Published online Jun 28, 2022. doi: 10.3748/wjg.v28.i24.2680
Peer-review started: January 15, 2022
First decision: April 12, 2022
Revised: April 17, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: June 28, 2022
Processing time: 159 Days and 23.6 Hours
Celiac disease (CD) is well recognized as a systemic, chronic autoimmune disease mainly characterized by gluten-sensitive enteropathy in genetically predisposed individuals but with various extraintestinal features. One of the affected organs in CD is the pancreas, consisting of both endocrine and exocrine alterations. Over the last decades there has been increasing interest in the pancreatic changes in CD, and this has been reflected by a great number of publications looking at this extraintestinal involvement during the course of CD. While pancreatic endocrine changes in CD, focusing on type 1 diabetes mellitus, are well documented in the literature, the relationship with the exocrine pancreas has been less studied. This review summarizes currently available evidence with regard to pancreatic exocrine alterations in CD, focusing on risk of pancreatitis in CD patients, association with autoimmune pancreatitis, prevalence and outcomes of pancreatic exocrine insufficiency in newly diagnosed and gluten-free diet treated CD patients, and the link with cystic fibrosis. In addition, we discuss mechanisms behind the associated pancreatic exocrine impairment in CD and highlight the recommendations for clinical practice.
Core Tip: Celiac disease (CD) is currently regarded as a systemic, chronic, immune-mediated disease triggered by gluten ingestion in genetically susceptible individuals. In the last decade there has been an increasing number of publications on extraintestinal involvement during the course of CD, some of which have assessed the pancreatic changes associated with this disease. This review summarizes currently available data with respect to exocrine pancreatic changes in CD, focusing on practices for clinicians.
- Citation: Balaban DV, Enache I, Ciochina M, Popp A, Jinga M. Pancreatic involvement in celiac disease. World J Gastroenterol 2022; 28(24): 2680-2688
- URL: https://www.wjgnet.com/1007-9327/full/v28/i24/2680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i24.2680
Celiac disease (CD) is an immune-mediated enteropathy that occurs in genetically predisposed individuals upon ingestion of gluten. Initially considered a small bowel disease, now it is widely recognized as a systemic illness, which accounts for its many protean manifestations. The systemic character along with its various clinical presentations make CD a sometimes difficult to recognize clinical chameleon. The wide spectrum of presenting features, sometimes subclinical, hinders case-finding strategies and delays diagnosis, as CD is often not considered among the differential diagnoses[1,2]. Moreover, there is also the further drawback of poor awareness among different medical specialties[3].
Among the extraintestinal features of CD[4-6], pancreatic involvement has rarely been reported compared to other organ-specific manifestations such as cutaneous, hematologic, liver-related, rheumatologic, cardiovascular, or neurological impairments[7-13]. Moreover, while some of these systemic features have been incorporated into case-finding and screening strategies such as anemia, osteoporosis or chronic liver disease[14], pancreatic-associated involvement in CD is scarcely reported in currently available guidelines[15-17]. The association between the pancreas and CD covered in guidelines is limited to the need to consider pancreatic exocrine insufficiency (PEI) as an alternative diagnosis in non-responsive CD and the fact that upper gastrointestinal surgery including pancreas-related indications may unmask subclinical CD[15,16]. This has also been highlighted by previous reports on the impact of CD on exocrine and endocrine pancreas, which also set the need for further research focused on this association[18]. Contrasting the well-documented pancreatic endocrine changes in CD, referring to type 1 diabetes mellitus in particular, the relationship with the exocrine pancreas has been less covered in the literature.
On the other hand, the wide spectrum of pancreatic diseases does not include CD as a risk factor or associated condition, except counting CD as a potential cause of PEI[19]. Pancreatic involvement can occur in patients with CD, either caused by the small-bowel disease or co-existing with it. The main mechanisms for this association are believed to be the impaired cholecystokinin (CCK) and secretin release, but also the chronic duodenal inflammation that can lead to secondary modifications of papillary mucosal area[20].
Our aim was to summarize currently available evidence with regard to exocrine pancreatic involvement in CD, looking at CD as a risk factor or associated condition with pathologies of the exocrine pancreas, and testing indications regarding the bidirectional association of the two diseases.
For this purpose, we searched PubMed in January 2022 for all publications on the association between pancreas and CD, using the medical subject headings (MeSH) terms–“pancreas” (ID: D010179) and “celiac disease” (ID: D002446), with the following search syntax (("pancreas" [Mesh]) OR (pancreas [Title/Abstract])) AND (("celiac disease" [Mesh]) OR (celiac disease [Title/Abstract])). We performed additional searches with pancreas-related terms, “Pancreas, exocrine” (ID: D046790), “pancreatitis” (ID: D010195), “pancreatitis, chronic” (ID: D050500), “autoimmune pancreatitis” (ID: D000081012) and “celiac disease” (ID: D002446). The extended search yielded a total of 889 results, of which 145 were duplicates. Search results were imported into Reference Citation Analysis (Baishideng Publishing Group, Inc.), which was used for article processing and selection. We filtered the search for reviews, editorials, comments and opinion articles (n = 140). We excluded papers referring to the association of CD with diabetes or alterations of the endocrine pancreas (n = 66). The remaining titles and consecutive abstracts were screened for pertinence to the topic. We selected relevant articles on exocrine pancreatic involvement in CD for full-text analysis and summarized findings according to significant associations. References and citing articles of selected papers were also analyzed for potentially relevant articles that might have been missed in the initial search. The process of article selection is detailed in Figure 1.
CD patients are at risk both for acute pancreatitis (AP) and chronic pancreatitis (CP)[21-23]. While some have described worse outcomes and increased medical burden among CD individuals[21], others have found lower morbidity and mortality among this patient group[24] (Table 1).
| Ref. | CD, n | Pancreatitis prevalence | OR of AP | Outcome |
| Alkhayyat et al[21], 2021 | 133400 | AP 1.06%; CP 0.52% | OR for AP = 2.66; OR for CP = 2.18 | Worse outcomes compared to non-CD |
| Osagiede et al[24], 2020 | 337201 | AP 2.2% | OR = 1.92 | Lower morbidity and mortality, attributed to less severe forms of AP or lower baseline comorbidities |
| Sadr-Azodi et al[22], 2012 | 28908 | Pancreatitis 1.4% | HR for gallstone-related AP = 1.59; HR for non-gallstone-related AP = 1.86; HR for CP = 3.33 | Increased risk of severe AP (gallstone-related: HR = 3.18; non-gallstone related: HR = 2.00) |
| Ludvigsson et al[23], 2007 | 14239 | Pancreatitis any type 0.66% | HR for pancreatitis of any type = 3.3; HR for CP = 19.8 | Patient population was represented by hospital inpatients, leaving out those managed as outpatients |
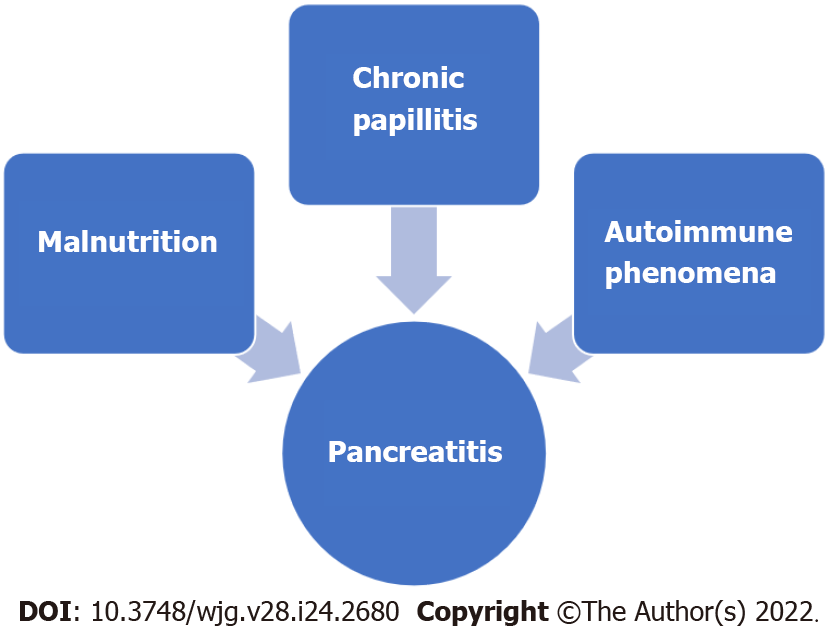
Several pathogenic mechanisms have been theorized to account for the elevated pancreatitis risk in CD–malnutrition, papillary stenosis, and immune phenomena[25] (Figure 2). Severe malnutrition affects pancreatic secretion and can cause pancreatic atrophy[26]. Also, chronic inflammation of the duodenal mucosa in CD patients can also involve the papillary area and lead to papillary stenosis and consequent pancreatic disease[20]. Finally, autoimmune pancreatitis (AIP) or other autoimmune phenomena such as islet-specific autoantibodies in CD-associated type 1 diabetes mellitus can contribute to the link between pancreatitis and CD[27,28].
With regard to CP, results are also discordant using similar diagnostic criteria; while some authors have reported CP features to be common in CD patients undergoing endoscopic ultrasound (EUS) assessment[29], others did not reveal significant structural alterations in the pancreatic parenchyma of CD individuals[30]. In their study using EUS criteria and elastography, Rana et al[30] concluded that PEI is functional and reversible after gluten-free diet (GFD). Supporting this finding, the pathognomonic pancreatic calcifications have been rarely reported in CD[31-35]. However, a biopsy-based study published as abstract has shown CD prevalence as high as 7.4% in established CP, recommending screening in this group[36].
Concerning CD screening in pancreatic diseases, there have been reports of asymptomatic hyperenzymemia, macrolipasemia, or macroamylasemia[37-42] in CD patients, but prevalence studies are missing. According to these reports, decrease or even resolution of macroamylasemia/macrolipasemia or elevated pancreatic enzyme levels can be seen on GFD. Of note, the occurrence of hyperenzymemia in CD can be a confounder for the reported elevated risk of AP associated with CD, by overdiagnosing AP in this scenario.
On the other hand, idiopathic recurrent pancreatitis and sphincter of Oddi dysfunction might be considered a testing indication for CD, given the mechanism of chronic papillitis[20,43]. Non-response to treatment in CP might warrant testing for CD, as suggested in some case reports[31].
CD is a well-recognized less common etiology of PEI[19,44,45]. This is well reported in currently available guidelines[46]. EPI has been reported with variable frequency in CD patients, depending on the test used to diagnose it. Early studies were based on direct pancreatic function testing (pancreatic enzyme or bicarbonate secretions measurements) and found that PEI is common in classical CD, but non-severe[47]. Fecal elastase (FE) is recommended for detecting PEI in newly diagnosed CD[48]. Impairment of pancreatic exocrine function can be seen both in newly diagnosed CD, in up to 80% of cases[19], and in treated CD, where it should be considered a cause of treatment failure in patients unresponsive to GFD[49,50]. In this latter group comprising GFD-treated CD patients with continuing diarrhea, EPI has been reported in 12%-18% of cases[51,52]. While CD patients improve with pancreatic enzyme replacement therapy (PERT), probably paralleling the restoring of mucosal architecture on GFD, some authors have reported that PERT could be discontinued in some patients who experience improvement in symptoms[53]. However, in CD patients with PEI, who report good adherence to GFD but experience continued malabsorption with adequate dosing PERT, additional pathogenic mechanisms such as enteric infections (e.g., Giardia), small intestinal bacterial overgrowth, or complications such as refractory CD and enteropathy associated T-cell lymphoma should be sought[54]. Moreover, gastroparesis in the setting of type 1 diabetes mellitus associated with CD could contribute to incomplete response to PERT. PEI should be readily recognized in slowly recovering children with CD on GFD, as it might accelerate weight increase with adequate enzyme supplementation[55].
Correlation of certain genetic polymorphisms with PEI has also been studied, but without conclusive associations in a small cohort of CD patients[56].
A concern with using FE is represented by the lower diagnostic accuracy compared to direct pancreatic function testing, variability among different tests and analytical processing of samples, taking into account the potential dilution due to diarrhea[57-59].
Despite the increased risk of pancreatitis in CD discussed above, changes in pancreatic enzyme secretion in these patients seen to be mainly functional, as reported by Rana et al[30], who found no correlation with structural parenchymal alterations, assessed by EUS. Another study, based on magnetic resonance imaging assessment, did not reveal morphological changes in CD patients with PEI[60]. This impaired function of the exocrine pancreas is also supported by the inverse correlation between mucosal damage and pancreatic enzyme levels, with reversal of PEI on GFD[19,61]. However, protein malnutrition, also present in CD patients, has been shown to be associated with acinar cell atrophy and fibrosis[18].
The main mechanism of PEI in CD patients is disruption of mucosal release of enteric hormones, mainly CCK and secretin, which represent a potent stimulus for pancreatic function. While exocrine pancreatic function is intrinsically normal, impaired CCK and secretin release from the atrophic mucosa leads to decreased secretion of enzymes into the duodenal lumen. The functional PEI that occurs in CD is reversible upon exogenous administration of CCK-pancreozymin[62]. Others have suggested that PEI can develop independent of this reduced entero-hormone release[63]. Another theorized mechanism is deficiency of amino acids, which leads to reduced protein synthesis for pancreatic enzymes. This mechanism is also backed up by the fact that correction of deficiencies alleviates PEI in CD patients[64]. Another hypothesis, but probably of less significance, is that of structural changes in the pancreatic parenchyma (acinar atrophy, fibrosis) seen in advanced malnutrition[65]. Mechanisms behind EPI in CD are summarized in Table 2[18,47,50,66].
| No. | |
| 1 | Impaired secretion of cholecystokinin and secretin from the diseased small bowel mucosa |
| 2 | Reduced amino acid uptake in the small bowel, which subsequently leads to reduction in precursors for synthesis of pancreatic enzymes |
| 3 | Morphologic alterations in pancreatic parenchyma secondary to protein malnutrition |
Another underrecognized, pancreas-related association for CD is cystic fibrosis (CF), an autosomal recessive disease characterized by mutations in the CF transmembrane conductance regulator gene, which encodes a chloride and bicarbonate channel expressed in epithelial cells in multiple organs[67]. While the pancreatic dysfunction in CF is well known, altered immune regulation has been described in these patients, which predisposes them to developing autoimmune phenomena. The systematic review and meta-analysis by Imrei et al[68] showed pooled prevalence of 1.8%-2.3% of biopsy-proven CD among CF patients, which is more than twice that of the general population[69]. Based on this observation, the authors recommend routine screening for CD in CF patients. This recommendation is supported by another group[70], who suggested performing CD screening in CF with persistent gastrointestinal symptoms or malabsorption (including improper weight and/or height gain in pediatric patients), despite adequate enzyme replacement therapy.
AIP is a chronic fibroinflammatory disease of the pancreas, with a relapsing steroid-responsive pattern. Given the common immune dysregulation background of both AIP and CD, researchers have looked whether there is an association of the two. While some isolated reports have shown a potential link between AIP and CD[71,72], a study looking at CD frequency in a cohort of AIP patients did not show an increased CD prevalence among this group (1.4%) and concluded that serologic screening for CD is not supported[73]. However, a murine model of AIP has provided insight that gliadin sensitization and challenge can induce pancreatitis and extrapancreatic inflammation in HLA-DQ8 transgenic mice[74]. On the other hand, immunoglobulin G4-positive cells, which have been searched for in duodenal/ampullary biopsies as an alternative to pancreatic biopsy for diagnosing AIP, have also been reported in 7 of 18 CD patients in the study by Cebe et al[75]. Given the scarce data on AIP and CD, further research is warranted to conclude if there is a link between the two beyond the plausible biologic mechanism. A recognized association is that of type 2 AIP and ulcerative colitis, and considering the also established connection between CD and inflammatory bowel diseases[76], linking the three diseases might provide some insight on the relationship between AIP and CD.
Pancreatic involvement, both endocrine and exocrine, is frequent in CD and should be searched for in the appropriate clinical setting. Conversely, certain pancreatic-related diseases should prompt looking for CD–CF with ongoing digestive symptoms, non-responsive CP, idiopathic recurrent pancreatitis. Concomitant pancreatic disease in a CD patient may contribute to aggravated malnutrition and should be readily recognized in order to improve nutritional status and prognosis. Also, there is increasing data that impaired pancreatic function in CD can improve on a GFD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society for Gastrointestinal Endoscopy, No. 45910264; Association for Pancreatic Pathology Romania; European Society for the Study of Coeliac Disease (ESsCD); Romanian Society of Gastroenterology and Hepatology; Romanian Society of Digestive Endoscopy; European Pancreatic Club; World Endoscopy Organization
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ashkar M, United States; Ghazanfar A, United Kingdom; Lee Y, South Korea A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Makharia GK, Singh P, Catassi C, Sanders DS, Leffler D, Ali RAR, Bai JC. The global burden of coeliac disease: opportunities and challenges. Nat Rev Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 2. | Pinto-Sanchez MI, Silvester JA, Lebwohl B, Leffler DA, Anderson RP, Therrien A, Kelly CP, Verdu EF. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat Rev Gastroenterol Hepatol. 2021;18:875-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Jinga M, Popp A, Balaban DV, Dima A, Jurcut C. Physicians' attitude and perception regarding celiac disease: A questionnaire-based study. Turk J Gastroenterol. 2018;29:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Therrien A, Kelly CP, Silvester JA. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J Clin Gastroenterol. 2020;54:8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Durazzo M, Ferro A, Brascugli I, Mattivi S, Fagoonee S, Pellicano R. Extra-Intestinal Manifestations of Celiac Disease: What Should We Know in 2022? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Balaban DV, Mihai A, Dima A, Popp A, Jinga M, Jurcut C. Celiac disease and Sjögren's syndrome: A case report and review of literature. World J Clin Cases. 2020;8:4151-4161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Dima A, Jurcut C, Jinga M. Rheumatologic manifestations in celiac disease: what should we remember? Rom J Intern Med. 2019;57:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Ciaccio EJ, Lewis SK, Biviano AB, Iyer V, Garan H, Green PH. Cardiovascular involvement in celiac disease. World J Cardiol. 2017;9:652-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Haggård L, Glimberg I, Lebwohl B, Sharma R, Verna EC, Green PHR, Ludvigsson JF. High prevalence of celiac disease in autoimmune hepatitis: Systematic review and meta-analysis. Liver Int. 2021;41:2693-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Mearns ES, Taylor A, Thomas Craig KJ, Puglielli S, Leffler DA, Sanders DS, Lebwohl B, Hadjivassiliou M. Neurological Manifestations of Neuropathy and Ataxia in Celiac Disease: A Systematic Review. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Rodrigo L, Beteta-Gorriti V, Alvarez N, Gómez de Castro C, de Dios A, Palacios L, Santos-Juanes J. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Elwenspoek MMC, Jackson J, O'Donnell R, Sinobas A, Dawson S, Everitt H, Gillett P, Hay AD, Lane DL, Mallett S, Robins G, Watson JC, Jones HE, Whiting P. The accuracy of diagnostic indicators for coeliac disease: A systematic review and meta-analysis. PLoS One. 2021;16:e0258501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 594] [Article Influence: 99.0] [Reference Citation Analysis (1)] |
| 16. | Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-76; quiz 677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1156] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 17. | Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA, Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL, Walker MM, Zingone F, Sanders DS; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 782] [Article Influence: 71.1] [Reference Citation Analysis (2)] |
| 18. | Freeman HJ. Pancreatic endocrine and exocrine changes in celiac disease. World J Gastroenterol. 2007;13:6344-6346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Singh VK, Haupt ME, Geller DE, Hall JA, Quintana Diez PM. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017;23:7059-7076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (6)] |
| 20. | Patel RS, Johlin FC Jr, Murray JA. Celiac disease and recurrent pancreatitis. Gastrointest Endosc. 1999;50:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Alkhayyat M, Saleh MA, Abureesh M, Khoudari G, Qapaja T, Mansoor E, Simons-Linares CR, Vargo J, Stevens T, Rubio-Tapia A, Chahal P. The Risk of Acute and Chronic Pancreatitis in Celiac Disease. Dig Dis Sci. 2021;66:2691-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Sadr-Azodi O, Sanders DS, Murray JA, Ludvigsson JF. Patients with celiac disease have an increased risk for pancreatitis. Clin Gastroenterol Hepatol. 2012;10:1136-1142.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Ludvigsson JF, Montgomery SM, Ekbom A. Risk of pancreatitis in 14,000 individuals with celiac disease. Clin Gastroenterol Hepatol. 2007;5:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Osagiede O, Lukens FJ, Wijarnpreecha K, Corral JE, Raimondo M, Kröner PT. Acute Pancreatitis in Celiac Disease: Has the Inpatient Prevalence Changed and Is It Associated With Worse Outcomes? Pancreas. 2020;49:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Basha J, Appasani S, Vaiphei K, Singh K, Kochhar R. Celiac disease presenting as recurrent pancreatitis and pseudocyst. JOP. 2012;13:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Brooks SE, Golden MH. The exocrine pancreas in kwashiorkor and marasmus. Light and electron microscopy. West Indian Med J. 1992;41:56-60. [PubMed] |
| 27. | Leeds JS, Sanders DS. Risk of pancreatitis in patients with celiac disease: is autoimmune pancreatitis a biologically plausible mechanism? Clin Gastroenterol Hepatol. 2008;6:951; author reply 951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Sasamori H, Fukui T, Hayashi T, Yamamoto T, Ohara M, Yamamoto S, Kobayashi T, Hirano T. Analysis of pancreatic volume in acute-onset, slowly-progressive and fulminant type 1 diabetes in a Japanese population. J Diabetes Investig. 2018;9:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Kumar S, Gress F, Green PH, Lebwohl B. Chronic Pancreatitis is a Common Finding in Celiac Patients Who Undergo Endoscopic Ultrasound. J Clin Gastroenterol. 2019;53:e128-e129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Rana SS, Dambalkar A, Chhabra P, Sharma R, Nada R, Sharma V, Rana S, Bhasin DK. Is pancreatic exocrine insufficiency in celiac disease related to structural alterations in pancreatic parenchyma? Ann Gastroenterol. 2016;29:363-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Rana SS, Bhasin DK, Sinha SK, Singh K. Coexistence of chronic calcific pancreatitis and celiac disease. Indian J Gastroenterol. 2007;26:150; author reply 150. [PubMed] |
| 32. | Pitchumoni CS, Thomas E, Balthazar E, Sherling B. Chronic calcific pancreatitis in association with celiac disease. Am J Gastroenterol. 1977;68:358-361. [PubMed] |
| 33. | Fitzgerald O, Fitzgerald P, Fennelly J, Mcmullin JP, Boland SJ. A clinical study of chronic pancreatitis. Gut. 1963;4:193-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Nanda R, Anand BS. Celiac disease and tropical calcific pancreatitis. Am J Gastroenterol. 1993;88:1790-1792. [PubMed] |
| 35. | Arya S, Rana SS, Sinha SK, Nagi B, Bhasin DK. Celiac disease and chronic calcific pancreatitis with pancreas divisum. Gastrointest Endosc. 2006;63:1080-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Nett A, Wamsterker E, DiMagno M. Should Patients With Established Chronic Pancreatitis Undergo Testing for Celiac Disease? Abstracts of Papers Submitted to the 47th Meeting of the American Pancreatic Association, October 26–29, 2016, Boston, Massachusetts. Pancreas 2016: 1494–551. |
| 37. | Rabsztyn A, Green PH, Berti I, Fasano A, Perman JA, Horvath K. Macroamylasemia in patients with celiac disease. Am J Gastroenterol. 2001;96:1096-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Bonetti G, Serricchio G, Giudici A, Bettonagli M, Vadacca GB, Bruno R, Coslovich E, Moratti R. Hyperamylasemia due to macroamylasemia in adult gluten enteropathy. Scand J Clin Lab Invest. 1997;57:271-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Bermejo JF, Carbone J, Rodriguez JJ, Macias R, Rodriguez M, Gil J, Marin MA, Torres F, Fernandez-Cruz E. Macroamylasaemia, IgA hypergammaglobulinaemia and autoimmunity in a patient with Down syndrome and coeliac disease. Scand J Gastroenterol. 2003;38:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Barera G, Bazzigaluppi E, Viscardi M, Renzetti F, Bianchi C, Chiumello G, Bosi E. Macroamylasemia attributable to gluten-related amylase autoantibodies: a case report. Pediatrics. 2001;107:E93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | La Villa G, Pantaleo P, Tarquini R, Cirami L, Perfetto F, Mancuso F, Laffi G. Multiple immune disorders in unrecognized celiac disease: a case report. World J Gastroenterol. 2003;9:1377-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Liu Z, Wang J, Qian J, Tang F. Hyperamylasemia, reactive plasmacytosis, and immune abnormalities in a patient with celiac disease. Dig Dis Sci. 2007;52:1444-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Rodrigo L, Alvarez N, Riestra S, de Francisco R, González Bernardo O, García Isidro L, López Vázquez A, López Larrea C. [Relapsing acute pancreatitis associated with gluten enteropathy. Clinical, laboratory, and evolutive characteristics in thirty-four patients]. Rev Esp Enferm Dig. 2008;100:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Kunovský L, Dítě P, Jabandžiev P, Eid M, Poredská K, Vaculová J, Sochorová D, Janeček P, Tesaříková P, Blaho M, Trna J, Hlavsa J, Kala Z. Causes of Exocrine Pancreatic Insufficiency Other Than Chronic Pancreatitis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Leeds JS, Hopper AD, Hurlstone DP, Edwards SJ, McAlindon ME, Lobo AJ, Donnelly MT, Morley S, Sanders DS. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Gheorghe C, Seicean A, Saftoiu A, Tantau M, Dumitru E, Jinga M, Negreanu L, Mateescu B, Gheorghe L, Ciocirlan M, Cijevschi C, Constantinescu G, Dima S, Diculescu M; Romanian Association for Pancreatic Pathology. Romanian guidelines on the diagnosis and treatment of exocrine pancreatic insufficiency. J Gastrointestin Liver Dis. 2015;24:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | DiMagno M. Exocrine Pancreatic Insufficiency and Pancreatitis Associated with Celiac Disease [Internet]. [cited 10 January 2022]. Available from: https://pancreapedia.org/sites/default/files/Exocrine-Pancreatic-Insufficiency-Pancreatitis-Celiac-Disease_0.pdf. |
| 48. | Pezzilli R. Exocrine pancreas involvement in celiac disease: a review. Recent Pat Inflamm Allergy Drug Discov. 2014;8:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Weizman Z, Hamilton JR, Kopelman HR, Cleghorn G, Durie PR. Treatment failure in celiac disease due to coexistent exocrine pancreatic insufficiency. Pediatrics. 1987;80:924-926. [PubMed] |
| 50. | Regan PT, DiMagno EP. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. 1980;78:484-487. [PubMed] |
| 51. | Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: do patients require long-term enzyme supplementation? Dig Dis Sci. 2010;55:2999-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency - Breaking the myths. BMC Med. 2017;15:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Carroccio A, Iacono G, Lerro P, Cavataio F, Malorgio E, Soresi M, Baldassarre M, Notarbartolo A, Ansaldi N, Montalto G. Role of pancreatic impairment in growth recovery during gluten-free diet in childhood celiac disease. Gastroenterology. 1997;112:1839-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Licul V, Cizmarević NS, Ristić S, Mikolasević I, Mijandrusić BS. CTLA-4 +49 and TNF-alpha-308 gene polymorphisms in celiac patients with exocrine pancreatic insufficiency. Coll Antropol. 2013;37:1191-1194. [PubMed] |
| 57. | Brydon WG, Kingstone K, Ghosh S. Limitations of faecal elastase-1 and chymotrypsin as tests of exocrine pancreatic disease in adults. Ann Clin Biochem. 2004;41:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Kampanis P, Ford L, Berg J. Development and validation of an improved test for the measurement of human faecal elastase-1. Ann Clin Biochem. 2009;46:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic Performance of Measurement of Fecal Elastase-1 in Detection of Exocrine Pancreatic Insufficiency: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1220-1228.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 60. | Vujasinovic M, Tepes B, Volfand J, Rudolf S. Exocrine pancreatic insufficiency, MRI of the pancreas and serum nutritional markers in patients with coeliac disease. Postgrad Med J. 2015;91:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Nousia-Arvanitakis S, Karagiozoglou-Lamboudes T, Aggouridaki C, Malaka-Lambrellis E, Galli-Tsinopoulou A, Xefteri M. Influence of jejunal morphology changes on exocrine pancreatic function in celiac disease. J Pediatr Gastroenterol Nutr. 1999;29:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | DiMagno EP, Go WL, Summerskill WH. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology. 1972;63:25-32. [PubMed] |
| 63. | Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991;32:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Tandon BN, Banks PA, George PK, Sama SK, Ramachandran K, Gandhi PC. Recovery of exocrine pancreatic function in adult protein-calorie malnutrition. Gastroenterology. 1970;58:358-362. [PubMed] |
| 65. | Tandon BN, George PK, Sama SK, Ramachandran K, Gandhi PC. Exocrine pancreatic function in protein--calorie malnutrition disease of adults. Am J Clin Nutr. 1969;22:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Buchan AM, Grant S, Brown JC, Freeman HJ. A quantitative study of enteric endocrine cells in celiac sprue. J Pediatr Gastroenterol Nutr. 1984;3:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet. 2021;397:2195-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 404] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 68. | Imrei M, Németh D, Szakács Z, Hegyi P, Kiss S, Alizadeh H, Dembrovszky F, Pázmány P, Bajor J, Párniczky A. Increased Prevalence of Celiac Disease in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 941] [Article Influence: 134.4] [Reference Citation Analysis (1)] |
| 70. | Emiralioglu N, Ademhan Tural D, Hizarcioglu Gulsen H, Ergen YM, Ozsezen B, Sunman B, Saltık Temizel İ, Yalcin E, Dogru D, Ozcelik U, Kiper N. Does cystic fibrosis make susceptible to celiac disease? Eur J Pediatr. 2021;180:2807-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Patel BJ, Cantor M, Retrosi G, Gheorghe R, Wrogemann J, Mujawar Q. Autoimmune Pancreatitis Masquerading as Celiac Disease. Pancreas. 2019;48:e53-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Masoodi I, Wani H, Alsayari K, Sulaiman T, Hassan NS, Nazmi Alqutub A, Al Omair A, H Al-Lehibi A. Celiac disease and autoimmune pancreatitis: an uncommon association. A case report. Eur J Gastroenterol Hepatol. 2011;23:1270-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | De Marchi G, Zanoni G, Conti Bellocchi MC, Betti E, Brentegani M, Capelli P, Zuliani V, Frulloni L, Klersy C, Ciccocioppo R. There Is No Association between Coeliac Disease and Autoimmune Pancreatitis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Moon SH, Kim J, Kim MY, Park do H, Song TJ, Kim SA, Lee SS, Seo DW, Lee SK, Kim MH. Sensitization to and Challenge with Gliadin Induce Pancreatitis and Extrapancreatic Inflammation in HLA-DQ8 Mice: An Animal Model of Type 1 Autoimmune Pancreatitis. Gut Liver. 2016;10:842-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Cebe KM, Swanson PE, Upton MP, Westerhoff M. Increased IgG4+ cells in duodenal biopsies are not specific for autoimmune pancreatitis. Am J Clin Pathol. 2013;139:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Pinto-Sanchez MI, Seiler CL, Santesso N, Alaedini A, Semrad C, Lee AR, Bercik P, Lebwohl B, Leffler DA, Kelly CP, Moayyedi P, Green PH, Verdu EF. Association Between Inflammatory Bowel Diseases and Celiac Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;159:884-903.e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |