Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1503
Peer-review started: March 26, 2021
First decision: June 14, 2021
Revised: July 12, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: April 21, 2022
Processing time: 384 Days and 9 Hours
Colorectal cancer (CRC) is one of the most prevalent cancers and the second leading cause of cancer-related deaths worldwide. The treatment strategy employed in CRC patients is becoming highly dependent on molecular characteristics present at diagnosis and during treatment. Liquid biopsy is an emerging field in the management of this cancer, and its relevance as a potential diagnostic, prognostic, monitoring, and therapeutic tool makes it a viable strategy in the clinical management of CRC patients. Liquid biopsy also has certain limitations, but these limitations seem to be at the reach of near-future technological development. In this letter, we focus on the clinical perspectives of liquid biopsy in CRC with particular regard to the various biomarkers recently identified that have been shown to be potentially useful in multiple aspects of early stage or metastatic CRC.
Core Tip: Liquid biopsy through analysis of biological components, such as circulating nuclear acids, circulating tumor cells, and more recently exosomes in body fluids, has shown good capacity to overcome several limitations faced by conventional tissue biopsies, in particular invasiveness and unrepeatability. Liquid biopsy has shown significant results in clinical applications in different types of cancer, especially colorectal cancer (CRC). Indeed, liquid biopsy can be used to detect CRC at an early stage, make treatment decisions, monitor response to treatment, predict relapses and metastases, reveal tumor heterogeneity, and detect minimal residual disease.
- Citation: Roviello G, Lavacchi D, Antonuzzo L, Catalano M, Mini E. Liquid biopsy in colorectal cancer: No longer young, but not yet old. World J Gastroenterol 2022; 28(15): 1503-1507
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1503
The strategy employed for the treatment of colorectal cancer (CRC) patients is becoming highly dependent on molecular characteristics at diagnosis and during the course of treatment. New therapeutic options have been shown to be effective for metastatic disease, including immune checkpoint inhibitors (ICIs), HER2-directed antibody-drug conjugates, chemotherapy-free regimens for BRAFV600-mutated tumors, and new agents targeting the RAS signaling pathway[1,2].
In recent years, peripheral blood has been extensively studied as a new source of information and alternative to tumor biopsy samples, but its potential has not yet been elucidated. Among the most studied components, circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and, more recently, exosomes have been considered promising biomarkers for monitoring treatment response, longitudinal molecular profiling, prognostication, and detection of minimal residual disease[3].
These advances coincided with the progressive increase in the accessibility and cost-effectiveness of next-generation sequencing (NGS) and with the development of new therapeutic approaches, including tumor-agnostic treatments[4].
CTC detection and enumeration have been studied as prognostic markers in several cancers. Sastre et al[5] evaluated the prognostic effect of the presence of CTCs detected using the CellSearch System in CRC patients (n = 97). The authors showed an association between CTC detection and stage. With a mean number of 3.4/7.5 mL CTCs, a higher rate of CTC positivity was observed in patients with stage IV disease (60.7%) than in those with stage II-III disease (20.7%-24.1%). Interestingly, no CTCs were detected in the healthy population[5]. Although CTC determination could represent a reliable surrogate for tumor burden, the possibility of longitudinal profiling of the disease offers far greater advantages for clinical practice than mere enumeration.
One of the most promising applications of liquid biopsy is the detection of early-stage CRC. For this purpose, CTC detection and quantification were studied with conflicting results regarding the discrimination of cancerous, precancerous, and other benign lesions[6]. Then, epigenetic changes were investigated to achieve a greater specificity. Methylated SEPT9 promoter DNA has been shown to be a potential biomarker with some limitations[7]. The interpretation of the results was, indeed, highly dependent on the choice of a favorable balance between sensitivity and specificity[8]. The detection of methylated SEPT9 promoter DNA through a real-time polymerase chain reaction assay was validated in a prospective study and recently received FDA approval for CRC screening[9]. However, several limitations deserve to be considered, including the rates of false-positive and negative results, reproducibility, need for confirmatory tests, and schedule of tests over time.
In the last few years, ctDNA has been extensively studied to identify minimal residual disease. A prospective multicenter study conducted by Henriksen et al[10] evaluated the preoperative and postoperative ctDNA status in stage I-III CRC. Overall, postoperative ctDNA was associated with relapse-free survival (P < 0.001). In addition, the detection of ctDNA made it possible to anticipate radiological relapse by approximately eight months[10]. Consistent with this finding, Kasi et al[11] evaluated the ctDNA status in a cohort of 250 patients using Signatera liquid biopsy. The ctDNA detection rate was significantly associated with stage and response to treatment[11].
Another application of liquid biopsy for the treatment of metastatic CRC involves the monitoring of RAS mutational status to select patients for anti-EGFR rechallenge. In the CRICKET trial, no responses were observed with cetuximab-based chemotherapy in RAS and RAF wild-type mCRC patients who had RAS-mutated ctDNA at the time of progression. Given that RAS mutations in ctDNA could be detected in approximately half of the patients at the beginning of third-line treatment[12], longitudinal monitoring of the disease is essential to offer potentially effective treatments to patients with few residual therapeutic opportunities. In addition, molecular selection in chemorefractory patients allows us to avoid unnecessary side effects for ineffective treatments. However, at the time of diagnosis, the molecular alterations in tissue biopsy cannot be replaced with those in liquid biopsy until the key issue of the concordance of RAS mutational status between plasma and tumor tissue is completely resolved. The results may differ depending on the methods used. To date, several prospective trials have demonstrated a high concordance between the two techniques that is approximately equal to 85%-95%[13,14].
Exosome quantification was initially studied as a prognostic factor associated with clinical and pathological parameters and, consequently, survival outcomes[15]. Then, several studies analyzed specific exosomal miRNAs. Among these, Matsumura et al[16] showed that CRC patients had higher levels of exosomal miR-17-92a than the control group. In addition, the authors identified the expression of exosomal miR-17-92a in peripheral blood as a prognostic factor for CRC patients[16]. Additionally, exosomal RNAs are used in the early diagnosis of cancers. For example, a panel consisting of two mRNAs (KRTAP5-4 and MAGEA3) and one lncRNA (BCAR4) serves as a promising candidate for CRC diagnosis[17-19].
Other studies are exploring the possibility of detecting other predictors of response, including microsatellite instability status and tumor mutational burden. The noninvasive detection of biomarkers predictive of response to ICIs is eagerly awaited to personalize treatments. However, their identification is profoundly limited by the amount of ctDNA and validation of specific assays. To date, there are no data to support their routine use[20,21].
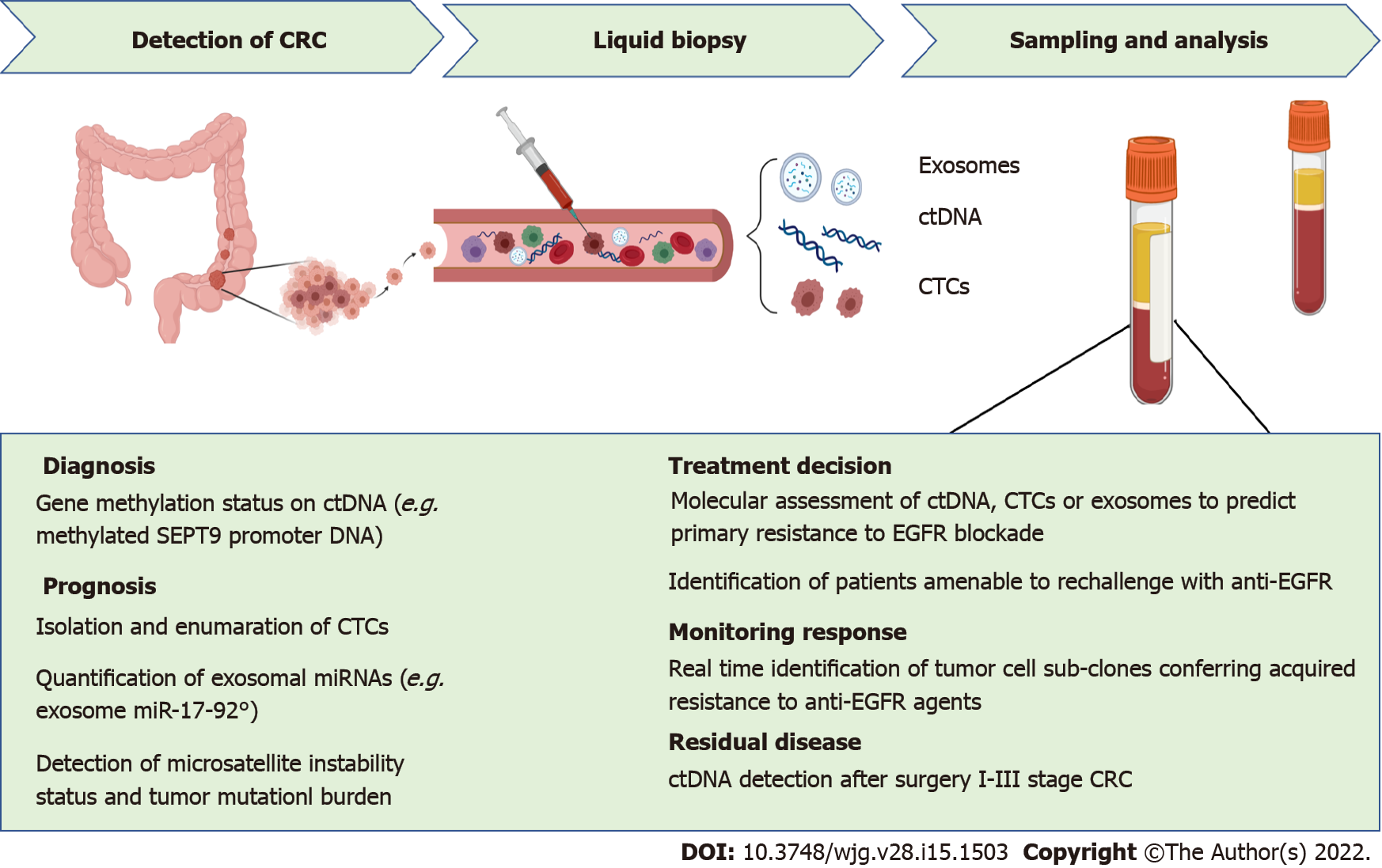
In conclusion, liquid biopsy is no longer considered a mere surrogate of tumor biopsy with minimal invasiveness (Figure 1). New perspectives could radically change clinical practice[22]. Further advances will include refining serum sequencing techniques to gain a deeper understanding of tumor temporal heterogeneity and promote accessibility to tumor-agnostic treatments. In addition, the real-time monitoring of drug resistance beyond RAS may offer the opportunity to guide and monitor new therapies, such as anti-RAS agents, BRAFV600-directed or HER2-directed treatments, and ICIs. In addition, monitoring plasma with amplicon-based NGS in CRC patients may offer high sensitivity in detecting low-frequency mutations and promote the identification of clones with potentially targetable alterations. All these aspects will be crucial to ensure the paradigm of a continuum of care for metastatic CRC patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Norčič G, Slovenia; Yuan Y, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 986] [Article Influence: 197.2] [Reference Citation Analysis (0)] |
| 2. | Roviello G, D'Angelo A, Petrioli R, Roviello F, Cianchi F, Nobili S, Mini E, Lavacchi D. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. Transl Oncol. 2020;13:100795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 699] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 4. | Lavacchi D, Roviello G, D'Angelo A. Tumor-Agnostic Treatment for Cancer: When How is Better than Where. Clin Drug Investig. 2020;40:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martín M, Arroyo M, Sanz-Casla MT, Díaz-Rubio E. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Parkinson DR, Dracopoli N, Petty BG, Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar P, Lee JSh, Liu MC, McCormack R, Mikulski S, Nagahara L, Pantel K, Pearson-White S, Punnoose EA, Roadcap LT, Schade AE, Scher HI, Sigman CC, Kelloff GJ. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Sun G, Meng J, Duan H, Zhang D, Tang Y. Diagnostic Assessment of septin9 DNA Methylation for Colorectal Cancer Using Blood Detection: A Meta-Analysis. Pathol Oncol Res. 2019;25:1525-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C, Habermann JK, Fleshner PR, Oubre BM, Day R, Sledziewski AZ, Lofton-Day C. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Potter NT, Hurban P, White MN, Whitlock KD, Lofton-Day CE, Tetzner R, Koenig T, Quigley NB, Weiss G. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem. 2014;60:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 10. | Henriksen TV, Tarazona N, Frydendahl A, Reinert T, Gimeno-Valiente F, Carbonell-Asins JA, Sharma S, Renner D, Hafez D, Roda D, Huerta M, Roselló S, Madsen AH, Løve US, Andersen PV, Thorlacius-Ussing O, Iversen LH, Gotschalck KA, Sethi H, Aleshin A, Cervantes A, Andersen CL. Circulating Tumor DNA in Stage III Colorectal Cancer, beyond Minimal Residual Disease Detection, toward Assessment of Adjuvant Therapy Efficacy and Clinical Behavior of Recurrences. Clin Cancer Res. 2022;28:507-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 11. | Kasi PM, Dayyani F, Morris VK, Kopetz S, Aleshin A. Tumor-informed assessment of molecular residual disease and its incorporation into practice for patients with early and advanced-stage colorectal cancer (CRC-MRD Consortia). J Clin Oncol. 2020;38(15_suppl):4108-4108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, Busico A, Pietrantonio F, Danesi R, Aprile G, Tamburini E, Barone C, Masi G, Pantano F, Pucci F, Corsi DC, Pella N, Bergamo F, Rofi E, Barbara C, Falcone A, Santini D. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019;5:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 13. | Bando H, Kagawa Y, Kato T, Akagi K, Denda T, Nishina T, Komatsu Y, Oki E, Kudo T, Kumamoto H, Yamanaka T, Yoshino T. A multicentre, prospective study of plasma circulating tumour DNA test for detecting RAS mutation in patients with metastatic colorectal cancer. Br J Cancer. 2019;120:982-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | García-Foncillas J, Alba E, Aranda E, Díaz-Rubio E, López-López R, Tabernero J, Vivancos A. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol. 2017;28:2943-2949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, Peña C, Herrera M, Diaz R, Mohammed N, Bonilla F. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano Y, Shinden Y, Eguchi H, Yamamoto H, Doki Y, Mori M, Ochiya T, Mimori K. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 17. | Dong L, Lin W, Qi P, Xu MD, Wu X, Ni S, Huang D, Weng WW, Tan C, Sheng W, Zhou X, Du X. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Galamb O, Barták BK, Kalmár A, Nagy ZB, Szigeti KA, Tulassay Z, Igaz P, Molnár B. Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors. World J Gastroenterol. 2019;25:5026-5048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, Shao Y, Zheng S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 20. | Cai Z, Wang Z, Liu C, Shi D, Li D, Zheng M, Han-Zhang H, Lizaso A, Xiang J, Lv J, Wu W, Zhang Z, Yuan F, He S, Sun J. Detection of Microsatellite Instability from Circulating Tumor DNA by Targeted Deep Sequencing. J Mol Diagn. 2020;22:860-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, Lieber DS, Lipson D, Silterra J, Amler L, Riehl T, Cummings CA, Hegde PS, Sandler A, Ballinger M, Fabrizio D, Mok T, Shames DS. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 869] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 22. | Salvianti F, Gelmini S, Mancini I, Pazzagli M, Pillozzi S, Giommoni E, Brugia M, Di Costanzo F, Galardi F, De Luca F, Castiglione F, Messerini L, Pinzani P, Antonuzzo L. Circulating tumour cells and cell-free DNA as a prognostic factor in metastatic colorectal cancer: the OMITERC prospective study. Br J Cancer. 2021;125:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |