Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3695
Peer-review started: April 6, 2022
First decision: May 29, 2022
Revised: May 30, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: July 28, 2022
Processing time: 111 Days and 14.1 Hours
Intrahepatic cholangiocarcinoma (ICC) is one of the most aggressive malign
To evaluate secular trends of ICC according to age, sex, and risk factors in Taiwan.
In this population-based study, we used the national Taiwan Cancer Registry database. Age-standardized and relative percent changes in incidence rates were used to describe secular trends in incidence rates and sex ratios of ICC in Taiwan.
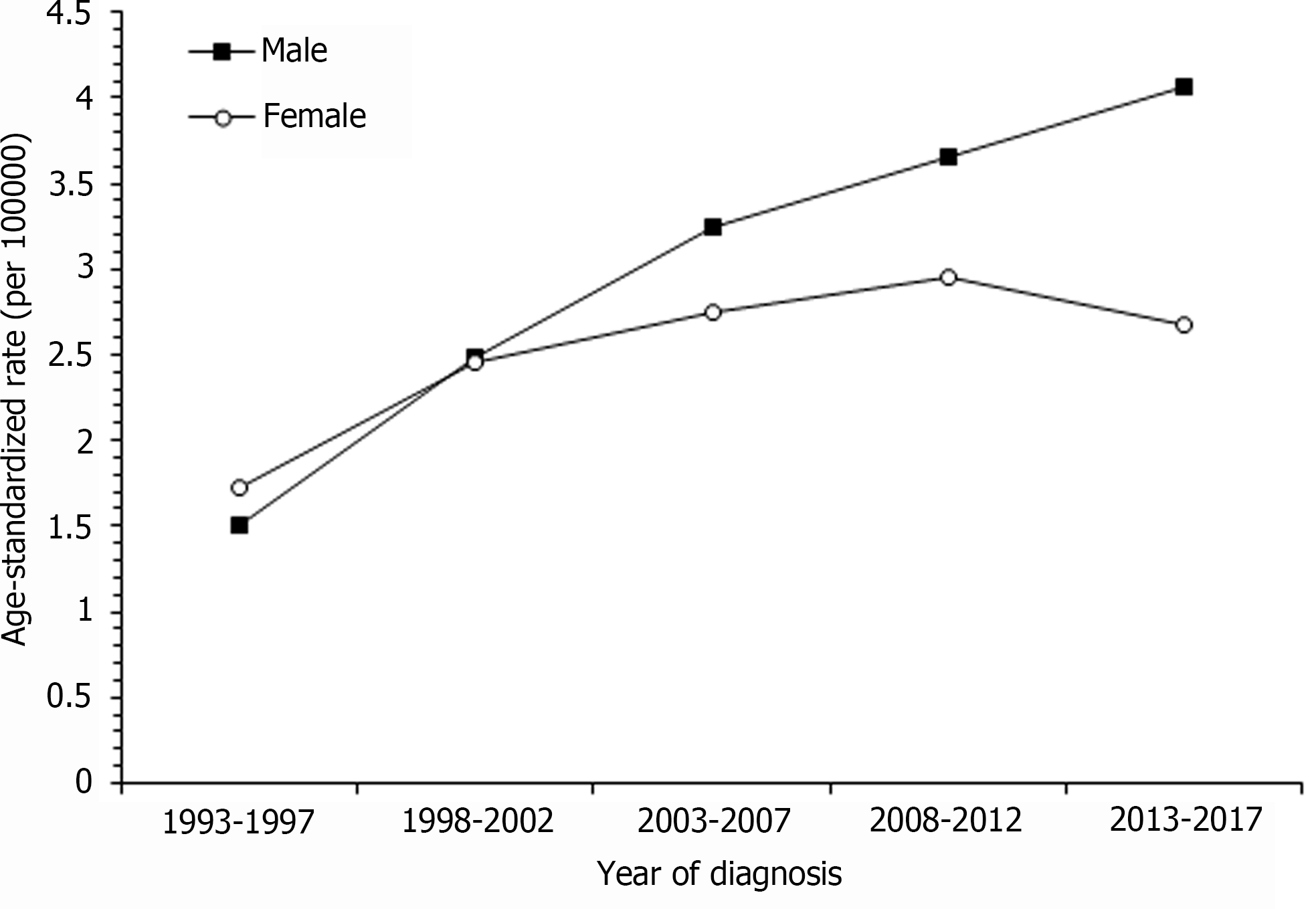
The age-standardized ICC incidence rate among males increased from 1.51 per 100000 in 1993-1997 to 4.07 per 100000 in 2013-2017 and among female from 1.73 per 100000 to 2.95 per 100000. The incidence in females tended to plateau after 2008-2012. For males, the ICC incidence increased as age increased. In the long-term incidence trend of ICC in females, the incidence of the four age groups (40-44, 45-49, 50-54 and 55-59 years) remained stable in different years; although, the incidence of the 60-64 group had a peak in 2003-2007, and the peak incidence of the 65-69 and 70-74 groups occurred in 2008-2012. Among males, beginning at the age of 65, there were increases in the incidence of ICC for the period of 2003-2017 as compared with females in the period of 2003-2017.
Increased incidence of ICC occurred in Taiwan over the past two decades. The increased incidence has progressively shifted toward younger people for both males and females.
Core Tip: It is important to evaluate the secular trends of intrahepatic cholangiocarcinoma (ICC) incidence and to determine insightful etiological clues in a population with a high incidence of liver cancer. Using the national Taiwan Cancer Registry, we observed an increased incidence of ICC for both males and females. Our observations should be taken in the context of other studies conducted on secular trends of ICC.
- Citation: Lin CR, Lee YK, Chiang CJ, Yang YW, Chang HC, You SL. Secular trends of intrahepatic cholangiocarcinoma in a high endemic area: A population-based study. World J Gastroenterol 2022; 28(28): 3695-3705
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3695
Intrahepatic cholangiocarcinoma (ICC) is one of the most aggressive malignancies, and its mortality rates are 1–2 per 100000[1]. After surgery, patients with ICC have 5-year survival rates of approximately 60%, and the rates of recurrence are 60%-65%[2]. In the United States, there were 1.18 new cases per 100000 in 2012[3]. ICC is the second most commonly diagnosed liver cancer[4], and the incidence of ICC continues to increase[5]. Florio et al[5] reported that the incidence rate of ICC has regional differences. For example, the incidence rate of ICC in 2008-2012 was 2.80 per 100000 in South Korea [95% confidence interval (CI): 2.68 to 2.93], 2.19 per 100000 in Thailand (95%CI: 2.01-2.36), 0.58 per 100000 in Italy (95%CI: 0.48-0.69), 0.78 per 100000 in the United States (95%CI: 0.74-0.82), and 1.15 per 100000 in the United Kingdom (95%CI: 1.12-1.18)[5]. The average annual percent change (AAPC) of ICC in 2008-2012 also exhibits regional differences. In particular, the AAPC of ICC in 2008-2012 was 4.5% in South Korea (95%CI: 3.0-5.9), -1.0% in Thailand (95%CI: -3.8-1.9), 3.3% in Italy (95%CI: 1.5-5.2), 2.0% in the United States (95%CI: -2.3-6.5), and 4.6% in the United Kingdom (95%CI: 3.2-6.1)[5]. Although these previous studies show that the threat of ICC is rising in many parts of the world[4,5], few studies have used nationwide incidence data to study the epidemiology of ICC.
Moreover, this population-based data facilitates an unbiased estimate of the burden of ICC into the near future. We aimed to evaluate the current trends of ICC in Taiwan. We investigated secular trends in the incidence of ICC to provide insightful etiological analyses, particularly for the variations by age and sex.
Incidence data from 1993–2017 were obtained from the Taiwan Cancer Registry (TCR). We extracted incidence data on ICC [topography code of International Classification of Diseases for Oncology (ICD-O-FT: T-155.1 before 2002 and ICD-O-3: C22.1, M code 9590-9993 was excluded)]. TCR is organized and funded by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan. In 1979, TCR began to register all cancers nationwide[6,7].
In 1995, Taiwan launched the National Health Insurance system[8]. Since that time, the health status of the Taiwanese population has been fully registered[7]. The incidence data were grouped into 17 5-year age groups (0-4, 5-9, 10-14, 15-19, … to 80+ years) and 5 periods (1993–1997, 1998–2002, …, and 2013–2017). Therefore, the data design comprised 14 birth cohorts (the oldest: 1908-1912 to the youngest: 1973-1977). In this study, we reasoned the choice of 1993-1997 as the beginning of the analytical year to avoid incomplete records of incidence data, and the quality of diagnosis in 1993 was similar to that after 1995.
We calculated the incidence rates by sex for each age group by dividing the number of cases by the corresponding population size[9]. Age-standardized incidence rates by sex were calculated using the direct method with the 2000 world standard population as reference[10]. The male-to-female incidence ratios were calculated by dividing the rate in males by that in females for each age group.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). The research protocol was approved by the Institutional Review Board of Fu-Jen Catholic University (No. C107099).
Figure 1 shows the trend of age-standardized rate (ASR) of ICC in men and women in Taiwan from 1993-1997 to 2013-2017. The incidence of ICC in men increased linearly. The ASR increased from 1.51 per 100000 in 1993-1997 to 4.07 per 100000 in 2013-2017, and its relative percent change was 169%. The incidence of ICC in women tended to plateau after 2008-2012. The ASR increased from 1.73 per 100000 population in 1993-1997 to 2.95 per 100000 in 2008-2012. The relative percentage change was 70%. From 1993-1997 to 2013-2017, the ASR incidence of ICC in men increased more rapidly than that in women.
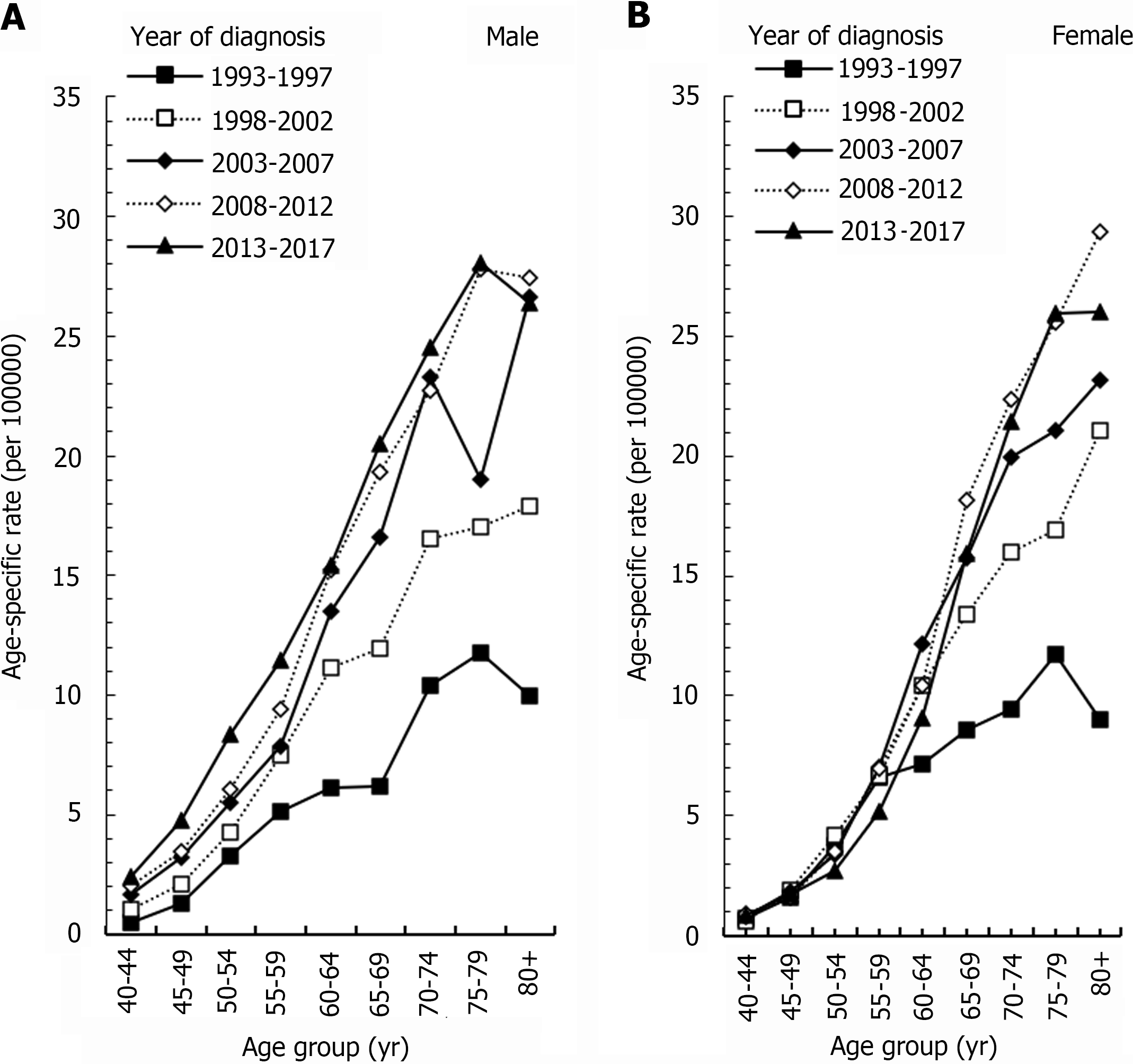
Figure 2A showed the age-specific rates of ICC per 100000 men in Taiwan. For males, the incidence of ICC increased as age increased. The age-specific rate of ICC in seven age groups (40-44, 45-49, 50-54, 55-59, 60-64, 65-69, and 70-74) increased steadily between 1993-1997 and 2013-2017. The respective relative percent changes were 372%, 265%, 156%, 124%, 153%, 231%, and 139%. The 70-74 age group had a larger growth between 1993-1997 and 2003-2007, and its relative percent change was 114%. Then, it increased more slowly between 2003-2007 and 2013-2017, and its relative percent change was only 5%. The 80+ age group showed a trend from rising to declining, with peaks occurring in 2008-2012. Compared to 1993-1997, its relative percent change was 175%. From 2008-2007 to 2013-2017, the incidence decreased 4%.
Figure 2B shows the age-specific rates of ICC per 100000 women in Taiwan. The age-specific rates of the four age groups of 40-44, 45-49, 50-54, and 55-59 remained stable in different years. The incidence of the 60-64 age group had a peak in 2003-2007, and the incidence increased by 69% compared with 1993-1997. The peak incidence of the 65-69 and 70-74 age groups occurred in 2008-2012 compared with the incidence of 1993-1997. The respective relative percent change was 111% and 136%. The incidence of the 75-79 age group increased steadily from 1993-1997 to 2013-2017, with an increase of 121%. The incidence of the 80+ age group peaked in 2008-2012 compared with that in 1993-1997, and the relative percentage change was 227%. Then, the incidence decreased by 11% between 2008-2012 and 2013-2017.
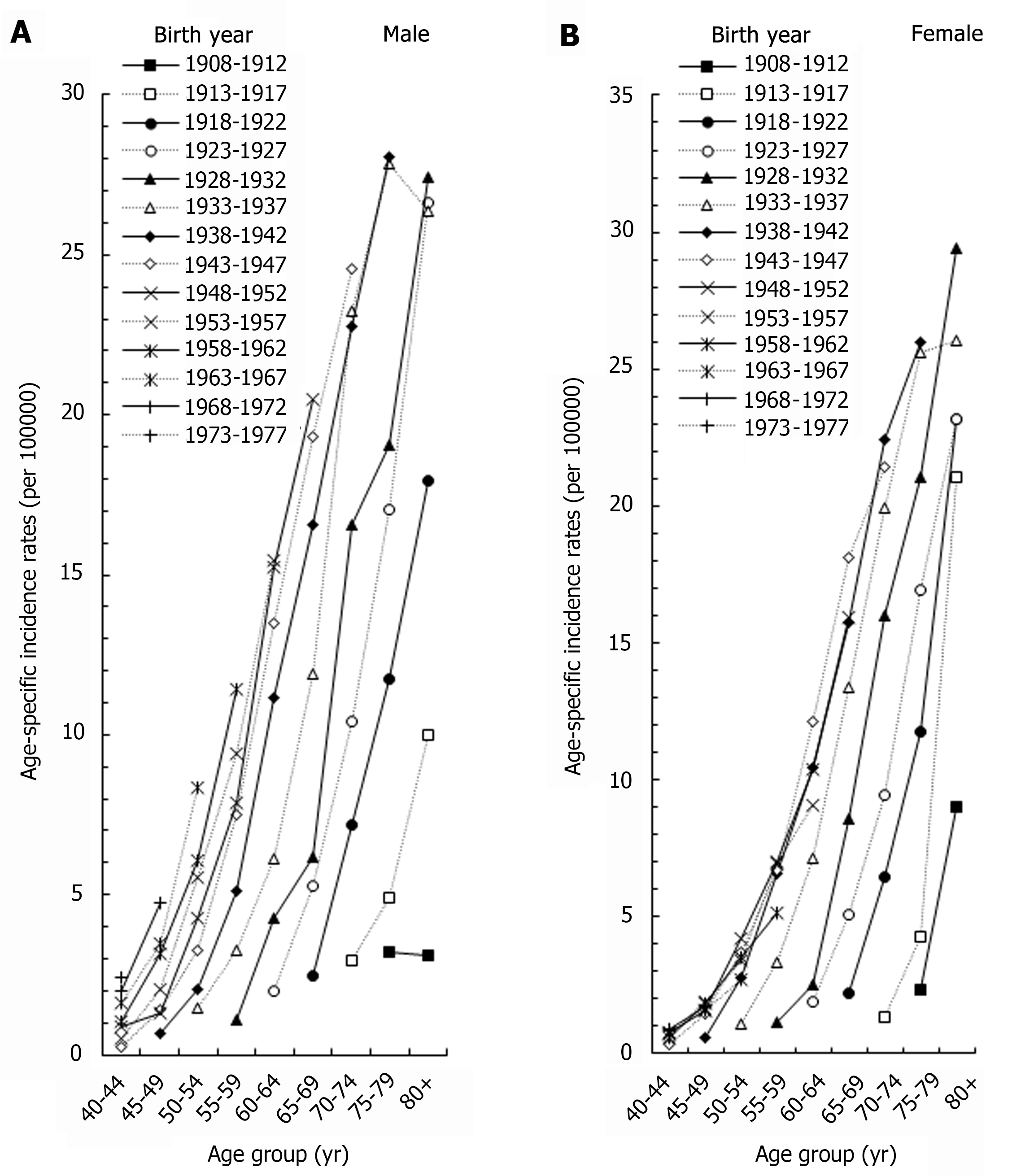
Figure 3 showed the age-specific rates of men and women in different birth cohorts. The incidence of ICC increased as the birth year increased for both males and females. For example, in the 70-74 age group, the incidence of males born in 1943-1947 was 8.41 times of those born in 1913-1917, and the incidence of women born in 1943-1947 was 16.11 times of those born in 1913-1917.
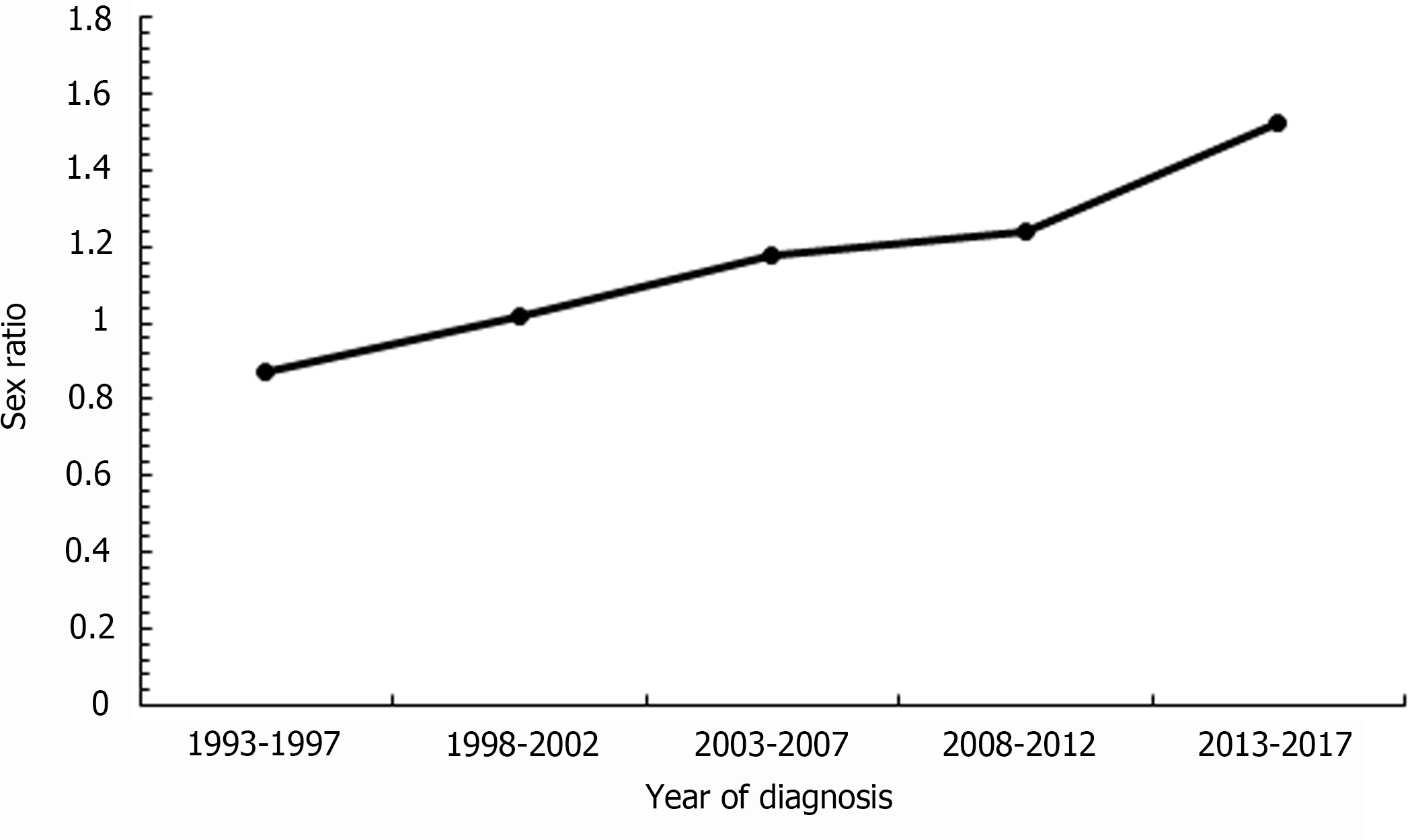
Figure 4 shows the sex ratio of the ASR of ICC in Taiwan from 1993 to 2017. A turning point was apparent at 1998-2002. Before that period, the incidence of ICC in women was higher than that in men. In 1998-2002, the sex ratio of the incidence of ICC was 1.02, which means that the incidence of ICC in men and women was almost the same. After 2002, the incidence of ICC in men was higher than that of women. From 1993-1997 to 2013-2017, the sex ratio of the incidence of ICC increased from 0.87 to 1.52, and the relative percent change was 75%, which indicates a trend of an increasing gap in the incidence of ICC between men and women.
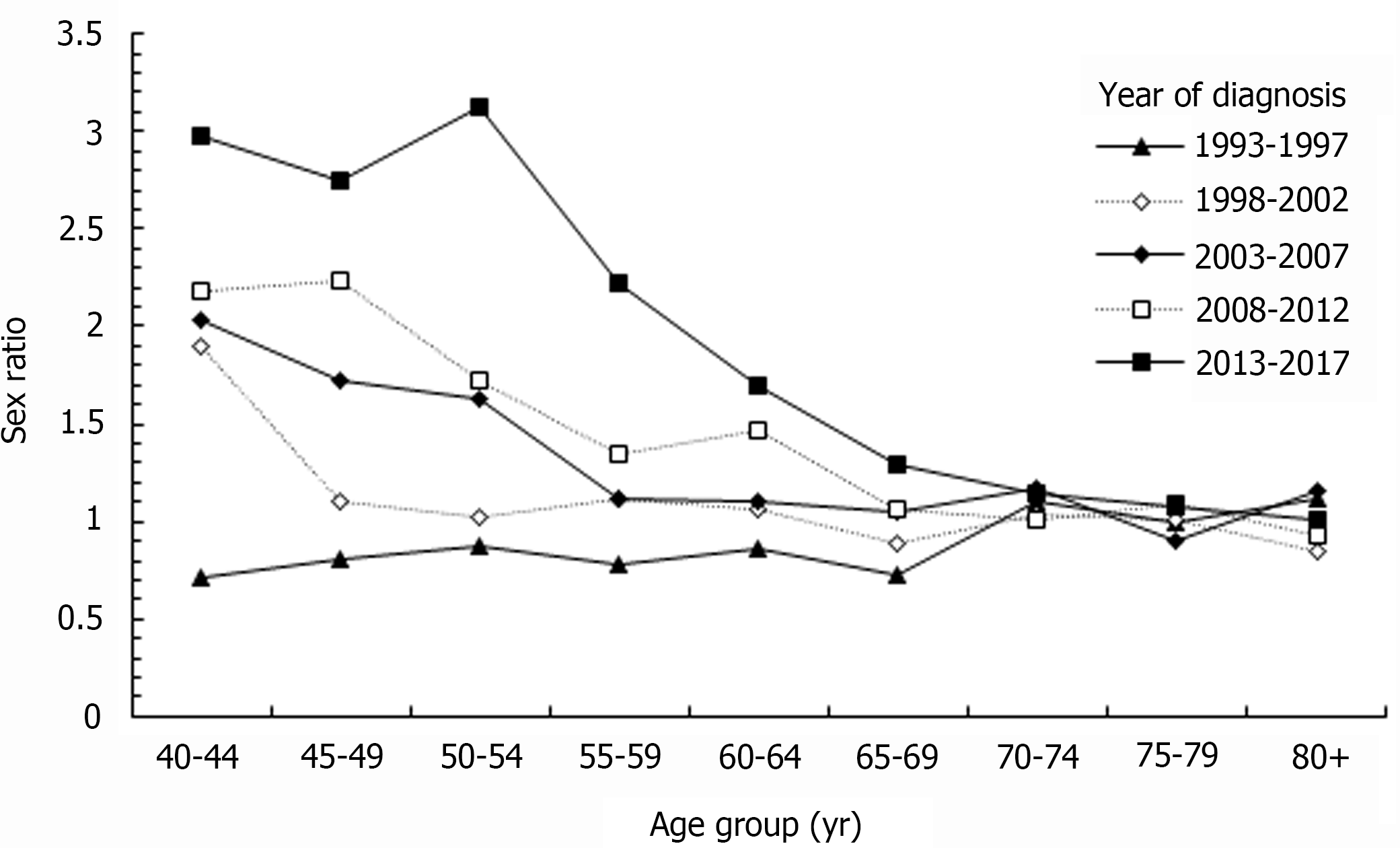
Figure 5 shows the sex ratio of the age-specific rates of ICC in Taiwan by diagnostic period. For ICC patients younger than 70-year-old, the sex ratio of age-specific rates increased with year. For example, in the 40-44 age group, the sex ratio was 0.72 in 1993-1997 and 2.98 in 2013-2017. Its relative percent change was 314% from 1993-1997 to 2013-2017. In the age group over 70-years-old, the sex ratio showed a stable baseline in different years, which meant that the incidence of ICC was similar in men and women.
This population-based study showed that the incidence of ICC has rapidly increased in Taiwan. This increase remained after stratification by age, sex, diagnosis period, and birth cohort. These results, which highlight the current and projected incidence of ICC, suggest that there is a need for more research to prevent the burden of this cancer in Asian countries.
The incidence rate of ICC varies among different regions of the world[4]. Globally, the ASR is 0.85 per 100000[11]. In Asia, the highest ASR was 2.80 per 100000 in South Korea, and the lowest was 0.26 per 100000 in Israel during 2008-2012[5]. According to our result, the ASR in Taiwan was about 3.3 per 100000 during 2008-2012, which means that Taiwan is a high-risk region of ICC. Higher incidence of ICC in Asian countries is likely due to the presence of more risk factors[4,12].
Changes in the incidence of ICC also differs among different countries[5]. Overall, the incidence of ICC has increased in most of the countries in the world[5]. However, the incidence of ICC was higher in Asia, and the increase-rate was higher in Europe and North America[13]. The AAPC of ICC incidence from 1993 to 2012 was 5.0% in Canada, 6.1% in Costa Rica, 6.5% in France, 7.5% in Germany, 8.7% in Poland, 10.5% in Ireland, and 20.1% in Latvia[5]. In Asian countries, the AAPC of ICC incidence was -0.7% in Japan, -1.0% in Thailand, -2.4% in Philippines, 4.5% in South Korea, and 11.1% in China[5]. In some Asian countries, such as Philippines, Thailand, and Japan, the incidence of ICC showed a nonsignificant decline[5]. In contrast to other studies, our results indicate a rising burden of ICC in the Asia-Pacific area.
A previous study attempted to report the population-based incidence rate of ICC[14]. A study conducted in the United States found that ICC incidence increased from 0.44 per 100000 in 1973 to 1.8 per 100000 in 2012. The AAPC from 2001 to 2012 was 2.3%, and AAPC was 4.36% from 2003 to 2012[3]. In this study, we used the national registry system, which provides comprehensive coverage of the entire population, to make an unbiased estimation of ICC incidence. We found that the incidence of ICC in Taiwan increased from 1.04 per 100000 to 3.36 per 100000 from 1988-1992 to 2013-2017.
An expert consensus document reported that ICC has several risk factors, such as aging of the population, smoking, obesity, diabetes mellitus (DM), hepatitis B virus (HBV) infection, and hepatitis C virus (HCV) infection[4], which may account for this projected increasing incidence. However, due to the number of risk factors of ICC, the phenomenon of the increasing trend of the ICC incidence in Taiwan cannot be explained by a single risk factor.
Aging is an established risk factor for ICC[4]. A study published in 2016 found that the median age at diagnosis among patients in the United States diagnosed with ICC was 67 years between 2008 and 2012[3]. More than 73% of those patients with ICC were older than 60 years[15], which was similar to our results. We found that the incidence rate was low before age 60 and increased dramatically thereafter. The proportion of people in the population aged 60 and above increased from 13.6% in 2007 to 23.2% in 2020 in Taiwan[9]. A previous study found that the aging of populations in developed countries contributes to the increasing incidence of ICC[15]. Nevertheless, we found the age-adjusted incidence increased gradually over time, and the trend remained consistently elevated, even after stratification by age. This implies that population aging has contributed to the increasing ICC incidence in Taiwan.
A previous meta-analysis showed that DM significantly increased the risk of ICC independent of various confounding factors (relative risk: 1.97, 95%CI: 1.57-2.46; P = 0.025 for heterogeneity)[16]. A previous study reported an increasing prevalence of DM for both sexes in Taiwan from 1992 to 1996[17], while the incidence remained stable at 6.9–7.7 per 1000 person-years from 1999 to 2004[18]. From 2000 to 2007, the ASR of type 2 DM remained high, at 8.7–9.8 per 1000[19]. In the elderly age group (≥ 65 years), the incidence rate in women (873.2/100000) was higher than that in men (721.4/100000)[20].
Smoking is the most comprehensive environmental factor responsible for ICC. A study based on the National Cancer Institute’s SEER 18 database reported that smoking increases the risk of ICC by 46% (95%CI: 1.28-1.66)[20]. A cohort study that included 1518741 individuals showed that current smokers have a 47% greater risk of ICC compared to non-smokers (95%CI: 1.07-2.02)[21]. Taiwan has a high prevalence of smoking. The smoking rate among adult men increased from 59% in 1986 to 63% in 1990, which resulted from the cigarette market opening[22]. A study based on the National Health Interview Survey of Taiwan was conducted in 2001. The prevalence of smokers was 46.8% and the prevalence of ex-smokers was 6.8% in Taiwanese adult males in 2001. The prevalence of smoking was 4.3% and the prevalence of ex-smokers was 0.5% in Taiwanese adult females in 2001[23]. In 2009, a new anti-smoking law was established in Taiwan. Despite the efforts of the Taiwan government, a cross-sectional study that included 961 adults found that up to 42% of sampled Taiwanese adults had smoked cigarettes after that new law had been implemented[24]. The high prevalence of smoking among the early birth cohort in Taiwan may partly explain its high incidence of ICC and seemed to have a cohort effect on ICC.
Obesity was found to increase the risk of ICC by a previous meta-analysis [odds ratio (OR): 1.56, 95%CI: 1.26-1.94][25]. Recently, the prevalence of obesity in Taiwan has increased rapidly. More specifically, a study based on the Nutrition and Health Surveys in Taiwan reported that the prevalence of obesity in men increased from 10.5% in 1993–1996 to 17.0% in 2005. The prevalence of obesity tripled for elementary school boys from 1993 to 2002. Due to the consumption of high-fat foods and lack of physical activity, the prevalence of obesity is likely to increase further in Taiwan[26]. The aforementioned phenomenon may contribute to the increasing prevalence of ICC.
A previous study reported that ICC incidence rates were higher in males than in females in most countries in the world[5]. The sex ratio of the incidence of ICC was 2.9 in Malta, 1.9 in South Korea, 1.9 in Thailand, 1.9 in Japan, 1.9 in Spain, 1.6 in Slovakia, and 1.6 in France[5]. A study based on the SEER database reported that the incidence of ICC was higher in males than in females, and the sex ratio of the age-specific rates of ICC decreased in the aging group[27]. Our results also showed similar outcomes, and this phenomenon may be due to the different hormone profiles of males and females[28]. A study reported that estrogen may modulate cholangiocyte proliferation in nude mice[29]. A previous study used chromatography-tandem mass spectrometry and competitive electrochemiluminescence immunoassay to analyze the relationship between sex steroid hormones and ICC. In that study, a high level of estradiol was found to increase the risk of ICC (OR: 1.40, 95%CI: 1.05-1.89)[30]. A previous study in Taiwan reported that women born in younger cohorts had a later age at natural menopause (hazard ratio: 0.87 per 10-year difference, 95%CI: 0.81-0.95)[31]. The lower ICC incidence of females in our study may be attributed to decreasing estrogen levels in elderly and postmenopausal women. The lower prevalence of smoking among women (4%-8%) than men (47% in 2001)[22] may also contribute to their lower incidence of ICC.
A previous study found that hepatitis B surface antigen (HBsAg) and HCV antibodies had a high association with ICC[32]. A meta-analysis reported that HBV and HCV infection increased the risk of ICC (OR: 3.17, 95%CI: 1.88-5.34 and OR: 3.42, 95%CI: 1.96-5.99, respectively)[33]. Previously, Taiwan was classified as a high-risk region for HBsAg[34]. A study found that HBV DNA could interrupt seven genes (TERT, CEACAM20, SPATA18, TRERF1, ZNF23, LINC01449, and LINC00486) in ICC tissue, which may indicate a potential mechanism for the increased risk of ICC in HBV carriers[35].
A retrospective multicenter study in Taiwan reported that the HBsAg prevalence in hepatocellular carcinoma from 1981 to 2001 decreased from 81.5% to 61.2% in males and decreased from 66.7% to 41.4% in females[36]. The sex ratio of HBsAg prevalence of hepatocellular carcinoma increased from 1.22 to 1.61[36], which was consistent with the increasing trend of the sex ratio of the ICC incidence in our study. A previous review hypothesized that HCV could induce the transformation of hepatocytes into cholangiocyte precursors[37]. The HCV antibody prevalence in hepatocellular carcinoma was 31.5% (95%CI: 30.4%-32.6%) in Taiwanese males and 56.7% (95%CI: 54.4%-59.0%) in Taiwanese females[36]. A retrospective cross-sectional study in Taiwan reported that in patients with HCV infection, visceral obesity was significantly associated with waist-to-height ratio, body fat percentage, fat-free mass/body weight, and muscle mass/body weight[38]. Thus, we assumed that HBV and/or HCV infection may be at least partially explain why the incidence of ICC in Taiwan was high.
We found that the sex ratio of the incidence of ICC increased as the diagnostic year increased. In the group over the age of 70 years, the sex ratio of the incidence of ICC between men and women was close to 1, which meant that there was almost no difference in the incidence of ICC between men and women. The proportion of women with ICC increases with age. Another population-based study in the United States showed the proportion of females with ICC was 38.9% for those aged 40-59 years, 45.8% for those aged 60-79 years, and 56.3% for those aged ≥ 80 years[27]. The aforementioned research results are consistent with our study outcome. However, another study based on the Italian National Institute of Statistics A in Italy, another country with a high risk of ICC, reported that the incidence of ICC in males was always higher than that in females[39], which was different from our study outcome. Due to the variety of risk factors of ICC, it is difficult to identify the cause of this difference. A study based on the Netherlands Cancer Registry reported that the incidence of ICC in a low endemic area significantly increased in older populations, especially in in the 45–59 years age group[40]. Interestingly, we also found a similar pattern.
Assessing the temporal trends in the sex difference of ICC incidence has useful implications. Any temporal changes in the sex difference reflect the contributions from environmental and extrinsic risk factors, while a stable sex difference indicates the role of intrinsic exposures or environmental risk. The increase of the sex difference in the incidence of ICC may be explained by the decreasing prevalence of smoking, particularly in men with a historically higher prevalence[23,24]. The decline in smoking prevalence in Taiwan, which has been more rapid for men than for women, may have also contributed to the decreased sex difference in ICC[23,24]. In addition, we noted an increased sex difference in the incidence of ICC since 2008, which warrants confirmation through continued monitoring and investigations.
We found that the gap in the sex ratio between the 40-44 age group and the 80+ age group increased as the diagnostic year increased. In age groups younger than 70 years, the sex ratio increased as the diagnostic year increased. However, in the age groups over 70 years, the sex ratio was similar during each diagnostic period. In our cohort, we found that the incidence of ICC in males and females was higher in the elder birth cohort, which resulted in the aforementioned phenomenon.
Surgery is the only curative treatment for ICC[4]. A previous study found the incidence of sarcopenia among ICC patients following receipt of liver resection to be 50%. Preoperative nutritional evaluation is important for ICC[41]. In addition, nutritional factors may affect the disease incidence. Indeed, a population-based cohort study found that regular use of oil supplement lowered the risk of total liver cancer to 44% (95%CI: 25%-59%) and risk of ICC to 40% (95%CI: 7%-61%)[42]. A meta-analysis suggested that vegetable and fruit consumption may reduce the risk of ICC; specifically, the reported ORs of mixed vegetables, mixed fruits, and combined fruits and vegetables were 0.61 (95%CI: 0.50-0.75), 0.79 (95%CI: 0.65-0.96), and 0.68 (95%CI: 0.57-0.80), respectively[43]. A multi-center study determined that high dietary fiber intake could be associated with a lower risk of intrahepatic bile duct cancer[44]. Finally, an animal model-based study in Taiwan provided further evidence of a relationship between ICC and nutrition; vitamin D supplementation lead to significant suppression of ICC initiation and progression in the rat model via regulation of gene expression (e.g., of lipocalin 2)[45].
This study has several limitations. As seen in other studies based on cancer registry databases[46], a substantial portion of cases did not receive histological verification. Old age and advanced tumor stage at presentation likely prohibited this verification. Nevertheless, integrity of the TCR database was 98.4%[47]. The morphological verification percentage of the TCR database is 93.0%[47], which minimizes the misdiagnosis of cancer incidence. In addition, quality and accuracy of clinical diagnosis in our databases has been validated, and the percentage of death certificate-only data is 1%[47]. In addition, we extracted the ICD-O-FT: T-155.1 before 2002 and ICD-O-3: C22.1 from 2003–2017, which may misclassify some diagnostic cases. However, due to the rarity of ICC cases in Taiwan, this deviation did not affect the statistical trend outcomes in our study. In addition, restriction to cases with histological proof would have introduced selection bias. Ultimately, although our study found that there are various risks associated with ICC, including sex, age, DM, HBV and/or HCV infection, obesity, nutritional factors, and smoking, the results need to be validated in larger cohorts, exploring the risk of ICC development in a high incidence area in particular.
The incidence of ICC continues to rise in Asia. The etiological role of ICC is still unclear. However, future prospective evaluations are warranted to explore potential risk factors of ICC. The assessment of their independent and/or interactive effects are suggested, which could lead to prevention of ICC, development of early detection methods for early curative surgery, and identification of potential prognostic factors related to improved therapy and outcomes.
Intrahepatic cholangiocarcinoma (ICC) is one of the most aggressive malignancies. However, because of its scarcity few population-based studies have explored its epidemiology. In Taiwan, we have a national cancer registry database, which can be used to evaluate the epidemiology of ICC.
To discover the secular incidence trends and associated risk factors of ICC in Taiwan.
To observe secular trends in ICC incidence according to age, sex, and risk factors in Taiwan.
In this population-based study, we used the national Taiwan Cancer Registry database. Relative percent change in incidence rates were used to describe secular trends in incidence rates and sex ratios of ICC in Taiwan.
The age-standardized ICC incidence rate among males increased from 1.51 per 100000 in 1993-1997 to 4.07 per 100000 in 2013-2017 and among females from 1.73 per 100000 to 2.95 per 100000. ICC incidence rates in females tended to plateau after 2008-2012. For males, the incidence of ICC increased as age increased. In the long-term incidence trend of ICC in females, the incidence of the four age groups of 40-44, 45-49, 50-54, and 55-59 years remained stable in different years, the incidence of the 60-64 age group had a peak in 2003-2007, and the peak incidence in the 65-69 and 70-74 age groups occurred in 2008-2012.
An increased incidence of ICC has occurred in Taiwan over the past two decades. The increased sex ratios has progressively shifted toward younger people.
Further long-term cohort studies are needed to investigate the relationship between ICC and its risk factors.
The authors would like to express their gratitude to the Fu Jen University Foundation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coppola A, Italy; Li JT, China S-Editor: Ma YJ L-Editor: A P-Editor: Cai YX
| 1. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 2. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 967] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 3. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 4. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 971] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 5. | Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126:2666-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 6. | Taiwan Cancer Registry, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Available: http://tcr.cph.ntu.edu.tw/main.php?Page=A1. Accessed 5 April 2013. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 8. | Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 521] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Department of Household Registration, MOI. Table-3. Population by Age, Sex and Marital Status and Marriage Type. 2021. [DOI] [Full Text] |
| 10. | Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Inoue M. Age Standardization of Rates: A New WHO Standard. GPE Discussion Paper Series: No.31. EIP/GPE/EBD. World Health Organization 2001. [DOI] [Full Text] |
| 11. | Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, Geschwind JF, Pawlik TM. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736-750.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 13. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 14. | Al Mahjoub A, Bouvier V, Menahem B, Bazille C, Fohlen A, Alves A, Mulliri A, Launoy G, Lubrano J. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. Eur J Gastroenterol Hepatol. 2019;31:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 17. | Tseng CH, Tseng CP, Chong CK, Huang TP, Song YM, Chou CW, Lai SM, Tai TY, Cheng JC. Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia. 2006;49:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Chang CH, Shau WY, Jiang YD, Li HY, Chang TJ, Sheu WH, Kwok CF, Ho LT, Chuang LM. Type 2 diabetes prevalence and incidence among adults in Taiwan during 1999-2004: a national health insurance data set study. Diabet Med. 2010;27:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Lin CC, Li CI, Hsiao CY, Liu CS, Yang SY, Lee CC, Li TC. Time trend analysis of the prevalence and incidence of diagnosed type 2 diabetes among adults in Taiwan from 2000 to 2007: a population-based study. BMC Public Health. 2013;13:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, Buring JE, Chan AT, Chong DQ, Doody MM, Gapstur SM, Gaziano JM, Giovannucci E, Graubard BI, Lee IM, Liao LM, Linet MS, Palmer JR, Poynter JN, Purdue MP, Robien K, Rosenberg L, Schairer C, Sesso HD, Sinha R, Stampfer MJ, Stefanick M, Wactawski-Wende J, Zhang X, Zeleniuch-Jacquotte A, Freedman ND, McGlynn KA. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. 2018;118:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 22. | Wen CP, Tsai SP, Chen CJ, Cheng TY, Tsai MC, Levy DT. Smoking attributable mortality for Taiwan and its projection to 2020 under different smoking scenarios. Tob Control. 2005;14 Suppl 1:i76-i80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Wen CP, Levy DT, Cheng TY, Hsu CC, Tsai SP. Smoking behaviour in Taiwan, 2001. Tob Control. 2005;14 Suppl 1:i51-i55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Wu IH, Essien EJ, Sansgiry SS, Peters RJ, Yang M, Abughosh SM. Cigarette Smoking among Taiwanese Adults. Epidemiol. 2011;1:107. [DOI] [Full Text] |
| 25. | Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? J Hepatol. 2012;57:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 26. | Pan WH, Lee MS, Chuang SY, Lin YC, Fu ML. Obesity pandemic, correlated factors and guidelines to define, screen and manage obesity in Taiwan. Obes Rev. 2008;9 Suppl 1:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Mosadeghi S, Liu B, Bhuket T, Wong RJ. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: An updated analysis of the 2000-2011 Surveillance, Epidemiology and End Results registry. Hepatol Res. 2016;46:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Antwi SO, Patel T. Increasing mortality of intrahepatic cholangiocarcinoma in the US: are gender-specific risk factors important? Hepatobiliary Surg Nutr. 2019;8:635-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Singsuksawat E, Thuwajit C, Charngkaew K, Thuwajit P. Increased ETV4 expression correlates with estrogen-enhanced proliferation and invasiveness of cholangiocarcinoma cells. Cancer Cell Int. 2018;18:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Petrick JL, Florio AA, Zhang X, Zeleniuch-Jacquotte A, Wactawski-Wende J, Van Den Eeden SK, Stanczyk FZ, Simon TG, Sinha R, Sesso HD, Schairer C, Rosenberg L, Rohan TE, Purdue MP, Palmer JR, Linet MS, Liao LM, Lee IM, Koshiol J, Kitahara CM, Kirsh VA, Hofmann JN, Guillemette C, Graubard BI, Giovannucci E, Gaziano JM, Gapster SM, Freedman ND, Engel LS, Chong DQ, Chen Y, Chan AT, Caron P, Buring JE, Bradwin G, Beane Freeman LE, Campbell PT, McGlynn KA. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Liver Cancer Among Postmenopausal Women. Hepatology. 2020;72:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Shen TY, Chen HJ, Pan WH, Yu T. Secular trends and associated factors of age at natural menopause in Taiwanese women. Menopause. 2019;26:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Matsumoto K, Onoyama T, Kawata S, Takeda Y, Harada K, Ikebuchi Y, Ueki M, Miura N, Yashima K, Koda M, Sakamoto T, Endo M, Horie Y, Murawaki Y. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Intern Med. 2014;53:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Li M, Du M, Cong H, Gu Y, Fang Y, Li J, Gan Y, Tu H, Gu J, Xia Q. Characterization of hepatitis B virus DNA integration patterns in intrahepatic cholangiocarcinoma. Hepatol Res. 2021;51:102-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lu SN, Su WW, Yang SS, Chang TT, Cheng KS, Wu JC, Lin HH, Wu SS, Lee CM, Changchien CS, Chen CJ, Sheu JC, Chen DS, Chen CH. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int J Cancer. 2006;119:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Navas MC, Glaser S, Dhruv H, Celinski S, Alpini G, Meng F. Hepatitis C Virus Infection and Cholangiocarcinoma: An Insight into Epidemiologic Evidences and Hypothetical Mechanisms of Oncogenesis. Am J Pathol. 2019;189:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Tsao YC, Chen JY, Yeh WC, Peng YS, Li WC. Association between visceral obesity and hepatitis C infection stratified by gender: a cross-sectional study in Taiwan. BMJ Open. 2017;7:e017117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R; AISF Cholangiocarcinoma committee. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Witjes CD, Karim-Kos HE, Visser O, de Vries E, IJzermans JN, de Man RA, Coebergh JW, Verhoef C. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB (Oxford). 2012;14:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Ardito F, Coppola A, Rinninella E, Razionale F, Pulcini G, Carano D, Cintoni M, Mele MC, Barbaro B, Giuliante F. Preoperative Assessment of Skeletal Muscle Mass and Muscle Quality Using Computed Tomography: Incidence of Sarcopenia in Patients with Intrahepatic Cholangiocarcinoma Selected for Liver Resection. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Jiang W, Li FR, Yang HH, Chen GC, Hua YF. Relationship Between Fish Oil Use and Incidence of Primary Liver Cancer: Findings From a Population-Based Prospective Cohort Study. Front Nutr. 2021;8:771984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Songserm N, Woradet S, Charoenbut P. Fruit and Vegetables Consumption: A Pointer for Cholangiocarcinoma Prevention in Northeast Thailand, the Highest Incidence Area in the World. Nutr Cancer. 2016;68:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Fedirko V, Lukanova A, Bamia C, Trichopolou A, Trepo E, Nöthlings U, Schlesinger S, Aleksandrova K, Boffetta P, Tjønneland A, Johnsen NF, Overvad K, Fagherazzi G, Racine A, Boutron-Ruault MC, Grote V, Kaaks R, Boeing H, Naska A, Adarakis G, Valanou E, Palli D, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HBA, Siersema PD, Peeters PH, Weiderpass E, Skeie G, Engeset D, Quirós JR, Zamora-Ros R, Sánchez MJ, Amiano P, Huerta JM, Barricarte A, Johansen D, Lindkvist B, Sund M, Werner M, Crowe F, Khaw KT, Ferrari P, Romieu I, Chuang SC, Riboli E, Jenab M. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol. 2013;24:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Chiang KC, Yeh CN, Lin KJ, Su LJ, Yen TC, Pang JH, Kittaka A, Sun CC, Chen MF, Jan YY, Chen TC, Juang HH, Yeh TS. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a Chemical-Induced animal model. Oncotarget. 2014;5:3849-3861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Kreng VB, Yang CT. The equality of resource allocation in health care under the National Health Insurance System in Taiwan. Health Policy. 2011;100:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Chiang CJ, Wang YW, Lee WC. Taiwan's Nationwide Cancer Registry System of 40 years: Past, present, and future. J Formos Med Assoc. 2019;118:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |