Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3346
Peer-review started: January 3, 2022
First decision: January 23, 2022
Revised: January 27, 2022
Accepted: June 23, 2022
Article in press: June 23, 2022
Published online: July 21, 2022
Processing time: 195 Days and 10.9 Hours
Liver cancer is the third leading cause of cancer-related death worldwide with primary type hepatocellular carcinoma (HCC). Factors, including carcinogens, infection of hepatitis viruses, alcohol abuse, and non-alcoholic fatty liver disease (NAFLD), can induce HCC initiation and promote HCC progression. The prevalence of NAFLD accompanying the increased incidence of obesity and type 2 diabetes becomes the most increasing factor causing HCC worldwide. However, the benefit of current therapeutic options is still limited. Intrahepatic immunity plays critically important roles in HCC initiation, development, and progression. Regulatory T cells (Tregs) and their associated factors such as metabolites and secreting cytokines mediate the immune tolerance of the tumor microenvironment in HCC. Therefore, targeting Tregs and blocking their mediated factors may prevent HCC progression. This review summarizes the functions of Tregs in HCC-inducing factors including alcoholic and NAFLD, liver fibrosis, cirrhosis, and viral infections. Overall, a better understanding of the role of Tregs in the development and progression of HCC provides treatment strategies for liver cancer treatment.
Core Tip: Liver cancer is the third leading cause of cancer-related death worldwide. Hepatocellular carcinoma (HCC) is the primary type of liver cancer. Factors, including carcinogenic infection of hepatitis viruses, alcohol abuse, and non-alcoholic fatty liver disease (NAFLD), can induce HCC initiation and promote HCC progression. The prevalence of NAFLD accompanying the increased incidence of obesity and type 2 diabetes becomes the most increasing factor causing HCC worldwide. However, the benefit of current therapeutic options is still limited. Intrahepatic immunity plays critically important roles in HCC initiation, development, and progression. Regulatory T cells (Tregs) and their associated factors such as metabolites and secreting cytokines mediate the immune tolerance of the tumor microenvironment in HCC. Therefore, targeting Tregs and blocking their mediated factors may prevent HCC progression. A better understanding of the role of Tregs in intrahepatic immunity is helpful to develop novel HCC treatment options.
- Citation: Zhang CY, Liu S, Yang M. Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy. World J Gastroenterol 2022; 28(27): 3346-3358
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3346
Liver cancer is the third leading cause of cancer-related death worldwide with 8.3% of death ratio, following lung and colorectal cancers[1]. The most common type of primary liver cancer is hepatocellular carcinoma (HCC) and the second type is cholangiocarcinoma[2]. Factors, including carcinogens (e.g., aflatoxin B1), infection of hepatitis viruses, alcohol abuse, and non-alcoholic fatty liver disease (NAFLD), can induce HCC and promote HCC progression[3-5]. In addition, accompanying the increasing incidence of obesity and type 2 diabetes (T2D), NAFLD becomes an increasing factor that causes HCC worldwide[6,7].
Surgical resection is a curative treatment option for the early stage of HCC. However, most cases in HCC were found in the late stage. In addition, other minimally invasive local therapies, such as radiofrequency ablation and microwave ablation, and systemic therapy, such as tyrosine kinase inhibitors, are treatment options for patients who are not suitable for surgery[8]. Furthermore, immunotherapy by targeting checkpoint inhibitors [e.g., anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) antibodies] shows benefits against advanced HCC in the clinic. A combination treatment by blocking both PD-L1 (e.g., atezolizumab) and vascular endothelial growth factor (VEGF) (e.g., bevacizumab) is one of the best first-line treatments for advanced HCC[9]. Other potential immunotherapy options including T cell-mediated therapy such as chimeric antigen receptor-engineered T cells[10-12], peptide-based vaccines[13-15], and micro ribonucleic acids (miRNAs)-mediated therapies[16], are undergoing investigations for HCC treatment.
Intrahepatic immunity including both innate and adaptive immune responses plays pivotal roles in the development and progression of HCC, especially for T cells[17]. Among them, the imbalance between effector CD4 and/or CD8 T cells and regulatory T cells (Tregs) induces immunotolerance and promotes HCC progression[18,19]. Factors impacting the balance of effector T cells and Tregs include gut microbiota, transforming growth factor-beta (TGF-β), and treatments such as trans-arterial chemoembolization[18-20], etc. The expression of cytokines such as interleukin (IL)-2, IL-5, interferon (IFN)-γ was increased with an increased ratio of cytotoxic T lymphocytes (CTLs)/Tregs with the treatment of Lenvatinib, a multiple kinase inhibitor, while the expression of T-cell immunoglobulin mucin-3 (Tim-3) and CTL-associated antigen-4 (CTLA-4) was decreased on Treg cells[21]. Therefore, modulating the Treg frequency and the expression of related cytokines are critically important for anti-tumor immunotherapy.
In this review, functions of Tregs on HCC causing factors such as alcoholic liver disease (ALD), NAFLD, liver fibrosis, and cirrhosis are discussed. In addition, molecules mediated Treg functions and therapeutic options by targeting Tregs are summarized. Moreover, clinical trials by targeting Tregs to modulate immune response were analyzed.
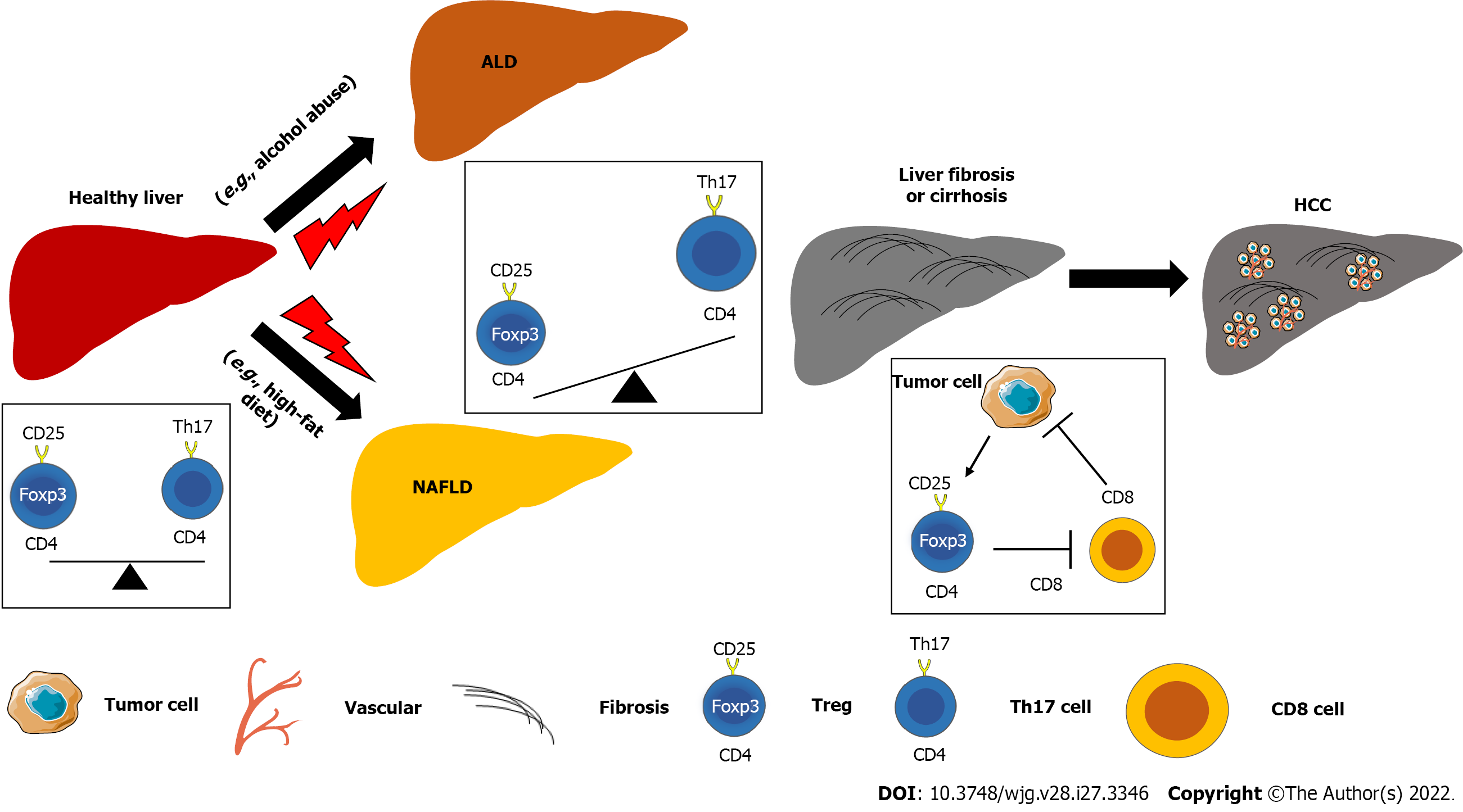
As immunosuppressive cells, Tregs play a pivotal role in chronic liver diseases, including ALD. For example, chronic-binge alcohol exposure in C57BL/6 mice induced the reduction of Treg cells, but increased T helper 17 cells (Th17) cells and the production of IL-17[22]. Treatment with ginsenoside F2 can ameliorate ALD by increasing the frequency of Foxp3+ Tregs and decreasing IL-17-producing Th17 cells compared to control groups[23]. However, the molecular mechanism of how Tregs impact the progression of ALD except for modulation of liver inflammation remains unclear.
The balance Th17 cells/Tregs plays an essential role in metabolic diseases by regulating immune response and glucose and lipid metabolism[8]. The lower Treg (forkhead box P3+/FOXP3+) and higher Th17 cell (IL-17-producing cells) numbers were found in portal or periportal tract in livers of adult NAFLD patients, whereas more Tregs were shown in pediatric NAFLD patients[24]. In addition, severe liver inflammation was positively associated with intralobular expression of FOXP3 in pediatric patients but was positively associated with higher expression of IL-17 and lower expression of FOXP3 in adult patients, indicating the role of Tregs in NAFLD is age-dependent. Intrahepatic imbalance of Th17/Treg cells promotes the progression of NAFLD, accompanying higher expression of inflammatory cytokines such as IL-6, IL-17, and IL-23 in both serum and liver[25]. Feeding a high-fat diet (HFD) can impact the balance of Th17/Treg cells and Th1/Th2 cells of CD4 T cells in mesenteric lymph nodes (MLN). In addition, those CD4 T cells can potentially migrate into the liver to promote liver inflammation to result in NAFLD progression[26]. The effects of CD4 T cells in MLN on liver inflammation and fat accumulation can be ameliorated by administration of antibiotics and probiotics, indicating an important role of gut microbiota in NAFLD pathogenesis[26].
Dywicki et al[27] showed intrahepatic Tregs were increased in high-fat high-carbohydrate (HF-HC) diet-induced nonalcoholic steatohepatitis (NASH) in BALB/c mice. In addition, depletion of adaptive immunity aggregated HF-HC diet-induced NASH in recombination activating 1-knockout BALB/c mice. Although Tregs showed an anti-inflammation effect in ALD[23], adoptive transfer of Tregs increased steatosis and serum level of alanine aminotransferase (ALT), indicating that Tregs enhance the progression of NAFLD[27]. Another study also showed that increasing Tregs in subcutaneous adipose tissue induced by adoptive transfer of Tregs from healthy C57BL/6J mice to high-fat HFD (HFHFD)-fed mice increased hepatic steatosis during NAFLD development[28].
Mechanistically, the formation of neutrophil extracellular traps during NASH progression can induce Treg differentiation from naïve CD4 T cells, which is dependent on Toll-like receptor 4 (TLR-4) and involved in NASH-HCC progression[29].
Progression of chronic liver disease, including ALD and NAFLD, can promote the development of liver fibrosis and its advanced stage liver cirrhosis. However, there are no currently available therapies that can treat or reverse liver cirrhosis. Deng et al[30] reported that co-infusion with human amniotic mesenchymal stromal cells (hAMSCs) and Tregs can prevent mild liver fibrosis. Tregs play a critical role in the secretion of hepatocyte growth factor (HGF) and cell differentiation of hAMSCs.
Furthermore, an imbalance of Th17 cells/Tregs was also shown in cirrhotic patients with hepatitis B virus (HBV) infection. The frequency of Tregs was reduced in peripheral blood, while the frequency of Th17 cells was increased, resulting in a decreased Treg/Th17 ratio as a potential diagnostic marker for decompensated liver cirrhosis[31]. Another study also showed that the frequencies of both Tregs and Th17 cells were increased in the blood of patients with HBV infection and cirrhotic livers but with a higher extent in Th17 cells, resulting in an increased ratio of Th17/Treg, compared to the control group[32]. In addition, the mRNA levels of proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor (TNF)-α, as well as the protein expression of nuclear factor κB in the liver were significantly increased in HBV-infected liver and cirrhotic liver compared to healthy controls. Another study also showed that HBV infection can induce IL-8/C-X-C motif chemokine receptor 1/TGF-β signaling to provoke Treg polarization, resulting in suppression of anti-tumor immunity and enhance of HCC metastasis[33]. Moreover, the frequency of Tregs in blood and plasma levels of IL-35 were increased and positively related to viral load in HCV infected patients with cirrhosis and HCC[34].
A meta-analysis showed that a higher infiltration of CD3 T cells, CD8 T cells, and natural killer cells was associated with better overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS). In contrast, a higher infiltration of Tregs and neutrophils indicated lower OS and DFS[35]. Another report also showed that an increase of Tregs or a decrease of M1 macrophages (proinflammatory phenotype) were associated with a poor prognosis of HCC patients[36]. C-C chemokine receptor type 4 (CCR4)+Tregs are predominant Tregs that are recruited in tumor tissue of HCC associated infection of hepatitis viruses, which is associated with HCC resistance to sorafenib treatment[37]. The frequency of CD127low, CD25+, CD4+, Tregs was increased significantly in the peripheral venous blood of HCC patients compared to healthy controls[38]. In addition, the serum levels of TGF-β1 and IL-10 in HCC patients were positively associated with the Treg population in the blood, which were decreased post-operation and chemotherapy treatments. C-C motif chemokine ligand (CCL) 5 expression on circulating tumor cells in HCC patients can attract Tregs to induce an immunosuppressive environment, one of the mechanisms for CTC escaping immune surveillance[39].
The expression of immune checkpoint proteins in the HCC microenvironment impacts Tregs and antitumor immunity. PD-L1+neutrophils, Tregs, and neutrophil to lymphocyte ratio were significantly increased in peripheral blood of patients with poorly differentiated HCC with a worse prognosis compared to that in patients with highly-moderately differentiated HCC[40]. Zhou et al[41] reported that tumor-associated neutrophils can induce the infiltration of the macrophages and Tregs from HCC mice or patients via producing CCL2 and CCL17, resulting in HCC progression and resistance to sorafenib. CTLA-4 on Tregs in HCC impacts dendritic cell function by downregulating CD80/CD86 on dendritic cells (DCs)[42]. Therefore, blockade of CTLA-4 in HCC can improve DC-mediated anti-tumor immunity.
Treatment with tivozanib, a tyrosine kinase inhibitor, can suppress Tregs by inhibiting receptor tyrosine kinase c-Kit (CD117)/stem cell factor (SCF) axis and increased CD4+PD-1+T cells, resulting in a significant improvement in OS of HCC patients[43]. Treatment with Lenvatinib also can inhibit IL-2 mediated Treg differentiation except for decreasing PD-L1 expression in HCC cells[44]. Overall, the balance between Tregs with other T cells plays a vital in liver diseases, including the initiation and progression of HCC (Figure 1).
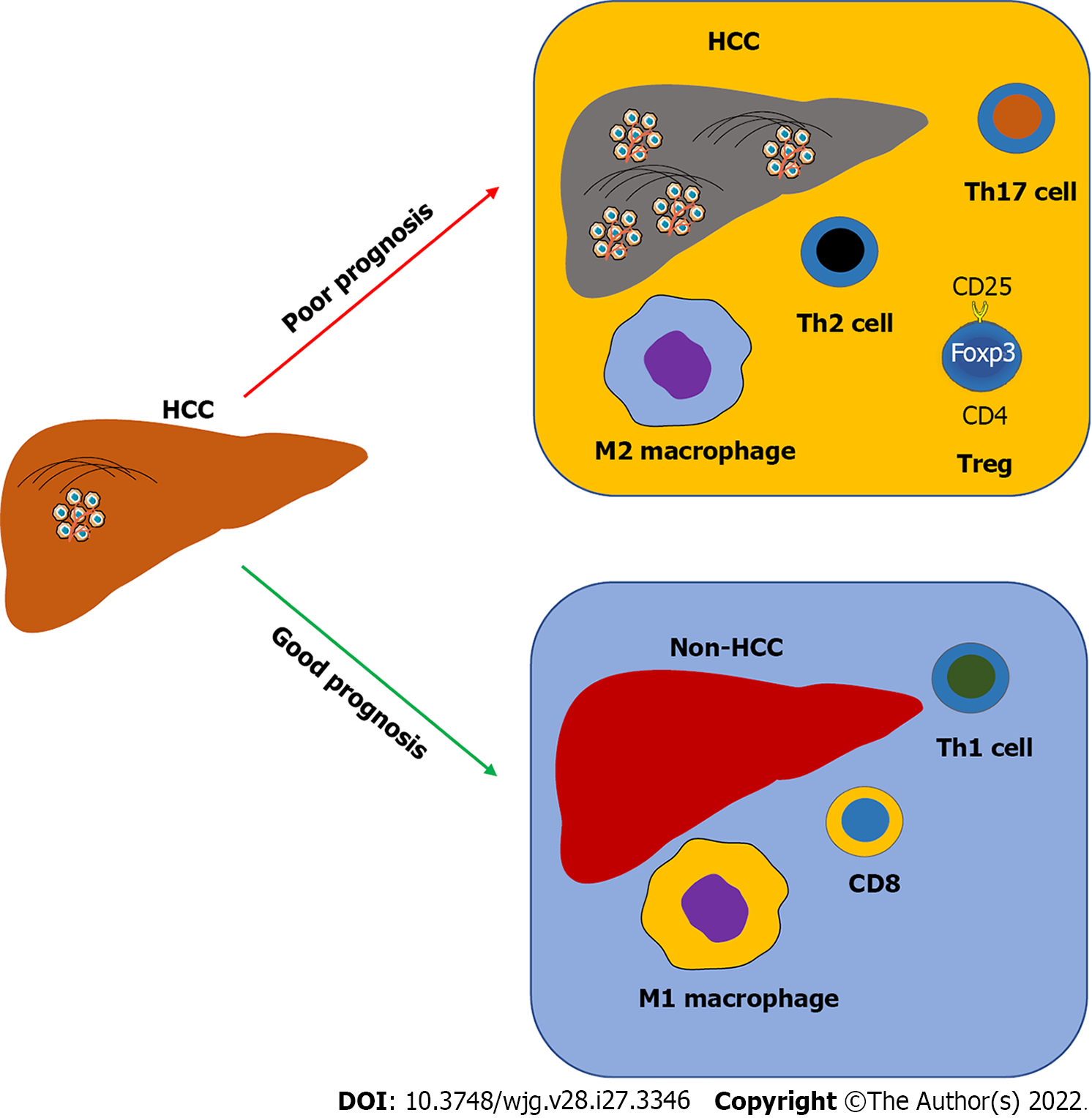
Furthermore, alteration of intrahepatic immunity is associated with HCC prognosis and treatment (Figure 2). An increase of Tregs, Th2, and Th17 T cells, as well as M2 macrophages, is usually and positively associated with HCC progression in patients, whereas an abundance of CD8 T cells, Th1 T cells, and M1 macrophages is associated with HCC therapy and good prognosis for HCC patients[45]. Single-cell RNA sequencing technologies have been applied to investigate the immune landscape of HCC samples to illustrate the subtypes of immune cells in HCC and their gene expressing profiles, as well as immune cell interactions, such as DCs with Tregs or CD8 T cells[46].
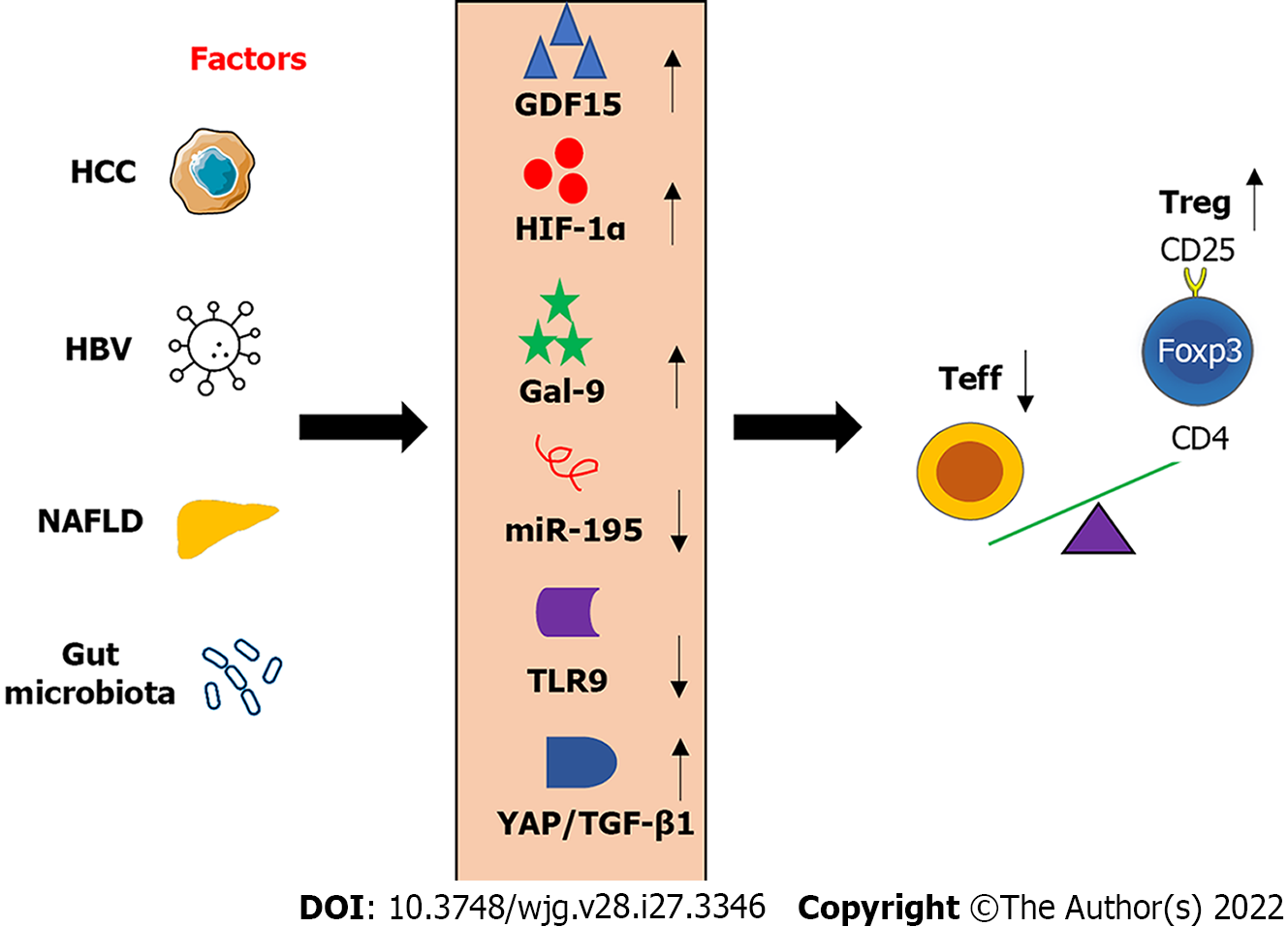
Hypoxia-inducible transcription factors (HIFs) regulate cell metabolism, proliferation, and migration in low oxygen or hypoxic environment, as well as angiogenesis[47]. It has been reported that the expression of HIF-1 alpha (HIF-1α) was higher in HCC tissues compared to that in corresponding adjacent tissues. In addition, overexpression of HIF-1α was associated with poor outcomes of HCC in human patients[48]. Chronic intermittent hypoxia can promote NASH progression via regulating the balance of Th17/Treg by inducing the expression of HIF-1α[49].
Tregs can be subclassified into inflamed-tissue related memory Tregs (mTregs) and non-related resting Treg (rTregs). During HBV infection, mTregs were increased accompanying liver inflammation and liver injury evidenced by an increase of serum ALT level, but not rTregs[50]. The S-type lectin galectin-9 (Gal-9) was increased in the HBV-infected liver, contributing to T cell depletion and exhaustion by binding Tim-3[51]. For example, activation of Gal-9/Tim-3 signaling in concanavalin A-induced mouse hepatitis suppressed the induction of effector T (Teff) cells and the production of IFN-γ[52]. In addition, the Gal-9/Tim-3 signaling pathway plays an important role in the expansion of mTregs[50].
The expression of growth differentiation factor 15 (GDF15) was positively related to the frequency of Tregs in HCC. GDF15 can promote the suppressive effect of natural Tregs via binding with its unrecognized receptor CD48 on T cells to inhibit the function of homology and U-box containing protein 1, which can degrade FOXP3[53]. Thus, neutralizing GDF15 by an antibody can eradicate HCC and enhance anti-tumor immunity.
Hepatic expression of microRNA-195 (miR-195) was reduced in NAFLD development, accompanying an increased ratio of Th17/Treg ratio in the blood, as well as the expression IL-17, CD40, and TNF-α in rat liver[54]. Overexpression of miR-195 can maintain the balance of Th17/Treg to ameliorate NAFLD and liver inflammation. Many miRNAs can regulate Th17/Treg cell balance in NAFLD such as miR-29c via interacting with insulin-like growth factor binding protein 1/IGFBP1)[55]. In addition, other microRNAs such as miR-155[56,57], miR-423-5p[58], and miR-1246[59] play important roles in modulating the balance of Tregs with Th17 cells and their functions in liver disease.
Activation of TLR signaling pathway can suppress the effect of Tregs on adaptive immune response, which is in part dependent on microbial production-induced expression of IL-6[60]. TLR9-deficiency increased the frequency of Treg cells in the intestine, resulting in a decrease of IL-17 and IFN-γ producing Teff cells[61]. The imbalance of Treg/Teff cells compromised immune response to oral infection, which can be reversed by reconstitution of gut flora deoxyribonucleic acid (DNA)[61]. In addition, the antibiotic treatment caused gut microbiota dysbiosis and recapitulated TLR9 deficiency-induced impaired immune response.
Yes-associated protein (YAP), a coactivator and a corepressor of the Hippo signaling pathway, plays a vital role in Tregs in vivo and in vitro[62]. Blocking YAP-mediated activation of activin can improve anti-tumor immunity via regulating TGF-β/mothers against decapentaplegic homolog (SMAD)[62]. Similarly, blockage of TGF-β signaling can compromise Treg function to improve anti-tumor immune response[63], which may expand the population of quiescent Tregs, CD4+CD25-Foxp3+.
The above-mentioned molecules can modulate Treg metabolism and function as potential molecular targets for HCC treatment. In addition, modulation of these molecules can potentially recover the balance of Tregs with other tumor-infiltrating immune cells to activate anti-tumor immunity (Figure 3).
Administration of miR-26a can reduce the frequency of Tregs and the concentrations of alpha-fetoprotein, des-gamma carboxyprothrombin, and VEGF in Balb/c mice with diethylnitrosamine-induced HCC[64]. The suppressive effects of miR-26a on HCC growth and angiogenesis are mediated by targeting IL-6/signal transducer and activator of transcription 3 (Stat3) signaling[65] and HGF/HGF receptor (HGFR/c-Met) signaling[66], respectively. In addition, miR-26a inversely regulated the expression of F-box protein 11 (FBXO11), which was upregulated and played an oncogenic role in HCC[67].
Adoptive transfer of Tregs attenuated triptolide-induced liver injury, while depletion of Tregs showed the opposite effect, indicating that Tregs contribute to the progression of liver injury[68]. Another study showed that adoptive transfer of hepatic stellate cell (HSC)-stimulated Tregs can significantly decrease liver injury in mice with autoimmune hepatitis by inducing the balance of Treg/Th17 ratio[69]. In addition, the adoptive transfer of HSCs promoted the differentiation of Tregs and decreased Th17 cells, resulting in amelioration of liver injury[70]. Deng et al[30] reported that co-infusion with hAMSCs and Tregs can prevent mild liver fibrosis. Tregs play a critical role in the secretion of HGF and cell differentiation of hAMSCs.
Depletion of Tregs in the intestine caused an increase in the abundance of Firmicutes and intestinal inflammation[71]. Supplementation of Lactobacillus rhamnosus GG or its culture supernatant can ameliorate chronic alcohol-induced liver injury by reducing TNF-α expression via inhibition of TLR4- and TLR5-mediated hepatic inflammation[72], as well as amelioration of intestinal barrier integrity and suppression of alcohol-induced endotoxemia[73]. In addition, the culture supernatant can balance the ratio of Treg and Th17 cells to reduce alcoholic-induced liver injury[22].
Treatment with Prohep, a novel probiotic mixture, significantly inhibited the HCC growth compared to the control group, resulting in an abundance of beneficial bacteria, such as Prevotella and Oscillibacter[74]. This study also showed that probiotic treatment regulated T-cell differentiation in the gut by reducing Th17 polarization and increasing the differentiation of anti-inflammatory Treg cells.
Dual anti-PD-1/VEGF receptor-2 therapy increased CD8 T cell infiltration and activation, reduced Tregs and infiltration of CCR2+monocytes, as well as the phenotype of tumor-associated macrophages (the M1/M2 ratio) in HCC tissue[75]. Another study also showed that Treg-mediated inhibition of IFN-γ production and cytotoxicity of CD8 T cells can be partially reduced by anti-PD-1 and anti-PD-L1 antibodies in HCC[76].
Treg depletion-mediated by anti-CTLA-4 monoclonal antibody (clone 9H10) restored the function of tumor antigen-specific CD8 T cells, with a synergetic effect with anti-PD-1 treatment[77].
CCR4 expression in Tregs accompanied with an increased expression IL-10 and IL-35, resulting in suppression of CD8 T cells and HCC progression. Administration of a CCR4 antagonist or N-CCR4-Fc, a neutralizing pseudo-receptor that can block Tregs accumulation in HCC, can enhance therapeutic efficacy to PD-1 blockade and sorafenib[37]. Treg depletion induced by anti-CCR4 antibody (mogamulizumab), in combination with anti-PD-1 antibody (nivolumab) showed antitumor activity and increased CD8+ T cell infiltration[78].
Treatment with resveratrol, a natural phenol, can inhibit H22 (a mouse HCC cell line)-induced orthotopic HCC tumor growth via decreasing the frequency of CD8+CD122+Tregs and M2-like macrophages in mice[79].
Ren et al[80] reported that Tregs were further increased in HCC patients compared to healthy and cirrhosis controls, as well as in HCC patients with Barcelona clinic liver cancer (BCLC) stage C compared to that in HCC patients with BCLC stage B. The authors also showed that treatment with microparticles-transarterial chemoembolization dramatically decreased Treg cell proportion at 1-2 wk post-treatment. Overall, the treatment options for HCC associated with Treg regulation were summarized in Table 1.
| Treatment | Targets | Functions | Ref. |
| CCR4 antagonist | CCR4 | Administration of a CCR4 antagonist or N-CCR4-Fc, a neutralizing pseudo-receptor that can block Tregs accumulation in HCC, can enhance therapeutic efficacy to PD-1 blockade and sorafenib | Gao et al[37], 2022 |
| miR-26a | IL6/Stat3 and HGF/c-Met | The suppressive effects of miR-26a on HCC growth and angiogenesis are mediated by targeting IL-6/signal transducer and activator of transcription 3 signaling and HGF/HGFR/c-Met signaling, respectively | Yang et al[65], 2013; Yang et al[66], 2014 |
| GDF15 neutralizing antibody | GDF15/CD48 | Inhibiting GDF15 function by a neutralizing antibody can effectively eradicate HCC and promote a tumoricidal immune response in mice | Wang et al[53], 2021 |
| Supplementation of Lactobacillus rhamnosus GG or its culture supernatant | The ratio of Treg and Th17 cells | Supplementation of Lactobacillus rhamnosus GG or its culture supernatant can ameliorate chronic alcohol-induced liver injury by reducing hepatic inflammation, enhancing intestinal barrier integrity, and inducing balance in the ratio of Treg and Th17 cells to reduce alcoholic-induced liver injury | Chen et al[22], 2016; Wang et al[72], 2013; Wang et al[73], 2012 |
| Prohep, a novel probiotic mixture | Gut microbiota and Treg differentiation | Probiotic treatment regulated T-cell differentiation in the gut by reducing Th17 polarization and increasing the differentiation of anti-inflammatory Treg cells, by increasing the abundance of beneficial bacteria, such as Prevotella and Oscillibacter | Li et al[74], 2016 |
| Anti-PD-1 and anti-PD-L1 antibodies | PD-1 and PD-L1 | Another study also showed that Treg-mediated inhibition of IFN-γ production and cytotoxicity of CD8 T cells can be partially reduced by anti-PD-1 and anti-PD-L1 antibodies in HCC | Langhans et al[76], 2019 |
| Dual anti-PD-1/VEGFR-2 therapy | VEGFR-2 and PD-1 | Dual therapies increased CD8 T cell infiltration and activation, reduced Tregs and infiltration of CCR2+monocytes, as well as the phenotype of tumor-associated macrophages (the M1/M2 ratio) in HCC tissue | Shigeta et al[75], 2020 |
| Anti-CTLA-4 monoclonal antibody | Tregs | Treg depletion-mediated by anti-CTLA-4 monoclonal antibody (clone 9H10) restored the function of tumor antigen-specific CD8 T cells, with a synergistic effect with anti-PD-1 treatment | Lee et al[77], 2020 |
| Resveratrol | Tregs and immunosuppressive cytokines including TGF-β1 and IL-10 | Treatment with resveratrol, a natural phenol, can inhibit H22 (a mouse HCC cell line)-induced orthotopic HCC tumor growth via decreasing the frequency of CD8+CD122+Tregs and M2-like macrophages in mice | Zhang et al[79], 2020 |
Tregs display multiple roles in the development and progression of HCC. The ratio of Treg/Th17 cells in peripheral blood can be applied to monitor immune tolerance as immune markers in liver transplantation[81]. The balance of Treg/Th17 cells or other effector T cells is essential for suppressing autoimmune diseases and cancers[82]. Therefore, treatments including diverse immunomodulatory therapies can regulate Tregs to enhance the antitumor immune response. In Table 2, potential therapies in clinical trials were summarized. Treatments including infusion of Tregs[83-85] and mesenchymal stromal cells (MSCs)[86], vaccines[87-89], and kinase inhibitors[90].
| Trial | Phase | Treatment | Results | Ref. |
| NCT02476123 | I | Anti-CCR4 antibody mogamulizumab | Treg depletion induced by anti-CCR4 antibody (mogamulizumab), in combination with anti-PD-1 antibody (nivolumab) showed antitumor activity and increased CD8+ T cell infiltration | Doi et al[78], 2019; |
| NCT02166177 | I | Intravenous infusion of ex vivo expanded Tregs | Treg transfer can transiently increase circulating Tregs and inhibit anti-donor T cell responses in patients with liver transplants | Fueyo et al[83], 2020 |
| NCT02166177 | I | Autologous Treg therapy | To defect safety and efficacy study of regulatory T cell therapy in liver transplant patients | Whitehouse et al[84], 2017 |
| NCT01624077 | I | Injection of Tregs | To defect safety and efficacy study of regulatory T cell therapy in liver transplant patients | Whitehouse et al[84], 2017 |
| NCT03654040 | I | A single dose of alloantigen-reactive Tregs (arTreg) (≥ 90 × 106 total cells) | It is a single-center, prospective, open-label, non-randomized clinical trial exploring cellular therapy to facilitate immunosuppression withdrawal in liver transplant recipients | Cvetkovski et al[85], 2021 |
| NCT03577431 | arTreg-CSB (2.5 × 106 cells) | |||
| NCT02260375 | I | Infusion of mesenchymal stromal cells | MSC infusion in liver transplant recipients slightly increased circulating Treg/memory Treg over baseline, without a statistically significant, but not in the control group | Casiraghi et al[86], 2021 |
| NCT02027116 | I | DNA vaccine GLS-6150 | GLS-6150 decreases Treg cell frequency and enhances HCV-specific T cell responses without significant side effects | Han et al[87], 2020 |
| NCT02174276 | II | GS-4774, a yeast-based therapeutic vaccine | Treatment with GS-4774 increased T-cell functions by increasing the production of IFN-γ and TNF and reducing the cell number of Tregs | Boni et al[88], 2019 |
| NCT02360592 | IV | Combined therapy with interferon plus IL-1 and hepatitis B Vaccine | Combination therapy increased the level of hepatitis B surface antigen with partial restoration of Tregs and NK cells | Wu et al[89], 2019 |
| NCT02072486 | None | Sorafenib, a multiple kinase inhibitor | Treatment with sorafenib can significantly suppress extracellular signal-regulated kinases+ FMS-like tyrosine kinase 3+ Tregs and myeloid-derived suppressor cells to benefit the survival of HCC patients | Kalathil et al[90], 2019 |
Tregs modulate the intestinal and intrahepatic immune response, contributing critically important roles in the gut-liver axis. Functional changes of Tregs are involved in the pathogenesis of chronic liver diseases, such as ALD and NAFLD, causing factors for HCC. Several important molecules investigated in recent studies are summarized and targeting them may potentially treat HCC by modulating Treg function and/or frequency. Clinical trials are undergoing to further explore the new treatments for HCC, which modulate the function of the frequency of Tregs. In the future, multi-omic analysis including metabolic and proteomic data for Treg metabolism and function during the progression of HCC is critical to illustrate the underlying mechanisms of Tregs in HCC pathogenesis and find out new therapeutic targets.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grassi G, Italy; Lu G, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64661] [Article Influence: 16165.3] [Reference Citation Analysis (176)] |
| 2. | Zhang C, Yang M. The Emerging Factors and Treatment Options for NAFLD-Related Hepatocellular Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Qi X, Yang M, Stenberg J, Dey R, Fogwe L, Alam MS, Kimchi ET, Staveley-O'Carroll KF, Li G. Gut microbiota mediated molecular events and therapy in liver diseases. World J Gastroenterol. 2020;26:7603-7618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | De Battista D, Zamboni F, Gerstein H, Sato S, Markowitz TE, Lack J, Engle RE, Farci P. Molecular Signature and Immune Landscape of HCV-Associated Hepatocellular Carcinoma (HCC): Differences and Similarities with HBV-HCC. J Hepatocell Carcinoma. 2021;8:1399-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Zhu Q, Ma Y, Liang J, Wei Z, Li M, Zhang Y, Liu M, He H, Qu C, Cai J, Wang X, Zeng Y, Jiao Y. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Liu S, Yang M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front Endocrinol (Lausanne). 2021;12:808526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Yang M, Ericsson AC. The Potential Gut Microbiota-Mediated Treatment Options for Liver Cancer. Front Oncol. 2020;10:524205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 856] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 10. | Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, Huang Y, Wu Q, Li X, He B, Li T, Liang B, Zhang J, Cao B, Liu M, Feng Y, Ye X, Chen X, Wang L, Tian Y, Li H, Li J, Hu H, He J, Hu Y, Zhi C, Tang Z, Gong Y, Xu F, Xu L, Fan W, Zhao M, Chen D, Lian H, Yang L, Li P, Zhang Z. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 11. | Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, Su L, Wu X, Zhou J, Mackay S, Kramer J, Duan Z, Yang H, Kolluri A, Hummer AM, Torres MB, Zhu H, Hall MD, Luo X, Chen J, Wang Q, Abate-Daga D, Dropulic B, Hewitt SM, Orentas RJ, Greten TF, Ho M. Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice. Gastroenterology. 2020;158:2250-2265.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 12. | Batra SA, Rathi P, Guo L, Courtney AN, Fleurence J, Balzeau J, Shaik RS, Nguyen TP, Wu MF, Bulsara S, Mamonkin M, Metelitsa LS, Heczey A. Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma. Cancer Immunol Res. 2020;8:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 13. | Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, Endo I, Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6:e1346764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Charneau J, Suzuki T, Shimomura M, Fujinami N, Nakatsura T. Peptide-Based Vaccines for Hepatocellular Carcinoma: A Review of Recent Advances. J Hepatocell Carcinoma. 2021;8:1035-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Yang M, Ericsson AC. Antimicrobial Peptides: Potential Application in Liver Cancer. Front Microbiol. 2019;10:1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Roy B, Ghose S, Biswas S. Therapeutic strategies for miRNA delivery to reduce hepatocellular carcinoma. Semin Cell Dev Biol. 2022;124:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Zhang C, Yang M. Targeting T Cell Subtypes for NAFLD and NAFLD-Related HCC Treatment: An Opinion. Front Med (Lausanne). 2021;8:789859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 292] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 19. | Dituri F, Mancarella S, Serino G, Chaoul N, Lupo LG, Villa E, Fabregat I, Giannelli G. Direct and Indirect Effect of TGFβ on Treg Transendothelial Recruitment in HCC Tissue Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, Boyton RJ, Altmann DM, Goldin RD, Akarca AU, Marafioti T, Mauri FA, Casagrande E, Grillo F, Giannini E, Bhoori S, Mazzaferro V. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Zhu J, Fang P, Wang C, Gu M, Pan B, Guo W, Yang X, Wang B. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. 2021;10:7977-7987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Chen RC, Xu LM, Du SJ, Huang SS, Wu H, Dong JJ, Huang JR, Wang XD, Feng WK, Chen YP. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett. 2016;241:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Kim MH, Kim HH, Jeong JM, Shim YR, Lee JH, Kim YE, Ryu T, Yang K, Kim KR, Jeon BM, Kim SC, Jung JK, Choi JK, Lee YS, Byun JS, Jeong WI. Ginsenoside F2 attenuates chronic-binge ethanol-induced liver injury by increasing regulatory T cells and decreasing Th17 cells. J Ginseng Res. 2020;44:815-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Cairoli V, De Matteo E, Rios D, Lezama C, Galoppo M, Casciato P, Mullen E, Giadans C, Bertot G, Preciado MV, Valva P. Hepatic lymphocytes involved in the pathogenesis of pediatric and adult non-alcoholic fatty liver disease. Sci Rep. 2021;11:5129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | He B, Wu L, Xie W, Shao Y, Jiang J, Zhao Z, Yan M, Chen Z, Cui D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol. 2017;18:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Su L, Wu Z, Chi Y, Song Y, Xu J, Tan J, Cong X, Liu Y. Mesenteric lymph node CD4+ T lymphocytes migrate to liver and contribute to non-alcoholic fatty liver disease. Cell Immunol. 2019;337:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Dywicki J, Buitrago-Molina LE, Noyan F, Davalos-Misslitz AC, Hupa-Breier KL, Lieber M, Hapke M, Schlue J, Falk CS, Raha S, Prinz I, Koenecke C, Manns MP, Wedemeyer H, Hardtke-Wolenski M, Jaeckel E. The Detrimental Role of Regulatory T Cells in Nonalcoholic Steatohepatitis. Hepatol Commun. 2022;6:320-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Van Herck MA, Vonghia L, Kwanten WJ, Vanwolleghem T, Ebo DG, Michielsen PP, De Man JG, Gama L, De Winter BY, Francque SM. Adoptive Cell Transfer of Regulatory T Cells Exacerbates Hepatic Steatosis in High-Fat High-Fructose Diet-Fed Mice. Front Immunol. 2020;11:1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, Shen C, Hu Z, Beane J, Ansa-Addo EA, Huang H, Tian D, Tsung A. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 30. | Deng Z, Zhou J, Mu X, Gu J, Li X, Shao Q, Li J, Yang C, Han G, Zhao J, Xia Y. Regulatory T Cells Improved the Anti-cirrhosis Activity of Human Amniotic Mesenchymal Stem Cell in the Liver by Regulating the TGF-β-Indoleamine 2,3-Dioxygenase Signaling. Front Cell Dev Biol. 2021;9:737825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Lan YT, Wang ZL, Tian P, Gong XN, Fan YC, Wang K. Treg/Th17 imbalance and its clinical significance in patients with hepatitis B-associated liver cirrhosis. Diagn Pathol. 2019;14:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Mou H, Wu S, Zhao G, Wang J. Changes of Th17/Treg ratio in the transition of chronic hepatitis B to liver cirrhosis and correlations with liver function and inflammation. Exp Ther Med. 2019;17:2963-2968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Zhang C, Gao Y, Du C, Markowitz GJ, Fu J, Zhang Z, Liu C, Qin W, Wang H, Wang F, Yang P. Hepatitis B-Induced IL8 Promotes Hepatocellular Carcinoma Venous Metastasis and Intrahepatic Treg Accumulation. Cancer Res. 2021;81:2386-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Abd El-Ghani EH, Afifi NA, Ibrahim MA, Zahran AM, El-Mokhtar MA, Mekky MA, Hetta HF. Regulatory T Cells and IL35 in Chronic Hepatitis C Related Cirrhosis and Hepatocellular Carcinoma. Egypt J Immunol. 2021;28:46-52. [PubMed] |
| 35. | Schoenberg MB, Li X, Han Y, Hao J, Miksch RC, Koch D, Börner N, Beger NT, Bucher JN, Schiergens TS, Guba MO, Werner J, Bazhin AV. The predictive value of tumor infiltrating leukocytes in Hepatocellular Carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47:2561-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Yu S, Wang Y, Hou J, Li W, Wang X, Xiang L, Tan D, Wang W, Jiang L, Claret FX, Jiao M, Guo H. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS One. 2020;15:e0231003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Gao Y, You M, Fu J, Tian M, Zhong X, Du C, Hong Z, Zhu Z, Liu J, Markowitz GJ, Wang FS, Yang P. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J Hepatol. 2022;76:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 38. | Zhou W, Deng J, Chen Q, Li R, Xu X, Guan Y, Li W, Xiong X, Li H, Li J, Cai X. Expression of CD4+CD25+CD127Low regulatory T cells and cytokines in peripheral blood of patients with primary liver carcinoma. Int J Med Sci. 2020;17:712-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Sun YF, Wu L, Liu SP, Jiang MM, Hu B, Zhou KQ, Guo W, Xu Y, Zhong Y, Zhou XR, Zhang ZF, Liu G, Liu S, Shi YH, Ji Y, Du M, Li NN, Li GB, Zhao ZK, Huang XY, Xu LQ, Yu QC, Peng DH, Qiu SJ, Sun HC, Dean M, Wang XD, Chung WY, Dennison AR, Zhou J, Hou Y, Fan J, Yang XR. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat Commun. 2021;12:4091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 40. | Zhou L, Wang J, Lyu SC, Pan LC, Shi XJ, Du GS, He Q. PD-L1+NEUT, Foxp3+Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma. Technol Cancer Res Treat. 2021;20:15330338211045820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646-1658.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 618] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 42. | Chen X, Du Y, Hu Q, Huang Z. Tumor-derived CD4+CD25+regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract. 2017;213:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Kalathil SG, Wang K, Hutson A, Iyer R, Thanavala Y. Tivozanib mediated inhibition of c-Kit/SCF signaling on Tregs and MDSCs and reversal of tumor induced immune suppression correlates with survival of HCC patients. Oncoimmunology. 2020;9:1824863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 45. | Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front Immunol. 2020;11:568759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 46. | Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, Peet GW, Zhong G, Lu S, Zhu W, Mao Y, Xiao M, Bergmann M, Hu X, Kerkar SP, Vogt AB, Pflanz S, Liu K, Peng J, Ren X, Zhang Z. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell. 2019;179:829-845.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1007] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 47. | Wilson GK, Tennant DA, McKeating JA. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J Hepatol. 2014;61:1397-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Guo Y, Xiao Z, Yang L, Gao Y, Zhu Q, Hu L, Huang D, Xu Q. Hypoxiainducible factors in hepatocellular carcinoma (Review). Oncol Rep. 2020;43:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Liu J, Li W, Zhu W, He W, Zhao H, Xiang Y, Liu C, Wu W. Chronic intermittent hypoxia promotes the development of experimental non-alcoholic steatohepatitis by modulating Treg/Th17 differentiation. Acta Biochim Biophys Sin (Shanghai). 2018;50:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Hu CC, Jeng WJ, Chen YC, Fang JH, Huang CH, Teng W, Hsieh YC, Lin YC, Chien RN, Sheen IS, Lin CY. Memory Regulatory T cells Increase Only In Inflammatory Phase of Chronic Hepatitis B Infection and Related to Galectin-9/Tim-3 interaction. Sci Rep. 2017;7:15280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 52. | Ju Y, Shang X, Liu Z, Zhang J, Li Y, Shen Y, Liu Y, Liu C, Liu B, Xu L, Wang Y, Zhang B, Zou J. The Tim-3/galectin-9 pathway involves in the homeostasis of hepatic Tregs in a mouse model of concanavalin A-induced hepatitis. Mol Immunol. 2014;58:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Wang Z, He L, Li W, Xu C, Zhang J, Wang D, Dou K, Zhuang R, Jin B, Zhang W, Hao Q, Zhang K, Wang S, Gao Y, Gu J, Shang L, Tan Z, Su H, Zhang Y, Zhang C, Li M. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 54. | Li Y, Jiang HT, Han LB, Xiao L, Gan JH. MiR-195 regulates CD40 to maintain Th17/Treg balance in rats with non-alcoholic fatty liver disease. Biomed Pharmacother. 2020;124:109930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Cai C, Chen DZ, Tu HX, Chen WK, Ge LC, Fu TT, Tao Y, Ye SS, Li J, Lin Z, Wang XD, Xu LM, Chen YP. MicroRNA-29c Acting on FOS Plays a Significant Role in Nonalcoholic Steatohepatitis Through the Interleukin-17 Signaling Pathway. Front Physiol. 2021;12:597449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Xia G, Wu S, Wang X, Fu M. Inhibition of microRNA-155 attenuates concanavalin-A-induced autoimmune hepatitis by regulating Treg/Th17 cell differentiation. Can J Physiol Pharmacol. 2018;96:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Liu N, Chang CW, Steer CJ, Wang XW, Song G. MicroRNA-15a/16-1 Prevents Hepatocellular Carcinoma by Disrupting the Communication Between Kupffer Cells and Regulatory T Cells. Gastroenterology. 2022;162:575-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 58. | Yu Z, Zhao H, Feng X, Li H, Qiu C, Yi X, Tang H, Zhang J. Long Non-coding RNA FENDRR Acts as a miR-423-5p Sponge to Suppress the Treg-Mediated Immune Escape of Hepatocellular Carcinoma Cells. Mol Ther Nucleic Acids. 2019;17:516-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 59. | Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1578] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 61. | Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 62. | Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, Zhang N, Lebid A, Ramaswamy A, Wei P, Zheng Y, Zhang X, Wu X, Vignali P, Yang CP, Li H, Pardoll D, Lu L, Pan D, Pan F. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018;8:1026-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 63. | Polanczyk MJ, Walker E, Haley D, Guerrouahen BS, Akporiaye ET. Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells. J Transl Med. 2019;17:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Badr AM, El-Ahwany E, Goda L, Nagy F, Helal N, El Deeb S. MicroRNA-26a systemic administration attenuates tumor formation in hepatocellular carcinoma mouse model. Pak J Pharm Sci. 2021;34:925-932. [PubMed] |
| 65. | Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 66. | Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, Ye QH, Qin LX. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 67. | Ma Y, Deng F, Li P, Chen G, Tao Y, Wang H. The tumor suppressive miR-26a regulation of FBXO11 inhibits proliferation, migration and invasion of hepatocellular carcinoma cells. Biomed Pharmacother. 2018;101:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Wang X, Sun L, Zhang L, Jiang Z. Effect of Adoptive Transfer or Depletion of Regulatory T Cells on Triptolide-induced Liver Injury. Front Pharmacol. 2016;7:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Huang H, Deng Z. Adoptive transfer of regulatory T cells stimulated by Allogeneic Hepatic Stellate Cells mitigates liver injury in mice with concanavalin A-induced autoimmune hepatitis. Biochem Biophys Res Commun. 2019;512:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Feng M, Wang Q, Jiang Z, Ding J, Wang H, Wang M, Lu L, Guan W. Adoptive transferred hepatic stellate cells attenuated drug-induced liver injury by modulating the rate of regulatory T cells/T helper 17 cells. Clin Immunol. 2016;165:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Kehrmann J, Effenberg L, Wilk C, Schoemer D, Ngo Thi Phuong N, Adamczyk A, Pastille E, Scholtysik R, Klein-Hitpass L, Klopfleisch R, Westendorf AM, Buer J. Depletion of Foxp3+ regulatory T cells is accompanied by an increase in the relative abundance of Firmicutes in the murine gut microbiome. Immunology. 2020;159:344-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32-G41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 74. | Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 75. | Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, Ochiai H, Bardeesy N, Huang P, Cobbold M, Zhu AX, Jain RK, Duda DG. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology. 2020;71:1247-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 76. | Langhans B, Nischalke HD, Krämer B, Dold L, Lutz P, Mohr R, Vogt A, Toma M, Eis-Hübinger AM, Nattermann J, Strassburg CP, Gonzalez-Carmona MA, Spengler U. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 77. | Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, Daud A, Bluestone JA. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 78. | Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2019;25:6614-6622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 79. | Zhang Q, Huang H, Zheng F, Liu H, Qiu F, Chen Y, Liang CL, Dai Z. Resveratrol exerts antitumor effects by downregulating CD8+CD122+ Tregs in murine hepatocellular carcinoma. Oncoimmunology. 2020;9:1829346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Ren Z, Yue Y, Zhang Y, Dong J, Liu Y, Yang X, Lin X, Zhao X, Wei Z, Zheng Y, Wang T. Changes in the Peripheral Blood Treg Cell Proportion in Hepatocellular Carcinoma Patients After Transarterial Chemoembolization With Microparticles. Front Immunol. 2021;12:624789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Jhun J, Lee SH, Lee SK, Kim HY, Jung ES, Kim DG, Choi J, Bae SH, Yoon SK, Chung BH, Yang CW, Cho ML, Choi JY. Serial Monitoring of Immune Markers Being Represented Regulatory T Cell/T Helper 17 Cell Ratio: Indicating Tolerance for Tapering Immunosuppression after Liver Transplantation. Front Immunol. 2018;9:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, Paulos CM. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 83. | Sánchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, Thirkell S, Lowe K, Fry L, Heward J, Kerr A, Ali J, Fisher C, Lewis G, Hope A, Kodela E, Lyne M, Farzaneh F, Kordasti S, Rebollo-Mesa I, Jose Lozano J, Safinia N, Heaton N, Lechler R, Martínez-Llordella M, Lombardi G. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 84. | Whitehouse GP, Hope A, Sanchez-Fueyo A. Regulatory T-cell therapy in liver transplantation. Transpl Int. 2017;30:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Cvetkovski F, Hexham JM, Berglund E. Strategies for Liver Transplantation Tolerance. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Casiraghi F, Perico N, Podestà MA, Todeschini M, Zambelli M, Colledan M, Camagni S, Fagiuoli S, Pinna AD, Cescon M, Bertuzzo V, Maroni L, Introna M, Capelli C, Golay JT, Buzzi M, Mister M, Ordonez PYR, Breno M, Mele C, Villa A, Remuzzi G; MSC-LIVER Study Group. Third-party bone marrow-derived mesenchymal stromal cell infusion before liver transplantation: A randomized controlled trial. Am J Transplant. 2021;21:2795-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Han JW, Sung PS, Hong SH, Lee H, Koh JY, White S, Maslow JN, Weiner DB, Park SH, Jeong M, Heo J, Ahn SH, Shin EC. IFNL3-adjuvanted HCV DNA vaccine reduces regulatory T cell frequency and increases virus-specific T cell responses. J Hepatol. 2020;73:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, Alfieri A, Brillo F, Fisicaro P, Acerbi G, Pedrazzi G, Andreone P, Cursaro C, Margotti M, Santoro R, Piazzolla V, Brunetto MR, Coco B, Cavallone D, Zhao Y, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Massetto B, Fung S, Ahn SH, Ma X, Mangia A, Ferrari C. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology. 2019;157:227-241.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 89. | Wu D, Wang P, Han M, Chen Y, Chen X, Xia Q, Yan W, Wan X, Zhu C, Xie Q, Jiang J, Wei L, Tan D, Dou X, Yu Y, Hou J, Luo X, Ning Q. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol Int. 2019;13:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Kalathil SG, Hutson A, Barbi J, Iyer R, Thanavala Y. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |