Published online Jul 7, 2022. doi: 10.3748/wjg.v28.i25.2782
Peer-review started: October 28, 2021
First decision: December 26, 2021
Revised: January 27, 2022
Accepted: May 26, 2022
Article in press: May 26, 2022
Published online: July 7, 2022
Processing time: 248 Days and 14.5 Hours
Dysregulated interactions between host inflammation and gut microbiota over the course of life increase the risk of colorectal cancer (CRC). While environmental factors and socio-economic realities of race remain predominant contributors to CRC disparities in African-Americans (AAs), this review focuses on the biological mediators of CRC disparity, namely the under-appreciated influence of inherited ancestral genetic regulation on mucosal innate immunity and its interaction with the microbiome. There remains a poor understanding of mechanisms linking immune-related genetic polymorphisms and microbiome diversity that could influence chronic inflammation and exacerbate CRC disparities in AAs. A better understanding of the relationship between host genetics, bacteria, and CRC pathogenesis will improve the prediction of cancer risk across race/ethnicity groups overall.
Core Tip: Studies largely examine either variations in microbiome composition or host immunity polymorphisms, often using genome-wide association studies comprised of populations mainly of European ancestry. There is, thus, a pressing need for studies that include, recruit, and account for more widely diverse cohorts. Identification of population-associated polymorphisms driving host/microbiome interactions linked to colorectal cancer (CRC) disparity may reveal genes or pathways that could be targeted for patient-specific CRC interception strategies.
- Citation: Ahmad S, Ashktorab H, Brim H, Housseau F. Inflammation, microbiome and colorectal cancer disparity in African-Americans: Are there bugs in the genetics? World J Gastroenterol 2022; 28(25): 2782-2801
- URL: https://www.wjgnet.com/1007-9327/full/v28/i25/2782.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i25.2782
Colorectal cancer (CRC) is the second leading cause of death amongst cancer patients, an estimated 53000 of whom will die in the United States from CRC in 2021[1]. Strikingly, although total CRC mortality has decreased over the last two decades, particularly in older individuals (age 64 +), CRC incidence has increased in individuals under 50[1]. Recent studies showed early-onset CRC patients were more likely to be African-Americans (AAs), who bear the highest CRC incidence rate between 20-year-old and 44-year-old (7.9/100000) as compared to Caucasian Americans (CAs) (6.7/100000) and Asian-Pacific Islanders (6.3/100000)[2]. AAs commonly display more aggressive types of CRC as well, and are generally diagnosed at more advanced stages of the disease, exhibiting survival rates 7% below those of CAs (58% vs 65% 5-year survival)[3]. Such statistics must be interpreted cautiously, since the noted increase of early-onset CRC may result from recently recommended and adopted early colonoscopy (40-45 years old) screening campaigns. Nevertheless, there are multiple proposed influences on CRC disparities in AAs, including differences in health care and treatment access, comorbidities and tumor characteristics[4-8]. Socioeconomic status (SES) also weighs heavily on the late diagnoses and prevention campaign efficacy observed in AA populations[2,6,9]. Undoubtedly the source of CRC disparity is multifactorial, and a layered perspective is imperative to address the alarming rise of early onset CRC, an otherwise preventable disease when detected early.
Herein, we aim to elucidate novel biological factors that may also contribute to the AA disparities in CRC mortality. Specifically, in addition to the genetic influence on CRC pathogenesis, an accumulating body of evidence connects CRC to dysregulated interactions between mucosal innate immunity and the microbiome[10]. Indeed, sustained inflammation promoted by chronic colorectal dysbiosis is an established driver of CRC pathogenesis[11]. Related to this notion is a recent gut microbiome profiling study that found that, in addition to diminished overall species diversity, pro-inflammatory Fusobacterium nucleatum (F. nucleatum) and Enterobacter species were significantly more abundant in AA CRC patients as compared to a CA cohort[12]. The presence of F. nucleatum has also been linked to inflammation-associated microsatellite alterations found more prevalently in AA rectal tumors, a finding linked to worsened CRC prognosis[13-15]. Furthermore, genetic landscape and microbiome composition have been shown to influence the occurrence of proximal colorectal tumors[16], which are more difficult to detect and are diagnosed nearly four times more often in AA than in CA CRC patients[17]. Nearly 80% of sessile serrated polyps are found in the proximal colon, a phenomenon associated with microbial biofilms and Fusobacteria, plus the frequency of BRAF mutations, CpG island hypermethylation phenotype, and microsatellite instability that increases from the distal to the proximal region[16,18-22]. Altogether, there is emerging research on the genetically tuned relationships between mucosal innate immunity, the microbiome, and disparate CRC development, but a functional understanding of how said relationships impact CRC pathology remains incomplete[23]. Additionally, despite the evidence that mucosal innate immunity and the microbiome are intimately connected, this review highlights how minority health research currently evaluates their contribution to CRC risk in a largely separate fashion[24].
Accordingly, we propose an integrated concept whereby a differential mucosal inflammatory response to gut microbiota, influenced by host genetic ancestry, represents an underappreciated factor affecting population susceptibility to CRC. In support of this concept, a recent study found that the most differentially expressed genes (DEGs) between AA and CA CRC tumors were related to the regulation of inflammatory immunity[25]. More broadly, transcriptional regulation of inflammation was deter
Given that SES and a variety of environmental factors associated with CRC pathogenesis and disparities are commonly discussed elsewhere[2,9], we are limiting the scope of the present review to the nascent literature associating CRC first to genetic polymorphisms related to innate immunity, second to those related to the microbiome, and finally explore how they may conjointly contribute to CRC disparities in the AA population. We also emphasize that, by considering admixture and genetic ancestry rather than self-reported race, population-specific risk studies including microbiome genome-wide association studies (GWAS) can more accurately capture human genetic diversity, thereby increasing the likelihood of identifying clinically relevant CRC risk factors associated with African ancestry[30-34]. Furthermore, polymorphisms related to innate immunity as well as the microbiome and contributing to links between CRC risk and African ancestry may have been left undiscovered by the longstanding genetic homogeneity of genomic research cohorts, a problem we discuss in our closing remark. Although technically challenging, expanding GWAS to more diverse “multi-ancestry” cohorts will reveal novel linkages between the microbiome, inflammation, and CRC risk that can build predictive polygenic risk scores adaptable to an equally diverse patient base[35,36]. Crucially, functional evaluation of CRC risk variants associated with African ancestry may offer insights into the trend of aggressive, earlier onset CRC in AA patients, paving the way towards personalized prevention and precision medicine.
To better appreciate how host genetics may impact CRC risk between populations of different ancestral origins by modulating innate immunity or the microbiome, we will first highlight how mucosal inflammation and the gut microbiome interact to affect CRC pathogenesis. The human gut contains up to 1013 bacteria that play critical roles in immune, metabolic, cardiovascular, and neurological development[37]. The composition and functions of this bacterial community (microbiota) and its associated genome (microbiome) are highly dynamic and influenced by both environmental factors and host genetic background to maintain immunological and metabolic functionality[38]. Meanwhile, a tightly regulated physical separation between the immune system and commensal bacteria is necessary to limit a chronic inflammatory response to the microbiota[39,40]. The integrity of the intestinal barrier and its epithelium are therefore essential elements of healthy host-microbiota mutualism[40]. To establish a “demilitarized zone” and keep microbes at bay, the epithelium uses different mechanisms including tight junctions between epithelial cells, protective mucus production, and the expression of a complex arsenal of innate receptors that trigger bactericidal mediator secretion[41]. Nevertheless, a permissible level of contact or bacterial penetrance is necessary to facilitate metabolic exchanges and immunity maturation for homeostatic equilibrium between dense microbial flora and the host[39,40].
In the case of a high-fat diet, the cumulative alteration of bacterial metabolites can disrupt this equilibrium, thereby promoting carcinogenic dysbiosis and mucosal inflammation[42]. Diet is, thus, a critical environmental factor when connecting inflammation and the microbiome to CRC risk, especially when considering CRC disparities in AAs compared to Native Africans[43-45]. However, CRC as impacted by the genetic origins of host inflammatory response remains understudied. Inflammatory bowel disease (IBD), a model of perturbed micro-immune crosstalk and a known influencing factor of CRC etiology, can be a useful departure point for this line of inquiry[46]. In fact, multiple GWAS have linked higher risk of IBD, CRC[47-50], and microbiotic dysbiosis to host genetic variations, but surprisingly little is known about how CRC disparities may be compounded by the genetic regulation of host inflammatory response to gut bacteria[51].
There are, however, documented relationships between the genetic regulation of innate inflammatory immunity, the microbiome, and colon carcinogenesis. For example, adenomatous polyposis coli (Apc)Min/+ mice knockout for toll-like receptor (TLR) 4 or its signaling adaptor partner myeloid differentiation primary response 88 demonstrated a decreased number of intestinal polyps[52]. Nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 6 and nucleotide binding oligomerization domain containing protein 2 (NOD2) knockout mice were shown to develop colon tumors following colitis, and fecal microbiota transplantation from these mice into wild type recipients triggered similar tumorigenesis, which interestingly attributed carcinogenic causality to the microbiota[53,54]. In the case of lipocaline-2 knockout mice, Alistipes spp. commensals thrived and drove proximal colon tumorigenesis[55]. The nature of host genetic events can therefore drive different microbiome shifts impacting CRC and its anatomical pathogenesis (i.e., distal vs proximal).
If inflammation is a mechanism connecting the microbiome and colorectal carcinogenesis, host genetic background, including immune-related single-nucleotide polymorphisms (irSNPs), could differentially regulate such associations based on ancestry. In humans, pattern recognition receptor polymorphisms are associated with both IBD and CRC risk[48]. GWAS have linked genetic loci to increased IBD risk and a variety of risk alleles affect immune response[56], including NOD2 or autophagy-related 16 like 1[57,58]. Such polymorphisms have been associated with microbial dysbiosis and an excessive inflammatory response[59,60]. Namely, Knights et al[59] found that among 474 individuals, NOD2 variants were associated with Enterobacteriaceae family enrichment, including Escherichia coli, a species notably enriched in IBD individuals. Also, Lavoie et al[60] described an increase of interleukin (IL)-17-producing CD4+ T (i.e., Th17) cells in the lamina propria of mice engineered to express the polymorphism T300A (rs2241880) in the Atg16 L1 gene. Although Th17-based colitis was associated with an increase of Bacteroides ovatus, T300A did not directly induce the increase of Bacteroides ovatus but rather induced the increase of IL23p19, an important cytokine for maintaining the Th17 lineage[61]. Th17 cells and their canonical cytokine IL-17 are critical pro-inflammatory contributors to epithelial homeostasis and mucosal immunity by orchestrating anti-bacterial defense and epithelial repair and regeneration as well as regulating barrier permeability by controlling the expression of occludin proteins[62,63]. When dysregulated in a chronic setting, sustained IL-17 production may promote colon tumorigenesis[64,65]. Several studies have now identified IL-23/Th17 pathway-associated polymorphisms linked to IBD susceptibility and the gut microbiome profile[66-68]. Presumably, genetic regulation of Th17-driven inflammation may impact IBD and ensuing CRC risk via the extent or nature of colonic dysbiosis. In sum, these GWAS suggest that by influencing the extent or nature of colonic dysbiosis, genetic regulation of inflammation represents a risk factors for both IBD and CRC. Next, we review how genetic ancestry contributes to this phenomenon, and how it may exacerbate CRC disparity in AAs.
The flow of genetic information across time and geography may contribute to current disparities in cancer incidence and progression[69]. Cancer is known to result from an accumulation of somatic genetic and epigenetic alterations that dysregulate the cell cycle but also depends on genetic background and polymorphisms that impact patient risk and predisposition[70,71]. Yet, few GWAS have implicated ancestral genetic variants in cancer predisposition amongst self-identified racial/ethnic groups[72,73]. Many such polymorphisms regulate gene expression via epigenetic or post-translational modification mechanisms, which affect noncoding sequences like microRNA (miRNA) binding sites[74,75]. Yet, the biological and clinical significance of most polymorphisms associated with cancer disparities remains unknown[72]. Mechanistic associations between chronic inflammation and carcinogenesis could link ancestral genetic diversity to cancer, whereby immune-related genetic regulatory variants have the potential to differentially modulate CRC across populations[27].
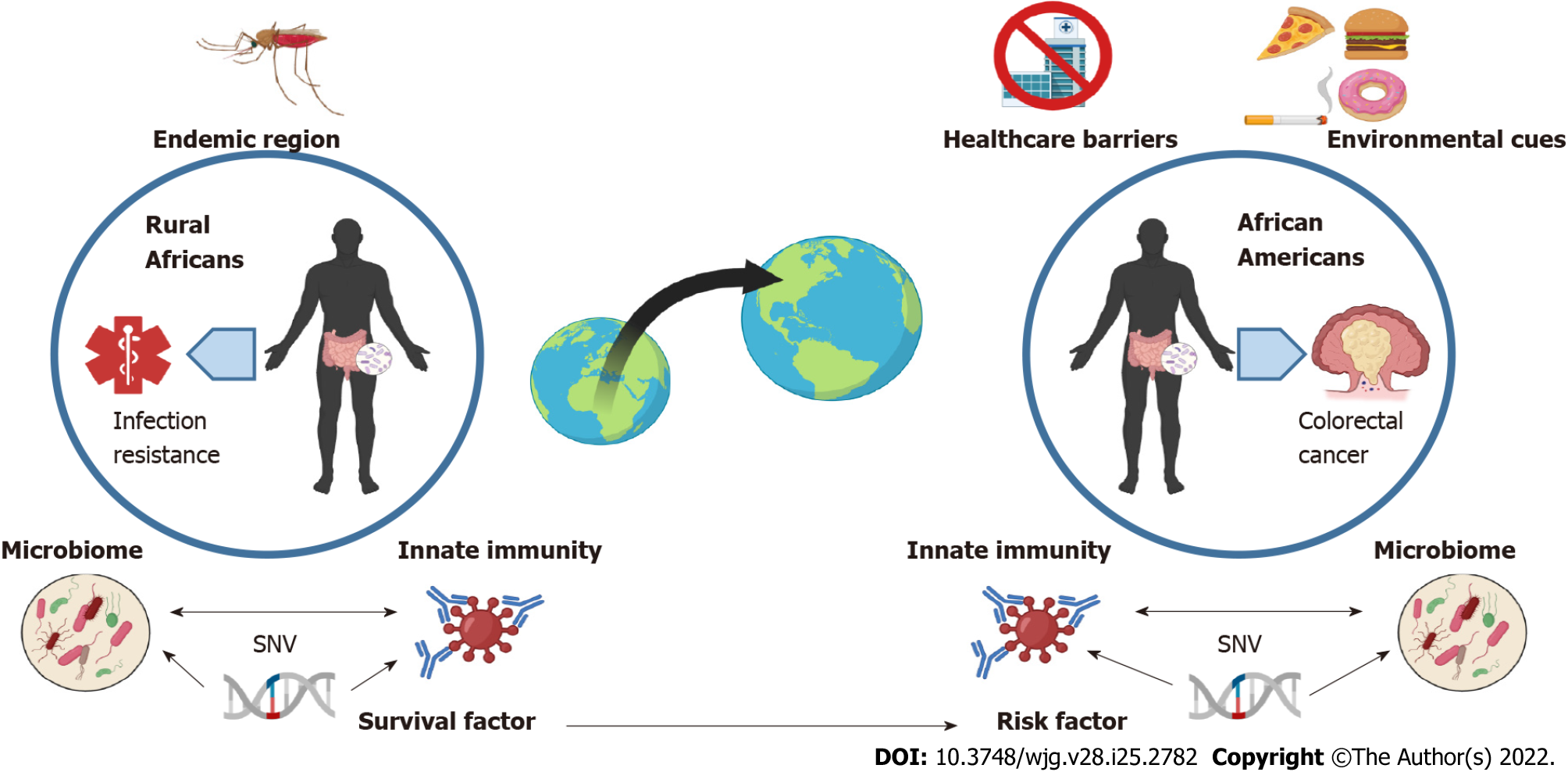
In fact, a large fraction of population-associated polymorphisms impact gene expression related to inflammation and innate immunity, which, being likely essential for surviving life-threatening infections, evolved under stronger selection pressures than other traits[76,77]. For instance, human genome diversification via archaic human genome introgression (i.e., admixture with Neanderthal genome) is a proposed adaptation of ancestral humans to infectious environments following “out of Africa” migration[77,78]. In non-African populations, Neanderthal-introgressed haplotypes reintroduced a splice variant (rs10774671) of the 2’-5’-oligoadenylate synthetase (OAS) 1 gene[79]. The OAS locus on chromosome 12 encodes three genes, OAS1, OAS2 and OAS3 that play an important role in virus defense. The elevated frequency of the Neanderthal-derived allele at the OAS locus was proposed to be the result of a positive selection in European and East Asian populations. This allele selection has a functional significance since it is associated with the production of a protein variant (OAS1 p46) characterized by higher enzymatic activity and improved resistance to West Nile virus and hepatitis virus C[80]. Therefore, the allele haplotype may provide a survival advantage to infectious agents in the non-African environment and represents an example of variety in baseline inflammation levels that may influence susceptibility to diseases like IBD and CRC in patients with insignificant African ancestry. Selection of genetic variants providing health and survival benefit in endemic areas may represent another means of adaptation and human genome diversification. In a cohort of 158 healthy individuals (distributed as European, Sub-Saharan African, and East-Asian), Barreiro et al[81] found that nucleotide diversity of the TLR family was shown to vary between African populations, suggesting pathogen-specific selection pressures. Specifically, the TLR10/TLR1/TLR6 locus showed signs of recent positive selection amongst non-African populations. Furthermore, of all SNPs in this region, a high frequency TLR1 single nucleotide variant (SNV) (non-synonymous T1805G variant) found in Europeans was the most significant population differentiator and was associated with a decrease in agonist-mediated nuclear factor-kappa B activation[81]. Although it is unclear if decreased TLR1-mediated immune response confers a selective advantage, it could potentially modulate otherwise harmful inflammatory responses to pathogens[82,83]. This finding suggested that a finely tuned balance between optimal defenses to pathogens and excessive inflammation may have been critical for evolutionary survival[78].
To investigate how ancestral immunity would impact pathogen response, Nédélec et al[27] studied interactions between macrophages and live bacteria (Listeria and Salmonella). Amongst the macrophages, they found that many of the DEGs; (30% of the 11914 genes analyzed) between AAs (n = 77) and CAs (n = 91) were involved in the regulation of the innate immunity. These results built off their previous findings that 9% of macrophage DEGs varied according to ancestry-associated regulation and that increased African ancestry could predict a stronger inflammatory response to infection[27]. Performing quantitative trait locus (QTL) analysis, the authors identified SNVs in 14% of DEGs or using alternative splicing between CA and AA individual-derived macrophages (either non-infected or infected with Listeria or Salmonella). A large fraction of DEGs were associated with expression QTL only in infected macrophages. In other words, SNVs in a significant fraction of inflammation-related genes were expressed in infected macrophages according to the level of African ancestry. Interestingly, the same authors also found that these DEGs included susceptibility genes previously reported by GWAS for rheumatoid arthritis, systemic sclerosis, or ulcerative colitis, all related to chronic inflammation and conditions with known AA disparity[78]. The interest of such a study, although performed on macrophages in vitro, is its illustration of the link between African genetic ancestry and inflammatory response to bacteria, one that could accelerate CRC by aggravating interactions between gut microbiota and the mucosal immune system. Reinforcing this concept is another GWAS that revealed that some of the most differentiating irSNPs between African and European populations were associated with genes regulating nuclear factor-kappa B or chemokine gene clusters[78]. Selected genetic variants may offer protection against infection in endemic regions for native/rural Africans but favor cancer development in descendants bearing the same variants in a western environment, a concept exemplifying the crossroad between host genetics and environmental factors that shapes cancer risk (Figure 1).
Regarding the possibility of a role of associations between irSNP and CRC risk into AA disparity, a recent study by Sanabria-Salas et al[33] studied links between pro-inflammatory IL1B haplotypes and CRC risk in patients from six Colombian cities. The authors associated the IL1B CGTC haplotype with CRC risk exclusively in patients from the coastal regions of Colombia who possessed the highest proportion of admixed African ancestry[33]. The same group has associated IL1B irSNPs (four SNPs -3737C/-1464G/-511T/-31C) with African ancestry and elevated cancer risk. The CGTC haplotype was most frequently found and highly expressed in AAs, establishing a functional link between IL1B irSNP and CRC risk[84]. Further studies, validating the connection between AA CRC patients and IL1B polymorphisms, will be required to confirm the IL1B SNP haplotype as a population-associated CRC risk marker; these findings nevertheless showcase a prime example of an exploitable connection between ancestry-related inflammation and cancer risk disparity.
Next, a cancer genomic meta-analysis using 48 GWAS within the National Cancer Institute GAME-ON Network (64591 cancer and 74467 control patients) across five common cancer sites (ovarian, lung, breast, colorectal, and prostate) found that genetic variants associated with inflammation and innate immune response were relevant to CRC risk, including SH2B adapter protein 3 (SH2B3) (rs3184504, P = 3.32 × 10-5), a negative regulator of growth factors and cytokine-induced signaling (Table 1). Unfortunately, irSNP associations with race/ethnicity and geographic distributions were not evaluated[85]. In contrast, Wang et al[47] demonstrated the merit of accounting for population diversity in their analysis of associations between innate immunity pathways and CRC risk. In two large studies (discovery and validation cohorts) across five distinct ethnic groups (AA, CA, Japanese-American, Latino, and Native Hawaiian), they found that among more than 600 common variants associated with 37 innate immunity-related genes, a SNV in the second intron of peroxisome proliferator-activated receptor gamma
| Ref. | Size | Analysis | Gene | SNP | Function | Ethnicity | Comment |
| Montazeri et al[89], 2020 | 6149 CRC 7337 controls | Meta-analysis | COLCA1/21 | rs3802842 (11q23.1) | Immune infiltration of LP | Europeans | Confirmed by Lu et al[49], 20191 |
| TGFB1, SMAD7,SMAD7 | rs1800469, rs12953717, rs4464148 | TGFB signaling inhibitor | |||||
| Law et al[50], 2019 | 34627 CRC 71379 controls | Meta-analysis | HLA-C | rs3131043 (6p21.33) | Adaptive immunity | Europeans | |
| HLA-DRB1/DQA1 | rs9271770 (6q21.33) | Adaptive immunity | |||||
| COLCA1/2 | rs3087967 (11q23.1) | Immune LP leukocytes | |||||
| FUT2 | rs12979278 (19q13.33) | Gut barrier | |||||
| Lu et al[49], 2019 | GWAS | NOD2 | rs2066847 | Innate immunity | East Asians | Confirmed by Montazeri et al[89], 20202 | |
| GATA3 | rs10795668 (10p14) | T cell transcription factor | |||||
| SMAD7 | rs7229639 | TGFB signaling inhibitor | |||||
| SMAD7 | rs4939827 | TGFB signaling inhibitor | |||||
| COLCA1/22 | rs3802842 (11q23.1) | Immune infiltration of LP | |||||
| Sanabria-Salas et al[33], 2017 | 391 CRC | GWAS | IL1B | CGTC haplotype (2q14) | Inflammation | Columbian Africans | Association with AA admixture |
| Hung et al[85], 2015 | 15414 CRC 17688 controls | GWAS | SH2B3 | rs3184504 (12q24) | Cytokine signaling | Europeans | Confirmed by Schumacher et al[135], 2015 |
| Schumacher et al[135], 2015 | 18299 CRC, 19656 controls (Europeans), 2098 cases, 6172 controls (Asian 1), 2627 cases, 3797 controls (Asian 2) | Meta-analysis | SH2B33 | rs3184504 (12q24.12) | Cytokine signaling | Europeans/Asians | Confirmed by Hung et al[85], 20153 |
| NOS1 | rs73208120 (12q24.22) | ROS production | |||||
| Wang et al[47], 2013 | 2535 CRC, 3915 controls (discovery), 2153 CRC, 2630 controls (validation) | GWAS | PPARG | rs9858822 | Monocyte activation | Multi-ethnic | High frequency in AA |
| Tsilidis et al[136], 2009 | CLUE II cohort, 208 CRC, 381 controls | GWAS | IL10 | rs1800896, rs1800890, rs3024496, rs3024498 | Increased IL-10 | ND |
Lastly, functional variants in the 3’-untranslated region (UTR) of inflammatory gene miRNA binding sites (miRSNPs) have been associated with CRC risk[75]. Four miRSNPs in the mannose binding lectin 2 (MBL2) gene 3’-UTR have been associated with increased CRC risk in the AA population. MBL2 codes for mannose binding lactose protein, a pattern recognition receptor that binds a wide range of pathogen-expressed sugars, leading to their phagocytosis. Although not assessed in this study, the modulation of the interactions between the mucosal inflammation and the microbiome, via MBL2 expression, could be a mechanistic link between African ancestry and higher CRC risk[74]. irSNPs associated with CRC risk are summarized in Table 1.
Ultimately, linking chronic inflammation risk loci to positive selection via resistance to past infectious agents and population displacement should be done with caution, as the merits are still debated[78]. Physiological interfacing of the immune system with other biological systems (reproduction and organ development) may also explain positive selection in a manner distinct from past pathogen resistance[78]. Yet, considering the evidence of host innate immunity regulation by population-enriched irSNPS[27], it is reasonable to speculate that the mucosal inflammation associated with commensalism is differentially tuned according to the level of African ancestry and could therefore influence CRC disparities. This view is supported by disparity research in other cancers, such as the finding that IL10 promoter SNPs enriched in AAs are also potential risk factors for prostate cancer development and progression[90]. Interestingly, one such IL10 polymorphism (rs1800871) was associated with Proteobacteria load in the gut microbiome (Table 2), but a connection between rs1800871, proteobacteria, and prostate cancer remains to be established[90]. In breast cancer, Jenkins et al[91] demonstrated how the ancestral selection of immune variants in the African continent can predispose AA women to ancestry-related differences in tumor immunogenicity. Specifically, the status of a “Duffy-null” polymorphism-regulated atypical chemokine receptor 1 (ACKR1) allele linked West African genetic ancestry to tumor immune infiltration. Thus, for breast cancer, duffy antigen receptor for chemokines/ACKR1 polymorphism may serve as a biomarker for precision medicine and immunotherapy in patients bearing significant West African ancestry[91]. This result highlights the potential that ancestry-associated irSNPS have for cancer screening and clinical care when paired with functional analyses and elevating the importance of similar studies for CRC.
| Ref. | Size | Analysis | Gene | SNP | Function | Bacteria | Ethnicity | Comment |
| Knights et al[59], 2014 | 474 IBD | 16S RNA | NOD2 | rs5743293, rs104895431, rs104895467, rs2068844, rs2068845, rs5743277, rs5743293 | Innate immunity | Enterobacteriaceae | Europeans | IBD |
| Blekhman et al[122], 2015 | 93 (HMP) | Metagenomic | LCT | rs2304371, rs3754689 | Metabolism | Bifidobacterium, SMB53 (Clostridiaceae) | Multi-ethnic | NS |
| GNA12 | rs1182182 | IBD | ||||||
| Goodrich et al[123], 2016 | 1126 | Fecal 16S rRNA | CD36 | rs1360741 | Immune-related | Blautia | Europeans | United Kingdom twins |
| Li et al[137], 2016 | 10523 IBD, 5726 IBD | Mucosal 16S rRNA | SLC39A8 | rs13107325 | Immune-related | Composition | ND | IBD |
| Bonder et al[68], 2016 | 1514 | Metagenomic | C11orf30-LRRC32 | rs2155219 | IBD | Coprococcus comes/Proteobacteria | AA1 PCa risk | |
| CCL2 | rs3091315, rs3091316 | Immune-related | Methonobacteria | |||||
| DAP2 | rs267939 | Innate immunity | Bifidobacterium | |||||
| IL23R | rs12141575 | Immune-related | Enterobacteriaceae/E. coli | |||||
| IL10 | rs18008711 | Immune-related | Proteobacteria/Sutterella | |||||
| MUC22 | rs3873352 | Barrier defense | ||||||
| NOD1 | rs12669082 | Innate immunity | Enterobacteriaceae/E.coli | |||||
| rs41524946 | Innate immunity | Enteroba cteriaceae/E. coli | ||||||
| rs55689059 | Innate immunity | Enterobacteriaceae/E. coli | ||||||
| rs55841603 | Innate immunity | Enterobacteriaceae/E. coli | ||||||
| NOD2 | rs8056611, rs2357792, | Innate immunity | Enterobacteriaceae/E. coli | |||||
| CD209 | rs1010046 | Innate immunity | Bacteroidetes | |||||
| Rühlemann et al[138], 2021 | 8956 | 16S rRNA/GWAS | FUT2 | rs602662 | Barrier defense | Bacteroides OTU97_27 | Europeans | |
| BLVRA | rs623108 | Innate immunity | Barnesiella spp. OTU99_55 |
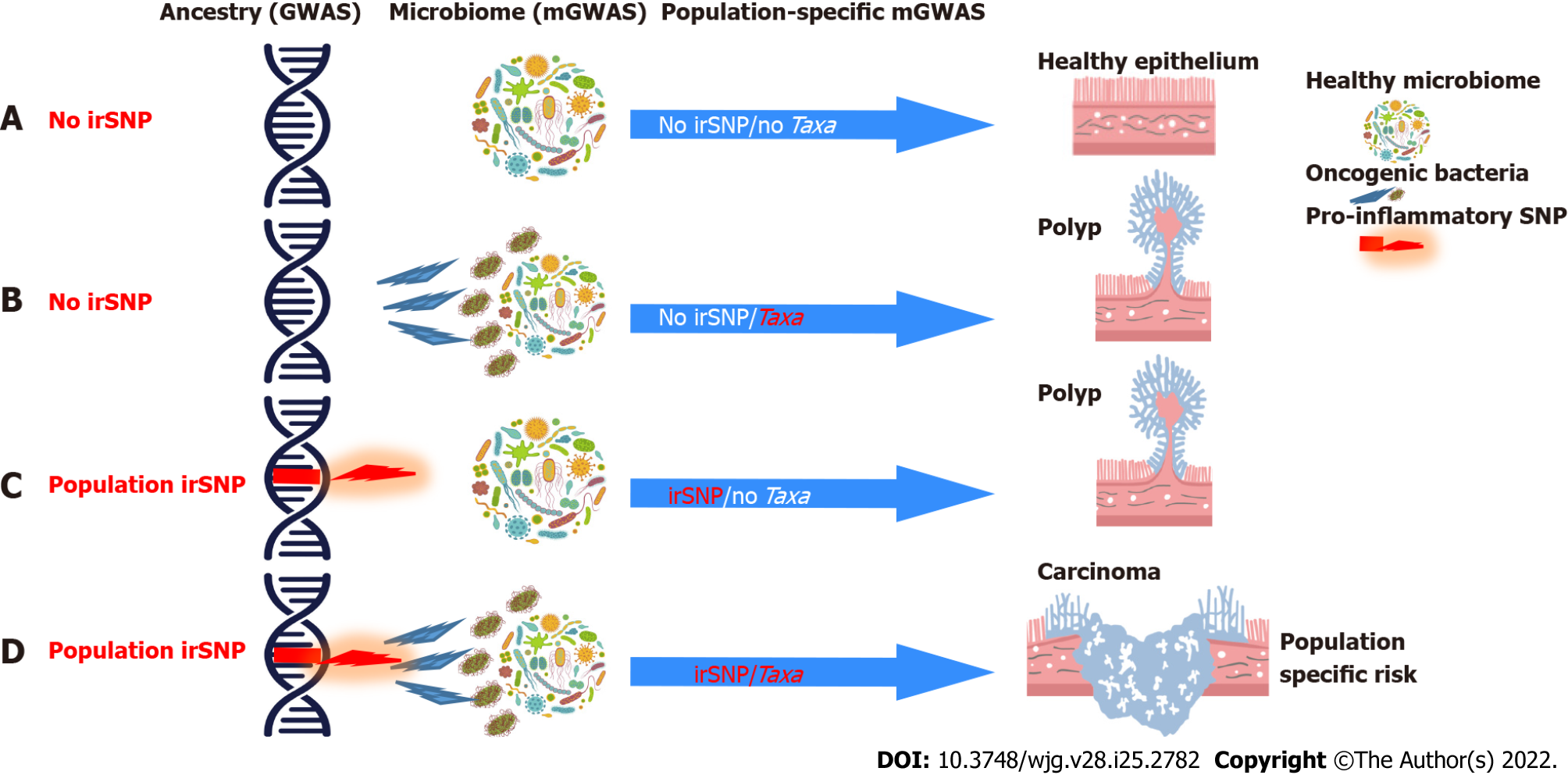
While host genetics may impact mucosal inflammation and CRC risk, other factors, including environmental factors such as diet, lifestyle, and antibiotic exposure, undoubtedly influence CRC susceptibility and treatment response by shaping gut microbiome composition[51,92,93]. Notwithstanding such findings, we propose that the predominant reliance of the microbiome on environmental cues in healthy individuals may conceal host genetic contributions (including genetic ancestry and somatic mutations) in disease contexts (e.g., CRC), driving the microbiome response to environmental fluctuations and defining, at least in part, differential susceptibility to cancer in AAs[94-96]. The numerous immune-related genetic variants that delineate chronic disease susceptibility between AAs and CAs (previously discussed) may then contribute to differential inflammatory responses to the microbiome dysbiosis and compound existing CRC disparities (Figure 2).
From this perspective, multiple metagenomic studies of fecal and mucosal samples have already found compositional and metabolomic differences between CRC and healthy patient microbiome[97-100]. Novel meta-analysis approaches combined these metagenome shotgun datasets across heterogeneous populations to explore relationships between the microbiome and CRC; associations were identified at both bacterial strain and gene levels[101-103]. A common core of 29 bacterial species was enriched in CRC cases, and choline metabolism was established as a reproducible biomarker of the CRC-associated microbiome[101,102]. While such findings are correlative and do not suggest causative links, several smaller studies have highlighted the role of specific bacteria[104-106] and microbial dysbiosis in triggering colorectal carcinogenesis in animal models[107]. However, microbiome GWAS (mGWAS) have not yet identified consistent associations between such carcinogenic bacteria and CRC risk[108]. Unfortunately, because population diversity was systematically underrepresented or not annotated in the metagenome datasets, it remains unknown how these results could translate into CRC risk factors or diagnostic biomarkers for specific racial/ethnic groups.
Meanwhile, several taxonomic and metagenomic studies have revealed an intriguing diversity in microbiome composition across racial/ethnic groups, but mechanistic understandings of associations between bacteria or groups of bacteria and race/ethnicity are sparse[34,109-113]. In particular, diversity of the microbiome is highly diet-driven. A study by O’Keefe et al[44] showed that a 2-wk food swap between AAs (received high fiber, low fat diet) and rural Africans (received low fiber, high fat diet) produced dramatic changes in mucosal biomarkers and a metabolome switch, illustrated by an increase in saccharolytic fermentation and anti-inflammatory butyrogenesis as well as suppression of secondary bile acid synthesis in AAs. In light of these results, it is critical that mGWAS take diet into consideration as a confounding factor, as its impact will inevitably interfere with the genetic/epigenetic influence on CRC risk[114]. Interestingly though, differences between CAs and AAs with respect to the mean alternate Healthy Eating Index (a measure of diet quality[115]) faded when adjusting for SES, implying that diet cannot entirely account for CRC disparities[116]. Multiple other factors besides diet are known to impact the composition and function of the microbiome, including smoking, alcohol consumption, as well as antibiotic exposure or metabolic condition such as diabetes or obesity, which are coincidentally also risk factors for CRC[117-119]. While these aspects of the microbiome biology have been extensively reviewed[120], we are paying much of our attention herein on the role of the host genetics and the ancestral genetic origin on the microbiome diversity and consequently CRC risk between ethnicities.
Interestingly, using the Healthy Life in an Urban Setting cohort and fecal 16S ribosomal RNA gene sequencing of over 2000 individuals, Deschasaux et al[110] found that microbiome diversity between racial/ethnic groups living in the same city was independent of metabolic health and only partially explained by SES, lifestyle, and diet factors. Yet, this finding was not always reproduced in other studies[45,111,121]. Overall, however, there is sufficient evidence to justify additional efforts to clarify if host genetics and population origins are taking part in shaping the microbiome[51,94]. The role of host genetic background has been suggested by mGWAS, which showed that SNPs such as rs4988235, associated with lactase persistence gene, contributed to microbiome composition[45,68,122,123]. A study performed with 416 twin pairs in the United Kingdom identified 26 “heritable” taxa, also suggesting that specific host genetic variants may participate in microbiome composition (Table 2)[124]. Notably, host genetic variants associated with microbiome composition were found to be enriched in immunity-related pathways[122]. Studies showing associations between metabolic and immune-related polymorphisms and microbiome composition are summarized in Table 2.
Finally, associations between the microbiome and somatic mutations in CRC have been described by Burns et al[95], who suggested that genetic determinants of the host and colon tumor mutations alter microbiome structure. The CRC mutanome could therefore help predict the composition and function of CRC-associated microbiomes and the clinical outcome or the response to therapies. To this end, of intrigue is the recent description by Ashktorab et al[125] and Brim et al[126] of genetic variation in tumor suppressor genes (APC), DNA mismatch repair genes, and other driver mutations (KRAS and PIK3C) in AAs with CRC, some of which were novel and not previously described in other populations. Loss of function mutations in APC were correlated with changes in 25 different microbial taxa, including an abundance of Finegolia or Christensenellaceae. Mutations in the zinc finger protein 717-coding gene were associated with an abundance of Akkermansia and Verrucomicrobiaceae, both colitis-associated species. Additionally, the same authors have isolated a novel Streptococcus spp. VT_162 from colon adenoma and CRC lesions in AAs[126]. Fecal Streptococcus spp. VT_162 was also confirmed in an advanced adenoma and CRC Chinese/Hong-Kong cohort[126]. An assessment of this bacterium’s relative prevalence in CAs compared to AAs will be necessary. Whether CA genetic background is more restrictive, while AA genetic background is more permissive to this species is not yet established. It may be postulated that, as seen in IBD patients, host genetic background drives the nature of the microbiome and alters the CRC risk posed by procarcinogenic “driver” bacteria[48,51].
Although there is a lack of direct causative links between such bacteria and colon carcinogenesis or growth promotion, this evidence at least signals that CRC disparities in AAs may be related to differential host inflammatory responses to similar bacterial communities (inflammation-driven disparities) and/or the contribution of ancestry-related factors in shaping microbiome diversity (bacteria-driven disparities). The molecular pathological epidemiology (MPE) that aims at uncovering an interactive relationship between environmental features and disease subtypes to understand disease incidence and mortality will provide etiologic and pathogenic insights in CRC disparities[127,128]. However, discoveries made in healthy donors often remain inconsistent from one study to another, perhaps due to multiple confounding environmental factors between heterogeneous cohorts[45,129]. More likely culprits for the inconsistency seen in GWAS that seek to identify the impact of host genetics on microbiome diversity are the homogeneity in sampled cohorts and differences in experimental approaches including stool vs mucosa sampling, sequencing approaches, and annotations. Future GWAS must address such issues in order to identify associations between genetic background and the microbiome that can reliably be applied to the question of disparities.
CRC disparity is far from an exclusively biological phenomenon but rather involves a complex interplay of SES, environmental and genetic components that collectively impact CRC risk and prognostics. Although there is a growing understanding of this complexity, studies examining the influence on CRC pathogenesis from ancestry-specific interactions between host genetics and the commensal microbiome are lacking. Such work is nevertheless urgently needed to appropriately mitigate CRC on a population basis and especially to help address alarming new trends, such as early-onset CRC amongst AAs. To facilitate this line of investigation, we reviewed irSNPs identified in CRC mGWAS and proposed that some of such variants (and others yet to be discovered) may alter microbiome composition and/or differential inflammatory responses to bacteria, thereby impacting CRC risk in a manner associated with genetic ancestry. However, testing the functional significance of such variants will require systematic studies that can incorporate the microbiome, mucosal immunity, and host genetics. A recent investigation by DeStefano Shields et al[130], although not related to cancer disparity, offers a potential experimental blueprint. Researchers introduced the BRAFV600E mutation to a MinApcΔ716/+ murine model of distal colon polyposis, then colonized BRAF mutant and MinApcΔ716/+ mice with Enterotoxigenic Bacteroides fragilis. Distal colon tumorigenesis was observed in MinApcΔ716/+ mice following colonization, whereas BRAFV600E MinApcΔ716/+ mice developed proximal colon tumors associated with immune signature and microbiome alterations plus sensitivity to anti-programmed death ligand 1[130]. These results suggested that host gene/bacteria interactions may drive CRC risk and pathogenesis, and demonstrate how such interactions can be disentangled mechanistically using experimental models with clinical implications. In the nascent field of precision medicine and multidisciplinary big data integration, the rapidly evolving MPE represents a successful model of integration of pathology, genomics, microbiome, immunology, epidemiology, and social science[127,128]. MPE will considerably improve precision medicine and prevention allowing, among other things, identifying SNPs that may impact microbiome and inflammation and serve as predictive markers. Genetic ancestry, however, remains to be integrated to this model to precisely address CRC disparities at the molecular level. Elucidating the mechanisms by which ancestry-associated variants impact CRC pathogenesis will be much more challenging, but models such as the one described by Lavoie et al[60], who used mice engineered to express the polymorphism T300A (rs2241880) in the Atg16 L1 gene known to increase IBD risk in humans, represent a promising approach to identifying mechanisms that can lead to personalized interventions applicable to minorities.
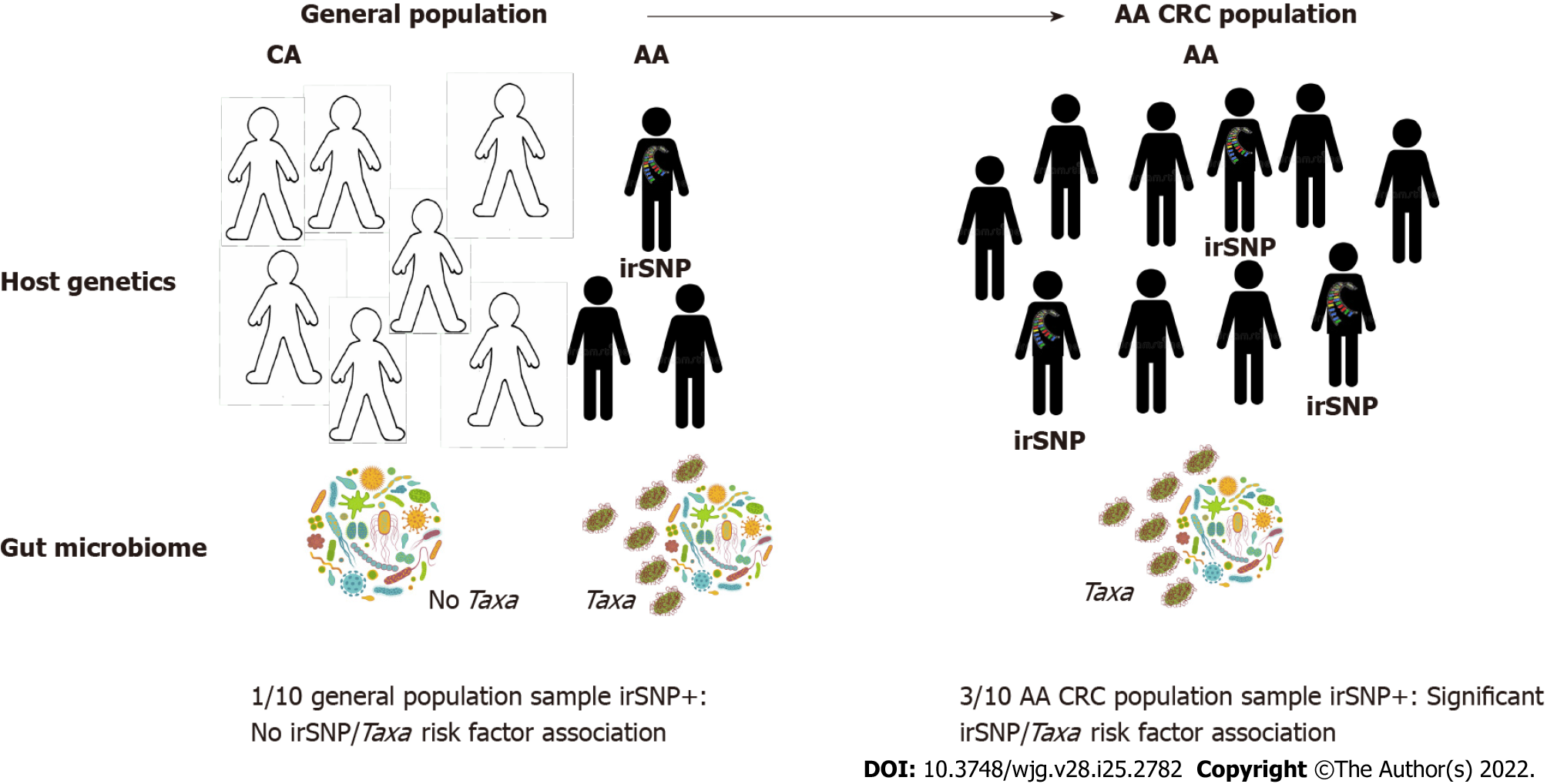
Yet, without addressing biases in genomics science, the ability of GWAS to detect variants of significance for AAs (or other minorities in general) will remain stunted[131]. To date, cancer GWAS have examined cohorts predominantly composed of Caucasian individuals, an homogeneity that limits the appreciation of how genetic ancestry impacts cancer risk[101,102]. Critically, the lack of diverse representation in sampling cohorts results in the increased likelihood that, even if identified, cancer-associated variants may be of limited clinical significance for non-Caucasian populations. While commenting on this issue, Davis[131] recently argued that the current state of genomic medicine is inadequately equipped to confront current oncological trends of disparate incidence and mortality in inclusive fashion and advocated for a persistent push towards the prioritization of patient diversity. Such an agenda is not only more harmonious with the principles of ethical human subjects research but is also scientifically meritable, as studies that account for diversity have already revealed novel genomic data that may improve our understanding of cancer etiology[132]. Therefore, for powered GWAS to detect and discern associations between population-enriched irSNPs, the microbiome, and CRC, proper accounting of population diversity and sufficient cohort size (estimated at > 4000 individuals)[45], or even population-specific CRC studies will be essential (illustrated in Figure 3). Encouragingly, recent methodological frameworks for multi-ancestry cohort GWAS have already yielded ancestry-related cancer variant risk factors[133,134]. Moreover, the recent initiative of the National Cancer Institute: Genetic Association and Mechanism in Oncology (GAME-ON; https://epi.grants.cancer.gov), which regrouped genomic data from more than 33 GWAS across five different cancers (CRC, lung, breast, ovary, and oral), is an example of the benefit of data sharing that will help identifying through meta-analysis data cancer risk loci in understudied populations, especially since 40% of the samples are from African, Asian, and Hispanic backgrounds. However, our integrated concept for CRC genomic research proposes that to represent accurately and capture the contribution of irSNP/microbiome interactions to CRC disparities, such diversified host genomic data must be paired with microbiome data.
In sum, population-related irSNP that regulate mucosal inflammation may modify the microbiota and its interaction with colon epithelium. Alternatively, a population-related SNP not involved in immune regulation may also alter the microbiome and trigger procarcinogenic chronic inflammation. Finally, a combination of two SNPs impacting inflammation and the microbiome with minor effects on CRC pathogenesis may trigger a strong procarcinogenic bacteria/inflammation interaction when combined in an at-risk population (Figure 2). By hunting for and characterizing such genetic factors using the emerging genetic admixture and ancestry paradigms, we believe scientists and clinicians will have a precision tool that enables a clearer understanding of the association between CRC with AA populations and the increasing trend of early onset CRC, ultimately to better mitigate CRC outcome disparities.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dabravolski SA, Belarus; Ogino S, United States A-Editor: Gao W, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (2)] |
| 2. | Carethers JM, Doubeni CA. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology. 2020;158:354-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Carethers JM. Clinical and Genetic Factors to Inform Reducing Colorectal Cancer Disparitites in African Americans. Front Oncol. 2018;8:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Polite BN, Sing A, Sargent DJ, Grothey A, Berlin J, Kozloff M, Feng S. Exploring racial differences in outcome and treatment for metastatic colorectal cancer: results from a large prospective observational cohort study (BRiTE). Cancer. 2012;118:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27:4109-4115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Johnston FM, Yeo HL, Clark C, Stewart JH 4th. Bias Issues in Colorectal Cancer Management: A Review. Ann Surg Oncol. 2022;29:2166-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Ciccone G, Prastaro C, Ivaldi C, Giacometti R, Vineis P. Access to hospital care, clinical stage and survival from colorectal cancer according to socio-economic status. Ann Oncol. 2000;11:1201-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Jepson C, Kessler LG, Portnoy B, Gibbs T. Black-white differences in cancer prevention knowledge and behavior. Am J Public Health. 1991;81:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, Davis M, de Smith AJ, Dutil J, Figueiredo JC, Fox R, Graves KD, Gomez SL, Llera A, Neuhausen SL, Newman L, Nguyen T, Palmer JR, Palmer NR, Pérez-Stable EJ, Piawah S, Rodriquez EJ, Sanabria-Salas MC, Schmit SL, Serrano-Gomez SJ, Stern MC, Weitzel J, Yang JJ, Zabaleta J, Ziv E, Fejerman L. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124:315-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 610] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 10. | Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 11. | Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiol. 2013;8:445-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Farhana L, Antaki F, Murshed F, Mahmud H, Judd SL, Nangia-Makker P, Levi E, Yu Y, Majumdar AP. Gut microbiome profiling and colorectal cancer in African Americans and Caucasian Americans. World J Gastrointest Pathophysiol. 2018;9:47-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Koi M, Okita Y, Carethers JM. Fusobacterium nucleatum Infection in Colorectal Cancer: Linking Inflammation, DNA Mismatch Repair and Genetic and Epigenetic Alterations. J Anus Rectum Colon. 2018;2:37-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Devaraj B, Lee A, Cabrera BL, Miyai K, Luo L, Ramamoorthy S, Keku T, Sandler RS, McGuire KL, Carethers JM. Relationship of EMAST and microsatellite instability among patients with rectal cancer. J Gastrointest Surg. 2010;14:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Carethers JM, Koi M, Tseng-Rogenski SS. EMAST is a Form of Microsatellite Instability That is Initiated by Inflammation and Modulates Colorectal Cancer Progression. Genes (Basel). 2015;6:185-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, Stein E, Vadivelu J, Roslani AC, Malik AA, Wanyiri JW, Goh KL, Thevambiga I, Fu K, Wan F, Llosa N, Housseau F, Romans K, Wu X, McAllister FM, Wu S, Vogelstein B, Kinzler KW, Pardoll DM, Sears CL. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321-18326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 533] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 17. | Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723-728. [PubMed] |
| 18. | Park CH, Han DS, Oh YH, Lee AR, Lee YR, Eun CS. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep. 2016;6:25271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Yu J, Chen Y, Fu X, Zhou X, Peng Y, Shi L, Chen T, Wu Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | DeDecker L, Coppedge B, Avelar-Barragan J, Karnes W, Whiteson K. Microbiome distinctions between the CRC carcinogenic pathways. Gut Microbes. 2021;13:1854641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Yang JF, Tang SJ, Lash RH, Wu R, Yang Q. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch Pathol Lab Med. 2015;139:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, Foti M, Gomez SL, Griggs JJ, Lathan CS, Li CI, Lichtenfeld JL, McCaskill-Stevens W, Paskett ED. Charting the future of cancer health disparities research: A position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. CA Cancer J Clin. 2017;67:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 2232] [Article Influence: 446.4] [Reference Citation Analysis (1)] |
| 25. | Jovov B, Araujo-Perez F, Sigel CS, Stratford JK, McCoy AN, Yeh JJ, Keku T. Differential gene expression between African American and European American colorectal cancer patients. PLoS One. 2012;7:e30168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Quach H, Rotival M, Pothlichet J, Loh YE, Dannemann M, Zidane N, Laval G, Patin E, Harmant C, Lopez M, Deschamps M, Naffakh N, Duffy D, Coen A, Leroux-Roels G, Clément F, Boland A, Deleuze JF, Kelso J, Albert ML, Quintana-Murci L. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell. 2016;167:643-656.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 27. | Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, Grenier JC, Freiman A, Sams AJ, Hebert S, Pagé Sabourin A, Luca F, Blekhman R, Hernandez RD, Pique-Regi R, Tung J, Yotova V, Barreiro LB. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell. 2016;167:657-669.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 28. | Banda Y, Kvale MN, Hoffmann TJ, Hesselson SE, Ranatunga D, Tang H, Sabatti C, Croen LA, Dispensa BP, Henderson M, Iribarren C, Jorgenson E, Kushi LH, Ludwig D, Olberg D, Quesenberry CP Jr, Rowell S, Sadler M, Sakoda LC, Sciortino S, Shen L, Smethurst D, Somkin CP, Van Den Eeden SK, Walter L, Whitmer RA, Kwok PY, Schaefer C, Risch N. Characterizing Race/Ethnicity and Genetic Ancestry for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics. 2015;200:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 29. | Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96:37-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 30. | Batai K, Hooker S, Kittles RA. Leveraging genetic ancestry to study health disparities. Am J Phys Anthropol. 2021;175:363-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Denny JC. Chapter 13: Mining electronic health records in the genomics era. PLoS Comput Biol. 2012;8:e1002823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Ruterbusch JJ, Levin AM, Kittles R, Rybicki BA, Bock CH. Abstract A66: Admixture and fine mapping in African Americans identifies a susceptibility locus for prostate cancer on chromosome 7. Cancer Epidemiol Biomarkers Prev. 2012;21:A66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Sanabria-Salas MC, Hernández-Suárez G, Umaña-Pérez A, Rawlik K, Tenesa A, Serrano-López ML, Sánchez de Gómez M, Rojas MP, Bravo LE, Albis R, Plata JL, Green H, Borgovan T, Li L, Majumdar S, Garai J, Lee E, Ashktorab H, Brim H, Margolin D, Fejerman L, Zabaleta J. IL1B-CGTC haplotype is associated with colorectal cancer in admixed individuals with increased African ancestry. Sci Rep. 2017;7:41920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Nieves Delgado A, Baedke J. Does the human microbiome tell us something about race? Humanit Soc Sci Commun. 2021;8:97. [DOI] [Full Text] |
| 35. | Gallagher S, Hughes E, Wagner S, Tshiaba P, Rosenthal E, Roa BB, Kurian AW, Domchek SM, Garber J, Lancaster J, Weitzel JN, Gutin A, Lanchbury JS, Robson M. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw Open. 2020;3:e208501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 36. | Thomas M, Sakoda LC, Hoffmeister M, Rosenthal EA, Lee JK, van Duijnhoven FJB, Platz EA, Wu AH, Dampier CH, de la Chapelle A, Wolk A, Joshi AD, Burnett-Hartman A, Gsur A, Lindblom A, Castells A, Win AK, Namjou B, Van Guelpen B, Tangen CM, He Q, Li CI, Schafmayer C, Joshu CE, Ulrich CM, Bishop DT, Buchanan DD, Schaid D, Drew DA, Muller DC, Duggan D, Crosslin DR, Albanes D, Giovannucci EL, Larson E, Qu F, Mentch F, Giles GG, Hakonarson H, Hampel H, Stanaway IB, Figueiredo JC, Huyghe JR, Minnier J, Chang-Claude J, Hampe J, Harley JB, Visvanathan K, Curtis KR, Offit K, Li L, Le Marchand L, Vodickova L, Gunter MJ, Jenkins MA, Slattery ML, Lemire M, Woods MO, Song M, Murphy N, Lindor NM, Dikilitas O, Pharoah PDP, Campbell PT, Newcomb PA, Milne RL, MacInnis RJ, Castellví-Bel S, Ogino S, Berndt SI, Bézieau S, Thibodeau SN, Gallinger SJ, Zaidi SH, Harrison TA, Keku TO, Hudson TJ, Vymetalkova V, Moreno V, Martín V, Arndt V, Wei WQ, Chung W, Su YR, Hayes RB, White E, Vodicka P, Casey G, Gruber SB, Schoen RE, Chan AT, Potter JD, Brenner H, Jarvik GP, Corley DA, Peters U, Hsu L. Genome-wide Modeling of Polygenic Risk Score in Colorectal Cancer Risk. Am J Hum Genet. 2020;107:432-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 37. | Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2407] [Article Influence: 200.6] [Reference Citation Analysis (0)] |
| 38. | Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019;17:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 39. | Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity. 2017;46:562-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 819] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 40. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3003] [Cited by in RCA: 2991] [Article Influence: 230.1] [Reference Citation Analysis (0)] |
| 41. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064-15069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1534] [Article Influence: 90.2] [Reference Citation Analysis (1)] |
| 42. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1970] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 43. | Ocvirk S, Wilson AS, Posma JM, Li JV, Koller KR, Day GM, Flanagan CA, Otto JE, Sacco PE, Sacco FD, Sapp FR, Newton K, Brouard F, DeLany JP, Behnning M, Appolonia CN, Soni D, Bhatti F, Methé B, Fitch A, Morris A, Gaskins HR, Kinross J, Nicholson JK, Thomas TK, O'Keefe SJD. A prospective cohort analysis of gut microbial co-metabolism in Alaska Native and rural African people at high and low risk of colorectal cancer. Am J Clin Nutr. 2020;111:406-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PG, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 693] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 45. | Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1842] [Article Influence: 263.1] [Reference Citation Analysis (0)] |
| 46. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1512] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 47. | Wang H, Taverna D, Stram DO, Fortini BK, Cheng I, Wilkens LR, Burnett T, Makar KW, Lindor NM, Hopper JL, Gallinger S, Baron JA, Haile R, Kolonel LN, Henderson BE, Newcomb PA, Casey G, Duggan D, Ulrich CM, Le Marchand L. Genetic variation in the inflammation and innate immunity pathways and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Turpin W, Goethel A, Bedrani L, Croitoru Mdcm K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm Bowel Dis. 2018;24:1133-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 49. | Lu Y, Kweon SS, Tanikawa C, Jia WH, Xiang YB, Cai Q, Zeng C, Schmit SL, Shin A, Matsuo K, Jee SH, Kim DH, Kim J, Wen W, Shi J, Guo X, Li B, Wang N, Zhang B, Li X, Shin MH, Li HL, Ren Z, Oh JH, Oze I, Ahn YO, Jung KJ, Conti DV, Schumacher FR, Rennert G, Jenkins MA, Campbell PT, Hoffmeister M, Casey G, Gruber SB, Gao J, Gao YT, Pan ZZ, Kamatani Y, Zeng YX, Shu XO, Long J, Matsuda K, Zheng W. Large-Scale Genome-Wide Association Study of East Asians Identifies Loci Associated With Risk for Colorectal Cancer. Gastroenterology. 2019;156:1455-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 50. | Law PJ, Timofeeva M, Fernandez-Rozadilla C, Broderick P, Studd J, Fernandez-Tajes J, Farrington S, Svinti V, Palles C, Orlando G, Sud A, Holroyd A, Penegar S, Theodoratou E, Vaughan-Shaw P, Campbell H, Zgaga L, Hayward C, Campbell A, Harris S, Deary IJ, Starr J, Gatcombe L, Pinna M, Briggs S, Martin L, Jaeger E, Sharma-Oates A, East J, Leedham S, Arnold R, Johnstone E, Wang H, Kerr D, Kerr R, Maughan T, Kaplan R, Al-Tassan N, Palin K, Hänninen UA, Cajuso T, Tanskanen T, Kondelin J, Kaasinen E, Sarin AP, Eriksson JG, Rissanen H, Knekt P, Pukkala E, Jousilahti P, Salomaa V, Ripatti S, Palotie A, Renkonen-Sinisalo L, Lepistö A, Böhm J, Mecklin JP, Buchanan DD, Win AK, Hopper J, Jenkins ME, Lindor NM, Newcomb PA, Gallinger S, Duggan D, Casey G, Hoffmann P, Nöthen MM, Jöckel KH, Easton DF, Pharoah PDP, Peto J, Canzian F, Swerdlow A, Eeles RA, Kote-Jarai Z, Muir K, Pashayan N; PRACTICAL consortium, Harkin A, Allan K, McQueen J, Paul J, Iveson T, Saunders M, Butterbach K, Chang-Claude J, Hoffmeister M, Brenner H, Kirac I, Matošević P, Hofer P, Brezina S, Gsur A, Cheadle JP, Aaltonen LA, Tomlinson I, Houlston RS, Dunlop MG. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun. 2019;10:2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 51. | Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18:690-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 340] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 52. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 53. | Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, Flavell RA. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110:9862-9867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 54. | Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nùñez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (1)] |
| 55. | Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, Hackl H, Pfister A, Schilling J, Moser PL, Kempster SL, Swidsinski A, Orth Höller D, Weiss G, Baines JF, Kaser A, Tilg H. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe. 2016;19:455-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 56. | Davies JM, Abreu MT. The innate immune system and inflammatory bowel disease. Scand J Gastroenterol. 2015;50:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 Leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3905] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 58. | Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17:665-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 59. | Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang H, Tyler AD, van Sommeren S, Imhann F, Stempak JM, Vangay P, Al-Ghalith GA, Russell C, Sauk J, Knight J, Daly MJ, Huttenhower C, Xavier RJ. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014;6:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 60. | Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, Dreyfuss JM, Glickman JN, Vlamakis H, Ananthakrishnan A, Kostic A, Garrett WS, Xavier RJ. The Crohn's disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 61. | Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3407] [Cited by in RCA: 3594] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 62. | Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 593] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 63. | Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 64. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1041] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 65. | Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, Taniguchi K, Feng Y, Fearon E, Grivennikov SI, Karin M. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 66. | Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, Ydegaard Turino S, Brodersen JB, Rashid S, Kaiser Rasmussen B, Avlund S, Bastholm Olesen T, Hoffmann HJ, Andersen Nexø B, Sode J, Vogel U, Andersen V. Polymorphisms in the Toll-Like Receptor and the IL-23/IL-17 Pathways Were Associated with Susceptibility to Inflammatory Bowel Disease in a Danish Cohort. PLoS One. 2015;10:e0145302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Newman WG, Zhang Q, Liu X, Amos CI, Siminovitch KA. Genetic variants in IL-23R and ATG16L1 independently predispose to increased susceptibility to Crohn's disease in a Canadian population. J Clin Gastroenterol. 2009;43:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, Deelen P, Vatanen T, Schirmer M, Smeekens SP, Zhernakova DV, Jankipersadsing SA, Jaeger M, Oosting M, Cenit MC, Masclee AA, Swertz MA, Li Y, Kumar V, Joosten L, Harmsen H, Weersma RK, Franke L, Hofker MH, Xavier RJ, Jonkers D, Netea MG, Wijmenga C, Fu J, Zhernakova A. The effect of host genetics on the gut microbiome. Nat Genet. 2016;48:1407-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 578] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 69. | Yeyeodu ST, Kidd LR, Kimbro KS. Protective Innate Immune Variants in Racial/Ethnic Disparities of Breast and Prostate Cancer. Cancer Immunol Res. 2019;7:1384-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Quan B, Qi X, Yu Z, Jiang Y, Liao M, Wang G, Feng R, Zhang L, Chen Z, Jiang Q, Liu G. Pathway analysis of genome-wide association study and transcriptome data highlights new biological pathways in colorectal cancer. Mol Genet Genomics. 2015;290:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, Ambs S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 393] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 72. | Özdemir BC, Dotto GP. Racial Differences in Cancer Susceptibility and Survival: More Than the Color of the Skin? Trends Cancer. 2017;3:181-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 73. | Yuan J, Hu Z, Mahal BA, Zhao SD, Kensler KH, Pi J, Hu X, Zhang Y, Wang Y, Jiang J, Li C, Zhong X, Montone KT, Guan G, Tanyi JL, Fan Y, Xu X, Morgan MA, Long M, Zhang R, Sood AK, Rebbeck TR, Dang CV, Zhang L. Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell. 2018;34:549-560.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 74. | Zanetti KA, Haznadar M, Welsh JA, Robles AI, Ryan BM, McClary AC, Bowman ED, Goodman JE, Bernig T, Chanock SJ, Harris CC. 3'-UTR and functional secretor haplotypes in mannose-binding lectin 2 are associated with increased colon cancer risk in African Americans. Cancer Res. 2012;72:1467-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Karimzadeh MR, Zarin M, Ehtesham N, Khosravi S, Soosanabadi M, Mosallaei M, Pourdavoud P. MicroRNA binding site polymorphism in inflammatory genes associated with colorectal cancer: literature review and bioinformatics analysis. Cancer Gene Ther. 2020;27:739-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Lazarus R, Vercelli D, Palmer LJ, Klimecki WJ, Silverman EK, Richter B, Riva A, Ramoni M, Martinez FD, Weiss ST, Kwiatkowski DJ. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol Rev. 2002;190:9-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 77. | Barreiro LB, Quintana-Murci L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet. 2010;11:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 78. | Brinkworth JF, Barreiro LB. The contribution of natural selection to present-day susceptibility to chronic inflammatory and autoimmune disease. Curr Opin Immunol. 2014;31:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Sams AJ, Dumaine A, Nédélec Y, Yotova V, Alfieri C, Tanner JE, Messer PW, Barreiro LB. Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome Biol. 2016;17:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 80. | Green R, Wilkins C, Thomas S, Sekine A, Hendrick DM, Voss K, Ireton RC, Mooney M, Go JT, Choonoo G, Jeng S, de Villena FP, Ferris MT, McWeeney S, Gale M Jr. Oas1b-dependent Immune Transcriptional Profiles of West Nile Virus Infection in the Collaborative Cross. G3 (Bethesda). 2017;7:1665-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Barreiro LB, Ben-Ali M, Quach H, Laval G, Patin E, Pickrell JK, Bouchier C, Tichit M, Neyrolles O, Gicquel B, Kidd JR, Kidd KK, Alcaïs A, Ragimbeau J, Pellegrini S, Abel L, Casanova JL, Quintana-Murci L. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 82. | Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am J Epidemiol. 2004;160:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D'Alfonso S. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 424] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 84. | Chen H, Wilkins LM, Aziz N, Cannings C, Wyllie DH, Bingle C, Rogus J, Beck JD, Offenbacher S, Cork MJ, Rafie-Kolpin M, Hsieh CM, Kornman KS, Duff GW. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 85. | Hung RJ, Ulrich CM, Goode EL, Brhane Y, Muir K, Chan AT, Marchand LL, Schildkraut J, Witte JS, Eeles R, Boffetta P, Spitz MR, Poirier JG, Rider DN, Fridley BL, Chen Z, Haiman C, Schumacher F, Easton DF, Landi MT, Brennan P, Houlston R, Christiani DC, Field JK, Bickeböller H, Risch A, Kote-Jarai Z, Wiklund F, Grönberg H, Chanock S, Berndt SI, Kraft P, Lindström S, Al Olama AA, Song H, Phelan C, Wentzensen N, Peters U, Slattery ML; GECCO, Sellers TA; FOCI, Casey G, Gruber SB; CORECT, Hunter DJ; DRIVE, Amos CI, Henderson B; GAME-ON Network. Cross Cancer Genomic Investigation of Inflammation Pathway for Five Common Cancers: Lung, Ovary, Prostate, Breast, and Colorectal Cancer. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Theodoropoulos G, Papaconstantinou I, Felekouras E, Nikiteas N, Karakitsos P, Panoussopoulos D, Lazaris ACh, Patsouris E, Bramis J, Gazouli M. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J Gastroenterol. 2006;12:5037-5043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 87. | Landi S, Moreno V, Gioia-Patricola L, Guino E, Navarro M, de Oca J, Capella G, Canzian F; Bellvitge Colorectal Cancer Study Group. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560-3566. [PubMed] |
| 88. | Koh WP, Yuan JM, Van Den Berg D, Ingles SA, Yu MC. Peroxisome proliferator-activated receptor (PPAR) gamma gene polymorphisms and colorectal cancer risk among Chinese in Singapore. Carcinogenesis. 2006;27:1797-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Montazeri Z, Li X, Nyiraneza C, Ma X, Timofeeva M, Svinti V, Meng X, He Y, Bo Y, Morgan S, Castellví-Bel S, Ruiz-Ponte C, Fernández-Rozadilla C, Carracedo Á, Castells A, Bishop T, Buchanan D, Jenkins MA, Keku TO, Lindblom A, van Duijnhoven FJB, Wu A, Farrington SM, Dunlop MG, Campbell H, Theodoratou E, Zheng W, Little J. Systematic meta-analyses, field synopsis and global assessment of the evidence of genetic association studies in colorectal cancer. Gut. 2020;69:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Abbas M, Mason T, Ibad A, Khraiwesh M, Apprey V, Kanaan Y, Wilson B, Dunston G, Ricks-Santi L, Brim H. Genetic Polymorphisms in IL-10 Promoter Are Associated With Smoking and Prostate Cancer Risk in African Americans. Anticancer Res. 2020;40:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Jenkins BD, Martini RN, Hire R, Brown A, Bennett B, Brown I, Howerth EW, Egan M, Hodgson J, Yates C, Kittles R, Chitale D, Ali H, Nathanson D, Nikolinakos P, Newman L, Monteil M, Davis MB. Atypical Chemokine Receptor 1 (DARC/ACKR1) in Breast Tumors Is Associated with Survival, Circulating Chemokines, Tumor-Infiltrating Immune Cells, and African Ancestry. Cancer Epidemiol Biomarkers Prev. 2019;28:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2636] [Cited by in RCA: 2256] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 93. | Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 940] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 94. | Xu F, Fu Y, Sun TY, Jiang Z, Miao Z, Shuai M, Gou W, Ling CW, Yang J, Wang J, Chen YM, Zheng JS. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 2020;8:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 95. | Burns MB, Montassier E, Abrahante J, Priya S, Niccum DE, Khoruts A, Starr TK, Knights D, Blekhman R. Colorectal cancer mutational profiles correlate with defined microbial communities in the tumor microenvironment. PLoS Genet. 2018;14:e1007376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 96. | Thyagarajan S, Zhang Y, Thapa S, Allen MS, Phillips N, Chaudhary P, Kashyap MV, Vishwanatha JK. Comparative analysis of racial differences in breast tumor microbiome. Sci Rep. 2020;10:14116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brünner N, Kristiansen K, Arumugam M, Sung JJ. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 785] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 98. | Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, Amiot A, Böhm J, Brunetti F, Habermann N, Hercog R, Koch M, Luciani A, Mende DR, Schneider MA, Schrotz-King P, Tournigand C, Tran Van Nhieu J, Yamada T, Zimmermann J, Benes V, Kloor M, Ulrich CM, von Knebel Doeberitz M, Sobhani I, Bork P. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol. 2014;10:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 859] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 99. | Baxter NT, Ruffin MT 4th, Rogers MA, Schloss PD. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016;8:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 100. | Zackular JP, Rogers MA, Ruffin MT 4th, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila). 2014;7:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 396] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 101. | Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, Beghini F, Manara S, Karcher N, Pozzi C, Gandini S, Serrano D, Tarallo S, Francavilla A, Gallo G, Trompetto M, Ferrero G, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Wirbel J, Schrotz-King P, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G, Cordero F, Dias-Neto E, Setubal JC, Tett A, Pardini B, Rescigno M, Waldron L, Naccarati A, Segata N. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 607] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 102. | Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, Sunagawa S, Coelho LP, Schrotz-King P, Vogtmann E, Habermann N, Niméus E, Thomas AM, Manghi P, Gandini S, Serrano D, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Waldron L, Naccarati A, Segata N, Sinha R, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25:679-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1007] [Cited by in RCA: 795] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 103. | Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH, Yu J. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 104. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1310] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 105. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1670] [Article Influence: 128.5] [Reference Citation Analysis (1)] |
| 106. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1919] [Article Influence: 159.9] [Reference Citation Analysis (0)] |
| 107. | Tomkovich S, Dejea CM, Winglee K, Drewes JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Mühlbauer M, Liu X, Fathi P, Anders RA, Besharati S, Perez-Chanona E, Yang Y, Ding H, Wu X, Wu S, White JR, Gharaibeh RZ, Fodor AA, Wang H, Pardoll DM, Jobin C, Sears CL. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J Clin Invest. 2019;129:1699-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 108. | Knippel RJ, Drewes JL, Sears CL. The Cancer Microbiome: Recent Highlights and Knowledge Gaps. Cancer Discov. 2021;11:2378-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 109. | Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16:e2006842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 110. | Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, Tremaroli V, Bakker GJ, Attaye I, Pinto-Sietsma SJ, van Raalte DH, Snijder MB, Nicolaou M, Peters R, Zwinderman AH, Bäckhed F, Nieuwdorp M. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. 2018;24:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 450] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 111. | Gupta VK, Paul S, Dutta C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front Microbiol. 2017;8:1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 674] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 112. | Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21:2759-2769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 113. | Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O'Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 114. | Jung SY, Papp JC, Sobel EM, Pellegrini M, Yu H, Zhang ZF. Pro-inflammatory cytokine polymorphisms in ONECUT2 and HNF4A and primary colorectal carcinoma: a post genome-wide gene-lifestyle interaction study. Am J Cancer Res. 2020;10:2955-2976. [PubMed] |
| 115. | Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1456] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 116. | Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 117. | Lu SSM, Mohammed Z, Häggström C, Myte R, Lindquist E, Gylfe Å, Van Guelpen B, Harlid S. Antibiotics Use and Subsequent Risk of Colorectal Cancer: A Swedish Nationwide Population-Based Study. J Natl Cancer Inst. 2022;114:38-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 118. | Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL, Yu MC. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. Br J Cancer. 2007;96:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Ali Khan U, Fallah M, Sundquist K, Sundquist J, Brenner H, Kharazmi E. Risk of colorectal cancer in patients with diabetes mellitus: A Swedish nationwide cohort study. PLoS Med. 2020;17:e1003431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 120. | Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21:1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 121. | Scepanovic P, Hodel F, Mondot S, Partula V, Byrd A, Hammer C, Alanio C, Bergstedt J, Patin E, Touvier M, Lantz O, Albert ML, Duffy D, Quintana-Murci L, Fellay J; Milieu Intérieur Consortium. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. 2019;7:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 122. | Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD, Keinan A, Ley RE, Gevers D, Clark AG. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015;16:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 515] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 123. | Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016;19:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 717] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 124. | Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 2151] [Article Influence: 215.1] [Reference Citation Analysis (0)] |
| 125. | Ashktorab H, Azimi H, Varma S, Lee EL, Laiyemo AO, Nickerson ML, Brim H. Driver genes exome sequencing reveals distinct variants in African Americans with colorectal neoplasia. Oncotarget. 2019;10:2607-2624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Brim H, Yooseph S, Lee E, Sherif ZA, Abbas M, Laiyemo AO, Varma S, Torralba M, Dowd SE, Nelson KE, Pathmasiri W, Sumner S, de Vos W, Liang Q, Yu J, Zoetendal E, Ashktorab H. A Microbiomic Analysis in African Americans with Colonic Lesions Reveals Streptococcus sp.VT162 as a Marker of Neoplastic Transformation. Genes (Basel). 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Ogino S, Nowak JA, Hamada T, Milner DA Jr, Nishihara R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol. 2019;14:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 128. | Nishi A, Milner DA Jr, Giovannucci EL, Nishihara R, Tan AS, Kawachi I, Ogino S. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn. 2016;16:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 129. | Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, Guttman DS, Griffiths A, Panaccione R, Otley A, Xu L, Shestopaloff K, Moreno-Hagelsieb G; GEM Project Research Consortium, Paterson AD, Croitoru K. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. 2016;48:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 130. | DeStefano Shields CE, White JR, Chung L, Wenzel A, Hicks JL, Tam AJ, Chan JL, Dejea CM, Fan H, Michel J, Maiuri AR, Sriramkumar S, Podicheti R, Rusch DB, Wang H, De Marzo AM, Besharati S, Anders RA, Baylin SB, O'Hagan HM, Housseau F, Sears CL. Bacterial-Driven Inflammation and Mutant BRAF Expression Combine to Promote Murine Colon Tumorigenesis That Is Sensitive to Immune Checkpoint Therapy. Cancer Discov. 2021;11:1792-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 131. | Davis MB. Genomics and Cancer Disparities: The Justice and Power of Inclusion. Cancer Discov. 2021;11:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Sherman RM, Forman J, Antonescu V, Puiu D, Daya M, Rafaels N, Boorgula MP, Chavan S, Vergara C, Ortega VE, Levin AM, Eng C, Yazdanbakhsh M, Wilson JG, Marrugo J, Lange LA, Williams LK, Watson H, Ware LB, Olopade CO, Olopade O, Oliveira RR, Ober C, Nicolae DL, Meyers DA, Mayorga A, Knight-Madden J, Hartert T, Hansel NN, Foreman MG, Ford JG, Faruque MU, Dunston GM, Caraballo L, Burchard EG, Bleecker ER, Araujo MI, Herrera-Paz EF, Campbell M, Foster C, Taub MA, Beaty TH, Ruczinski I, Mathias RA, Barnes KC, Salzberg SL. Assembly of a pan-genome from deep sequencing of 910 humans of African descent. Nat Genet. 2019;51:30-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 133. | Peterson RE, Edwards AC, Bacanu SA, Dick DM, Kendler KS, Webb BT. The utility of empirically assigning ancestry groups in cross-population genetic studies of addiction. Am J Addict. 2017;26:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 134. | Adedokun B, Du Z, Gao G, Ahearn TU, Lunetta KL, Zirpoli G, Figueroa J, John EM, Bernstein L, Zheng W, Hu JJ, Ziegler RG, Nyante S, Bandera EV, Ingles SA, Press MF, Deming-Halverson SL, Rodriguez-Gil JL, Yao S, Ogundiran TO, Ojengbede O, Blot W, Troester MA, Nathanson KL, Hennis A, Nemesure B, Ambs S, Fiorica PN, Sucheston-Campbell LE, Bensen JT, Kushi LH, Torres-Mejia G, Hu D, Fejerman L, Bolla MK, Dennis J, Dunning AM, Easton DF, Michailidou K, Pharoah PDP, Wang Q, Sandler DP, Taylor JA, O'Brien KM, Kitahara CM, Falusi AG, Babalola C, Yarney J, Awuah B, Addai-Wiafe B; GBHS Study Team, Chanock SJ, Olshan AF, Ambrosone CB, Conti DV, Ziv E, Olopade OI, Garcia-Closas M, Palmer JR, Haiman CA, Huo D. Cross-ancestry GWAS meta-analysis identifies six breast cancer loci in African and European ancestry women. Nat Commun. 2021;12:4198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Schumacher FR, Schmit SL, Jiao S, Edlund CK, Wang H, Zhang B, Hsu L, Huang SC, Fischer CP, Harju JF, Idos GE, Lejbkowicz F, Manion FJ, McDonnell K, McNeil CE, Melas M, Rennert HS, Shi W, Thomas DC, Van Den Berg DJ, Hutter CM, Aragaki AK, Butterbach K, Caan BJ, Carlson CS, Chanock SJ, Curtis KR, Fuchs CS, Gala M, Giovannucci EL, Gogarten SM, Hayes RB, Henderson B, Hunter DJ, Jackson RD, Kolonel LN, Kooperberg C, Küry S, LaCroix A, Laurie CC, Laurie CA, Lemire M, Levine D, Ma J, Makar KW, Qu C, Taverna D, Ulrich CM, Wu K, Kono S, West DW, Berndt SI, Bezieau S, Brenner H, Campbell PT, Chan AT, Chang-Claude J, Coetzee GA, Conti DV, Duggan D, Figueiredo JC, Fortini BK, Gallinger SJ, Gauderman WJ, Giles G, Green R, Haile R, Harrison TA, Hoffmeister M, Hopper JL, Hudson TJ, Jacobs E, Iwasaki M, Jee SH, Jenkins M, Jia WH, Joshi A, Li L, Lindor NM, Matsuo K, Moreno V, Mukherjee B, Newcomb PA, Potter JD, Raskin L, Rennert G, Rosse S, Severi G, Schoen RE, Seminara D, Shu XO, Slattery ML, Tsugane S, White E, Xiang YB, Zanke BW, Zheng W, Le Marchand L, Casey G, Gruber SB, Peters U. Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat Commun. 2015;6:7138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 136. | Tsilidis KK, Helzlsouer KJ, Smith MW, Grinberg V, Hoffman-Bolton J, Clipp SL, Visvanathan K, Platz EA. Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer Causes Control. 2009;20:1739-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 137. | Li D, Achkar JP, Haritunians T, Jacobs JP, Hui KY, D'Amato M, Brand S, Radford-Smith G, Halfvarson J, Niess JH, Kugathasan S, Büning C, Schumm LP, Klei L, Ananthakrishnan A, Aumais G, Baidoo L, Dubinsky M, Fiocchi C, Glas J, Milgrom R, Proctor DD, Regueiro M, Simms LA, Stempak JM, Targan SR, Törkvist L, Sharma Y, Devlin B, Borneman J, Hakonarson H, Xavier RJ, Daly M, Brant SR, Rioux JD, Silverberg MS, Cho JH, Braun J, McGovern DP, Duerr RH. A Pleiotropic Missense Variant in SLC39A8 Is Associated With Crohn's Disease and Human Gut Microbiome Composition. Gastroenterology. 2016;151:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 138. | Rühlemann MC, Hermes BM, Bang C, Doms S, Moitinho-Silva L, Thingholm LB, Frost F, Degenhardt F, Wittig M, Kässens J, Weiss FU, Peters A, Neuhaus K, Völker U, Völzke H, Homuth G, Weiss S, Grallert H, Laudes M, Lieb W, Haller D, Lerch MM, Baines JF, Franke A. Genome-wide association study in 8,956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat Genet. 2021;53:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |