Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1536
Peer-review started: November 25, 2021
First decision: January 11, 2022
Revised: January 18, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 21, 2022
Processing time: 140 Days and 21.6 Hours
Crohn's disease (CD) is a chronic nonspecific intestinal inflammatory disease. The aetiology and pathogenesis of CD are still unclear. Anal fistula is the main complication of CD and is a difficult problem to solve at present. The main limitation of developing new therapies is bound up with the short of preclinical security and effectiveness data. Therefore, an ideal animal model is needed to establish persistent anal fistula and an inflamed rectal mucosa.
To improve the induction method of colitis and establish a reliable and reproducible perianal fistulizing Crohn’s disease animal model to evaluate new treatment strategies.
Twenty male New Zealand rabbits underwent rectal enema with different doses of 2,4,6-trinitrobenzene sulfonic acid to induce proctitis. Group A was treated with an improved equal interval small dose increasing method. The dosage of group B was constant. Seven days later, the rabbits underwent surgical creation of a transsphincteric fistula. Then, three rabbits were randomly selected from each group every 7 d to remove the seton from the fistula. The rabbits were examined by endoscopy every 7 days, and biopsy forceps were used to obtain tissue samples from the obvious colon lesions for histological analysis. The disease activity index (DAI), colonoscopy and histological scores were recorded. Perianal endoscopic ultrasonography (EUS) was used to evaluate the healing of fistulas.
Except for the DAI score, the colonoscopy and histological scores in group A were significantly higher than those in group B (P < 0.05). In the ideal model rabbit group, on the 7th day after the removal of the seton, all animals had persistent lumens on EUS imaging, showing continuous full-thickness high signals. Histological inspection of the fistula showed acute and chronic inflammation, fibrosis, epithelialization and peripheral proctitis of the adjoining rectum.
The improved method of CD colitis induction successfully established a rabbit perianal fistula CD preclinical model, which was confirmed by endoscopy and pathology.
Core Tip: In this work, we improved the method of Crohn's disease (CD) colitis induction and successfully established a rabbit perianal fistula CD preclinical model, which was confirmed by endoscopy and pathology. The anatomy of this mid- to large-sized animal can simulate the human intestinal environment and tolerate examination and operation. This model may be used to assess perianal fistulizing CD treatments and their effectiveness.
- Citation: Lu SS, Liu WJ, Niu QY, Huo CY, Cheng YQ, Wang EJ, Li RN, Feng FF, Cheng YM, Liu R, Huang J. Establishing a rabbit model of perianal fistulizing Crohn’s disease. World J Gastroenterol 2022; 28(15): 1536-1547
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1536
Crohn's disease (CD) is a chronic, nonspecific intestinal inflammatory disease. The aetiology and pathogenesis of CD are still unclear[1]. Since the 1950s, the incidence rate of CD has been steady-state growth of industrialization nations. CD is a common digestive disease with an incidence of 12.7/100000 residents very year in Europe[2]. In the course of CD, there are different types of perianal diseases, including fistula, abscess, anal fissure, stricture and dermatophyte. These lesions may appear prior to or accompanied by CD intestinal symptoms and are factors affecting the prognosis of CD[3]. Anal fistula is the main complication of CD. Studies have shown that 15%-45% of CD patients have perianal lesions such as anal fistula[1].
Fistulizing anoperineal lesions represent a complex disease phenotype for which the treatment requires a multidisciplinary approach[4]. Modern medical concepts describe that patients with CD anal fistula should be treated with drugs first, and surgical treatment should be considered when necessary to control intestinal inflammation[5]. The main therapeutic drugs used are antibiotics, immunosuppressants, biological agents, etc[6-9]. In recent years, many studies have shown that the use of mesenchymal stem cells can be a new treatment for specific cases of complex fistulas[10,11]. In addition, some scholars have suggested other new treatments, such as hyperbaric oxygen therapy, as a potential adjuvant treatment for patients with inflammatory bowel diseases (IBDs)[12,13]. However, these new treatments have not been fully developed into routine and safe technical procedures. Major constraints on the development of update therapeutic schedules is obviously correlated with the short of preclinical security and effectiveness data. Up to now, an ideal animal model that can reproduce sustaining anal fistula and an inflamed rectal mucosa is needed.
The main purpose of this research is to improve the colitis induction method and develop a simple, reliable and reproducible fistula animal model to assess new treatment strategies.
Twenty male New Zealand rabbits, weighing about 2.0 kg, were chosen and numbered after weighing (the grouping methods are listed in Table 1). They were raised and placed under the condition of no special pathogen. The laboratory was clean, with good light and ventilation. The indoor temperature was controlled between 24 and 28 ℃, and the relative humidity was maintained between 50% and 70%, with 10-15 air changes per hour and 12 h of light each day. On the day before and the day of the operation, the rabbits were fed formula and drank freely. Cages of rabbits were disinfected and kept separate. Sufficient water and food were given. The rabbits were kept in cages for 7 d to adapt to the environment. The rabbits were weighed on the day of operation and then every 7 d. This study was approved by the ethics committee of Changzhou University.
| Day 1 | Day 7 | Day 14 | |
| Group A (n = 9) | 4.0 mL | 5.0 mL | 6.0 mL |
| Group B (n = 9) | 5.0 mL | 5.0 mL | 5.0 mL |
| Group C (n = 2) | - | - | - |
For each procedure (enema, surgery or endoscopy), 1.5% pentobarbital sodium (3.5 mL/kg) was used for ear vein anaesthesia, and dyclonine hydrochloride mucilage was locally applied around the anus to reduce the pain associated with surgery.
Proctitis: A total of 100 mg/kg 2,4,6-trinitrobenzenesulfonic acid solution (TNBS) was dissolved in 50% ethanol (the total volume of solution is shown in Table 1) and was used for the induction of CD[14,15]. After 7 d of adaptive feeding, the experimental rabbits were fasted for 48 h and injected with 1.5% pentobarbital sodium through the ear vein. After anaesthesia, the rabbits were administered enemas with a TNBS + ethanol mixture by a 5 mL syringe through a central venous catheter every week according to the dose in Table 1 and then injected with air in a section of approximately 0.5 mm in length to remove the drug adhering to the syringe and enema tube wall as much as possible. Then, the rabbits were assigned to intervention groups A, B or C, where group C was used as a control.
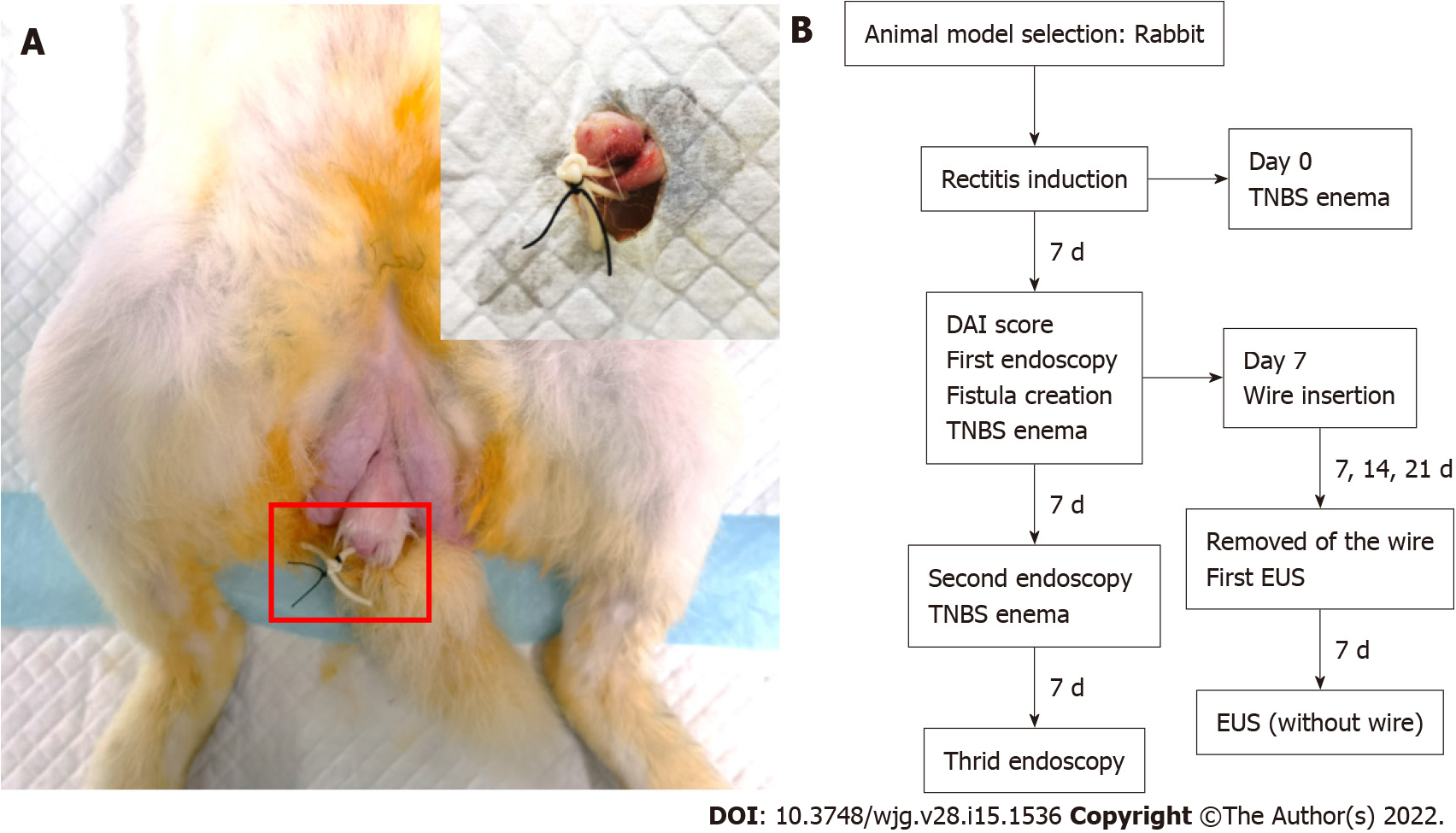
Perianal fistula: On the 7th day after enema with TNBS, an anal fistula was caused by a minor operation. in the state of anaesthesia, the rabbits was fixed supine. Their perianal hair was shaved, and the area was disinfected with iodophor solution and then smeared with dyclonine hydrochloride mucilage. The elastic surgical seton (rubber band, diameter = 1.2 mm), soaked with TNBS solution in advance, was inserted into the needle core. For the experimental group, the seton was placed 1 cm from the anal margin at the same site, and a straight needle with a rubber band was used to puncture the rectum and then remove the punctured tissue from the body. A needle holder was used to clip the rubber band from the outside of the anus through the whole tunnel to make the rubber band pass through the perianal puncture opening, and a thin thread was used to fix the rubber band to prevent slippage and anal congestion. The external orifice is approximately 1 cm from the anus, as shown in Figure 1A. The surgical loop must be released without any tension. Finally, after the operation, the rabbits were returned to the feeding room, where they were observed and their vital signs were monitored until they woke up.
After the operation, a 1-mL syringe was used to inject TNBS mixed solution (diluted with 5% TNBS and absolute ethanol 1:1, total volume of 200 μL) into the fistula. Different doses of TNBS mixed solution (Table 1) were infused into the intestine once a week for three weeks, three times in total. To determine the best surgical procedure and reproducibility, 3 rabbits were randomly selected from each of groups A and B every 7 d, and the fistula setons were removed for endoscopic ultrasonography (EUS) assessment to evaluate the lumen. By the 28th day, the setons of all rabbits were removed. The characteristics of the two intervention groups and the different stages of the study are summarized in Figure 1B.
Clinical assessment: Clinical observation included: (1) recording the changes in daily activity, food intake, stool characteristics and body weight of the experimental animals and determining the disease activity index (DAI) score (Supplementary Table 1)[16]; (2) recording the number of deaths of the experimental animals in each group every day; and (3) checking whether the operation seton existed every day. In autonomous shedding, the new seton was inserted into the primary lumen again.
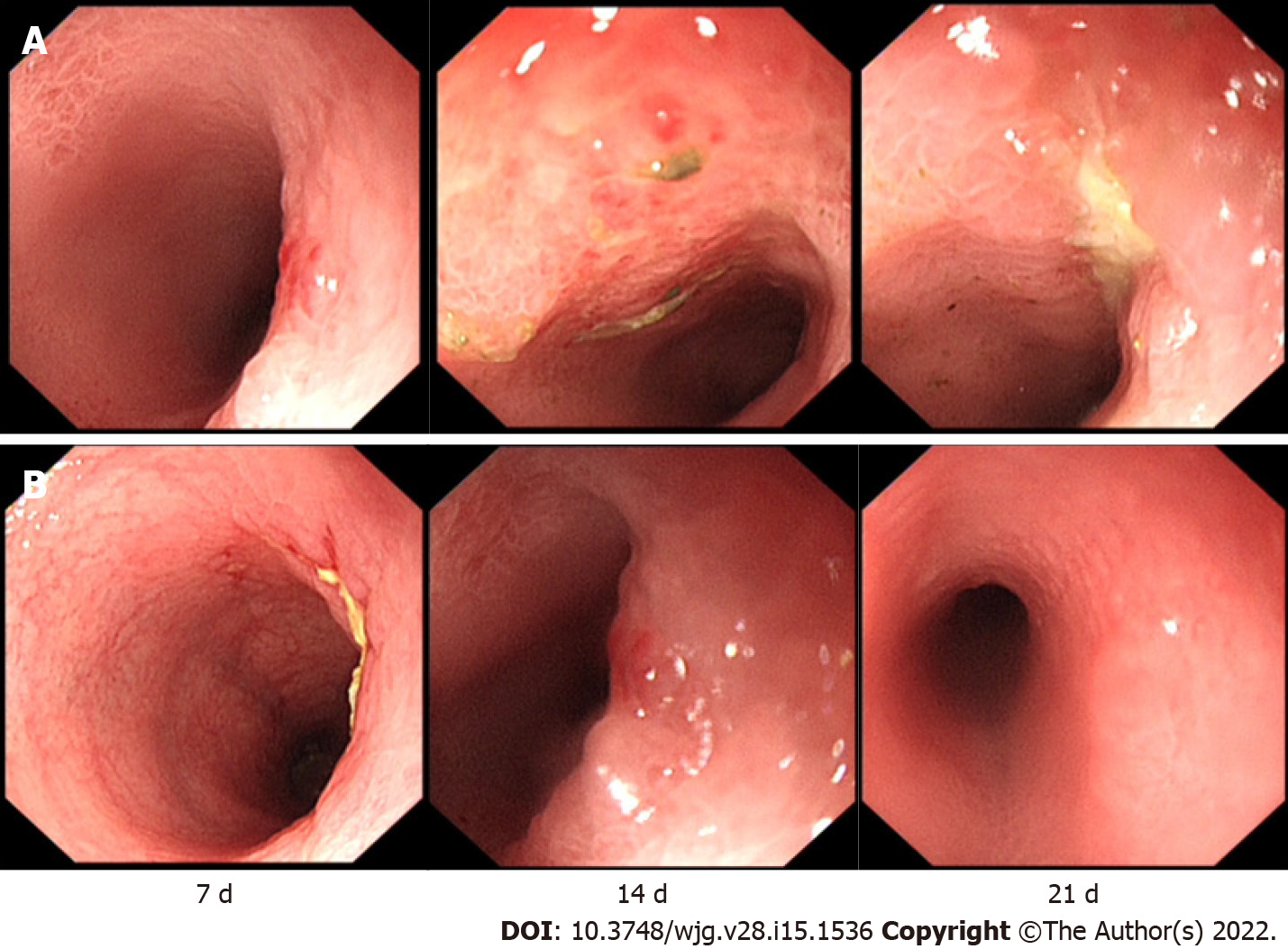
Endoscopic assessment: The colon macroscopic damage index (CMDI) was used for endoscopic assessment[17]. The CMDI was assessed according to the criteria described in Supplemental Table 2. Before the start of the study (TNBS enema administration), we performed an endoscopic examination of the rabbits to determine that the colon before treatment was normal, and these results were not included in the final statistics. After the study, the first intestinal endoscopy was performed on the 7th day (the day of surgical seton insertion). Morphological damage to the intestinal wall after the first intestinal administration was observed and scored. Then, endoscopy was performed every 7 d, and intestinal injury was observed and recorded. The last endoscopy was performed 21 d after the first enema. Endoscopy and scoring were performed by two experienced gastroenterologists (19 and 22 years of experience in the diagnosis and treatment of IBD, respectively).
| Group | Day 7 | P value | Day 14 | P value | Day 21 | P value | Day 28 | P value | |
| DAI | A | 4.22 ± 2.33 | 0.026 | 6.88 ± 0.93 | 0.020 | 8.00 ± 2.00 | 0.810 | 7.78 ± 1.56 | 0.021 |
| B | 7.13 ± 2.53 | 8.75 ± 1.91 | 8.25 ± 2.25 | 5.75 ± 1.67 | |||||
| Endoscopy scores | A | 1.11 ± 0.78 | 0.002 | 2.44 ± 0.88 | 0.319 | 3.89 ± 0.93 | < 0.001 | ||
| B | 2.88 ± 1.13 | 2.88 ± 0.83 | 1.63 ± 0.92 | ||||||
| Histology scores | A | 1.44 ± 0.88 | < 0.001 | 3.11 ± 0.93 | 0.017 | 4.11 ± 0.78 | < 0.001 | ||
| B | 3.50 ± 0.93 | 2.00 ± 0.76 | 1.88 ± 0.99 | ||||||
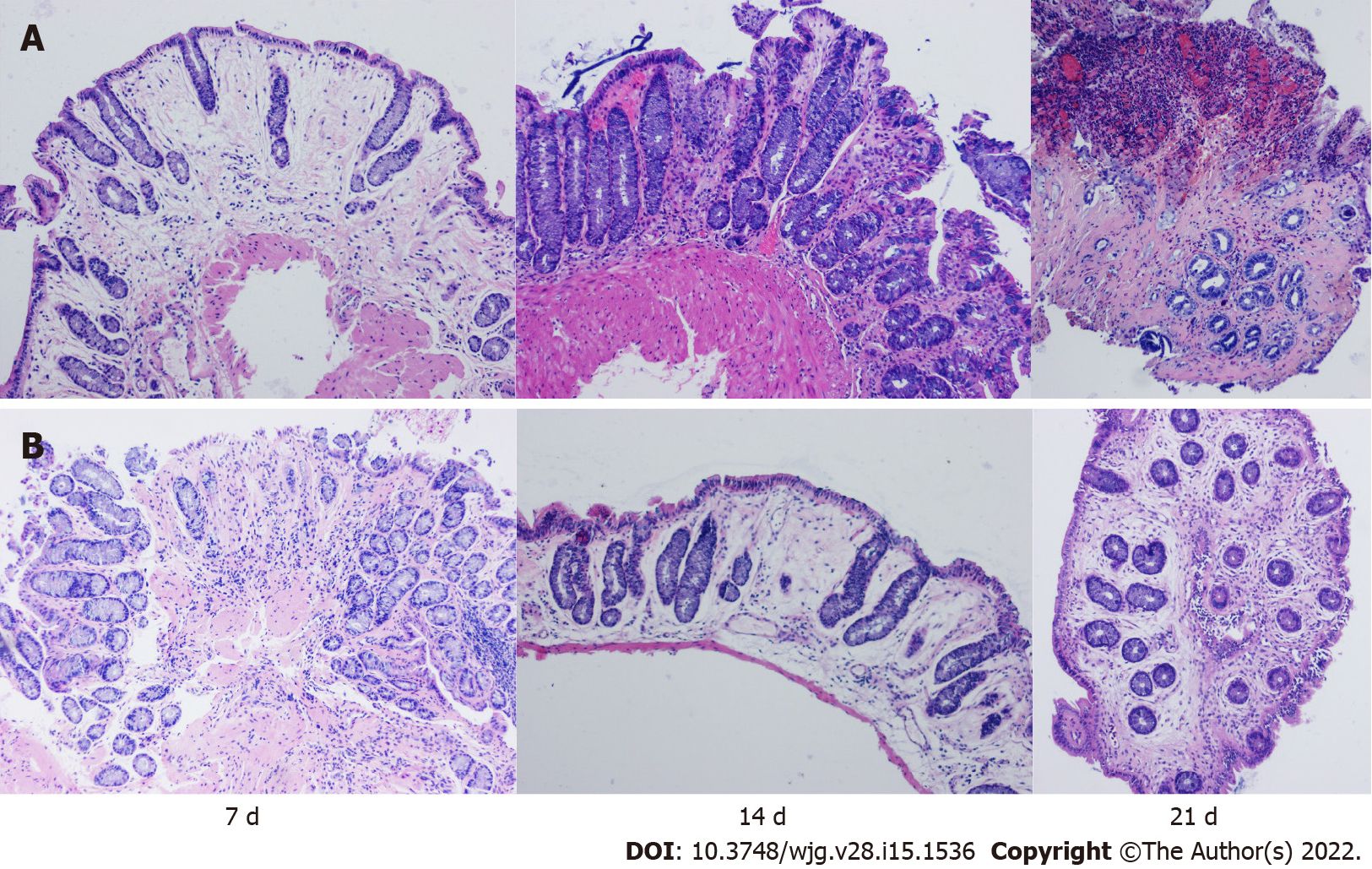
Histological examination: The tissue damage index (TDI) was used for the histological examination. The TDI was assessed using a modified version of the histological grading system described by MacPherson et al[18], as shown in Supplementary Table 3. At the same time as the endoscopic examination, 2-4 pieces of tissue with obvious inflammation and/or ulcers were clipped with biopsy forceps, fixed with neutral formaldehyde solution for 24 h, and stored at -4 ℃. Then, the specimens were embedded in paraffin, sliced continuously with a slicer, stained with haematoxylin-eosin, and finally scored histologically. Two experienced gastrointestinal pathologists performed blinded histological analyses.
The histological diagnosis of fistulas was ground on the following criteria: the internal orifice of the lumen is located on the rectal mucosa, and the external orifice of the lumen is located on the perineal skin. At the same time, it has the histological characteristics of proctitis (neutrophils, B and T lymphocytes, macrophages) were present. The feature of fistulas was decided by the presence or absence of epithelialization, fibration, and inflammation[19].
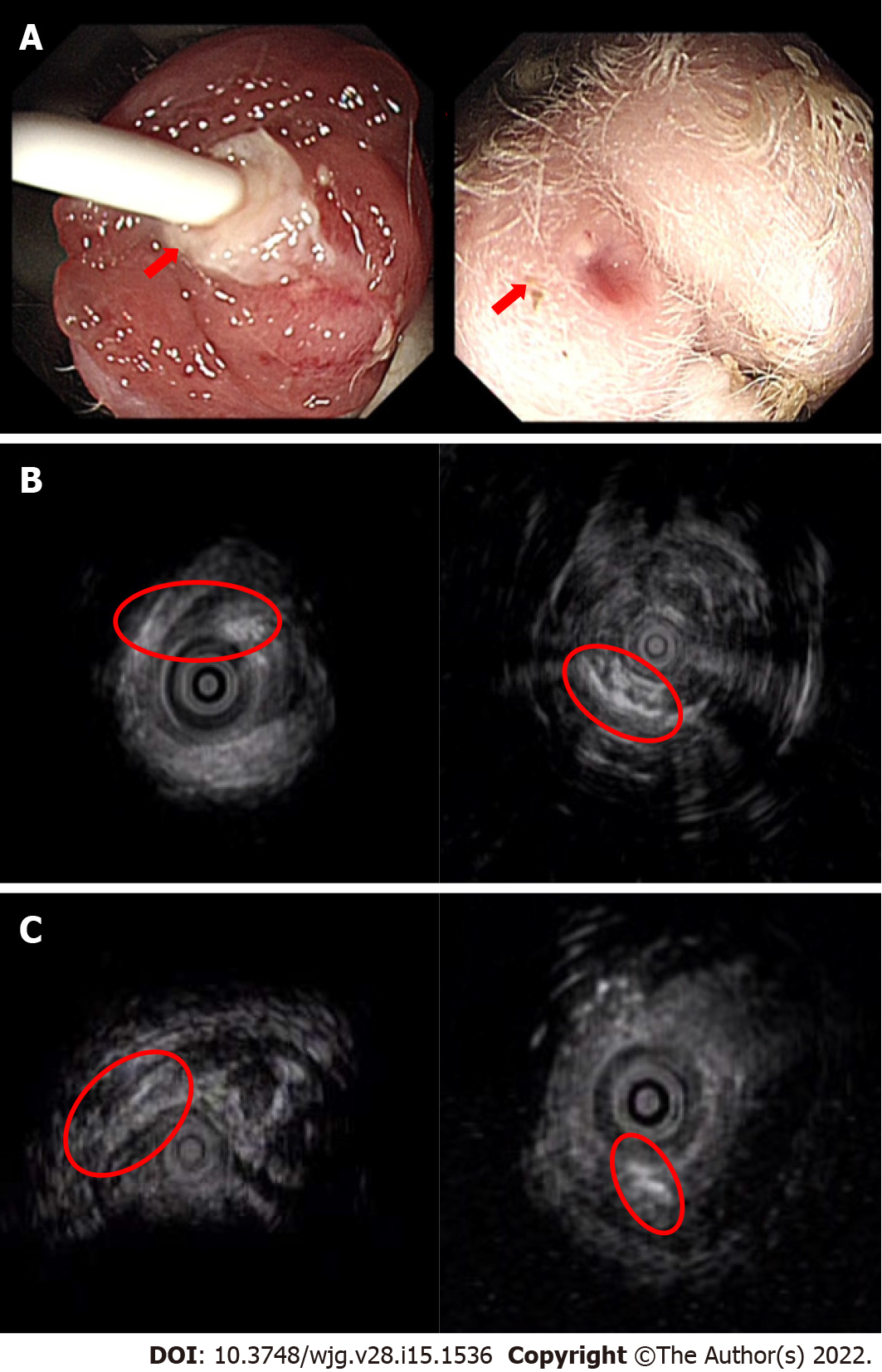
EUS assessment: The time of the insertion of the anal fistula operation thread was different in each group. After anal fistula formation, on the day of the removal of the thread inserted into the fistula, the perianal fistula of experimental rabbits in each group was examined by EUS for the first time, including mainly the observation of the fistula inner mouth, outer mouth, and course and the inflammation of the surrounding mucosa. The second EUS was performed on the 7th day after the removal of the thread. Spontaneous healing of the fistula was observed and recorded. Image recording and parameter interpretation were accomplished by a gastroenterologist (12 years of experience in diagnosis and treatment in IBD) and a ultrasound engineer (14 years of experience in interpreting ultrasound imaging). While they were blinded to groups of animals and histological results.
All the data were handled and analyzed by statistical software (SPSS 19.0), and the results are rendered as the mean ± SD. P < 0.05 was have been viewed as statistically critical.
Clinical examination: In groups A and B, there were different degrees of loose stool and bloody stool visible to the naked eye. Rabbits ate less and were slow, low spirited, and occasionally irritable. Their weight gradually decreased with time. In group C, the body weight increased significantly with time, the activity was normal, and although there was occasional diarrhoea, there was no bloody stool. This condition was followed by the expected gradual weight recovery phase after the discontinuation of TNBS, which confirmed the healing of the colon injury. No rectal prolapse was observed. One week after the operation, the seton was removed from the perianal area of the experimental rabbits, and all the experimental rabbits showed two visible healing holes, which demonstrated the existence of the inner and outer holes. The rate of spontaneous seton shedding was approximately 17.6% (3/17) in each group. Also new setons were inserted again in the primary lumen of each animal. A total of 1 experimental rabbit died one week after surgery (group B) throughout the duration of experiments.
Endoscopy and pathology: Endoscopy was used to assess the modelling results. The process of colitis induction was smooth, and all rabbits underwent anal fistula surgery. One rabbit died one week after surgery. The rabbit was excluded from the results analysis. The remaining 17 rabbits were marked according to the length of insertion time.
After colitis was induced in the intervention group, the scores were determined, and the results are shown in Table 2. According to the statistical analysis (Table 2), except for the DAI score, the scores in group A were significantly higher than the scores in group B (P < 0.05).
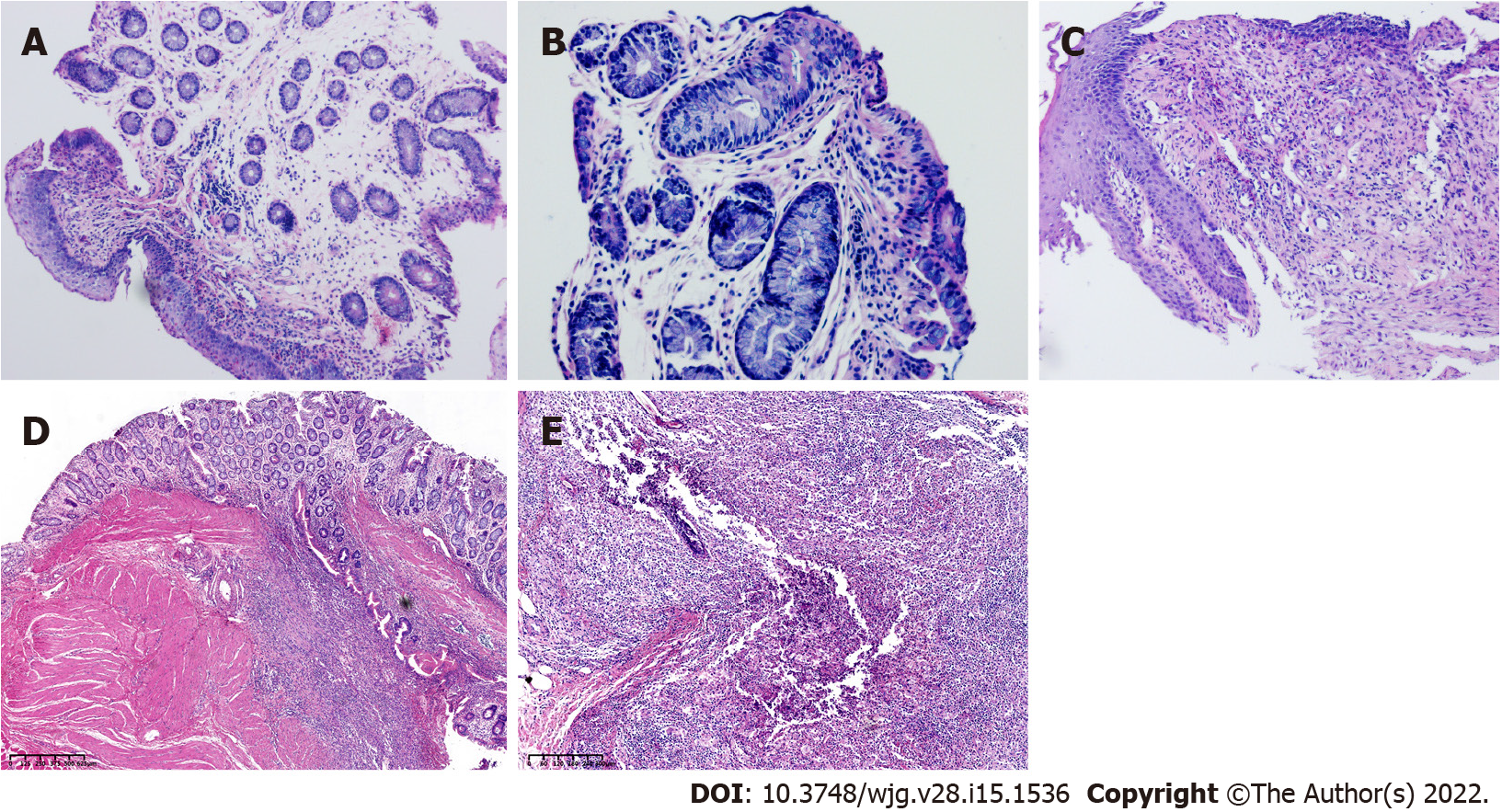
In addition, we performed endoscopy in the process of colitis induction in rabbits of groups A and B and used biopsy forceps to obtain intestinal specimens for histological analysis. We found that although the rabbits in group B had obvious intestinal inflammation on the 7th day after the first TNBS enema, the intestinal inflammation at the last endoscopic examination was weaker than the intestinal inflammation in group A (Figures 2 and 3), showing that the inflammation of group A was higher than the inflammation of the other groups, and the modelling method of a TNBS dose increase in group A was better than the modelling method of other groups.
EUS: All rabbits in groups A and B underwent two EUS scans of perianal fistulas. At the first EUS scan, all 17 rabbits (100%) had visible fistulas, also the external and internal orifice were noticeable in just about the greater part rabbits. At the second perianal EUS, that is, 7 d after the surgical seton was removed from the rabbits, the healing of the fistula in each group was different, as shown in Figure 4. Fistula was observed in 100% (6/6) of 6 rabbits with a surgical seton insertion time of 21 d. A scar was seen at the outer mouth of the fistulas, and granulation tissue hyperplasia was seen at the inner mouth. Other rabbits showed spontaneous healing of the fistula lumen and the disappearance of the fistula inner and outer orifices (Figure 4).
Pathology: Fibrosis has been distinguished in the connective tissue contiguous of the fistula 7 d after the insertion of the surgical seton. In addition, there were signs of acute (neutrophil infiltration and abscess formation) and chronic inflammation (lymphoplasmacyte infiltration, granuloma). On the 21st day after the insertion of the suture, granulation tissue was identified on the perianal orifice of the fistula. The pathologist positioned the rectal mucosa and thread through the anal sphincter as the fistula,which shown in Figure 5.
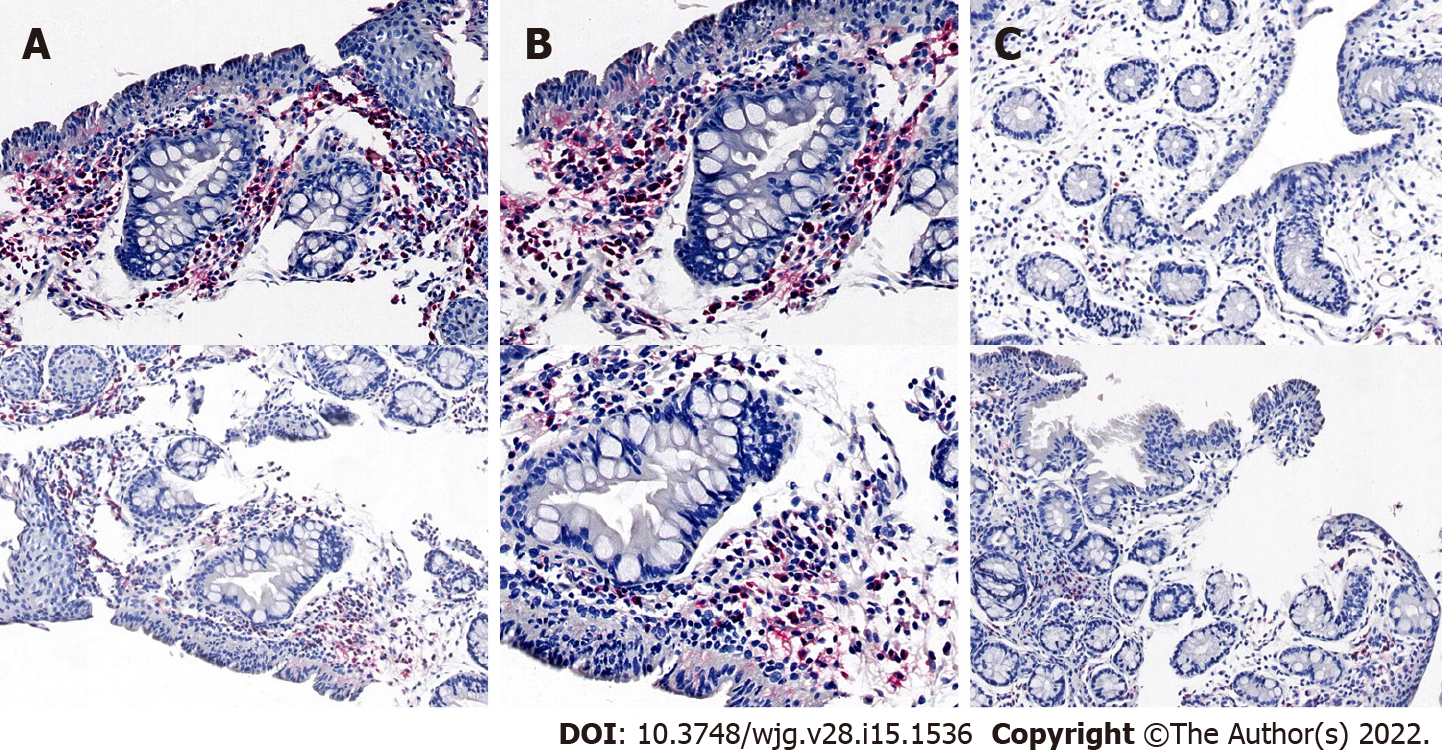
Immunohistochemistry confirmed that group A was acute inflammation. Neutrophils and other inflammatory cells infiltrated in the fistula (Figure 6).
One of the most challenging phenotypes of CD is perianal fistula. The combination of perianal disease and CD predicts a significantly worse course[20]. The pathogenesis of CD and its complications are unclear. At present, there is no ideal curative treatment[21]. It is difficult to treat perianal fistulizing CD, which usually requires more active medical and surgical intervention.
Compared with rats, rabbits are mid- to large-sized animals. The rabbit anal and rectal anatomy is similar to that of humans and is of appropriate size[22,23]. The rabbit anatomy can maximize and tolerate the simulation of human perianal fistulizing CD-related auxiliary examinations, such as endoscopy, EUS, computed tomography, and magnetic resonance imaging (MRI). A preclinical model of rectal histological inflammation with perianal sphincteric fistula was established and observed continuously under endoscopy and confirmed by EUS. The diagnosis of fistula depends on EUS and histology.
As to the improvements in CD animal modelling through the use of this method involving TNBS[24], the method started with the administration of a small dose of TNBS, and then an increasing dose was administered at the same intervals. This procedure not only simulated the characteristics human CD recurrence through repeated stimulation of the intestinal mucosa but also prolonged the duration of inflammation, showing that TNBS could induce a stronger inflammatory response by being administered at an increasing dose in a step-by-step manner. Group B showed improvements in bowel inflammation, possibly due to the initially established tolerable doses, but further investigation is needed. In our model, persistent inflammation is induced, and slight changes may occur with longer healing time, with histological changes replicating the unpredictability of CD. In the process of making an anal fistula, we used an elastic rubber band, which ensured the tension-free state of the fistula. At the end of the study, all rabbit models still had visible fistulas 7 d after the removal of the seton, and EUS showed continuous full-thickness high signal. However, the rabbits in which setons were inserted by 7 and 14 d had fistula healing, and 50% and 67% of the rabbits had fistulas visible on EUS after setons were removed 7 d later. Pathology revealed the same results for the longest seton insertion. The best option to obtain a preclinical model of perianal fistula consisted of low-dose incremental administration of TNBS and 21-d seton insertion. The induced fistula might has been described by mucosal ulcers extending to the perianal dermis. Pathologic examination showed augmented chronic submucosal infiltration of inflammatory cells, granuloma formation, and neutrophilic aggregation of colonic mucosa. At the same time, ultrasound endoscopy revealed continuous high signals in the skin around the anus, indicating the presence of an inflammatory response. These features are similar to those found in human perianal fistula CD and there is active inflammation in the fistula[19].
Perianal fistulizing CD models have been described differently by several authors. For example, the SAMP1/YitFc mouse model[25] or anal furunculosis model in canines[26] generate fistulas at random. A unprompted model similar to CD terminal ileitis was found in SAMP1/YitFc mice[25]. In this study, 5% of mice were found to form anal fistula naturally, which may be partially similar to human CD anal fistula. Whereas, only a small proportion of mice form fistula, which restricts application of this model in the study of CD. Size considerations make the murine model[27] more suitable for testing medical therapies than surgical interventions. For the canine model, considering the unpredictable onset time and the difficulty of inducing fistula, the applicability of the model is limited. A previous study developed another experimental model in 16 rabbits[28] by the surgical creation of a high transsphincteric fistula. Another study developed a pig anal fistula model[29] (6 pigs) to test a new biological plug, which was confirmed by histology. A recent article in 2019[19] described induced proctitis in rats by rectal enema using TNBS. Seven days later, the sphincter fistula was established using a surgical seton. In all these cited studies, unlike our research, previously published models either did not have proctitis or fistula inflammation, or a single high-dose enema caused high mortality. In addition, it is to some extent unethical that tissues can be obtained from these animals only after they are killed.
According to the current literature, we first applied endoscopy and EUS to the study of animal models of anal fistula in CD. Previous reports have used endoscopy to study oesophageal diseases in rabbits[30]. We performed colonoscopy with a small-diameter endoscope on rabbits, which can clearly show intestinal mucosa lesions. In addition, biopsy was performed in this mid- to large-sized animal for pathology assessment. In recent years, less invasive imaging modalities, such as pelvic MRI and EUS, have been used for fistula evaluation[31]. According to the latest available guidelines, contrast-enhanced pelvic MRI is generally considered the initial procedure for assessing perianal fistulizing CD[32]. However, EUS is considered a good alternative to MRI and has good accuracy when anal stenosis is excluded[32]. Although this method cannot obtain complete fistula tissue for histological analysis, we believe that, to a certain extent, this method is more in line with ethical requirements. Moreover, this simple, reproducible animal model can be used to evaluate the new treatment and efficacy of CD anal fistula.
With that in mind, we chose a small dose of TNBS as the initial stimulus and then repeated administration at the same intervals with dose increases. This procedure mimics to some extent the characteristics of human CD recurrence with repeated stimulation of the intestinal mucosa, prolongs the duration of inflammation, and imitates the process of CD recurrence. Second, the longest period of seton insertion was 21 d, an ideal result. Although in other studies, fistula-related thread insertion times were longer than ours[28],the prolongation of the seton insertion time may lead to longer fistula removal. However, given that our study required continuous weekly bowel administration, extending the duration of the study increased the damage to the animals. Moreover, the criteria for the difference between the two experimental conditions (constant-dose or increased-dose TNBS) introduced in the study still need to be verified by larger animal experiments, and the molecular mechanism involved should be investigated. Thus, there are some limitations to this study.
In this study, a simple preclinical animal model of perianal fistulizing CD in rabbits was established by using an improved method of CD colitis induction. The model can simulate the human condition, and the intestinal and fistula lesions induced can be evaluated by EUS, endoscopic and histological examinations to assess new therapeutic strategies.
Crohn's disease (CD) is a chronic nonspecific intestinal inflammatory disease. The aetiology and pathogenesis of CD are still unclear. Anal fistula is the main complication of CD and is a difficult problem to solve at present. In recent years, there has been an increasing number of potential treatments for patients with inflammatory bowel diseases. However, these new treatments have not been fully developed into routine and safe technical procedures.
The main limitation in developing new therapies for CD with anal fistula is connected with the deficiency of preclinical safety and credible experimental data records. Therefore, an ideal animal model is needed to establish models of persistent anal fistula and an inflamed rectal mucosa.
The aim of this study was to improve the induction method of colitis and establish a reliable and reproducible perianal fistulizing CD animal model to evaluate new treatment strategies.
Twenty male New Zealand rabbits underwent rectal enema with different doses of 2,4,6-trinitrobenzene sulfonic acid (TNBS) to induce proctitis. Group A was treated with an improved equal interval small dose increasing method. The dosage of group B was constant. Seven days later, the rabbits underwent surgical creation of a transsphincteric fistula. Then, three rabbits were randomly selected each group every 7 d to remove the seton from the fistula. The rabbits were examined by endoscopy every 7 d, and biopsy forceps were used to obtain tissue samples from the obvious colon lesions for histological analysis. The disease activity index (DAI), colonoscopy and histological scores were recorded. Perianal endoscopic ultrasonography (EUS) was used to evaluate the healing of fistulas.
Except for the DAI score, the colonoscopy and histological scores in group A were significantly higher than those in the other groups (P < 0.05). In the ideal model rabbit group, on the 7th day after the removal of the seton, all animals had persistent lumens on EUS imaging, showing continuous full-thickness high signals. Acute and chronic inflammation, epithelialization, fibrosis, and peripheral proctitis of consecutive rectum are the histological features of fistula.
A preclinical model of perianal fistulizing CD in rabbits was established by using an improved method of CD colitis induction. The model can simulate the human environment, and intestinal and fistula lesions can be evaluated by EUS, endoscopic and histological examinations to assess new therapeutic strategies.
The establishment of a model of fistula associated with colitis allows the evaluation of different therapeutic approaches. However, fistula formation in animal models does not fully reflect the condition in humans. We hope that the simple, reliable and repeatable fistula animal model established by this improved colitis induction method can be used to evaluate new treatment strategies. The criteria for the difference between the two experimental conditions (constant-dose or increased-dose TNBS) introduced in the study still need to be verified by larger animal experiments, and the molecular mechanism involved should be investigated. The optimal animal model should include genetically mediated development of CD with anal fistula. However, an ideal model for preclinical research is difficult to establish due to the long experimental period required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gangl A, Austria; Saraiva MM, Portugal S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 712] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 3. | Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut. 1980;21:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 324] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Hedin CRH, Sonkoly E, Eberhardson M, Ståhle M. Inflammatory bowel disease and psoriasis: modernizing the multidisciplinary approach. J Intern Med. 2021;290:257-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Bislenghi G, Wolthuis A, Van Assche G, Vermeire S, Ferrante M, D'Hoore A. Cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn's disease. Expert Opin Biol Ther. 2019;19:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Herrlinger KR, Stange EF. Twenty-five years of biologicals in IBD: What´s all the hype about? J Intern Med. 2021;290:806-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 8. | Kamiński JP, Zaghiyan K, Fleshner P. Increasing experience of ligation of the intersphincteric fistula tract for patients with Crohn's disease: what have we learned? Colorectal Dis. 2017;19:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn's disease. Ann Surg. 2014;260:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 11. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 413] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 12. | Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: The safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Lansdorp CA, Gecse KB, Buskens CJ, Löwenberg M, Stoker J, Bemelman WA, D'Haens GRAM, van Hulst RA. Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn's disease. Aliment Pharmacol Ther. 2021;53:587-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Shibata Y, Taruishi M, Ashida T. Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid--colonoscopic and histopathologic studies. Gastroenterol Jpn. 1993;28:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Mohammad Jafari R, Shayesteh S, Ala M, Yousefi-Manesh H, Rashidian A, Hashemian SM, Sorouri M, Dehpour AR. Dapsone Ameliorates Colitis through TLR4/NF-kB Pathway in TNBS Induced Colitis Model in Rat. Arch Med Res. 2021;52:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 313] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 232] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Flacs M, Collard M, Doblas S, Zappa M, Cazals-Hatem D, Maggiori L, Panis Y, Treton X, Ogier-Denis E. Preclinical Model of Perianal Fistulizing Crohn's Disease. Inflamm Bowel Dis. 2020;26:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Kotze PG, Magro DO, Saab B, Saab MP, Pinheiro LV, Olandoski M, Ayrizono MLS, Martinez CAR, Coy CSR. Comparison of time until elective intestinal resection regarding previous anti-tumor necrosis factor exposure: a Brazilian study on patients with Crohn's disease. Intest Res. 2018;16:62-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Rackovsky O, Hirten R, Ungaro R, Colombel JF. Clinical updates on perianal fistulas in Crohn's disease. Expert Rev Gastroenterol Hepatol. 2018;12:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | de la Portilla F, López-Alonso M, Borrero JJ, Díaz-Pavón J, Gollonet JL, Palacios C, Vázquez-Monchul J, Sánchez-Gil JM. The rabbit as an animal model for proctology research: anatomical and histological description. J Invest Surg. 2011;24:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Aungst MJ, Fischer JR, Bonhage MR, Albright TS, Noel KA, Wright J. Rectovaginal fistula model in the New Zealand white rabbit. Int Urogynecol J. 2010;21:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Ferreira-Duarte M, Rodrigues-Pinto T, Sousa T, Faria MA, Rocha MS, Menezes-Pinto D, Esteves-Monteiro M, Magro F, Dias-Pereira P, Duarte-Araújo M, Morato M. Interaction between the Renin-Angiotensin System and Enteric Neurotransmission Contributes to Colonic Dysmotility in the TNBS-Induced Model of Colitis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Rivera-Nieves J, Bamias G, Vidrich A, Marini M, Pizarro TT, McDuffie MJ, Moskaluk CA, Cohn SM, Cominelli F. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Ferrer L, Kimbrel EA, Lam A, Falk EB, Zewe C, Juopperi T, Lanza R, Hoffman A. Treatment of perianal fistulas with human embryonic stem cell-derived mesenchymal stem cells: a canine model of human fistulizing Crohn's disease. Regen Med. 2016;11:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Bruckner RS, Nissim-Eliraz E, Marsiano N, Nir E, Shemesh H, Leutenegger M, Gottier C, Lang S, Spalinger MR, Leibl S, Rogler G, Yagel S, Scharl M, Shpigel NY. Transplantation of Human Intestine Into the Mouse: A Novel Platform for Study of Inflammatory Enterocutaneous Fistulas. J Crohns Colitis. 2019;13:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Benlice C, Yildiz M, Baghaki S, Erguner I, Olgun DC, Batur S, Erdamar S, Ambarcioglu P, Hamzaoglu I, Karahasanoglu T, Baca B. Fistula tract curettage and the use of biological dermal plugs improve high transsphincteric fistula healing in an animal model. Int J Colorectal Dis. 2016;31:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Dryden GW, Boland E, Yajnik V, Williams S. Comparison of Stromal Vascular Fraction with or Without a Novel Bioscaffold to Fibrin Glue in a Porcine Model of Mechanically Induced Anorectal Fistula. Inflamm Bowel Dis. 2017;23:1962-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Huang J, Shuang J, Xiong G, Wang X, Zhang Y, Tang X, Fan Z, Shen Y, Song H, Liu Z. Establishing a rabbit model of malignant esophagostenosis using the endoscopic implantation technique for studies on stent innovation. J Transl Med. 2014;12:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Lahat A, Assulin Y, Beer-Gabel M, Chowers Y. Endoscopic ultrasound for perianal Crohn's disease: disease and fistula characteristics, and impact on therapy. J Crohns Colitis. 2012;6:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, Levesque BG, D'Haens G; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |