Published online Mar 28, 2022. doi: 10.3748/wjg.v28.i12.1187
Peer-review started: July 1, 2021
First decision: July 26, 2021
Revised: July 30, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 28, 2022
Processing time: 267 Days and 3.9 Hours
Despite a decline in incidence and mortality during the last decades, stomach cancer is one of the main health challenges worldwide. According to the GLOBOCAN 2020 estimates, stomach cancer caused approximately 800000 deaths (accounting for 7.7% of all cancer deaths), and ranks as the fourth leading cause of cancer deaths in both genders combined. About 1.1 million new cases of stomach cancer were diagnosed in 2020 (accounting for 5.6% of all cancer cases). About 75% of all new cases and all deaths from stomach cancer are reported in Asia. Stomach cancer is one of the most lethal malignant tumors, with a five-year survival rate of around 20%. There are some well-established risk factors for stomach cancer: Helicobacter pylori infection, dietary factors, tobacco, obesity, and radiation. To date, the most important way of preventing stomach cancer is reduced exposure to risk factors, as well as screening and early detection. Further research on risk factors can help identify various opportunities for more effective prevention. Screening programs for stomach cancer have been implemented in a few countries, either as a national or opportunistic screening of high-risk individuals only. Generally, due to its high aggressiveness and heterogeneity, stomach cancer still remains a severe global health problem.
Core Tip: Despite the decline in incidence and mortality during the last decades, stomach cancer is one of the main health challenges worldwide. According to the GLOBOCAN 2020 estimates, stomach cancer caused approximately 800000 deaths, and ranks as the fourth leading cause of deaths from cancer in both genders combined. Around 1.1 million new stomach cancer cases were diagnosed in 2020. There are some well-established risk factors for stomach cancer: Helicobacter pylori infection, dietary factors, tobacco, obesity, and radiation. To date, the most important way of preventing stomach cancer is reduced exposure to risk factors, as well as screening and early detection.
- Citation: Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol 2022; 28(12): 1187-1203
- URL: https://www.wjgnet.com/1007-9327/full/v28/i12/1187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i12.1187
Stomach cancer was the fifth most common malignant tumor in the world in 2020 with approximately 1.1 million new cases, and is the fourth leading cause of cancer death, with around 800000 deaths[1,2]. Over 85% of stomach cancer cases are registered in countries with high and very high Human Developing Index (590000 and 360000 cases, respectively)[1]. The highest number of cases of stomach cancer (almost 820000 new cases and 580000 deaths) was registered in Asia (mainly in China)[1,2-4]. The estimated five-year survival rate is lower than 20%[2,5-8].
Worldwide, stomach cancer incidence and mortality correlate with increasing age and are relatively rare in persons of both gender younger than 45 years[2,4,7]. The frequency of stomach cancer in men is approximately double that in women[1-3]. In men, stomach cancer was the most commonly diagnosed cancer in 2020 in seven countries (all countries were in Asia: Iran, Afghanistan, Turkmenistan, Uzbekistan, Tajikistan, Kyrgyzstan, and Bhutan) and the leading cause of death from cancer in ten countries (Iran, Afghanistan, Tajikistan, Kyrgyzstan, and Bhutan in Asia, Mali and Cape Verde in Africa, Colombia and Peru in South America, and Costa Rica in Central America)[1,2]. Although stomach cancer was not the most diagnosed cancer in women in any country, stomach cancer was the leading cause of death from cancer among females in three countries (Tajikistan, Bhutan, and Peru)[1,2]. The incidence and mortality rates from stomach cancer were generally low in Northern America and Northern Europe in 2020 and equivalent to rates registered across most of the African regions[1,2].
In the first half of the 20th century, gastric cancer was the leading cause of death from malignant tumors in the United States and Europe[9,10]. Over the past decades, the incidence and mortality due to stomach cancer have substantially declined in many countries[1,2].
Stomach cancer is a multifactorial disease[9-12], including both lifestyle and environmental risk factors Helicobacter pylori (H. pylori) infection, low socioeconomic status, dietary factors, such as high intake of salty and smoked food and low consumption of fruits and vegetables, fiber intake, in addition to tobacco smoking, alcohol use, low physical activity, obesity, radiation, gastroesophageal reflux disease, positive family history and inherited predisposition. However, the etiology of stomach cancer has not yet been sufficiently elucidated.
Topographically, stomach cancer is classified into two subsites: cardia stomach cancer (arising from the upper stomach) and noncardia stomach cancer (arising from the other parts of the stomach), which differ in epidemiologic patterns and etiology[13]. The majority of all stomach cancers (approximately 90%) are adenocarcinomas, while other types (including lymphoma, sarcoma, neuroendocrine tumors) are rare[12,14]. Two major histologic types of stomach cancer adenocarcinomas are diffuse and intestinal, which differ in epidemiological peculiarities, such as age at diagnosis, gender ratio, etc.[15,16].
Despite the strong declining trends in incidence and mortality, stomach cancer remains an important part of the global burden of cancer. Many of the risk factors remain insufficiently understood and need to be the focus of further research in order to achieve more specific, targeted prevention measures.
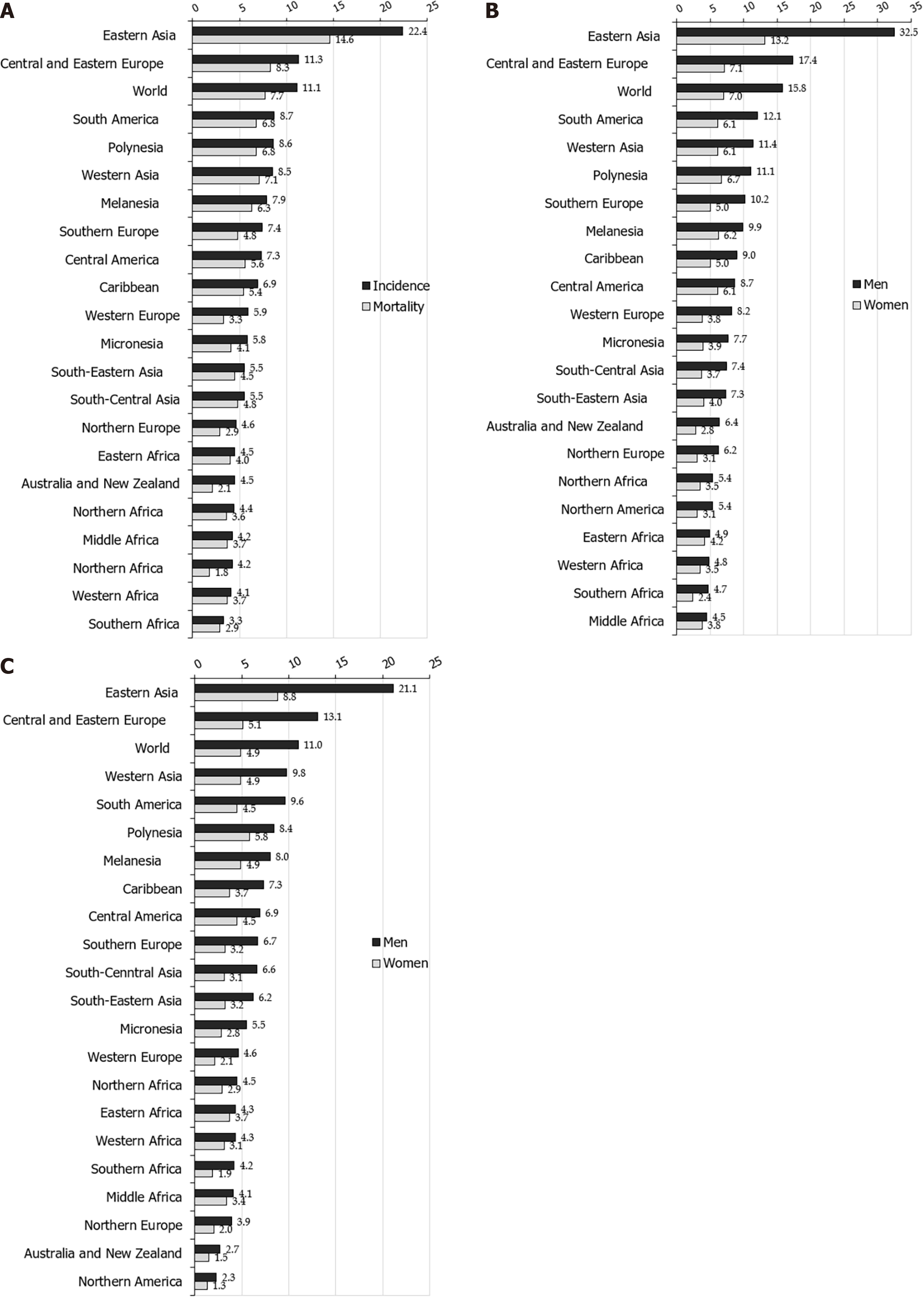
Worldwide, there is a considerable geographic variation in stomach cancer incidence. Stomach cancer incidence rates in 2020 were highest in Eastern Asia (22.4 per 100000 people), followed by Central and Eastern Europe (11.3 per 100000 people), and South America, Polynesia and Western Asia (equally about 8.6 per 100000 people) (Figure 1A)[2]. The lowest rate (3.3 per 100000 people) was registered in Southern Africa.
More than three quarters (75.3%; 819944) of all stomach cancer cases are residents of Asia[2]. Most (86.7%; 944591 cases) stomach cancer cases were residents of more developed regions. The least number of stomach cancer cases was recorded in Micronesia/Polynesia.
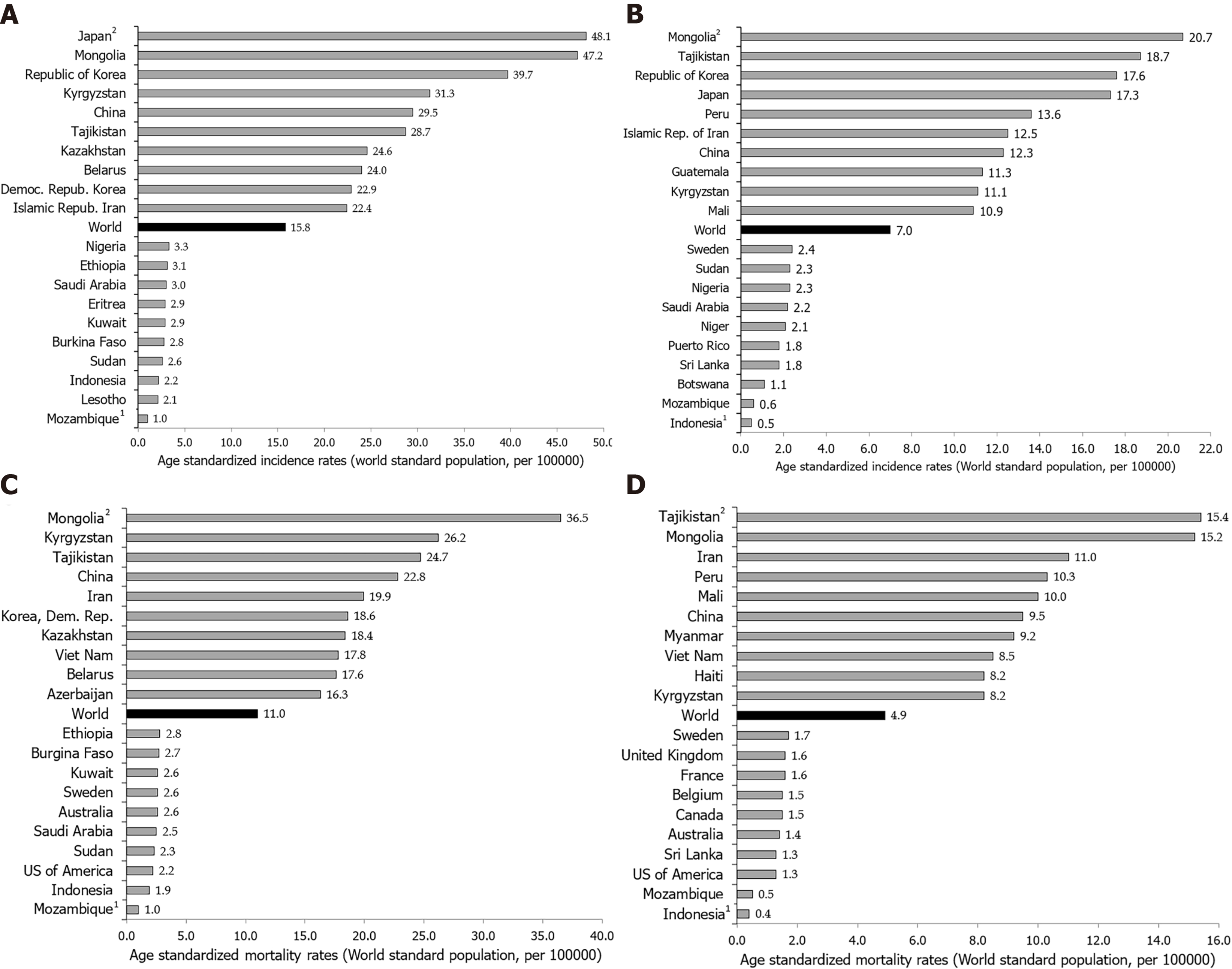
Notable variations in the incidence of stomach cancer in 2020, as well as for mortality, exist around the world (Figures 1-4)[2]. The highest incidence rates were recorded in countries of eastern Asia (Mongolia, Japan, Republic of Korea), while the highest death rates were observed in countries of western Asia (Tajikistan, Kyrgyzstan, Iran). The lowest incidence and mortality rates of stomach cancer were recorded in Northern America and Northern Europe, Australia/New Zealand and some African countries. Patterns in females were broadly similar to those observed in males, but large differences were observed between sexes and throughout different countries/regions (Figures 1-4)[2]. Globally, the incidence rate of stomach cancer in males was 15.8 per 100000 in 2020, and in females 7.0 per 100000 (Figure 1B)[2]. The gastric cancer incidence rates were about 2 to 3 times higher in males than in females (ranging from 32.5 per 100000 in Eastern Asia to 4.5 per 100000 in Middle Africa for men, and in women ranging from 13.2 in Eastern Asia to 2.4 in Southern Africa) (Figure 1B)[2]. By countries, the differences were fifty-fold: the incidence rates of gastric cancer in men ranged from 48.1 per 100000 in Japan to 1.0 per 100000 in Mozambique in 2020 (Figure 2A). Also, similar differences were observed by regions: the highest incidence rates were reported in Eastern Asia (Japan: 48.1, Mongolia: 47.2, Republic of Korea: 39.7), while the lowest rates were recorded in South Africa (Mozambique: 1.0, Lesotho: 2.1). The incidence rates of stomach cancer in women ranged from 20.7 per 100000 inhabitants in Mongolia (followed by Tajikistan: 18.7, Republic of Korea: 17.6 and Japan: 17.3) to about 0.5 in Indonesia and Mozambique in 2020 (Figure 2B).
However, the distribution of stomach cancer did not have a clear geographical pattern: namely, even though the highest risk populations in the world are in Asian countries (e.g. Japan, Mongolia, Republic of Korea), some other countries in Asia register relatively low rates (such as Sri Lanka, Indonesia, Thailand) (Figure 2A and B)[2]. On the other hand, in some low-risk populations, there are some high-risk groups for stomach cancer, such as Koreans and Japanese who live in the United States[17,18].
Also, rates varied across races. Stomach cancer incidence in men in the United States was highest in blacks, followed by Asians/Pacific Islanders, Hispanics, and American Indian/Alaska natives[7]. In women, the highest rates were registered in Hispanics, followed by blacks and Asian/Pacific Islanders, and American Indian/Alaska natives. In the United States, for both sexes, the lowest rates were recorded in whites.
Also, incidence and mortality of gastric cancer in all indigenous groups exceeded the frequency among their non-indigenous counterparts: the highest gastric cancer rates were registered in Indigenous Siberians, Mapuche in Chile and among Alaskan Inuit[19]. Additionally, increasing incidence trends were observed in some indigenous groups, especially in Inuit residing in the circumpolar region and in Maori in New Zealand.
Although differences in stomach cancer incidence in different parts of the world are still not fully clear, most of the variation in stomach cancer incidence worldwide is due to variations in exposure to environmental or lifestyle related risk factors[20-22]. Additionally, migrant studies[23] and secular trends of gastric cancer rates also indicate that environmental factors have an important role in the etiology of gastric cancer[24]. The most important established risk factor for gastric cancer is infection with H. pylori[25]. Internationally, variations in H. pylori infection prevalence show similarities with variations in stomach cancer prevalence; in developing countries, H. pylori infection prevalence in adults is 76% vs 58% in developed countries[26]. The prevalence was estimated to be 77.6% in South Africa, 55.8% in China, 52.2% in Mexico, 24.6% in Australia and 22.1% in Denmark[27]. In the United States of America, the prevalence in non-Hispanic blacks was 53%, in Mexican Americans was 62%, but was 26% among non-Hispanic whites[28]. In part, the geographical variation of H. pylori infection rates correlate with the frequency of stomach cancer across populations. On the other hand, certain highly infected populations (e.g. in Africa and South Asia), unlike the East Asian countries, do not have a high incidence of stomach cancer, which can be explained, at least in part, by the differences in prevalence of genotypes of H. pylori (in East Asian the vacA m1 genotype is predominant, whereas the m2 genotype predominated in Africa, South Asia, and Europe)[29].
Additionally, several other environmental factors are also considered as contributors to gastric cancer occurrence[21,22,30]. Differences between sexes and international variations could likely be due to tobacco smoking[31]. Based on the Global Burden of Disease study[22], the drop in burden of stomach cancer was associated with improved Socio-demographic Index, then to high-sodium diet in both genders combined, as well as to smoking in males, in particular in east Asian populations.
In addition, some research points to the role of aging[24] and hereditary and genetic factors[6] in stomach cancer burden. Incidence differences by sex have never been fully explained, but some theories have suggested a protective role of female sex-specific hormones[15,32]. A higher stomach cancer incidence in males than in females may be due to differences in the incidence of different subtypes of adenocarcinoma according to histology (intestinal or diffuse) and location (proximal or distal)[12,13,33]. Diffuse adenocarcinoma is more common in younger and female patients, whereas intestinal adenocarcinoma is more common in males and the elderly[15]. Intestinal adenocarcinoma dominates high-risk areas and is considered responsible for much of the international variation in incidence. The observed differences in stomach cancer incidence worldwide could be due to diagnostic capacity and changes in the quality of registries, where coverage, completeness and accuracy vary by country[34].
Nearly three quarters of stomach cancer deaths (74.8%; 575206 deaths) were registered in Asia[2]. Most (83.7%, 643609 deaths) of those who died due to stomach cancer were residents of more developed regions. The least number of deaths were recorded in Micronesia/Polynesia.
Stomach cancer mortality varies greatly across populations and regions. Mortality rates for stomach cancer in 2020 in both genders were highest in the Eastern Asia region (14.6 per 100000 people), followed by South America, Polynesia, Western Asia and Central and Eastern Europe (equally about 8.5 per 100000 people) (Figure 1A)[2]. The lowest mortality rates (about 2.0 per 100000 people) were registered in Northern Africa and Australia. The differences in mortality rates were thirty-fold between the population with the highest rate (Mongolia - 24.6), and the one with the lowest rate (Mozambique - 0.7).
Stomach cancer mortality by gender shows significant geographic variations[1,2,6-8]. Globally, the mortality rate of stomach cancer in males in 2020 was 11.0 per 100000, and in females 4.9 per 100000 (Figure 1C)[2]. The region with the highest mortality rates due to stomach cancer in 2020 in both genders was Eastern Asia (21.3 and 8.8 per 100000, respectively) (Figure 1C)[2]. The lowest rates of stomach cancer mortality in both sexes were in North America (2.3 and 1.3 per 100000, respectively). In men, the risk of dying from stomach cancer was highest in Mongolia (36.5), followed by Kyrgyzstan, Tajikistan and China (approximately 25.0 per 100000) (Figure 2C). By contrast, the risk of death from stomach cancer was lowest in men in Mozambique (1.0) and Indonesia (1.9). Women living in Tajikistan and Mongolia had the greatest risk (approximately 15.0 per 100000) of death from stomach cancer, while the risk for women in Indonesia and Mozambique was lowest (less than 1.0 per 100000) (Figure 2D).
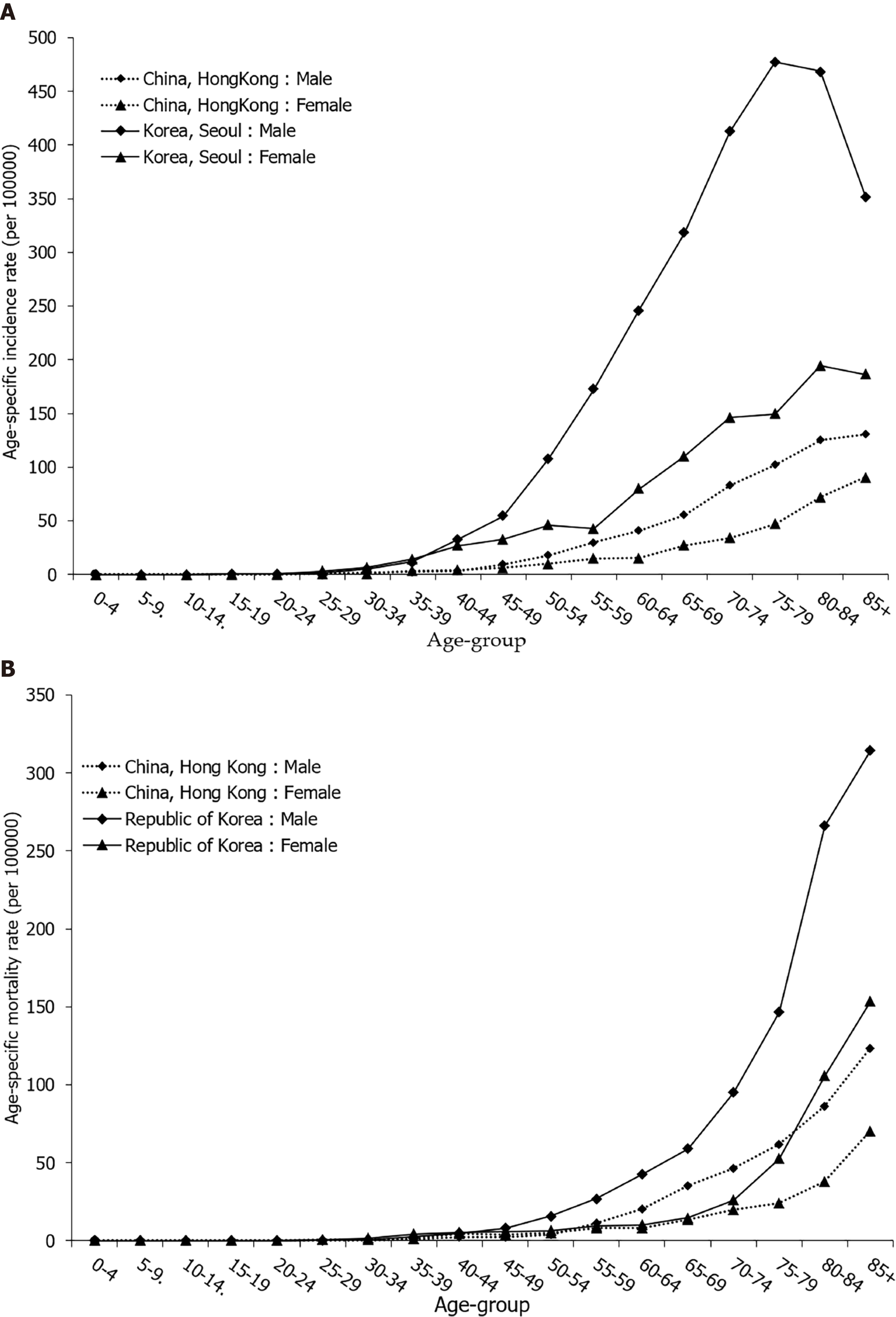
Gastric cancer mortality rates begin to rise in middle-aged persons, with the highest rates observed in the elderly (aged 75 years and older) age group for both males and females (Figure 4B).
Stomach cancer mortality showed apparent geographical variability. Generally, the large differences in mortality rates are between developing and developed countries. Considering developed countries, this mortality pattern could be explained by increased hygiene standards, dissemination of food refrigeration, better preservation of food, high intake of fresh fruits and vegetables and eradication of H. pylori[11,22,35]. In the second decade of the 21st century in Japan, mortality due to stomach cancer reached the levels of Western countries (Figure 4B), which could be attributed to the introduction of gastric cancer screening and to changes in lifestyle, such as the reduction in salt use and an increase in the consumption of fresh fruits and vegetables, improvement in food storage, smoking reduction and prevention of infection with H. pylori[22,36,37]. However, reasons for the significant international variations in stomach cancer mortality rates are not fully clear. Diffuse adenocarcinoma is more common in females, while intestinal adenocarcinoma is dominant in males, this subtype being responsible for most of the international variations[15].
There is a wide variation in the relative contribution of cardia and noncardia cancers to the overall number of stomach cancer cases, with a higher proportion of cardia cancers in countries with lower stomach cancer incidence and mortality rates (such as the United States, Canada and Denmark)[38]. In males in Europe, the proportion of cardia and noncardia stomach cancers ranged between 11.6% (Belarus) and 72.0% (Finland), with higher proportions observed in Northern Europe and lower proportions in Eastern Europe. Among other countries worldwide, the proportion of cardia stomach cancers ranged between 5.8% (Republic of Korea) and 64.8% (Iran). In females, a similar geographic pattern was observed, although rates were lower: in Europe, the proportion of cardia and noncardia stomach cancers ranged between 10.6% (Italy) and 44.5% (United Kingdom), while worldwide it ranged from 4.3% (Republic of Korea) to 31.5% (Australia).
Low incidence rates of stomach cancer, which notably became a rare diagnosis among the white United States population, are attributed to the “unplanned triumph” of prevention, which involves a decreased H. pylori prevalence and improved food storage and preservation[9,39].
Cancer mortality data are influenced by data on incidence, as well as the success of treatment. Although the World Health Organization estimates present detailed and high-quality information on the incidence and mortality of stomach cancer recorded by cancer registries (regional or national) around the world, these estimates should be interpreted with considerable caution, due to the limited quality and coverage of cancer data worldwide, especially in low- and middle-income countries, due to issues of local data quality, registry coverage, and analytical capacity[2,40,41]. The effect of the coronavirus disease 2019 pandemic on cancer burden is not yet clear, particularly taking into consideration the geographical variations and evolution of the pandemic across countries (because of the lockdown, possible delays in cancer diagnoses, etc.)[2].
The differences in availability of improvements in stomach cancer diagnosis and treatment may have had some role in the observed variations in mortality rates worldwide, but this contribution remains open to further quantification[42]. Screening programs and early detection of stomach cancer which have been implemented in Japan[43] and in Korea[44] can partly explain the differences in mortality rates. Also, in Japan, advancements were made in the surgical treatment of early disease, resulting in a better survival rate compared to other countries[45]. However, stomach cancer survival remains unacceptably low in most areas of the world[46,47].
The high prevalence of H. pylori infection is widely recognized as the key contributor to high rates of stomach cancer mortality[48]. There is abundant evidence that exposure to other risk factors (tobacco, diet, alcohol use, etc.) may have contributed to the apparent international differences in mortality rates of gastric cancer[49-51]. Also, disparities in socio-economic status could have an influence on stomach cancer mortality rates, mediated by varying exposures to infection, environmental factors, as well as barriers in accessing medical care[22,52].
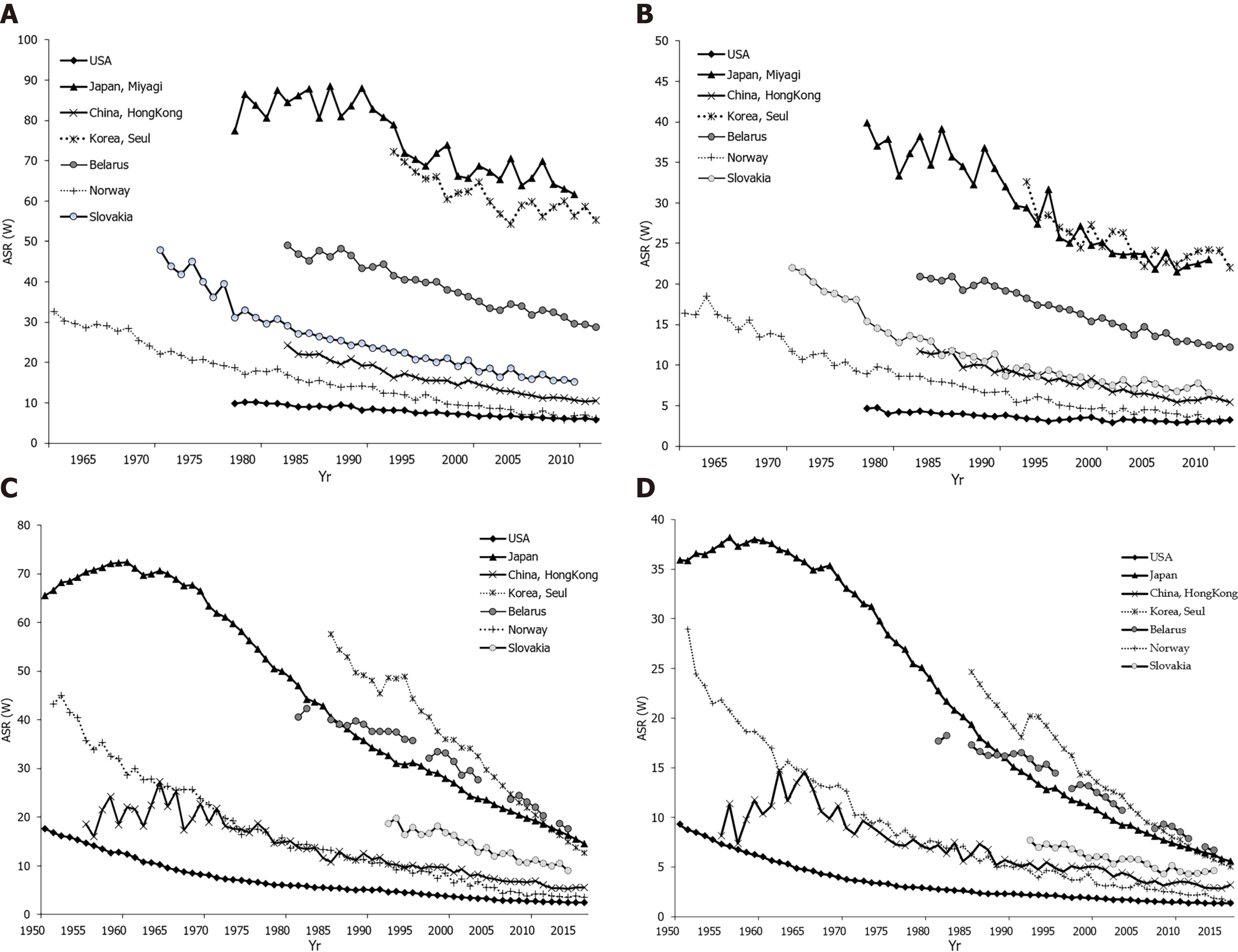
Declining gastric cancer incidence rates are the dominant epidemiological pattern globally[1,2]. Figures 3A and B show data, for males and females, on stomach cancer incidence secular trends for selected populations. In both sexes, the underlying pattern was a rapid decline in incidence rates over the whole considered time period, regardless of the background stomach cancer risk. There were two exceptions to this pattern. The first exception was seen in the Japanese population (Miyagi prefecture) where, particularly in males, very high rates were observed until the 1990s, and then declined but remained high. The second exception was for the United States population where over the entire time period the rates were constantly very low. The exact reason for the decrease in the incidence of gastric cancer in the last few decades is not completely known, but it most likely includes improvements in diet, food storage and declining prevalence of infection with H. pylori due to a general improvement in sanitation and increased use of antibiotics[53]. Eradication of H. pylori can be achieved with antibiotic therapy; but, the treatment of asymptomatic carriers is not practical because many countries have a very high infection burden (e.g., over 75% of adult persons living in sub-Saharan Africa have H. pylori infection) and reinfection is relatively easy[54,55]. Figure 3C and D represent secular trends for stomach cancer mortality, for males and females, in selected countries over the period 1961 to 2016[2]. Also, downward trends for stomach cancer mortality rates show a very similar pattern as well as incidence trends. In men, the steep decreasing trends for stomach cancer mortality were observed in all selected countries continuously over the observed period. Two exceptions to the mortality pattern were seen. The first exception was for Slovakia where, particularly in women, the rates showed a slower downward trend up to the 2000s, with a flattening of the mortality trend from the 2010s onwards. The second exception was for the United States population where mortality rates remained constantly very low over the entire time period. Stomach cancer mortality in both women and men has shown a significant declining trend in most developed countries over the past 50 years[1,2]. A similar trend, although starting later, has been seen in some countries in Asia, such as Japan and China[37,50]. Factors that led to a decline in mortality involve increased availability of fresh fruits and vegetables, reduced use of salt, reduced incidence of H. pylori infection due to improved hygiene and use of antibiotics, and the implementation of screening programs[56,57].
By age, incidence and mortality patterns for stomach cancer in women were broadly similar to those in men, regardless of the background stomach cancer risk being high or low (Figure 4). In selected countries, stomach cancer was predominantly a disease of the elderly, and almost 90% of all cases were diagnosed after the age of 55 years (Figure 4A). For both sexes, stomach cancer mortality continuously increases with age, and is two times higher in those older than 70 years (Figure 4B).
The favorable trend of stomach cancer incidence in developed countries could largely be attributed to a decrease in H. pylori prevalence: this is reflected by the “birth cohort effect” where in some countries (including Korea, Japan, the United States) rates of H. pylori have been declining in younger generations[24,34,38]. Intestinal adenocarcinoma dominates high-risk areas and is considered responsible for much of the variation in incidence. Recent studies indicate an increase in gastric cancer incidence (cardia and noncardia stomach cancers combined) in persons under the age of 50 in both low- and high-risk countries (such as the United Kingdom, the United States, Canada, Belarus, Chile)[58]. The increasing prevalence of autoimmune gastritis and dysbiosis of the stomach microbiome could have contributed to the increase in stomach cancer incidence among younger generations[59].
In both men and women, trends in the prevalence of cigarette smoking are related to trends in incidence and mortality of stomach cancer with a lag of roughly several decades[22,60]. Besides, stomach cancer mortality trends were minimally influenced by changes in the coding of this disease in the second half of the twentieth century[61].
In general, survival for patients with stomach cancer is poor[5,62]. In addition, there is global variation in stomach cancer survival[34,47]. Worldwide, with the exception of Japan and Korea, most areas have an overall 5-year relative survival of stomach cancer of about 20%-30%[63]. A 5-year relative stomach cancer survival rate of about 20% is observed in Western developed countries and in developing countries according to an international comparison of data from population based cancer registries[64-66]. In contrast to North America and Europe[66], stomach cancer survival is higher in East Asia: e.g., 5-year survival rate is 67% in Korea and 69% in Japan[67,68], followed by Jordan (56%) and Costa Rica (46%) in 2010-2014[69]. Also, a notable increase in stomach cancer survival was seen in China (from 30.2% to 35.9%) in recent years[69]. These differences are in part explained by the early stomach cancer detection due to the screening programs implemented in East Asia[63]. The relatively high overall survival for stomach cancer in Japan is the result of a high proportion of patients being diagnosed in the early stage of the disease: in 1995-2000, 53% of stomach cancers were diagnosed at an early stage in Japan[70] in contrast to about 27% in the United States[71]. Differences in tumor biology and stomach cancer subtype (with East/Central Asia and Eastern Europe having a larger proportion of noncardia stomach cancers than North America and Western Europe) may also contribute to survival differences[8,46,72,73]. Worldwide, the proportion of cardia stomach cancers, indicating worse prognosis, ranged from 6% in South Korea to 72% in Finland for men, and for women it ranged from 4% in South Korea to 52% in Serbia[38]. Similarly, cases with the intestinal subtype had a higher survival rate than patients with diffuse tumors[70]. Many factors influence the survival of stomach cancer, including the type of cancer, stage at diagnosis, age, sex, race, overall health, and lifestyle[74-76]. Generally, due to high aggressiveness and heterogeneity, stomach cancer still remains a severe global health problem[47,77].
Stomach cancer is a multifactorial disease. The notable international variation, time trends, and the migratory effect on stomach cancer frequency suggest that environmental and lifestyle factors are very important in the development of this disease.
In 1994, the International Agency for Research on Cancer has classified H. pylori infection as a carcinogen in humans due to the evidence which links H. pylori infection and risk of gastric cancer[48]. H. pylori as a carcinogen most likely acts indirectly, by causing gastritis, which is a precursor to stomach atrophy, metaplasia, and dysplasia. While the risk of stomach cancer correlated with the duration of H. pylori infection, no association was found for the histologic subtype of stomach cancer (intestinal or diffuse), or sex. Based on a meta-analysis of cohort studies, the risk of stomach cancer in people with H. pylori infection was 2.36[78]. Chronic or recurrent H. pylori infection is a major cause of stomach cancer; the relative risk is estimated to be 2.7-3.8 for cancer of cardia, and 1.1-11.1 for noncardia stomach cancer[20]. H. pylori infection is attributed to 592000 (63.4%) of all cases of stomach cancer globally[26]. H. pylori is a stomach colonizing bacterium; how H. pylori is transmitted has not been elucidated definitely, but the person-to-person pathway is most likely a contact pathway[48]. H. pylori infection is acquired in childhood, and population prevalence is associated with socioeconomic status[79]. High prevalence of H. pylori infection, and little international variation, suggest that other factors are important in the etiology of gastric cancer[53]. The main risk factors for noncardia stomach cancer are H. pylori infection, tobacco smoking and dietary factors, while gastroesophageal reflux disease, obesity and possibly tobacco smoking play an important role in the development of cardia gastric cancer[6,9-13,22].
The main cofactors responsible for the development of stomach cancer are smoking and diet[30,31,80-82]. After adjusting for alcohol intake or the presence of chronic H. pylori infection in the stomach, an independent association with smoking was confirmed[83,84]. Over 45 case-control studies and 27 cohort studies confirmed the association of tobacco with stomach cancer, with the average relative risk being RR = 1.5-2.0[85]. One recent meta-analysis of prospective observational studies suggests that the summary relative risk was higher in men (1.63) than in women (1.30)[31]. The risk of stomach cancer increases significantly with cigarette smoking (40% for smokers and 82% for heavy smokers) and alcohol consumption[86]. It is estimated that in developing countries the gastric cancer risk attributable to smoking is 11% in men and 4% in women, while in developed countries the risk is 17% in men and 11% in women[85,87].
While some authors believe that diet has no role in the etiology of gastric cancer, the American Cancer Society states that smoked foods, salted fish and meat, and pickled vegetables represent risk factors for gastric cancer[88]. Some bacteria, like H. pylori, can convert nitrates and nitrites (commonly found in meat products) to substances which are shown to cause gastric cancer in animals[48,89]. It is also known that adherence to the Mediterranean diet is significantly inversely correlated with gastric cancer[89,90].
The correlation between salt intake (high in salt, smoked foods, salted fish and meat) and stomach cancer risk has been indicated in several epidemiological studies[91-94]. Sodium chloride is known to increase gastrocarcinogenesis using N-methyl-N-nitro-N-nitrosoguanidine in a rat experiment[95], as well as in a human study[96]. The mucin layer which covers and protects the stomach epithelium is damaged by high doses of salt, which also cause high osmotic pressure that further damages epithelial cells. Prolonged damage to the mucous membrane leads to chronic atrophic gastritis and intestinal metaplasia, which are precursors for stomach cancer.
Higher consumption of fruits and vegetables has been associated with a lower risk of malignant tumors in a number of epidemiological studies (over 200 case-control and cohort studies)[97,98], while results are particularly numerous and consistent for stomach cancer[99]. The intake of fresh fruits and vegetables, which contain antioxidant vitamins (e.g. vitamins A and C), reduces gastric cancer risk. In a cohort of 900000 adults (404576 men and 495477 women) who were not diagnosed with malignancy at the time of enrollment, 57145 people died after 16 years, with the highest weight subjects having a higher mortality rate from malignant tumors in general: men had a 52% higher rate, and women a 62% higher rate, compared to people with normal body weight[100]. Higher mortality was found for esophageal, colon, liver, gallbladder, pancreatic and kidney cancer, but also for non-Hodgkin's lymphoma and multiple myeloma.
Patients with gastroesophageal reflux disease (GERD), especially with the long-standing forms, had a significantly increased risk for cardia stomach cancer and the majority of studies noted a 2-4-fold increase in risk[10,101,102], although not all[103]. One of the explanations for the association between GERD and cardia gastric cancer is that GERD may cause metaplasia with potential progression to adenocarcinoma[104]. On the other hand, a lack of association between GERD and noncardia stomach cancer might be explained, at least in part, by the association with atrophic gastritis which might be associated with a decrease in gastric acid secretion and lower risk of GERD[105].
Of the demographic factors, socio-economic status, older age and male gender play an important role[9-11]. Socio-economic deprivation, within any population, is consistently linked with increased gastric cancer risk[106,107]. The risk of developing stomach cancer increases with age[7,34]. Stomach cancer rarely develops before the age of 40, more than 80% of stomach cancers occur between 60 and 80 years of age. Stomach cancer affects men more than women[1-3]. The consistency of risk difference by sex has never been adequately explained although possible explanations included differences in environmental exposures and lifestyle factors, as well as the theory regarding the potentially protective role of female sex-specific hormones[108]. According to the results of studies conducted in the United States, stomach cancer occurs more often in African Americans compared to the white population[7]. Possible reasons for this increased risk include socioeconomic factors, prevalence of H. pylori infection, cigarette smoking, and obesity[7,22,109,110].
It is estimated that around 10% of stomach cancer cases aggregate in families, and only 1%-3% are hereditary[10,13,111]. A positive family history of stomach cancer in a first-degree relative is a risk factor for stomach cancer, but the magnitude of risk varies with different ethnic groups and geographic regions, ranging from 2 to 10[112,113]. Although familial aggregation could be a risk factor because of shared genetic factors, the influence of shared environment cannot be ruled out, e.g., passage of H. pylori infection from parents to children, the same dietary factors, etc. Although migrant studies indicate a significant reduction in the risk of gastric cancer in Japanese immigrants, the results of many studies point out that exposure to environmental factors in childhood is important for determining gastric cancer risk[48,53,79]. Namely, migrant studies show that exposure in childhood is important in stomach cancer etiology: e.g. infection with H. pylori often occurs before the age of 10, i.e. often before the migration, also children born in the immigrant country are likely to acquire the infection from family members who have migrated from their native country[114].
Gastric cancer risk is increased in many genetic disorders, such as hereditary diffuse gastric cancer, Peutz-Jeghers syndrome, and familial adenomatous polyposis[10,112]. Persons who have mutations or deletion in genes such as p53, BRCA2, MSH2, and MLH1, have an increased risk of stomach cancer[10,111].
Some studies found a link between stomach cancer and antioxidant use, non-steroidal anti-inflammatory drugs, statins, physical activity, and radiation[6,9-13]. Some researchers have suggested a correlation between the excess or deficit of iodine, goiter, and stomach cancer, as well as a decrease in stomach cancer mortality after performing effective iodine prophylaxis[115].
Other potential risk factors in relation to stomach cancer include poor oral hygiene and tooth loss[116], hookah and opium use[117], Epstein-Barr virus infection[118], and consumption of pickled vegetables[119], but the results are not convincing, at least not yet. In addition, in many persons with stomach cancer there is no one specific stomach cancer risk factor.
During the past century, Western developed countries experienced a major reduction in stomach cancer incidence and mortality, without the introduction of specific primary and secondary prevention measures. Generally, favorable trends in the frequency of stomach cancer are thought to be an important part a consequence of changes such as the reduction in the use of salt and an increase in the consumption of fruit and fresh vegetables due to improvements in food storage (refrigerators, freezers). This phenomenon has been dubbed the “unplanned triumph” of prevention[9,39].
Primary and secondary prevention strategies are the focus of stomach cancer prevention.
Primary prevention measures involve improvements in environment and lifestyle habits such as tobacco control/smoking cessation, reducing salt intake, increasing fruit and vegetable intake, developing other healthy behaviors (such as Mediterranean diet, higher intake of fiber, physical activity), H. pylori eradication, other medications (intake of non-steroidal anti-inflammatory drugs, statins), refraining from high alcoholic beverages, sanitation and hygiene improvements. The WHO has set a global goal of reducing the intake of salt to less than 5 g (2000 mg of sodium) per person per day by the year 2025[120]. A meta-analysis of randomized trials (all trials were performed in areas with a high incidence of stomach cancer, mostly in Asia), in a total of 6695 participants followed from 4 to 10 years showed that the risk of stomach cancer can be reduced by 35% with the treatment of H. pylori[121]. In addition to endoscopic and histological surveillance, the American and European guidelines recommend eradication of H. pylori in all persons who have atrophy and/or intestinal metaplasia and all persons who are first-degree relatives of stomach cancer patients[122,123]. According to the Asian Pacific Gastric Cancer Consensus, population-based screening and treatment of H. pylori infection is recommended in regions which have an annual stomach cancer incidence of more than 20/100000[124]. Eradication of H. pylori can be achieved with antibiotic therapy; but, the treatment of asymptomatic carriers is not practical as many countries have a very high infection burden (e.g., over 75% of adult persons living in sub-Saharan Africa have H. pylori infection) and reinfection is relatively easy[54].
Japan has had a national endoscopic surveillance program since the early 1970s because of the high stomach cancer risk[125]. It is recommended that all people older than 40 years undergo screening with a double-contrast barium X-ray radiography and endoscopy every year[126]. A study in China demonstrated that a preventive intervention which included eradication of H. pylori, nutritional supplements, and screening (with double-contrast radiography and endoscopy) resulted in a 49% reduction in relative risk for overall mortality in a high-risk group of individuals[127].
Upper gastrointestinal endoscopy is the gold standard for stomach cancer diagnosis and due to its high detection rate it is used for stomach cancer screening in high-risk areas (such as Japan, Korea, Venezuela and other areas), but the available evidence shows that endoscopic surveillance of premalignant gastric lesions showed conflicting results[128]. Besides, the procedure is expensive, unpleasant for the patient and carries a risk of hemorrhage and perforation[129,130].
Stomach cancer screening might be possible via the detection of potential markers of gastric atrophy (a stomach cancer precursor lesion)[125,131,132], including serum pepsinogens, serum ghrelin, H. pylori serum antibodies, gastrin-17, or antigastric parietal cell antibodies, but the results are not convincing, at least not yet.
Worldwide, stomach cancer incidence and mortality have declined significantly during the past five decades. However, stomach cancer remains a global health problem as the fifth leading cancer and fourth most common cause of cancer-related deaths in the world. Further illumination of risk factors can help identify various opportunities for prevention. Primary and secondary prevention strategies with more effectiveness are needed in order to reduce stomach cancer incidence and mortality, particularly in populations with a high burden of stomach cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Public, environmental and occupational health
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang X S-Editor: Zhang H L-Editor: Webster JR P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2969] [Article Influence: 742.3] [Reference Citation Analysis (7)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 4. | Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 6. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 7. | Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2018, National Cancer Institute. 2021 Apr 15 [cited 22 June 2021]. Bethesda, MD, based on November 2020 SEER data submission, posted to the SEER web sit. Available from: https://seer.cancer.gov/csr/1975_2018/. |
| 8. | Asplund J, Kauppila JH, Mattsson F, Lagergren J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann Surg Oncol. 2018;25:2693-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Collatuzzo G, Pelucchi C, Negri E, López-Carrillo L, Tsugane S, Hidaka A, Shigueaki Hamada G, Hernández-Ramírez RU, López-Cervantes M, Malekzadeh R, Pourfarzi F, Mu L, Zhang ZF, Lunet N, La Vecchia C, Boffetta P. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: A pooled analysis in the Stomach cancer Pooling (StoP) Project. Int J Cancer. 2021;149:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 10. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1328] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 11. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 12. | Deng W, Jin L, Zhuo H, Vasiliou V, Zhang Y. Alcohol consumption and risk of stomach cancer: A meta-analysis. Chem Biol Interact. 2021;336:109365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 13. | Lv L, Liang X, Wu D, Wang F, Zhang Y, Cang H, Deng X, Li M. Is cardia cancer a special type of gastric cancer? J Cancer. 2021;12:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Avital I, Stojadinovic A, Pisters PWT, Kelsen DP, Willett CG. Cancer of the Stomach. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 10th ed. Philadelphia, PA: Wolters Kluwer; 2015, 613-642. |
| 15. | Yao Q, Qi X, Xie SH. Sex difference in the incidence of cardia and non-cardia gastric cancer in the United States, 1992-2014. BMC Gastroenterol. 2020;20:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Jin H, Pinheiro PS, Xu J, Amei A. Cancer incidence among Asian American populations in the United States, 2009-2011. Int J Cancer. 2016;138:2136-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Shah SC, McKinley M, Gupta S, Peek RM Jr, Martinez ME, Gomez SL. Population-Based Analysis of Differences in Gastric Cancer Incidence Among Races and Ethnicities in Individuals Age 50 Years and Older. Gastroenterology. 2020;159:1705-1714.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut. 2014;63:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1-441. [PubMed] |
| 21. | Lyons K, Le LC, Pham YT, Borron C, Park JY, Tran CTD, Tran TV, Tran HT, Vu KT, Do CD, Pelucchi C, La Vecchia C, Zgibor J, Boffetta P, Luu HN. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev. 2019;28:397-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 23. | Pabla BS, Shah SC, Corral JE, Morgan DR. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:347-359.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer. 2017;141:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Eslick GD. Helicobacter pylori infection causes gastric cancer? World J Gastroenterol. 2006;12:2991-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2599] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 27. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2051] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 28. | Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 31. | Li WY, Han Y, Xu HM, Wang ZN, Xu YY, Song YX, Xu H, Yin SC, Liu XY, Miao ZF. Smoking status and subsequent gastric cancer risk in men compared with women: a meta-analysis of prospective observational studies. BMC Cancer. 2019;19:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 32. | Watts EL, Perez-Cornago A, Knuppel A, Tsilidis KK, Key TJ, Travis RC. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. Int J Cancer. 2021;149:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Lui FH, Tuan B, Swenson SL, Wong RJ. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992-2009 SEER data. Dig Dis Sci. 2014;59:3027-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Balakrishnan M, George R, Sharma A, Graham DY. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep. 2017;19:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 35. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 858] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 36. | Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S; JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118:2315-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 39. | Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 544] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 40. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4902] [Article Influence: 700.3] [Reference Citation Analysis (1)] |
| 41. | Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171-177. [PubMed] [DOI] [Full Text] |
| 42. | Shibata A, Parsonnet J. Stomach cancer. In: Schottenfeld D, FraumeniJF. Cancer epidemiology and prevention, 3rd ed. New York, USA: Oxford University Press, 2006. |
| 43. | Zhang X, Li M, Chen S, Hu J, Guo Q, Liu R, Zheng H, Jin Z, Yuan Y, Xi Y, Hua B. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology. 2018;155:347-354.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 44. | Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, Jung KW, Lee CW, Choi IJ, Park EC, Lee D. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology. 2017;152:1319-1328.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 45. | Tanaka K, Kiyohara Y, Kubo M, Matsumoto T, Tanizaki Y, Okubo K, Ninomiya T, Oishi Y, Shikata K, Iida M. Secular trends in the incidence, mortality, and survival rate of gastric cancer in a general Japanese population: the Hisayama study. Cancer Causes Control. 2005;16:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Anderson LA, Tavilla A, Brenner H, Luttmann S, Navarro C, Gavin AT, Holleczek B, Johnston BT, Cook MB, Bannon F, Sant M; EUROCARE-5 Working Group:. Survival for oesophageal, stomach and small intestine cancers in Europe 1999-2007: Results from EUROCARE-5. Eur J Cancer. 2015;51:2144-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 48. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Helicobacter Pylori. IARC Monographs-100B. [cited 2 June 2021]. Available from: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-15.pdf. |
| 49. | Lindblad M, Rodríguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 50. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 51. | Plummer M, Franceschi S, Muñoz N. Epidemiology of gastric cancer. IARC Sci Publ. 2004;311-326. [PubMed] |
| 52. | Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, Cook P, Anderson G, Morgan DR, Baker LH, Greenberg ER, Herrero R. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013;24:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Parkin DM. International variation. Oncogene. 2004;23:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 54. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 55. | Fall K, Ye W, Nyrén O. Antibiotic treatment and risk of gastric cancer. Gut. 2006;55:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 57. | Ness-Jensen E, Bringeland EA, Mattsson F, Mjønes P, Lagergren J, Grønbech JE, Waldum HL, Fossmark R. Hypergastrinemia is associated with an increased risk of gastric adenocarcinoma with proximal location: A prospective population-based nested case-control study. Int J Cancer. 2021;148:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? Gut. 2020;69:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 59. | Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr, Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst. 2018;110:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 60. | Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 61. | Pérez-Gómez B, Aragonés N, Pollán M, Suárez B, Lope V, Llácer A, López-Abente G. Accuracy of cancer death certificates in Spain: a summary of available information. Gac Sanit. 2006;20 Suppl 3:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 63. | Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 395] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 64. | Verdecchia A, Corazziari I, Gatta G, Lisi D, Faivre J, Forman D; EUROCARE Working Group. Explaining gastric cancer survival differences among European countries. Int J Cancer. 2004;109:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Strong VE, Wu AW, Selby LV, Gonen M, Hsu M, Song KY, Park CH, Coit DG, Ji JF, Brennan MF. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 66. | Lepage C, Sant M, Verdecchia A, Forman D, Esteve J, Faivre J; EUROCARE working group. Operative mortality after gastric cancer resection and long-term survival differences across Europe. Br J Surg. 2010;97:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 68. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 69. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3429] [Article Influence: 489.9] [Reference Citation Analysis (1)] |
| 70. | Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, Clarke CA. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol. 2012;30:3507-3515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK. SEER Cancer Statistics Review, 1975-2001, National Cancer Institute. [cited 2 June 2021]. Available from: https://seer.cancer.gov/csr/1975_2001/. |
| 72. | Lagergren J, Mattsson F. Diverging trends in recent population-based survival rates in oesophageal and gastric cancer. PLoS One. 2012;7:e41352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 74. | Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol. 2013;24:2699-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Tao L, Wang R, Gao YT, Yuan JM. Impact of postdiagnosis smoking on long-term survival of cancer patients: the Shanghai cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22:2404-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Li H, Wei Z, Wang C, Chen W, He Y, Zhang C. Gender Differences in Gastric Cancer Survival: 99,922 Cases Based on the SEER Database. J Gastrointest Surg. 2020;24:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 77. | Gao JP, Xu W, Liu WT, Yan M, Zhu ZG. Tumor heterogeneity of gastric cancer: From the perspective of tumor-initiating cell. World J Gastroenterol. 2018;24:2567-2581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (4)] |
| 78. | Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 79. | Alvarado-Esquivel C. Seroepidemiology of Helicobacter pylori Infection in Tepehuanos Aged 15 Years and Older in Durango, Mexico. J Pathog. 2013;2013:243246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Ferro A, Morais S, Rota M, Pelucchi C, Bertuccio P, Bonzi R, Galeone C, Zhang ZF, Matsuo K, Ito H, Hu J, Johnson KC, Yu GP, Palli D, Ferraroni M, Muscat J, Malekzadeh R, Ye W, Song H, Zaridze D, Maximovitch D, Aragonés N, Castaño-Vinyals G, Vioque J, Navarrete-Muñoz EM, Pakseresht M, Pourfarzi F, Wolk A, Orsini N, Bellavia A, Håkansson N, Mu L, Pastorino R, Kurtz RC, Derakhshan MH, Lagiou A, Lagiou P, Boffetta P, Boccia S, Negri E, La Vecchia C, Peleteiro B, Lunet N. Tobacco smoking and gastric cancer: meta-analyses of published data versus pooled analyses of individual participant data (StoP Project). Eur J Cancer Prev. 2018;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Du S, Li Y, Su Z, Shi X, Johnson NL, Li P, Zhang Y, Zhang Q, Wen L, Li K, Chen Y, Zhang X, Fei Y, Ding X. Index-based dietary patterns in relation to gastric cancer risk: a systematic review and meta-analysis. Br J Nutr. 2020;123:964-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology. 2011;22:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | González CA, López-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scand J Gastroenterol. 2010;45:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Moy KA, Fan Y, Wang R, Gao YT, Yu MC, Yuan JM. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2010;19:2287-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 85. | Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 86. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1-1438. [PubMed] |
| 87. | Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, Epstein S, Belpomme D. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 88. | American Cancer Society. Stomach cancer, 2021. Atlanta, GA: American Cancer Society; 2021. [cited 2 June 2021]. Available from: http://www.cancer.org/acs/groups/cid. |
| 89. | Assaad S, Chaaban R, Tannous F, Costanian C. Dietary habits and Helicobacter pylori infection: a cross sectional study at a Lebanese hospital. BMC Gastroenterol. 2018;18:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Willett WC. Diet and cancer. Oncologist. 2000;5:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Fang X, Wei J, He X, An P, Wang H, Jiang L, Shao D, Liang H, Li Y, Wang F, Min J. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 92. | Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90:128-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 94. | Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 95. | Velmurugan B, Bhuvaneswari V, Burra UK, Nagini S. Prevention of N-methyl-N'-nitro-N-nitrosoguanidine and saturated sodium chloride-induced gastric carcinogenesis in Wistar rats by lycopene. Eur J Cancer Prev. 2002;11:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Thapa S, Fischbach LA, Delongchamp R, Faramawi MF, Orloff M. The Association between Salt and Potential Mediators of the Gastric Precancerous Process. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res. 2015;59:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Wan Q, Li N, Du L, Zhao R, Yi M, Xu Q, Zhou Y. Allium vegetable consumption and health: An umbrella review of meta-analyses of multiple health outcomes. Food Sci Nutr. 2019;7:2451-2470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Bae JM, Kim EH. Dietary intakes of citrus fruit and risk of gastric cancer incidence: an adaptive meta-analysis of cohort studies. Epidemiol Health. 2016;38:e2016034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 101. | Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari AA, McColl KE. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, Webb PM, Green AC; Australian Cancer Study. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 103. | Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF Jr. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 104. | Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 105. | Figueroa JD, Terry MB, Gammon MD, Vaughan TL, Risch HA, Zhang FF, Kleiner DE, Bennett WP, Howe CL, Dubrow R, Mayne ST, Fraumeni JF Jr, Chow WH. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control. 2009;20:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Naess O, Claussen B, Thelle DS, Smith GD. Four indicators of socioeconomic position: relative ranking across causes of death. Scand J Public Health. 2005;33:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 108. | Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 109. | Epplein M, Signorello LB, Zheng W, Peek RM Jr, Michel A, Williams SM, Pawlita M, Correa P, Cai Q, Blot WJ. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev. 2011;20:826-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 110. | Gupta S, Tao L, Murphy JD, Camargo MC, Oren E, Valasek MA, Gomez SL, Martinez ME. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology. 2019;156:59-62.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 111. | Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114-33; discussion 133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 112. | Foschi R, Lucenteforte E, Bosetti C, Bertuccio P, Tavani A, La Vecchia C, Negri E. Family history of cancer and stomach cancer risk. Int J Cancer. 2008;123:1429-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Yaghoobi M, McNabb-Baltar J, Bijarchi R, Hunt RH. What is the quantitative risk of gastric cancer in the first-degree relatives of patients? World J Gastroenterol. 2017;23:2435-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 114. | Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, Yamaoka Y, Berenson GS. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 115. | Gołkowski F, Szybiński Z, Rachtan J, Sokołowski A, Buziak-Bereza M, Trofimiuk M, Hubalewska-Dydejczyk A, Przybylik-Mazurek E, Huszno B. Iodine prophylaxis--the protective factor against stomach cancer in iodine deficient areas. Eur J Nutr. 2007;46:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Shakeri R, Malekzadeh R, Etemadi A, Nasrollahzadeh D, Abedi-Ardekani B, Khoshnia M, Islami F, Pourshams A, Pawlita M, Boffetta P, Dawsey SM, Kamangar F, Abnet CC. Association of tooth loss and oral hygiene with risk of gastric adenocarcinoma. Cancer Prev Res (Phila). 2013;6:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Sadjadi A, Derakhshan MH, Yazdanbod A, Boreiri M, Parsaeian M, Babaei M, Alimohammadian M, Samadi F, Etemadi A, Pourfarzi F, Ahmadi E, Delavari A, Islami F, Farzadfar F, Sotoudeh M, Nikmanesh A, Alizadeh BZ, de Bock GH, Malekzadeh R. Neglected role of hookah and opium in gastric carcinogenesis: a cohort study on risk factors and attributable fractions. Int J Cancer. 2014;134:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 118. | Tavakoli A, Monavari SH, Solaymani Mohammadi F, Kiani SJ, Armat S, Farahmand M. Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20:493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 119. | Yoo JY, Cho HJ, Moon S, Choi J, Lee S, Ahn C, Yoo KY, Kim I, Ko KP, Lee JE, Park SK. Pickled Vegetable and Salted Fish Intake and the Risk of Gastric Cancer: Two Prospective Cohort Studies and a Meta-Analysis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Cappuccio FP, Capewell S, Lincoln P, McPherson K. Policy options to reduce population salt intake. BMJ. 2011;343:d4995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 122. | Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Mégraud F, Xiao SD, Sugano K, Nyrén O; Lejondal H. pylori-Gastric Cancer Task Force. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 123. | Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 124. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, Jung HC, Hoang BH, Kachintorn U, Goh KL, Chiba T, Rani AA; Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 125. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 126. | Kakizoe T. Cancer Statistics in Japan. Tokyo, Japan: Foundation for Promotion of Cancer Research, 1999. |
| 127. | Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS, Yuan Y. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol. 2003;9:432-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Areia M, Carvalho R, Cadime AT, Rocha Gonçalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013;18:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 129. | Yao MD, von Rosenvinge EC, Groden C, Mannon PJ. Multiple endoscopic biopsies in research subjects: safety results from a National Institutes of Health series. Gastrointest Endosc. 2009;69:906-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 130. | Ismaila BO, Misauno MA. Gastrointestinal endoscopy in Nigeria--a prospective two year audit. Pan Afr Med J. 2013;14:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Abnet CC, Zheng W, Ye W, Kamangar F, Ji BT, Persson C, Yang G, Li HL, Rothman N, Shu XO, Gao YT, Chow WH. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women's Health Study Cohort. Br J Cancer. 2011;104:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Murphy G, Kamangar F, Dawsey SM, Stanczyk FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Albanes D, Freedman ND. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst. 2011;103:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |