Published online Mar 28, 2022. doi: 10.3748/wjg.v28.i12.1239
Peer-review started: September 21, 2021
First decision: November 7, 2021
Revised: November 22, 2021
Accepted: February 16, 2022
Article in press: February 16, 2022
Published online: March 28, 2022
Processing time: 184 Days and 21 Hours
Inflammatory bowel disease (IBD) is a chronic disease with recurrent intestinal inflammation. Although the exact etiology of IBD remains unknown, the accepted hypothesis of the pathogenesis to date is that abnormal immune responses to the gut microbiota are caused by environmental factors. The role of the gut microbiota, particularly the bidirectional interaction between the brain and gut microbiota, has gradually attracted more attention.
To investigate the potential effect of spinal anesthesia on dextran sodium sulfate (DSS)-induced colitis mice and to detect whether alterations in the gut microbiota would be crucial for IBD.
A DSS-induced colitis mice model was established. Spinal anesthesia was administered on colitis mice in combination with the methods of cohousing and fecal microbiota transplantation (FMT) to explore the role of spinal anesthesia in IBD and identify the potential mechanisms involved.
We demonstrated that spinal anesthesia had protective effects against DSS-induced colitis by alleviating clinical symptoms, including reduced body weight loss, decreased disease activity index score, improved intestinal permeability and colonic morphology, decreased inflammatory response, and enhanced intestinal barrier functions. Moreover, spinal anesthesia significantly increased the abundance of Bacteroidetes, which was suppressed in the gut microbiota of colitis mice. Interestingly, cohousing with spinal anesthetic mice and FMT from spinal anesthetic mice can also alleviate DSS-induced colitis by upregulating the abundance of Bacteroidetes. We further showed that spinal anesthesia can reduce the increase in noradrenaline levels induced by DSS, which might affect the gut microbiota.
These data suggest that microbiota dysbiosis may contribute to IBD and provide evidence supporting the protective effects of spinal anesthesia on IBD by modulating the gut microbiota, which highlights a novel approach for the treatment of IBD.
Core Tip: Inflammatory bowel disease (IBD) is a chronic inflammation with rising trends, but the pathogenesis is still not well understood. The effects of the gut microbiota, particularly the bi-directional interaction between brain and gut microbiota, have gradually attracted increasing attention. In the present study, we found that spinal anesthesia, a regional sympathetic block, alleviated the intestinal inflammation, maintained immunological function, and improved intestinal barrier function by modulating the gut microbiota. And reducing the increase of noradrenaline level in dextran sodium sulfate-treated mice by spinal anesthesia could be one of the mechanisms. The study highlights a novel approach for the treatment of IBD.
- Citation: Hong Y, Zhao J, Chen YR, Huang ZH, Hou LD, Shen B, Xin Y. Spinal anesthesia alleviates dextran sodium sulfate-induced colitis by modulating the gut microbiota. World J Gastroenterol 2022; 28(12): 1239-1256
- URL: https://www.wjgnet.com/1007-9327/full/v28/i12/1239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i12.1239
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract that includes Crohn’s disease (CD) and ulcerative colitis (UC). Over the past decades, the incidence of IBD has rapidly increased globally with consistently rising trends, particularly in Asian countries[1,2]. However, the pathogenesis of IBD is still not well understood. Multiple factors, such as environmental factors, the host’s genetics, immune responses, and the intestinal microbiome, might participate in the progression of the disease[3]. Currently, the available clinical treatments for IBD include corticosteroids, salicylates, and biologics. However, as a life-long disease, IBD still lacks a radical cure due to its multiple mechanisms.
With the development of genomics, people have gained a better understanding of a “forgotten organ”, the gut microbiota. The imbalance of the gut microbiota damages intestinal epithelial barrier function and the immune response, which are related to the occurrence and progression of IBD[4,5]. Intestinal dysbiosis, which means a compositional imbalance of commensal bacteria, is the central characteristic of the gut microbiota in IBD[6]. Probiotics can effectively improve intestinal symptoms and suppress inflammation in IBD[7,8]. More importantly, recent studies have shown that intestinal dysbiosis can be regulated through a central system called the “brain-gut axis”[9]. The brain-gut axis is a bidirectional communication network that links the central nervous system and the gastrointestinal tract. The central nervous system communicates with intestinal targets, such as the muscle layer, gut mucosa, permeability, mucus secretion, immunity, and enteric microbiota, when is activated in response to environmental factors, such as pain and stress[10].
Neuraxial anesthesia includes spinal, epidural, and combined spinal-epidural anesthesia to maintain regional sympathetic blocks. The postganglionic sympathetic neurons, which are located in the celiac, superior mesenteric, and inferior mesenteric ganglia, interact with the intestines at the serosal surface and innervate the vascular beds as well as the central nervous system. Neuraxial anesthesia has been shown to exert a positive effect on intestinal microvascular perfusion[11,12]. In an animal model of sepsis, thoracic epidural anesthesia was demonstrated to ameliorate perfusion deficits in the muscularis and mucosal layer of the gut[13]. However, whether neuraxial anesthesia affects intestinal inflammation in IBD is still unclear.
In this study, a dextran sodium sulfate (DSS)-induced colitis mouse model was established to explore the role of spinal anesthesia in IBD and identify the potential mechanisms involved. Moreover, cohousing and fecal microbiota transplantation (FMT) were used to help mice from separate lines share microbes across coached individuals[14,15]. This may open up an opportunity to regulate the distribution of the gut microbiota and prevent IBD.
Male C57BL/6 WT mice were purchased from the Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, China). All animal procedures were conducted in accordance with guidelines for laboratory animal care after approval by the Laboratory Animals Ethics Committee of Zhejiang University. These mice were housed in the standard animal care facility for 1 wk with free access to food and water at the Laboratory Animal Center of Zhejiang University with conventional housing circumstances of a 12 h/12 h light/dark cycle and 22 °C. All animal experiments were completed at the Laboratory Animal Center of Zhejiang University. Efforts were made to minimize any discomfort or pain, and the minimum number of animals was used.
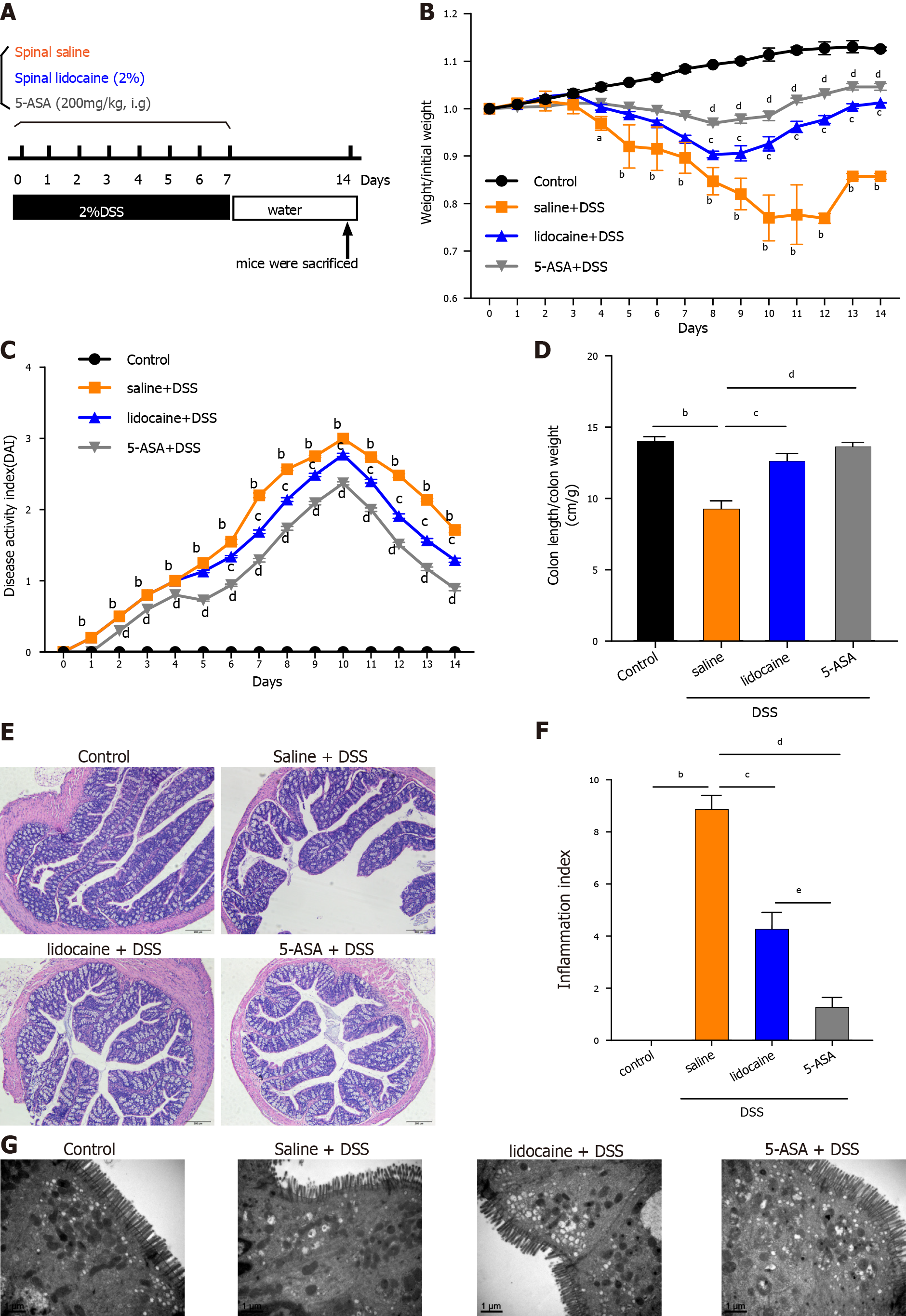
Acute colitis was induced by administration of 2% DSS (MW: 36000-50000 Da, Sigma–Aldrich) in drinking water for 1 wk in male C57BL/6 mice (6-8 wk old). Mice were randomly divided into four groups with 10 mice per group: Untreated normal controls, mice receiving DSS + spinal saline (saline, subarachnoid administration), mice receiving DSS + spinal lidocaine (2% lidocaine, subarachnoid administration), or those receiving DSS + 5-aminosalicylic acid (5-ASA) [200 mg/kg body weight (BW) in saline, i.g.]. After cessation of DSS exposure and lidocaine or 5-ASA treatment, the mice were given water ad libitum for an additional 1 wk (n = 10). The weight, food intake, and disease activity index (DAI) were recorded every day, and at the end of the experiments, the mice were sacrificed. At autopsy, the colon was rinsed with saline and excised. After the length of the colon was measured, it was cut and fixed in 10% formalin before paraffin embedding as previously described[16]. In addition to protein extracts, the colon was snap-frozen immediately in liquid nitrogen and stored at -70 °C before the preparation of soluble extracts[16].
For paraffin-embedded tissue, colon sections cut at 3 μm were stained with hematoxylin and eosin (HE). The inflammatory index was measured by the histological score, which is the sum of scores of four individual inflammatory parameters: Ulceration (0 or 1), inflammation severity (0, 1, 2, or 3), inflammation area involved (0, 1, 2, 3, or 4), and hyperplasia and dysplasia (0, 1, 2, or 3) as detailed previously[16].
The tissues from the colon were fixed with 2.5% glutaraldehyde overnight at 4 °C and then postfixed with 1% osmium tetraoxide for 2 h. After three rinses with phosphate buffer solution (PBS), the tissues were then rinsed with distilled water, followed by a graded ethanol dehydration series ending with propylene oxide. The tissues were embedded in resin after infiltration in a mixture of one-half propylene oxide and one-half resin. Sections (120 nm) were cut and stained with 4% uranyl acetate for 20 min and with 0.5% lead citrate for 5 min. Microvilli in the colon were observed by transmission electron microscopy (Philips Tecnai 10, Holland) at the Center of Cryo-Electron Microscopy at Zhejiang University.
Immunohistochemistry (IHC) of colon sections from mice was performed using formalin-fixed paraffin-embedded tissue as described previously[17]. Sections were stained using primary antibody against CD4 (1:1000; HuaBio, ER1706-80) and appropriate horseradish peroxidase-conjugated secondary antibody (1:1000; MXB, KIT-5006). Images were captured under a light microscope (Olympus BX41, Shanghai, China). Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, United States) was used to analyze the staining intensity. The semiquantitative results of IHC were based on the average value from three mice per group. Three separate slides from each mouse were analyzed. Five microscopic fields at 100 × magnification were randomly selected, and the integral optical density of the protein of interest was calculated.
Colon tissues from each group were homogenized in RIPA buffer (Beyotime, P0013B) with 1 × protease inhibitor cocktail (Beyotime, P1010). The supernatant was collected by centrifugation at 13000 × g for 10 min, and the protein concentration was detected with a bicinchoninic acid protein assay kit (Beyotime, P0012S). An aliquot of 50 μg protein from each sample was separated using SDS-PAGE, transferred to a nitrocellulose membrane, and then blocked with 5% nonfat milk in PBS (pH 7.4). The membranes were incubated with a primary antibody against claudin-1 (1:5000, Proteintech, 130501-1-AP), occludin (1:500, Huabio, ET1701-76), or β-actin (1:5000, ABcam, ab8227) at 4 °C overnight. Blots were incubated in a secondary antibody against rabbit or mouse IgG (1:5000, CST, 7071, and 7072) for 2 h at room temperature and then subjected to chemiluminescent detection using ChemiDoc (BioRad). Digital images were quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, United States).
The slices of colon tissues were immersed in xylene twice for dewaxing. After the gradient in water, the slices were incubated in Alcian blue reagent for 20 min and distilled water for 3 min. The sample was immersed in 0.5%-1% periodic acid solution and oxidized for 5 min, and it was washed in distilled water at a rapid rate. After applying Schiff reagent staining for 30-60 min (at 37 °C boxes), the slices were washed 2-3 times with sulfate water and distilled water twice. After redying the cell nucleus with hematoxylin solution, the samples were dehydrated with 95% alcohol and 100% alcohol for 1 min and xylene. After sealing with neutral gum, the samples were observed under a microscope.
Total RNA from different groups was extracted using a TRIzol RNA Kit (TransGen Biotech) based on the manufacturer’s protocol. Subsequently, cDNA synthesis and antisense RNA amplification were performed using HiScript II Q Select RT SuperMix (Vazyme). Polymerase chain reaction (PCR) was conducted on a StepOne Real-Time PCR System (ABI StepOnePlus, Applied Biosystems; Thermo Fisher Scientific, Inc.) using Hieff UNICON qPCR SYBR Green Master Mix (Shanghai Yeasen Biotechnology Co., Ltd.). The primers were synthesized by BioSun Biotechnology. The following primers were used: Interleukin (IL)-1β forward, 5’-AGTTGACGGACCCCAAAAG-3’ and reverse, 5’-TTGAAGCTGGATGCTCTCAT-3’; IL-6 forward, 5’-AGTCCTTCCTACCCCAATTTCC-3’ and reverse, 5’- GGTCTTGGTCCTTAGCCACT-3’; inducible nitric oxide synthase (iNOS) forward, 5’- CTCACCTACTTCCTGGACATTAC-3’ and reverse, 5’- CAATCTCTGCCTATCCGTCTC-3’; β-actin forward, 5’- CCACCATGTACCCAGGCATT-3’ and reverse, 5’-AGGGTGTAAAACGCAGCTCA-3’. Each assay was performed in triplicate. Fold changes were calculated after normalizing the change in expression of β-actin using the 2-ΔΔcycle threshold method.
Serum IL-1β, IL-6, tumor necrosis factor-alpha (TNF-α), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) concentrations in plasma were measured using an RSGELISA kit (Affymetrix, United States) following the manufacturer’s instructions.
Alterations in CD4+ T cell and regulatory T cell (Treg) populations in the intestine were analyzed by staining cells with specific antibodies using flow cytometry performed on a BD FACS S ORP ARIA II (BD Biosciences, Mountain View, CA, United States) according to a method previously described with slight modifications.
Briefly, after DSS and lidocaine treatment, about 1 × 106 cells were collected, and 10 μL of PerCP-Cy5.5-conjugated monoclonal mouse anti-human CD4 antibody and 10 μL of PE-conjugated monoclonal mouse anti-human CD25 antibody (BD Biosciences) were added after washing once with PBS (pH 7.4) (Gibco; Thermo Fisher Scientific, Waltham, MA, United States). To exclude the amount of nonspecific binding, 10 μL of PerCP-Cy5.5-conjugated and 10 μL of PE-conjugated mouse IgG1κ isotype control (BD Biosciences) were used and evaluated as blank controls. After incubation in the dark at 37 °C for 20 min, the cells were washed with PBS and resuspended in diluted Foxp3 buffer A (BD Biosciences), followed by incubation in the dark at room temperature for 10 min. Then, the cells were washed once with PBS, resuspended in 0.15 mL of Foxp3 buffer C composed of 49 parts of Foxp3 buffer A and one part of Foxp3 buffer B (BD Biosciences), and incubated in the dark at room temperature for 30 min. After incubation, the cells were washed once with PBS, and 10 μL of Alexa Fluor 488-conjugated mouse anti-mouse Foxp3 antibody or 10 μL of Alexa Fluor 488-conjugated mouse IgG1 isotype control was added, followed by incubation for 30 min in the dark at 37 °C. The cells were suspended in 0.4 mL staining buffer [0.4% (v/v) formaldehyde neutral buffer solution in PBS (pH 7.4)]. After washing once with PBS, the cells were analyzed by flow cytometry, and the data were further analyzed using FlowJo software (BD Biosciences). CD4+ T cells in the lymphocyte fraction were gated, and the percentages of CD4+ T and Tregs in the CD4+ T cells were calculated.
Each group of mice were fasted for 8 h before being sacrificed. After 3 h of administration with FITC-dextran (MW: 4K, R-FD-001, XINQIAO Biotechnology, China, 400 mg/kg in PBS), 300 μL of heart blood was harvested, and plasma was separated. Different concentrations of standard products were prepared with mixed mouse plasma without FITC-dextran gastric filling, PBS, and FITC-dextran solutions. The intracellular fluorescence was measured with a microplate reader at an excitation wavelength of 492 nm and an emission wavelength of 525 nm (DTX880, Beckman Coulter, United States). The FITC-dextran content was calculated according to the standard curve. The fluorescence intensity of FITC-dextran in the blood of mice was positively correlated with the permeability of their intestines.
At the end of the experiment, overnight fasted mice were sacrificed. Fecal samples were collected from the mice, and gut microbiota DNA was extracted. The V3-V4 region of 16S rDNA was amplified with universal primers. The PCR products were then quantified by electrophoresis on a 1.5% agarose gel followed by cDNA purification with the QIAquick Gel Extraction kit (Qiagen). Sequencing and data analysis were subsequently performed on an Illumina HiSeq platform by Novogene (Beijing, China). Briefly, after the raw sequences were identified by their unique barcodes, Ribosomal Database Project Classifier 2.8 was used to perform the assignment of all sequences at 50% confidence. Operational Taxonomic Units (OTUs) present in 50% or more of the colon content samples were identified as core OTUs. PLS-DA of core OTUs was performed using Simca-P version 12 (Umetrics), and to visualize and cluster the bacterial community into different groups, a heatmap was generated using Multi-Experiment Viewer software. Community diversity was measured by the Shannon-Weiner biodiversity index (Shannon index), alpha diversity index (Ace index), and Chao1 richness estimator (Chao 1 index).
For cohousing experiments, siblings (male C57BL/6 mice, 4-12 wk, n = 12) were divided into four groups consisting of a group of WT mice (n = 3), a noncohousing DSS group (n = 3), a cohousing DSS group (n = 3), and a cohousing lidocaine + DSS group (n = 3). The DSS group and lidocaine + DSS group were treated as described above. The cohousing DSS group and the cohousing lidocaine group were transferred to fresh cages to start the cohousing experiments in the same experimental room. The mice remained together for 2 wk with free access to water and chow diet.
Bacterial strains that were previously isolated from lidocaine mice were mixed and resuspended in PBS. The mice were exposed to DSS for 7 d and then randomly separated into either an FMT group or a DSS group. The FMT and DSS groups were gavaged with fecal microbiota (0.3 mL) or PBS (0.3 mL). After observation for 30 min, all mice were housed separately.
A method for the determination of noradrenaline was established by high-performance liquid chromatography-tandem mass spectrometry. The noradrenaline standard was detected and analyzed by gradient. After blood samples were collected, the serum was separated and frozen at -80 °C. After thawing, methanol was added, and then acetonitrile was added after the internal standard solution was vortex centrifuged again. After stratification, the upper organic phase was placed in another tube, and 10 μL of the organic phase was taken for determination. Chromatographic conditions are as follows: Agilent Zorbax sb-c8 column (2.1 mm × 100 mm, 1.8 μm); mobile phase, water (containing 0.1% formic acid, 2 mmol/L ammonium acetate): Acetonitrile (containing 0.1% formic acid) = 55:45; flow rate, 0.4 mL/min; column temperature, 30 °C; injection volume, 2 μL; autosampler temperature, 10 °C. For mass spectrometry, the ionization mode was electrospray ionization, multireaction ion detection MRM was used in the positive ion mode, the capillary voltage was 3.5 kV, the temperature of the dryer was 350 °C, the flow rate of the dryer was 5 L/min, the atomization gas was 60 psi, the sheath gas temperature was 350 °C, and the sheath gas flow rate was 11 L/min.
Experiments were performed in triplicate and repeated at least three times. Data are presented as the mean ± SD or SEM from independent experiments. Statistical analyses involved one-way ANOVA and two-way ANOVA performed with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, United States) or SPSS 22 (IBM Corp., Armonk, NY, United States). P < 0.05 was considered statistically significant.
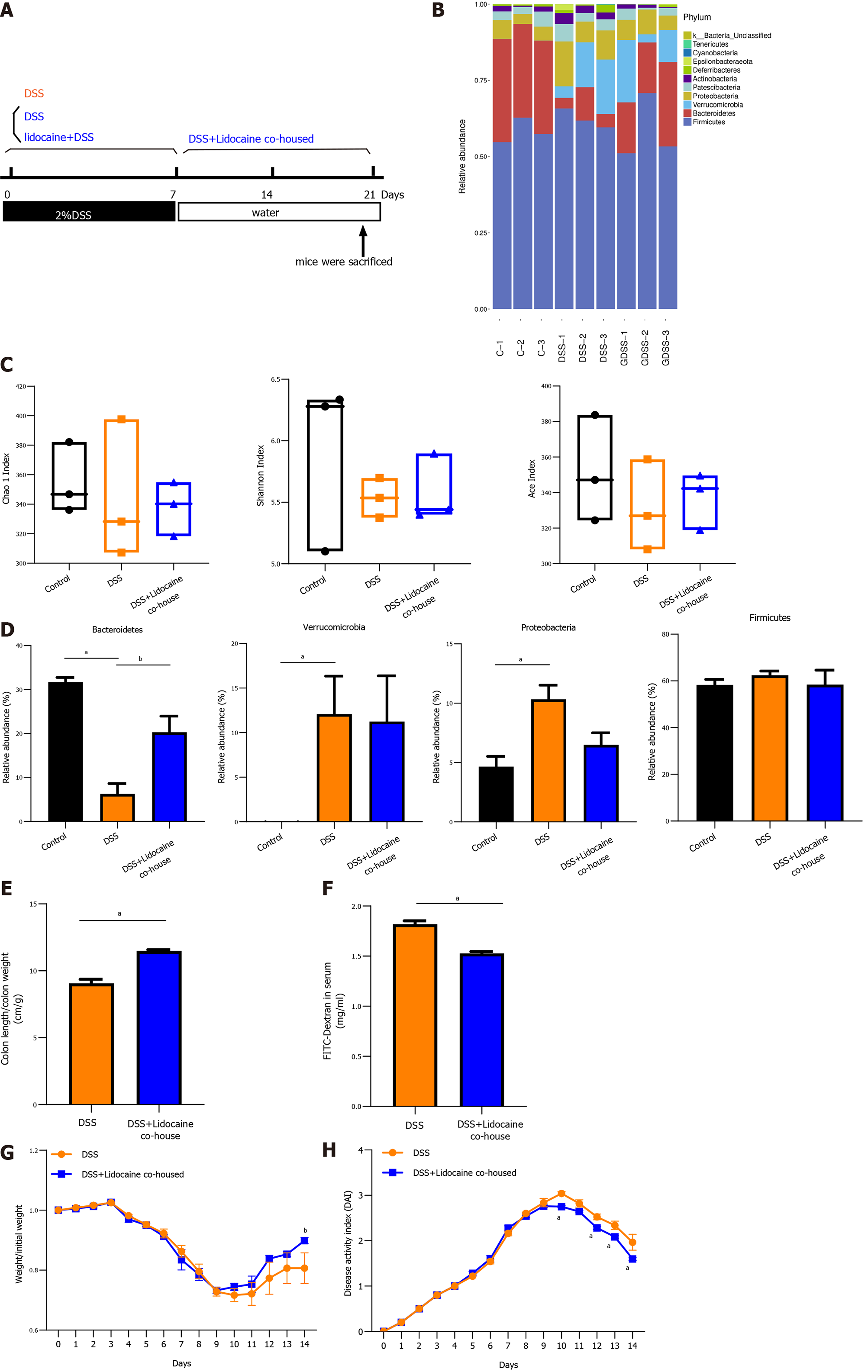
Acute colitis was induced in C57BL/6 mice by the administration of 2% DSS in drinking water for 1 wk. To test whether spinal anesthesia could relieve colitis, colitis mice were given lidocaine (subarachnoid administration), saline (vehicle, subarachnoid administration), and 5-ASA (positive control, i.g.) daily until the termination of the experiment after 7 d. The experimental protocol is shown in Figure 1A. The DSS-treated colitis mice exhibited an increasing DAI level with dramatic body weight loss, colon shortening, rectal bleeding, and stool consistency reduction. In contrast, colitis mice treated with lidocaine or 5-ASA showed obvious improvements in body weight (Figure 1B), DAI score (Figure 1C), and colon length (Figure 1D). H&E staining images of colonic tissues of the DSS-treated mice showed obvious intestinal injury, including the loss of crypts and discontinuous brush borders and large lumens. However, these damage signs were noticeably ameliorated by lidocaine treatment, which appeared to protect the colonic mucosal structure by limiting multifocal inflammation (Figures 1E and 1F). Furthermore, SEM showed that the microvilli of colitis mice became shorter and uneven, and the arrangement was disordered. Interestingly, the lidocaine group had more microvilli than the saline group (Figure 1G). Taken together, these results suggested that spinal anesthesia with lidocaine relieved the symptoms of colitis and colonic epithelial injury in a DSS-induced IBD mouse model.
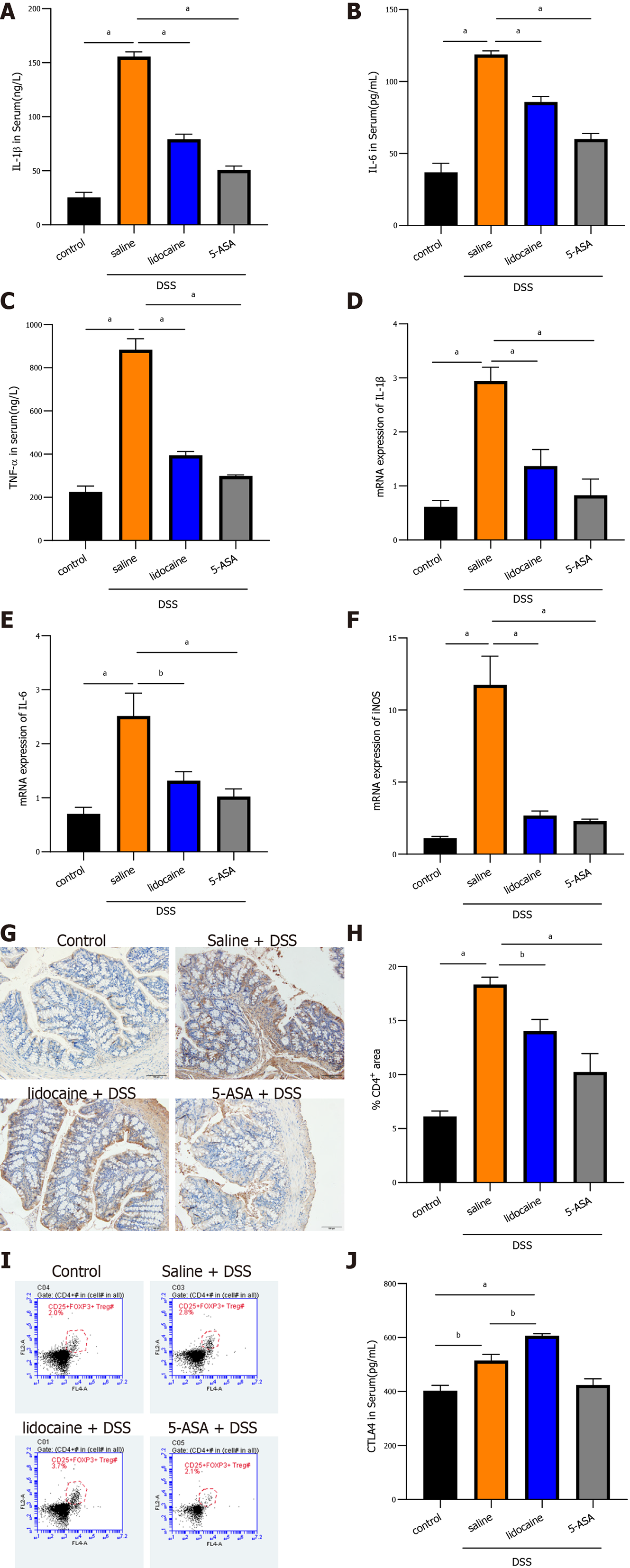
To better understand the alleviation of colitis by spinal anesthesia, the inflammatory response was determined by detecting proinflammatory cytokines and infiltration of immune cells in the colon. As expected, in colitis mice, enzyme-linked immunosorbent assay (ELISA) analysis showed that the levels of IL-1β, IL-6, and TNF-α in plasma were dramatically higher than those in the control mice (Figures 2A, 2B and 2C). The levels of these cytokines were lower in the 5-ASA group than in the saline + DSS group. Interestingly, spinal anesthesia with lidocaine also significantly suppressed the expression of IL-1β, IL-6, and TNF-α in plasma of DSS-induced mice (Figures 2A, 2B and 2C). Meanwhile, the transcriptional levels of cytokines were detected by real-time quantitative PCR (qRT-PCR), and the results revealed that spinal anesthesia also decreased the mRNA levels of IL-1β, IL-6, and iNOS in DSS-induced mice (Figures 2D, 2E and 2F). CD4+/CD25+/FoxP3+ Tregs and CTLA4 cells play an important role in maintaining the healthy intestinal immune state. We then focused on those immune cells. IHC analysis showed that DSS treatment dramatically increased the area of infiltration of CD4+ T cells in the colon, whereas spinal anesthesia or treatment with 5-ASA significantly inhibited the production of CD4+ T cells (Figures 2G and 2H). Importantly, flow cytometry assays showed that spinal anesthesia led to an increase in Tregs (Figure 2I), thereby inducing a change in the proportion of T lymphocytes. Similar results were also obtained when we determined the level of CTLA4 in serum. The lidocaine + DSS group had a higher serum CTLA4 level than the saline + DSS group (Figure 2J). Together, these data suggested that spinal anesthesia may downregulate proinflammatory cytokines and upregulate the levels of CTL4 and CD4+/CD25+/Foxp3+ Tregs to alleviate the inflammatory activity in DSS-induced mice.
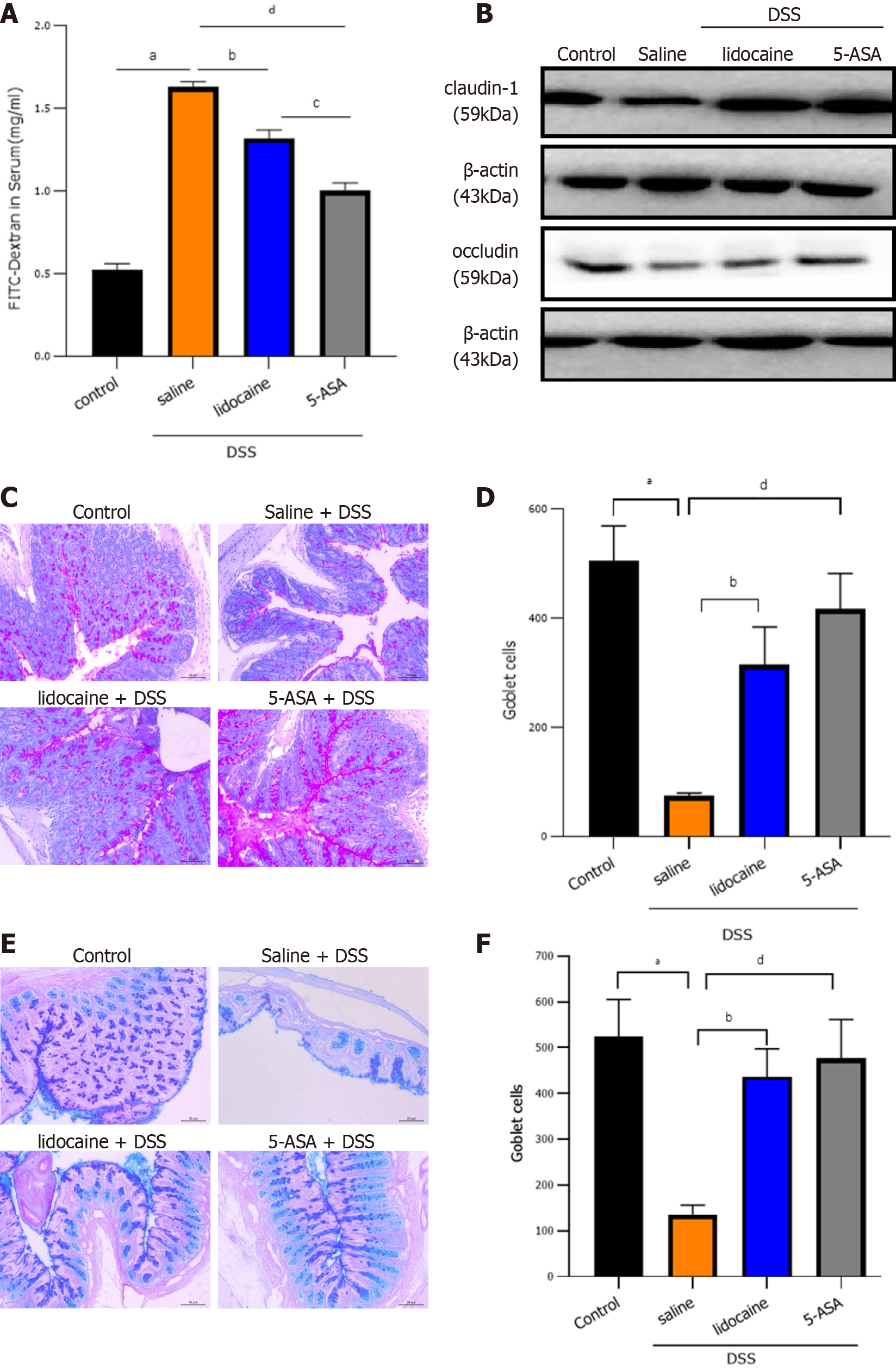
To examine intestinal barrier function, we first detected intestinal permeability. After spinal anesthesia, the concentration of FITC-dextran in the serum of colitis mice decreased significantly, which indicated the strengthening of intestinal physical barrier function (Figure 3A). Intercellular tight junctions (TJs), whose integrity is a key determinant of paracellular permeability, are important for maintaining epithelial barrier function. Western blot analysis showed that claudin-1 and occludin, two representative TJ proteins, were significantly lower in the DSS groups than in the control group (Figure 3B). However, spinal anesthesia with lidocaine reversed the levels of claudin-1 and occludin in the positive control 5-ASA group. Goblet cells secrete gel-forming mucins to lubricate and protect the intestine. Alcian blue/periodic acid-Schiff staining showed that there was a clear trend of decreasing neutral (Figures 3C and 3D) and acidic (Figures 3E and 3F) mucins after DSS treatment. Spinal anesthesia with lidocaine resulted in a significant increase in mucins. Together, these results showed that spinal anesthesia with lidocaine can protect intestinal barrier function in the DSS-induced IBD mouse model.
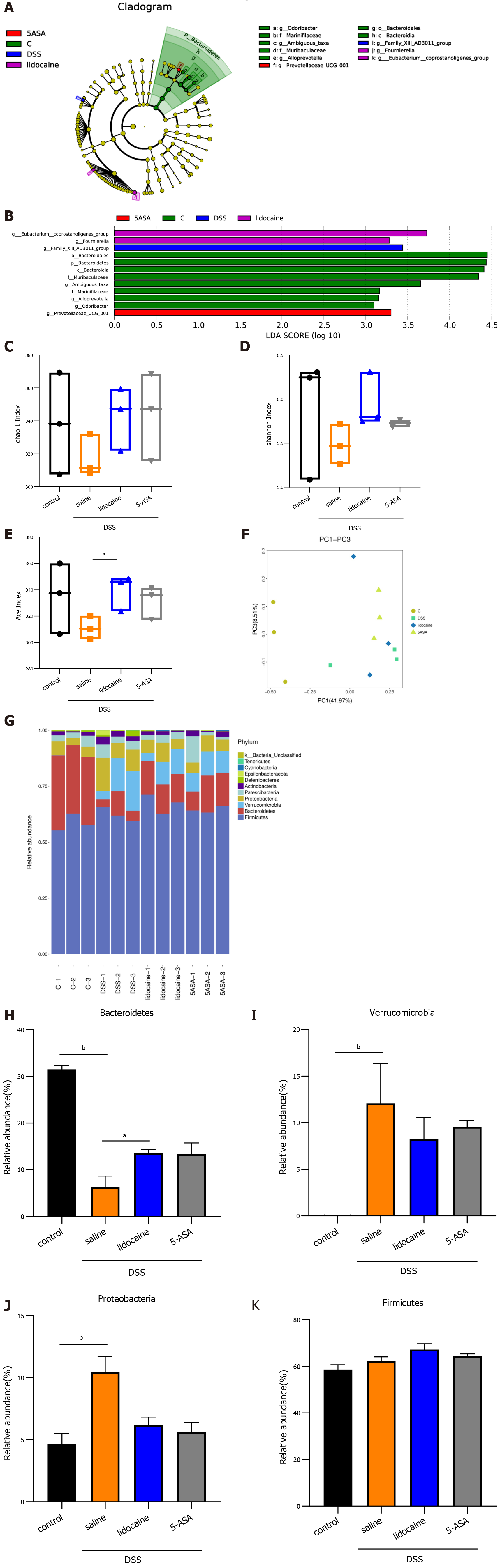
A wealth of information has been generated regarding the altered microbiota composition, or dysbiosis, at different taxonomic levels that manifests in patients with IBD and animal models of IBD[18,19]. The composition of the fecal microbiota was determined by analysis of -30000 paired reads per mouse of the -250 bp v3-v4 region of the 16S rRNA gene by Illumina MiSeq sequencing (Figures 4A and 4B). The PCoA analysis revealed that specific bacterial alterations occurred during colitis. Ace index varied between the saline + DSS group and the lidocaine + DSS group (Figure 4C). Interestingly, spinal anesthesia significantly rescued the abundance of Bacteroidetes, which was suppressed by DSS (Figures 4D and 4E). Other types of bacteria, including Verrucomicrobia and Proteobacteria, were also affected by spinal anesthesia, although the difference did not reach statistical significance. Together, these data demonstrated that spinal anesthesia with lidocaine can alter the intestinal flora in the IBD mouse model.
To certify whether the gut microbiota contributes to relieving colitis by spinal anesthesia, DSS mice and spinal anesthesia with lidocaine mice were cohoused (Figure 5A). Using microbiota sequencing analysis, we compared the gut microbiota in the cohousing and noncohousing groups. Figure 5B reveals that cohousing altered the relative abundance of intestinal flora. There was no significant difference in various measures, including the Chao1 index, Shannon index, and Ace index (Figure 5C). However, cohousing significantly increased the abundance of Bacteroidetes (Figure 5D), which was consistent with the change affected by spinal anesthesia. Meanwhile, cohousing rescued the shortened colon and intestinal permeability in DSS-induced mice (Figures 5E and 5F), and the body weight and DAI of the cohousing spinal anesthesia and DSS mice were better than those of the DSS mice (Figures 5G and 5H).
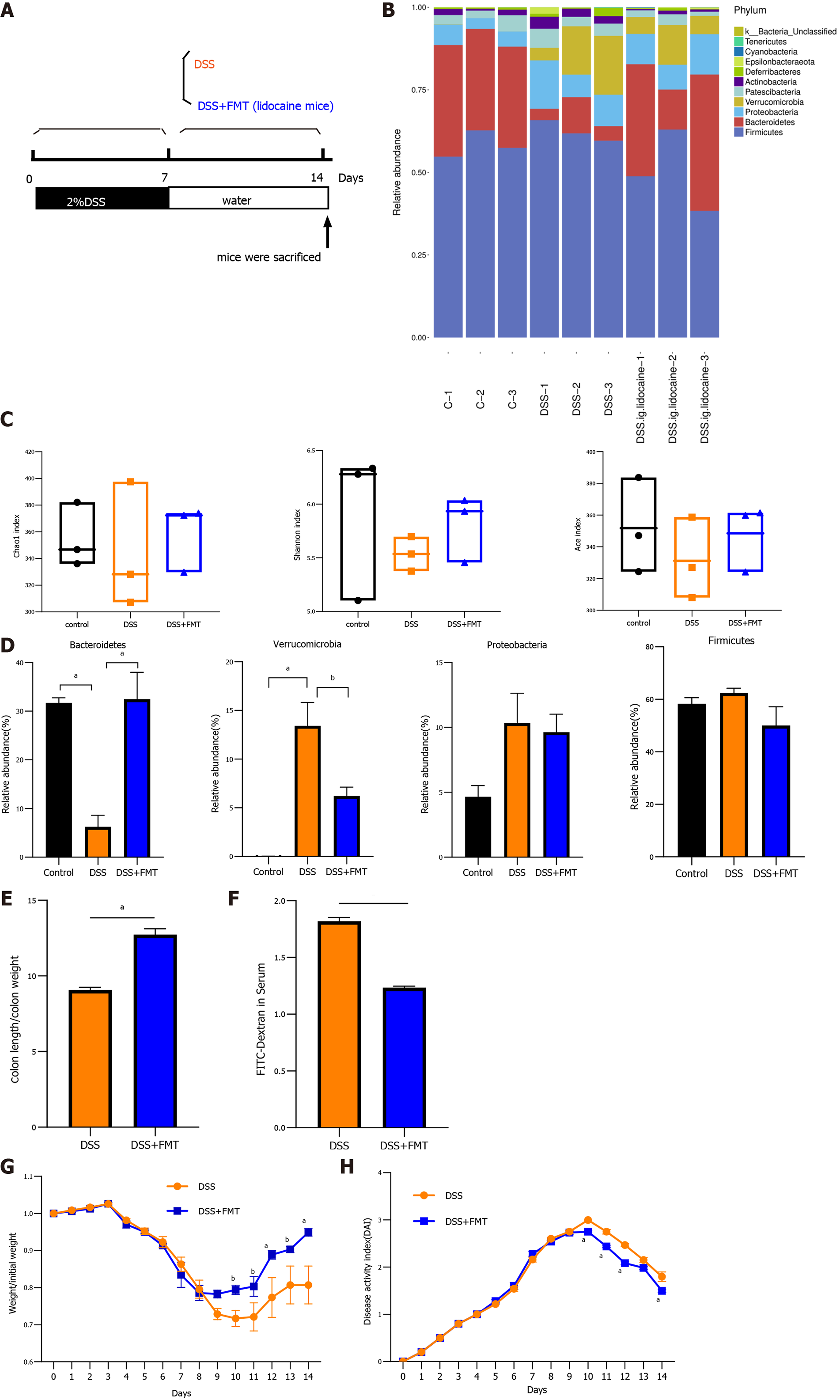
FMT is generally accepted as a promising experimental treatment for patients suffering from gut dysbiosis. We were interested in determining whether transplantation of the intestinal flora from the spinal anesthesia group has an effect on DSS-induced colitis (Figure 6A). FMT also altered the relative abundance of intestinal flora (Figure 6B). Various measures, including the Chao1 index, Shannon index, and Ace index, varied between the cohousing group and the noncohousing group (Figure 6C). FMT significantly increased the abundance of Bacteroidetes and decreased the abundance of Verrucomicrobia, recovering the change in DSS-induced mice (Figure 6D). Similarly, FMT reduced colon swelling and relieved intestinal permeability (Figures 6E and 6F). The body weight and DAI of the DSS mice treated by FMT were better than those of the DSS mice (Figures 6G and 6H), which was consistent with changes by cohousing treatment. Taken together, these results suggested that the gut microbiota was implicated in relieving colitis by spinal anesthesia due to the key role that it plays in intestinal inflammation.
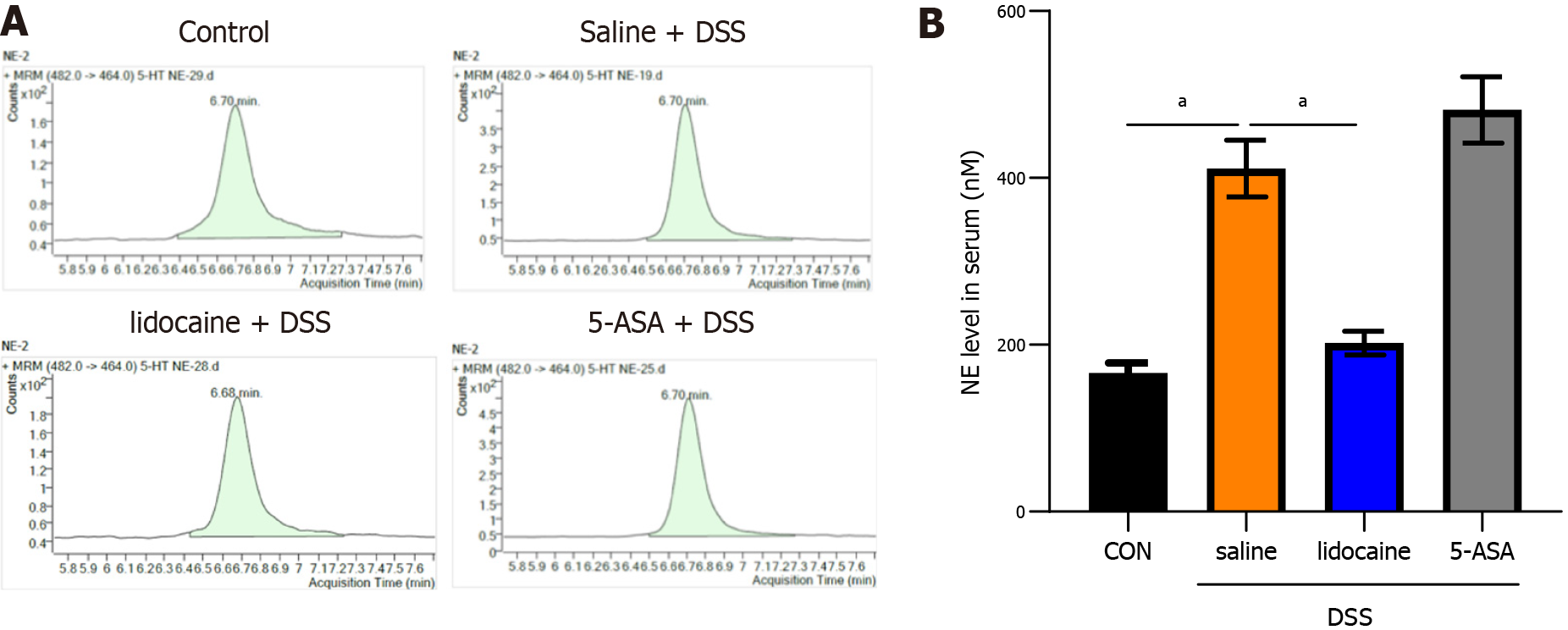
Several studies[12,13] have indicated that the sympathetic system primarily exerts an inhibitory influence on the gut by the release of noradrenaline. To verify that spinal anesthesia modulates the gut microbiota via noradrenaline, we used liquid chromatography to detect the level of noradrenaline in our model (Figure 7A). The results indicated that the level of noradrenaline was increased in the saline + DSS group compared with the control group (Figures 7A and 7B). Spinal anesthesia with lidocaine decreased the noradrenaline level in DSS-induced mice. The altered level of noradrenaline could be one of the mechanisms underlying the effect of spinal anesthesia on the gut microbiota.
The pathogenesis of IBD is not fully understood, and changes in intestinal microbes, impairment of the epithelial barrier, and the chronic dysregulated immune response in the gastrointestinal tract are strongly implicated in the development of IBD[20,21]. In particular, increasing evidence from animal and human studies has shown that gut microbes are key factors contributing to IBD by acting on different parts of the gut-brain-microbiota axis[22]. Therefore, our study explored the possibility of improving the clinical outcomes of IBD by acting on this axis. Interestingly, we found that IBD mice treated by lidocaine spinal injection, which is a regional sympathetic block, showed a significant amelioration of symptoms and intestinal permeability. Moreover, this treatment increased the abundance of specific genera, such as Bacteroidetes, and regulated gut immunity. Here, we, for the first time, directly highlighted the therapeutic effects of spinal anesthesia on DSS-induced colitis mice.
Neuraxial anesthesia (i.e., spinal, epidural, and combined spinal-epidural techniques) is widely used to induce analgesia for lower extremities and lower abdominal surgery. In addition, the specific beneficial effect of neuraxial anesthesia attributed to sympathetic nerve blockade, which increases gastrointestinal track microvascular perfusion and function, has been observed in animal and clinical studies[23,24]. Recently, several reports have shown that spinal or epidural anesthesia may improve the ischemic and insufficient oxygenation stage of organs[11,12]. In the present study, we found that spinal anesthesia with lidocaine not only relieved intestinal permeability but also increased the diversity of the gut microbiota and changed the composition of microbiota species in colitis mice, which suggests that spinal anesthesia affects part of the gut-brain-microbiota axis to alleviate colitis in DSS-induced mice.
Microbial predominance includes four different phyla, Bacteroidetes, Prodominace, Firmicutes, and Verrucomycrobia[25], in healthy people, and an alteration of the intestinal microbiota occurs in IBD patients. Prior studies have noted that polysaccharides, a component of Bacteroidetes, can prevent intestinal inflammatory disease, and Akkermansia exerts beneficial effects on colitis[26]. In the DSS model, we found that the abundance of Proteobacteria and Verrucomycrobia increased, but the abundance of Bacteroidetes decreased, which might lead to abnormal intestinal immune responses and intestinal imbalance. After spinal anesthesia, we identified that the abundance of Bacteroidetes with health-promoting functions significantly increased and the abundance of Proteobacteria and Verrucomycrobia decreased. Furthermore, our cohousing and FMT experiments revealed that the microbiota of spinal anesthesia mice transferred to DSS colitis mice can effectively relieve disease. These results confirmed that altered gut microbiota caused IBD and that Bactericides and Verrucomycrobia species are critical in keeping the gut microbiota healthy.
Extensive studies have found that behavioral disorders, such as anxiety, stress, or depression, change the composition of the intestinal flora and influence the recurrence of CD[27,28]. The sympathetic nerve innervated to the gut plays a critical role in affecting the composition of the gut microbiota and maintaining immune homeostasis via the hypothalamus-pituitary-adrenocortical axis mainly by secreting catecholamine[9,29,30]. In the stress model, the sympathetic system primarily exerts an inhibitory influence on the gut, decreasing intestinal motor function and secretion via the release of neurotransmitters, such as noradrenaline[31]. Noradrenaline, one of the main catecholamines, can influence the microbiota in the gut, leading to the altered release of cytokines and bacterial molecules. Noradrenaline can also increase the growth of several bacteria in nutrient-deficient environments, including Campylobacter jejuni, Escherichia coli, Helicobacter pylori, Pseudomonas aeruginosa, and Salmonella enterica spp[32]. Catecholamines were found to facilitate the removal of iron from human lactoferrin and transferrin in a dose- and time-dependent manner, which also correlated with bacterial growth[33]. In our study, the level of noradrenaline was increased after DSS treatment, which may influence the microbiota present in the colitis gut. Moreover, spinal anesthesia with lidocaine recovered the level of noradrenaline. This could be one of the mechanisms underlying the effect of spinal anesthesia on the gut microbiota.
It is evident that the sympathetic nervous system has the potential to serve as a therapeutic target for inflammatory disease. Increasing sympathetic tone has a deteriorating effect on IBD, including the development of intestinal inflammation and inhibition of immune defenses[34,35]. This is in line with the finding in the present study that thoracic sympathetic block through spinal anesthesia in colitis mice increased the expression of FoxP3+ Treg cells, which are a subset of CD4+ T lymphocytes and plays an important role in maintaining the immune response[36]. Moreover, the levels of caludin-1 and occludin, the main tight junction proteins of the intestinal epithelial barrier, were changed. All these results indicated that immune function and colon barrier function were impaired, which may be caused by increased noradrenaline levels and altered gut microbiota.
In recent years, diet and short-chain fatty acids have been recommended to replace or increase conventional IBD therapies[37]. Some peptides named food protein-derived bioactive peptides against intestinal inflammation have attracted increasing attention, but their mechanism and effect are under exploration[38]. Experts have said “as we enter a new era of patient-centered health care, treating the ‘brain’ is as important as the ‘gut’ for comprehensive, whole-person IBD management”[39]. However, it is difficult to verify the bidirectional relationship of the gut-brain axis, and here, we for the first time used spinal anesthesia to block thoracic nerve in IBD mice, and we luckily found that it was effective for IBD.
In conclusion, this study clearly revealed that spinal anesthesia inhibited the development of DSS-induced colitis in mice. We demonstrated that spinal anesthesia alleviated intestinal inflammation, maintained immunological function, and improved intestinal barrier function by modulating the gut microbiota. Reducing the increase in noradrenaline levels in DSS-treated mice by spinal anesthesia could be one of the mechanisms underlying the effect on the gut microbiota. The present study provided evidence supporting the protective effects of spinal anesthesia on IBD by modulating the gut microbiota, which highlights a novel approach for the treatment of IBD.
Neuraxial anesthesia has been shown to exert a positive effect on intestinal microvascular perfusion. In an animal model of sepsis, thoracic epidural anesthesia was demonstrated to ameliorate perfusion deficits in the muscularis and mucosal layers of the gut. However, whether spinal aneathesia as a neuraxial anesthesia affects intestinal inflammation in inflammatory bowel disease (IBD) is still unclear.
The exact etiology of IBD remains unknown, and the imbalance of the gut microbiota is related to the occurrence and progression of IBD. The bidirectional between the brain and gut microbiota has gradually attracted more attention. Finding interventions on the brain-gut axis will be a new vision.
A dextran sodium sulfate (DSS)-induced colitis mouse model was established to explore the role of spinal anesthesia in IBD and to identify the potential mechanisms involved.
A DSS-induced colitis mice model was established, and then we used spinal anesthesia on colitis mice to explore the role of spinal anesthesia in IBD and identify the potential mechanisms involved. Moreover, cohousing and fecal microbiota transplantation were used to help mice from separate lines share microbes across cocaged individuals.
This study clearly revealed that spinal anesthesia inhibited the development of DSS-induced colitis in mice. We demonstrated that spinal anesthesia alleviated intestinal inflammation, maintained immunological function, and improved intestinal barrier function by modulating the gut microbiota. Reducing the increase in noradrenaline levels in DSS-treated mice by spinal anesthesia could be one of the mechanisms underlying the effect on the gut microbiota.
The study implied a positive effect of spinal anesthesia in relieving intestinal inflammation, protecting intestinal barrier function, and regulating the intestinal microflora in an IBD mouse model. Decreasing the noradrenaline level would be a possible mechanism of spinal anesthesia.
The present study provided evidence supporting the protective effects of spinal anesthesia on IBD by modulating gut microbiota, which highlights a novel approach for the treatment of IBD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu Q, Yang M S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Guo X
| 1. | Yang Y, Owyang C, Wu GD. East Meets West: The Increasing Incidence of Inflammatory Bowel Disease in Asia as a Paradigm for Environmental Effects on the Pathogenesis of Immune-Mediated Disease. Gastroenterology. 2016;151:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Liu M, Sun T, Li N, Peng J, Fu D, Li W, Li L, Gao WQ. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat Commun. 2019;10:4614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Vemuri R, Gundamaraju R, Shastri MD, Shukla SD, Kalpurath K, Ball M, Tristram S, Shankar EM, Ahuja K, Eri R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. Biomed Res Int. 2018;2018:4178607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1296] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 6. | Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 460] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 7. | Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;CD005573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol (Oxf). 2016;217:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 10. | Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 797] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 11. | Adolphs J, Schmidt DK, Korsukewitz I, Kamin B, Habazettl H, Schäfer M, Welte M. Effects of thoracic epidural anaesthesia on intestinal microvascular perfusion in a rodent model of normotensive endotoxaemia. Intensive Care Med. 2004;30:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Schäper J, Ahmed R, Perschel FH, Schäfer M, Habazettl H, Welte M. Thoracic epidural anesthesia attenuates endotoxin-induced impairment of gastrointestinal organ perfusion. Anesthesiology. 2010;113:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Daudel F, Freise H, Westphal M, Stubbe HD, Lauer S, Bone HG, Van Aken H, Sielenkämper AW. Continuous thoracic epidural anesthesia improves gut mucosal microcirculation in rats with sepsis. Shock. 2007;28:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Caruso R, Ono M, Bunker ME, Núñez G, Inohara N. Dynamic and Asymmetric Changes of the Microbial Communities after Cohousing in Laboratory Mice. Cell Rep. 2019;27:3401-3412.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Robertson SJ, Lemire P, Maughan H, Goethel A, Turpin W, Bedrani L, Guttman DS, Croitoru K, Girardin SE, Philpott DJ. Comparison of Co-housing and Littermate Methods for Microbiota Standardization in Mouse Models. Cell Rep. 2019;27:1910-1919.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Zheng Z, Li C, Pan Y, Tang X, Wang XJ. Synthetic Imine Resveratrol Analog 2-Methoxyl-3,6-Dihydroxyl-IRA Ameliorates Colitis by Activating Protective Nrf2 Pathway and Inhibiting NLRP3 Expression. Oxid Med Cell Longev. 2019;2019:7180284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Zheng Z, Chen Y, Huang J, Deng H, Tang X, Wang XJ. Mkp-1 is required for chemopreventive activity of butylated hydroxyanisole and resveratrol against colitis-associated colon tumorigenesis. Food Chem Toxicol. 2019;127:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Verdugo-Meza A, Ye J, Dadlani H, Ghosh S, Gibson DL. Connecting the Dots Between Inflammatory Bowel Disease and Metabolic Syndrome: A Focus on Gut-Derived Metabolites. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Maronek M, Link R, Ambro L, Gardlik R. Phages and Their Role in Gastrointestinal Disease: Focus on Inflammatory Bowel Disease. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1068] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 21. | Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 743] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 22. | Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 23. | Guay J, Nishimori M, Kopp S. Epidural local anaesthetics vs opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev. 2016;7:CD001893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Steinbrook RA. Epidural anesthesia and gastrointestinal motility. Anesth Analg. 1998;86:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1071] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 26. | Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X, Shi D, Li L. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front Microbiol. 2019;10:2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 27. | Ananthakrishnan AN, Khalili H, Pan A, Higuchi LM, de Silva P, Richter JM, Fuchs CS, Chan AT. Association between depressive symptoms and incidence of Crohn's disease and ulcerative colitis: results from the Nurses' Health Study. Clin Gastroenterol Hepatol. 2013;11:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Million M, Taché Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol. 1999;276:G1027-G1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Willemze RA, Welting O, van Hamersveld HP, Meijer SL, Folgering JHA, Darwinkel H, Witherington J, Sridhar A, Vervoordeldonk MJ, Seppen J, de Jonge WJ. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol Motil. 2018;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288-G1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 31. | Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 32. | Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O'Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J Cell Physiol. 2017;232:2359-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 33. | Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol. 2000;182:6091-6098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Poulakos M, Machin JD, Pauly J, Grace Y. Vedolizumab: A New Opponent in the Battle Against Crohn's Disease and Ulcerative Colitis. J Pharm Pract. 2016;29:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 36. | Smids C, Horjus Talabur Horje CS, Drylewicz J, Roosenboom B, Groenen MJM, van Koolwijk E, van Lochem EG, Wahab PJ. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J Crohns Colitis. 2018;12:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 671] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 38. | Zhu W, Ren L, Zhang L, Qiao Q, Farooq MZ, Xu Q. The Potential of Food Protein-Derived Bioactive Peptides against Chronic Intestinal Inflammation. Mediators Inflamm. 2020;2020:6817156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Click BH, Greer JB, Regueiro MD, Hartman DJ, Davis PL, Siegel CA, Herfarth HH, Rosh JR, Shah SA, Koltun WA, Binion DG, Baidoo L, Szigethy E. IBD LIVE Series-Case 7: The Brain-Gut Connection and the Importance of Integrated Care in IBD. Inflamm Bowel Dis. 2017;23:681-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |