Published online Jul 7, 2022. doi: 10.3748/wjg.v28.i25.2843
Peer-review started: January 8, 2022
First decision: March 9, 2022
Revised: March 10, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 7, 2022
Processing time: 176 Days and 19.6 Hours
Patients with inflammatory bowel disease (IBD) are more likely to have concurrent immune-mediated inflammatory diseases (IMIDs) than those without IBD. IMIDs have been observed to alter the phenotype and outcomes of IBD in recent studies. Several studies have found that IBD patients with concurrent IMIDs may have more extensive or severe disease phenotypes, and are considered to be at increased risk of requiring biologics and IBD-related surgeries, suggesting that having multiple IMIDs is a poor prognostic factor for IBD. Furthermore, IBD patients with primary sclerosing cholangitis and Takayasu arteritis are reported to have unique endoscopic phenotypes, suggesting concurrent IMIDs can influence IBD phenotype with specific intestinal inflammatory distributions. In this review, we discuss the pathogenesis, disease phenotypes, and clinical outcomes in IBD patients with concomitant IMIDs.
Core Tip: Patients with inflammatory bowel disease (IBD) are more likely to acquire other immune-mediated inflammatory diseases (IMIDs). IBD patients with concurrent IMIDs were more likely to require biologics and IBD-related surgeries than non-IBD patients due to extensive disease phenotypes according to recent studies. As a result, when treating IBD patients, we must be aware of the concurrence of other IMIDs and understand its pathogenesis to select biologic and small molecule therapies that treat multiple concomitant diseases.
- Citation: Akiyama S, Fukuda S, Steinberg JM, Suzuki H, Tsuchiya K. Characteristics of inflammatory bowel diseases in patients with concurrent immune-mediated inflammatory diseases. World J Gastroenterol 2022; 28(25): 2843-2853
- URL: https://www.wjgnet.com/1007-9327/full/v28/i25/2843.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i25.2843
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic relapsing intestinal inflammatory disorder[1]. IBD is a complex disease that is linked to both hereditary and environmental variables, although the etiology is unknown[2-4]. Previous genome-wide association studies (GWASs) identified 99 loci associated with UC and revealed a minimum of 28 shared associations between UC and CD[5]. GWASs have revealed 71 unique loci related to CD and significant genetic overlap with loci related to other immune-mediated inflammatory diseases (IMIDs)[6].
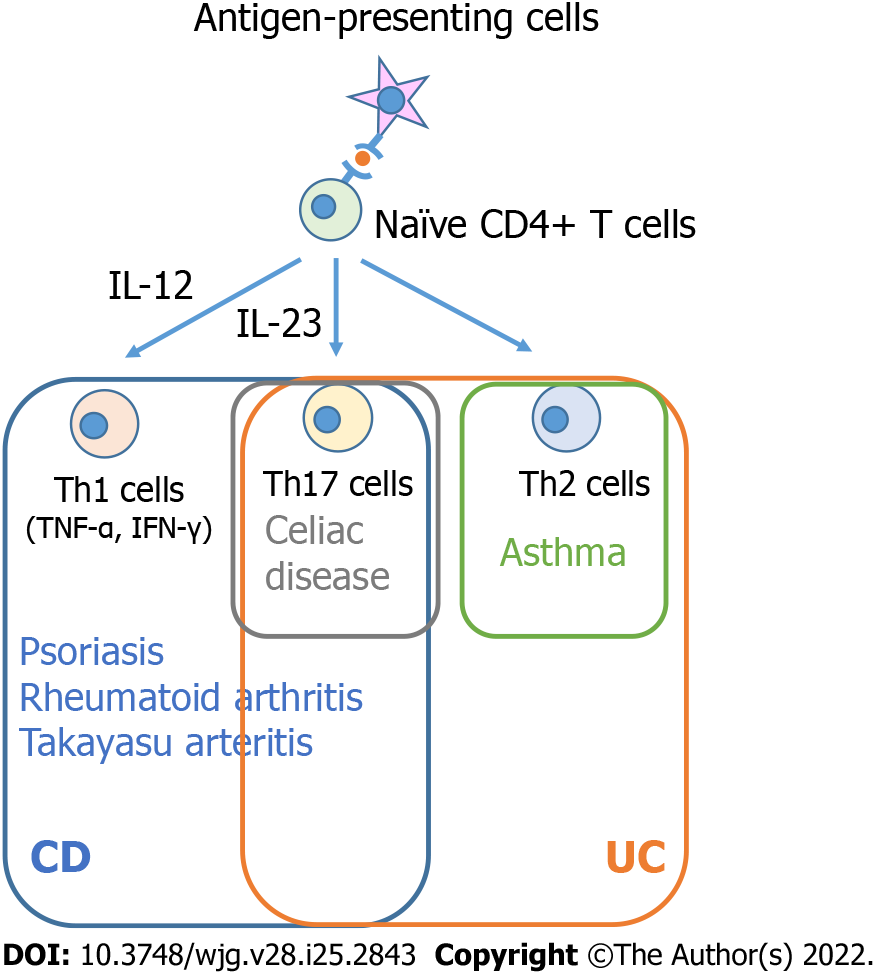
In the pathophysiology of intestinal inflammation in IBD, antigen-presenting cells (APCs) and effector T cells, such as T helper (Th) 1, Th2, and Th17 cells play a pathogenic role[4]. T-cell-mediated immune responses are triggered and modulated by APCs, such as dendritic cells and macrophages. Interleukin (IL)-12 activates Th1 cells that produce interferon-γ, tumor necrosis factor (TNF)-α, and IL-12[7] (Figure 1). TNF-α is released from activated macrophages in response to interferon, leading to the differentiation of stromal cells into myofibroblasts and the synthesis of matrix metalloproteinases that destroy tissue[8]. While aberrant Th1 responses are thought to involve in CD intestinal inflammation, Th2 cell-mediated inflammation is suggested to be fundamental to the pathogenesis of UC[8]. Th17 cells are characterized by a distinct subset of CD4+ T cells promoting the expression of IL-17 and maintained by IL-23[9,10] (Figure 1). Th17 cells and their cytokines play important roles in the inflammatory pathways of both UC and CD[11]. Innate lymphoid cells (ILCs), which do not exhibit antigen receptors to distinguish T cells from B cells, contribute to the pathogenesis in IBD as well, particularly in CD[12]. ILCs are divided into three groups based on the cytokine profiles of helper T-cell subsets, with ILC1, ILC2, and ILC3 serving as the counterparts of Th1, Th2, and Th17 cells, respectively[12].
Given that IBD patients are more likely to have additional IMIDs than nonIBD patients in various studies[13-15], multiple immune pathways may be shared between IBD and other IMIDs. Among recent cross-sectional research of members of a Northern California managed care organization, 17% of IBD patients had at least one IMID diagnosis, whereas the proportion of concurrent IMIDs was 10% in nonIBD individuals[13]. While another study found no significant differences in CD phenotype between individuals with and without IMID, UC patients with another IMID were more likely to have a pancolitis phenotype than those who did not. Furthermore, IBD patients with another IMID had a higher percentage of patients who needed TNF inhibitors or surgery than those without[14]. Of note, previous research found that IBD patients with primary sclerosing cholangitis (PSC) or Takayasu arteritis (TAK) have distinct endoscopic IBD phenotypes[16,17]. IBD can affect the phenotypes and disease courses of concurrent IMIDs as well, according to a recent meta-analysis[18]. All these studies reveal that the presence of other IMIDs in IBD patients can influence disease phenotypes and outcomes and vice versa.
We evaluate the pathogenesis, disease phenotypes, and clinical outcomes in IBD patients with concurrent IMIDs in this review, which includes IMIDs with a high prevalence among patients with IBD and IMIDs that alter IBD phenotypes and outcomes.
Asthma, psoriasis, and rheumatoid arthritis (RA) were the most common IMIDs among IBD patients, according to the data from Northern California managed care organizations[13]. Another study based on prospective registry data found that 21% of IBD patients also had another IMID. Asthma (9.9%), psoriasis (6.1%), and RA (2.0%) were consistently reported to coexist with IBD[14].
Asthma is a prevalent chronic airway disease characterized by respiratory symptoms such as coughing and shortness of breath[19]. Asthma is a Th2 cell-mediated disease. Dendritic cells capture inhaled allergens and present them to naïve CD4+ T cells, resulting in the polarization of Th2 cells. These cells produce IL-4, IL-5, and IL-13, promoting eosinophil migration to the airway, bronchial hyperresponsiveness, mucus overproduction, and immunoglobulin E synthesis[19]. ILC2 also plays a role in the pathogenesis of asthma. ILC2 is activated by IL-25 and IL-33 and can produce Th2 cell-associated cytokines, causing airway hypersensitivity and tissue remodeling[19]. Th2 cell-mediated inflammatory pathways are also implicated in IBD patients, particularly UC patients (Figure 1 and Table 1). Macrophages or dendritic cells acquire luminal antigens in the intestine and present them to naïve CD4+ T cells, boosting their differentiation into Th2 cells in the immunological pathways involved in UC[20]. Given the high transcript levels of IL-17A that are detected in both of CD and UC, Th17 cells are also linked to the development of UC[11]. Although ILC1 and ILC3 are considered to be involved in the pathogenesis of CD, it is unclear if ILC2 contributes to the development of UC[12,21].
| IMIDs with high prevalence among patients with IBD | Possible shared immune cells contributing to pathogenesis | IBD phenotypes | IBD outcomes | Possible shared therapies |
| Asthma | Th2 cells | Indefinite | Indefinite | Indefinite |
| Psoriasis | Th1 cells, Th17 cells, and ILC3 | Indefinite | Indefinite | Ustekinumab; TNF inhibitors |
| Rheumatoid arthritis | Th1 cells, Th17 cells, ILC1, and ILC3 | Indefinite | Indefinite | Tofacitinib; TNF inhibitors |
| IMIDs affecting phenotypes or outcomes of IBD | ||||
| PSC | Indefinite (leaky gut theory) | Severe right-sided colitis, rectal sparing, and backwash ileitis | PSC-IBD patients have fewer or no symptoms, and are less likely to require immunosuppressants, hospitalization, and surgery than IBD patients alone | Indefinite |
| Celiac disease | Th17 cells | CD patients with celiac disease are less likely to have ileocolonic involvement than CD patients alone | CD patients with celiac disease are less likely to require TNF inhibitors or azathioprine than CD patients alone. UC patients with celiac disease have an increased risk of colectomy | Indefinite |
| Takayasu arteritis | Th1 cells and Th17 cells | Discontinuous aphthous erosions/ulcers or focal mucosal inflammation | Indefinite | Tofacitinib; Ustekinumab; TNF inhibitors |
Psoriasis is an immune-mediated skin disorder that affects the skin and/or joints[22] resulting in pruritic and painful skin plaques. Psoriasis is caused by the activation of plasmacytoid dendritic cells, which release various proinflammatory cytokines including TNF, interferon-γ, IL-12, and IL-23. IL-12 aids in the development of Th1 cells from naïve T cells, while IL-23 aids in the maintenance of IL-17-producing Th17 cells. Th17 cells generate IL-17A, IL-17F, and IL-22, while activated Th1 cells release interferon-γ and TNF-α, causing keratinocyte hyperproliferation and other psoriasis clinical features[23]. Similarly, Th1 and Th17 cells are involved in the major immunological mechanism of IBD, particularly CD[24]. ILC3, the counterpart of Th17 cells, is an important source of IL-17 in both psoriasis and CD[21]. Indeed, a systematic review and meta-analysis found that psoriasis patients had an increased risk of IBD, particularly CD[25]. A multicenter retrospective study found that 61% of IBD patients who started ustekinumab, an antagonist of the p40 subunit of IL-12 and IL-23, for concomitant active psoriasis achieved clinical remission of IBD[26]. Although TNF inhibitors are effective in the treatment of both psoriasis and IBD, they can also cause psoriasis[27]. TNF-α inhibition is speculated to cause uncontrolled production of interferon-γ by plasmacytoid dendritic cells, resulting in the recruitment of Th1 or Th17 cells into the dermis, which produces proinflammatory cytokines, such as IL-12 and IL-23[28]. Thus, these data support the notion that the immune mechanism involving Th1 cells, Th17 cells, and ILC3 may be the common pathway between psoriasis and IBD (Figure 1 and Table 1).
RA is a chronic inflammatory disease that affects the cartilage and bone[29] resulting in painful and disfiguring joint deformities. Dendritic cells or macrophages uptake antigens and present them to T cells in the pathogenesis of RA, resulting in Th1 or Th17 cell-mediated diseases in the synovial tissue. Subsequently, the stimulated T cells activate macrophages, B cells, fibroblasts, and osteoclasts. TNF, IL-1, and IL-6 are secreted by the activated macrophages. Inflammatory mediators including matrix metalloproteinases are then produced by fibroblast-like synoviocytes, resulting in cartilage destruction. The activation of osteoclasts induces bone erosions as well[30]. A lymph node activation in RA patients is associated with an imbalance of ILCs, and ILC1 and ILC3 within the lymph node microenvironment can cause inflammation[21]. As a result, RA may have similar immune mechanisms with a CD rather than UC (Figure 1 and Table 1).
Ustekinumab was found to be ineffective in clinical trials of RA patients, whereas TNF, IL-6, and Janus kinase (JAK) inhibitors such as tofacitinib significantly improved RA disease activity when compared to placebo[31]. As a result, among medications approved for RA and IBD, TNF and JAK inhibitors may be effective for both IMIDs. Given that JAKs bind to cytokine receptors and transmit various extracellular cytokine signals such as IL-6, IL-12, IL-23, and interferons, JAK inhibitors are therefore expected to be effective for various IMIDs[28,32] (Table 1).
Several recent population-based studies and meta-analyses have shown that the presence of IMIDs may influence the disease course of IBD. According to a nationwide study of the Danish population, 22.5% of IBD patients had at least one concurrent IMID. This study found that risk factors such as older age and methotrexate use were associated with an increased risk of concurrent IMIDs, whereas male gender, a high socioeconomic status, and infliximab use were associated with a reduced risk of concurrent IMIDs. The impact of at least one concurrent IMID on the disease course of IBD was also investigated in this study. The occurrence of IMIDs increased the risk of surgery in CD patients but not in UC patients in the subgroup who developed IMIDs after the onset of IBD. Both UC and CD patients who had concurrent IMIDs were more likely to require biologics than those who did not[33]. IBD patients with concurrent IMIDs had an increased risk of extensive colitis/pancolitis and IBD-related surgeries compared with those without IMIDs, according to a recent systematic review and meta-analysis involving 16064 IBD patients with concurrent IMIDs and 3451414 IBD patients without IMIDs. Furthermore, those with concurrent IMIDs were at an increased risk of requiring immunomodulators and biologics[34]. Overall, these findings suggest that the coexistence of IMIDs is associated with severe IBD phenotypes.
Another recent systematic review and meta-analysis found that CD patients with concurrent IMIDs were more likely to have upper gastrointestinal involvement and nonstricturing and nonpenetrating phenotypes than those without IMIDs. Given that this meta-analysis suggests that CD patients with concurrent IMIDs may have a milder disease course, which contradicts the findings of the previous meta-analysis, more research is needed to better understand CD patients’ behaviors if they develop other IMIDs and require medication[35].
While it is unclear how different IMIDs affect IBD phenotypes and outcomes, previous research has consistently suggested that IMIDs such as PSC or TAK can influence the endoscopic findings of IBD to specific phenotypes[16,17,36,37]. On the other hand, recent studies have shown that there is an association between celiac disease and IBD and that IBD patients with celiac disease can have distinct phenotypic characteristics and outcomes[38,39].
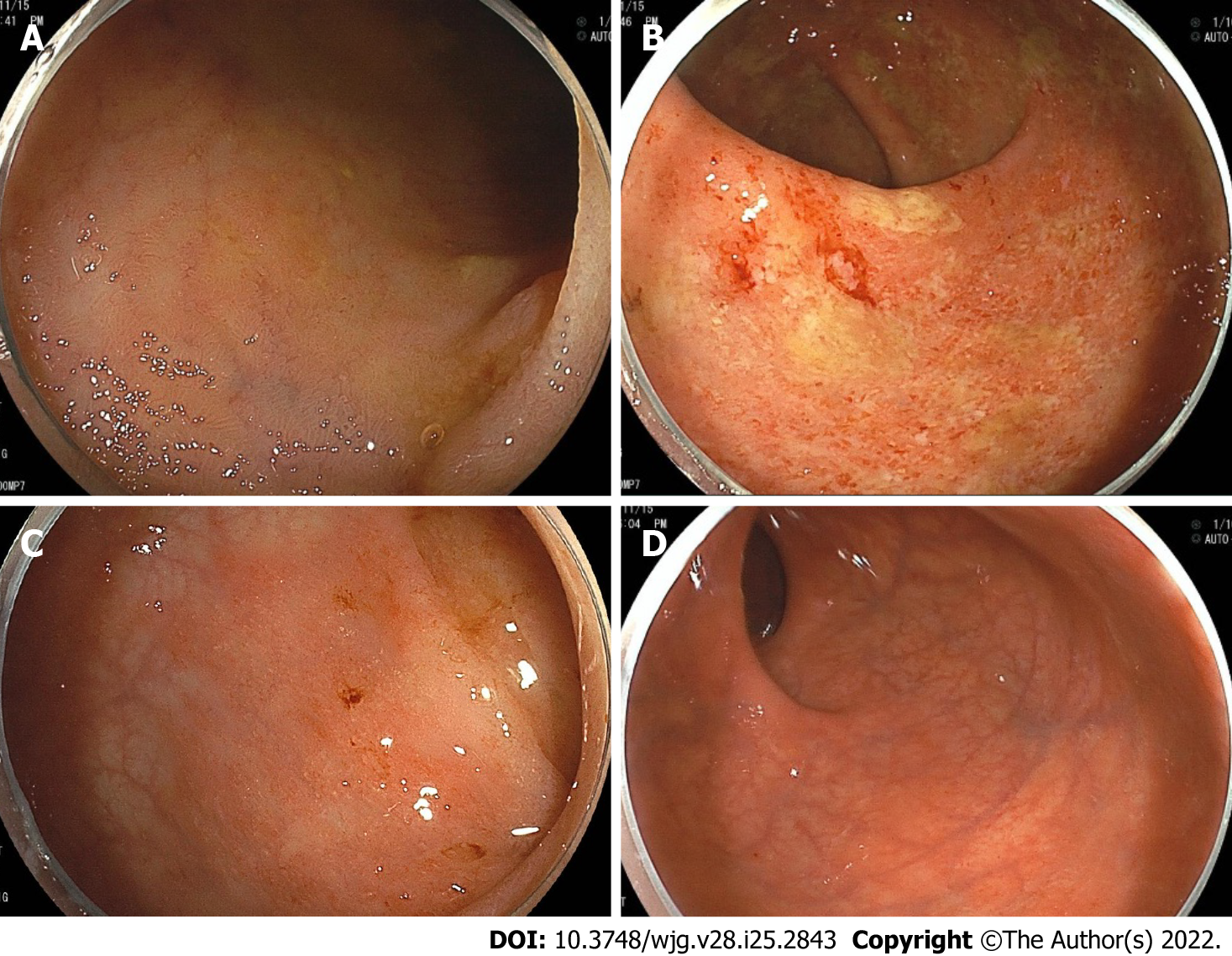
PSC is a chronic and progressive cholestatic disease that affects the intrahepatic and extrahepatic bile ducts[40]. PSC was found to be present in 2.2% of IBD patients. In one study, PSC was found to be present in 2.5% of UC patients and 0.96% of CD patients, respectively[41]. Patients with “extensive UC” had a higher prevalence of PSC than those with left-sided colitis. PSC was more common in CD patients with ileocolonic or colonic involvement than in those with ileal involvement[35,41]. PSC-IBD patients have a predilection for more severe right-sided colitis according to several studies[42,43]. Furthermore, patients with IBD who have PSC frequently develop a distinct IBD phenotype characterized by “rectal sparing” and “backwash ileitis”[16]. According to a systematic review of 11406 PSC-IBD patients, the pooled rate of rectal sparing and backwash ileitis was 9.9% and 12.3%, respectively[36]. Based on this systematic review, a meta-analysis found that the pooled event rate of pancolitis and rectal sparing was significantly higher in PSC-IBD patients than in IBD patients without PSC, but the rate of backwash ileitis was not statistically significant between the two groups[43].
Although PSC-IBD patients frequently develop pancolonic involvement with significant right-sided colitis, several studies have shown that their disease course was mild[43]. Previous studies found that PSC-IBD patients had fewer or no symptoms[44] and were less likely to require immunosuppressants such as steroids, thiopurines, hospitalization[45], and surgery than IBD patients without PSC[46]. In addition, patients with PSC-CD had a higher risk of nonstricturing and nonpenetrating behavior than those without PSC, as well as being less likely to undergo CD-related surgeries, according to a recent meta-analysis[35]. All of these findings suggest that PSC-IBD patients may have better IBD outcomes, suggesting that PSC-IBD is a distinct disease phenotype from IBD phenotypes in patients with other IMIDs (Table 1).
The cause of the disparity between endoscopic disease activity and clinical outcomes in patients with PSC-IBD is unknown. In a retrospective study, immunosuppressant use and colonic or ileal surgical resection were found to be less common in PSC-IBD patients than in IBD patients without PSC. However, this study discovered that a higher percentage of PSC-IBD patients used 5-aminosalicylates, suggesting that PSC-IBD patients are more likely to use 5-aminosalicylates to lower their risk of colorectal cancer or dysplasia and that their use can be associated with mild disease activity[46-48]. Meanwhile, a case-controlled study found that PSC-UC patients had a milder disease course than those with UC alone, even though the number of patients taking 5-aminosalicylates or sulfasalazine was the same[49]. Hence, more research is needed to understand if differences in the pathogenesis or medical therapies between PSC-IBD patients and patients with IBD alone can explain the unique disease phenotype of PSC-IBD.
Many studies have been carried out to better understand the mechanisms of PSC-IBD, and it has been discovered that there is less genetic overlap between PSC and IBD[50]. Thus, environmental factors such as the gut microbiome have been highlighted. According to the “leaky gut” theory, IBD-related mucosal injury allows the colonic bacteria to cross the gut barrier and into the liver, resulting in the development of PSC[43]. Although the exact mechanism is unknown, PSC-IBD patients were more likely to develop colorectal cancer than IBD patients without PSC[43], so UC guidelines recommend colorectal cancer screening at the time of PSC diagnosis[51].
Celiac disease is a gluten-induced disease that frequently coexists in patients with IMIDs[52]. Patients with IBD have a higher risk of celiac disease compared to controls according to a systematic review and meta-analysis (risk ratio 3.96; 95% confidence interval: 2.23-7.02). Patients with celiac disease have a significantly increased risk of IBD compared to controls (risk ratio 9.88; 95% confidence interval: 4.03-24.2), suggesting the shared pathogenesis between both diseases[38]. A case–control study based on a national registry of pediatric IBD patients found that CD patients with celiac disease were less likely to have ileocolonic involvement and require medical therapies such as TNF inhibitors or azathioprine than CD patients alone. While disease distribution and medical treatments were not significantly different between UC patients with and without celiac disease, UC patients with celiac disease had a higher risk of colectomy[39], suggesting that CD patients with celiac disease may have better outcomes, whereas the concurrence of celiac disease can potentially worsen UC outcomes (Table 1).
Some genetic, environmental, and immunological factors are shared by celiac disease and IBD. GWASs revealed that celiac disease and CD share genetic risk loci including PTPN2, IL18RAP, TAGAP, and PUS10[53]. Both diseases have been linked to microbial factors[54,55]. Celiac disease is a CD4+ T-cell-dependent disease associated with chronic intestinal inflammation, according to the immunological mechanism[56]. APCs present gliadin peptide to CD4+ T cells, which then produce proinflammatory cytokines such as interferon-γ and IL-21, increasing intraepithelial lymphocytes (IELs)[56]. The IELs such as CD8+ T cells carrying αβ T-cell receptor (TCR) or CD4−CD8− γδ TCR+ T cells cause cytolysis or apoptosis in intestinal epithelial cells[57,58]. Patients with celiac disease have mucosal Th17 cells, and an increased level of IL-17A expression is associated with villous atrophy, a hallmark pathologic feature of celiac disease[59,60]. Th17 cells produce IL-17, interferon-γ, and IL-21, which play a role in celiac disease pathogenesis[61]. In terms of ILCs, it remains unclear how these cells are associated with the disease initiation and progression[62]. These findings support that Th17 cells may be involved in both IBD and celiac disease (Figure 1 and Table 1).
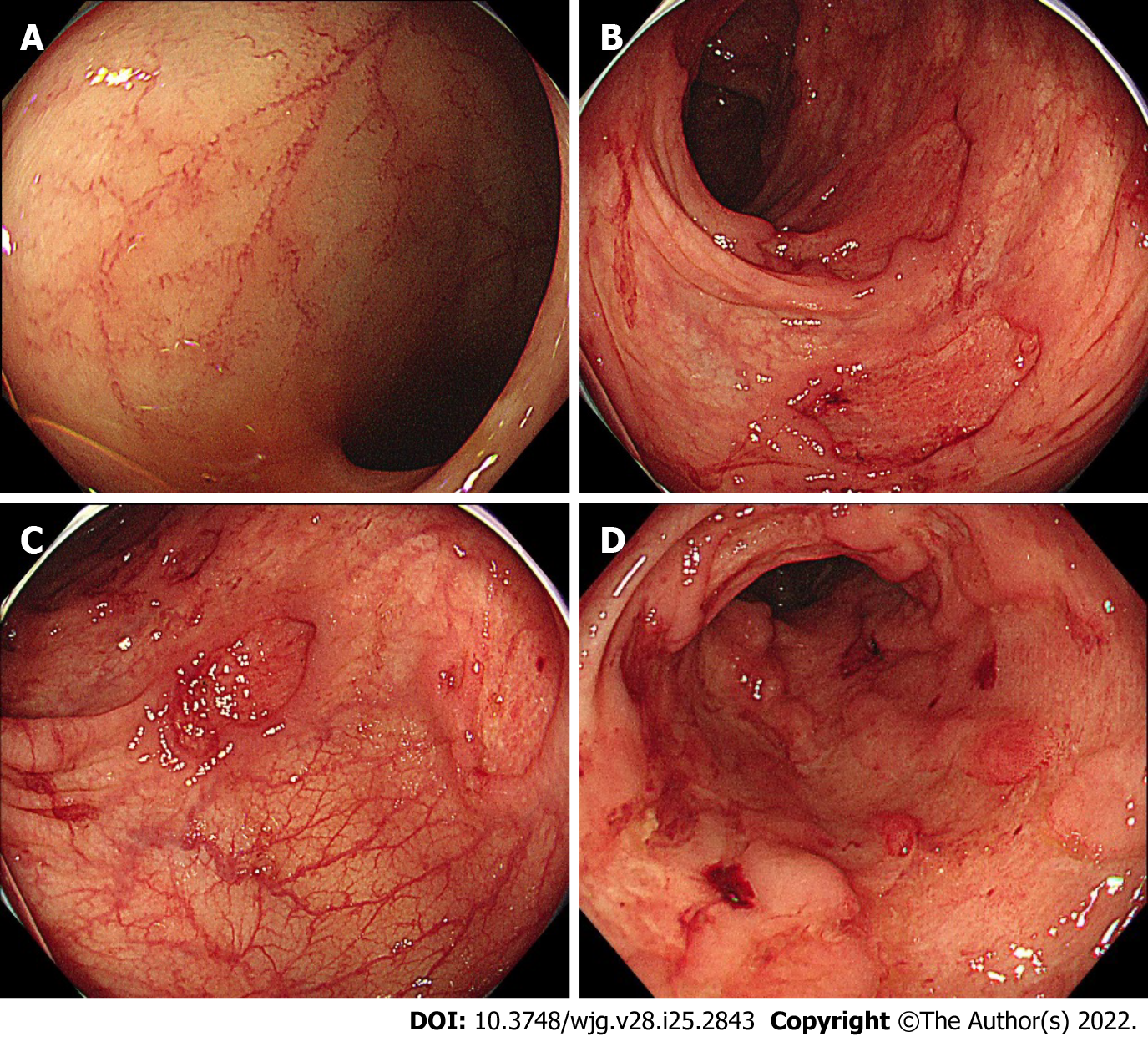
TAK is a type of chronic large-vessel vasculitis that primarily affects the aorta, and its major branches, as well as pulmonary arteries[63]. The prevalence of UC and CD among TAK patients was reported to be 6.4% (30/470) and 9.0% (4/44), respectively[64,65]. Endoscopic features of IBD in TAK patients were found to be atypical for UC or CD in a previous retrospective study[17]. This study, which included 142 Japanese TAK patients, identified 13 TAK-IBD patients (9.2%), and their endoscopic findings at the initial time of IBD diagnosis were assessed. As a result, 7/8 (87.5%) of TAK patients had “discontinuous aphthous erosions/ulcers or focal mucosal inflammation” with only one patient having continuous inflammation typical of UC[17], suggesting that TAK-IBD patients have a distinct endoscopic IBD phenotype. This study also discovered that patients with TAK-IBD were more likely to have the HLA haplotype, which has a susceptible effect on UC, when compared with TAK patients without IBD, implying that TAK-IBD may have a similar immune mechanism to UC. A recent case study describing the evolution of colonic inflammation in a TAK-IBD patient during 10-year observation found that colonic inflammation was initially discontinuous and more severe in the proximal colon than in the remaining colon[37], supporting the presence of a distinct IBD phenotype in TAK-IBD patients (Table 1).
Th1 and Th17 cells are predominant in the pathophysiology of TAK just as they are in the pathogenesis of CD[66] (Figure 1 and Table 1). TAK patients’ aorta expresses higher levels of IL-6, IL-12, IL-17, and interferon-γ[67], and a single nucleotide polymorphism encoding a common component of IL-12 and IL-23 is associated with the development of TAK[64]. In a previous study, ustekinumab was shown to reduce inflammatory markers and the dose of steroid needed in the treatment of TAK patients[68]. Many case studies have described TAK-IBD patients’ therapeutic approaches to date. Tofacitinib, a JAK inhibitor that was recently approved for UC[69], was found to be effective for both inflammatory conditions[70,71], implying that it can inhibit multiple inflammatory signals involved in TAK and IBD, suggesting that JAK pathways could be a promising therapeutic target. To better understand the prognosis of IBD in such patients with TAK, further studies are needed.
Figures 2 and 3 show representative endoscopic findings of PSC-IBD and TAK-IBD, respectively.
Recent research has revealed that IBD and other IMIDs share overlapping genetic and immunological etiologies. Indeed, IBD patients frequently develop concurrent IMIDs, and these patients have a higher risk of severe IBD phenotypes, implying that the presence of IMIDs is a poor prognostic factor for IBD. As a result, when treating IBD patients, we should be aware of the presence of other IMIDs, as biologics and small molecule therapies may be able to treat multiple disease states simultaneously. Multidisciplinary care with rheumatologists or dermatologists is also important when deciding the therapeutic strategy for IBD patients with concurrent IMIDs.
Furthermore, patients with IMIDs such as PSC or TAK can have distinct endoscopic phenotypes of IBD, suggesting that the immune mechanisms of these IMIDs may affect how intestinal inflammation is distributed. Even though IBD patients with concurrent IMIDs are more likely to require biologics or surgery, the disease course of PSC-IBD patients is not often associated with poor IBD outcomes, although these patients are more likely to develop pancolonic inflammation. Further research involving various concurrent IMIDs is needed in light of these findings to better understand how each IMID and its medication may affect the phenotype and natural history of IBD.
In the future, clinicians and researchers will face a challenge in identifying therapeutic targets to improve patient outcomes. Previous studies have shown that Th1 and Th17 cells (rather than Th2 cells) are more likely to be involved in the concurrence of other IMIDs in IBD patients, implying that these effector T cells and their cytokines could be promising therapeutic targets. Future research is needed to gain further insights into the shared immune mechanisms involved in the pathophysiology of IBD and other IMIDs to develop appropriate therapeutic targets to treat both diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aloi M, Italy; Bozkurt HS, Turkey; Knudsen T, Denmark A-Editor: Gao W, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 521] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 2. | Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 633] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 3. | Younis N, Zarif R, Mahfouz R. Inflammatory bowel disease: between genetics and microbiota. Mol Biol Rep. 2020;47:3053-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1883] [Article Influence: 134.5] [Reference Citation Analysis (2)] |
| 5. | Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1048] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 6. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2007] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 7. | Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 396] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 706] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 9. | Akiyama S, Sakuraba A. Distinct roles of interleukin-17 and T helper 17 cells among autoimmune diseases. J Transl Autoimmun. 2021;4:100104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1235] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 11. | Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, Hibi T. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 12. | Panda SK, Colonna M. Innate Lymphoid Cells in Mucosal Immunity. Front Immunol. 2019;10:861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 13. | Weng X, Liu L, Barcellos LF, Allison JE, Herrinton LJ. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organization. Am J Gastroenterol. 2007;102:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Conway G, Velonias G, Andrews E, Garber JJ, Yajnik V, Ananthakrishnan AN. The impact of co-existing immune-mediated diseases on phenotype and outcomes in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (3)] |
| 16. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Akiyama S, Fujii T, Matsuoka K, Yusuke E, Negi M, Takenaka K, Nagahori M, Ohtsuka K, Isobe M, Watanabe M. Endoscopic features and genetic background of inflammatory bowel disease complicated with Takayasu arteritis. J Gastroenterol Hepatol. 2017;32:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Attauabi M, Wewer MD, Bendtsen F, Seidelin JB, Burisch J. Inflammatory Bowel Diseases Affect the Phenotype and Disease Course of Coexisting Immune-Mediated Inflammatory Diseases: A Systematic Review With Meta-Analysis. Inflamm Bowel Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity. 2019;50:975-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 736] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 20. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1547] [Article Influence: 119.0] [Reference Citation Analysis (5)] |
| 21. | Mohammadi H, Sharafkandi N, Hemmatzadeh M, Azizi G, Karimi M, Jadidi-Niaragh F, Baradaran B, Babaloo Z. The role of innate lymphoid cells in health and disease. J Cell Physiol. 2018;233:4512-4529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 23. | Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182:840-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 24. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1804] [Article Influence: 225.5] [Reference Citation Analysis (111)] |
| 25. | Fu Y, Lee CH, Chi CC. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018;154:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 26. | Pugliese D, Daperno M, Fiorino G, Savarino E, Mosso E, Biancone L, Testa A, Sarpi L, Cappello M, Bodini G, Caprioli F, Festa S, Laino G, Maconi G, Mazzuoli S, Mocci G, Sartini A, D'Amore A, Alivernini S, Gremese E, Armuzzi A. Real-life effectiveness of ustekinumab in inflammatory bowel disease patients with concomitant psoriasis or psoriatic arthritis: An IG-IBD study. Dig Liver Dis. 2019;51:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Pugliese D, Guidi L, Ferraro PM, Marzo M, Felice C, Celleno L, Landi R, Andrisani G, Pizzolante F, De Vitis I, Papa A, Rapaccini GL, Armuzzi A. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharmacol Ther. 2015;42:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Akiyama S, Lin A, Traboulsi C, Rubin DT. Treatment of Crohn's Disease and Concomitant Alopecia Areata With Tofacitinib. ACG Case Rep J. 2021;8:e00690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3395] [Cited by in RCA: 3870] [Article Influence: 276.4] [Reference Citation Analysis (0)] |
| 30. | Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 695] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 31. | Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewé R. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:744-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 404] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 33. | Burisch J, Jess T, Egeberg A. Incidence of Immune-Mediated Inflammatory Diseases Among Patients With Inflammatory Bowel Diseases in Denmark. Clin Gastroenterol Hepatol. 2019;17:2704-2712.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Attauabi M, Zhao M, Bendtsen F, Burisch J. Systematic Review with Meta-analysis: The Impact of Co-occurring Immune-mediated Inflammatory Diseases on the Disease Course of Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2021;27:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Attauabi M, Zhao M, Bendtsen F, Burisch J. Systematic review and meta-analysis: the impact of co-occurring immune-mediated inflammatory diseases on the disease localization and behavior of Crohn's disease. Therap Adv Gastroenterol. 2021;14:17562848211004839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Wada A, Higashiyama M, Hirata D, Ito S, Tanemoto R, Nishii S, Mizoguchi A, Inaba K, Sugihara N, Hanawa Y, Horiuchi K, Akita Y, Narimatsu K, Komoto S, Tomita K, Hokari R. Changes in Colonic Inflammation Related with Takayasu Arteritis during a 10-year Observation Period. Intern Med. 2022;61:475-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Pinto-Sanchez MI, Seiler CL, Santesso N, Alaedini A, Semrad C, Lee AR, Bercik P, Lebwohl B, Leffler DA, Kelly CP, Moayyedi P, Green PH, Verdu EF. Association Between Inflammatory Bowel Diseases and Celiac Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;159:884-903.e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Bramuzzo M, Lionetti P, Miele E, Romano C, Arrigo S, Cardile S, Di Nardo G, Illiceto MT, Pastore M, Felici E, Fuoti M, Banzato C, Citrano M, Congia M, Norsa L, Pozzi E, Zuin G, Agrusti A, Bianconi M, Grieco C, Giudici F, Aloi M, Alvisi P. Phenotype and Natural History of Children With Coexistent Inflammatory Bowel Disease and Celiac Disease. Inflamm Bowel Dis. 2021;27:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Beheshti-Maal A, Tamimi A, Iravani S, Memarnejadian A, Sorouri M, Aghdaei HA, Zali MR, Hossein Khannazer N, Vosough M. PSC associated inflammatory bowel disease: a distinct entity. Expert Rev Gastroenterol Hepatol. 2022;16:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Barberio B, Massimi D, Cazzagon N, Zingone F, Ford AC, Savarino EV. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology. 2021;161:1865-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Krugliak Cleveland N, Rubin DT, Hart J, Weber CR, Meckel K, Tran AL, Aelvoet AS, Pan I, Gonsalves A, Gaetano JN, Williams KM, Wroblewski K, Jabri B, Pekow J. Patients With Ulcerative Colitis and Primary Sclerosing Cholangitis Frequently Have Subclinical Inflammation in the Proximal Colon. Clin Gastroenterol Hepatol. 2018;16:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Ricciuto A, Kamath BM, Griffiths AM. The IBD and PSC Phenotypes of PSC-IBD. Curr Gastroenterol Rep. 2018;20:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 45. | Moayyeri A, Daryani NE, Bahrami H, Haghpanah B, Nayyer-Habibi A, Sadatsafavi M. Clinical course of ulcerative colitis in patients with and without primary sclerosing cholangitis. J Gastroenterol Hepatol. 2005;20:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 48. | Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 49. | Lundqvist K, Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Ellinghaus D, Folseraas T, Holm K, Ellinghaus E, Melum E, Balschun T, Laerdahl JK, Shiryaev A, Gotthardt DN, Weismüller TJ, Schramm C, Wittig M, Bergquist A, Björnsson E, Marschall HU, Vatn M, Teufel A, Rust C, Gieger C, Wichmann HE, Runz H, Sterneck M, Rupp C, Braun F, Weersma RK, Wijmenga C, Ponsioen CY, Mathew CG, Rutgeerts P, Vermeire S, Schrumpf E, Hov JR, Manns MP, Boberg KM, Schreiber S, Franke A, Karlsen TH. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology. 2013;58:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1042] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 52. | Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 53. | Festen EA, Goyette P, Green T, Boucher G, Beauchamp C, Trynka G, Dubois PC, Lagacé C, Stokkers PC, Hommes DW, Barisani D, Palmieri O, Annese V, van Heel DA, Weersma RK, Daly MJ, Wijmenga C, Rioux JD. A meta-analysis of genome-wide association scans identifies IL18RAP, PTPN2, TAGAP, and PUS10 as shared risk loci for Crohn's disease and celiac disease. PLoS Genet. 2011;7:e1001283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 54. | Caminero A, Meisel M, Jabri B, Verdu EF. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol. 2019;16:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 55. | Harris KG, Chang EB. The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: new insights into complex disease. Clin Sci (Lond). 2018;132:2013-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Hisamatsu T, Erben U, Kühl AA. The Role of T-Cell Subsets in Chronic Inflammation in Celiac Disease and Inflammatory Bowel Disease Patients: More Common Mechanisms or More Differences? Inflamm Intest Dis. 2016;1:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Sollid LM. Intraepithelial lymphocytes in celiac disease: license to kill revealed. Immunity. 2004;21:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 618] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 59. | Fernández S, Molina IJ, Romero P, González R, Peña J, Sánchez F, Reynoso FR, Pérez-Navero JL, Estevez O, Ortega C, Santamaría M. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol. 2011;106:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Lahdenperä AI, Fälth-Magnusson K, Högberg L, Ludvigsson J, Vaarala O. Expression pattern of T-helper 17 cell signaling pathway and mucosal inflammation in celiac disease. Scand J Gastroenterol. 2014;49:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Monteleone I, Sarra M, Del Vecchio Blanco G, Paoluzi OA, Franzè E, Fina D, Fabrizi A, MacDonald TT, Pallone F, Monteleone G. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol. 2010;184:2211-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Yu X, Vargas J, Green PHR, Bhagat G. Innate Lymphoid Cells and Celiac Disease: Current Perspective. Cell Mol Gastroenterol Hepatol. 2021;11:803-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Alibaz-Oner F, Direskeneli H. Update on Takayasu's arteritis. Presse Med. 2015;44:e259-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Terao C, Matsumura T, Yoshifuji H, Kirino Y, Maejima Y, Nakaoka Y, Takahashi M, Amiya E, Tamura N, Nakajima T, Origuchi T, Horita T, Matsukura M, Kochi Y, Ogimoto A, Yamamoto M, Takahashi H, Nakayamada S, Saito K, Wada Y, Narita I, Kawaguchi Y, Yamanaka H, Ohmura K, Atsumi T, Tanemoto K, Miyata T, Kuwana M, Komuro I, Tabara Y, Ueda A, Isobe M, Mimori T, Matsuda F. Takayasu arteritis and ulcerative colitis: high rate of co-occurrence and genetic overlap. Arthritis Rheumatol. 2015;67:2226-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 65. | Reny JL, Paul JF, Lefèbvre C, Champion K, Emmerich J, Blétry O, Piette JC, Fiessinger JN. Association of Takayasu's arteritis and Crohn's disease. Results of a study on 44 Takayasu patients and review of the literature. Ann Med Interne (Paris). 2003;154:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 66. | Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, Terrier B, Geri G, Rosenzwajg M, Klatzmann D, Fourret P, Cluzel P, Chiche L, Gaudric J, Koskas F, Cacoub P. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. 2015;67:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Savioli B, Abdulahad WH, Brouwer E, Kallenberg CGM, de Souza AWS. Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev. 2017;16:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Gon Y, Yoshifuji H, Nakajima T, Murakami K, Nakashima R, Ohmura K, Mimori T, Terao C. Long-term outcomes of refractory Takayasu arteritis patients treated with biologics including ustekinumab. Mod Rheumatol. 2021;31:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1189] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 70. | Kuwabara S, Tanimura S, Matsumoto S, Nakamura H, Horita T. Successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis. Ann Rheum Dis. 2020;79:1125-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Sato S, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Furuya M, Gunji N, Fujiwara T, Asano T, Onizawa M, Kobayashi H, Watanabe H, Ohira H, Migita K. A case of Takayasu arteritis complicated by refractory ulcerative colitis successfully treated with tofacitinib. Rheumatology (Oxford). 2020;59:1773-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |