Published online Jan 14, 2022. doi: 10.3748/wjg.v28.i2.263
Peer-review started: August 5, 2021
First decision: November 7, 2021
Revised: November 17, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: January 14, 2022
Processing time: 159 Days and 2.5 Hours
Prisoners are at risk of hepatitis C virus (HCV) infection, especially among the people who inject drugs (PWID). We implemented an outreach strategy in combination with universal mass screening and immediate onsite treatment with a simplified pan-genotypic direct-acting antivirals (DAA) regimen, 12 wk of sofosbuvir/velpatasvir, in a PWID-dominant prison in Taiwan.
To implement an outreach strategy in combination with universal mass screening and immediate onsite treatment with a simplified pan-genotypic DAA regimen in a PWID-dominant prison in Taiwan.
HCV-viremic patients were recruited for onsite treatment program for HCV micro-elimination with a pangenotypic DAA regimen, 12 wk of sofosbuvir/ velpatasvir, from two cohorts in Penghu Prison, either identified by mass screen or in outpatient clinics, in September 2019. Another group of HCV-viremic patients identified sporadically in outpatient clinics before mass screening were enrolled as a control group. The primary endpoint was sustained virological response (SVR12, defined as undetectable HCV ribonucleic acid (RNA) 12 wk after end-of-treatment).
A total of 212 HCV-viremic subjects were recruited for HCV micro-elimination campaign; 91 patients treated with sofosbuvir/Ledipasvir or glecaprevir/ pibrentasvir before mass screening were enrolled as a control. The HCV micro-elimination group had significantly lower proportion of diabetes, hypertension, hyperlipidemia, advanced fibrosis and chronic kidney diseases, but higher levels of HCV RNA. The SVR12 rate was comparable between the HCV micro-elimination and control groups, 95.8% (203/212) vs 94.5% (86/91), respectively, in intent-to-treat analysis, and 100% (203/203) vs 98.9% (86/87), respectively, in per-protocol analysis. There was no virological failure, treatment discontinuation, and serious adverse event among sofosbuvir/velpatasvir-treated patients in the HCV micro-elimination group.
Outreach mass screening followed by immediate onsite treatment with a simplified pangenotypic DAA regimen, sofosbuvir/velpatasvir, provides successful strategies toward HCV micro-elimination among prisoners.
Core Tip: We implemented an outreach strategy in combination with universal mass screening and immediate onsite treatment with a simplified pangenotypic direct-acting antivirals egimen, 12 wk of sofosbuvir/velpatasvir, in a people who inject drugs (PWID)-dominant prison. Our study achieved high sustained virological response rate in HCV-infected PWID-dominant prisoners. We provided successful strategies toward HCV micro-elimination among prisoners.
- Citation: Chen CT, Lu MY, Hsieh MH, Tsai PC, Hsieh TY, Yeh ML, Huang CI, Tsai YS, Ko YM, Lin CC, Chen KY, Wei YJ, Hsu PY, Hsu CT, Jang TY, Liu TW, Liang PC, Hsieh MY, Lin ZY, Huang CF, Huang JF, Dai CY, Chuang WL, Shih YL, Yu ML. Outreach onsite treatment with a simplified pangenotypic direct-acting anti-viral regimen for hepatitis C virus micro-elimination in a prison. World J Gastroenterol 2022; 28(2): 263-274
- URL: https://www.wjgnet.com/1007-9327/full/v28/i2/263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i2.263
Hepatitis C virus (HCV) infection is a progressive and blood-borne infectious disease that can lead to end stage liver diseases, such as hepatic decompensation, liver cirrhosis, and hepatocellular carcinoma[1,2]. Iatrogenic transmission of HCV, such as blood transfusion and surgery, has decreased in developed countries. Whereas people who inject drugs (PWID) has become the major population of HCV transmission, which could consist of approximately 80% of HCV-infected patients[3]. Given that lack of vaccine available, “treatment as prevention” for HCV transmission in PWID is very important for HCV elimination.
Prisoners are at high risk of HCV infection, with prevalence rates ranging from 3.1% to 38%[4,5]. The high prevalence of HCV infection in prisoners is resulted from unsafe lifestyles, psychiatric disorders, and social problems before they are incarcerated. Recently, PWID has been the most important risk factor of HCV infection in prisoners[6]. The anti-HCV prevalence rate could be as high as 91% among PWID prisoners[7]. Screening and eliminating HCV infection in prisoners is therefore an important social health issue.
According to the American Association for the Study of Liver Diseases and European Association for the Study of the Liver (EASL) guidelines, all HCV viremic patients should be treated if life span is expected more than one year[8,9]. HCV therapeutic strategies have been revolutionized significantly because of the availability of direct-acting antivirals (DAA)[10]. Interferon (IFN)-based regimens for HCV infection have serious side effects, long therapeutic duration, and contraindications, leading to the huge gaps in HCV care cascade[11]. The current IFN-free DAA regimens provide shorter treatment duration, very high treatment efficacy and safety profiles, not only for general population[12], but also for special populations[13], such as HCV/human immunodeficiency virus (HIV) coinfected patients, hepatitis B virus (HBV)/HCV coinfected patients and patients with chronic kidney diseases in real-world clinical settings[14,15].
World Health Organization (WHO) set a global goal of HCV elimination by 2030[16], and Taiwan authority is even ambitious by 2025[17]. To achieve the goal, imple
Recently, the latest EASL HCV guideline recommended simplified, genotyping/ subtyping-free, pangenotypic anti-HCV treatment, either sofosbuvir/ velpatasvir or glecaprevir/pibrentasvir, to increase the accessibility and global cure rates among patients with > 12 years, chronic hepatitis C without cirrhosis or with compensated cirrhosis, with or without HIV co-infection, whatever treatment-naïve or IFN-experienced[8].
Since HCV treatment is not frequently administered to prisoners due to unawa
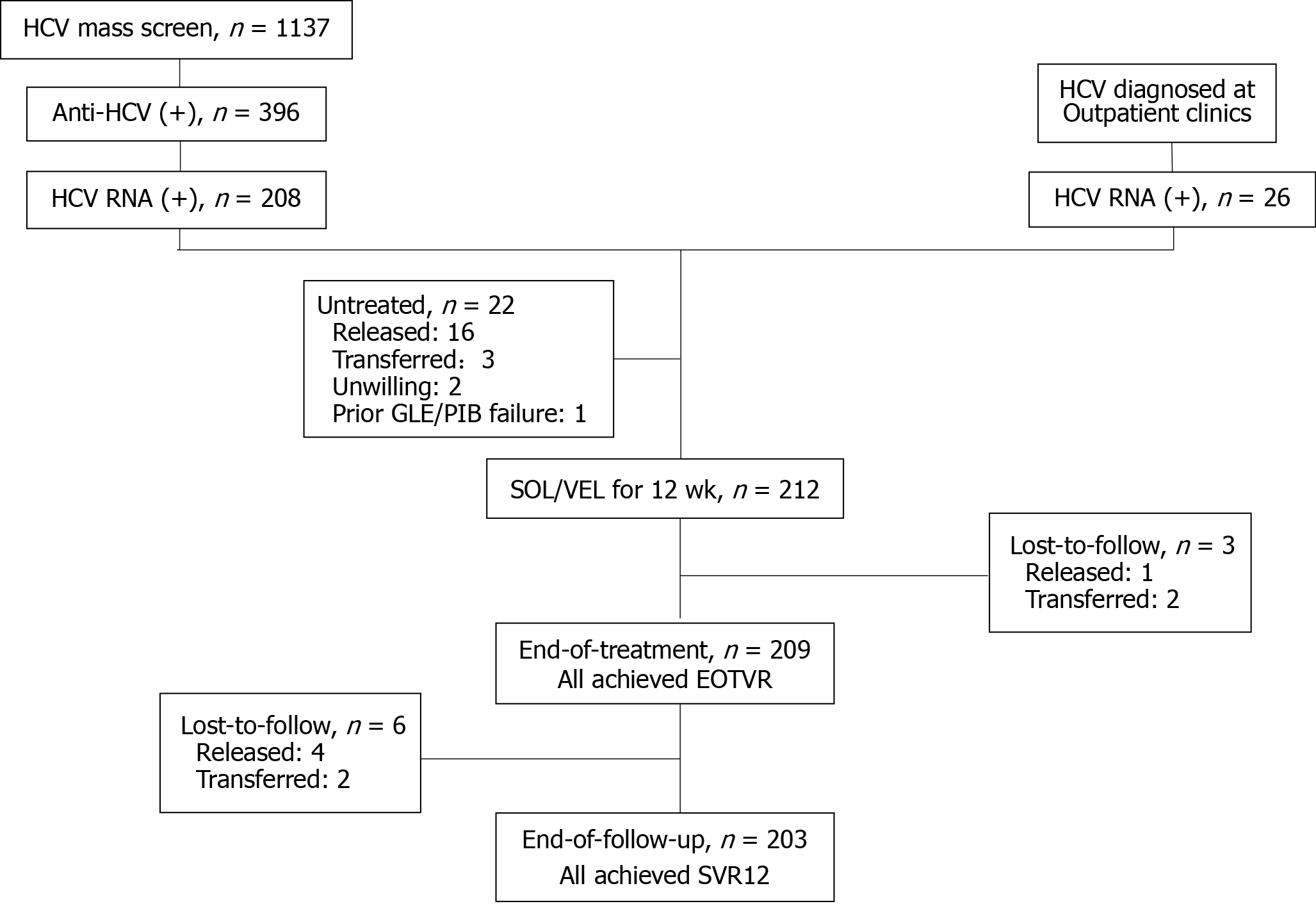
HCV-viremic patients were recruited from two cohorts in Penghu Prison (Agency of Corrections, Ministry of Justice, Taiwan), a PWID-dominant prison (Figure 1).
In September 2019, we conducted a 5 d universal mass screening of viral hepatitis in Penghu Prison. These inclusion criteria were prisoners, who were at least 20 years old, being willing to enter the study for screening of viral hepatitis. The study of mass screening was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (IRB: KMUHIRB-SV(I)-20190033). All participants provided written informed consents. A total of 1137 subjects from 1697 inmates participated the mass screening[21]. Among them, 396 (34.8%) subjects had anti-HCV seropositivity; 208 (52.5%) of the 396 subjects were seropositive for HCV ribonucleic acid (RNA) and linked to the onsite HCV treatment program with universal sofosbuvir/velpatasvir regimen.
Another 26 HCV-viremic subjects identified in outpatient clinics of Penghu Prison between August to December 2019 were also linked to the onsite HCV treatment program with universal sofosbuvir/velpatasvir regimen.
All patients received pretreatment evaluation in December 2019, including medical history, liver and renal function tests, complete blood cell counts, HCV viral loads and genotyping, abdominal sonography and assessment of potential drug–drug interactions. A 12 wk, oral pan-genotypic regimen of sofosbuvir/velpatasvir 400/100 mg fixed-dose combination once daily was initiated in January-February 2020.
A total of 91 HCV-viremic patients identified in outpatient clinics of Penghu Prison and treated with DAA before mass screening from 2017 to 2019 were enrolled as a control. The selection of DAA regimens were based on physician’s discretion acc
All participants signed informed consent forms. These enrolled inmates of our study were protected according to the guidelines of the Declaration of Helsinki. The current study of DAA therapy was approved by the Institutional Review Board of Tri-Service General Hospital (IRB: TSGHIRB 2-107-05-080).
Anti-HCV antibody was determined by the third generation, commercially available immunoassay (Ax SYM HCV III; Abbott Laboratories, North Chicago, IL). HCV RNA viral loads and genotype were determined by real-time PCR assays [RealTime HCV; Abbott Molecular, Des Plaines IL, United States; detection limit: 12 IU/mL])[22]. Liver cirrhosis was defined by the presence of clinical, radiological, endoscopic or laboratory evidence of cirrhosis and/or portal hypertension or fibrosis-4 index (FIB-4) (> 6.5). Laboratory data monitoring and assessment of side effects were performed at treatment wk 2, 4, 8 and end-of-treatment (EOT), and 12 wk after EOT.
The primary endpoint was sustained virological response (SVR12, defined as undetectable HCV RNA throughout 12 wk of the post-treatment follow-up period).
The efficacy of all DAA regimens was determined in a intent-to-treat (ITT) population (all enrolled patients with at least one dose of DAA) and a per-protocol (PP) population (subjects receiving at least one dose of DAA and retained in Penghu Prison throughout the DAA treatment and follow-up period). Safety assessments reported adverse event (AE), serious adverse event (SAE) and laboratory abnormalities in the ITT population. Continuous variables are expressed as means ± standard deviation (SD), and categorical variables are expressed as percentages. The differences of continuous variables are estimated by the Student’s t test. The differences in categorical variables are analyzed using the Chi-square test. The on-treatment and off-treatment virological response rates were analyzed in number and percentages with 95% confidence interval (CI). All data analyses were performed using the SPSS software version 18.0 (SPSS Inc., Chicago, Illinois, United States).
The patient flowchart of HCV mass screen, assessment and treatment was shown in Figure 1. A total of 234 HCV-viremic patients, 208 from mass screening and 26 from outpatient clinics in Penghu Prison were assessed for eligibility of group therapy with sofosbuvir/velpatasvir in December 2019. Twenty-two patients were excluded from anti-HCV therapy due to scheduled to be released from jail (n = 16) or transferred to other jails (n = 3) within 6 mo, unwilling to receive therapy (n = 2) and prior glecaprevir/pibrentasvir treatment failure (n = 1). Finally, 212 patients were recruited for sofosbuvir/velpatasvir therapy initiated in January-February 2020.
The baseline characteristics of 303 HCV-viremic patients, including 212 in HCV micro-elimination campaign and 91 sporadic controls from outpatient clinics before micro-elimination campaign were listed in Table 1. They mean age was 48.4 years with male dominant (99.7%). Thirty (9.9%) had HBV coinfection. The mean FIB-4 was 1.3, with 20 (6.6%) had advanced fibrosis (FIB-4 > 3.25). Only one patient (0.3%) had liver cirrhosis. The mean HCV RNA levels was 6.5 Logs IU/mL, dominant with HCV genotype 1 (HCV-GT1, 42.2%), followed by HCV-GT6 (35.3%), HCV-GT3 (11.6%) and HCV-GT2 (10.6%). Three (1%) patients were prior IFN-experienced. The two groups had comparable characteristics in terms of age, gender, HBV co-infection, liver and renal function tests, FIB-4 score, HCV genotype distribution, and prior history of IFN-based therapy. However, the sporadic patients identified in outpatient clinics had significantly higher proportion of comorbidities, including diabetes, hypertension, hyperlipidemia and an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, but significantly lower HCV viral loads. None of patient had decompensated cirrhosis nor liver cancer.
| Total | Sporadic HCV therapy in outpatient clinics (January 1, 2019 - December 31, 2019) | Campaign of HCV micro-elimination (January 1, 2020 - March 31, 2020) | P value | |
| n | 303 | 91 | 212 | - |
| Age (yr) | 48.4 ± 8.2 | 47.6 ± 8.7 | 48.7 ± 8.0 | 0.271 |
| Male | 303 (99.7) | 90 (98.9) | 212 (100.0) | 0.126 |
| 1BMI, kg/m2 | 23.9 ± 3.2 | 23.9 ± 3.3 | 23.9 ± 3.2 | 0.986 |
| > 27 kg/m2 | 34 (13.8) | 11 (13.9) | 23 (13.4) | 0.960 |
| Diabetes | 10 (3.3) | 8 (8.8) | 2 (0.9) | 0.0005a |
| Hypertension | 59 (19.5) | 25 (27.5) | 34 (16.0) | 0.021a |
| Hyperlipidemia | 8 (2.6) | 7 (7.7) | 1 (0.5) | 0.0003a |
| Cardiovascular disease | 2 (0.7) | 1 (1.1) | 1 (0.5) | 0.537 |
| HBsAg (+) | 30 (9.9) | 9 (9.9) | 21 (9.9) | 0.997 |
| AST, IU/L | 41.3 ± 35.5 | 45.9 ± 38.9 | 39.4 ± 33.8 | 0.168 |
| ALT, IU/L | 65.4 ± 77.4 | 71.6 ± 69.8 | 62.7 ± 80.4 | 0.329 |
| Abnormal AST or ALT | 159 (52.5) | 54 (59.3) | 105 (49.5) | 0.117 |
| White cell count, × 103/ìL | 6.6 ± 1.9 | 6.4 ± 2.0 | 6.7 ± 1.8 | 0.188 |
| Hemoglobin concentration, g/dL | 15.9 ± 1.3 | 16.0 ± 1.3 | 15.9 ± 1.3 | 0.762 |
| Platelet count, × 103u/L | 227.6 ± 67.4 | 219.4 ± 72.1 | 231.2 ± 65.1 | 0.181 |
| Albumin, g/dl | 4.5 ± 0.3 | 4.5 ± 0.4 | 4.5 ± 0.2 | 0.233 |
| Total bilirubin, mg/dL | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.003a |
| LC | 1 (0.3) | 1 (1.1) | 0 (0.0) | 0.300 |
| FIB-4 | 1.3 ± 1.0 | 1.5 ± 1.4 | 1.2 ± 0.8 | 0.096 |
| > 3.25 | 20 (6.6) | 10 (11.0) | 10 (4.7) | 0.044a |
| eGFR, mL/min/1.73 m2 | 99.9 ± 17.7 | 99.1 ± 21.0 | 100.3 ± 16.4 | 0.624 |
| < 60 | 4 (1.3) | 3 (3.3) | 1 (0.4) | 0.048a |
| HCV RNA, log10 IU/mL | 6.5 ± 1.1 | 6.0 ± 1.0 | 6.7 ± 1.1 | < 0.001a |
| HCV genotype, 1/2/1+2/3/6 | 128 (42.2)/32 (10.6)/1 (0.3)/35 (11.6)/107 (35.3) | 38 (41.8)/9 (9.9)/0/11 (12.1)/33 (36.2) | 90 (42.5)/23 (10.8)/1 (0.5)/24 (11.3)/74 (34.9) | 0.968 |
| DAA regimen | ||||
| SOF/VEL | 212 (70.0) | 0 (0.0) | 212 (100.0) | < 0.001a |
| SOF/LDV | 78 (25.7) | 78 (85.7) | 0 (0.0) | |
| GLE/PIB | 13 (4.3) | 13 (14.3) | 0 (0.0) | |
| Prior treatment history | ||||
| Naïve | 300 (99.0) | 89 (97.8) | 211 (99.5) | 0.216 |
| Experienced-IFN | 3 (1.0) | 2 (2.2) | 1 (0.5) |
All of 212 patients in HCV micro-elimination campaign received sofosbuvir/ velpatasvir treatment; while among 91 sporadic patients with DAA therapy before HCV micro-elimination campaign, 78 (85.7%) received 12 wk of sofosbuvir/Ledipasvir and 13 (14.3%) received 8-12 wk of glecaprevir/pibrentasvir according to the Taiwan HCV guideline[12,13].
In ITT analysis, the overall SVR12 rate was 95.4% (289/303) with comparable SVR12 rates between sporadic HCV control group (94.5%, 86/91) and HCV micro-elimination group (95.8%, 203/212, P = 0.126, Table 2).
| Undetectable HCV RNA, n/N (%) | Total | Sporadic HCV therapy in outpatient clinics (January 1, 2019 - December 31, 2019) | Campaign of HCV micro-elimination with simplified pan-genotypic SOF/VEL regimen (January 1, 2020 - March 31, 2020) | P value |
| Intention-to-treat population | ||||
| Treatment 4 wk | 284/303 (93.7) | 85/91 (93.4) | 199/212 (93.9) | 0.879 |
| End-of-treatment | 300/303 (99.0) | 91/91 (100.0) | 209/212 (98.6) | 0.557 |
| End-of 12 wk follow-up | 289/303 (95.4) | 86/91 (94.5) | 203/212 (95.8) | 0.126 |
| Per-protocol population | ||||
| Treatment 4 wk | 284/301 (94.4) | 85/901 (94.4) | 199/2112 (94.3) | 0.964 |
| End-of-treatment | 300/300 (100.0) | 91/91 (100.0) | 209/2093 (100.0) | - |
| End-of 12 wk follow-up | 289/290 (99.7) | 86/874 (98.9)e5 | 203/2036 (100.0) | 0.126 |
During DAA treatment period, all of patients in sporadic HCV control group completed DAA therapy, while 3 patients in HCV micro-elimination group lost-to-follow (2 transferred; 1 released). During the post-treatment follow-up period, 4 patients in sporadic HCV control group lost-to-follow (4 released), while 6 patients in HCV micro-elimination group lost-to-follow (2 transferred; 4 released). In PP analysis, the overall SVR12 rate was 99.7% (289/290) with comparable SVR12 rates between sporadic HCV control group (98.9%, 86/87) and HCV micro-elimination group (100%, 203/203, P = 0.126, Table 2). Only one patient experienced virological failure (54 years old male, treatment-naïve, HCV-GT3 infection with baseline viral loads of 62,883 IU/mL and FIB-4 of 2.37; relapsed from a 12 wk regimen of glecaprevir/pibrentasvir).
The safety profiles of both groups were shown in Table 3. None of patients had treatment discontinuation other than released or transferred. None experienced serious adverse event. The frequency of adverse events was 4.3% (4/91) and 1.4% (3/212), respectively, among patients in sporadic control group and HCV micro-elimination group. The most reported adverse events were rash in 3 of 13 (23.1%) patients treated with glecaprevir/pibrentasvir and pruritus in 2 of 212 (0.9%) patients treated with sofosbuvir/velpatasvir. None of patients experienced grade 3 or 4 Laboratory abnormality.
| n (%) | Total | Sporadic HCV therapy in outpatient clinics (January 1, 2019 - December 31, 2019) | Campaign of HCV micro-elimination with simplified pan-genotypic SOF/VEL regimen (January 1, 2020 - March 31, 2020) |
| n | 303 | 91 | 212 |
| Treatment discontinuation other than released or transferred | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious adverse events | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse events | 7 (2.3) | 4 (4.3) | 3 (1.4) |
| Fatigue | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus | 2 (0.7) | 0 (0.0) | 2 (0.9) |
| Rash | 3 (1.0) | 3 (3.2) | 0 (0.0) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dizziness | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Insomnia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Headache | 1 (0.3) | 0 (0.0) | 1 (0.5) |
| Others | 1 (0.3) | 1 (1.0) | 0 (0.0) |
| Grade 3 or 4 laboratory abnormalities | |||
| Total blood bilirubin | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alanine aminotransferase | 0 (0.0) | 0 (0.0) | 0 (0.0) |
In the current study, we demonstrated that mass screening combined with onsite group therapy by using a simplified pan-genotypic DAA regimen, 12 wk of sofosbuvir/velpatasvir, provides an “one-size fits all” solution toward the achie
Recent advance in the development of IFN-free pan-genotypic DAA regimens has remarkably improved the treatment efficacy with an overall SVR rates of > 90%. Therefore, WHO set the global of HCV elimination by 2030, through the achievement of > 90% diagnosis rate and > 80% treatment rate for eligible patients[16]. Nevertheless, there are many barriers in each HCV care cascade toward HCV elimination at the population level[11,23]. To overcome the barriers, combining the concept of micro-elimination and an outreach strategy with immediate onsite treatment would be a more efficient and practical approach to achieve that goal[18,24]. The current study compared the HCV-infected inmates identified sporadically in outpatient clinics of Penghu Prison from 2017 to 2019 before mass screening and the patients identified by mass screening. We found that mass screening identified 208 HCV-viremic patients in a 5 d screening program from 1137 inmates (encountered around two-third of total inmates in Penghu Prison), compared to 91 HCV-viremic inmates treated in outpatient clinics from 2017 to September 2019. Our results demonstrated that mass screening with immediate onsite treatment provide much more efficient and practical solution to overcome the gaps of disease awareness and link-to-care in the HCV care cascades toward HCV micro-elimination in prisoners. In addition, we implemented “HCV reflex testing” in the mass screening program to scale-up and speed-up the diagnosis and link-to-care for treatment uptake of HCV infections[25].
PWID is known as the major risk factor of HCV infection and transmission. Although the anti-HCV prevalence in PWID prisoners decreased from 91% in 2014 to 34.8% in 2019 by the strategy of safe injection in Taiwan[21], almost all (97.6%) of HCV-infected prisoners were PWID. Given the lack of vaccine available and high risk of transmission, the strategy of universal screening and concept of “treatment as prevention” are the keys to HCV elimination in prison as well as PWID.
We observed that the sporadic HCV-infected prisoners identified in outpatient clinics had significantly higher proportion of comorbidities, including diabetes, hypertension, hyperlipidemia and eGFR, than those participating in the HCV micro-elimination campaign. It implicated that a great proportion of identified sporadically in outpatient clinics were due to concomitant morbidities; by contrast, many HCV-infected patients were unaware to their HCV diseases. In our mass screening, only 36.6% (145/396) of HCV-infected prisoners were aware of HCV infection before screening[21]. It indicates that the implementation of an outreach strategy with universal mass screen is necessary for HCV micro-elimination in prison.
Despite of the advances in the management of HCV infections, DAA therapy in incarcerated HCV-infected people remains many obstacles to be resolved, including disease unawareness, lack of updated information about the benefits of new DAA treatment, uncertainty of treatment right[26], poor accessibility due to of onsite treatment facilities or HCV treaters. Another difficulty for HCV treatment in prisoners is the unexpected or scheduled releasing from prison or transferring to other prisons, which frequently leads to the interruption of treatment or lost-to- follow up[20,27]. We are lucky that the Taiwan Health Insurance covered all incarcerated people, including all of the laboratory tests and ultrasound sonography and the cost of DAA regimens. Each prison has a contracted hospital providing point-of-care facility. Before initiating DAA therapy, we excluded the patients with expected release or transfer within 24 wk, and negotiated with the authority to avoid unnecessary transferring to other prisons during the period of HCV treatment and follow-up once the inmates entering the DAA course. Eventually we achieved a high treatment rate of 90.6% (212/234) and a high treatment complete rate of 95.8% (203/212), with a high cure rate at 100% (212/212).
Before the IFN-free DAA available, the lower SVR rate, much longer treatment duration and frequent adverse events of IFN-based treatment discouraged HCV-infected prisoners from receiving treatment[10]. IFN-free DAA regimens revolutionized HCV treatment which has largely extended the indication for various HCV-infected patients. Nevertheless, the application of typical DAA regimens are based on HCV genotype, presence of decompensated cirrhosis, renal function, and prior treatment experience. The two pangenotypic DAA regimens, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir, have achieved very high SVR rates of > 95%, regardless of HCV GTs, except for treatment-experienced cirrhotic HCV GT3 patients or GT3b patients[8,12,13]. Recently, to improve the access to anti-HCV therapy, reduce the cost of laboratory tests and the relative complexity of genotype-based treatment strategies, simplified treatment without many information needed for treatment decision are recommended to facilitate the care cascade among populations who are historically less engaged in healthcare, such as PWIDs and prisoners[8]. EASL recommends simplified, genotyping/subtyping-free regimens for IFN-free DAA treatment-naïve (except sofosbuvir plus ribavirin), HCV-infected or HCV-HIV coinfected adolescent and adult patients without cirrhosis or with compensated cirrhosis, regardless of HCV genotypes[8]. These recommendations are a universal 12 wk regimen of sofos
In our study, none of prisoners had DAA treatment discontinuation due to adverse events. None experienced serious adverse event. These data indicated that the simplified, genotyping/subtyping-free regimen, sofosbuvir/velpatasvir, was safe and well tolerated for HCV-infected PWID-dominant prisoners. Very few adverse events were reported in both groups, whatever using sofosbuvir/Ledipasvir, glecaprevir/ pibrentasvir and sofosbuvir/velpatasvir, when compared to the data from clinical trials[30,31]. It might be due to that current population was younger and less patients with advanced fibrosis or chronic kidney diseases.
There were some limitations in our study. First, not all inmates in Penghu Prison participated our mass screening. Strategies and policy to encourage inmates to receive HCV screening is mandatory to achieve the goal of WHO. Second, unexpected prisoners’ transferral and release could not be completely avoided, which caused incomplete treatment and follow-up. Successfully linking the released or transferred people to another HCV treaters could help completing HCV treatment and follow-up. Third, there was no reimbursement for the retreatment of prior DAA failed patients in Taiwan at the time of the current study.
Well-designed strategies for mass screening and treatment for HCV-infected prisoners can be implemented successfully by the collaboration between physicians and prison authorities. We demonstrated that mass screening followed by immediate onsite treatment with a simplified pangenotypic DAA regimen, sofosbuvir/velpatasvir, provides successful strategies toward HCV micro-elimination among prisoners.
Prisoners are at high risk of hepatitis C virus (HCV) infection. To screen and treat HCV infection in prisoners is an important social health issue. It can be the start for HCV micro-elimination.
HCV treatment is not frequently administered to prisoners due to multiple factors. Therefore, we implemented an outreach strategy in combination with universal mass screen and onsite treatment in a prison.
To implement an outreach strategy. HCV-infected prisoners received a simplified pan-genotypic direct-acting antivirals (DAA) regimen, 12 wk of sofosbuvir/velpatasvir. The primary endpoint was sustained virological response (SVR12, defined as undetectable HCV RNA throughout 12 wk of the post-treatment follow-up period).
All participants received blood tests. We used reflex testing. All HCV-infected prisoners received DAA therapy. Laboratory data monitoring and assessment of side effects were performed at treatment wk 2, 4, 8 and end-of-treatment (EOT), and 12 wk after EOT.
DAA regimen with sofosbuvir/velpatasvir achieved high SVR12 rate. There was no virological failure, treatment discontinuation, and serious adverse event among sofosbuvir/velpatasvir-treated patients in the HCV micro-elimination group.
Well-designed strategies for mass screening and treatment for HCV-infected prisoners can be implemented successfully by the collaboration between physicians and prison authorities.
Our study provided evidence for the concept that simplified, genotyping/subtyping-free regimens can achieve high SVR12 rate in HCV-infected prisoners. In the future, it is possible to implement the strategy to all prisoners in our country.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ballestín SS, Chang TS S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A, Sharma BC, Hamid SS, Dokmeci AK, Al-Mahtab M, McCaughan GW, Wasim J, Crawford DH, Kao JH, Yokosuka O, Lau GK, Sarin SK. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Yu ML, Chuang WL. Path from the discovery to the elimination of hepatitis C virus: Honoring the winners of the Nobel Prize in Physiology or Medicine 2020. Kaohsiung J Med Sci. 2021;37:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Prevost TC, Presanis AM, Taylor A, Goldberg DJ, Hutchinson SJ, De Angelis D. Estimating the number of people with hepatitis C virus who have ever injected drugs and have yet to be diagnosed: an evidence synthesis approach for Scotland. Addiction. 2015;110:1287-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Santos BF, de Santana NO, Franca AV. Prevalence, genotypes and factors associated with HCV infection among prisoners in Northeastern Brazil. World J Gastroenterol. 2011;17:3027-3034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Reekie JM, Levy MH, Richards AH, Wake CJ, Siddall DA, Beasley HM, Kumar S, Butler TG. Trends in HIV, hepatitis B and hepatitis C prevalence among Australian prisoners - 2004, 2007, 2010. Med J Aust. 2014;200:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Rosa Fd, Carneiro M, Duro LN, Valim AR, Reuter CP, Burgos MS, Possuelo L. Prevalence of anti-HCV in an inmate population. Rev Assoc Med Bras (1992). 2012;58:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hsieh MH, Tsai JJ, Hsieh MY, Huang CF, Yeh ML, Yang JF, Chang K, Lin WR, Lin CY, Chen TC, Huang JF, Dai CY, Yu ML, Chuang WL. Hepatitis C virus infection among injection drug users with and without human immunodeficiency virus co-infection. PLoS One. 2014;9:e94791. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members.. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 778] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 9. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 10. | Yu ML. Hepatitis C treatment from "response-guided" to "resource-guided" therapy in the transition era from interferon-containing to interferon-free regimens. J Gastroenterol Hepatol. 2017;32:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Yu ML, Yeh ML, Tsai PC, Huang CI, Huang JF, Huang CF, Hsieh MH, Liang PC, Lin YH, Hsieh MY, Lin WY, Hou NJ, Lin ZY, Chen SC, Dai CY, Chuang WL, Chang WY. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan. Medicine (Baltimore). 2015;94:e690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, Peng CY, Lin HC, Kao JH, Chuang WL. 2020 Taiwan consensus statement on the management of hepatitis C: part (I) general population. J Formos Med Assoc. 2020;119:1019-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, Peng CY, Lin HC, Kao JH, Chuang WL. 2020 Taiwan consensus statement on the management of hepatitis C: Part (II) special populations. J Formos Med Assoc. 2020;119:1135-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Yeh ML, Huang CF, Huang CI, Holmes JA, Hsieh MH, Tsai YS, Liang PC, Tsai PC, Hsieh MY, Lin ZY, Chen SC, Huang JF, Dai CY, Chuang WL, Chung RT, Yu ML. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. 2020;73:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Martin NK, Jansen K, An der Heiden M, Boesecke C, Boyd A, Schewe K, Baumgarten A, Lutz T, Christensen S, Thielen A, Mauss S, Rockstroh JK, Skaathun B, Ingiliz P. Eliminating Hepatitis C Virus Among Human Immunodeficiency Virus-Infected Men Who Have Sex With Men in Berlin: A Modeling Analysis. J Infect Dis. 2019;220:1635-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Lancaster K, Rhodes T, Rance J. "Towards eliminating viral hepatitis": Examining the productive capacity and constitutive effects of global policy on hepatitis C elimination. Int J Drug Policy. 2020;80:102419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wu GH, Pwu RF, Chen SC, Chen DS. Taiwan is on track of accelerating hepatitis C elimination by 2025. Liver Int. 2020;40:1506-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, Hatzakis A, Jadoul M, Prestileo T, Razavi H, Rockstroh JK, Wiktor SZ, Colombo M. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin Liver Dis. 2018;38:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 19. | Yu ML, Huang CF, Wei YJ, Lin WY, Lin YH, Hsu PY, Hsu CT, Liu TW, Lee JJ, Niu SW, Huang JC, Hung TS, Yeh ML, Huang CI, Liang PC, Hsieh MY, Chen SC, Huang JF, Chang JM, Chiu YW, Dai CY, Hwang SJ, Chuang WL; FORMOSA-LIKE investigators. Establishment of an outreach, grouping healthcare system to achieve microelimination of HCV for uremic patients in haemodialysis centres (ERASE-C). Gut. 2021;70:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Lloyd AR, Clegg J, Lange J, Stevenson A, Post JJ, Lloyd D, Rudge G, Boonwaat L, Forrest G, Douglas J, Monkley D. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis. 2013;56:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Lu MY, Chen CT, Shih YL, Tsai PC, Hsieh MH, Huang CF, Yeh ML, Huang CI, Wang SC, Tsai YS, Ko YM, Lin CC, Chen KY, Wei YJ, Hsu PY, Hsu CT, Jang TY, Liu TW, Liang PC, Hsieh MY, Lin ZY, Chen SC, Huang JF, Dai CY, Chuang WL, Yu ML, Chang WY. Changing epidemiology and viral interplay of hepatitis B, C and D among injecting drug user-dominant prisoners in Taiwan. Sci Rep. 2021;11:8554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Vermehren J, Yu ML, Monto A, Yao JD, Anderson C, Bertuzis R, Schneider G, Sarrazin C. Multi-center evaluation of the Abbott RealTime HCV assay for monitoring patients undergoing antiviral therapy for chronic hepatitis C. J Clin Virol. 2011;52:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Huang CF, Yu ML. Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy. Clin Mol Hepatol. 2020;26:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Huang CF, Wu PF, Yeh ML, Huang CI, Liang PC, Hsu CT, Hsu PY, Liu HY, Huang YC, Lin ZY, Chen SC, Huang JF, Dai CY, Chuang WL, Yu ML. Scaling up the in-hospital hepatitis C virus care cascade in Taiwan. Clin Mol Hepatol. 2021;27:136-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Daniels AM, Studdert DM. Hepatitis C Treatment in Prisons - Incarcerated People's Uncertain Right to Direct-Acting Antiviral Therapy. N Engl J Med. 2020;383:611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Yap L, Carruthers S, Thompson S, Cheng W, Jones J, Simpson P, Richards A, Thein HH, Haber P, Lloyd A, Butler T. A descriptive model of patient readiness, motivators, and hepatitis C treatment uptake among Australian prisoners. PLoS One. 2014;9:e87564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lampertico P, Mauss S, Persico M, Barclay ST, Marx S, Lohmann K, Bondin M, Zhang Z, Marra F, Belperio PS, Wedemeyer H, Flamm S. Real-World Clinical Practice Use of 8 wk Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis. Adv Ther. 2020;37:4033-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Liu CH, Yu ML, Peng CY, Hsieh TY, Huang YH, Su WW, Cheng PN, Lin CL, Lo CC, Chen CY, Chen JJ, Ma Q, Brooks-Rooney C, Kao JH. Comorbidities, concomitant medications and potential drug-drug interactions with interferon-free direct-acting antiviral agents in hepatitis C patients in Taiwan. Aliment Pharmacol Ther. 2018;48:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Chuang WL, Chien RN, Peng CY, Chang TT, Lo GH, Sheen IS, Wang HY, Chen JJ, Yang JC, Knox SJ, Gao B, Garrison KL, Mo H, Pang PS, Hsu YC, Hu TH, Chu CJ, Kao JH. Ledipasvir/sofosbuvir fixed-dose combination tablet in Taiwanese patients with chronic genotype 1 hepatitis C virus. J Gastroenterol Hepatol. 2016;31:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |