Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3297
Peer-review started: January 16, 2022
First decision: April 11, 2022
Revised: April 22, 2022
Accepted: June 19, 2022
Article in press: June 19, 2022
Published online: July 21, 2022
Processing time: 182 Days and 13 Hours
Pancreatic ductal adenocarcinoma is one of the most aggressive and lethal cancers. Surgical resection is the only curable treatment option, but it is available for only a small fraction of patients at the time of diagnosis. With current therapeutic regimens, the average 5-year survival rate is less than 10% in pancreatic cancer patients. Immunotherapy has emerged as one of the most promising treatment options for multiple solid tumors of advanced stage. However, its clinical efficacy is suboptimal in most clinical trials on pancreatic cancer. Current studies have suggested that the tumor microenvironment is likely the underlying barrier affecting immunotherapy drug efficacy in pancreatic cancer. In this review, we discuss the role of the tumor microenvironment in pancreatic cancer and the latest advances in immunotherapy on pancreatic cancer.
Core Tip: Despite advances in basic and translational research, pancreatic cancer remains one of the most lethal cancers. Recent breakthroughs in immunotherapy have revolutionized cancer therapy and have shown great potential to transform pancreatic cancer treatment. However, due to the barrier related to the tumor microenvironment, pancreatic cancer has shown inferior treatment outcomes toward various immunotherapy regimens. Further efforts, such as combinatory immunotherapy or molecular tumor subtyping, are warranted to overcome immunotherapy resistance in pancreatic cancer.
- Citation: Smith C, Zheng W, Dong J, Wang Y, Lai J, Liu X, Yin F. Tumor microenvironment in pancreatic ductal adenocarcinoma: Implications in immunotherapy. World J Gastroenterol 2022; 28(27): 3297-3313
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3297
Pancreatic ductal adenocarcinoma (PDAC) develops in the exocrine compartment of the pancreas and accounts for approximately 90% of pancreatic malignancies, making it the most common pancreatic neoplasm. Due to the lack of early diagnosis and limited treatment response, PDAC remains a highly aggressive and lethal malignancy and is the fourth leading cause of cancer-related death worldwide[1]. Although there has been notable progress in understanding tumor biology and the development of novel therapeutic regimens, the average 5-year survival rate is still less than 5%-10% in PDAC patients[1,2]. The clinical manifestations of pancreatic cancers are generally nonspecific, including weight loss, abdominal pain, thromboembolic disease, and type 2 diabetes[3,4]. In approximately 60%-70% of PDAC cases, the tumor arises from the head of the pancreas and could present as pancreatitis and obstructive jaundice[5]. Tumors of the pancreatic body and tail frequently have a poor prognosis due to their late presentation and associated advanced tumor stage[6].
The standard of care for resectable PDAC is surgical resection followed by adjuvant chemotherapy. Surgical resection remains the only curative therapy, but it is available for merely 10%-20% of patients at the time of diagnosis. Moreover, even with curative surgical resection, local recurrence and distal meta
The difficulties in treating pancreatic cancer lie at the cellular and genetic levels[12]. Mutational changes in pancreatic tumors lead to gene instability, tumor growth, and resistance to treatments[13]. In addition to the characteristic molecular landmarks, including oncogenic KRAS mutation and inactivation of the tumor suppressor genes CDKN2A/P16, TP53, and SMAD4, PDAC also frequently harbors mutations involving diverse cell signaling pathways[14]. The molecular heterogeneity likely accounts for its drug resistance in chemotherapy[15]. In addition, pancreatic cancer stem cells, accounting for approximately 1% of all pancreatic cancer cells, have the capacity for self-renewal and exhibit chemoresistance properties[16].
Immunotherapy has emerged as one of the most promising treatment options for advanced solid tumors, including lung, kidney, bladder, liver, and colorectal cancers[17]. Unfortunately, PDAC is notoriously resistant to immunotherapy, and thus far, most phase I/II clinical trials on PDAC have failed to demonstrate the desirable clinical efficacy of immunotherapy[18]. Of note, microsatellite instability (MSI), one of the predictive biomarkers for immune checkpoint blockade therapy, is only detected in a rare small portion of PDAC patients (less than 1%)[19,20]. On the other hand, emerging evidence has pinpointed the tumor microenvironment (TME) in PDAC as a critical component of treatment resistance toward immunotherapy[21,22].
In this review, we discuss the role of the tumor microenvironment and the latest advances in immunotherapy on pancreatic cancer through the search of peer-reviewed clinical and basic research articles related to this topic on PubMed, as well as the publicly accessible information on relevant clinical trials through ClinicalTrials.gov.
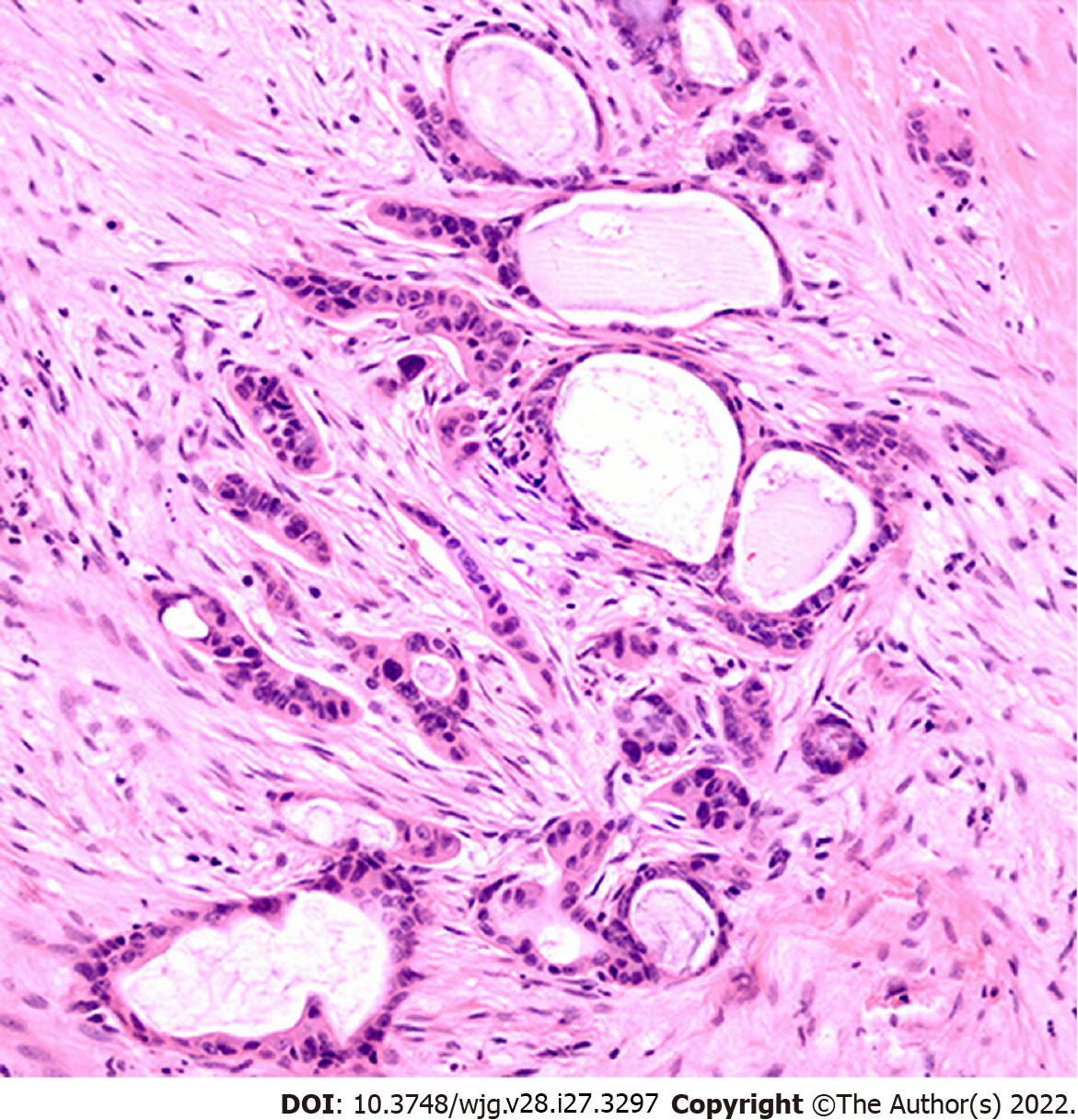
PDAC is a type of stromal-rich cancer that frequently presents with a prominent desmoplastic reaction and is characterized by fibrogenic connective stromal tissue surrounding invasive carcinoma[23] (Figure 1). Desmoplastic reaction, or desmoplasia, is considered as the morphological basis of the TME. In general, the TME in PDAC demonstrates extensive desmoplasia, decreased stromal vascularization, and altered immune cell infiltration that lead to reduced drug activity and advancement of tumor progression. This process is characterized by an increase in the deposition of noncellular components, such as extracellular matrix (ECM), as well as an increase in the proliferation of cellular components, such as cancer-associated fibroblasts (CAFs) and immune cells[24,25]. Various cytokines, including interferons, interleukins, tumor necrosis factor (TNF), and transforming growth factor β (TGF-β), also play essential roles linking the TME cellular and noncellular components to regulate tumor growth, metastases, and drug resistance. Of note, the overall stroma is responsible for most of the tumor mass, but the stromal cellular components make up a relatively small fraction, approximately 10%-30%, of the tumor mass[26].
The ECM is a significant factor in the initiation and progression of PDAC, and its deposition is associated with tumor migration, invasion, and poor prognosis[27]. The ECM is predominantly produced by cancer-associated pancreatic stellate cells (PSCs), a subtype of CAFs[28]. In PDAC, the ECM comprises most of the tumor mass and various matrix proteins, including collagen, fibronectin, proteoglycans, hyaluronan, proteolytic matrix metalloproteinases (MMPs), and tissue inhibitors of MMP[29]. Among ECM components of particular interest are hyaluronan and MMPs in tumor progression and prognosis in PDAC.
In general, ECM provides a rigid barrier leading to increased tumor pressure, decreased vascularization, and reduced drug delivery. A significant cause of drug resistance is the inability of conventional chemotherapeutic drugs such as gemcitabine to penetrate the thick stromal layer[30]. Therefore, it is rational to propose a combinatory therapeutic strategy for PDAC by targeting the tumor ECM. Hyaluronan, or hyaluronic acid (HA), is a glycosaminoglycan polymer and a major component of the ECM. Increased deposition of HA is associated with tumor metastases, drug resistance, and poor prognosis in PDAC[27,31]. Since stromal HA levels are dynamically regulated by synthases (to produce HA) and hyaluronidases (to degrade HA), hyaluronidase-based drug development has been a promising field in targeted therapy against the TME. The enzymatic depletion of hyaluronan through recombinant hyaluronidase (PEGPH20) has led to significantly increased overall survival when combined with neoadjuvant chemotherapy[32]. This is attributed mainly to improved delivery of systemic therapy through degradation of HA and remodeling of the TME. However, a recent phase IB/II randomized study (NCT01959139) of FOLFIRINOX plus pegylated recombinant PEGPH20 showed increased toxicity with this combination therapy and decreased overall survival (OS) (7.7 mo vs 14.4 mo) compared with FOLFIRINOX monotherapy[33]. Moreover, despite promising results of PEGPH20 in phase I-II studies, in a recent phase III randomized study (HALO 109-301), the addition of PEGPH20 to nab-paclitaxel/gemcitabine did not improve OS and progression-free survival (PFS) in patients with hyaluronan-high metastatic PDAC, and additional development of PEHPH20 in metastatic PDAC was halted[34].
MMPs are calcium-dependent metalloproteinases responsible for ECM protein degradation and are implicated in cancer initiation, growth, and metastasis. Clinical trial results with broad-spectrum MMP inhibitors were discouraging due to lack of specificity, associated toxicity, and insufficient clinical benefit[35], warranting further basic and translational studies to classify the role of individual MMPs in PDAC. Among MMP family members, the expression levels of MMP-2, MMP-7, MMP-9, and MMP-11 were significantly elevated in PDAC tumor tissues compared with normal pancreas samples[36,37]. Increased MMP-2 expression in PDAC leads to tumor invasion and progression[38-40]. MMP-7 expression is also associated with PDAC initiation and progression[41] and has been shown to be an independent prognostic factor for PDAC in a multivariate analysis. MMP-9 is significantly associated with pancreatic cancer progression and poor prognosis[37] and has emerged as a prognostic biomarker and potential therapeutic target. Highly selective and potent MMP-9 inhibitory antibodies have been developed for ulcerative colitis and colorectal cancer[42]. However, in a preclinical study, systemic ablation of MMP-9 facilitated pancreatic cancer growth and metastasis by creating a tumor-promoting TME[43]. This study has suggested a controversial role for MMP-9 in pancreatic cancer progression.
Additional studies have also demonstrated conflicting results in drugs targeting the tumor stroma. Olive et al[44] demonstrated that depletion of ECM in PDAC, through inhibition of the Sonic Hedgehog signaling pathway, promoted gemcitabine efficacy and improved survival. However, the involvement of the Sonic Hedgehog-dependent tumor stroma in PDAC has been controversial, as evidence shows that some components of the tumor stroma could actually act to restrain, rather than support, tumor growth[45]. All these failures indicate that targeting desmoplasia alone is insufficient for treating advanced PDAC. The tumor stroma has both tumor-promoting and tumor-suppressing functions, which are probably context dependent. The stromal heterogeneity should be considered for the development of targeted therapy.
PDAC displays unique immunologic hallmarks. The TME in PDAC consists of diverse cellular components, including CAFs, regulatory and cytotoxic lymphocytes, macrophages, and endothelial cells[46]. CAFs are the major TME cellular component responsible for the production and deposition of ECM proteins. The involvement of CAFs in the progression of PDAC has been a hot and controversial topic. Similar to the observations made with tumor stroma, CAFs also play dual functions in regulating PDAC progression. On the one hand, CAFs promote cancer progression and drug resistance through the deposition of dense ECM, the release of exosomes (extracellular vesicles), and metabolic support[47-49]. On the other hand, depletion of CAFs leads to accelerated PDAC progression and reduced survival in multiple preclinical studies[50,51]. These discrepancies are likely associated with the heterogeneity of CAFs[52,53], a concept supported by recent studies demonstrating the existence of multiple distinct and mutually exclusive CAF subtypes in pancreatic cancer[54,55]. CAF subtypes with diverse biomarkers, including α-smooth muscle actin (αSMA), fibroblast activation protein (FAP), S100A4, and platelet-derived growth factor receptor-β (PDGFRβ), have been identified[56]. Specifically, FAP-positive active CAFs have been linked to tumor-promoting functions by maintaining an immunosuppressive TME[57]. FAP is a type-II transmembrane serine protease, and its expression has been detected in both the tumor stroma and cancer cells in PDAC, with the highest expression in the tumor stroma at the tumor front[58]. FAP-positive CAFs potently shape the immune landscape in the TME by secreting TGF-β, VEGF, and multiple matrix processing enzymes[59,60], recruiting circulating myeloid-derived suppressor cells (MDSCs) into the tumor stroma[57], and inhibiting natural killer cell (NK) cytotoxicity and cytokine production[61]. FAP has been suggested as an ideal target for the TME, and its specific therapeutic reagents are in development[62].
In addition to CAFs, the TME also consists of multiple types of immunosuppressive cells, including regulatory T cells (Tregs), MDSCs, and tumor-associated macrophages (TAMs)[63]. These cells correlate to provide an immunosuppressive TME and have been under extensive preclinical and clinical investigation.
Tregs, defined as CD4+/CD25+/FOXP3+ T cells, are a subtype of repressive T cells that play an essential role in maintaining immune tolerance and preventing autoimmune disorders. Tregs can be found in PDAC and premalignant lesion intraductal papillary mucinous neoplasms (IPMNs). The prevalence of Tregs in CD4+ T lymphocytes correlates significantly with the progression and invasion of IPMNs and is associated with poor prognosis in PDAC. The immunosuppressive function of Tregs has been attributed to the secretion of suppressive cytokines, including IL-10 and TGF-β1, and the induction of CD4+ T-cell death[64,65]. Preoperative chemoradiation therapy has been shown to decrease Tregs in PDAC[66]. However, in a recent study, depletion of Tregs in a mouse model caused accelerated tumor progression due to unexpected crosstalk between Tregs and CAFs in PDAC[67]. This study has challenged the current view and posed uncertainties in developing Treg-based targeted therapy.
MDSCs and TAMs have also been suggested as potential therapeutic targets against the TME. Even though these two cell types are considered as separate entities, they have no demarcated boundaries and share many common characteristics[68]. MDSCs are a group of heterogeneous immature myeloid cells and can potently suppress T-cell function in tumors[69]. The levels of MDSCs correlate with the progression of PDAC and have been proposed as a predictive biomarker of chemotherapy failure[70,71]. TAMs are circulating monocyte-derived macrophages in the tumor stroma and represent a significant population of immune cells within the TME. TAMs can be further subclassified into the M1 and M2 subtypes, with M1 being proinflammatory (antitumorigenic) and M2 being anti-inflammatory (protumorigenic)[72]. M2-polarized TAMs are associated with an unfavorable prognosis in PDAC[73]. Liu et al[74] revealed progressive accumulations of MDSCs and M2-polarized TAMs accompanied by dynamic reductions in cytotoxic T cells (CTLs) and helper T cells (Ths) in PDAC progression. Gemcitabine affects the TME by inhibiting the expansion of MDSCs and the induction of Th2 cells while promoting M2-polarized TAMs[74]. M2-polarized TAMs can also be induced by other chemotherapeutic agents, such as carboplatin and cisplatin, leading to increased secretion of interleukin-6 (IL-6), IL-10, and prostaglandin E2[75]. In addition, interferon-γ upregulates the expression of programmed death-ligand 1 (PD-L1) in MDSCs, resulting in an immunosuppressive environment[76]. Further investigations and clinical trials are needed to test the efficacy of targeting MDSCs and TAMs in pancreatic cancer.
Current treatment options for PDAC have limited effects on patient survival. The recent development of immunotherapy has improved clinical outcomes for various types of solid tumors[17] and can revolutionize cancer treatment in PDAC. Activating the patient's T cells is the principal basis for cancer immunotherapy. This is accomplished through multiple mechanisms, such as decreased tumor-specific antigen presentation, T-cell activation, T-cell infiltration into the pancreatic tumor, and elimination of cancer cells by T cells[77]. Multiple cancer immunotherapies have been introduced, including immune checkpoint inhibitors, cancer vaccines, and adoptive cell transfer.
Immune checkpoint molecules are a group of surface receptors expressed on various immune cells that transduce inhibitory signals to T cells upon ligand binding. These molecules play an important role in preventing an autoimmune attack against self-antigens. Due to strong immune selective pressure, cancer cells frequently adopt the power of immune checkpoint molecules to avoid immune destruction. Initially approved for the treatment of metastatic melanoma, immune checkpoint inhibitors (ICIs) have been cleared to treat various solid tumors, including advanced or metastatic urothelial carcinoma, non-small-cell lung cancer, colorectal cancer, triple-negative breast cancer, and head and neck squamous cell carcinoma[78,79]. Currently, FDA-approved immune checkpoint inhibitors (ICIs) include anti-CTLA-4 agents (ipilimumab), anti-PD-1 agents (nivolumab, pembrolizumab, cemiplimab) and anti-PD-L1 agents (atezolizumab, avelumab, durvalumab)[79].
ICIs have emerged as a new therapeutic option for pancreatic cancer. Unfortunately, most phase I and II clinical trials on ICI treatment have failed to show the desired beneficial effect in PDAC. Two independent phase II clinical trials have demonstrated unsatisfactory clinical outcomes on monotherapy with anti-CTLA-4 mAb (Table 1). Single-agent ipilimumab, an anti-CTLA-4 mAb, was ineffective for the treatment of advanced PDAC (NCT00112580) (https://clinicaltrials.gov/ct2/show/NCT00112580)[80,81]. Monotherapy with tremelimumab, another anti-CTLA-4 mAb, also yielded poor clinical outcomes in PDAC, with 18 out of 20 patients demonstrating progressive disease and a poor median OS of 4 mo (95%CI: 2.83-5.42 mo) (NCT02527434) (https://clinicaltrials.gov/ct2/show/NCT02527434).
| Strategy | Treatment | Phase | Number | Cancer stage | Outcomes |
| Immune checkpoint inhibitor (target) monotherapy | Tremelimumab (CTLA-4) | II | NCT02527434 | Advanced/metastatic PDAC | Tremelimumab monotherapy is ineffective for metastatic PDAC. |
| Ipilimumab (CTLA-4) | II | NCT00112580 | Advanced PDAC | Ipilimumab monotherapy is ineffective for advanced PDAC. | |
| Atezolizumab (PD-L1) | I/II | NCT03829501 | Advanced PDAC | No results reported yet | |
| Immune checkpoint inhibitor (target) + immune checkpoint inhibitor(target) | Tremelimumab (CTLA-4) + Durvalumab (PD-L1) | II | NCT02558894 | Metastatic PDAC | ORR 3.1% for combination therapy. (ORR 0% for monotherapy). |
| Nivolumab (PD-1) + Ipilimumab (CTLA-4) | I/II | NCT01928394 | Advanced/metastatic PDAC | No results reported yet | |
| Immune checkpoint inhibitor (target) + chemotherapy | Tremelimumab (CTLA-4) + Gemcitabine | I | NCT00556023 | Advanced PDAC | Median OS 7.4 mo (95%CI: 5.8-9.4 mo) |
| Ipilimumab(CTLA-4) + Gemcitabine | Ib | NCT01473940 | Advanced/metastatic PDAC | Median OS 6.90 mo (95%CI: 2.63–9.57 mo) | |
| Pembrolizumab (PD-1) + Gemcitabine and Nab-paclitaxel | Ib/II | NCT02331251 | Advanced/metastatic PDAC | Median OS 15.0 mo (95%CI: 6.8–22.6 mo) | |
| Immune Checkpoint Inhibitor (Target) + Target therapy | Durvalumab (PD-L1) + Galunisertib | I | NCT02734160 | Metastatic PDAC | Median PFS 1.9 mo (95%CI: 1.5-2.2 mo); median OS was NR (95%CI: 3.6 mo, NR) |
| Durvalumab (PD-L1) + Pexidartinib | I | NCT02777710 | Advanced/metastatic PDAC | No results reported yet | |
| Immune Checkpoint Inhibitor (Target) + Radiation Therapy | Tremelimumab (CTLA-4) + Durvalumab (PD-1) + SBRT | I/II | NCT02311361 | Advanced/metastatic PDAC | ORR of 9.6% including 2 patients who achieved a durable partial response lasting over 12 mo |
Combination therapy with ipilimumab and gemcitabine, on the other hand, has demonstrated promising results due to the increased immune response by enhancing naïve T-cell activation[82]. In a phase 1b clinical trial (NCT01473940) (https://clinicaltrials.gov/ct2/show/NCT01473940), initial results on combination therapy with ipilimumab and gemcitabine showed that the treatment was tolerable, with a median PFS of 2.5 mo (95%CI: 0.8-4.8 mo) and a median OS of 8.5 mo (95%CI: 2.2-10.3 mo). In this study, five out of the 11 patients had stable disease, while two had a partial response. An ongoing clinical trial (NCT01928394) (https://clinicaltrials.gov/ct2/show/NCT01928394) is comparing nivolumab (anti-PD-1 mAb) monotherapy and combination therapy with nivolumab plus ipilimumab in patients with advanced or metastatic PDAC, and the results will be released in 2023.
Notably, in a phase I clinical trial (NCT00556023) (https://clinicaltrials.gov/ct2/show/NCT00556023), a tolerable and safe profile was demonstrated by combination therapy with tremelimumab plus gemcitabine, warranting further study in patients with metastatic PDAC. Thirty-four patients were enrolled in the study, and the median OS was 7.4 mo (95%CI: 5.8-9.4 mo). Two patients achieved a partial response at the end of treatment[83]. A phase Ib/II study (NCT02331251) (https://clinicaltrials.gov/ct2/show/NCT02331251) was performed to evaluate the safety and efficacy of pembrolizumab, an anti-PD-1 mAb, in combination with gemcitabine plus nab-paclitaxel chemotherapy. The median PFS and OS were 9.1 and 15.0 mo for chemotherapy naïve-treated patients, respectively, and changes in tumor cell-free DNA copy number instability were considered to be a potential prognostic factor for OS[84].
A phase I study on atezolizumab, an engineered IgG1 mAb targeting PD-L1, showed tolerability at doses up to 20 mg/kg every three weeks in a Japanese cohort[85]. In a phase II randomized clinical trial (NCT02558894) (https://clinicaltrials.gov/ct2/show/NCT02558894), evaluation of durvalumab, an anti-PD-L1 agent, with or without tremelimumab in patients with metastatic PDAC was evaluated following the failure of 5-FU and gemcitabine-based chemotherapy[86]. No patients in the study responded to durvalumab monotherapy, and the efficacy analysis demonstrated an objective response rate (ORR) of 3.1% (95%CI: 0.08-16.22) with the combination therapy of durvalumab plus tremelimumab[86].
A high tumor mutational burden (TMB) in cancer cells tends to produce more immunogenic neoantigens and may predict immunotherapy response[87]. A phase II clinical trial (NCT05093231) (https://clinicaltrials.gov/ct2/show/NCT05093231) investigating the efficacy of pembrolizumab plus olaparib in metastatic pancreatic adenocarcinoma patients exhibiting high tumor mutation burden is ongoing, and the results will be released in 2026.
Based on the results from current clinical trials, further studies need to focus on the combined approaches using ICIs with different therapeutic approaches, including chemotherapy, radiotherapy, or additional innovative platforms of immunotherapy, such as cancer vaccine and adoptive cell transfer.
Therapeutic cancer vaccines include whole-cell vaccines, dendritic cells, DNA, and peptide vaccines that activate cancer antigen-specific cytotoxic T lymphocytes (CTLs), eliciting immunogenic antigen presentation and leading to an anticancer response[88]. One such pancreatic cancer vaccine is GVAX, which is generated from irradiated pancreatic cancer cells expressing granulocyte-macrophage colony-stimulating factor (GM-CSF)[89] (Table 2). Upon vaccination, GVAX secretes GM-CSF, induces subsequent activation of antigen-presenting cell and T-cell priming, and stimulates the patient’s immune system against pancreatic cancer cells[90]. GVAX was tolerable even at high doses, and the vaccination-induced increased delayed-type hypersensitivity response to autologous tumor cells[91]. In a phase II clinical trial on GVAX (NCT00084383) (https://clinicaltrials.gov/ct2/show/NCT00084383), sixty patients received GVAX 8-10 wk after surgical intervention, followed by adjuvant 5-FU-based chemoradiotherapy. The median PFS was 17.3 mo (95%CI: 14.6-22.8 mo), with a median OS of 24.8 mo (95%CI: 21.2-31.6 mo), which compares favorably with published data for resected PDAC[92]. Combinatory immunotherapy has aimed to induce a much more sustained antitumor T-cell response[93]. In a phase Ib trial for locally advanced, unresectable or metastatic PDAC (NCT00836407) (https://clinicaltrials.gov/ct2/show/NCT00836407), thirty patients received either ipilimumab monotherapy or ipilimumab plus GVAX cancer vaccine, and the median OS was 3.6 mo for the ipilimumab monotherapy group, compared to 5.7 mo in the group with combination therapy[94]. Although combinatory immunotherapy has shown its potential for advanced PDAC, more studies are needed to fully explore this novel therapeutic strategy’s capability.
| Treatment | Phase | Number | Cancer stage | Outcomes |
| GVAX, 5-FU, chemoradiation | II | NCT00084383 | Resected stage I/II PDAC | Median OS 24.8 mo (95%CI: 21.2-31.6 mo) |
| GVAX, cyclophosphamide, CRS-207 | II | NCT01417000 | Metastatic PDAC | Cy/GVAX and CRS-207 extended OS for PDAC patients, with minimal toxicity |
| GVAX, cyclophosphamide, CRS-207 | II | NCT02004262 | Metastatic PDAC | Cy/GVAX and CRS-207 did not show survival benefit over chemotherapy in patients with previously treated metastatic PDAC |
| GVAX, Ipilimumab, FOLFIRINOX | II | NCT01896869 | Metastatic PDAC | Ipilimumab + GVAX group did not show survival benefit over chemotherapy [median OS 9.38 mo (95% CI, 5.0-12.2 mo) vs 14.7 mo (95%CI: 11.6-20.0 mo)] |
| Algenpantucel-L | II | NCT00569387 | Surgically resected PDAC | The addition of algenpantucel-L to standard adjuvant therapy for resected pancreatic cancer may improve survival (12-mo DFS 62%, 12-mo OS 86%) |
| Gemcitabine, 5FU Chemoradiation, Algenpantucel-L | III | NCT01072981 | Surgically Resected PDAC | No results reported yet |
| Dendritic cells pulsed with MUC-1/WT-1 | I/II | NCT03114631 | PDAC | Dendritic cells immunotherapy provided a favorable outcome in PDAC patents (12-mo OS 78.2% vs 33.8%) |
| GI-4000 (KRAS), Gemcitabine | II | NCT00300950 | Non-metastatic, Post-resection PDAC | Overall, GI-4000 group showed a similar pattern of recurrence-free survival and OS compared with the placebo group. For stratified R1 resection subgroup, there was a trend in 1 year OS (72% vs 56%), an improvement in OS (523.5 vs 443.5 d (hazard ratio: 1.06; 95%CI: 0.53–2.13, P = 0.872), and increased frequency of immune responders (40% vs 8%; P = 0.062) for GI-4000 vs placebo. |
| Ras-peptide vaccine, IL-2, GM-CSF | II | NCT00019331 | Metastatic PDAC | No results reported yet |
| GV1001 (telomerase peptide vaccine), Gemcitabine, Capecitabine | III | NCT00425360 | Locally Advanced or Metastatic PDAC | Adding GV1001 vaccination to chemotherapy did not improve OS. |
Few human leukocyte antigen (HLA)-A(*)2402-restricted tumor-associated antigens, including the KIF20A-10-66 peptide, have been identified in PDAC[95]. A phase I/II clinical trial in Japan showed a better prognosis in patients with metastatic PDAC and HLA-A*2402-positive status who received KIF20A-10-66 peptide vaccination as second-line treatment after failure of gemcitabine chemotherapy[96]. In two separate phase II clinical trials, KIF20A-derived peptide was evaluated in combination with two antiangiogenic cancer vaccines targeting vascular endothelial growth factor receptor 1 (VEGFR1) and VEGFR2. In the HLA-A*2402-matched group, patients with peptide-specific CTL induction had improved prognosis and increased OS[97,98]. Another HLA-A24-restricted antigenic peptide, SVN-2B, also functions as an immunogenic molecule. A vaccination protocol of SVN-2B in combination with interferon-α has demonstrated effective clinical and immunological responses for advanced PDAC[99].
Algenpantucel-L is a whole-cell pancreatic cancer vaccine with two irradiated allogenic human pancreatic cell lines (HAPa-1 and HAPa-2) expressing the murine enzyme (1,3)-galactosyltransferase (αGT)[100]. Of note, the αGT enzyme is the critical barrier to xenotransplantation due to hyperacute rejection[101]. As a result, Algenpantucel-L will induce a hyperacute rejection of the allograft cells through rapid activation of antibody-dependent cell-mediated cytotoxicity (ADCC), leading to a response against the patient’s pancreatic cancer cells through epitope spreading[102]. A phase II, open-label trial (NCT00569387) (https://clinicaltrials.gov/ct2/show/NCT00569387) evaluated the use of the Algenpantucel-L tumor vaccine in combination with gemcitabine plus 5-FU chemoradiotherapy in patients with resected PDAC. Seventy patients were recruited in the study, and the 12-mo disease-free survival (DFS) and OS were 63% and 86%, respectively, suggesting that the Algenpantucel-L tumor vaccine could be administered with standard chemotherapy following surgical resection of pancreatic cancer[101]. Unfortunately, in a recent phase 3, open-label, randomized clinical trial (NCT01836432) (https://clinicaltrials.gov/ct2/show/NCT01836432), Algenpantucel-L failed to improve survival on borderline resectable or locally advanced PDAC receiving neoadjuvant chemoradiation therapy[103].
Overexpression of Mucin 1 (MUC-1), a type I transmembrane protein with O-linked glycosylation, plays a crucial role in oncogenic signaling to promote metastasis, angiogenesis, and invasion[104]. MUC-1 has served as a target for cancer vaccine immunotherapy[105]. Following surgical resection, a phase I/II study of a MUC1 peptide-loaded dendritic cell vaccine was conducted in 12 pancreaticobiliary cancer patients. Four out of twelve (33.3%) patients who received this MUC-1-based tumor vaccine were alive after four years without evidence of recurrence[106]. An optimized construct with MUC-1-variable number tandem repeats has been designed with much more potent immunogenicity[107].
Dendritic cell vaccines have been introduced to enhance the antitumor immune response through the stimulation of naïve T cells[108]. In a study evaluating the effectiveness of a dendritic cell vaccine in patients with advanced PDAC (NCT01410968) (https://clinicaltrials.gov/ct2/show/NCT01410968), autologous dendritic cells were isolated in HLA-A2-positive patients, loaded with three A-2 restricted peptides, and readministered as a cellular vaccine. The results were promising with the generation of antigen-specific T cells in three patients, as well as tolerable adverse effects[109]. In a phase I study in Japan, a Wilms' tumor 1 (WT1)-pulsed dendritic cell vaccine combined with chemotherapy showed safety and potential acquisition of immunity in resected PDAC[110]. Multiple associated studies have further supported the clinical benefits of dendritic cell-based vaccines in PDAC[111-113].
Approximately 95% of PDAC patients have mutations in the KRAS oncogene. Despite an early study suggesting an unproven efficacy by targeting mutated KRAS in PDAC[114], multiple subsequent clinical studies have demonstrated the clinical potential for such a therapeutic approach. A phase I/II clinical trial (NCT02261714) (https://clinicaltrials.gov/ct2/show/NCT02261714) evaluated the efficacy of a synthetic mutant RAS peptide vaccine with GM-CSF in PDAC. TG01, a mixture of 7 synthetic RAS peptides representing the most common KRAS mutations, combined with GM-CSF and gemcitabine was well tolerated with a robust immune response and improved clinical outcome[115]. One study demonstrated a long-term immune response and improved survival in patients with resected PDAC after KRAS vaccination[116]. An alternative KRAS-based tumor vaccine is GI-4000, a recombinant heat-inactivated Saccharomyces cerevisiae yeast-derived vaccine expressing mutated KRAS proteins. A phase I trial revealed a favorable safety profile and immunogenicity of the GI-4000 cancer vaccine[117]. A subsequent phase II trial (NCT00300950) (https://clinicaltrials.gov/ct2/show/NCT00300950) compared GI-4000 plus gemcitabine with placebo plus gemcitabine alone in patients with resected PDAC carrying KRAS mutation. GI-4000 was well tolerated. It led to a similar median OS compared with placebo. However, compared with the placebo group, the GI-4000 group had a trend of improved OS (523.5 vs 443.5 d) and an increased frequency of immune responders (40% vs 8%) in the stratified R1 resection subgroup[118].
The GV1001 tumor vaccine consists of a fragment (16 amino acids) of human telomerase reverse transcriptase (hTERT) found in a high proportion in PDAC cancer cells and has been introduced as a novel therapeutic regimen[119]. In a phase I/II clinical trial evaluating the clinical outcomes in patients with unresectable PDAC, GV1001 plus GM-CSF elicited an immune response in 63% of patients, resulting in a median OS of 7.2 mo for immune responders compared to 2.9 mo for nonimmune responders[120]. However, in a randomized phase III study of patients with locally advanced and metastatic PDAC, combination therapy consisting of GV1001, gemcitabine, and capecitabine chemotherapy showed no improvement in OS compared to chemotherapy alone [6.9 mo (95%CI: 6.4–7.6 mo) vs 7.9 mo (95%CI: 7.1–8.8 mo)] (NCT00425360) (https://clinicaltrials.gov/ct2/show/NCT00425360)[121]. Another GV1001-based phase III clinical trial (NCT00358566) (https://clinicaltrials.gov/ct2/show/NCT00358566) was terminated early because of a lack of survival advantage.
Adoptive cell transfer, also known as cellular immunotherapy, includes chimeric antigen receptor T-cell (CAR T cell) therapy and tumor-infiltrating lymphocyte (TIL) therapy[122,123]. CAR T-cell therapy is the most common type of adoptive cell transfer. Generally, it involves harvesting the patient’s T cells, genetic modification to express surface chimeric antigen receptor, ex vivo expansion, and then transferring the cells back to enhance tumor immunity. In forty-three patients with PDAC who underwent radical pancreatectomy, gemcitabine plus adoptive cell transfer with T cells stimulated by the MUC1-expressing human pancreatic cancer cell line demonstrated a median OS of 14.7 mo[124]. Mesothelin is a tumor antigen highly expressed in PDAC[125]. In a preclinical study, CAR T-cell therapy targeting mesothelin demonstrated promising tumor-suppressive effects[126]. Amatuximab (MORab-009), a chimeric mAb targeting mesothelin, also led to reduced growth of mesothelin-expressing tumors, including PDAC[127]. In a phase I trial, the efficacy of MORAb-009 was tested in seven PDAC patients, and one patient had disease control for greater than six months[128]. However, in a phase II randomized placebo-controlled clinical trial (NCT00570713) (https://clinicaltrials.gov/show/NCT00570713) evaluating the efficacy of MORAb-009 plus gemcitabine, no significantly improved clinical outcome was observed [median OS: 6.5 mo (95%CI: 4.5–8.10 mo) vs 6.9 mo (95%CI: 5.4–8.8 mo)]. Compared with the development of ICIs and cancer vaccines, adoptive cell transfer therapy is still in the early development phase against pancreatic cancer (Table 3); more preclinical and clinical studies are needed to further explore its full clinical potential.
| Treatment | Phase | Number | Cancer stage | Outcomes |
| MORAb-009, Gemcitabine | II | NCT00570713 | Advanced PDAC | MORAb-009 did not show survival benefit over placebo group [median OS 6.5 mo, 95%CI: 4.5–8.10 mo vs 6.9 mo 95%CI: 5.4–8.8 mo] |
| MORAb-009 | I | NCT00325494 | PDAC | No results reported yet |
| Radiolabeled Amatuximab (MORAb-009) | I | NCT01521325 | PDAC | No results reported yet |
| Autologous Redirected RNA Mesothelin CAR T cells | I | NCT01897415 | PDAC | No results reported yet |
| CART-133 T cells | I/II | NCT02541370 | Relapsed and/or Chemotherapy Refractory Advanced PDAC | No results reported yet |
Immunotherapy has thus far failed to fulfill its promise in PDAC. The underlying mechanisms appear to be complex and multifactorial primarily due to their unique genetic signatures, metabolic features, and immunosuppressive TME. Pancreatic cancers carry unique molecular genetic backgrounds. MSI in pancreatic cancer is extremely rare (approximately 1%). Oncogenic KRAS mutations, the most common mutation in PDAC, have also contributed to PDAC initiation and maintenance by producing an immunosuppressive TME[129].
Furthermore, altered metabolism of glucose, amino acids, and lipids and their crosstalk with the TME play essential roles in PDAC tumor progression[130]. Multiple lines of evidence have pinpointed the TME as one of the significant barriers to developing effective immunotherapy for PDAC. It is of great clinical interest to sensitize PDAC to immunotherapy through modification of the TME.
One such effort has been focused on CAFs in the TME. As an immunosuppressive component of the TME, FAP-positive CAFs potentially account for the ineffectiveness of immunotherapy in PDAC[131]. Another subtype of CAFs, characterized by the expression of the leucine-rich repeat-containing 15 (LRRC15) protein, could only be detected in pancreatic cancer tissue and is associated with poor response to anti-PD-L1 therapy[132]. Notably, FAP-positive CAFs are the only CAF subtype that expresses CXC motif chemokine ligand 12 (CXCL12). Ablation of FAP-positive CAFs or inhibition of CXCL12 uncovers the antitumor activity of CTLA-4 and PD-L1-based immunotherapy[133]. A phase I/II clinical trial (NCT03168139) (https://clinicaltrials.gov/ct2/show/NCT03168139) was conducted to evaluate the treatment effect of pembrolizumab in patients receiving docetaxel (NOX-A12), an agent targeting CXCL12 and TME in metastatic PDAC. No results have been reported yet.
Cellular components in the TME, including MDSCs and TAMs, are also promising targets in the combinatory strategy for immunotherapy. MDSCs and TAMs induce an immunosuppressive TME, partially through colony-stimulating factor 1 receptor (CSF1R) and focal adhesion kinase (FAK)[134]. Small molecular inhibitors of CSF1R or FAK can reprogram the TME and improve T lymphocyte-mediated pancreatic cancer destruction[135,136]. Multiple clinical trials with CSF1R or FAK inhibitors combined with immunotherapy are currently ongoing (Table 4).
| Strategy | Treatment | Phase | Number | Cancer stage |
| Immune checkpoint inhibitor (target) + CAFs/CXCL12 targeted agents | Pembrolizumab(PD-1) + Olaptesed pegol | I/II | NCT03168139 | Metastatic PDAC |
| Immune checkpoint inhibitor (target)+ CSF1R targeted agent | Durvalumab (PD-L1) + Pexidartinib | I | NCT02777710 | Metastatic/Advanced PDAC |
| Nivolumab (PD-1) + Cabiralizumab | I | NCT02526017 | Advanced PDAC | |
| Nivolumab (PD-1) + Cabiralizumab + Gemcitabine | II | NCT03697564 | Advanced PDAC (Stage IV) | |
| Immune checkpoint inhibitor (target) + FAK targeted agent | Pembrolizumab(PD-1) + Defactinib | I/IIa | NCT02758587 | Advanced PDAC |
| Pembrolizumab(PD-1) + Defactinib + Gemcitabine | I | NCT02546531 | Advanced PDAC | |
| Pembrolizumab (PD-1) + Defactinib | II | NCT03727880 | Resectable PDAC |
Despite advances in translational research, PDAC remains a highly lethal malignancy. Recent breakthroughs in immunotherapy have revolutionized cancer therapy and have shown great potential to transform future PDAC treatment. However, PDAC has shown inferior treatment outcomes toward various immunotherapy regimens compared to other cancer types. The TME has been considered as the fundamental underlying barrier to therapy resistance. To overcome this therapeutic resistance, further investigations with innovative treatment strategies will be needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Plougmann JI, Denmark; Tantau AI, Romania; Zhao CF, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11933] [Article Influence: 2983.3] [Reference Citation Analysis (4)] |
| 2. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3539] [Article Influence: 393.2] [Reference Citation Analysis (0)] |
| 3. | Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S, Alguacil J, Corominas JM, Solà R, Salas A, Real FX. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | De Souza A, Khawaja KI, Masud F, Saif MW. Metformin and pancreatic cancer: Is there a role? Cancer Chemother Pharmacol. 2016;77:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Corbo V, Tortora G, Scarpa A. Molecular pathology of pancreatic cancer: from bench-to-bedside translation. Curr Drug Targets. 2012;13:744-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 6. | Sperti C, Pasquali C, Pedrazzoli S. Ductal adenocarcinoma of the body and tail of the pancreas. J Am Coll Surg. 1997;185:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, Evans RG. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1670] [Article Influence: 334.0] [Reference Citation Analysis (1)] |
| 9. | Ettrich TJ, Seufferlein T. Systemic Therapy for Metastatic Pancreatic Cancer. Curr Treat Options Oncol. 2021;22:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 11. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 12. | Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 264] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (13)] |
| 13. | Storz P, Crawford HC. Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2020;158:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 14. | Grant TJ, Hua K, Singh A. Molecular Pathogenesis of Pancreatic Cancer. Prog Mol Biol Transl Sci. 2016;144:241-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Lee CJ, Li C, Simeone DM. Human pancreatic cancer stem cells: implications for how we treat pancreatic cancer. Transl Oncol. 2008;1:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Pham T, Roth S, Kong J, Guerra G, Narasimhan V, Pereira L, Desai J, Heriot A, Ramsay R. An Update on Immunotherapy for Solid Tumors: A Review. Ann Surg Oncol. 2018;25:3404-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Katayama ES, Hue JJ, Bajor DL, Ocuin LM, Ammori JB, Hardacre JM, Winter JM. A comprehensive analysis of clinical trials in pancreatic cancer: what is coming down the pike? Oncotarget. 2020;11:3489-3501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Goggins M, Offerhaus GJ, Hilgers W, Griffin CA, Shekher M, Tang D, Sohn TA, Yeo CJ, Kern SE, Hruban RH. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am J Pathol. 1998;152:1501-1507. [PubMed] |
| 20. | Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJ, Hruban RH, Kern SE. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol. 2000;156:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front Immunol. 2018;9:1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 23. | Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 790] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 25. | Hessmann E, Buchholz SM, Demir IE, Singh SK, Gress TM, Ellenrieder V, Neesse A. Microenvironmental Determinants of Pancreatic Cancer. Physiol Rev. 2020;100:1707-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 26. | Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 27. | Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 28. | Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 29. | Ferrara B, Pignatelli C, Cossutta M, Citro A, Courty J, Piemonti L. The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 30. | Binenbaum Y, Na'ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2015;23:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 31. | Kultti A, Zhao C, Singha NC, Zimmerman S, Osgood RJ, Symons R, Jiang P, Li X, Thompson CB, Infante JR, Jacobetz MA, Tuveson DA, Frost GI, Shepard HM, Huang Z. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed Res Int. 2014;2014:817613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1640] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 33. | Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, Saad F, Shore ND, Chen L, Heery CR, Gerritsen WR, Priou F, Langkilde NC, Novikov A, Kantoff PW. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2019;37:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, Li CP, Hingorani SR, de la Fouchardiere C, Kasi A, Heinemann V, Maraveyas A, Bahary N, Layos L, Sahai V, Zheng L, Lacy J, Park JO, Portales F, Oberstein P, Wu W, Chondros D, Bullock AJ; HALO 109-301 Investigators. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020;38:3185-3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 35. | Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2074] [Cited by in RCA: 2088] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 36. | Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Xu Y, Li Z, Jiang P, Wu G, Chen K, Zhang X, Li X. The co-expression of MMP-9 and Tenascin-C is significantly associated with the progression and prognosis of pancreatic cancer. Diagn Pathol. 2015;10:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, Möller P, Gschwend JE, Seufferlein T, Gress T, Adler G, Bachem MG. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Ellenrieder V, Alber B, Lacher U, Hendler SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, Adler G, Gress TM. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, Lee M, O'Sullivan C, Barry-Hamilton V, Ghermazien H, Mikels-Vigdal A, Garcia CA, Jorgensen B, Velayo AC, Wang R, Adamkewicz JI, Smith V. Selective Allosteric Inhibition of MMP9 Is Efficacious in Preclinical Models of Ulcerative Colitis and Colorectal Cancer. PLoS One. 2015;10:e0127063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 43. | Grünwald B, Vandooren J, Gerg M, Ahomaa K, Hunger A, Berchtold S, Akbareian S, Schaten S, Knolle P, Edwards DR, Opdenakker G, Krüger A. Systemic Ablation of MMP-9 Triggers Invasive Growth and Metastasis of Pancreatic Cancer via Deregulation of IL6 Expression in the Bone Marrow. Mol Cancer Res. 2016;14:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2541] [Article Influence: 158.8] [Reference Citation Analysis (0)] |
| 45. | Cheng X, Kim JY, Ghafoory S, Duvaci T, Rafiee R, Theobald J, Alborzinia H, Holenya P, Fredebohm J, Merz KH, Mehrabi A, Hafezi M, Saffari A, Eisenbrand G, Hoheisel JD, Wölfl S. Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Mol Oncol. 2016;10:806-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Truong LH, Pauklin S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 47. | Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 592] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 48. | Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezène P, Rubis M, Secq V, Garcia S, Moutardier V, Lombardo D, Iovanna JL, Tomasini R, Guillaumond F, Vander Heiden MG, Vasseur S. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 49. | Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 853] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 50. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1911] [Article Influence: 173.7] [Reference Citation Analysis (1)] |
| 51. | Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A. 2014;111:E3091-E3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 52. | Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101:147-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 767] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 53. | Helms E, Onate MK, Sherman MH. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020;10:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 54. | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1020] [Cited by in RCA: 1756] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 55. | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 2449] [Article Influence: 489.8] [Reference Citation Analysis (0)] |
| 56. | Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2209] [Cited by in RCA: 2942] [Article Influence: 326.9] [Reference Citation Analysis (0)] |
| 57. | Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, Chu Y, Fan J, He R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76:4124-4135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 530] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 58. | Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, Ni CR, Zhu MH. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18:840-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (7)] |
| 59. | Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol. 2018;9:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 60. | Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 399] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 61. | Li T, Yi S, Liu W, Jia C, Wang G, Hua X, Tai Y, Zhang Q, Chen G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013;30:663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 62. | Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39:783-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 63. | Aliru ML, Schoenhals JE, Venkatesulu BP, Anderson CC, Barsoumian HB, Younes AI, K Mahadevan LS, Soeung M, Aziz KE, Welsh JW, Krishnan S. Radiation therapy and immunotherapy: what is the optimal timing or sequencing? Immunotherapy. 2018;10:299-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 613] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 66. | Tsuchikawa T, Hirano S, Tanaka E, Matsumoto J, Kato K, Nakamura T, Ebihara Y, Shichinohe T. Novel aspects of preoperative chemoradiation therapy improving anti-tumor immunity in pancreatic cancer. Cancer Sci. 2013;104:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Zhang Y, Lazarus J, Steele NG, Yan W, Lee HJ, Nwosu ZC, Halbrook CJ, Menjivar RE, Kemp SB, Sirihorachai VR, Velez-Delgado A, Donahue K, Carpenter ES, Brown KL, Irizarry-Negron V, Nevison AC, Vinta A, Anderson MA, Crawford HC, Lyssiotis CA, Frankel TL, Bednar F, Pasca di Magliano M. Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis. Cancer Discov. 2020;10:422-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 68. | Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125:3365-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 69. | De Cicco P, Ercolano G, Ianaro A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front Immunol. 2020;11:1680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 70. | Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, Abrams Z, Luedke E, Lesinski GB, Mundy-Bosse B, Bekaii-Saab T, Carson WE 3rd. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A, Allavena P, Mantovani A, Marchesi F. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2016;65:1710-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 72. | Lankadasari MB, Mukhopadhyay P, Mohammed S, Harikumar KB. TAMing pancreatic cancer: combat with a double edged sword. Mol Cancer. 2019;18:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37:8657-8664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 74. | Liu Q, Li Y, Niu Z, Zong Y, Wang M, Yao L, Lu Z, Liao Q, Zhao Y. Atorvastatin (Lipitor) attenuates the effects of aspirin on pancreatic cancerogenesis and the chemotherapeutic efficacy of gemcitabine on pancreatic cancer by promoting M2 polarized tumor associated macrophages. J Exp Clin Cancer Res. 2016;35:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 76. | Young K, Hughes DJ, Cunningham D, Starling N. Immunotherapy and pancreatic cancer: unique challenges and potential opportunities. Ther Adv Med Oncol. 2018;10:1758835918816281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | Zhang J, Wolfgang CL, Zheng L. Precision Immuno-Oncology: Prospects of Individualized Immunotherapy for Pancreatic Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293-12297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2103] [Cited by in RCA: 2454] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 79. | Twomey JD, Zhang B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021;23:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 80. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 977] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 81. | Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 82. | Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 84. | Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schütz E, Khemka V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 85. | Mizugaki H, Yamamoto N, Murakami H, Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T, Takahashi T. Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34:596-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | O'Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, Takahashi O, Yang Y, Fitts D, Philip PA. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 518] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 87. | Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell. 2021;39:154-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 747] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 88. | Salman B, Zhou D, Jaffee EM, Edil BH, Zheng L. Vaccine therapy for pancreatic cancer. Oncoimmunology. 2013;2:e26662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Rosenberg A, Mahalingam D. Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response. J Gastrointest Oncol. 2018;9:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Laheru D, Biedrzycki B, Jaffee EM. Development of a cytokine-modified allogeneic whole cell pancreatic cancer vaccine. Methods Mol Biol. 2013;980:175-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O'Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 92. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 93. | Chung V, Kos FJ, Hardwick N, Yuan Y, Chao J, Li D, Waisman J, Li M, Zurcher K, Frankel P, Diamond DJ. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin Transl Oncol. 2019;21:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 94. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 95. | Osawa R, Tsunoda T, Yoshimura S, Watanabe T, Miyazawa M, Tani M, Takeda K, Nakagawa H, Nakamura Y, Yamaue H. Identification of HLA-A24-restricted novel T Cell epitope peptides derived from P-cadherin and kinesin family member 20A. J Biomed Biotechnol. 2012;2012:848042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med. 2013;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Suzuki N, Hazama S, Iguchi H, Uesugi K, Tanaka H, Hirakawa K, Aruga A, Hatori T, Ishizaki H, Umeda Y, Fujiwara T, Ikemoto T, Shimada M, Yoshimatsu K, Shimizu R, Hayashi H, Sakata K, Takenouchi H, Matsui H, Shindo Y, Iida M, Koki Y, Arima H, Furukawa H, Ueno T, Yoshino S, Nakamura Y, Oka M, Nagano H. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2017;108:73-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 98. | Miyazawa M, Katsuda M, Maguchi H, Katanuma A, Ishii H, Ozaka M, Yamao K, Imaoka H, Kawai M, Hirono S, Okada KI, Yamaue H. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int J Cancer. 2017;140:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 99. | Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y, Tamura Y, Tsukahara T, Kanaseki T, Sato N, Hirata K. Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2013;104:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Algenpantucel-L immunotherapy in pancreatic adenocarcinoma. Immunotherapy. 2016;8:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94-100; discussion p. 100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 102. | McCormick KA, Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Pancreatic cancer: Update on immunotherapies and algenpantucel-L. Hum Vaccin Immunother. 2016;12:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Hewitt DB, Nissen N, Hatoum H, Musher B, Seng J, Coveler AL, Al-Rajabi R, Yeo CJ, Leiby B, Banks J, Balducci L, Vaccaro G, LoConte N, George TJ, Brenner W, Elquza E, Vahanian N, Rossi G, Kennedy E, Link C, Lavu H. A Phase 3 Randomized Clinical Trial of Chemotherapy With or Without Algenpantucel-L (HyperAcute-Pancreas) Immunotherapy in Subjects With Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer. Ann Surg. 2022;275:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 104. | Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 105. | Behrens ME, Grandgenett PM, Bailey JM, Singh PK, Yi CH, Yu F, Hollingsworth MA. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene. 2010;29:5667-5677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, Finn OJ, Ramanathan RK. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955-964. [PubMed] |
| 107. | Gong YF, Zhou QB, Liao YD, Mai C, Chen TJ, Tang YQ, Chen RF. Optimized construction of MUC1-VNTRn DNA vaccine and its anti-pancreatic cancer efficacy. Oncol Lett. 2017;13:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 109. | Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, Scurti G, Salem ML, Nelson MH, Thomas MB, Paulos CM, Salazar AM, Nishimura MI, Rubinstein MP, Li Z, Cole DJ. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 110. | Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H, Shimodaira S. WT1-pulsed Dendritic Cell Vaccine Combined with Chemotherapy for Resected Pancreatic Cancer in a Phase I Study. Anticancer Res. 2018;38:2217-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, Homma S, Okamoto M. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 112. | Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S, Yusa S, Ishidao T, Kishimoto J, Shibamoto Y, Nagaya M, Yonemitsu Y; DC Vaccine Study Group at the Japan Society of Innovative Cell Therapy (J-SICT). Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother. 2014;63:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Takakura K, Koido S, Kan S, Yoshida K, Mori M, Hirano Y, Ito Z, Kobayashi H, Takami S, Matsumoto Y, Kajihara M, Misawa T, Okamoto M, Sugiyama H, Homma S, Ohkusa T, Tajiri H. Prognostic markers for patient outcome following vaccination with multiple MHC Class I/II-restricted WT1 peptide-pulsed dendritic cells plus chemotherapy for pancreatic cancer. Anticancer Res. 2015;35:555-562. [PubMed] |
| 114. | Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, O'Reilly EM. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 115. | Palmer DH, Valle JW, Ma YT, Faluyi O, Neoptolemos JP, Jensen Gjertsen T, Iversen B, Amund Eriksen J, Møller AS, Aksnes AK, Miller R, Dueland S. TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01-01): a single-arm, phase 1/2 trial. Br J Cancer. 2020;122:971-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 116. | Wedén S, Klemp M, Gladhaug IP, Møller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 117. | Cohn A, Morse MA, O'Neil B, Whiting S, Coeshott C, Ferraro J, Bellgrau D, Apelian D, Rodell TC. Whole Recombinant Saccharomyces cerevisiae Yeast Expressing Ras Mutations as Treatment for Patients With Solid Tumors Bearing Ras Mutations: Results From a Phase 1 Trial. J Immunother. 2018;41:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Muscarella P, Bekaii-Saab T, McIntyre K, Rosemurgy A, Ross SB, Richards DA, Fisher WE, Flynn PJ, Mattson A, Coeshott C, Roder H, Roder J, Harrell FE, Cohn A, Rodell TC, Apelian D. A Phase 2 Randomized Placebo-Controlled Adjuvant Trial of GI-4000, a Recombinant Yeast Expressing Mutated RAS Proteins in Patients with Resected Pancreas Cancer. J Pancreat Cancer. 2021;7:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Suehara N, Mizumoto K, Muta T, Tominaga Y, Shimura H, Kitajima S, Hamasaki N, Tsuneyoshi M, Tanaka M. Telomerase elevation in pancreatic ductal carcinoma compared to nonmalignant pathological states. Clin Cancer Res. 1997;3:993-998. [PubMed] |
| 120. | Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 121. | Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 122. | Morotti M, Albukhari A, Alsaadi A, Artibani M, Brenton JD, Curbishley SM, Dong T, Dustin ML, Hu Z, McGranahan N, Miller ML, Santana-Gonzalez L, Seymour LW, Shi T, Van Loo P, Yau C, White H, Wietek N, Church DN, Wedge DC, Ahmed AA. Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer. 2021;124:1759-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 123. | Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 124. | Matsui H, Hazama S, Sakamoto K, Shindo Y, Kanekiyo S, Nakashima M, Matsukuma S, Tokuhisa Y, Iida M, Suzuki N, Yoshimura K, Takeda S, Ueno T, Yoshino S, Oka M, Nagano H. Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy. Pancreas. 2017;46:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Chen Y, Ayaru L, Mathew S, Morris E, Pereira SP, Behboudi S. Expansion of anti-mesothelin specific CD4+ and CD8+ T cell responses in patients with pancreatic carcinoma. PLoS One. 2014;9:e88133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 126. | Watanabe K, Luo Y, Da T, Guedan S, Ruella M, Scholler J, Keith B, Young RM, Engels B, Sorsa S, Siurala M, Havunen R, Tähtinen S, Hemminki A, June CH. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 127. | Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, Chao Q, Jacob S, Turchin H, Gibbs L, Phillips MD, Mudali S, Iacobuzio-Donahue C, Jaffee EM, Moreno M, Pastan I, Sass PM, Nicolaides NC, Grasso L. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PubMed] |
| 128. | Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132-6138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 129. | Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 130. | Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, Zhao F, You L, Wang W, Zhao Y. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 131. | Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 950] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 132. | Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A, Senbabaoglu Y, Modrusan Z, Liang Y, Junttila MR, Klijn C, Bourgon R, Turley SJ. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020;10:232-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 133. | Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212-20217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1555] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 134. | Osipov A, Saung MT, Zheng L, Murphy AG. Small molecule immunomodulation: the tumor microenvironment and overcoming immune escape. J Immunother Cancer. 2019;7:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 135. | Li M, Li M, Yang Y, Liu Y, Xie H, Yu Q, Tian L, Tang X, Ren K, Li J, Zhang Z, He Q. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 136. | Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 774] [Article Influence: 86.0] [Reference Citation Analysis (0)] |