Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3334
Peer-review started: December 30, 2021
First decision: April 16, 2022
Revised: April 26, 2022
Accepted: June 23, 2022
Article in press: June 23, 2022
Published online: July 21, 2022
Processing time: 200 Days and 4.1 Hours
The morbidity and mortality of hepatocellular carcinoma (HCC) rank 6th and 4th, respectively, among malignant tumors worldwide. Traditional diffusion-weighted imaging (DWI) uses the apparent diffusion coefficient (ADC) obtained by applying the monoexponential model to reflect water molecule diffusion in active tissue; however, the value of ADC is affected by microcirculation perfusion. Using a biexponential model, intravoxel incoherent motion (IVIM)-DWI quantitatively measures information related to pure water molecule diffusion and microcirculation perfusion, thus compensating for the shortcomings of DWI. The number of studies examining the application of IVIM-DWI in patients with HCC has gradually increased over the last few years, and many results show that IVIM-DWI has vital value for HCC differentiation, pathological grading, and predicting and evaluating the treatment response. The present study principally reviews the principle of IVIM-DWI and its research progress in HCC differentiation, patho
Core Tip: Intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) quantitatively measures information related to pure water molecule diffusion and microcirculation perfusion using a biexponential model, thus compensating for the shortcomings of traditional DWI. The number of studies assessing IVIM-DWI in patients with hepatocellular carcinoma (HCC) has gradually increased over the last few years. This review, which is based on the literature, identified an imaging biomarker for HCC differentiation, pathological grading, predicting and evaluating the treatment response, predicting postoperative recurrence and predicting gene expression prediction. The results of this analysis show that IVIM-DWI has important value in the treatment and diagnosis of HCC.
- Citation: Zhou Y, Zheng J, Yang C, Peng J, Liu N, Yang L, Zhang XM. Application of intravoxel incoherent motion diffusion-weighted imaging in hepatocellular carcinoma. World J Gastroenterol 2022; 28(27): 3334-3345
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3334
The morbidity and mortality of hepatocellular carcinoma (HCC) rank 6th and 4th, respectively, among malignant tumors worldwide[1,2]. The apparent diffusion coefficient (ADC) obtained using traditional diffusion-weighted imaging (DWI) reflects water molecule diffusion in tissue[3]. However, many studies have shown that a voxel contains other diffusion information in addition to the diffusion movement of pure water molecules, namely, the microcirculation perfusion of the capillary network in tissue[3-6]. One limitation of traditional DWI is that it is unable to distinguish water molecule diffusion and microcirculation perfusion information. Le Bihan et al[6,7] proposed that intravoxel incoherent motion (IVIM) quantitatively obtains information on both pure water molecule diffusion and diffusion related to microcirculation perfusion, thus compensating for the shortcoming of traditional DWI. Therefore, IVIM has become a research hotspot in recent years. The liver is suitable for IVIM research because of its relatively large blood supply. Since the first application of IVIM-DWI for the study of liver disease by Yamada et al[8], the number of studies assessing IVIM-DWI in patients with HCC has progressively increased, and significant progress has been achieved. This study mainly reviews IVIM-DWI research progress in the diagnosis and treatment of HCC.
DWI is currently the only functional magnetic resonance imaging (MRI) technology that noninvasively measures water molecule diffusion in living tissue[3]. According to the theory of traditional DWI, the movement of free water molecules is considered to conform to a Gaussian distribution. However, due to the presence of complex cell structures and multiple diffusion barriers, such as cell membranes, in biological tissues, the diffusion behavior of water in biological tissues is more complicated. Therefore, the displacement of water in tissue may substantially deviate from a Gaussian distribution, challenging the validity of the monoexponential model to varying degrees. Additionally, the ADC, which reflects the water molecule diffusion, is affected simultaneously by microcirculation perfusion[3,5,7]. IVIM-DWI uses sufficient b values and biexponential models for the acquisition and analysis of images and distinguishes information regarding the pure tissue diffusion of water molecules and diffusion related to microcirculation perfusion. Among IVIM-DWI parameters, the simple diffusion coefficient, which is also referred to as the slow apparent diffusion coefficient (D), reflects the diffusion movement of pure water molecules in the tissue in a region of interest (ROI). The false diffusion coefficient, which is also called the fast apparent diffusion coefficient (D*), reflects diffusion movement related to microcirculation perfusion in the capillary network in an ROI. The fraction of the fast apparent diffusion coefficient (f) represents the volume ratio of the local microcirculation perfusion-related effect to the total diffusion effect, which may reflect the blood volume in an ROI. The formula for determining the relationship between IVIM-DWI signal attenuation and b values in tissue is as follows:
Sb/S0 = (1 - f) × exp (-bD) + f × exp [-b (D + D*)]
Where b is the diffusion sensitivity coefficient with units of s/mm2, while the unit for both D and D* is mm2/s, and S represents intravoxel signal intensity, with S0 and Sb representing the signal intensities when b = 0 and b has other values, respectively. The b value determines the effect of microcirculation perfusion on DWI signal attenuation[6]. When the b value is low (b < 200 s/mm2), the effect of microcirculation perfusion accounts for a large proportion of the total diffusion effect, whereas when the b value is high (b ≥ 200 s/mm2), signal attenuation basically reflects only the pure water molecule diffusion in a voxel.
To date, many studies have shown that IVIM-DWI has important value in differentiating HCC from other liver lesions[8-22]. In theory, malignant tumors restrict the movement of water molecules to a significantly greater extent than benign tumors; thus, the ADC and D values of malignant tumors are lower than those of benign tumors[23]. Studies have confirmed that the ADC and D values of malignant liver lesions are remarkably lower than those of benign liver lesions and are useful to distinguish benign from malignant liver lesions, with the D value showing higher differentiation efficiency[8,10-15,17]. Yoon et al[14] conducted an IVIM study on 169 lesions in 142 patients. The values of ADC and D in malignant liver lesions [1.14 ± 0.24, 0.95 ± 0.21, (× 10-3 mm2/s), respectively] were significantly lower than those in benign liver lesions (1.72 ± 0.37, 1.61 ± 0.34, (× 10-3 mm2/s), respectively). The area under the receiver operating characteristic (ROC) curve (AUC) values for the ADC value and D value in differentiating liver malignancies and hypervascular malignancies were 0.933 and 0.971, respectively, and 0.919 and 0.961, respectively (all P < 0.0005).
However, research results for D* values and f values in differentiating benign and malignant liver nodules are inconsistent. A study by Ichikawa et al[16] showed that the D* value of malignant liver lesions was significantly lower than that of benign lesions, but no difference in f values was observed between benign and malignant lesions. A study by Luo et al[10] also revealed that the D* values of HCC were obviously lower than those of focal nodular hyperplasia, with no significant difference in the f value between the two groups. However, another study[11] by the same group showed that the f value in the malignant group was significantly lower than that in the benign group, and the D* value did not differ significantly between the two groups. The studies by Klauss et al[12] and Doblas et al[18] both showed no significant differences in the D* and f values between benign and malignant liver lesions and no significance in the values of D* and f in differentiating those two types of liver lesions. Recently, Podgórska et al[24] reported that the perfusion-diffusion ratio was more effective than IVIM-DWI parameters in differentiating solid benign and malignant primary liver lesions.
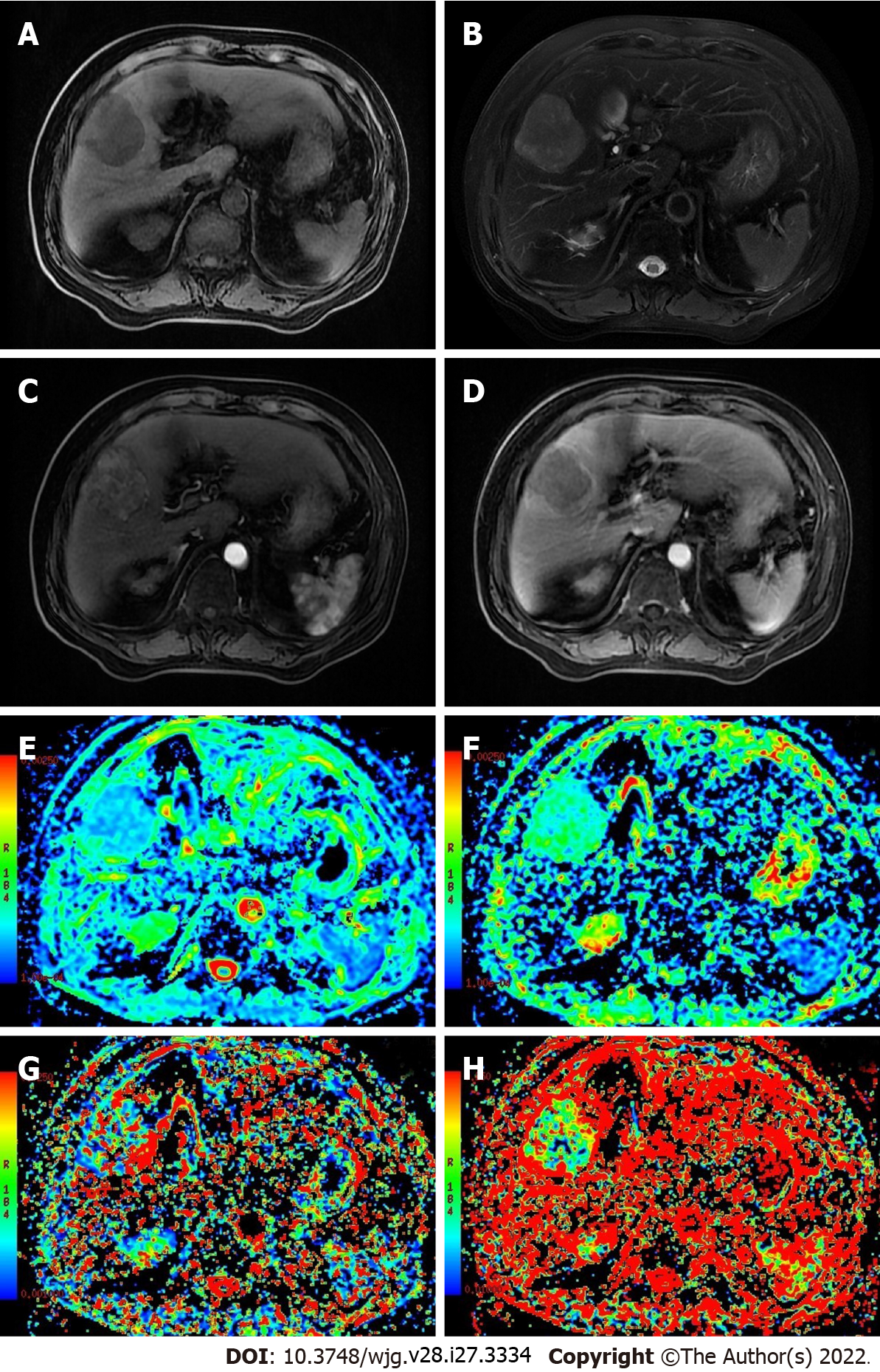
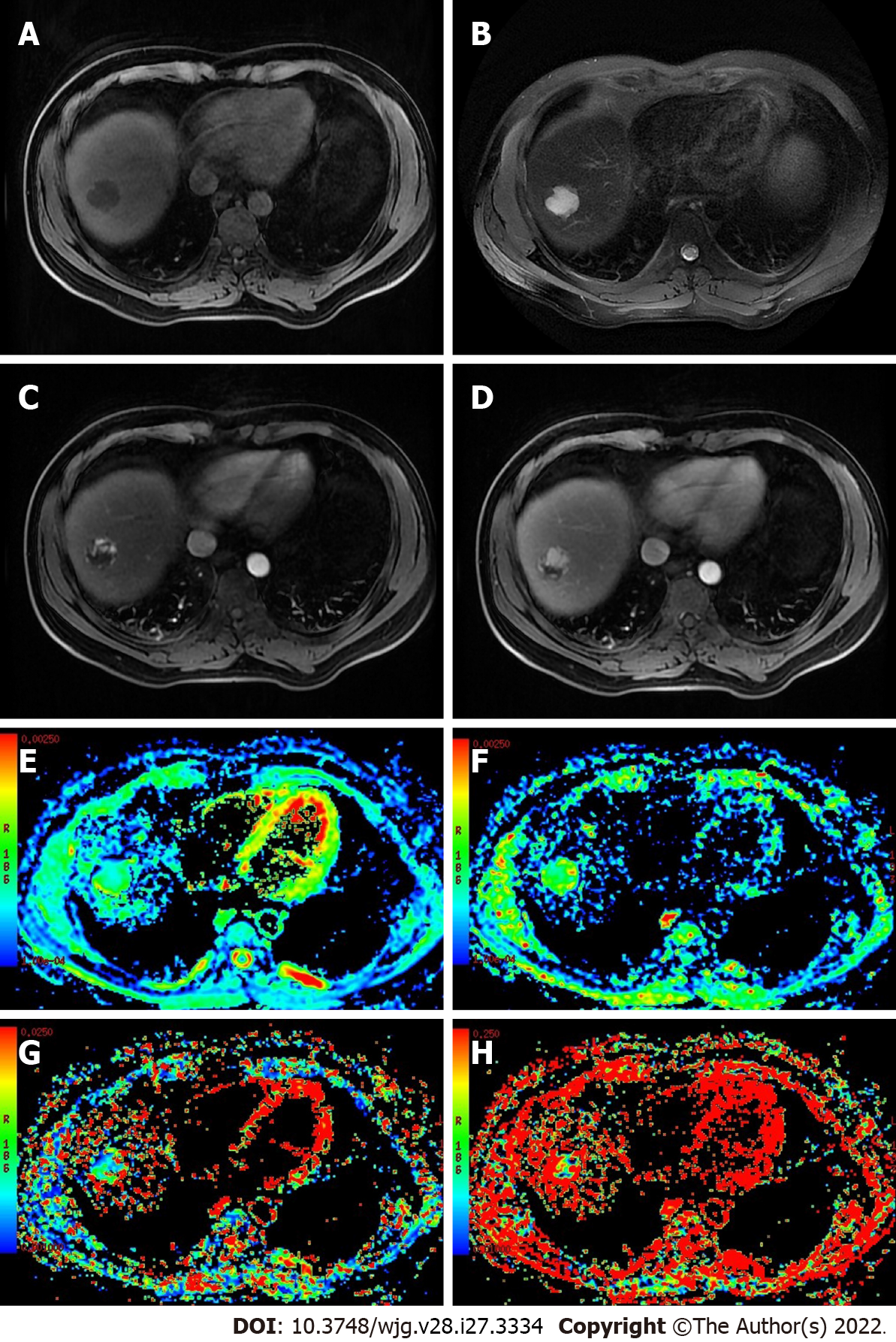
The inconsistencies in the results described above may be related to the following factors: (1) D* and f values not only reflect blood perfusion information but may also reflect other information, such as particle or gland excretion[10,15,25]; (2) the D* values of hepatic hemangioma with different blood supply types exhibit a range of fluctuation range[13]; and (3) interference from other factors, such as the b value distribution, ROI settings, and differences in tumor composition, may occur[16,23,26] (Figures 1 and 2).
Relatively few studies assessing the differentiation of malignant nodules with different pathological characteristics in the liver have been published. The few available studies have involved differentiating HCC from intrahepatic cholangiocarcinoma (ICC) and/or metastasis[9,27-29]. Our previous study[27] showed significantly lower ADC and D values for HCC than for ICC, a significantly higher D* value for HCC than for ICC, higher diagnostic efficiency for D* [AUC = 0.896], and no significant difference in f values between the two groups. According to other studies, ADC and D for HCC are obviously lower than those for ICC. However, the results for the IVIM-DWI parameters D* and f are not consistent. Choi et al[9] and Shao et al[28] reported that the f values of HCC were higher than those of ICC, while the D* values did not differ significantly between the two studied groups. Wei et al[29] did not document significant differences in either D* or f values between HCC and ICC groups.
The results of studies analyzing the differentiation between HCC and liver metastases differ substantially. Choi et al[9] found that the f value of HCC was significantly higher than that of liver metastases, but no significant differences were detected in other IVIM-DWI parameters. The study by Wu et al[30] indicated no obvious differences in the values of ADC, D, D*, and f for HCC from those for hepatic metastases, which may be due to changes in microcirculation and cell density caused by metastatic tumors of different origins. Dividing liver metastases into different subgroups according to different origins may be a more reasonable approach.
Preoperative prediction of the pathological grade of HCC has important value in formulating guidelines for individualized treatments. Studies have shown that IVIM helps predict the pathological grade of HCC before surgery[23,26,31-44]. A meta-analysis by Yang et al[32] included 16 studies (1428 cases of HCC) using IVIM-DWI for pathological grading and found that ADC and D values showed high accuracy for the pathological grading of noninvasive HCC and better differentiation efficiency for D values than for ADC values. The explanation for this result may be that a higher tumor pathological grade indicates greater cellularity, a higher nuclear-to-cytoplasmic ratio, and a smaller extracellular space, resulting in more restricted water diffusion, which ultimately leads to lower ADC and D values[23,33,35]. A potential explanation for the superiority of D values compared to ADC values may be that D values theoretically avoid the effect of microcirculation perfusion information. The results for the correlation between D* and f values and pathological grading are inconsistent. In addition to reporting results for ADC and D values and tumor tissue grading consistent with those of previous studies, Li et al[36] used rat models and showed that the D* and f values of higher-grade lesions were higher than those of lower-grade lesions and that D* and f values were positively correlated with the tumor tissue grade. Sokmen et al[26] and Granata et al[38] found that f values were significantly correlated with the HCC pathological grade, while a significant correlation was not identified between D* values and the pathological grade. On the other hand, multiple studies by Zhu et al[23,33,35] showed that neither D* values nor f values were significantly correlated with the HCC pathological grade.
IVIM-DWI accurately reflects microstructures and blood perfusion changes before and after tumor treatment and is particularly suitable for the prediction and evaluation of the responses to and efficacy of tumor locoregional therapy (LRT)[45]. Many researchers have investigated the value of IVIM-DWI in the prediction and evaluation of transarterial chemoembolization (TACE) efficacy in patients with HCC[46-52]. Park et al[50] reported that the preoperative D* value of HCC with better lipiodol deposition was markedly higher than that of HCC with poor lipiodol deposition, suggesting that the D* value may predict lipiodol deposition after TACE for patients with HCC. As shown in our previous study[46], the ADC and D values of HCC were obviously elevated four weeks after TACE treatment, the D* value was significantly reduced, and the f value did not change significantly.
Hectors et al[53] investigated the effect of yttrium 90 radioembolization on diffusion and perfusion in HCC. The authors performed multiparametric MRI including IVIM-DWI on 24 patients with HCC before (n = 24) and six weeks (n = 21) after radioembolization. The ADC and D values of HCC were significantly increased at six weeks after radioembolization (P < 0.0004), while the D* values were significantly reduced (P = 0.014), and no significant change was detected in f values (P = 0.765).
Server et al[54] administered LRT to 15 patients with HCC (11 patients underwent transarterial radioembolization, and four patients underwent TACE, and IVIM-DWI was performed before and eight weeks after LRT). Their results indicated that the ADC and D values obtained after LRT were significantly higher than those recorded before treatment, but the f values were significantly lower than those recorded before LRT. In the responder group, the ADC and D values were significantly increased, and the f values were significantly reduced after LRT. No significant differences were detected in the nonresponder group. Based on these findings, IVIM effectively predicts and evaluates the response to and efficacy of LRT. In these studies, the potential explanation for the inconsistency in the blood perfusion parameters D* and f may be that they represent different types of perfusion in various aspects, with the former usually reflecting the blood flow rate in capillaries and the latter mainly reflecting blood volume (Table 1).
| Ref. | Country | Subject number | Key findings |
| Juan et al[46], 2019 | China | 20 | After TACE, the ADC and Dslow values of HCC increased significantly, and the value of Dfast decreased significantly; the f value did not change markedly. |
| Wu et al[47], 2019 | China | 55 | Compared with nonresponders, the mean, median, and 25th percentile of the PF, ADC, and ADCtotal were higher in responders, while the skewness and kurtosis of PF, kurtosis of ADC and ADCtotal were lower |
| Lin et al[48], 2016 | China | 118 | Before TACE, significant differences in ADC, Dslow and Dfast were observed between the effective and ineffective groups. After TACE, the effective group exhibited lower Dfast and higher ADC and Dslow values than the ineffective group. The tumor regression rate was negatively correlated with Dfast but positively correlated with ADC and Dslow |
| Park et al[50], 2014 | Korea | 44 | The preoperative D* value was significantly increased in the good lipiodol uptake group compared with the poor uptake group after TACE, while the ADC, D, and f values were not significantly different between these two groups |
| Jia F et al[51], 2020 | China | 56 | ROC curve analyses revealed that the combined model including APT SIs and D values was better able to predict the tumor response to TACE than the individual parameters |
| Wu L et al[52], 2017 | China | 30 | The ADCtotal ratio and D ratio measured 24-48 h after TACE were independent predictors of the response to TACE of patients with HCC and showed stronger associations with PFS than mRECIST criteria |
| Hectors et al[53], 2020 | United States | 24 | The ADC and D values of HCC were significantly increased at six weeks after radioembolization, while the D* values were significantly reduced, with no significant change in f values |
| Server et al[54], 2019 | Turkey | 15 | The ADC and D values after LRT were significantly higher than those measured before treatment, while the f values were significantly lower than those recorded before LRT. In the responder group, the ADC and D values were significantly increased after LRT, whereas the f values were significantly reduced |
Changes in volume are often not obvious in the early stage of targeted therapy for HCC. Compared to traditional imaging methods, IVIM-DWI has more advantages in the early evaluation of the HCC response to targeted therapy[55-59]. Wagner et al[56] revealed that the D value of viable tumor regions in malignant liver tumors was significantly lower than that of necrotic tumor tissue but higher than that of fibrotic tumor regions. Joo et al[55] used IVIM-DWI to evaluate the efficacy of the vascular blocker CKD-516 against rabbit VX2 liver tumors and found that the D* and f values of the treatment group were noticeably reduced at four hours. Based on these studies, IVIM-DWI is useful to assess early responses to tumor vascular blockers[56]. Yang et al[57] investigated the values of IVIM-DWI in evaluating the response of 35 nude mice with HCC to sorafenib. Compared to the values recorded at baseline and in the control group, the ADC and D values of the treatment group at each time point were significantly higher, while the f value was significantly decreased at 7 d and increased at 21 d. The values of ADC, D, and f were significantly correlated with the necrotic fraction. Lewin et al[58] used IVIM parameters to evaluate the efficacy of sorafenib treatment in patients with advanced HCC. The f values of responder patients were obviously increased at two weeks and two months after treatment, while these values decreased in nonresponder patients. Therefore, the authors propose that the f value is useful as a reliable marker to evaluate sorafenib treatment efficacy in patients with advanced HCC. Shirota et al[59] used IVIM-DWI to evaluate the responses of patients with HCC undergoing sorafenib treatment and found that the D value at baseline was significantly higher in the responder group than in the nonresponder group. The sensitivity and specificity of the D value for evaluating the treatment response were 100% and 67%, respectively (Table 2).
| Ref. | Country | Subject number | Key findings |
| Joo et al[55], 2014 | Korea | 21 | The D* and f values of the treatment group were significantly decreased at four hours. The sizes of the decreases in f and fD* at four hours were negatively correlated with the tumor size at 7 d of follow-up |
| Wagner et al[56], 2012 | France | 48 | The D values of viable tumor regions in malignant liver tumors were significantly lower than those of necrotic tumor tissue but higher than those of fibrotic tumor regions |
| Yang et al[57], 2017 | China | 35 | Compared with the values at baseline and those recorded in the control group, the ADC and D values in the treatment group were significantly higher at each time point, whereas the f values decreased significantly at 7 d and increased at 21 d |
| Lewin et al[58], 2011 | France | 12 | The f values increased significantly in responder patients at two weeks and two months after treatment but decreased in nonresponder patients. The f value was useful as a reliable marker to evaluate sorafenib treatment efficacy in patients with advanced HCC |
| Shirota et al[59], 2016 | Japan | 9 | The D values at baseline were significantly higher in the responder group than in the nonresponder group. The sensitivity and specificity of the D values for evaluating treatment response were 100% and 67%, respectively |
Immunotherapy for HCC is an emerging method with promising results; it exerts an antitumor effect without affecting tumor size. Functional imaging methods will play an important role in evaluating the response to immunotherapy. Andersson et al[60] evaluated the response of patients with HCC to intratumoral injections of the immune primer ilixadencel using IVIM-DWI and a histogram analysis. Seventeen patients with HCC were enrolled in this study. Their results showed that the 10th percentile of D* decreased significantly after treatment to baseline, and significant correlations were identified between the 10th percentile and median D value. Chen et al[61] reported that both pretreatment IVIM-DWI and DCE-MRI parameters, especially ADC and slope, may predict progression-free survival and overall survival in patients with HCC receiving lenalidomide (a dual antiangiogenic and immunomodulatory agent) as second-line therapy. Recently, the application of immune checkpoint inhibitors in tumors has become a new research hotspot. Subsequently, IVIM-DWI will inevitably play a valuable role in predicting the response to and evaluating the efficacy of tumor immunotherapy.
Some researchers have reported the value of IVIM-DWI in predicting recurrence and prognosis after the surgical resection of HCC. Microvascular invasion (MVI) is one of the main factors affecting postoperative recurrence or survival among patients with HCC. IVIM-DWI predicts HCC MVI prior to surgery[62]. Wei et al[63] prospectively studied the predictive value of preoperative IVIM-DWI and conventional imaging characteristics for MVI. The results of the univariate analysis showed that the characteristics that were significantly correlated with HCC MVI were decreased ADC values, decreased D values, and irregular circumferential enhancement. In the multivariate analysis, only the D value was an independent risk factor for HCC MVI (the AUC was 0.815). Zhao et al[64] and Li et al[65] reported similar results. Zhang et al[66] studied 157 patients undergoing HCC resection and found that D values might serve as a marker for predicting HCC recurrence after hepatectomy and that the predictive capability would be improved if it was combined with age and the alpha-fetoprotein level.
Recently, some studies have indicated that IVIM-DWI predicts gene expression in patients with HCC[67-69]. We recently investigated the correlations of HCC IVIM-DWI parameters with angiopoietin-2 (Ang-2) and transketolase (TKT) expression[67]. We observed significantly higher D* and f values in the high Ang-2 expression group than in the low Ang-2 expression group, and the values of D* and f were positively correlated with the Ang-2 expression levels . On the other hand, the ADC and D values of the TKT high expression group were significantly lower than those of the TKT low expression group, and the ADC and D values were negatively correlated with TKT expression. Based on this result, IVIM-DWI noninvasively predicts Ang-2 and TKT expression in HCC before surgery. Shi et al[68] performed IVIM-DWI of 52 patients with HCC and identified four IVIM-derived histogram metrics with the capability for differentiating Ki67 expression, with P values less than 0.05. By establishing a diagnostic model based on a logistic regression model, the AUC value for diagnosing high Ki67 expression was 0.861. The results of this study suggest that the histogram indicators obtained from IVIM-DWI scanning can accurately predict the Ki67 expression status.
IVIM-DWI accurately and precisely reflects tissue structures and pathophysiological changes and has important application value for HCC differentiation, pathological grading, and predicting and evaluating the treatment response. However, the factors affecting IVIM-DWI measurement results are complicated and diverse[8,20,25,70-76]. Factors including the number, distribution, and fitting methods of b values, other physiological activities (such as gland secretion and flow), ROI settings, scanning devices and field strength, imaging acquisition methods, and motion artifacts all affect data quality[23,25,34,41]. Increased standardization of data acquisition and analysis is imperative to facilitate the generation of reliable IVIM-DWI biomarker measures that are broadly applicable[74,77,78]. In addition, recent studies have shown that IVIM-DWI modeling of the perfusion component is constrained by the diffusion component, and a reduced D slow measure leads to artificially higher PF and D fast measures[75,76]. These results should also be considered in the design of future IVIM-DWI studies. We postulate that with advances in imaging technology and further in-depth research, IVIM-DWI will certainly play a more prominent role in the diagnosis and treatment of HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marusic M, Croatia; Nath L, India; Sato T, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2902] [Article Influence: 483.7] [Reference Citation Analysis (17)] |
| 2. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2962] [Article Influence: 370.3] [Reference Citation Analysis (0)] |
| 3. | Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 609] [Article Influence: 40.6] [Reference Citation Analysis (2)] |
| 4. | Lewis S, Dyvorne H, Cui Y, Taouli B. Diffusion-weighted imaging of the liver: techniques and applications. Magn Reson Imaging Clin N Am. 2014;22:373-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 531] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 6. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2444] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 7. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2634] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 8. | Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Choi IY, Lee SS, Sung YS, Cheong H, Lee H, Byun JH, Kim SY, Lee SJ, Shin YM, Lee MG. Intravoxel incoherent motion diffusion-weighted imaging for characterizing focal hepatic lesions: Correlation with lesion enhancement. J Magn Reson Imaging. 2017;45:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Luo M, Zhang L, Jiang XH, Zhang WD. Intravoxel incoherent motion: application in differentiation of hepatocellular carcinoma and focal nodular hyperplasia. Diagn Interv Radiol. 2017;23:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Luo M, Zhang L, Jiang XH, Zhang WD. Intravoxel Incoherent Motion Diffusion-weighted Imaging: Evaluation of the Differentiation of Solid Hepatic Lesions. Transl Oncol. 2017;10:831-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Klauss M, Mayer P, Maier-Hein K, Laun FB, Mehrabi A, Kauczor HU, Stieltjes B. IVIM-diffusion-MRI for the differentiation of solid benign and malign hypervascular liver lesions-Evaluation with two different MR scanners. Eur J Radiol. 2016;85:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Zhu L, Cheng Q, Luo W, Bao L, Guo G. A comparative study of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters for the characterization of common solid hepatic tumors. Acta Radiol. 2015;56:1411-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging. 2014;39:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Watanabe H, Kanematsu M, Goshima S, Kajita K, Kawada H, Noda Y, Tatahashi Y, Kawai N, Kondo H, Moriyama N. Characterizing focal hepatic lesions by free-breathing intravoxel incoherent motion MRI at 3.0 T. Acta Radiol. 2014;55:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Araki T. Intravoxel incoherent motion imaging of focal hepatic lesions. J Magn Reson Imaging. 2013;37:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Penner AH, Sprinkart AM, Kukuk GM, Gütgemann I, Gieseke J, Schild HH, Willinek WA, Mürtz P. Intravoxel incoherent motion model-based liver lesion characterisation from three b-value diffusion-weighted MRI. Eur Radiol. 2013;23:2773-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Doblas S, Wagner M, Leitao HS, Daire JL, Sinkus R, Vilgrain V, Van Beers BE. Determination of malignancy and characterization of hepatic tumor type with diffusion-weighted magnetic resonance imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived measurements. Invest Radiol. 2013;48:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Regini F, Colagrande S, Mazzoni LN, Busoni S, Matteuzzi B, Santini P, Wyttenbach R. Assessment of Liver Perfusion by IntraVoxel Incoherent Motion (IVIM) Magnetic Resonance-Diffusion-Weighted Imaging: Correlation With Phase-Contrast Portal Venous Flow Measurements. J Comput Assist Tomogr. 2015;39:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Dyvorne H, Jajamovich G, Kakite S, Kuehn B, Taouli B. Intravoxel incoherent motion diffusion imaging of the liver: optimal b-value subsampling and impact on parameter precision and reproducibility. Eur J Radiol. 2014;83:2109-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Ai Z, Han Q, Huang Z, Wu J, Xiang Z. The value of multiparametric histogram features based on intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) for the differential diagnosis of liver lesions. Ann Transl Med. 2020;8:1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Granata V, Fusco R, Amato DM, Albino V, Patrone R, Izzo F, Petrillo A. Beyond the vascular profile: conventional DWI, IVIM and kurtosis in the assessment of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2020;24:7284-7293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology. 2014;270:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Podgórska J, Pasicz K, Skrzyński W, Gołębiewski B, Kuś P, Jasieniak J, Kiliszczyk A, Rogowska A, Benkert T, Pałucki J, Grabska I, Fabiszewska E, Jagielska B, Kukołowicz P, Cieszanowski A. Perfusion-Diffusion Ratio: A New IVIM Approach in Differentiating Solid Benign and Malignant Primary Lesions of the Liver. Biomed Res Int. 2022;2022:2957759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kakite S, Dyvorne H, Besa C, Cooper N, Facciuto M, Donnerhack C, Taouli B. Hepatocellular carcinoma: short-term reproducibility of apparent diffusion coefficient and intravoxel incoherent motion parameters at 3.0T. J Magn Reson Imaging. 2015;41:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Sokmen BK, Sabet S, Oz A, Server S, Namal E, Dayangac M, Dogusoy GB, Tokat Y, Inan N. Value of Intravoxel Incoherent Motion for Hepatocellular Carcinoma Grading. Transplant Proc. 2019;51:1861-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Peng J, Zheng J, Yang C, Wang R, Zhou Y, Tao YY, Gong XQ, Wang WC, Zhang XM, Yang L. Intravoxel incoherent motion diffusion-weighted imaging to differentiate hepatocellular carcinoma from intrahepatic cholangiocarcinoma. Sci Rep. 2020;10:7717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Shao S, Shan Q, Zheng N, Wang B, Wang J. Role of Intravoxel Incoherent Motion in Discriminating Hepatitis B Virus-Related Intrahepatic Mass-Forming Cholangiocarcinoma from Hepatocellular Carcinoma Based on Liver Imaging Reporting and Data System v2018. Cancer Biother Radiopharm. 2019;34:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Wei Y, Gao F, Zheng D, Huang Z, Wang M, Hu F, Chen C, Duan T, Chen J, Cao L, Song B. Intrahepatic cholangiocarcinoma in the setting of HBV-related cirrhosis: Differentiation with hepatocellular carcinoma by using Intravoxel incoherent motion diffusion-weighted MR imaging. Oncotarget. 2018;9:7975-7983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Wu H, Liang Y, Jiang X, Wei X, Liu Y, Liu W, Guo Y, Tang W. Meta-analysis of intravoxel incoherent motion magnetic resonance imaging in differentiating focal lesions of the liver. Medicine (Baltimore). 2018;97:e12071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Shan Q, Kuang S, Zhang Y, He B, Wu J, Zhang T, Wang J. A comparative study of monoexponential versus biexponential models of diffusion-weighted imaging in differentiating histologic grades of hepatitis B virus-related hepatocellular carcinoma. Abdom Radiol (NY). 2020;45:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Yang D, She H, Wang X, Yang Z, Wang Z. Diagnostic accuracy of quantitative diffusion parameters in the pathological grading of hepatocellular carcinoma: A meta-analysis. J Magn Reson Imaging. 2020;51:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Wei Y, Gao F, Wang M, Huang Z, Tang H, Li J, Wang Y, Zhang T, Wei X, Zheng D, Song B. Intravoxel incoherent motion diffusion-weighted imaging for assessment of histologic grade of hepatocellular carcinoma: comparison of three methods for positioning region of interest. Eur Radiol. 2019;29:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Ichikawa S, Motosugi U, Hernando D, Morisaka H, Enomoto N, Matsuda M, Onishi H. Histological Grading of Hepatocellular Carcinomas with Intravoxel Incoherent Motion Diffusion-weighted Imaging: Inconsistent Results Depending on the Fitting Method. Magn Reson Med Sci. 2018;17:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Zhu SC, Liu YH, Wei Y, Li LL, Dou SW, Sun TY, Shi DP. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging for predicting histological grade of hepatocellular carcinoma: Comparison with conventional diffusion-weighted imaging. World J Gastroenterol. 2018;24:929-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Li M, Zheng XJ, Huang ZX, Song B. [Predicting Histological Grade of HCC in Rats using Intravoxel Incoherent Motion Imaging]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49:243-247. [PubMed] |
| 37. | Shan Q, Chen J, Zhang T, Yan R, Wu J, Shu Y, Kang Z, He B, Zhang Z, Wang J. Evaluating histologic differentiation of hepatitis B virus-related hepatocellular carcinoma using intravoxel incoherent motion and AFP levels alone and in combination. Abdom Radiol (NY). 2017;42:2079-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Granata V, Fusco R, Catalano O, Guarino B, Granata F, Tatangelo F, Avallone A, Piccirillo M, Palaia R, Izzo F, Petrillo A. Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for Hepatocellular carcinoma: correlation with histologic grade. Oncotarget. 2016;7:79357-79364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, Nakanishi K, Kamiyama T, Kubota K, Haga H, Matsuno Y, Onodera Y, Kato M, Asaka M. Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:1302-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011;196:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 41. | Granata V, Fusco R, Filice S, Catalano O, Piccirillo M, Palaia R, Izzo F, Petrillo A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect Agent Cancer. 2018;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Wu B, Jia F, Li X, Li L, Wang K, Han D. Comparative Study of Amide Proton Transfer Imaging and Intravoxel Incoherent Motion Imaging for Predicting Histologic Grade of Hepatocellular Carcinoma. Front Oncol. 2020;10:562049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Zhou Y, Yang G, Gong XQ, Tao YY, Wang R, Zheng J, Yang C, Peng J, Yang L, Li JD, Zhang XM. A study of the correlations between IVIM-DWI parameters and the histologic differentiation of hepatocellular carcinoma. Sci Rep. 2021;11:10392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 44. | Song Q, Guo Y, Yao X, Rao S, Qian C, Ye D, Zeng M. Comparative study of evaluating the microcirculatory function status of primary small HCC between the CE (DCE-MRI) and Non-CE (IVIM-DWI) MR Perfusion Imaging. Abdom Radiol (NY). 2021;46:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Hussein RS, Tantawy W, Abbas YA. MRI assessment of hepatocellular carcinoma after locoregional therapy. Insights Imaging. 2019;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 46. | Peng J, Yang C, Zheng J, Wang R, Zhou Y, Wang WC, Yang L, Zhang XM, Miao ND, Ren YJ, Xu H and Min XL. Intravoxel Incoherent Motion Diffusion Weighted Imaging for the Therapeutic Response of Transarterial Chemoembolization for Hepatocellular Carcinoma. J Cancer Ther. 2019;10:591-601. [DOI] [Full Text] |
| 47. | Wu LF, Rao SX, Xu PJ, Yang L, Chen CZ, Liu H, Huang JF, Fu CX, Halim A, Zeng MS. Pre-TACE kurtosis of ADCtotal derived from histogram analysis for diffusion-weighted imaging is the best independent predictor of prognosis in hepatocellular carcinoma. Eur Radiol. 2019;29:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Lin M, Tian MM, Zhang WP, Xu L, Jin P. Predictive values of diffusion-weighted imaging and perfusion-weighted imaging in evaluating the efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma. Onco Targets Ther. 2016;9:7029-7037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Yang K, Zhang XM, Yang L, Xu H, Peng J. Advanced imaging techniques in the therapeutic response of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2016;22:4835-4847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Park YS, Lee CH, Kim JH, Kim IS, Kiefer B, Seo TS, Kim KA, Park CM. Using intravoxel incoherent motion (IVIM) MR imaging to predict lipiodol uptake in patients with hepatocellular carcinoma following transcatheter arterial chemoembolization: a preliminary result. Magn Reson Imaging. 2014;32:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Jia F, Wu B, Yan R, Li L, Wang K, Han D. Prediction Model for Intermediate-Stage Hepatocellular Carcinoma Response to Transarterial Chemoembolization. J Magn Reson Imaging. 2020;52:1657-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Wu L, Xu P, Rao S, Yang L, Chen C, Liu H, Fu C, Zeng M. ADCtotal ratio and D ratio derived from intravoxel incoherent motion early after TACE are independent predictors for survival in hepatocellular carcinoma. J Magn Reson Imaging. 2017;46:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Hectors SJ, Lewis S, Kennedy P, Bane O, Said D, Segall M, Schwartz M, Kim E, Taouli B. Assessment of Hepatocellular Carcinoma Response to 90Y Radioembolization Using Dynamic Contrast Material-enhanced MRI and Intravoxel Incoherent Motion Diffusion-weighted Imaging. Radiol Imaging Cancer. 2020;2:e190094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Server S, Sabet S, Bilgin R, Inan N, Yuzer Y, Tokat Y. Intravoxel Incoherent Motion Parameters for Assessing the Efficiency of Locoregional Bridging Treatments before Liver Transplantation. Transplant Proc. 2019;51:2391-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Joo I, Lee JM, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for monitoring the therapeutic efficacy of the vascular disrupting agent CKD-516 in rabbit VX2 liver tumors. Radiology. 2014;272:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Wagner M, Doblas S, Daire JL, Paradis V, Haddad N, Leitão H, Garteiser P, Vilgrain V, Sinkus R, Van Beers BE. Diffusion-weighted MR imaging for the regional characterization of liver tumors. Radiology. 2012;264:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Yang SH, Lin J, Lu F, Han ZH, Fu CX, Lv P, Liu H, Gao DM. Evaluation of antiangiogenic and antiproliferative effects of sorafenib by sequential histology and intravoxel incoherent motion diffusion-weighted imaging in an orthotopic hepatocellular carcinoma xenograft model. J Magn Reson Imaging. 2017;45:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Lewin M, Fartoux L, Vignaud A, Arrivé L, Menu Y, Rosmorduc O. The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol. 2011;21:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Shirota N, Saito K, Sugimoto K, Takara K, Moriyasu F, Tokuuye K. Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imaging. 2016;16:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Andersson M, Jalnefjord O, Montelius M, Rizell M, Sternby Eilard M, Ljungberg M. Evaluation of response in patients with hepatocellular carcinoma treated with intratumoral dendritic cell vaccination using intravoxel incoherent motion (IVIM) MRI and histogram analysis. Acta Radiol. 2021;2841851211065935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Chen BB, Shao YY, Lin ZZ, Hsu CH, Cheng AL, Hsu C, Liang PC, Shih TT. Dynamic Contrast-Enhanced and Intravoxel Incoherent Motion MRI Biomarkers Are Correlated to Survival Outcome in Advanced Hepatocellular Carcinoma. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Zeng Q, Liu B, Xu Y, Zhou W. An attention-based deep learning model for predicting microvascular invasion of hepatocellular carcinoma using an intra-voxel incoherent motion model of diffusion-weighted magnetic resonance imaging. Phys Med Biol. 2021;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Wei Y, Huang Z, Tang H, Deng L, Yuan Y, Li J, Wu D, Wei X, Song B. IVIM improves preoperative assessment of microvascular invasion in HCC. Eur Radiol. 2019;29:5403-5414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 64. | Zhao W, Liu W, Liu H, Yi X, Hou J, Pei Y, Feng D, Liu L, Li W. Preoperative prediction of microvascular invasion of hepatocellular carcinoma with IVIM diffusion-weighted MR imaging and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2018;13:e0197488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Li H, Zhang J, Zheng Z, Guo Y, Chen M, Xie C, Zhang Z, Mei Y, Feng Y, Xu Y. Preoperative histogram analysis of intravoxel incoherent motion (IVIM) for predicting microvascular invasion in patients with single hepatocellular carcinoma. Eur J Radiol. 2018;105:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol. 2019;29:5791-5803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Zheng J, Gong XQ, Tao YY, Wang R, Yang G, Li JD, Ren T, Li ZM, Yang C, Wang WC, Yang L, Zhang XM. A Correlative Study Between IVIM-DWI Parameters and the Expression Levels of Ang-2 and TKT in Hepatocellular Carcinoma. Front Oncol. 2020;10:594366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Shi G, Han X, Wang Q, Ding Y, Liu H, Zhang Y, Dai Y. Evaluation of Multiple Prognostic Factors of Hepatocellular Carcinoma with Intra-Voxel Incoherent Motions Imaging by Extracting the Histogram Metrics. Cancer Manag Res. 2020;12:6019-6031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Guo Y, Chen J, Zhang Y, Guo Y, Jiang M, Dai Y, Yao X. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models. Eur J Radiol. 2022;150:110237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Andreou A, Koh DM, Collins DJ, Blackledge M, Wallace T, Leach MO, Orton MR. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol. 2013;23:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 71. | Dyvorne HA, Galea N, Nevers T, Fiel MI, Carpenter D, Wong E, Orton M, de Oliveira A, Feiweier T, Vachon ML, Babb JS, Taouli B. Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters--a pilot study. Radiology. 2013;266:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 72. | Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010;31:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 73. | Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, Laurent A, Deux JF, Brugieres P, Rahmouni A. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008;249:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 519] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 74. | Wang YXJ, Huang H, Zheng CJ, Xiao BH, Chevallier O, Wang W. Diffusion-weighted MRI of the liver: challenges and some solutions for the quantification of apparent diffusion coefficient and intravoxel incoherent motion. Am J Nucl Med Mol Imaging. 2021;11:107-142. [PubMed] |
| 75. | Wáng YXJ. Mutual constraining of slow component and fast component measures: some observations in liver IVIM imaging. Quant Imaging Med Surg. 2021;11:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Wáng YXJ. Observed paradoxical perfusion fraction elevation in steatotic liver: An example of intravoxel incoherent motion modeling of the perfusion component constrained by the diffusion component. NMR Biomed. 2021;34:e4488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Tao YY, Zhou Y, Wang R, Gong XQ, Zheng J, Yang C, Yang L, Zhang XM. Progress of intravoxel incoherent motion diffusion-weighted imaging in liver diseases. World J Clin Cases. 2020;8:3164-3176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Tramontano L, Cavaliere C, Salvatore M, Brancato V. The Role of Non-Gaussian Models of Diffusion Weighted MRI in Hepatocellular Carcinoma: A Systematic Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |