chronic HBV patients reduced HCC mortality by 37%[66]. An estimated 70% of liver cancer cases are preventable with risk factor modification and screening, thus judicious screening of high-risk populations with use of evidence-based guidelines can significantly reduce mortality from chronic HBV-related HCC[40]. The the American Association for the Study of Liver Diseases (AASLD) recommends HCC surveillance with ultrasound with or without AFP for all patients with diagnosed cirrhosis, and chronic HBV carriers who are high risk including African Americans older than 20 years, Asians older than 40 years, and those with family history of HCC. However, less than 1 in 5 high-risk patients are regularly screened[67]. Although greater than 90% of patients with acute HBV experience resolution of disease and the small minority develop chronic HBV infection, 40% of chronic HBV patients go on to develop cirrhosis, liver failure and HCC, and up to 25% of patients with chronic HBV end up dying from cirrhosis or HCC; thus HCC screening has been found cost-effective in patients with HBV even without cirrhosis when incidence of HCC is greater than 0.2% per year[67,68].
Lack of health literacy is particularly apparent in vulnerable and underserved populations such as immigrant populations, Mexican Americans, and African Americans, who already disproportionately bear the burden of chronic HBV and HCC[69,70]. Increasing social awareness of risk factors and protective factors of chronic HBV can be an avenue to improve health literacy. Social media is used by much of the developed world. For example, World Hepatitis Day is an annual health education campaign led by the WHO in July that heavily utilizes social media to make a call to action to bolster efforts in prevention, screening, treatment and to spread awareness of viral hepatitis[71]. Culturally sensitive approaches are necessary with an increasingly diverse population of the United States, to effectively communicate with various ethnic groups. One such example is “photo novels” developed to cater to specific cultures which have been shown to be effective in increasing HBV awareness and screening in underserved populations[72].
In many developing countries, such as those in Sub-Saharan Africa and the Asia-Pacific region where the majority of global chronic HBV and HCC cases occur, there is limited knowledge of hepatitis B and the benefits of the HBV vaccine[1,11,12,73]. Only two percent of the patients in African and Southeast Asian regions were aware of their chronic HBV status in 2019[1]. There is a dire need for informational campaigns in these endemic regions to increase awareness and health education amongst vulnerable populations in addition to the national immunization programs that must be initiated at the federal level with support from international health organizations.
Chronic HBV infection continues to have significant impact globally despite escalating vaccine coverage, screening techniques and availability of anti-viral medications. The close geographic relationship between endemic chronic HBV and increased burden of HCC remains to this day in Africa and the Asia-Pacific regions comprising the vast majority of HBV-related HCC in the world. HBV and HCC disproportionately affect certain ethnic groups within the United States and worldwide, many of which are of low SES. Men and the elderly are disproportionally affected at greater rates. To mitigate this largely preventable disease, enhanced access to screening, vaccinations, surveillance, and treatment must be achieved to reduce the burden of chronic HBV and HCC worldwide.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu DF, China; Venegas M, Chile S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021: actions for impact: web annex 2: data methods. July 15, 2021. [Cited 2 January 2022]. Available from: https://www.who.int/publications/i/item/9789240027077. [DOI] [Full Text] |
| 2. | Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1999] [Article Influence: 199.9] [Reference Citation Analysis (4)] |
| 4. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 5. | McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Ni YH, Chen DS. Hepatitis B vaccination in children: the Taiwan experience. Pathol Biol (Paris). 2010;58:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 7. | Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 8. | Kuang XJ, Jia RR, Huo RR, Yu JJ, Wang JJ, Xiang BD, Li LQ, Peng Z, Zhong JH. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat. 2018;25:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 10. | Vallet-Pichard A, Pol S. Review article: immunisation against hepatitis B virus infection and the prevention of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:1166-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 12. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 13. | Petruzziello A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol J. 2018;12:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 14. | Ringelhan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci. 2017;372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 15. | Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. J Hepatol. 2017;66:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1326] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 17. | Upala P, Apidechkul T, Tamornpark R, Chomchoei C, Yeemard F. Seroprevalence and factors associated with hepatitis B infection among the hill tribe adult population in Thailand: a cross-sectional study. BMC Infect Dis. 2020;20:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Naveira MCM, Badal K, Dhakal J, Mayer NA, Pokharel B, Del Prado RF. Seroprevalence of hepatitis B and C in Nepal: a systematic review (1973-2017). Hepatol Med Policy. 2018;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, Chen W, Bai S, Wu J. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 20. | Leroi C, Adam P, Khamduang W, Kawilapat S, Ngo-Giang-Huong N, Ongwandee S, Jiamsiri S, Jourdain G. Prevalence of chronic hepatitis B virus infection in Thailand: a systematic review and meta-analysis. Int J Infect Dis. 2016;51:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections. July 15, 2021. [Cited 2 January 2022]. Available from: http://apps.who.int/iris/bitstream/handle/10665/342808/9789240030985-eng.pdf. |
| 22. | Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis. 2016;20:607-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 23. | Chang ET, Keegan TH, Gomez SL, Le GM, Clarke CA, So SK, Glaser SL. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109:2100-2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Cohen C, Evans AA, London WT, Block J, Conti M, Block T. Underestimation of chronic hepatitis B virus infection in the United States of America. J Viral Hepat. 2008;15:12-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, Ruhl C, Unalp-Arida A. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;24:1052-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 27. | Ajayi F, Jan J, Singal AG, Rich NE. Racial and Sex Disparities in Hepatocellular Carcinoma in the USA. Curr Hepatol Rep. 2020;19:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Flores YN, Datta GD, Yang L, Corona E, Devineni D, Glenn BA, Bastani R, May FP. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: A Large Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2021;30:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Li J, Hansen BE, Peppelenbosch MP, De Man RA, Pan Q, Sprengers D. Factors associated with ethnical disparity in overall survival for patients with hepatocellular carcinoma. Oncotarget. 2017;8:15193-15204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Ren F, Zhang J, Gao Z, Zhu H, Chen X, Liu W, Xue Z, Gao W, Wu R, Lv Y, Hu L. Racial disparities in the survival time of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma between Chinese patients and patients of other racial groups: A population-based study from 2004 to 2013. Oncol Lett. 2018;16:7102-7116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Njei B, Ditah I, Lim JK. Persistent racial disparities in survival among u.s. Adults with hepatocellular carcinoma after liver transplantation: the paradox of all-cause and cause-specific mortality. Gastrointest Cancer Res. 2013;6:73-74. [PubMed] |
| 32. | Koc ÖM, Robaeys G, Yildirim B, Posthouwer D, Hens N, Koek GH. The influence of ethnicity on disease outcome in patients with chronic hepatitis B infection. J Med Virol. 2019;91:623-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Burton A, Balachandrakumar VK, Driver RJ, Tataru D, Paley L, Marshall A, Alexander G, Rowe IA; HCC-UK/BASL/NCRAS Partnership, Palmer DH, Cross TJS. Regional variations in hepatocellular carcinoma incidence, routes to diagnosis, treatment and survival in England. Br J Cancer. 2022;126:804-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Chu JJ, Wörmann T, Popp J, Pätzelt G, Akmatov MK, Krämer A, Reintjes R. Changing epidemiology of hepatitis B and migration--a comparison of six Northern and North-Western European countries. Eur J Public Health. 2013;23:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Roman S, Jose-Abrego A, Fierro NA, Escobedo-Melendez G, Ojeda-Granados C, Martinez-Lopez E, Panduro A. Hepatitis B virus infection in Latin America: a genomic medicine approach. World J Gastroenterol. 2014;20:7181-7196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Organization PAH. Hepatitis B and C in the Spotlight: A public health response in the Americas. 2016. [Cited 2 January 2022]. Available from: https://iris.paho.org/bitstream/handle/10665.2/31449/9789275119297-eng.pdf?sequence=5&isAllowed=y. |
| 37. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 38. | Debes JD, Chan AJ, Balderramo D, Kikuchi L, Gonzalez Ballerga E, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Diaz Ferrer J, Yang JD, Carrera E, Garcia JA, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Chan AJ, Balderramo D, Kikuchi L, Ballerga EG, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Ferrer JD, Yang JD, Carrera E, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR, Debes JD. Early Age Hepatocellular Carcinoma Associated With Hepatitis B Infection in South America. Clin Gastroenterol Hepatol. 2017;15:1631-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. |
American Cancer Society.
Cancer Facts and Figures |
| 41. | Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 42. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 879] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 43. | Wu EM, Wong LL, Hernandez BY, Ji JF, Jia W, Kwee SA, Kalathil S. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 44. | Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 45. | Yip TC, Wong GL, Chan HL, Tse YK, Liang LY, Hui VW, Lee HW, Lui GC, Kong AP, Wong VW. Elevated testosterone increases risk of hepatocellular carcinoma in men with chronic hepatitis B and diabetes mellitus. J Gastroenterol Hepatol. 2020;35:2210-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, Phan AT, Hawk E, Javle M, Lee JS, Torres HA, Rashid A, Lenzi R, Hassabo HM, Abaza Y, Shalaby AS, Lacin S, Morris J, Patt YZ, Amos CI, Khaderi SA, Goss JA, Jalal PK, Kaseb AO. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2017;15:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: epidemiology and pathogenic role of viral factors. J Chin Med Assoc. 2007;70:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Zhang H, Spencer K, Burley SK, Zheng XFS. Toward improving androgen receptor-targeted therapies in male-dominant hepatocellular carcinoma. Drug Discov Today. 2021;26:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Nguyen MH, Lim JK, Burak Ozbay A, Fraysse J, Liou I, Meyer N, Dusheiko G, Gordon SC. Advancing Age and Comorbidity in a US Insured Population-Based Cohort of Patients With Chronic Hepatitis B. Hepatology. 2019;69:959-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 50. | Lai JC, Wong VW, Yip TC, Hui VW, Tse YK, Lee HW, Liang LY, Lui GC, Chan HL, Wong GL. Secular trend of treatment uptake in patients with chronic hepatitis B: A territory-wide study of 135 395 patients from 2000 to 2017. J Gastroenterol Hepatol. 2021;36:3487-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Lim JK, Nguyen MH, Kim WR, Gish R, Perumalswami P, Jacobson IM. Prevalence of Chronic Hepatitis B Virus Infection in the United States. Am J Gastroenterol. 2020;115:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 52. | Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol. 2013;12:173-182. [PubMed] |
| 53. | World Health Organization. Immunization coverage. 15 July 2021. [cited 11 Jan 2022]. Available from: https://www.who.int/publications. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Goh MJ, Kang W, Kim KM, Sinn DH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Incidence and risk factors for development of hepatocellular carcinoma at young age in patients with chronic hepatitis B. Scand J Gastroenterol. 2022;57:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 456] [Cited by in RCA: 500] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 56. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 57. | Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, Homan CE, Snowball MM, Cagle HH, Williams JL, Chulanov VP. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 58. | Wong GL, Chan HL, Yiu KK, Lai JW, Chan VK, Cheung KK, Wong EW, Wong VW. Meta-analysis: The association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Ohikere K, Chitnis AS, Hahambis TA, Singal A, Wong RJ. Ethnic Minorities and Low Socioeconomic Status Patients With Chronic Liver Disease Are at Greatest Risk of Being Uninsured. Gastroenterology Res. 2021;14:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Quaglia A, Lillini R, Mamo C, Ivaldi E, Vercelli M; SEIH (Socio-Economic Indicators, Health) Working Group. Socio-economic inequalities: a review of methodological issues and the relationships with cancer survival. Crit Rev Oncol Hematol. 2013;85:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Verdecchia A, Baili P, Quaglia A, Kunkler I, Ciampichini R, Berrino F, Micheli A. Patient survival for all cancers combined as indicator of cancer control in Europe. Eur J Public Health. 2008;18:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Flores YN, Yee HF Jr, Leng M, Escarce JJ, Bastani R, Salmerón J, Morales LS. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. 2008;103:2231-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Stewart SL, Kwong SL, Bowlus CL, Nguyen TT, Maxwell AE, Bastani R, Chak EW, Chen MS Jr. Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988-2012. World J Gastroenterol. 2016;22:8584-8595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 945] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 67. | Frenette CT, Isaacson AJ, Bargellini I, Saab S, Singal AG. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin Proc Innov Qual Outcomes. 2019;3:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 68. | Chang MS, Nguyen MH. Epidemiology of hepatitis B and the role of vaccination. Best Pract Res Clin Gastroenterol. 2017;31:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol. 2021;12:S361-S373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 70. | Hyun S, Ko O, Kim S, Ventura WR. Sociocultural barriers to hepatitis B health literacy in an immigrant population: a focus group study in Korean Americans. BMC Public Health. 2021;21:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | World Hepatitis Day - July 28, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 72. | Lee S, Yoon H, Chen L, Juon HS. Culturally appropriate photonovel development and process evaluation for hepatitis B prevention in Chinese, Korean, and Vietnamese American communities. Health Educ Behav. 2013;40:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Machmud PB, Glasauer S, Gottschick C, Mikolajczyk R. Knowledge, Vaccination Status, and Reasons for Avoiding Vaccinations against Hepatitis B in Developing Countries: A Systematic Review. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |