Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1563
Peer-review started: September 14, 2021
First decision: November 16, 2021
Revised: November 25, 2021
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: April 21, 2022
Processing time: 212 Days and 16.5 Hours
Identifying hepatic fibrosis is crucial for nonalcoholic fatty liver disease (NAFLD) management. The fibrosis-8 (FIB-8) score, recently developed by incorporating four additional variables into the fibrosis-4 (FIB-4) score, showed better performance in predicting significant fibrosis in NAFLD.
To validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 and FIB-4 scores and NAFLD fibrosis score (NFS) for predicting significant fibrosis.
We collected the data of biopsy-proven NAFLD patients from three Asian centers in three countries. All the patients with available variables for the FIB-4 score (age, platelet count, and aspartate and alanine aminotransferase levels) and FIB-8 score (the FIB-4 variables plus 4 additional parameters: The body mass index (BMI), albumin to globulin ratio, gamma-glutamyl transferase level, and presence of diabetes mellitus) were included. The fibrosis stage was scored using nonalcoholic steatohepatitis CRN criteria, and significant fibrosis was defined as at least fibrosis stage 2.
A total of 511 patients with biopsy-proven NAFLD and complete data were included for validation. Of these 511 patients, 271 (53.0%) were female, with a median age of 51 (interquartile range: 41, 58) years. The median BMI was 29 (26.3, 32.6) kg/m2, and 268 (52.4%) had diabetes. Among the 511 NAFLD patients, 157 (30.7%) had significant fibrosis (≥ F2). The areas under the receiver operating characteristic curves of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively. The FIB-8 score demonstrated significantly better performance for predicting significant fibrosis than the NFS (P = 0.001) and was also clinically superior to FIB-4, although statistical significance was not reached (P = 0.073). The low cutoff point of the FIB-8 score for predicting significant fibrosis of 0.88 showed 92.36% sensitivity, and the high cutoff point of the FIB-8 score for predicting significant fibrosis of 1.77 showed 67.51% specificity.
We demonstrated that the FIB-8 score had significantly better performance for predicting significant fibrosis in NAFLD patients than the NFS, as well as clinically superior performance vs the FIB-4 score in an Asian population. A novel simple fibrosis score comprising commonly accessible basic laboratories may be beneficial to use for an initial assessment in primary care units, excluding patients with significant liver fibrosis and aiding in patient selection for further hepatologist referral.
Core Tip: Noninvasive diagnosis of hepatic fibrosis is crucial for nonalcoholic fatty liver disease (NAFLD). The fibrosis-8 (FIB-8) score was recently developed by incorporating four additional variables into the fibrosis-4 (FIB-4) score. The diagnostic performance of the FIB-8 score exhibited higher accuracy in diagnosing significant fibrosis (≥ F2) than the NAFLD fibrosis score but was not superior to the FIB-4 score in our Asian cohort population. We postulated that gamma-glutamyl transferase might be an additional variable that predicts significant fibrosis in NAFLD patients.
- Citation: Prasoppokakorn T, Chan WK, Wong VWS, Pitisuttithum P, Mahadeva S, Nik Mustapha NR, Wong GLH, Leung HHW, Sripongpun P, Treeprasertsuk S. Validation model of fibrosis-8 index score to predict significant fibrosis among patients with nonalcoholic fatty liver disease . World J Gastroenterol 2022; 28(15): 1563-1573
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1563
Nonalcoholic fatty liver disease (NAFLD) is a global health issue and has become the most common liver disease in Western countries, accounting for an estimated 25% of the adult population[1] and affecting an estimated 25%–30% of the adult population in the Asia Pacific region[2]. A meta-analysis in Asia during 1999 to 2019, described the overall pooled incidence rate was 50.9 per 1000 person-years[3]. According to our previous study, the prevalence of significant fibrosis (defined as ≥ F2 fibrosis) is 18.4% in asymptomatic NAFLD patients[4]. Nonalcoholic steatohepatitis (NASH) has emerged as the most common cause of cryptogenic cirrhosis and hepatocellular carcinoma worldwide. The presence of hepatic fibrosis is the major determinant of future risk of mortality and liver-related morbidity[5], and detecting significant fibrosis is crucial for NAFLD because no well-accepted and proven therapy is available for this disease to date[6]. However, patients with F2 or higher are at a higher risk of long-term liver-related death than patients with F0-1. Those with significant fibrosis should be intensively followed up or considered to participate in the therapeutic trial for NAFLD.
Liver biopsy remains the gold standard for evaluating hepatic fibrosis. However, because of several drawbacks, including invasiveness, the risk of bleeding complications, intrinsic sampling and pathologist reader variability[7], and cost, noninvasive tests are more practical. Thus, the 2018 American Association for the Study of Liver Diseases (AASLD) practice guidance recommends the use of the fibrosis-4 (FIB-4) score, the NAFLD fibrosis score (NFS), vibration-controlled transient elastography, and magnetic resonance elastography[8] to identify those at low or high risk for advanced fibrosis [bridging fibrosis (F3) or cirrhosis (F4)]. Noninvasive tests using only clinical and routine laboratory parameters are inexpensive and particularly important in primary care or resource-limited settings where the pretest probability of advanced fibrosis is low because these scores have good negative predictive values (NPVs) to exclude advanced fibrosis[9]. Therefore, using simple fibrosis scores as an initial assessment in primary care is reasonable. The FIB-4 score comprises four parameters, age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelets, while the NFS score comprises six parameters in addition to those comprising the FIB-4 score, such as the body mass index (BMI), presence of diabetes, and serum albumin level[10].
According to Sripongpun et al[11], their AASLD 2019 abstract reported a new model for a fibrosis-8 score (FIB-8) score developed by incorporating the following four additional variables: BMI, albumin/globulin (A/G) ratio, gamma-glutamyl transferase (GGT) level, and diabetes. The subjects were enrolled in the PIVENS and FLINT trials, of which 522 participants all had histologically confirmed NASH[12,13]. The optimal low and high cutoffs for the FIB-8 score to exclude and include F ≥ 2 were < 0.88 and ≥ 1.77, respectively, with a sensitivity of 95.3% and a specificity of 79.2%. The areas under the receiver operating characteristic curves (AUROCs) of the FIB-8 score were 0.79 and 0.78 in the training and validation datasets, respectively. The FIB-8 score provided significantly better AUROCs than the FIB-4 score (P < 0.001) and NFS (P = 0.005) in the validation dataset for predicting significant and advanced fibrosis in NAFLD patients. Following the study, the field test and validation of the FIB-8 score in a real-world cohort of NAFLD patients revealed that the AUROCs of the FIB-8 score were 0.84 with imputed data (n = 130) and 0.91 when only patients with complete data without imputation were included (n = 31). The FIB-8 score again outperformed the FIB-4 score and NFS, with AUROCs of 0.86 vs 0.80 and 0.77, respectively, for diagnosing advanced fibrosis (F3)[14].
To our best knowledge, no validation of the FIB-8 score has been reported in a larger cohort. Therefore, this study was to validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis.
We collected the data of biopsy-proven NAFLD patients from the following three Asian centers in three countries: (1) Chulalongkorn University, Thailand; (2) The Chinese University of Hong Kong, Hong Kong; and (3) University of Malaya, Malaysia. The data from Thailand were collected from April 2008 to May 2019, those from Hong Kong were collected from July 2006 to November 2017, and those from Malaysia were collected from November 2012 to October 2015.
NAFLD was diagnosed based on ultrasonographic findings of fatty liver as well as transient elastography and the exclusion of viral hepatitis B and C infection, significant alcohol intake, and current usage of medications causing hepatic steatosis. Only patients with biopsy-proven NAFLD were included. Patients with other causes of chronic liver disease, incomplete histological data, and without significant hepatic steatosis were excluded. The laboratory data for the FIB-4 score (age, platelet count, and aspartate and ALT levels), FIB-8 score [the FIB-4 variables plus 4 additional parameters: The BMI, albumin to globulin ratio, gamma-glutamyl transferase level, and presence of diabetes mellitus (DM)], and the NFS were collected. The time interval between the enrolled laboratories and the date of liver biopsy was within 1 year. The fibrosis stage was scored using the NASH Clinical Research Network (CRN) criteria, and significant fibrosis was defined as at least fibrosis stage 2 (F ≥ 2).
We validated the noninvasive methods from the FIB-8 score, FIB-4 score, and NFS and the test variables for predicting significant fibrosis (Table 1)[11,15,16].
| Index | Number of parameters and variables | Formula | Ref. |
| FIB-8 index | 8; age, AST, ALT, platelets, BMI, albumin/globulin, GGT, diabetes | FIB4 + 0.025 × BMI (kg/m2) - 0.702 × (albumin/globulin ratio) + 0.004 × GGT (U/L) + 0.858 × diabetes (yes = 1, no = 0)1 | Sripongpun et al[11], 2019 |
| FIB-4 index | 4; age, AST, ALT, platelets | Age (years) × AST (U/L)/[platelet count (109/L) × √ALT (U/L)] | Sterling et al[15], 2006 |
| NFS | 6; age, BMI, diabetes, AST/ALT, platelets, albumin | -1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio - 0.013 × platelet count (× 109/L) - 0.66 × albumin (g/dL) | Angulo et al[16], 2007 |
We aimed to validate the FIB-8 score in a biopsy-proven NAFLD cohort and compare the diagnostic performance of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis (≥ F2) in an Asian cohort.
The study was reviewed and approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB number 238/59). This is a retrospective study, and signed informed consent was waived by the Ethics Committee. The analysis used anonymous clinical data after each patient agreed to treatment by written consent.
Categorical and continuous variables were compared between patients with and without significant fibrosis using Chi-squared and Student’s t-test or the Wilcoxon rank-sum test (according to the distribution of the data), respectively. Most of the numerical values did not follow a normal distribution and were expressed as medians and interquartile ranges. The diagnostic performance of each scoring system was then evaluated using receiver operating characteristic curves, and comparisons between the correlated AUROCs were performed using DeLong’s test[17]. The sensitivities (Sens) and specificities (Spec) of each scoring system were analyzed using the given low and high cutoffs for predicting F2, as reported previously-i.e., 0.88 and 1.77 for the FIB-8 score, 0.81 and 1.81 for the FIB-4 score, and -2.45 and 0.03 for the NFS, respectively[11,18]. All statistical analyses were performed using the SPSS statistical analysis package (version 18.0.0; SPSS Inc., Chicago, Illinois, United States), Stata (version 15; StataCorp), and R program version 4.1.1. A P value < 0.05 was considered statistically significant.
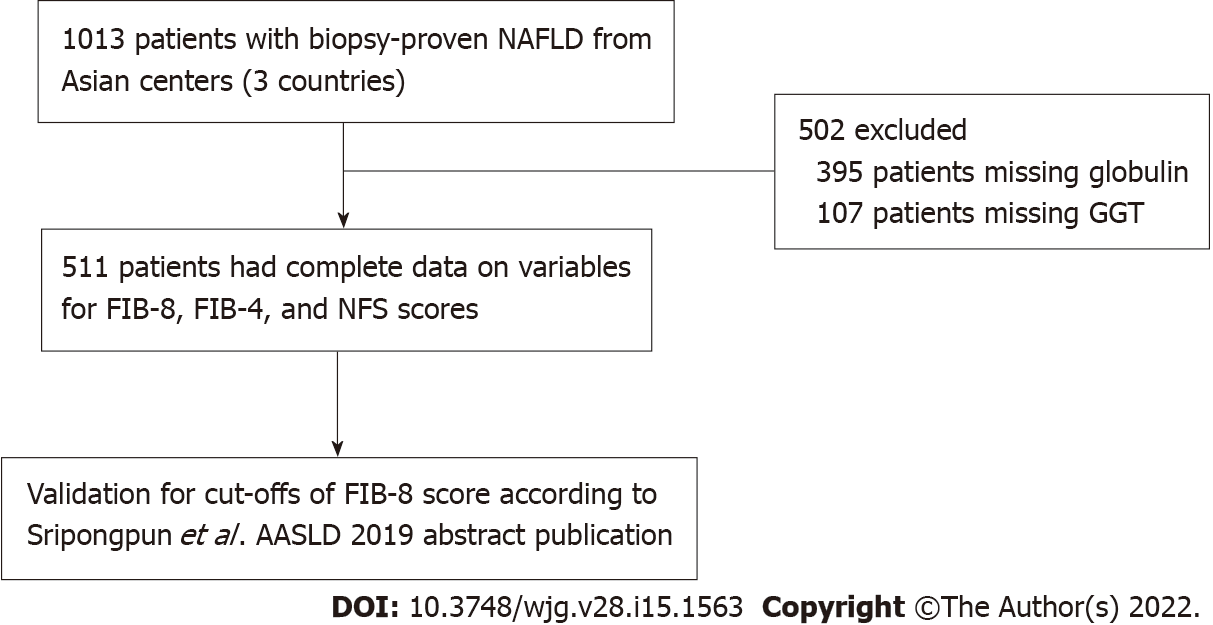
A total of 1013 patients with biopsy-proven NAFLD were included in the database. Of those, 511 patients had complete data on variables, including the NFS and FIB-4 and FIB-8 scores, and were eligible for the current study (Figure 1). Of the 511 patients, 271 (53.0%) were female, with a median age of 51 [interquartile range (IQR): 41, 58] years. The median BMI was 29 (26.3, 32.6) kg/m2, and 268 (52.4%) had diabetes. Among the 511 NAFLD patients, 157 (30.7%), 88 (17.2%), and 16 (3.1%) patients had significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4), respectively. The baseline characteristics comparing NAFLD F0–1 and significant fibrosis (F ≥ 2) are shown in Table 2. The significant factors associated with significant fibrosis were an older age [55 (48, 61) vs 49.5 (39, 57) years; P < 0.001], the presence of diabetes (71.3% vs 44.0%; P < 0.001), higher levels of AST [53.5 (36, 75) vs 35 (26, 52) U/L; P < 0.001], ALT [75 (50, 111) vs 59.5 (40, 98) U/L; P < 0.001] and GGT [81 (48, 151) vs 56.5 (35, 92) U/L; P < 0.001], a lower platelet count [230 (189, 277) vs 266 (226.8, 302) × 109/cu.mm; P < 0.001], lower levels of total cholesterol [182 (159, 209) vs 193 (170, 220) mg/dL; P = 0.004] and LDL-cholesterol [107 (85, 132) vs 116 (96, 143) mg/dL; P = 0.003], and a higher median Controlled Attenuation Parameter (CAP) [324 (294, 347) vs 299 (211, 339) dB/m] (Table 2).
| Variables | Total (n = 511) | Fibrosis stage F0-1 (n = 354) | Fibrosis stage ≥ F2 (n = 157) | P value |
| Age (yr), median (IQR) | 51 (41, 58) | 49.5 (39, 57) | 55 (48, 61) | < 0.001 |
| Sex | 0.138 | |||
| Male, n (%) | 240 (47.0) | 174 (49.2) | 66 (42.0) | |
| Female, n (%) | 271 (53.0) | 180 (50.8) | 91 (58.0) | |
| BMI (kg/m2), median (IQR) | 29.0 (26.3, 32.6) | 28.8 (26.2, 31.9) | 29.5 (26.3, 33.8) | 0.099 |
| Diabetes, n (%) | 268 (52.4) | 156 (44.0) | 112 (71.3) | < 0.001 |
| Albumin (g/dL), median (IQR) | 4.4 (4.1, 4.6) | 4.4 (4.2, 4.6) | 4.30 (4.0, 4.6) | 0.053 |
| Globulin (g/dL), median (IQR) | 3.4 (3.0, 3.8) | 3.4 (3.0, 3.8) | 3.5 (3.1, 3.8) | 0.21 |
| AST (U/L), median (IQR) | 39 (28, 60) | 35 (26, 52) | 53.5 (36, 75) | <0.001 |
| ALT (U/L), median (IQR) | 65 (42, 101) | 59.5 (40, 98) | 75 (50, 111) | < 0.001 |
| GGT (U/L), median (IQR) | 63 (37, 108) | 56.5 (35, 92) | 81 (48, 151) | < 0.001 |
| Platelet (× 109/μL), median (IQR) | 254 (213, 297) | 266 (226.8, 302) | 230 (189, 277) | < 0.001 |
| Hemoglobin (g/dL), median (IQR) | 14.2 (13.3, 15.2) | 14.2 (13.4, 15.2) | 14.1 (13.3, 15.2) | 0.393 |
| White blood cells (cells/μL), median (IQR) | 7430 (6060, 8700) | 7400 (6100, 8725) | 7500 (5950, 8695) | 0.768 |
| INR, median (IQR) | 1.01 (0.96, 1.06) | 1.00 (0.95, 1.07) | 1.01 (0.97, 10.6) | 0.625 |
| Total cholesterol (mg/dL), median (IQR) | 189.5 (166, 217) | 193 (170, 220) | 182 (159, 209) | 0.004 |
| LDL-cholesterol (mg/dL), median (IQR) | 115 (92, 139) | 116 (96, 143) | 107 (85, 132) | 0.003 |
| HDL-cholesterol (mg/dL), median (IQR) | 46 (39, 52) | 46 (39, 53) | 44 (38, 50) | 0.168 |
| Triglyceride (mg/dL), median (IQR) | 120 (77, 157) | 120 (77, 155) | 119 (80, 159) | 0.483 |
| HbA1C (%), median (IQR) | 6.1 (5.6, 7.2) | 5.9 (5.5, 6.8) | 6.8 (5.8, 7.6) | < 0.001 |
| Fibrosis stage, n (%) | < 0.001 | |||
| 0 | 151 (29.5) | 151 (42.7) | 0 (0) | |
| 1 | 203 (39.7) | 203 (57.3) | 0 (0) | |
| 2 | 69 (13.5) | 0 (0) | 69 (43.9) | |
| 3 | 72 (14.1) | 0 (0) | 72 (45.9) | |
| 4 | 16 (3.1) | 0 (0) | 16 (10.2) | |
| Median CAP (dB/m), median (IQR) | 308.5 (230, 342) | 299 (211, 339) | 324 (294, 347) | < 0.001 |
| Median TE (kPa), median (IQR) | 7.6 (5.6, 10.9) | 6.6 (5.1, 8.8) | 11.1 (8.6, 15.5) | < 0.001 |
| FIB-8, median (IQR) | 2.0 (1.2, 2.9) | 1.8 (1.1, 2.4) | 3.0 (2.2, 4.0) | < 0.001 |
| FIB-4, median (IQR) | 1.0 (0.7, 1.5) | 0.8 (0.6, 1.2) | 1.5 (1.0, 2.1) | < 0.001 |
| NFS, mean ± SD | -1.8 ± 1.5 | -2.0 ± 1.4 | -1.2 ± 1.3 | < 0.001 |
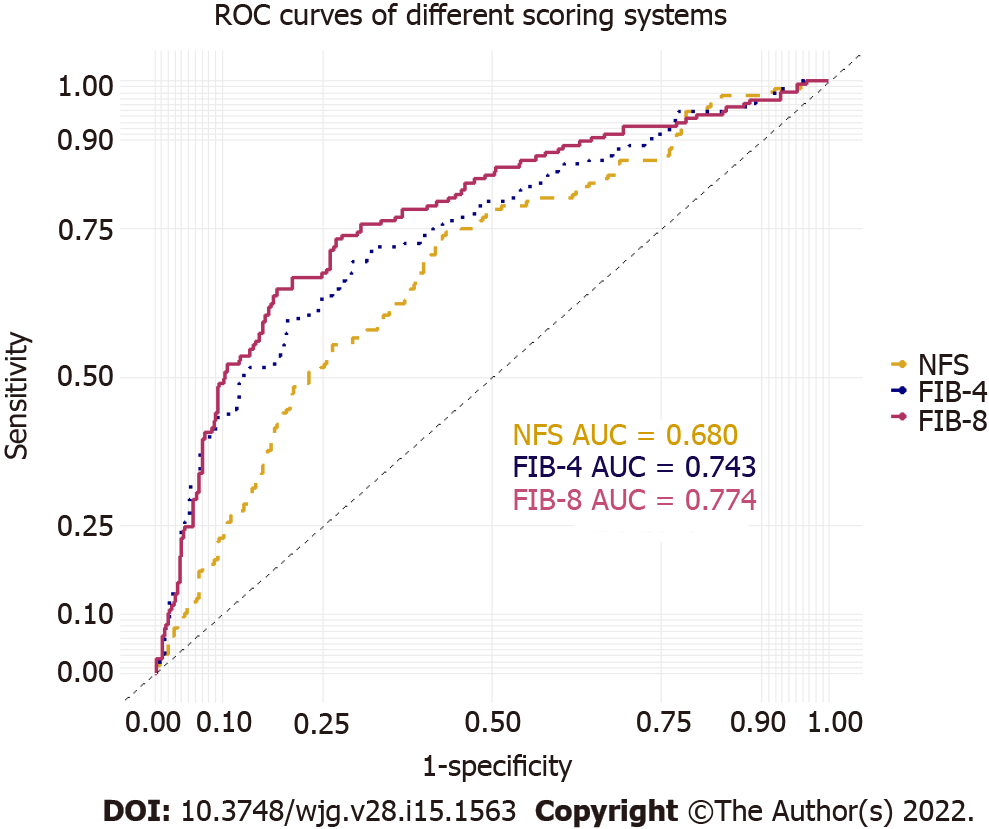
The AUROCs of the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis were 0.774 (95%CI: 0.729-0.820), 0.743 (95%CI: 0.695-0.791), and 0.680 (95%CI: 0.630-0.730), respectively (Figure 2). The FIB-8 score showed a significantly better performance for predicting significant fibrosis (≥ F2) than the NFS (P = 0.001) and was numerically higher than the FIB-4 score, but the difference was not statistically significant (P = 0.073). The sensitivities and specificities of the cutoffs specified to exclude and include significant fibrosis for each score are reported in Table 3.
| FIB-8 score | FIB-4 score | NFS | |
| AUC for predicting ≥ F2 fibrosis | 0.77a,b | 0.74 | 0.68 |
| 95% confidence interval | 0.73-0.82 | 0.70-0.79 | 0.63-0.73 |
| Low and high cutoffs for ≥ F2 fibrosis | 0.88 and 1.77 | 0.81 and 1.81 (17) | -2.45 and 0.03 (17) |
| Sensitivity (according to the low cutoff) | 92.36% | 80.25% | 80.89% |
| Specificity (according to the high cutoff) | 67.51% | 93.50% | 93.20% |
| Proportion of patients in low/indeterminate/high group | 18.8/35.4/45.8% | 38.2/47.1/14.7% | 31.9/58.1/10% |
The cohort was stratified by age into three groups: Age < 35 (n = 66), 35-65 (n = 412), and > 65 years (n = 33). The AUROCs of the FIB-8 score, FIB-4 score, and NFS in patients aged 35-65 years for predicting significant fibrosis were 0.79, 0.76, and 0.68, respectively. This patient group comprised most of the cohort and had similar diagnostic performance results as the entire cohort. However, the FIB-8 score, FIB-4 score, and NFS were poor in patients aged < 35 years (AUROC: 0.55, 0.59, and 0.70, respectively) and > 65 years (AUROC: 0.66, 0.71, and 0.54, respectively). The number of patients in each age group and center is shown in Supplementary Table 1. A detailed summary of the AUROC, sensitivity, specificity, positive predictive value, and NPV for the FIB-8 score, FIB-4 score, and the NFS is shown in Supplementary Table 2.
Based on the results of the present study, we validated the diagnostic performance of the FIB-8 score, FIB-4 score, and NFS score in 511 biopsy-proven NAFLD patients for predicting significant fibrosis. The main issue affecting the diagnostic ability of new methods for detecting liver fibrosis in NAFLD patients is the prevalence of fibrosis among the particular population. Our results demonstrated that the overall prevalence rates of significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4) were 157 (30.7%), 88 (17.2%), and 16 (3.1%), respectively. The mean incidence rates of significant fibrosis from previous publications were 52.5% and 35.4% in the PIVENS plus FLINT trials and a Stanford University trial, respectively[11,14] (Table 4). The remarkable aspects were as follows: (1) Our study had a lower incidence of fibrosis than the first cohort; (2) Among the noninvasive methods, the FIB-8 score and NFS included the BMI in their models, and our cohort had a lower mean BMI than previous reports (30.4 kg/m2 vs 34.0 and 31.5 kg/m2), which might have resulted in lower percentages of sensitivity and specificity in our cohort than those previously reported; and (3) GGT is a uniquely incorporated variable in the new FIB-8 scoring system. Some reported studies have demonstrated that a higher GGT level is a risk factor for advanced fibrosis in NAFLD[19,20]. Additionally, considering NAFLD patients with type 2 DM, a serum GGT level over 82 U/L was independently associated with advanced fibrosis using noninvasive methods in multivariate analysis (P = 0.004)[21]. In our study, the baseline characteristics correlatively showed that a higher level of median GGT was a significant factor associated with significant fibrosis [81 (IQR: 48, 151) vs 56.5 (35, 92); P < 0.001]. We postulated that GGT may be an additional variable predicting significant fibrosis in NAFLD patients. The diagnostic performance of the FIB-8 score exhibited higher accuracy for diagnosing significant fibrosis (≥ F2) than the NFS but was not superior to the FIB-4 score in previous studies or our study; the AUROCs for the FIB-8 score, FIB-4 score, and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively (FIB-8 vs NFS, P = 0.001; FIB-8 vs FIB-4, P = 0.073). The sensitivities of the low cutoff of FIB-8 score to exclude significant fibrosis was 92.36%. Consequently, the high sensitivity and NPV for excluded significant fibrosis may be beneficial in primary care units and to select patients for further hepatologist referral. However, the limited specificity of the high cutoff of FIB-8 score to include significant fibrosis may require further step assessment instance transient elastography.
| Variable | Data from AASLD 2019, n = 522 | FIB-8 score validation (EASL 2020), n = 130 | FIB-8 score validation (Our cohort), n = 511 |
| Population | Mean age: 49 ± 12. Female: 62.5%; BMI: 34 ± 7 kg/m2; DM: 30%; ≥ F2: 52.5% | Mean age: 52.4; Female: 53.1%; BMI: 31.5 kg/m2; DM: 34%; ≥ F2: 35.4% | Mean age: 49.3 ± 11.9; Female: 53.0%; BMI: 30.4 ± 7.1 kg/m2; DM: 52.4%; ≥ F2: 30.7% |
| Sensitivity and specificity % | 86.7% and 82.7%, respectively, Validation set | > 90%, 80.6% | 92.3%, 67.5% |
| AUC for predicting > F2 fibrosis | 0.78, Validation set | 0.84 | 0.77 |
| Performance superior to | > FIB-4 score; P < 0.001; > NFS; P = 0.005 | > FIB-4 score (AUC 0.80); > NFS (AUC 0.77) | > FIB-4 score; P = 0.073; > NFS; P = 0.001 |
Furthermore, our results demonstrated that the FIB-4 score offered better diagnostic performance than the NFS score (P < 0.001). According to meta-analysis results from Castera[10], the FIB-4 score and NFS showed the best diagnostic performance for detecting advanced fibrosis compared with other blood-based models. However, this meta-analysis included studies that used different cut-off thresholds. Furthermore, a recent meta-analysis from Castellana et al[22] reported a head-to-head comparison of the FIB-4 score and NFS from 18 studies that used consistent cutoffs. The FIB-4 score offered higher performance for including and NFS for excluding advanced fibrosis. However, our studies used different cutoffs and aimed to predict significant fibrosis, not advanced fibrosis. Consequently, our cohort was not suitable to compare the FIB-4 score and NFS.
Additionally, our results demonstrated the performance of the FIB-8 score, FIB-4 score, and NFS in patients aged > 65 years (AUROC: 0.66, 0.71, and 0.54, respectively). The performance was poor in patients aged < 35 years (AUROC: 0.55, 0.59, and 0.70, respectively). Thus, these scores have insufficient accuracy for use in NAFLD patients in extreme age groups. Similarly, McPherson et al[23] demonstrated age as a confounding factor for the accurate noninvasive scoring system predicting advanced fibrosis[23]. The FIB-8 score has low accuracy for predicting significant fibrosis in NAFLD patients, similar to the FIB-4 score and NFS in patients aged < 35 and > 65 years.
Our study had limitations. First, we had limited complete data for half of our database because of the lack of either globulin or GGT. In usual clinical practice, clinicians do not routinely check both laboratory parameters, and no added value exists for observing or monitoring these values in patients. The second limitation of our study was the lower incidence of fibrosis in our cohort vs other cohorts. The differences in fibrosis may have diagnostic value for novel fibrosis scores for validation. Validations in larger cohorts are needed.
To our best knowledge, our study is the first to report a new validation model of the FIB-8 score for predicting significant fibrosis among patients with NAFLD in an Asian population. The FIB-8 score yielded higher accuracy in diagnosing significant fibrosis than the NFS. Additionally, the FIB-8 score was non-inferior but insignificantly superior to the FIB-4 score. A novel simple fibrosis score comprising commonly accessible basic laboratories may be additionally used to add previous fibrosis scores for an initial assessment in primary care units and to select patients for further hepatologist referral.
The new, simple fibrosis FIB-8 score had significantly better performance for predicting significant fibrosis in NAFLD patients than the NFS and was non-inferior but insignificantly superior to the FIB-4 score in the Asian population. A simple fibrosis score comprising commonly accessible basic laboratories may be used for an initial assessment in primary care units and to select patients for further hepatologist referral.
In the nonalcoholic fatty liver disease (NAFLD) population, noninvasive fibrosis scores, such as the fibrosis-4 (FIB-4) score and NAFLD fibrosis score (NFS), are generally applied in clinical practice guidelines. The novel fibrosis-8 (FIB-8) score yielded higher accuracy in diagnosing significant fibrosis in a previously reported cohort. A larger cohort may provide more reliability and benefit in clinical practice.
A noninvasive fibrosis score in NAFLD patients using only routine laboratory parameters is particularly important in initial assessment in the primary care unit or resource-limited conditions. We proposed the novel FIB-8 score, which incorporates the additional variables body mass index (BMI), the A/G ratio, gamma-glutamyl transferase (GGT), and diabetes into the FIB-4 score. The additional variables, particularly GGT, may provide better diagnostic accuracy for predicting significant fibrosis in NAFLD patients.
We aimed to validate the FIB-8 score among patients with a biopsy-proven NAFLD cohort and to compare the diagnostic performance of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis.
This was a retrospective study involving 1013 biopsy-proven NAFLD patients from 3 Asian centers in 3 countries in an Asian population. All the patients with available baseline biochemical tests for the FIB-8 score calculation and all related variables for predicting liver fibrosis were included.
A total of 1013 patients were included in the final analysis. Of those, 511 patients had complete data on the variables, including the NFS and FIB-4 and FIB-8 scores. One hundred fifty-seven (30.7%) patients had significant fibrosis (≥ F2). The areas under the receiver operating characteristic curves of the FIB-8 and FIB-4 scores and NFS for predicting significant fibrosis were 0.774, 0.743, and 0.680, respectively. The FIB-8 score had significantly better performance for predicting significant fibrosis than the NFS (P = 0.001) but was not superior to the FIB-4 score (P = 0.073). The low cutoff point of the FIB-8 score for predicting significant fibrosis of 0.88 showed 92.36% sensitivity, and the high cutoff point of the FIB-8 score for predicting significant fibrosis of 1.77 had 67.51% specificity.
The FIB-8 score, which incorporates the additional variables of the BMI, A/G ratio, GGT level, and diabetes into the FIB-4 score, yielded better performance for predicting significant fibrosis in NAFLD patients than the NFS but was not superior to the FIB-4 score in the Asian population. A simple fibrosis score comprising commonly accessible basic laboratories may be used for an initial assessment in primary care units.
Future prospective studies are needed to compare the diagnostic accuracy of various noninvasive scores for predicting significant fibrosis and staging fibrosis.
We would like to thank the supporting team of the Gut and Obesity in Asia workgroup for the database. Additionally, we would like to thank the research coordinator and statistician, Kanokwan Sornsiri, and Chonlada Phathong, from the Division of Gastroenterology, King Chulalongkorn Memorial Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dietrich CG, Germany; Ji G, China; Tarantino G, Italy; Ulasoglu C, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1460] [Article Influence: 243.3] [Reference Citation Analysis (1)] |
| 2. | Chan WK, Treeprasertsuk S, Imajo K, Nakajima A, Seki Y, Kasama K, Kakizaki S, Fan JG, Song MJ, Yoon SK, Dan YY, Lesmana L, Ho KY, Goh KL, Wong VW. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther. 2018;47:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 703] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 4. | Teeratorn N, Piyachaturawat P, Thanapirom K, Chaiteerakij R, Sonsiri K, Komolmit P, Tangkijvanich P, Rerknimitr R, Adams L, Treeprasertsuk S. Screening for non-alcoholic fatty liver disease in community setting: A cohort study using controlled attenuation parameter-transient elastography. JGH Open. 2020;4:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 739] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 6. | Tarantino G, Citro V, Capone D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J Clin Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 8. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 9. | Mahady SE, Macaskill P, Craig JC, Wong GLH, Chu WCW, Chan HLY, George J, Wong VWS. Diagnostic Accuracy of Noninvasive Fibrosis Scores in a Population of Individuals With a Low Prevalence of Fibrosis. Clin Gastroenterol Hepatol. 2017;15:1453-1460.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Castera L. Non-invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healthcare and liver clinics. Liver Int. 2020;40 Suppl 1:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Sripongpun P, Ajitha Mannalithara DK, Alexis Touros and W. Ray Kim. FIB-8 score: A model incorporating additional common variables into the FIB-4 score affords better prediction of significant fibrosis in non-alcoholic fatty liver disease. AASLD Abstract publication. 2019;1723. |
| 12. | Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, Tonascia J; NASH CRN Research Group. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1802] [Article Influence: 180.2] [Reference Citation Analysis (3)] |
| 14. | Sripongpun P, Donghee Kim AM, W. Ray Kim. The FIB-8 score: validation of a model to screen patients with non-alcoholic fatty liver disease for significant fibrosis. EASL The Digital Inter Liver Con 2020. 2020;FRI072. |
| 15. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 16. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2285] [Article Influence: 126.9] [Reference Citation Analysis (1)] |
| 17. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 18. | Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, Brandman D, Tonascia J, Chalasani N, Neuschwander-Tetri B, Sanyal AJ; NASH Clinical Research Network. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin Gastroenterol Hepatol. 2019;17:1877-1885.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 19. | Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, Hatemi I, Olgac V, Sonsuz A, Ozbay G, Yurdakul I, Senturk H. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55:1433-1438. [PubMed] |
| 20. | Fujii H, Doi H, Ko T, Fukuma T, Kadono T, Asaeda K, Kobayashi R, Nakano T, Doi T, Nakatsugawa Y, Yamada S, Nishimura T, Tomatsuri N, Sato H, Okuyama Y, Kimura H, Kishimoto E, Nakabe N, Shima T. Frequently abnormal serum gamma-glutamyl transferase activity is associated with future development of fatty liver: a retrospective cohort study. BMC Gastroenterol. 2020;20:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Arab JP, Barrera F, Gallego C, Valderas JP, Uribe S, Tejos C, Serrano C, Huete Á, Liberona J, Labbé P, Quiroga T, Benítez C, Irarrázaval P, Riquelme A, Arrese M. High prevalence of undiagnosed liver cirrhosis and advanced fibrosis in type 2 diabetic patients. Ann Hepatol. 2016;15:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Castellana M, Donghia R, Guerra V, Procino F, Castellana F, Zupo R, Lampignano L, Sardone R, De Pergola G, Romanelli F, Trimboli P, Giannelli G. Fibrosis-4 Index vs Nonalcoholic Fatty Liver Disease Fibrosis Score in Identifying Advanced Fibrosis in Subjects With Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Am J Gastroenterol. 2021;116:1833-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |