Published online Jul 7, 2022. doi: 10.3748/wjg.v28.i25.2910
Peer-review started: February 20, 2022
First decision: May 9, 2022
Revised: May 18, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 7, 2022
Processing time: 133 Days and 22.2 Hours
Cholinergic nerves are widely distributed throughout the human body and participate in various physiological activities, including sensory, motor, and visceral activities, through cholinergic signaling. Cholinergic signaling plays an important role in pancreatic exocrine secretion. A large number of studies have found that cholinergic signaling overstimulates pancreatic acinar cells through muscarinic receptors, participates in the onset of pancreatic diseases such as acute pancreatitis and chronic pancreatitis, and can also inhibit the progression of pancreatic cancer. However, cholinergic signaling plays a role in reducing pain and inflammation through nicotinic receptors, but enhances the proliferation and invasion of pancreatic tumor cells. This review focuses on the progression of cholinergic signaling and pancreatic diseases in recent years and reveals the role of cholinergic signaling in pancreatic diseases.
Core Tip: The pancreas is a nerve-rich organ that lies behind the peritoneum and is surrounded by many nerve plexuses. Studies have found that cholinergic signaling is involved in the physiological function of the pancreas and the pathological process of pancreatic diseases due to its action on different receptors. Perhaps starting with cholinergic receptors could uncover potential therapeutic targets for pancreatic diseases.
- Citation: Yang JM, Yang XY, Wan JH. Multiple roles for cholinergic signaling in pancreatic diseases. World J Gastroenterol 2022; 28(25): 2910-2919
- URL: https://www.wjgnet.com/1007-9327/full/v28/i25/2910.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i25.2910
Acetylcholine is a neurotransmitter released by all cholinergic neurons that plays an important role in the peripheral and central nervous systems. The vagus nerve is the longest nerve in the human body and provides innervation to most organs, especially the organs of the digestive system. Cholinergic signaling released by vagus nerve activation mainly acts on cholinergic receptors. According to pharmacological properties, cholinergic receptors are divided into muscarinic receptors (M receptors) and nicotinic receptors (N receptors). Among them, M receptors are widely distributed in smooth muscles and glands and participate in the secretion of glands. M receptors are divided into 5 subtypes of M1-M5, among which M1, M3, and M5 receptors are coupled to Gq protein and M2 and M4 receptors are coupled to Gi protein[1,2]. Each of these five subtypes of M receptors has a unique distribution pattern and is expressed in many areas of the central nervous system and peripheral tissues[1,3-5]. N receptors are ligand-gated ion channels, a pentamer made up of 5 identical homologous subunits (including α1-10, β1-β5, γ, δ, and ε) and retain the potential to be activated by the appropriate agonist[6-8].
The pancreas is an important digestive organ of the human body and plays an important role in the digestion and absorption of food. Pancreatic diseases include acute pancreatitis (AP), chronic pancreatitis (CP), and pancreatic cancer (PCa). Recently, the incidence of pancreatic disease has been increasing year by year, posing a serious threat to human life and health[9-11]. Recent studies have found that cholinergic neuromodulation is involved in the occurrence and development of pancreatic diseases[12-14]. This article reviews the relationship between the three main types of pancreatic diseases (AP, CP, PCa) and cholinergic signaling.
The exocrine system of the pancreas includes three different stages, namely, the cephalic stage, the stomach stage, and the intestinal stage[15]. The total amount of daily exocrine activity of the human pancreas is 1-2 L, and approximately 20% occurs in the cephalic stage, which is under the control of the vagus nerve[16-18]. Studies on humans and animals have shown that the cholinergic system regulates pancreatic exocrine secretion through the vagus nerve reflex. These reflexes originate in the dorsal motor nucleus of the vagus nerve in the medulla oblongata. The cranial preganglionic nerve fibers exit the vagus nerve and terminate at the intrapancreatic ganglia to form synapses through cholinergic preganglionic fibers[19,20].
The intrapancreatic ganglion are the integration center of pancreatic exocrine secretion, and terminal axons from these ganglia innervate approximately every acinar artery. Although preganglionic neurotransmission is mediated by acetylcholine through nicotinic and muscarinic receptors, postpancreatic innervation can be mediated by a variety of neurotransmitters, including acetylcholine, which acts on muscarinic receptors in pancreatic acinar cells[21,22]. Cholinergic agonists produce a pancreatic secretory response similar to that of cephalic stimulation, whereas cholinergic antagonists or resection of the vagus nerve can block the cephalic response. These results show that the acetylcholine released by the efferents of the vagus nerve is the primary mechanism by which sensory input leads to the regulation of pancreatic exocrine secretion[23-26]. The cholinergic system also plays an important role in the gastric and intestinal stages of pancreatic secretion regulation[27,28], which are regulated by nerves and bodily fluids. In the intestinal phase, exocrine secretion of the pancreas is mainly regulated by cholecystokinin (CCK) and other gastrointestinal hormones. However, the cholinergic system also plays a role in the secretion of human pancreatic enzymes stimulated by CCK[21,29]. The postprandial physiological dose of CCK mainly acts on the afferent pathway of the vagus nerve in the gastric and duodenal mucosa and stimulates pancreatic exocrine secretion through the cholinergic efferent nerve[19].
Studies have found that human pancreatic acinar cells preferentially express the M3 receptor[30] and are highly expressed in acinar cells[31]. Acetylcholine acts on the M3 receptor and couples to the Gq protein to activate phospholipase C and promote the release of intracellular calcium ions by initiating the phosphatidyl C-inositol triphosphate cascade and promoting the secretion of pancreatic acinar cells[2,32,33]. According to drug blocking studies, the M3 receptor is a muscarinic receptor that stimulates exocrine secretion form the pancreas[34-36]. However, some studies have shown that the M1 receptor can also control pancreatic exocrine secretion[37,38]. The inhibitory effect of drugs blocking the M1 receptor on the secretion of amylase in isolated pancreatic acinar cells was significantly greater than that of drugs blocking the M3 receptor[39]. Carbachol-induced amylase secretion was significantly impaired in the isolated acinars with M1 and M3 muscarinic receptor single knockout (KO) mice, and amylase secretion was eliminated in the acinar preparation of M1 and M3 muscarinic receptor double KO mice. Therefore, it is proposed that cholinergic signaling stimulates the secretion of pancreatic amylase and is mediated by the combination of M1 and M3 receptors[40].
AP is a common pancreatic disease in clinical practice. AP has a rapid onset and progress, and high morbidity and mortality worldwide[11]. There are many causes of AP, such as biliary, hypertriglyceridemia, and chronic alcohol consumption, among which biliary is the most common factor[41]. However, the specific mechanism underlying the cause of pancreatitis is not clear. When the human body is exposed to cholinergic agonists, such as scorpion stings or organophosphorus pesticide poisoning, some patients will develop AP. This is direct evidence that cholinergic signaling stimulation is associated with the occurrence of human AP[42-45]. Organophosphorus pesticides can inhibit cholinesterase activity and cause a large amount of acetylcholine to accumulate in nerve endings. Similarly, scorpion toxin is a neurotoxic protein that can cause AP by activating the nerve pathway that releases acetylcholine[46,47].
Pancreatic duct ligation in the rat model is often used to simulate clinical AP caused by gallstone obstruction[48], but the severity of experimental pancreatitis is low except when used in the possum model. However, some researchers have found in a pancreatic duct ligation rat model that cholinergic signaling stimulation can aggravate AP inflammation, suggesting that cholinergic signaling may be involved in the pathogenesis of AP caused by cholangiopancreatic duct obstruction[49]. This may be due to an increase in M3 receptor expression induced by rat pancreatic duct ligation, which amplifies the overstimulation of cholinergic signaling on acinar cells and aggravates the intracellular stress response[50]. Before the first clinical onset of acute alcoholic pancreatitis, patients usually have a history of alcohol abuse for many years. Chronic alcohol intake can affect the exocrine regulation of the pancreas by interfering with the cholinergic and trypsin pathways[51]. Long-term use of ethanol feeding can significantly reduce pancreatic acetylcholinesterase activity in rats, while the expression level of pancreatic cholinergic M receptors has not changed, which increases the level and duration of acetylcholine in the pancreas and leads to excessive cholinergic signaling stimulation and damage to pancreatic acinar cells[52,53]. Ethanol-treated pancreatic acinar cells can aggravate the pancreatic injury response caused by the cholinergic agonist carbachol, and its effect may be mediated by protein kinase C downstream signaling of cholinergic receptors[54]. The cholinergic receptor antagonist atropine can improve pancreatitis induced by the combination of alcohol and cerulein suggesting that the cholinergic signaling pathway is involved in the pathogenesis of pancreatitis[52]. Therefore, acetylcholine may play a key role in the pathogenesis of acute alcoholic pancreatitis[12].
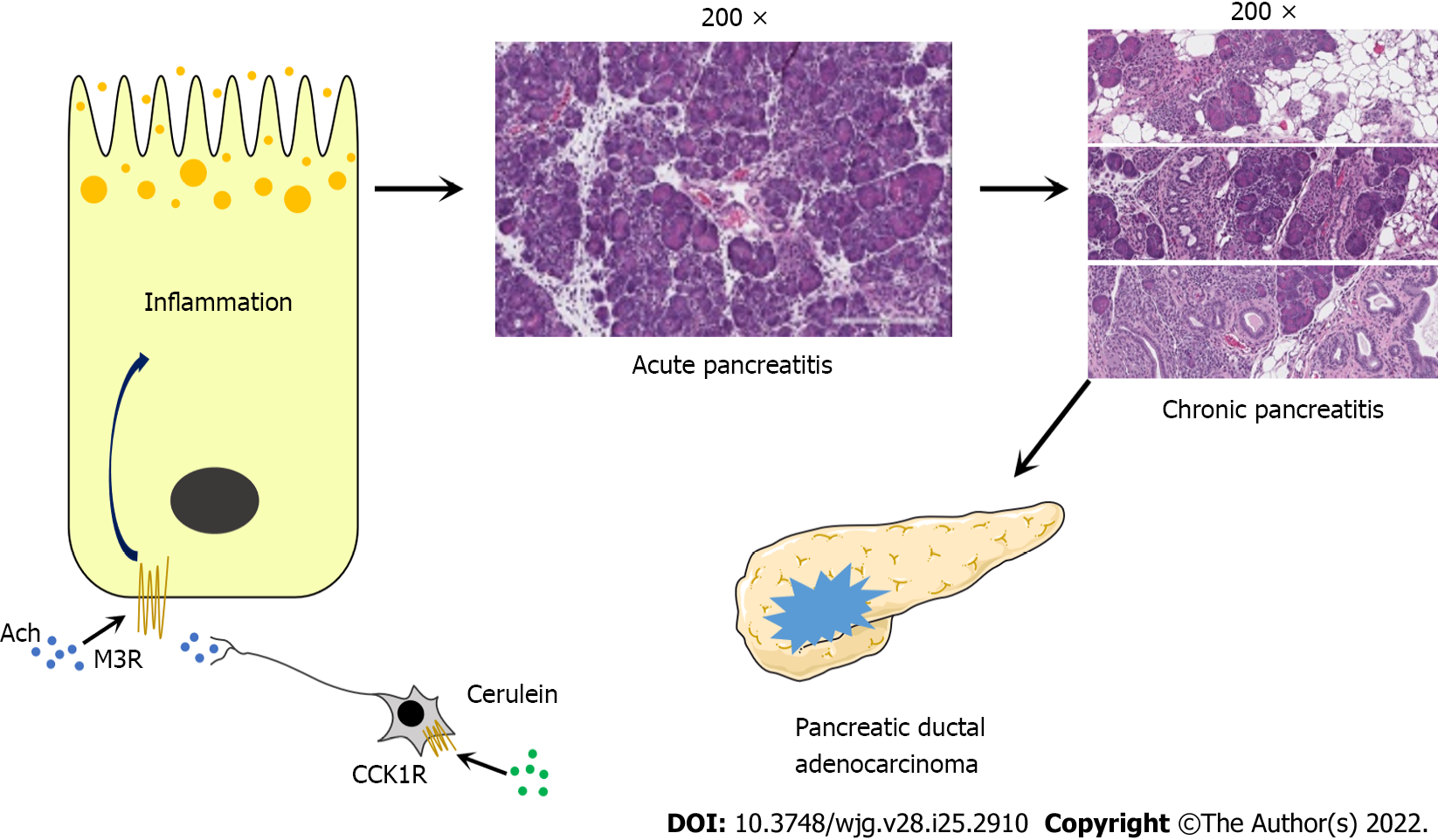
The M3 receptor is highly expressed in human pancreatic acinar cells[31]. Wan et al[55] used chemical genetic technology, in which designer receptors are exclusively activated by designer drugs, to express mutant M3 receptors in mouse acinar cells, causing them to lose their response to acetylcholine but can be activated by the specific drug clozapine-N-oxide (CNO). CNO can induce AP in mutant M3 receptor mice and cause more extensive acinar cell necrosis and inflammation. In addition, the use of M3 receptor antagonists can improve the severity of AP induced by cerulein in wild-type mice (Figure 1)[55].
These results indicate that the activation of the M3 receptor by cholinergic nerve terminals releasing acetylcholine may be one of the pathogeneses of AP. Muscarinic receptor agonists stimulate the activation of trypsinogen and nuclear factor-kappaB, which are two key signaling pathways in the pathogenesis of pancreatitis[56]. Reducing inflammation has always been a major goal in the treatment of AP, and many anti-inflammatory drugs have shown beneficial responses in experimental pancreatitis[57]. However, due to the lack of an in-depth understanding of its pathogenesis, there is currently no effective prevention and treatment strategy[58]. Through in-depth research on the cholinergic signaling pathway, it may be possible to block cholinergic signaling early to treat AP, especially in the prevention of pancreatitis after endoscopic retrograde cholangiopancreatography.
CP is characterized by chronic inflammation and fibrosis of the pancreas caused by multiple factors. The incidence and prevalence of CP are increasing each year, but there is no current specific treatment[10]. The most common causes of CP are excessive drinking, smoking or genetic mutations[59]. Alcohol is considered to be one of the main risk factors for CP. A total of 40%-70% of CP patients become sick due to excessive alcohol consumption[10]. At the same time, excessive alcohol consumption increases the risk of PCa in individuals[60].
Recurrent episodes of AP have been associated with the progression of CP, which is more common in patients with alcoholism. It has been reported that a certain extent of chronic pancreatic damage was already present at the time of AP episodes[41]. The dose-response relationship between alcohol consumption for AP and CP is linear in males[61]. Alcohol-induced pancreatitis may be caused by the alcohol-induced increased viscosity of pancreatic secretions, which blocks the pancreatic duct, and by premature activation of trypsinogen in acinar cells[62].
The hypertonicity of intrapancreatic cholinergic neurons caused by chronic alcoholism may be involved in the pathogenesis of CP[13]. In CP patients, ethanol can cause excessive sensitivity of the pancreatic parasympathetic nerve pathways after a meal[63]. This may be due to chronic alcoholism interrupting the autonomic nerve suppression reflex, leading to the decentralization of the intermediate autonomic nerve (located in the gastric antrum and duodenum) and the intrapancreatic ganglia. Due to an increase in the activity of these autonomic nerves, cholinergic signaling in the pancreas increases, resulting in excessive protein secretion and obstruction of pancreatic juice flow[64,65]. Histopathological analysis of pancreatic tissue samples from patients with CP (including alcoholic pancreatitis) demonstrated that the density of cholinergic fibers in the pancreas of patients with CP was slightly increased compared with normal pancreatic tissue samples[66]. Therefore, the impaired interaction between cholinergic signaling and their receptors on pancreatic acinar cells may be a mechanism of the pathogenesis of alcoholic pancreatitis.
Compared with AP, few CP models use injury mechanisms that may be related to the pathogenesis of human diseases, and the clinical relevance of the pathogenesis of most CP models is unclear[48]. However, Wan et al[55] used a mutant M3 receptor mouse-induced CP model and observed typical CP features, such as extensive chronic inflammation, fibrosis, adipose tissue infiltration, and pancreatic atrophy (Figure 1)[55]. The use of this M3 receptor model may increase our understanding of the pathophysiological process of human CP and identify specific treatments.
Long-term and recurrent pain is a common characteristic of CP[10,67]. A large number of studies have shown that cholinergic nerves have a significant analgesic effect on chronic neuropathic pain, inflammatory pain, and visceral pain[68-70]. Choline transporter (CHT1) is considered to be the rate-limiting step of neuronal acetylcholine synthesis and is essential for the effective recovery of acetylcholine[71,72]. CHT1 is upregulated in CP-induced pain models. CHT1 specific inhibitor, hemicholinium-3, can significantly enhance CP-induced hyperalgesia and reduce the amount of acetylcholine in the dorsal root ganglia of the pancreas in a dose-dependent manner[73]. Further research found that acetylcholine reduces pain and inflammation through α7 nicotinic acetylcholine receptors (α7 nAChRs)[70,74-77]. The activation of α7 nAChRs enhances the autophagic flux of acinar cells mediated by the transcription factor EB pathway and promotes lysosomal degradation to inhibit acinar cell damage, thereby protecting experimental pancreatitis. It has been suggested that cholinergic signaling activation of endogenous α7 nAChRs in the pancreas may be an endogenous protective mechanism in the process of pancreatitis[78]. Therefore, the use of α7 nAChRs to develop analgesic and anti-inflammatory drugs will be a promising target, especially for the treatment of CP.
PCa is a gastrointestinal tumor with a poor prognosis, and more than 90% of PCa are pancreatic ductal adenocarcinoma (PDAC). According to reports, the 5-year survival rate for PCa patients in the United States from 2009 to 2015 was only 9%[79]. With the continuous increase in morbidity and mortality, PCa is expected to become the second leading cause of cancer-related deaths by 2030[80].
CP has been identified as a risk factor for PCa[81,82]. A meta-analysis found that CP increases the risk of PCa, and five years after the diagnosis of CP the risk of PCa increased nearly eightfold[83]. Pain is a characteristic feature of CP and PCa. Studies have found that increased nerve fiber density and hypertrophy are typical features of CP and PCa, and this pathological change appears to enhance and produce pancreatic neuropathic pain[84]. In vitro, myenteric plexus and dorsal root ganglia neurons were isolated from neonatal rats with CP and PCa, and they exhibited strong neurite outgrowth, more complex branching patterns, and somatic hypertrophy. They findings suggest that the intrapancreatic microenvironment in CP and PCa appears to be a key factor in the generation of pancreatic neuropathy and neural plasticity[85].
In-depth research on the tumor microenvironment found that perineural invasion is an important feature of PCa, which can lead to local tumor recurrence and poor prognosis[86]. As the degree of invasion increases, the survival rate of PCa patients is significantly reduced[87]. However, cholinergic signaling appears to play a tumor-suppressing role in PCa[14,88]. Regarding the relationship between heart rate variability (HRV) as an indicator of vagus nerve activity and the overall survival of patients with advanced PCa, it was found that higher vagus nerve activity represented by a higher initial HRV was significantly associated with a lower risk of death from PCa and was not affected by confounding factors such as age and cancer treatment[89]. The activation of M receptors by cholinergic signals can inhibit the progression of pancreatic tumors. Inhibition of the downstream EGFR/MAPK and PI3K/AKT signaling pathways of PCa cells through the M1 receptor signaling pathway inhibits tumor stem cells, CD11b+ cells, tumor necrosis factor-α levels, and liver metastasis[14].
However, M3 receptor expression seems to be a biomarker for the poor prognosis of PDAC. Compared with patients with low M3 expression, patients with high M3 expression have a worse prognosis and shorter survival time, but the study did not find a statistically significant relationship between M3 receptor expression and peripheral nerve infiltration in PCa[90]. The presence of nonneuronal acetylcholine secreted by fibroblasts and pancreatic stellate cells in the microenvironment of pancreatic tumors activates the M3 receptor, leading to further tumor progression[91].
Oxidative stress and inflammatory signaling contribute to the development of pancreatitis. Español et al[92] reported that long-term inflammatory stimulation by LPS plus interferon-γ (IFN-γ) could induce muscarinic receptor expression which mainly involved the M3 and M5 receptors. It could also upregulate the expression of NOS and COX-2 to enhance the effect of carbachol on NO and PGE2 production[92]. Transgenic overexpression of COX-2 in the pancreas induces CP and the formation of preinvasive ductal tumors[93,94]. Muscarinic receptors could serve as promising therapeutic targets for pancreatic inflammation and could prevent the transformation of CP to PCa.
The increase in acetylcholine levels by nAChRs can inhibit histone deacetylase 1-mediated CCL5 chemokines. This could weaken the ability of PDAC cells to recruit CD8+ T cells and directly inhibit the production of IFN-γ by CD8+ T cells. This effect is conducive to Th2 differentiation and could thereby promot tumor growth[86]. Smoking is one of the main causes of PCa[95], and approximately 21% of PCa deaths are attributed to smoking[96]. Studies have shown that nicotine (nAChR agonist) can increase the proliferation activity and self-renewal ability of PCa stem cells by activating the sonic hedgehog signaling pathway[97]. α7 nAChRs have also been shown to upregulate mucin-4 by coactivating the JAK2/STAT3 downstream signaling cascade via the MEK/ERK1/2 pathway, thereby increasing the migration and invasion capabilities of PCa cells[98]. Because the pancreas is widely innervated by many different neurons, the relationship between PCa and the nervous system is complicated[99]. Cholinergic signaling produces different effects based on the different receptor mechanisms involved and may become a potential therapeutic target for PCa in the future.
Cholinergic signaling participates in the physiological function of the pancreas and the pathological process of pancreatic diseases. Although the mechanism by which cholinergic signaling regulates pancreatic diseases is still unclear, an increasing number of studies have shown that cholinergic signaling plays a key role in the occurrence and development of pancreatic diseases. There are many animal models of pancreatitis that can be used to help us study the pathogenesis and patho
| Pancreatic diseases | Receptor type | Relevant mechanism | Effect | Ref. |
| Acute pancreatitis | M3 | Receptor overexpression | Acinar cell hypersecretion | [49,50,52,55] |
| Chronic pancreatitis | M3 | Cholinergic signaling increases | Acinar cell hypersecretion | [13,64-66] |
| M3/M5 | Receptor overexpression | Induce fibroblast proliferation | [92] | |
| α7 | Enhances the autophagic flux of acinar cells | Inhibit acinar cell damage | [70,74-77] | |
| Pancreatic cancer | M1 | Inhibition of the EGFR/MAPK and PI3K/AKT signaling pathways | Inhibit the progression of pancreatic tumors | [14] |
| M3 | Receptor overexpression | Induction of preinvasive ductal tumor formation | [90,92-94] | |
| α7 | Activating the JAK2/STAT3 signaling pathway | Increasing the migration and invasion capabilities of tumor cells | [86,97,98] |
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aktekin A, Turkey; Kitamura K, Japan; Luyer MDP, Netherlands; Matsuo Y, Japan; Susak YM, Ukraine S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279-290. [PubMed] |
| 2. | Qin K, Dong C, Wu G, Lambert NA. Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers. Nat Chem Biol. 2011;7:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 369] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 425] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Wang J, Lindstrom J. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br J Pharmacol. 2018;175:1805-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Wonnacott S, Bermudez I, Millar NS, Tzartos SJ. Nicotinic acetylcholine receptors. Br J Pharmacol. 2018;175:1785-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137:22-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 391] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 9. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1674] [Article Influence: 334.8] [Reference Citation Analysis (1)] |
| 10. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (1)] |
| 11. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 528] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 12. | Grönroos JM, Kaila T, Aho HJ, Nevalainen TJ. Decrease in the number of muscarinic receptors in rat pancreas after chronic alcohol intake. Pharmacol Toxicol. 1989;64:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Tiscornia OM, Dreiling DA. Physiopathogenic hypothesis of alcoholic pancreatitis: supranormal ecbolic stimulation of the "pancreon" units secondary to the loss of the negative component of pancreas innervation. Pancreas. 1987;2:604-612. [PubMed] |
| 14. | Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018;8:1458-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 15. | Konturek SJ, Pepera J, Zabielski K, Konturek PC, Pawlik T, Szlachcic A, Hahn EG. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol. 2003;54:293-317. [PubMed] |
| 16. | Anagnostides A, Chadwick VS, Selden AC, Maton PN. Sham feeding and pancreatic secretion. Evidence for direct vagal stimulation of enzyme output. Gastroenterology. 1984;87:109-114. [PubMed] |
| 17. | Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Defilippi C, Solomon TE, Valenzuela JE. Pancreatic secretory response to sham feeding in humans. Digestion. 1982;23:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Owyang C. Physiological mechanisms of cholecystokinin action on pancreatic secretion. Am J Physiol. 1996;271:G1-G7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol. 2001;52:523-538. [PubMed] |
| 22. | Singer MV, Niebergall-Roth E. Secretion from acinar cells of the exocrine pancreas: role of enteropancreatic reflexes and cholecystokinin. Cell Biol Int. 2009;33:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Becker S, Niebel W, Singer MV. Nervous control of gastric and pancreatic secretory response to 2-deoxy-D-glucose in the dog. Digestion. 1988;39:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Holtmann G, Singer MV, Kriebel R, Stäcker KH, Goebell H. Differential effects of acute mental stress on interdigestive secretion of gastric acid, pancreatic enzymes, and gastroduodenal motility. Dig Dis Sci. 1989;34:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Chariot J, de la Tour J, Anglade P, Rozé C. Cholinergic mechanisms in the pancreas after extrinsic denervation in the rat. Am J Physiol. 1987;252:G755-G761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Park HS, Lee YL, Kwon HY, Chey WY, Park HJ. Significant cholinergic role in secretin-stimulated exocrine secretion in isolated rat pancreas. Am J Physiol. 1998;274:G413-G418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | You CH, Rominger JM, Chey WY. Effects of atropine on the action and release of secretin in humans. Am J Physiol. 1982;242:G608-G611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Blair EL, Brown JC, Harper AA, Scratcherd T. A gastric phase of pancreatic secretion. J Physiol. 1966;184:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Gabryelewicz A, Kulesza E, Konturek SJ. Comparison of loxiglumide, a cholecystokinin receptor antagonist, and atropine on hormonal and meal-stimulated pancreatic secretion in man. Scand J Gastroenterol. 1990;25:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Lugea A, Waldron RT, Mareninova OA, Shalbueva N, Deng N, Su HY, Thomas DD, Jones EK, Messenger SW, Yang J, Hu C, Gukovsky I, Liu Z, Groblewski GE, Gukovskaya AS, Gorelick FS, Pandol SJ. Human Pancreatic Acinar Cells: Proteomic Characterization, Physiologic Responses, and Organellar Disorders in ex Vivo Pancreatitis. Am J Pathol. 2017;187:2726-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Wakui M, Osipchuk YV, Petersen OH. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990;63:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 221] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Louie DS, Owyang C. Muscarinic receptor subtypes on rat pancreatic acini: secretion and binding studies. Am J Physiol. 1986;251:G275-G279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Iwatsuki K, Horiuchi A, Yonekura H, Homma N, Haruta K, Chiba S. Subtypes of muscarinic receptors in pancreatic exocrine secretion in anesthetized dog. Pancreas. 1989;4:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | van Zwam AJ, Willems PH, Rodrigues de Miranda JF, de Pont JJ, van Ginneken CA. Binding characteristics of the muscarinic receptor subtype in rabbit pancreas. J Recept Res. 1990;10:119-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Singer MV, Teyssen S, Küppers U. Influence of the M1-receptor antagonists telenzepine and pirenzepine on pancreatic secretory response to intraduodenal tryptophan in dogs. Digestion. 1991;48:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Teyssen S, Niebergall E, Chari ST, Singer MV. Comparison of two dose-response techniques to study the pancreatic secretory response to intraduodenal tryptophan in the absence and presence of the M1-receptor antagonist telenzepine. Pancreas. 1995;10:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Schmid SW, Modlin IM, Tang LH, Stoch A, Rhee S, Nathanson MH, Scheele GA, Gorelick FS. Telenzepine-sensitive muscarinic receptors on rat pancreatic acinar cells. Am J Physiol. 1998;274:G734-G741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther. 2005;313:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 823] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 42. | Bartholomew C. Acute scorpion pancreatitis in Trinidad. Br Med J. 1970;1:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Singh S, Bhardwaj U, Verma SK, Bhalla A, Gill K. Hyperamylasemia and acute pancreatitis following anticholinesterase poisoning. Hum Exp Toxicol. 2007;26:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Roeyen G, Chapelle T, Jorens P, de Beeck BO, Ysebaert D. Necrotizing pancreatitis due to poisoning with organophosphate pesticides. Acta Gastroenterol Belg. 2008;71:27-29. [PubMed] |
| 45. | Marsh WH, Vukov GA, Conradi EC. Acute pancreatitis after cutaneous exposure to an organophosphate insecticide. Am J Gastroenterol. 1988;83:1158-1160. [PubMed] |
| 46. | Becerril B, Marangoni S, Possani LD. Toxins and genes isolated from scorpions of the genus Tityus. Toxicon. 1997;35:821-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Fletcher PL Jr, Fletcher MD, Possani LD. Characteristics of pancreatic exocrine secretion produced by venom from the Brazilian scorpion, Tityus serrulatus. Eur J Cell Biol. 1992;58:259-270. [PubMed] |
| 48. | Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 49. | Samuel I, Chaudhary A, Fisher RA, Joehl RJ. Exacerbation of acute pancreatitis by combined cholinergic stimulation and duct obstruction. Am J Surg. 2005;190:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 50. | Samuel I, Zaheer S, Fisher RA, Zaheer A. Cholinergic receptor induction and JNK activation in acute pancreatitis. Am J Surg. 2003;186:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Grönroos JM, Aho HJ, Nevalainen TJ. Cholinergic hypothesis of alcoholic pancreatitis. Dig Dis. 1992;10:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Lugea A, Gong J, Nguyen J, Nieto J, French SW, Pandol SJ. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res. 2010;34:1768-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Grönroos JM, Kaila T, Hietaranta AJ. Alcohol, pancreatic muscarinic receptors and acute pancreatitis. Exp Toxicol Pathol. 1994;45:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Satoh A, Gukovskaya AS, Reeve JR Jr, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-kappaB activation in pancreatic acinar cells through effects on protein kinase C-epsilon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G432-G438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Wan J, Wang J, Wagner LE 2nd, Wang OH, Gui F, Chen J, Zhu X, Haddock AN, Edenfield BH, Haight B, Mukhopadhyay D, Wang Y, Yule DI, Bi Y, Ji B. Pancreas-specific CHRM3 activation causes pancreatitis in mice. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Zhan X, Wang F, Bi Y, Ji B. Animal models of gastrointestinal and liver diseases. Animal models of acute and chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2016;311:G343-G355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Kambhampati S, Park W, Habtezion A. Pharmacologic therapy for acute pancreatitis. World J Gastroenterol. 2014;20:16868-16880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 59. | Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322:2422-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 60. | Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 61. | Samokhvalov AV, Rehm J, Roerecke M. Alcohol Consumption as a Risk Factor for Acute and Chronic Pancreatitis: A Systematic Review and a Series of Meta-analyses. EBioMedicine. 2015;2:1996-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Klochkov A, Kudaravalli P, Lim Y, Sun Y. Alcoholic Pancreatitis. StatPearls. Treasure Island (FL), 2021. |
| 63. | Hirano H, Shimosegawa T, Meguro T, Shiga N, Koizumi M, Toyota T. Effects of ethanol on meal-stimulated secretion of pancreatic polypeptide and cholecystokinin: comparison of healthy volunteers, heavy drinkers, and patients with chronic pancreatitis. J Gastroenterol. 1996;31:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Gregg JA, Sharma MM. Endoscopic measurement of pancreatic juice secretory flow rates and pancreatic secretory pressures after secretin administration in human controls and in patients with acute relapsing pancreatitis, chronic pancreatitis, and pancreatic cancer. Am J Surg. 1978;136:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Renner IG, Rinderknecht H, Valenzuela JE, Douglas AP. Studies of pure pancreatic secretions in chronic alcoholic subjects without pancreatic insufficiency. Scand J Gastroenterol. 1980;15:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Ceyhan GO, Demir IE, Rauch U, Bergmann F, Müller MW, Büchler MW, Friess H, Schäfer KH. Pancreatic neuropathy results in "neural remodeling" and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104:2555-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Di Sebastiano P, di Mola FF, Bockman DE, Friess H, Büchler MW. Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut. 2003;52:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Zhang XL, Albers KM, Gold MS. Inflammation-induced increase in nicotinic acetylcholine receptor current in cutaneous nociceptive DRG neurons from the adult rat. Neuroscience. 2015;284:483-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Joshi SK, Mikusa JP, Weaver B, Honore P. Morphine and ABT-594 (a nicotinic acetylcholine agonist) exert centrally mediated antinociception in the rat cyclophosphamide cystitis model of visceral pain. J Pain. 2008;9:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013;65:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Okuda T, Haga T. High-affinity choline transporter. Neurochem Res. 2003;28:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Yamamura HI, Snyder SH. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972;178:626-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 230] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Luo D, Chen L, Yu B. Inhibition of the high affinity choline transporter enhances hyperalgesia in a rat model of chronic pancreatitis. Biochem Biophys Res Commun. 2017;488:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 299] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 75. | Schneider L, Jabrailova B, Soliman H, Hofer S, Strobel O, Hackert T, Büchler MW, Werner J. Pharmacological cholinergic stimulation as a therapeutic tool in experimental necrotizing pancreatitis. Pancreas. 2014;43:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Li B, Wu J, Bao J, Han X, Shen S, Ye X, Dai J, Wu Z, Niu M, He Y, Ni J, Wen L, Wang X, Hu G. Activation of α7nACh receptor protects against acute pancreatitis through enhancing TFEB-regulated autophagy. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15319] [Article Influence: 3063.8] [Reference Citation Analysis (4)] |
| 80. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5140] [Article Influence: 467.3] [Reference Citation Analysis (0)] |
| 81. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1139] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 82. | Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 83. | Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 84. | Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Müller MW, Giese T, Büchler MW, Giese NA, Friess H. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177-186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 85. | Demir IE, Ceyhan GO, Rauch U, Altintas B, Klotz M, Müller MW, Büchler MW, Friess H, Schäfer KH. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil. 2010;22:480-490, e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Yang MW, Tao LY, Jiang YS, Yang JY, Huo YM, Liu DJ, Li J, Fu XL, He R, Lin C, Liu W, Zhang JF, Hua R, Li Q, Jiang SH, Hu LP, Tian GA, Zhang XX, Niu N, Lu P, Shi J, Xiao GG, Wang LW, Xue J, Zhang ZG, Sun YW. Perineural Invasion Reprograms the Immune Microenvironment through Cholinergic Signaling in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020;80:1991-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 87. | Liebl F, Demir IE, Mayer K, Schuster T, DʼHaese JG, Becker K, Langer R, Bergmann F, Wang K, Rosenberg R, Novotny AR, Feith M, Reim D, Friess H, Ceyhan GO. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg. 2014;260:900-7; discussion 907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 88. | Kamiya A, Hiyama T, Fujimura A, Yoshikawa S. Sympathetic and parasympathetic innervation in cancer: therapeutic implications. Clin Auton Res. 2021;31:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | De Couck M, Maréchal R, Moorthamers S, Van Laethem JL, Gidron Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2016;40:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | Zhang L, Xiu D, Zhan J, He X, Guo L, Wang J, Tao M, Fu W, Zhang H. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco Targets Ther. 2016;9:6719-6726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Pfitzinger PL, Fangmann L, Wang K, Demir E, Gürlevik E, Fleischmann-Mundt B, Brooks J, D'Haese JG, Teller S, Hecker A, Jesinghaus M, Jäger C, Ren L, Istvanffy R, Kühnel F, Friess H, Ceyhan GO, Demir IE. Indirect cholinergic activation slows down pancreatic cancer growth and tumor-associated inflammation. J Exp Clin Cancer Res. 2020;39:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Español AJ, Maddaleno MO, Lombardi MG, Cella M, Martínez Pulido P, Sales ME. Treatment with LPS plus INF-γ induces the expression and function of muscarinic acetylcholine receptors, modulating NIH3T3 cell proliferation: participation of NOS and COX. Br J Pharmacol. 2014;171:5154-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Colby JK, Klein RD, McArthur MJ, Conti CJ, Kiguchi K, Kawamoto T, Riggs PK, Pavone AI, Sawicki J, Fischer SM. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia. 2008;10:782-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Müller-Decker K, Fürstenberger G, Annan N, Kucher D, Pohl-Arnold A, Steinbauer B, Esposito I, Chiblak S, Friess H, Schirmacher P, Berger I. Preinvasive duct-derived neoplasms in pancreas of keratin 5-promoter cyclooxygenase-2 transgenic mice. Gastroenterology. 2006;130:2165-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 96. | GBD 2017 Pancreatic Cancer Collaborators. . The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 97. | Al-Wadei MH, Banerjee J, Al-Wadei HA, Schuller HM. Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signalling. Eur J Cancer. 2016;52:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, Ouellette MM, Batra SK. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32:1384-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 99. | Hutchings C, Phillips JA, Djamgoz MBA. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim Biophys Acta Rev Cancer. 2020;1874:188411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |