Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1798
Peer-review started: October 28, 2021
First decision: December 12, 2021
Revised: December 21, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 7, 2022
Processing time: 182 Days and 19.3 Hours
The occurrence and development of acute liver failure (ALF) is closely related to a series of inflammatory reactions, such as the production of reactive oxygen species (ROS). Hypoxia inducible factor 1α (HIF-1α) is a key factor that regulates oxygen homeostasis and redox, and the stability of HIF-1α is related to the ROS level regulated by Sirtuin (Sirt) family. The activation of Sirt1 will lead to a powerful antioxidant defense system and therapeutic effects in liver disease. However, little is known about the relationship between HIF-1α and Sirt1 in the process of ALF and the molecular mechanism.
To investigate whether HIF-1α may be a target of Sirt1 deacetylation and what the effects on ALF are.
Mice were administrated lipopolysaccharide (LPS)/D-gal and exposed to hypoxic conditions as animal model, and resveratrol was used as an activator of Sirt1. The cellular model was established with L02 cells stimulated by LPS. N-acetyl-L-cysteine was used to remove ROS, and the expression of Sirt1 was inhibited by nicotinamide. Western blotting was used to detect Sirt1 and HIF-1α activity and related protein expression. The possible signaling pathways involved were analyzed by immunofluorescent staining, co-immunoprecipitation, dihydroethidium staining, and Western blotting.
Compared with mice stimulated with LPS alone, the expression of Sirt1 decreased, the level of HIF-1α acetylation increased in hypoxic mice, and the levels of carbonic anhydrase 9 and Bcl-2-adenovirus E1B interacting protein 3 increased significantly, which was regulated by HIF-1α, indicating an increase of HIF-1α activity. Under hypoxia, the down-regulation of Sirt1 activated and acetylated HIF-1α in L02 cells. The inhibition of Sirt1 significantly aggravated this effect and the massive production of ROS. The regulation of ROS was partly through peroxisome proliferator-activated receptor alpha or AMP-activated protein kinase. Resveratrol, a Sirt1 activator, effectively relieved ALF aggravated by hypoxia, the production of ROS, and cell apoptosis. It also induced the deacetylation of HIF-1α and inhibited the activity of HIF-1α.
Sirt1 may have a protective effect on ALF by inducing HIF-1α deacetylation to reduce ROS.
Core Tip: Hypoxia inducible factor 1α (HIF-1α) is a transcription factor that regulates oxygen homeostasis. Under hypoxic conditions, HIF-1α translocates to the nucleus and binds to β-subunits, resulting in transcription of target genes. In acute liver failure, HIF-1α contributes to early liver cell necrosis. The activation of Sirtuin1 (Sirt1) will result in a powerful antioxidant defense system. This study examined the influence of Sirt1-mediated pathways on HIF-1α expression in vivo and in vitro, explored the relationship between Sirt1 and HIF-1α, and further explored its potential mechanism.
- Citation: Cao P, Chen Q, Shi CX, Wang LW, Gong ZJ. Sirtuin1 attenuates acute liver failure by reducing reactive oxygen species via hypoxia inducible factor 1α. World J Gastroenterol 2022; 28(17): 1798-1813
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1798
Acute liver failure (ALF) refers to a large number of necrosis of liver cells or severe liver damage caused by various reasons[1]. ALF is often accompanied by coagulation dysfunction and progressive multiple organ failure due to liver metabolism disorders and decreased immune function[2]. The occurrence and development of ALF is closely related to a series of inflammatory reactions, such as the release of inflammatory cytokines and the production of reactive oxygen species (ROS)[3].
Hypoxia-inducible factor (HIF)-1 consists of an oxygen-regulated subunit HIF-1α and a constitutive expression subunit HIF-1β. The activity and stability of the alpha subunit of HIF are regulated by its post-translational modifications such as acetylation[4]. Under hypoxic conditions, HIF-1α acts as a primary transcription factor to regulate hypoxia-related anti-inflammatory responses[5]. HIF-1α is a key factor that regulates oxygen homeostasis and redox and promotes effective adaptation to hypoxia[6]. During the development of liver diseases such as liver cancer, hypoxia is a common finding. Hypoxia promotes the stabilization of HIF-1α. HIF signal in innate immune cells and liver cancer cells is beneficial to the recruitment and maintenance of primordial tumorigenic immune cells and promotes immune evasion[7].
The monitoring of HIF-1α activity by members of the Sirtuin (Sirt) family has been a topic of interest in recent years[8-10]. According to reports, HIF-1α has been confirmed to be related to Sirt1, Sirt2, and Sirt3 in the Sirt family, and the stability of HIF-1α is related to the ROS level regulated by Sirt3 and the oxygen level regulated by Sirt6[11-14]. Sirt2 causes protein hydroxylation and ubiquitination by increasing the binding of HIF-1α to propylamine hydroxylase[8]. However, the regulation mechanism of Sirt1 on HIF-1α activity has always been a controversial topic.
Sirt1 in the sirtuin family is a nicotinamide adenine dinucleotide-dependent protein lysine deacetylase with diverse physiological functions such as anti-inflammation, neuronal signaling, DNA repair, and stress response. Sirt1 has been shown to be an important target for the treatment of various diseases[15,16], and its activation will lead to a powerful antioxidant defense system and therapeutic effects in liver ischemia reperfusion[17]. Studies have shown that Sirt1 regulates HIF-1α through the formation of physical complexes between proteins, and Sirt1 may have a negative regulatory effect on HIF-1α[18]. Sirt1 has also been reported to regulate HIF-1α actively by stabilizing the protein[19]. Whether Sirt1 is used as a negative regulator or a positive regulator of HIF-1α or depends on the experimental conditions or experimental models remains to be further studied.
In this study, we examined the regulation of Sirt1 on HIF-1α activity in ALF and explored its possible molecular mechanisms.
Male C57BL/6J wild-type mice aged 5-6 wk were purchased from Wuhan Biomedical Research Institute of Wuhan University. All mice were raised in the specific pathogen free animal facility of Renmin Hospital of Wuhan University with conditions of light-controlled, room temperature 25 °C, and humidity 55 ± 5%, and they were free to eat and drink. All animal operations were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University, China (Approval No. WDRY2021-K016).
The mice were randomly divided into six groups with 6 mice in each group: Saline control group; Hypoxia group; Lipopolysaccharide (LPS) group; Hypoxia + LPS group; Resveratrol group; and LPS + Hypoxia + Resveratrol group. Hypoxia group and Hypoxia + LPS group were cultured in COY Vinyl Anaerobic Chambers (COY, Grass Lake, MI, United States). To avoid pulmonary and cerebral edema caused by a rapid drop in oxygenation, the fraction of inspired oxygen (FiO2) (1%/d) was gradually decreased from 21% normoxia (room-air oxygen) to 8% oxygen (severe hypoxia) over the course of 2 wk, followed by continual exposure to 8% oxygen for an additional 2 wk. On the 14th d after being exposed to 8% oxygen, Resveratrol (10 mg/kg; Sigma–Aldrich, St. Louis, MI, United States)[20] was given intragastrically in Resveratrol group and LPS + Hypoxia + Resveratrol group while LPS (100 μg/kg; Sigma–Aldrich) was administrated by intraperitoneal injection combined with D-Gal (400 mg/ kg) in LPS group and LPS + Hypoxia + Resveratrol group[21]. Twenty-four hours after LPS administration, animals were quickly euthanized with inhaled CO2, followed by the collection of blood samples and liver tissues[21].
Human embryonic liver cell line L02 was purchased from China Center for Type Culture Collection (Wuhan, China). N-acetyl-L-cysteine (NAC) (Beyotime, Shanghai, China) (5 mmol/L)[22], nicotinamide (NAM) (Beyotime) (5 mmol/L)[23], GW6471 (Sigma-Aldrich) (3 μM)[24] or Compound C (Sigma-Aldrich) (10 μM)[25], which were dissolved in dimethyl sulfoxide (Sigma-Aldrich), were used to pretreat L02 cells for 1 h, followed by LPS (5 μg/mL)[26] treatment. Hypoxic conditions (1% O2) were obtained using humidified variable aerobic workstation InVivo2 400 (Ruskinn, Pencoed, United Kingdom)[27]. For transient transfection, cells were transfected with 2 μg plasmid of pECE-flag-Sirt1 (Addgene, Cambridge, MA, United States) and pECE empty vector (Addgene).
Blood samples were collected after mice were anesthetized. The level of malondialdehyde (Cat. No. GM1134), superoxide dismutase (Cat. No. GM1133), and glutathione peroxidase (Cat. No. GM1135) were determined with commercial kits (Servicebio, Wuhan, China), respectively, according to the manufacturer's instructions.
The liver tissues were sliced completely and stained with hematoxylin-eosin. The pathological changes of liver tissue were observed and evaluated by light microscope (Olympus, Tokyo, Japan). The degree of liver damage in the ALF models were assessed by the liver histology score.
Liver tissue sections were intact, and L02 cell suspensions were fixed on glass slides. Sections were fixed with 4% paraformaldehyde for 30 min, and 50-100 μL membrane rupture working solution and 3 % hydrogen peroxide solution were added in sequence according to the manufacturer's instructions. Primary antibody against acetyl-lysine or HIF-1α (Santa Cruz Biotechnologies, Dallas, TX, United States) diluted 1:100 with 5% bovine serum albumin was added on the slides and tissue sections, and the slides were incubated overnight at 4 °C in a wet box. Then, slides were incubated with secondary antibody (1:50 dilution, Beyotime), and they were imaged using a fluorescent microscope (Olympus).
Approximately 1 mg of total protein was incubated with anti-Sirt1 antibody (Servicebio) or anti-HIF-1α antibody (Servicebio) overnight at 4 °C followed by precipitation with 20 µl of protein A/G-Plus-Agarose (Servicebio) for 4 h at 4 °C. The precipitated complex was immunoblotted with anti-Sirt1, anti-HIF-1α, or anti-acetyl-lysine.
L02 cell suspensions were fixed on glass slides. Cell culture fluid (2 mL) was added and the culture was continued for about 6 h. Dihydroethidium (1 mL) (Cat. No. GDP1018), which was dissolved in dimethyl sulfoxide at a ratio of 1:1000, was added to each well, and the samples were incubated in the dark. An appropriate amount of DAPI solution was added to the wells and stained. Then, a drop of anti-fluorescence quenching medium was added into the hole; the slides were imaged under a fluorescent microscope.
Proteins were extracted from cells and tissues as directed by the radioimmunoprecipitation assay kit (Sigma-Aldrich). An appropriate amount of concentrated sodium dodecyl sulfate polyacrylamide gel electrophoresis protein loading buffer was added to the collected protein samples, and then 5-10 μL of the sample was loaded in the sodium dodecyl sulfate polyacrylamide gel electrophoresis gel sample holes. Low voltage constant pressure electrophoresis for the upper gel and high voltage constant voltage electrophoresis were applied, when bromophenol blue entered the lower gel. After electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes. The following primary antibodies were used: Sirt1 (Cat. No. 9475, Cell Signaling Technology, Danvers, MA, United States), peroxisome proliferator-activated receptor alpha (PPARα, Cat. No. 23398R, Bioss, Woburn, MA, United States), HIF-1α (Cat. No. 20398R, Bioss), AMP-activated protein kinase (AMPK, Cat. No. 32047, Abcam, Cambridge, United Kingdom), p-AMPK (Cat. No. 131357, Abcam), Bnip3 (Cat. No. 109414, Abcam), and glyceraldehyde-3-phosphate dehydrogenase (Cat. No. 8245, Abcam). Image Lab statistical software (Bio-Rad, Hercules, CA, United States) was used to evaluate band intensities on Western blots.
Statistical analysis was performed using GraphPad Prism software version 8.0 (San Diego, CA, United States). The Y axis was labeled as fold of control mean. Data were expressed as the means ± standard deviations. Differences among multiple groups were evaluated using conventional Student’s t test or analysis of variance. Statistical significance was considered at P < 0.05.
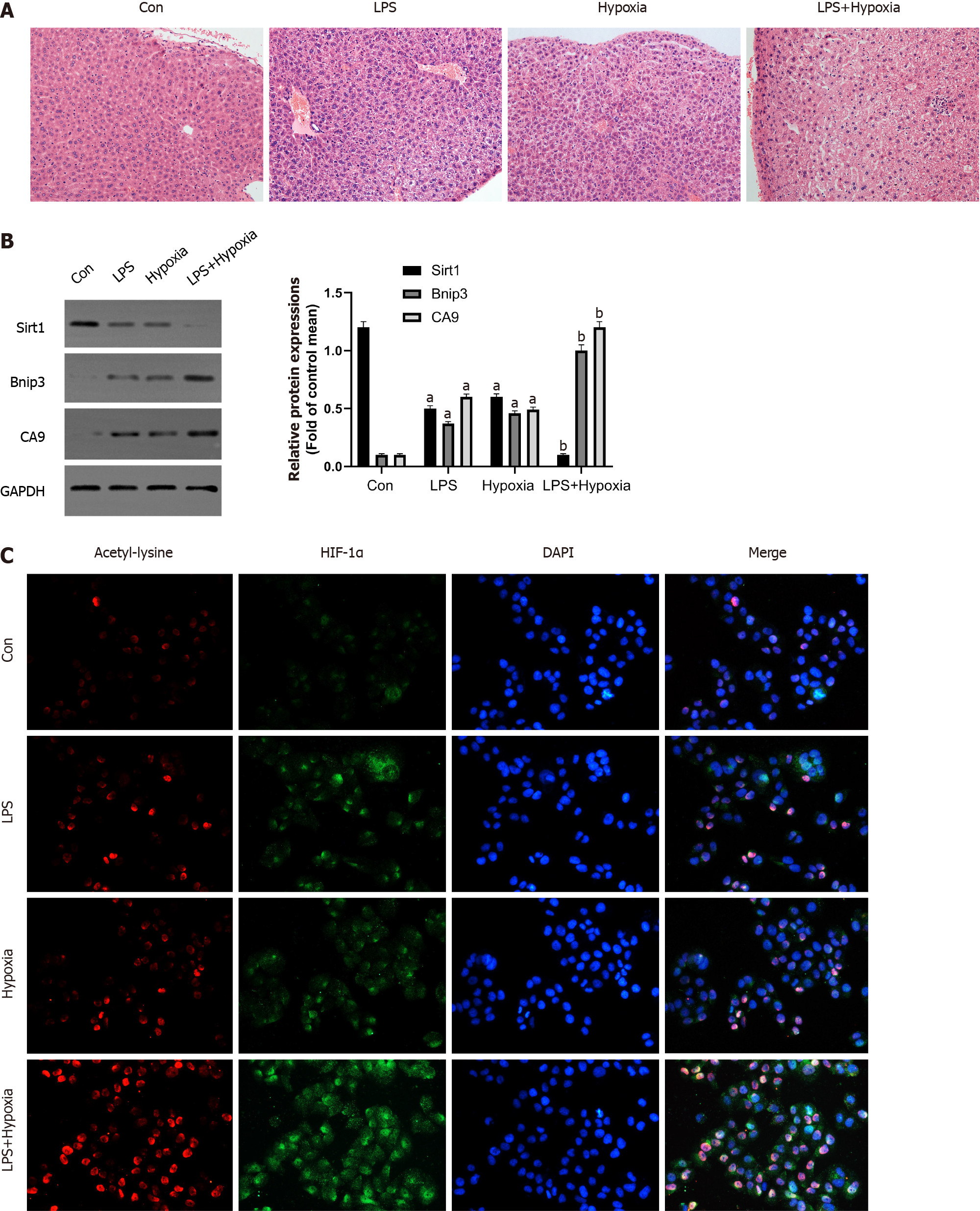
The liver structure of each group was shown by histopathological examination. Compared with the control group, large-scale hepatocyte necrosis in the LPS and Hypoxia groups and the number of infiltrating inflammatory cells were significantly increased, while the inflammatory response was significantly more severe in the LPS + Hypoxia group (Figure 1A). Next, we tested the expression of some key proteins in ALF. As shown in Figure 1B, compared with the control group, the expression of Sirt1 in the LPS group was significantly reduced, and hypoxia aggravated this effect. The expression of Bcl-2 adenovirus E1B-interacting protein 3 (Bnip3) in the LPS + hypoxia group was significantly increased, as was carbonic anhydrase 9 (CA9), both of which are regulated by HIF-1α, suggesting that hypoxia significantly increased the activity of HIF-1α in the LPS group. Of note, the expression of HIF-1α in the LPS + Hypoxia group was significantly increased in the form of acetylation. LPS significantly increased HIF-1α acetylation induced by hypoxia (Figure 1C).
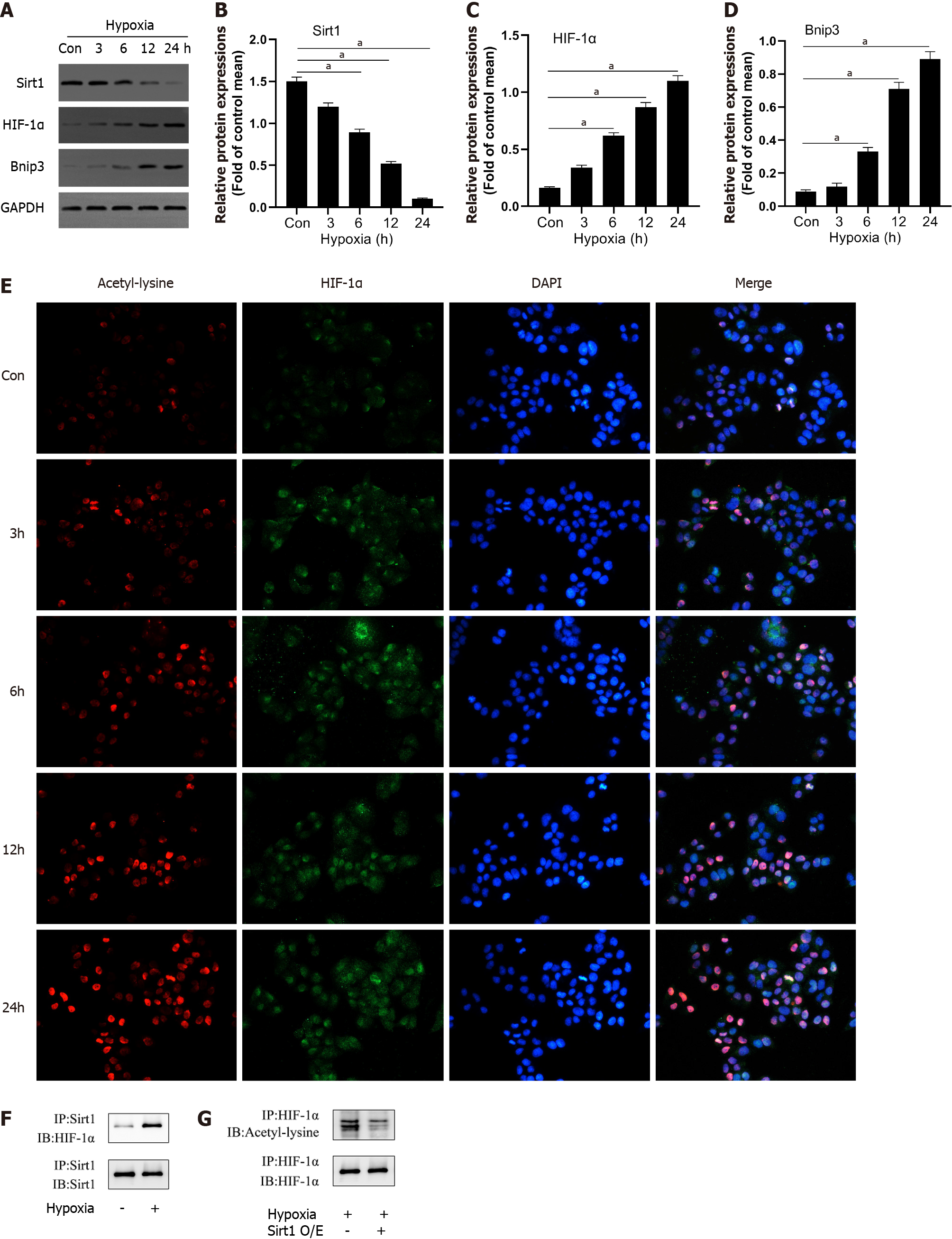
To detect changes in the expression of Sirt1 in L02 cells during hypoxia, we measured the expression levels of Sirt1, HIF-1α, and Bnip3 using Western blotting. Compared to the control group, hypoxia reduced Sirt1 expression and upregulated HIF-1α and Bnip3 expression in a time-dependent manner (Figure 2A-D). Through immunofluorescence experiments, we found that as the duration of hypoxia increased, the expression of HIF-1α increased significantly in the form of acetylation (Figure 2E). We then analyzed the interaction between Sirt1 and HIF-1α. After hypoxia induced endogenous HIF-1α, Sirt1-HIF-1α binding was observed (Figure 2F). We next examined whether Sirt1 deacetylates HIF-1α. Immunoblotting with anti-acetyl-lysine in HIF-1α immunoprecipitates was used to detect the lysine acetylation level of HIF-1α. As shown in Figure 2G, Sirt1 overexpression significantly decreased HIF-1α acetylation, suggesting that Sirt1 regulated lysyl acetylation of HIF-1α. These results suggested that hypoxia-induced enhancement of HIF-1α activity and lysine acetylation were related to the down-regulation of Sirt1.
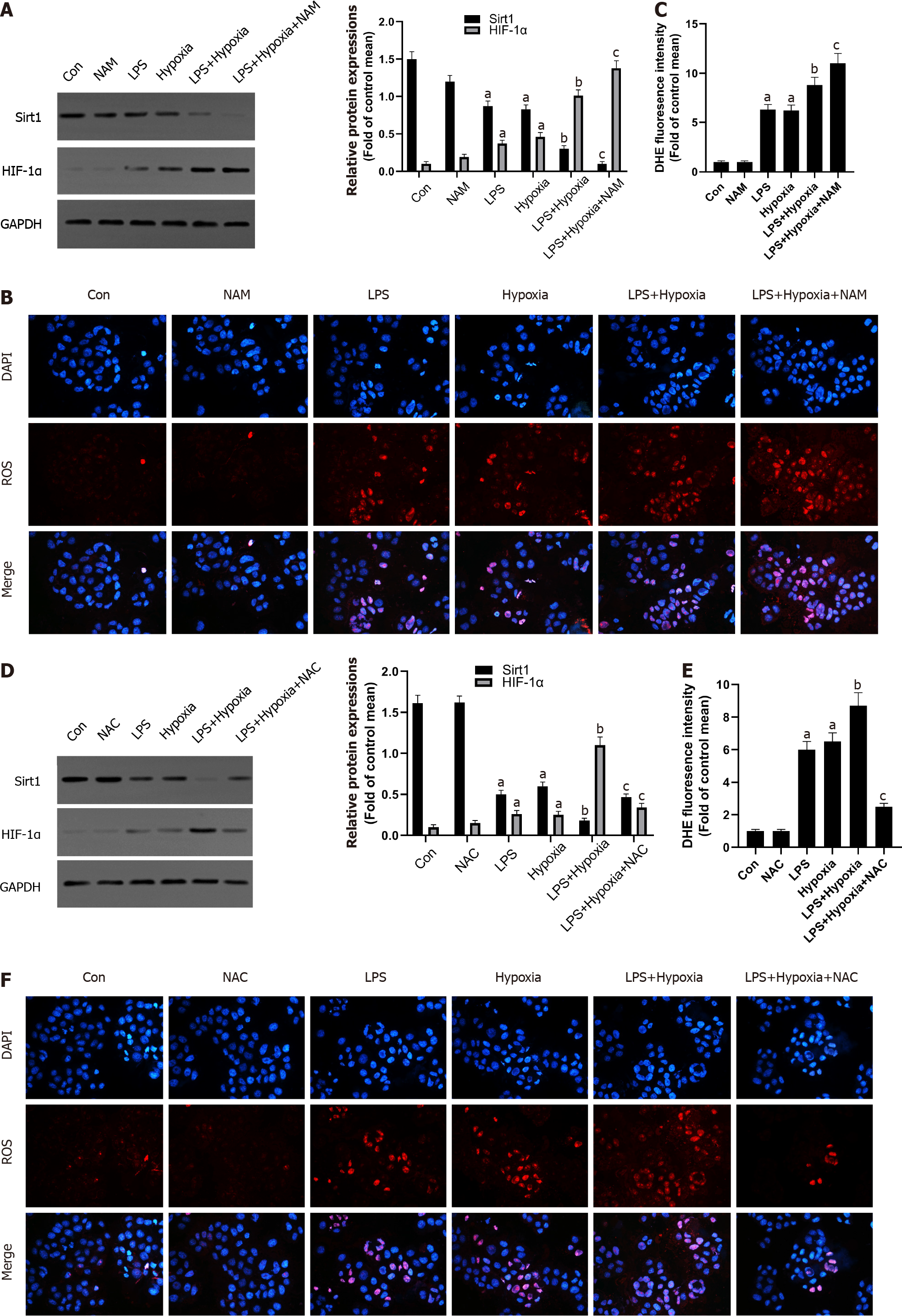
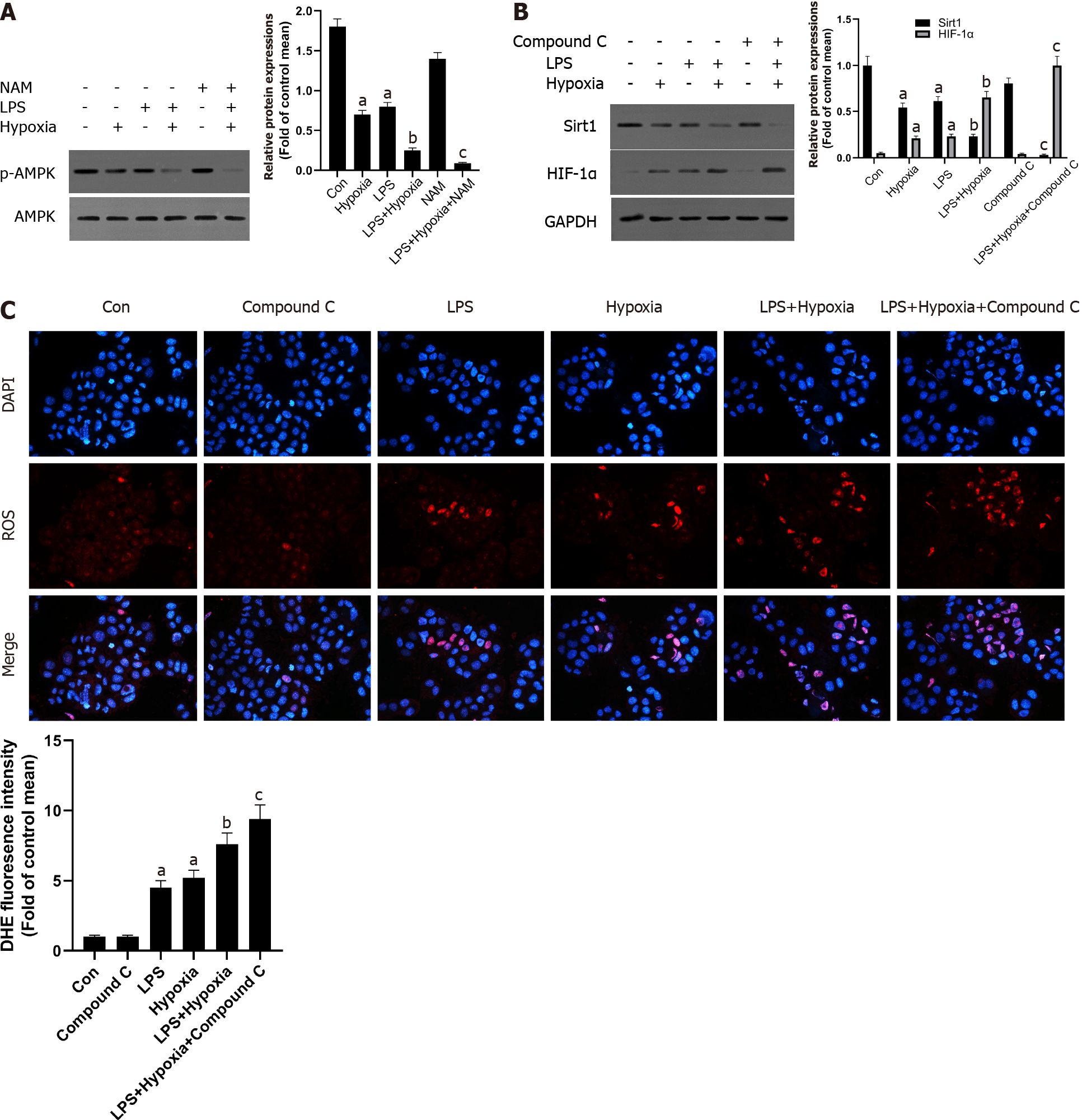
Next, we explored the possible molecular mechanisms of the interaction between Sirt1 and HIF-1α. As shown in the Figure 3A, LPS increased the expression of HIF-1α, and the expression of Sirt1 was further reduced after HIF-1α was increased by hypoxia in L02 cells. At the same time, the use of a specific Sirt1 inhibitor NAM to inhibit Sirt1 further aggravated this effect. Sirt1 appear to interact with HIF-1α in L02 cells. Studies have found that excessive production of ROS is considered harmful and related to hypoxia[28]. Oxidative stress has been shown to promote inflammation during ALF[29]. How oxidative stress is involved in inflammation during ALF remains unclear. Therefore, we examined the antioxidant effect of Sirt1 during hypoxia. DHE staining showed that the level of ROS stimulated by LPS was significantly increased by hypoxia, and this effect was enhanced when NAM was used to inhibit the Sirt1 signaling pathway (Figure 3B and C). Next, we found that the expression of Sirt1 was increased and HIF-1α was opposite when NAC was used, which is an effective ROS scavenger (Figure 3D). At the same time, LPS-induced levels of ROS were significantly reversed by NAC (Figure 3E and F).
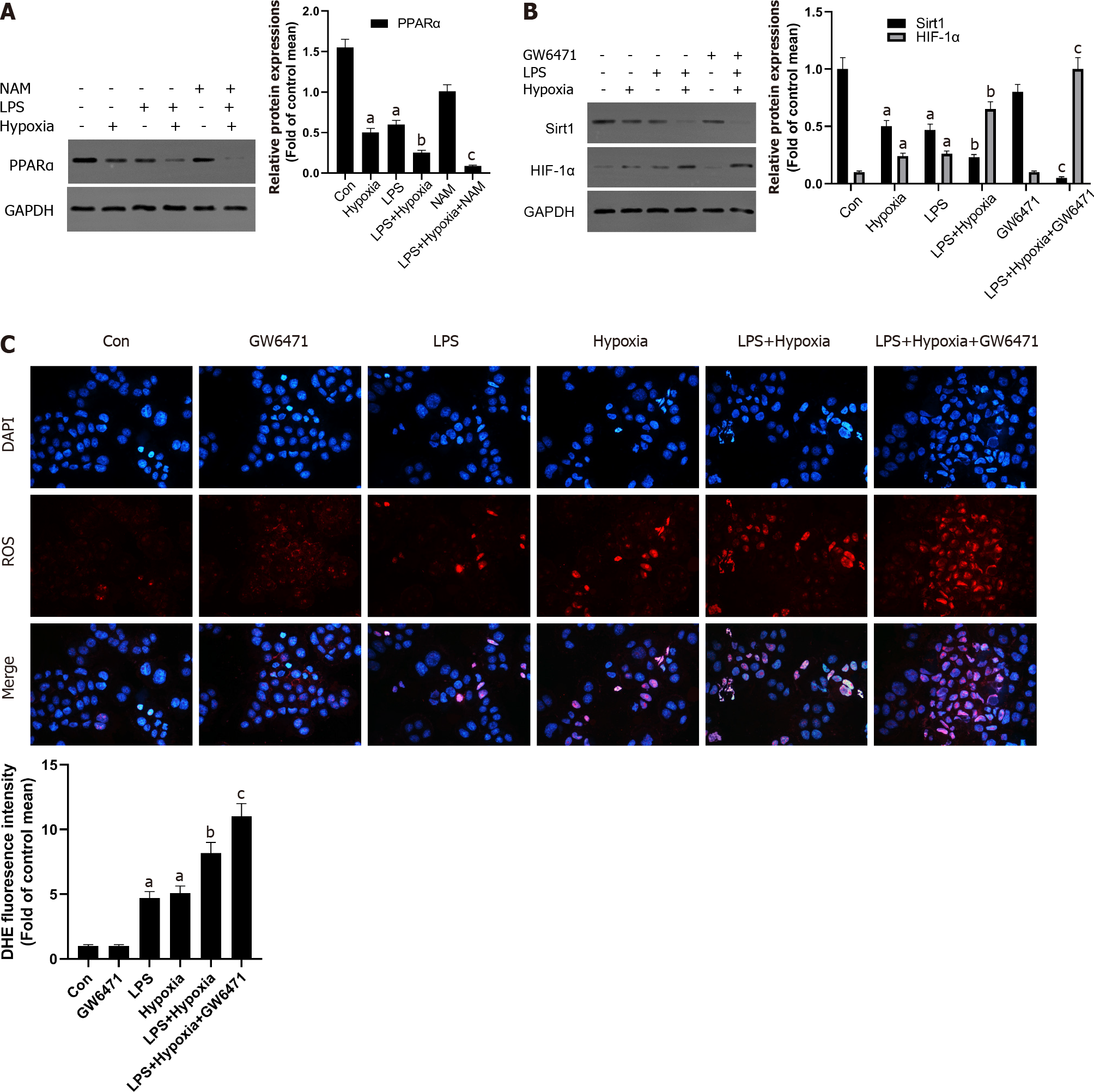
Some studies have shown that liver PPARα expression is lower in patients with hepatitis C and advanced nonalcoholic fatty liver disease, perhaps due to the inhibitory effect of multiple cytokines[30]. This also shows that increasing PPARα may help reduce liver inflammation. In our study, as shown in Figure 4A, in the L02 cells stimulated by LPS, PPARα expression was decreased and aggravated after hypoxia intervention, and its effect was further aggravated when NAM was used to inhibit the Sirt1 signaling pathway, suggesting that hypoxia-induced PPARα inhibition was closely related to Sirt1. In addition, Sirt1 expression was further reduced by the PPARα inhibitor GW6471, while HIF-1α was opposite (Figure 4B) and the levels of ROS were also improved (Figure 4C), suggesting that the inhibition of Sirt1/PPARα signaling pathway might increase hypoxia-induced ROS production in L02 cells.
AMPK acts as a regulator of cellular energy metabolism and redox homeostasis. More and more evidence shows that AMPK plays a protective role by regulating the redox system[31]. Next, we further studied whether Sirt1 can regulate AMPK and its role in cell hypoxia in L02 cells. As shown in Figure 5A, the phosphorylation level of AMPK in L02 cells induced by LPS after hypoxia treatment was significantly reduced, while NAM pretreatment aggravated this effect, indicating AMPK could be modulated by hypoxia via Sirt1. In addition, AMPK inhibitor Compound C further reduced the expression of Sirt1, the expression of HIF-1α was further increased (Figure 5B), and the levels of ROS were also improved (Figure 5C). Therefore, these results suggested that Sirt1/AMPK signaling pathway might be involved in modulating ROS in LPS-stimulated L02 cells during hypoxia.
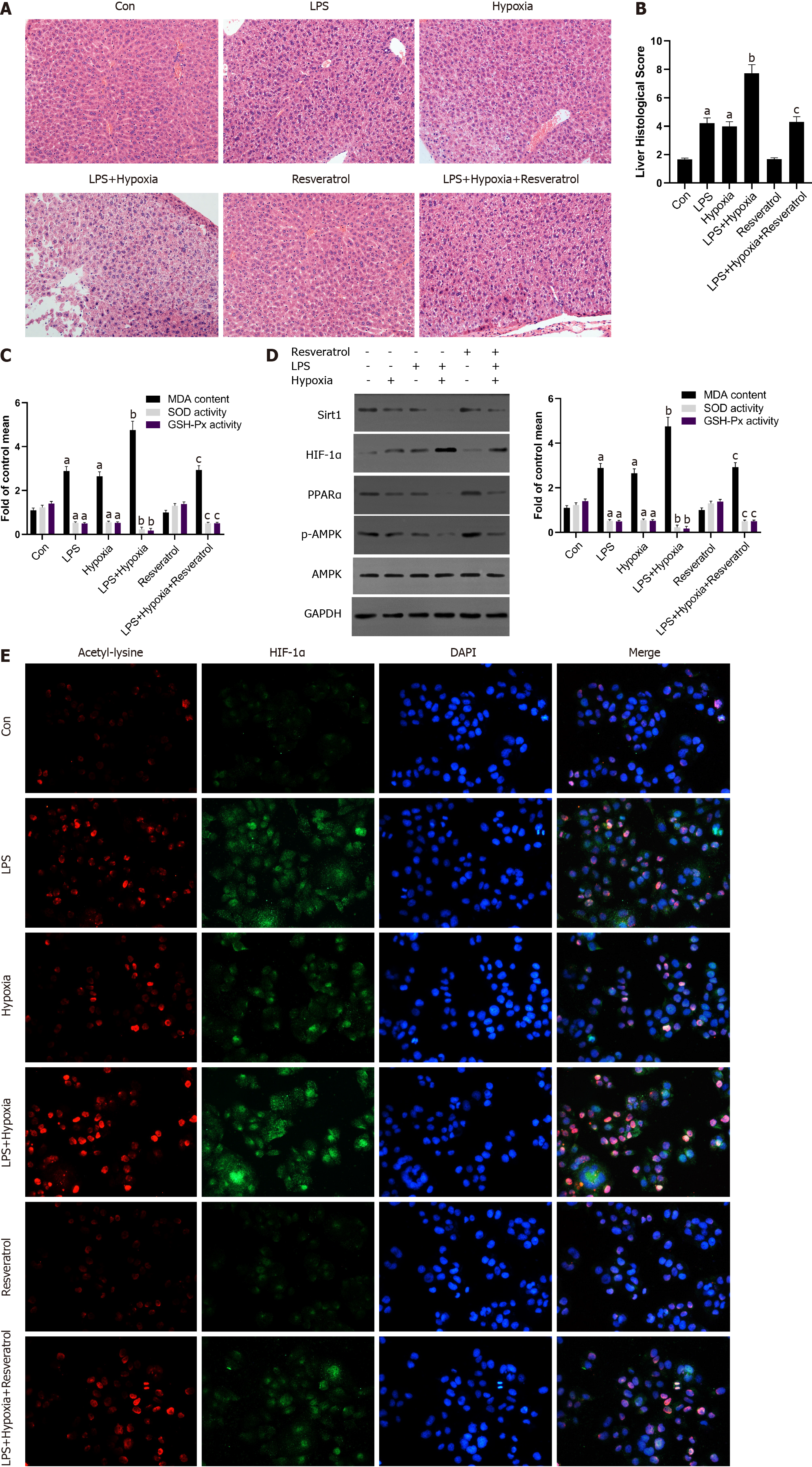
Finally, to determine further whether Sirt1 attenuated the progressive aggravation of ALF induced by hypoxia through the Sirt1/AMPK or the Sirt1/PPARα pathway, LPS-stimulated mice were exposed to hypoxia with or without resveratrol treatment, which is a Sirt1 activator. Compared with the LPS group, activation of Sirt1 by resveratrol alleviated the more sever liver tissue damage in the LPS + Hypoxia group (Figure 6A and B). LPS + Hypoxia group mice showed lower activity of superoxide dismutase and glutathione peroxidase, while malondialdehyde levels were increased, indicating that hypoxia led to decreased antioxidant activity. However, resveratrol treatment could significantly improve the activity (Figure 6C). As shown in Figure 6D, resveratrol dramatically alleviated hypoxia-induced reduction levels of PPARα protein and the phosphorylation of AMPK in LPS-stimulated mice, suggesting that Sirt1 was a key regulator on the activation of PPARα and the phosphorylation of AMPK during hypoxia in ALF. Finally, we demonstrated with animals whether Sirt1 has a regulatory effect on hypoxia-induced HIF-1α lysine acetylation and HIF-1α activity. As shown in Figure 6E, with the intervention of resveratrol, the expression of HIF-1α and the level of acetylation decreased significantly. These findings indicate that the activation of Sirt1 induced HIF-1α inactivation and deacetylation, thereby alleviating the progressive aggravation of ALF induced by hypoxia.
Recently, more and more studies have confirmed the effect of Sirt1 in liver disease. Sirt1 has been confirmed to have a protective effect in a variety of disease models, including liver fibrosis[32], drug-induced liver injury[33], non-alcoholic fatty liver disease[34], and fatty liver[35]. As well known, HIF-1α is a transcription factor that can promote the adaptive response of cells to hypoxia. Some reports have mentioned the connection between Sirt1 and HIF protein, but there are still many controversies about the results. According to reports, in hypoxic Hep3B or HEK293 cells, Sirt1 targeted HIF-2α and increased the transcriptional activity of HIF-2α but not HIF-1α[36]. On the contrary, another group of studies showed that Sirt1 interacted with HIF-1α, causing HIF-1α deacetylation to promote its activity in Hep3B and Huh7 cells[19]. Therefore, the regulation of Sirt1 on the activity of HIF-1α and its expression seems to be cell-type-specific, which is currently unclear. It has not been reported that the beneficial effect of Sirt1 activation is related to its HIF-1α deacetylation against ALF.
In our research, we found that the activity of HIF-1α increased after acetylation and promoted hepatocyte apoptosis in ALF models and hypoxia models in vitro. In addition, we demonstrated that the expression of Sirt1 in L02 cells decreased in a time-dependent manner due to hypoxia, which was closely related to the activation and acetylation of HIF-1α. During hypoxia, with the decrease of the level of nicotinamide adenine dinucleotide, the activity of Sirt1 decreased and HIF-1α transcription activity further increased[18,19]. Therefore, the insufficient expression of Sirt1 in the liver or the acetylation of HIF-1α might be the key mediators of ALF.
Next, we carefully evaluated Sirt1's regulatory effect on HIF-1α activity in ALF and explored its possible molecular mechanisms. ROS are by-products of normal metabolism in living cells, but excessive ROS accumulation can damage organelles, leading to increased oxidative stress[37,38]. ALF produces excessive amounts of ROS due to insufficient detoxification of toxic substances in the liver[39]. Sirt1 has been reported to play an important role in anti-inflammatory and antioxidant processes[40]. Here, we demonstrated that HIF-1α was over-activated in hypoxia due to increased level of ROS in the absence of Sirt1, and the effect was inhibited by the antioxidant NAC, indicating that ROS was involved in this activation.
In particular, PPARα is reported to be a potent inhibitor of NF-κB signaling pathway and inflammation[41]. The positive effect of Sirt1 on the inflammatory pathway may be related to PPARα[42], and the interference of PPAR transcriptional activity may disturb estrogen/androgen receptor expression and impair steroidogenesis and ROS metabolism[43]. In addition, PPARα contributes to the protection of redox homeostasis[44]. Previous studies have confirmed that Sirt1 can regulate AMPK, which is an important energy sensor[45]. AMPK acts as a regulator of cellular energy metabolism and redox homeostasis. More and more evidence shows that AMPK plays a cardiovascular protective role by regulating the redox system[46]. In diabetes, the activation of AMPK increases the expression of mitochondrial antioxidant enzymes and leads to a decrease in the production of mitochondrial ROS in endothelial cell[47].
Our experiments revealed that the inhibition of PPARα and the phosphorylation of AMPK induced by hypoxia were closely related to Sirt1, and the inhibition of Sirt1/PPARα or Sirt1/AMPK signaling pathway might increase hypoxia-induced ROS production in L02 cells. In order to determine whether the activation of Sirt1 induced inactivation of HIF-1α, the progressive aggravation of ALF induced by hypoxia in vivo was rescued in mice treated with resveratrol. As expected, the activation of Sirt1 significantly alleviated the degree of liver damage in ALF and enhanced antioxidant activity. Resveratrol dramatically alleviated the hypoxia-induced reduction level of PPARα protein and the phosphorylation of AMPK in LPS-stimulated mice. In addition, the activation of Sirt1 induced the deacetylation of HIF-1α compared to LPS-stimulated mice exposed to hypoxia; the expression of HIF-1α and the level of acetylation decreased significantly.
One limitation of our study is that we did not use HIF-1α overexpressing mice in vivo to test whether the increase of Sirt1 activity can rescue ALF. We need to conduct further experiments to solve this problem.
In summary, we have demonstrated that Sirt1 reduced oxidative stress in ALF by regulating the activity and acetylation of HIF-1α, achieved by normalizing the Sirt1/PPARα and Sirt1/AMPK pathway. Our research showed that the deacetylation and inactivation of HIF-1α induced by the activation of Sirt1 might have therapeutic benefits in reducing liver damage during ALF.
Acute liver failure (ALF) is a life-threatening disease that can rapidly develop into multiple organ failure. The mortality rate is high. If effective treatment measures are not taken, various complications will occur, including cerebral edema, sepsis, renal failure, gastrointestinal bleeding, and respiratory failure. Hypoxia inducible factor 1α (HIF-1α) is a transcription factor that regulates oxygen homeostasis. In ALF, HIF-1α contributes to early liver cell necrosis. Sirtuin1 (Sirt1) plays a key role in health by deacetylating target proteins in many tissues, including the liver. The activation of Sirt1 will result in a powerful antioxidant defense system. However, the role of Sirt1 in ALF and the relationship between Sirt1 and HIF-1α remain unclear and require further investigation.
The results of this study might provide a basis for the application of Sirt1 in the treatment of ALF and further understanding of the mechanism of Sirt1 and HIF-1α in the process of ALF.
This study detected the changes in the expression of Sirt1 and HIF-1α in liver tissues and hepatocytes under hypoxia during the ALF process as well as the differences in the expression levels of key enzymes. In addition, this study further explored the relationship and mechanism of Sirt1 signaling pathway and HIF-1α expression.
Western blotting was used to detect the expression levels of Sirt1 and HIF-1α related proteins in mouse liver tissues, and immunofluorescence staining was used to observe the acetylation level of HIF-1α. Detection of HIF-1α and reactive oxygen species (ROS) levels and the correlation analysis between Sirt1 and HIF-1α were performed. Finally, Sirt1 was activated to observe the influence of the Sirt1 signaling pathway and HIF-1α on ALF, and changes in the expression levels of related markers were detected.
The expression of Sirt1 decreased and the level of HIF-1α acetylation increased in hypoxic mice, and the levels of carbonic anhydrase 9 and Bcl-2-adenovirus E1B interacting protein 3 increased significantly, which was regulated by HIF-1α, indicating an increase of HIF-1α activity. Under hypoxia, the down-regulation of Sirt1 activated and acetylated HIF-1α in L02 cells. The inhibition of Sirt1 significantly aggravated this effect and the massive production of ROS. The regulation of ROS was partly through peroxisome proliferator-activated receptor alpha or AMP-activated protein kinase (AMPK). The activation of Sirt1 effectively relieved ALF aggravated by hypoxia, the production of ROS, and cell apoptosis. It also induced the deacetylation of HIF-1α and inhibited the activity of HIF-1α.
The inhibition of peroxisome proliferator-activated receptor alpha and the phosphorylation of AMPK induced by hypoxia were closely related to Sirt1, and the inhibition of Sirt1/PPARα or Sirt1/AMPK signaling pathway might increase hypoxia-induced ROS production. The activation of Sirt1 reduced oxidative stress in ALF by regulating the activity and acetylation of HIF-1α.
The results of this study showed that the deacetylation and inactivation of HIF-1α induced by the activation of Sirt1 might have therapeutic benefits in reducing liver damage during ALF. This study preliminarily clarified the role of Sirt1 and HIF-1α in ALF, so as to deepen the understanding of the mechanism of ALF, and provided guidance for the selection of ALF treatment targets. The results of this study indicate that Sirt1 activator may have a certain prospective application as a therapeutic drug for ALF.
The authors would like to thank the central laboratory at Renmin Hospital of Wuhan University (Wuhan, Hubei Province, China) for their support of our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Renmin Hospital of Wuhan University, Renmin Hospital of Wuhan University.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao L, China; Shamseldeen AA, Egypt; Subramaniyan V, Malaysia S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Dong V, Nanchal R, Karvellas CJ. Pathophysiology of Acute Liver Failure. Nutr Clin Pract. 2020;35:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Larson-Nath C, Vitola BE. Neonatal Acute Liver Failure. Clin Perinatol. 2020;47:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Manakkat Vijay GK, Ryan JM, Abeles RD, Ramage S, Patel V, Bernsmeier C, Riva A, McPhail MJ, Tranah TH, Markwick LJ, Taylor NJ, Bernal W, Auzinger G, Willars C, Chokshi S, Wendon JA, Ma Y, Shawcross DL. Neutrophil Toll-Like Receptor 9 Expression and the Systemic Inflammatory Response in Acetaminophen-Induced Acute Liver Failure. Crit Care Med. 2016;44:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Baumeister J, Chatain N, Hubrich A, Maié T, Costa IG, Denecke B, Han L, Küstermann C, Sontag S, Seré K, Strathmann K, Zenke M, Schuppert A, Brümmendorf TH, Kranc KR, Koschmieder S, Gezer D. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Tak E, Jung DH, Kim SH, Park GC, Jun DY, Lee J, Jung BH, Kirchner VA, Hwang S, Song GW, Lee SG. Protective role of hypoxia-inducible factor-1α-dependent CD39 and CD73 in fulminant acute liver failure. Toxicol Appl Pharmacol. 2017;314:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Xu E, Ji Z, Jiang H, Lin T, Ma J, Zhou X. Hypoxia-Inducible Factor 1A Upregulates HMGN5 by Increasing the Expression of GATA1 and Plays a Role in Osteosarcoma Metastasis. Biomed Res Int. 2019;2019:5630124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Yuen VW, Wong CC. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest. 2020;130:5052-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 8. | Seo KS, Park JH, Heo JY, Jing K, Han J, Min KN, Kim C, Koh GY, Lim K, Kang GY, Uee Lee J, Yim YH, Shong M, Kwak TH, Kweon GR. SIRT2 regulates tumour hypoxia response by promoting HIF-1α hydroxylation. Oncogene. 2015;34:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | De Matteis S, Scarpi E, Granato AM, Vespasiani-Gentilucci U, La Barba G, Foschi FG, Bandini E, Ghetti M, Marisi G, Cravero P, Gramantieri L, Cucchetti A, Ercolani G, Santini D, Frassineti GL, Faloppi L, Scartozzi M, Cascinu S, Casadei-Gardini A. Role of SIRT-3, p-mTOR and HIF-1α in Hepatocellular Carcinoma Patients Affected by Metabolic Dysfunctions and in Chronic Treatment with Metformin. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, Zhang Z, Liu M, Ni Q, Yu X, Xu X. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett. 2019;452:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Li A, Peng R, Sun Y, Liu H, Peng H, Zhang Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α signaling. Cell Death Dis. 2018;9:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 12. | Lee SD, Kim W, Jeong JW, Park JW, Kim JE. AK-1, a SIRT2 inhibitor, destabilizes HIF-1α and diminishes its transcriptional activity during hypoxia. Cancer Lett. 2016;373:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Wang X, Shen K, Wang J, Liu K, Wu G, Li Y, Luo L, Zheng Z, Hu D. Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1α/SIRT3/HIF-1α signaling. Free Radic Biol Med. 2020;159:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Yang Z, Yu W, Huang R, Ye M, Min Z. SIRT6/HIF-1α axis promotes papillary thyroid cancer progression by inducing epithelial-mesenchymal transition. Cancer Cell Int. 2019;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Alves-Fernandes DK, Jasiulionis MG. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 16. | Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz-Leutz E, Cathou W, Gentek R, Perrin P, Leutz A, Berruyer C, Sieweke MH. SIRT1 regulates macrophage self-renewal. EMBO J. 2017;36:2353-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Mahmoud AR, Ali FEM, Abd-Elhamid TH, Hassanein EHM. Coenzyme Q10 protects hepatocytes from ischemia reperfusion-induced apoptosis and oxidative stress via regulation of Bax/Bcl-2/PUMA and Nrf-2/FOXO-3/Sirt-1 signaling pathways. Tissue Cell. 2019;60:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 19. | Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, Keogh A, Tschan MP, Candinas D, Vorburger SA, Stroka D. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS One. 2012;7:e33433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Wang G, Xie X, Yuan L, Qiu J, Duan W, Xu B, Chen X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors. 2020;46:441-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Yang F, Jiao FZ, Chen Q, Zhang WB, Wang LW, Gong ZJ. Modulations of Histone Deacetylase 2 Offer a Protective Effect through the Mitochondrial Apoptosis Pathway in Acute Liver Failure. Oxid Med Cell Longev. 2019;2019:8173016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Oh SI, Park JK, Park SK. Lifespan extension and increased resistance to environmental stressors by N-acetyl-L-cysteine in Caenorhabditis elegans. Clinics (Sao Paulo). 2015;70:380-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Gazanion E, Vergnes B, Seveno M, Garcia D, Oury B, Ait-Oudhia K, Ouaissi A, Sereno D. In vitro activity of nicotinamide/antileishmanial drug combinations. Parasitol Int. 2011;60:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Luo D, Zhang Y, Yuan X, Pan Y, Yang L, Zhao Y, Zhuo R, Chen C, Peng L, Li W, Jin X, Zhou Y. Oleoylethanolamide inhibits glial activation via moudulating PPARα and promotes motor function recovery after brain ischemia. Pharmacol Res. 2019;141:530-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 847] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 26. | Chen J, Wang B, Lai J, Braunstein Z, He M, Ruan G, Yin Z, Wang J, Cianflone K, Ning Q, Chen C, Wang DW. Trimetazidine Attenuates Cardiac Dysfunction in Endotoxemia and Sepsis by Promoting Neutrophil Migration. Front Immunol. 2018;9:2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986-2996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Yu LM, Zhang WH, Han XX, Li YY, Lu Y, Pan J, Mao JQ, Zhu LY, Deng JJ, Huang W, Liu YH. Hypoxia-Induced ROS Contribute to Myoblast Pyroptosis during Obstructive Sleep Apnea via the NF-κB/HIF-1α Signaling Pathway. Oxid Med Cell Longev. 2019;2019:4596368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Tian Z, Chen Y, Yao N, Hu C, Wu Y, Guo D, Liu J, Yang Y, Chen T, Zhao Y, He Y. Role of mitophagy regulation by ROS in hepatic stellate cells during acute liver failure. Am J Physiol Gastrointest Liver Physiol. 2018;315:G374-G384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Kersten S, Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. 2017;136:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 288] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 31. | Chang C, Su H, Zhang D, Wang Y, Shen Q, Liu B, Huang R, Zhou T, Peng C, Wong CC, Shen HM, Lippincott-Schwartz J, Liu W. AMPK-Dependent Phosphorylation of GAPDH Triggers Sirt1 Activation and Is Necessary for Autophagy upon Glucose Starvation. Mol Cell. 2015;60:930-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 32. | Song L, Chen TY, Zhao XJ, Xu Q, Jiao RQ, Li JM, Kong LD. Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. Br J Pharmacol. 2019;176:1619-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Nagappan A, Kim JH, Jung DY, Jung MH. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 34. | Li CX, Gao JG, Wan XY, Chen Y, Xu CF, Feng ZM, Zeng H, Lin YM, Ma H, Xu P, Yu CH, Li YM. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J Gastroenterol. 2019;25:5120-5133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017;13:852-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 36. | Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 37. | Roux C, Jafari SM, Shinde R, Duncan G, Cescon DW, Silvester J, Chu MF, Hodgson K, Berger T, Wakeham A, Palomero L, Garcia-Valero M, Pujana MA, Mak TW, McGaha TL, Cappello P, Gorrini C. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci U S A. 2019;116:4326-4335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 38. | He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 1240] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 39. | Hwang Y, Kim JC, Tae G. Significantly enhanced recovery of acute liver failure by liver targeted delivery of stem cells via heparin functionalization. Biomaterials. 2019;209:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Liu TF, Vachharajani V, Millet P, Bharadwaj MS, Molina AJ, McCall CE. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J Biol Chem. 2015;290:396-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Shavva VS, Mogilenko DA, Nekrasova EV, Trulioff AS, Kudriavtsev IV, Larionova EE, Babina AV, Dizhe EB, Missyul BV, Orlov SV. Tumor necrosis factor α stimulates endogenous apolipoprotein A-I expression and secretion by human monocytes and macrophages: role of MAP-kinases, NF-κB, and nuclear receptors PPARα and LXRs. Mol Cell Biochem. 2018;448:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2011;90:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 43. | Wang G, Cheng S, Zhang S, Zhu Y, Xiao Y, Ju L. LPS impairs steroidogenesis and ROS metabolism and induces PPAR transcriptional activity to disturb estrogen/androgen receptor expression in testicular cells. Mol Biol Rep. 2020;47:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Huang Y, Lang H, Chen K, Zhang Y, Gao Y, Ran L, Yi L, Mi M, Zhang Q. Resveratrol protects against nonalcoholic fatty liver disease by improving lipid metabolism and redox homeostasis via the PPARα pathway. Appl Physiol Nutr Metab. 2020;45:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628-27635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 657] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 46. | Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52:1607-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med. 2007;42:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |